Abstract
The ability to resist copper toxicity is important for microbial pathogens to survive attack by innate immune cells. A sur7Δ mutant of the fungal pathogen Candida albicans exhibits decreased virulence that correlates with increased sensitivity to copper, as well as defects in other stress responses and morphogenesis. Previous studies indicated that copper kills sur7Δ cells by a mechanism distinct from the known resistance pathways involving the Crp1 copper exporter or the Cup1 metallothionein. Since Sur7 resides in punctate plasma membrane domains known as MCC/eisosomes, we examined overexpression of SUR7 and found that it rescued the copper sensitivity of a mutant that fails to form MCC/eisosomes (pil1Δ lsp1Δ), indicating that these domains act to facilitate Sur7 function. Genetic screening identified new copper-sensitive mutants, the strongest of which were similar to sur7Δ in having altered plasma membranes due to defects in membrane trafficking, cortical actin, and morphogenesis (rvs161Δ, rvs167Δ, and arp2Δ arp3Δ). Consistent with the mutants having altered plasma membrane organization, they were all more readily permeabilized by copper, which is known to bind phosphatidylserine and phosphatidylethanolamine and cause membrane damage. Although these phospholipids are normally localized to the intracellular leaflet of the plasma membrane, their exposure on the surface of the copper-sensitive mutants was indicated by increased susceptibility to membrane damaging agents that bind to these phospholipids. Increased copper sensitivity was also detected for a drs2Δ mutant, which lacks a phospholipid flippase that is involved in maintaining phospholipid asymmetry. Copper binds phosphatidylserine with very high affinity, and deleting CHO1 to prevent phosphatidylserine synthesis rescued the copper sensitivity of sur7Δ cells, confirming a major role for phosphatidylserine in copper sensitivity. These results highlight how proper plasma membrane architecture protects fungal pathogens from copper and attack by the immune system, thereby opening up new avenues for therapeutic intervention.
Author summary
The transition metal copper is used by the innate immune system to attack microbial pathogens. To better understand how the human fungal pathogen Candida albicans resists this type of stress, we screened for mutants that were more susceptible to killing by copper. Interestingly, we identified a new class of copper-sensitive mutants whose plasma membranes are more readily permeabilized by copper. The common characteristic of these new copper-sensitive mutants is that they have an altered cell surface, which weakened their resistance to copper. These results help to explain the toxic effects of copper and suggest novel therapeutic strategies for fungal infections.
Introduction
The human fungal pathogen C. albicans typically grows as a commensal organism on human mucosa. However, C. albicans can cause severe mucosal infections or lethal systemic infections when the immune system is impaired [1, 2]. Serious infections also occur when conditions promote an overgrowth of C. albicans that overwhelms the immune system. This can happen as a consequence of the use of antibacterial antibiotics that disrupt the microbiota or as the result of biofilm formation on medical devices and catheters. In order to survive in a human host, C. albicans must be able to resist a wide range of stressful conditions promoted by the immune system. This includes elevated temperature, antimicrobial peptides, oxidation, and nitrosylation [3–5]. Copper has recently been recognized as a form of stress encountered by microbes in vivo [6–8], and C. albicans cells have been reported to experience copper stress at sites of infection [9, 10]. For example, stimulation of macrophages with interferon-γ leads to increased expression of the copper importer CTR1 and translocation of the ATP7A copper transporter to the phagosomal membrane where it pumps copper into the phagosome [11]. Copper can react with H2O2 produced by the oxidative burst in phagosomes to form a broader array of damaging reactive oxygen species [6, 12, 13].
Cells have a complex relationship with copper in that excess copper is toxic, but too little copper is also bad, as cells require this metal as an essential cofactor for many enzymes. The ability of copper to transition between cuprous (Cu1+) and cupric (Cu2+) oxidation states facilitates the catalysis of many important electron transfer reactions in the cell. Fungal cells therefore use several mechanisms to tightly regulate the levels of copper such that there appears to be less than one free copper molecule per cell [14, 15]. One mechanism is that excess copper inactivates the Mac1 transcription factor to turn off expression of the copper importer CTR1, thereby decreasing copper uptake [16]. Once inside the cell, intracellular copper is typically sequestered by a chaperone protein for delivery to an appropriate metalloprotein, such as the role of Ccs1 in delivering copper to the Sod1 superoxide dismutase [17]. Excess cytoplasmic copper is bound by scavenger proteins, such as the Cup1 and Crd2 metallothionein proteins in C. albicans, or stored in the vacuole [18–21]. In Cryptococcus neoformans, metallothioneins play a key role in copper resistance and are important for virulence [7, 22]. In C. albicans, the major role in resisting copper is carried out by Crp1, a plasma membrane exporter which pumps excess copper out of the cell, while metallothioneins play a less important role [18, 19]. Consistent with this, CRP1 is important for virulence of C. albicans [9]. The C. albicans copper resistance genes CUP1 and CRP1 are induced in response to excess copper by the Cup2 transcription factor [18, 19, 23, 24], and copper resistance also appears to be regulated by the PKA pathway [25].
Recent studies revealed that the Sur7 plasma membrane protein promotes resistance of C. albicans to copper by a mechanism that is distinct from the known pathways mediated by Crp1 and Cup1 [26]. Sur7 is a tetraspan integral membrane protein that localizes to punctate patches in the plasma membrane known as MCC domains, each of which is associated with a complex of cytoplasmic proteins known as an eisosome [27–29]. These domains correspond to ~250 nm long furrows in the plasma membrane created by the Pil1 and Lsp1 proteins [30–32]. The sur7Δ mutant showed strong defects in virulence and a decreased ability to grow in macrophages that correlated with increased sensitivity to copper [26]. The sur7Δ mutant has additional defects in morphogenesis, plasma membrane, and cell wall composition that likely contribute to the virulence defects [31, 33–35].
Many of the toxic effects of copper are attributed to its redox properties, which can lead to oxidation of protein, lipids and DNA [6–8, 36]. Excess copper can also inhibit growth of microbes by having direct toxic effects on a wide range of macromolecules, especially those containing thiols, such as the iron sulfur cluster proteins [6, 7, 37]. Previous studies showed that copper can permeabilize the plasma membranes of diverse cell types, including bacterial, fungal, plant, and animal [12, 36, 38–41]; however, the mechanism is not understood. To better define the novel process by which Sur7 promotes resistance to copper, we used genetic approaches to screen for C. albicans mutants that are more susceptible to killing by copper. The strongest mutants identified are similar to sur7Δ cells in having defects in membrane trafficking and cortical actin organization that cause broad changes in plasma membrane architecture (pil1Δ lsp1Δ, rvs161Δ, rvs167Δ, and arp2Δ arp3Δ). Interestingly, these mutants were all permeabilized more readily by copper. Analysis of cell surface lipids implicated exposure of phosphatidylserine (PS) in copper sensitivity, which correlates with studies on model membranes demonstrating that copper binds PS with high affinity and promotes membrane damage [42–44]. These results provide important new insight into how plasma membrane architecture is organized to protect the cell surface from attack by copper. These results also have significance for the development of novel therapeutic approaches and for the effective use of metallic copper surfaces as an antimicrobial strategy [45, 46].
Results
MCC/eisosomes facilitate the role of Sur7 in resistance to copper
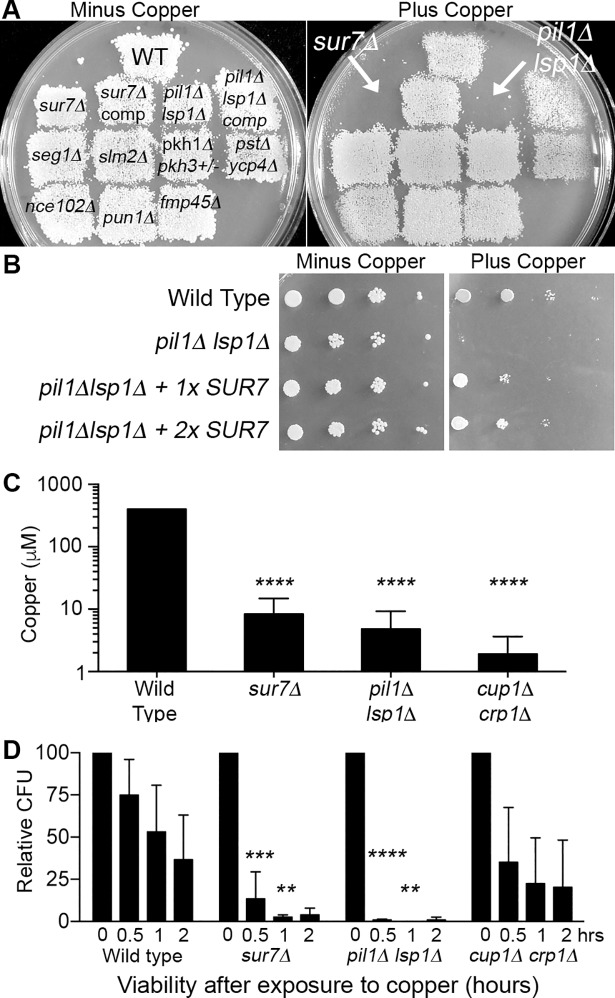
To determine whether other proteins present in the MCC/eisosome domains of the C. albicans plasma membrane contribute to copper resistance, we tested 8 different mutant strains for their ability to grow on agar medium containing 500 μM CuSO4 (Fig 1A). All of the mutants exhibited wild-type growth, except the pil1Δ lsp1Δ mutant, which was similar to the sur7Δ strain in failing to show detectable growth. Pil1 and Lsp1 are membrane binding BAR domain proteins that function to form the eisosome domains [27, 30, 32]. MCC/eisosome domains facilitate Sur7 function by promoting its stability and localization in the plasma membrane [31]. We therefore tested the effects of overexpressing SUR7 in the pil1Δ lsp1Δ mutant and found that it strongly rescued the growth of pil1Δ lsp1Δ cells on copper-containing medium (Fig 1B), similar to the way that overexpression of SUR7 was found previously to rescue many of the cell wall and morphogenesis mutant phenotypes of pil1Δ lsp1Δ cells [31]. These results indicate that Sur7 plays the key role in copper resistance, and that Pil1 and Lsp1 act to facilitate this role.
Fig 1. MCC/eisosome proteins Sur7, Pil1, and Lsp1 are important for resistance of Candida albicans to copper.
(A) C. albicans strains lacking the indicated MCC/eisosome genes were replica-plated onto minimal medium containing either 0 μM or 500 μM CuSO4 and incubated at 30°C for 48 hr. Only the sur7Δ and the pil1Δ lsp1Δ double deletion demonstrated strong sensitivity. (B) SUR7 overexpression partially rescued the copper sensitivity of the pil1Δ lsp1Δ strain. pil1Δ lsp1Δ strains containing zero, one, or two extra copies of the SUR7 gene were spotted onto minimal medium in the absence or presence of 1 mM CuSO4 and incubated for 48 hr at 30°C. (C) The highest concentration of copper at which the indicated strains could grow after incubation in synthetic medium in 96-well plates for 48 hr at 37°C with different concentrations of copper. The sur7Δ, pil1Δ lsp1Δ, and known copper sensitive mutant cup1Δ crp1Δ could only grow at lower concentrations of copper than the wild-type strain, indicating increased sensitivity. (D) Time course study of viability after exposure to copper. Cells were incubated in H2O or 0.5 μM CuSO4 at 37°C for the indicated time and then an aliquot was spread on YPD medium to determine viable colony forming units (CFU). All deletion mutants exhibited lower viability after incubation in CuSO4. Images in panel A are representative of three independent experiments, and those in panel B of two independent experiments, all performed on different days. The results in panel C represent averages of three independent experiments and those in panel D of four independent experiments, all performed on different days. Error bars indicate SD. ****, P<0.001. ***, P<0.01. **, P<0.01 by two-way ANOVA. Strains used were: WT, wild-type DIC185; sur7Δ (YJA11); sur7Δ comp. (YJA12); pil1Δ lsp1Δ (YHXW21-1); pil1Δ lsp1Δ comp (+ LSP1, YHXW23-1); seg1Δ (YLD182-9-10-2); slm2Δ (YHXW36-1); pkh1Δ pkh3Δ/PKH3 (YLD146-11-2-5); pst1Δ pst2Δ pst3Δ ycp4Δ (LLF060); nce102Δ (YHXW14); pun1Δ (YLD204-2); fmp45Δ (YHXW3), pil1Δ lsp1Δ + 1xSUR7 (YHXW45-1), pil1Δ lsp1Δ + 2xSUR7 (YHXW46-1), and cup1Δ crp1Δ (KC25).
To quantify the sensitivity to copper, mutant strains were grown in liquid cultures containing different concentrations of copper. Both the sur7Δ and the pil1Δ lsp1Δ mutants showed greatly increased sensitivity to copper, as they were inhibited by about a 100-fold lower dose of copper under these growth conditions (Fig 1C). Significantly, they were nearly as susceptible to copper as the highly sensitive cup1Δ crp1Δ mutant, which lacks both the metallothionein and the copper exporter genes [18]. The concentrations of copper needed to inhibit cell growth were affected by the growth medium, as the inclusion of components such as amino acids or yeast extract elevated the concentration of copper needed to kill cells, presumably because these media additives can chelate the copper. Therefore, these tests were done with minimal medium.
Time course studies were carried out by treating cells with 0.5 μM copper in water and then measuring the viable colony forming units (CFUs). Interestingly, whereas wild type cells slowly lost viability over a 2 hr incubation, the sur7Δ and pil1Δ lsp1Δ mutant cells rapidly lost viability as most of the cells were dead after 30 min (Fig 1D). The cup1Δ crp1Δ strain appeared to give intermediate sensitivity in this short-term assay, although variability in the CFU assays limited the statistical significance of these results. This was more readily observed in an independent set of time course assays performed under more acidic conditions that prolong the time course (S1 Fig).
Genes that function in membrane trafficking and morphogenesis affect C. albicans copper resistance
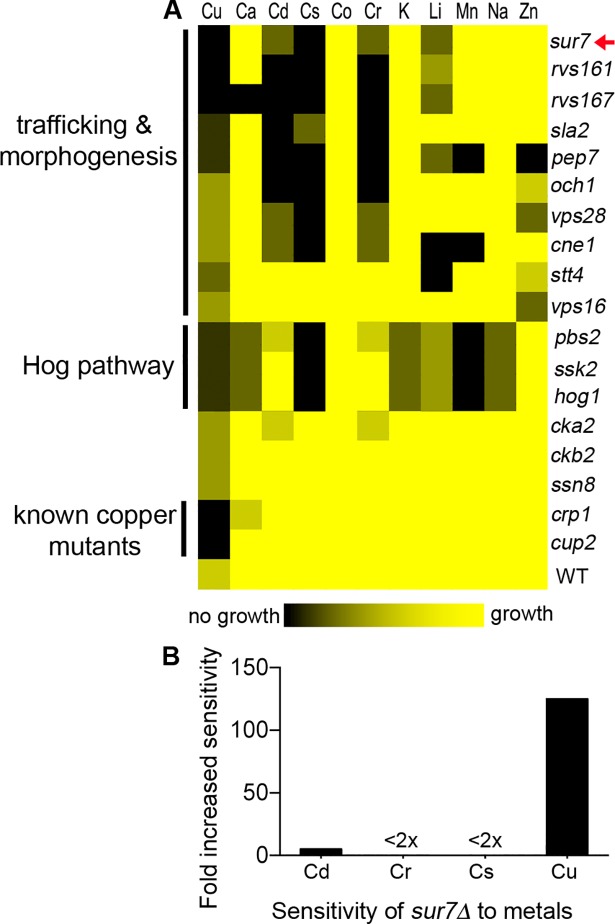
To better define how cells resist copper toxicity, we screened libraries of C. albicans mutant strains [23, 47, 48], along with deletion strains in our own collection, by replica-plating cells onto copper-containing medium. The most sensitive mutants were also tested for growth in the presence of 10 other metals to examine the specificity of the copper phenotype. A heat map summary (Fig 2A) of the 18 most copper-sensitive mutants revealed that they mainly fell into three categories. One expected category included mutants lacking known copper resistance genes (crp1Δ and cup2Δ) that were characterized by high sensitivity to copper, but not the other metals. A second group contained HOG MAP kinase pathway mutants (pbs2Δ, ssk2Δ, hog1Δ), and the third was comprised of sur7Δ and other mutants with defects in morphogenesis and membrane trafficking (Fig 2A). These latter two mutant categories could be readily distinguished by their sensitivity to other metals. For example, the HOG pathway mutants were inhibited by calcium and manganese, whereas many of the morphogenesis mutants were sensitive to cadmium and chromium (Fig 2A). Although sur7Δ cells appeared more sensitive to cadmium, chromium, and cesium when assayed for growth by replica-plating onto agar plates, growth in liquid cultures containing different concentrations of metals indicated that the sur7Δ mutant was only weakly more susceptible to these other metals (Fig 2B). Thus, the sur7Δ mutant is preferentially affected by copper.
Fig 2. Genes that function in stress response, morphogenesis, and membrane trafficking influence copper resistance.
(A) C. albicans strains were replica-plated onto minimal synthetic media containing copper or another indicated metal, and then the extent of growth was recorded and summarized in a heat map. The mutant strains clustered into three major groups. The sur7Δ mutant clustered with other mutants known to be defective in trafficking and morphogenesis, a separate cluster included the HOG1 pathway mutants that are sensitive to stress, and the known copper mutants crp1Δ and cup2Δ clustered. The results represent a compilation of three experiments for each metal performed on different days. (B) The sensitivity of sur7Δ to cadmium (Cd), chromium, (Cr), and cesium (Cs) was further tested by incubating cells in different concentrations of these metals in synthetic medium a 96-well plate, since replica-plating indicated some degree of growth inhibition in response to these metals. Results indicated that sur7Δ cells only showed strong sensitivity to copper. The graph represents the averages of two to three independent experiments performed on different days.
Sensitivity to oxidative stress is not the chief cause of copper toxicity for sur7Δ cells
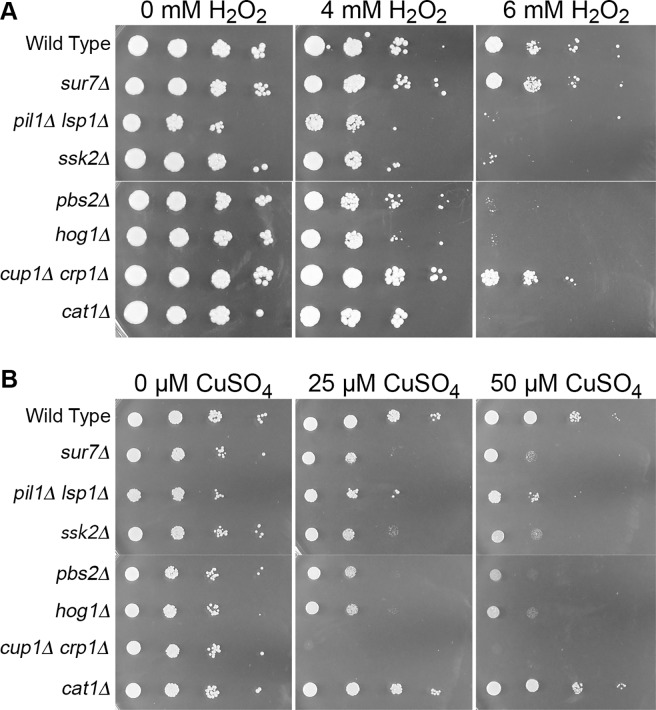
We next examined whether the copper sensitivity of sur7Δ and other C. albicans mutants identified in the screen was due to oxidative stress generated by copper. Copper is known to react with compounds such as H2O2 to generate diverse reactive oxygen species (ROS) that damage cells [6]. This is due in part to the ability of copper to transition between cuprous (Cu1+) and cupric (Cu2+) oxidation states, enabling it to undergo Fenton-like chemical reactions. Interestingly, the sur7Δ mutant showed at most a weak increase in sensitivity to oxidative stress when grown on medium containing H2O2 (Fig 3A). In contrast, control strains showed that a catalase mutant (cat1Δ) and the HOG pathway mutants (pbs2Δ, ssk2Δ,and hog1Δ) displayed increased sensitivity to H2O2, as expected based on previously published data [49, 50]. The cat1Δ catalase mutant grew well on medium containing copper, indicating that the presence of this metal in the medium did not cause high levels of oxidative stress (Fig 3B). In addition, the cup1Δ crp1Δ strain, which is very sensitive to copper, grew similar to the wild type control strain on medium containing H2O2. Interestingly, the pil1Δ lsp1Δ mutant was more sensitive to H2O2, which is likely to be due to effects on a family of four related antioxidant proteins that localize to eisosomes [51]. Altogether, these results indicate that the copper sensitivity of the sur7Δ mutant is not due to increased susceptibility to oxidative stress.
Fig 3. Copper sensitivity does not correlate with sensitivity to oxidative stress.
Dilutions of the indicated cells were spotted onto (A) YPD containing H2O2 or (B) synthetic medium with CuSO4. A cat1Δ catalase deletion served as a control as it is highly sensitive to oxidative stress, but grew to wild type levels on CuSO4 media. Conversely, the known copper-sensitive mutant cup1Δ crp1Δ grew well on H2O2, but failed to grow on either CuSO4 concentration. Hog1 pathway strains pbs2Δ, sskΔ 2, and hog1Δ all displayed sensitivity to increasing concentrations of both H2O2 and CuSO4, as did pil1Δ lsp1Δ. sur7Δ was inhibited by copper but grew similar to wild type on H2O2 media. Images are representative of two independent experiments performed on different days. Strains used were wild type, DIC185, sur7Δ (YJA11), pil1Δ lsp1Δ (YHXW21-1), ssk2Δ (YLD185-7), pbs2Δ (YLD197-1), hog1Δ (YLD184-3), cat1Δ (MT505-A), and cup1Δ crp1Δ (KC25).
Actin organization promotes resistance of C. albicans to copper
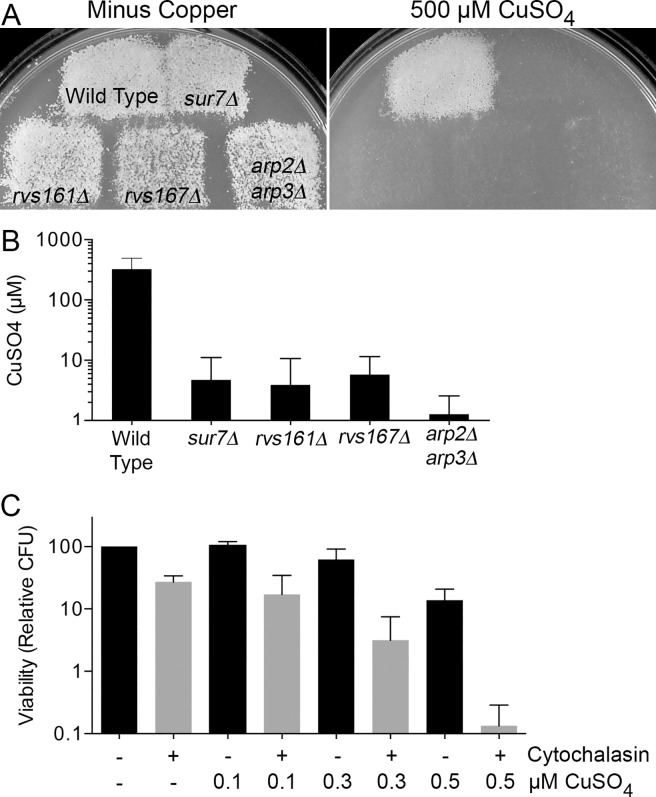
Since the members of the largest group of copper-sensitive mutants we identified were similar to sur7Δ in having defects in membrane trafficking and morphogenesis, we examined three other mutants that are known to have strong defects in these processes and to also show altered plasma membrane organization. Two mutants, rvs161Δ and rvs167Δ, lack BAR domain proteins that are needed for proper cortical actin organization and play an important role in the scission phase of endocytosis [52]. In addition, we tested a double arp2Δ arp3Δ mutant of the actin-related proteins Arp2 and Arp3 that form a complex that is needed for cortical actin localization and efficient endocytosis [53]. All three strains showed increased inhibition of growth when replica-plated onto agar medium containing copper (Fig 4A). Incubation in a range of copper concentrations in liquid media showed that all three mutants were similar to sur7Δ in that they could only resist low concentrations of copper. The arp2Δ arp3Δ strain was the most sensitive, as it could only grow in copper concentrations up to 3.2 μM, while the wild type grew at ≥400 μM (Fig 4B).
Fig 4. Actin defects contribute to copper sensitivity in C. albicans.
(A) To further analyze the effects of copper on morphogenesis and trafficking mutants, rvs161Δ, rvs167Δ, and arp2Δ arp3Δ were replica-plated onto synthetic medium with 500 μM CuSO4. Only the wild type DIC185 was able to grow, indicating the extreme sensitivity of the deletion mutant strains. Images are representative of two independent experiments performed on different days. (B) Quantification of sensitivity by incubating mutant cells in a dilution series of copper concentrations in synthetic medium demonstrated that the arp2Δ arp3Δ mutant was the most sensitive, being inhibited by concentrations above 3.2 μM. The wild type, DIC185, was able to grow in 400 μM CuSO4. The graph represents the averages of four independent experiments performed on different days. (C) To determine whether an intact actin cytoskeleton is essential for resistance to copper, wild type DIC185 cells were treated with cytochalasin A in synthetic medium for 2 hr to depolarize the actin cytoskeleton, and then viability was assayed following incubation in the indicated concentration of CuSO4 for 2 hr. Relative colony-forming units (CFUs) decreased significantly with depolymerization of actin and exposure to increasing levels of copper. The graph represents averages of four independent experiments performed on different days. Strains used were DIC185, sur7Δ (YJA11), rvs161Δ (YLD14-3), rvs167Δ (YLD16), and arp2Δ arp3Δ (CaEE27).
A common feature of the copper-sensitive mutants sur7Δ, pil1Δ lsp1Δ, rvs161Δ, rvs167Δ, and arp2Δ arp3Δ is that they have abnormal actin organization [33, 52, 53]. We therefore examined the possibility that resistance to copper is dependent upon an intact actin cytoskeleton. This was tested by treating the wild type control strain DIC185 with the actin polymerization inhibitor cytochalasin A for 2 hr to disrupt the actin cytoskeleton, and then incubating the cells in the presence or absence of different concentrations of copper. Interestingly, over the range of copper concentrations used, cells incubated in water showed about a 5-fold decrease in viability, whereas cytochalasin A-treated cells showed about a 100-fold decrease in viability (Fig 4C). This synergistic effect on the loss of viability when cytochalasin A-treated cells were exposed to copper supports the conclusion that normal actin cytoskeleton and plasma membrane organization promote resistance of C. albicans to copper.
Copper permeabilizes the plasma membrane of sur7Δ and other morphogenesis mutants
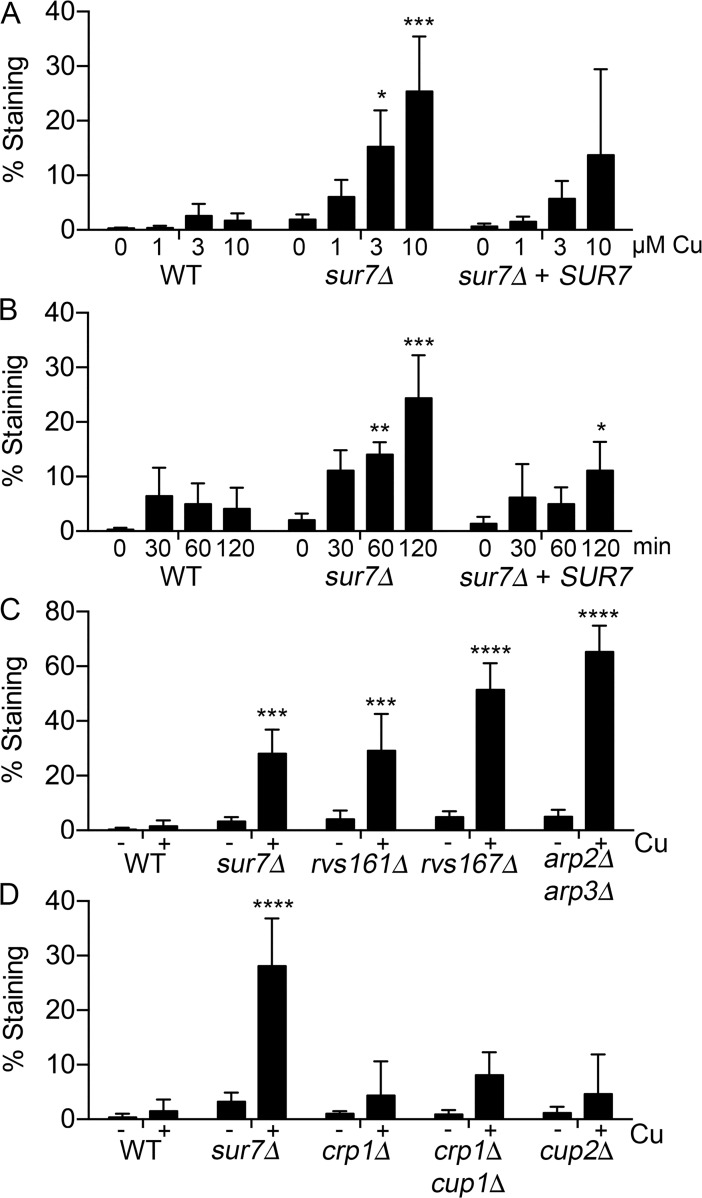
The observation that sur7Δ cells have altered plasma membrane architecture, including mislocalization of cortical actin, septins, and the lipid PI4,5P2 [26, 31, 33], suggested the possibility that copper reduces the viability of these cells by permeabilizing the plasma membrane. Copper has been shown to permeabilize the plasma membranes of a wide range of cell types [38–41], but the mechanism is not known and no mutants have been described previously that are hypersensitive to this process. To examine how copper affects the plasma membrane barrier function, we incubated C. albicans cells with different concentrations of CuSO4 for 2 hr, and then treated the cells with the membrane impermeable dye SYTOX Green that can only stain intracellular nucleic acids if the plasma membrane has been compromised. Interestingly, the sur7Δ cells showed significantly increased staining in a dose-dependent manner compared to the wild type and sur7Δ + SUR7 complemented strains (Fig 5A and S2 Fig). A time course analysis demonstrated that sur7Δ cells experienced more membrane damage than other strains even after a short 30 min incubation in copper (Fig 5B). Similar results were obtained with other membrane-impermeable stains, such as propidium iodide and FM4-64, and copper reduced the fluorescence of sur7Δ cells carrying the plasma membrane localized Pma1-GFP fusion protein, further supporting the conclusion that copper was permeabilizing the plasma membrane and having toxic effects (S3 Fig).
Fig 5. Copper permeabilizes plasma membranes of C. albicans copper-sensitive strains with morphogenesis defects.
(A) Log phase sur7Δ cells were incubated in water containing the indicated concentrations of CuSO4 for 2 hr at 37°C and then stained with SYTOX Green to assess the integrity of the plasma membrane. The sur7Δ mutant displayed increased permeabilization of the plasma membrane with increased copper concentration compared to the wild type and Sur7 complemented strains. (B) Time course assay in which log phase sur7Δ cells were incubated in 10 μM CuSO4 for the indicated time at 37°C and then stained with SYTOX Green. The sur7Δ cells suffered greater membrane damage as early as 30 min after addition of copper.(C) Log phase cells of the morphogenesis mutants rvs161Δ, rvs167Δ, and arp2Δ arp3Δ strains were incubated in 10 μM CuSO4 for 2 hr at 37°C and then stained with SYTOX Green. All mutants demonstrated increased staining, indicative of membrane permeabilization. (D) Log phase cells of known copper mutants were incubated in 10 μM CuSO4 for 2 hr at 37°C and then stained with SYTOX Green. The crp1Δ, crp1Δ cup1Δ, and cup2Δ strains all showed significantly less staining than the sur7Δ strain, indicating a low level of membrane permeabilization caused by CuSO4. Error bars indicate SD. ****, P<0.001. ***, P<0.01. **, P<0.01, *, P<0.05 by two-way ANOVA in comparison to the corresponding wild type values. The results represent the average of three independent assays (panels A, C, D) or six independent assays (Panel B) carried out on different days. Strains used included DIC185, sur7Δ (YJA11), crp1Δ (KC16), cup1Δ crp1Δ (KC25), and cup2Δ (YLD117-1).
The other copper-sensitive morphogenesis mutants rvs161Δ, rvs167Δ, and arp2Δ arp3Δ showed strong SYTOX Green staining after incubation in copper, with arp2Δ arp3Δ exhibiting the highest level of staining (Fig 5C). This correlated with arp2Δ arp3Δ being the most copper-sensitive strain (Fig 4B). In contrast, the mutant strains that lack the Crp1 copper exporter or the Cup1 metallothionein that sequesters intracellular copper (crp1Δ, cup1Δ crp1Δ, and cup2Δ) showed only a small increase in SYTOX Green staining after copper treatment, which was significantly lower than that seen for sur7Δ and the other morphogenesis mutants (Fig 5D). This indicates that plasma membrane permeabilization by copper is separate from the toxic effects of intracellular copper.
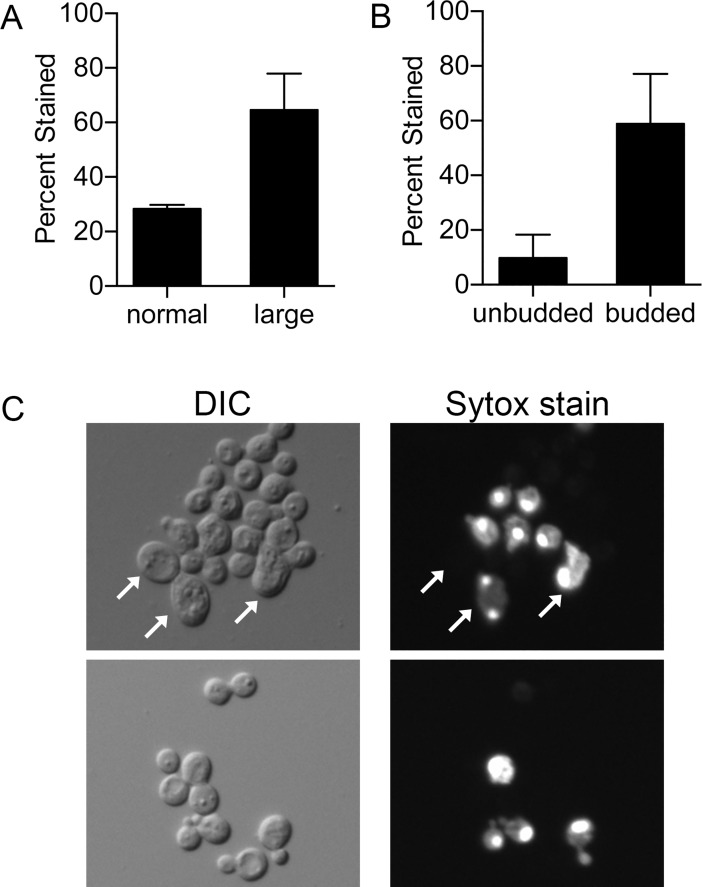
The relationship between cell morphology and SYTOX Green staining was examined since sur7Δ cells are known to range from typical-looking budding cells to abnormally large cells [33]. Interestingly, about 65% of large cells (≥ 7 μm) stained with SYTOX Green after copper treatment compared to only about 25% of normal sized cells (Fig 6A and 6C). Although the larger cells were permeabilized more readily, it is significant that the typical-sized cells were also susceptible to copper. The cell cycle stage of the sur7Δ cells also affected permeabilization by copper, as about 60% of budded cells stained compared to only 10% of unbudded cells (Fig 6B and 6C). This correlates with increased function of the actin cytoskeleton and vesicle trafficking during polarized bud morphogenesis.
Fig 6. Cell morphology affects plasma membrane permeabilization by copper.
Log phase cells were incubated in a solution of 10 μM CuSO4 for 2 hr at 37°C and then stained with SYTOX Green. (A) A greater percentage of large sur7Δ cells (≥ 7 μm) stained compared to those of normal size. (B) More budded sur7Δ cells stained with SYTOX Green than unbudded cells.(C) Representative photographs of SYTOX Green stained sur7Δ cells with arrows indicating large cells (>7 μm; upper right panel). Some cells of normal size also stained. Budded cells that stained are shown in the lower right panel. DIC images appear on left. The graphs represent averages of four independent experiments performed on different days. Strains included wild type DIC185 and sur7Δ (YJA11).
Increased susceptibility to antibiotics indicates copper-sensitive morphogenesis mutants have altered surface distribution of plasma membrane lipids
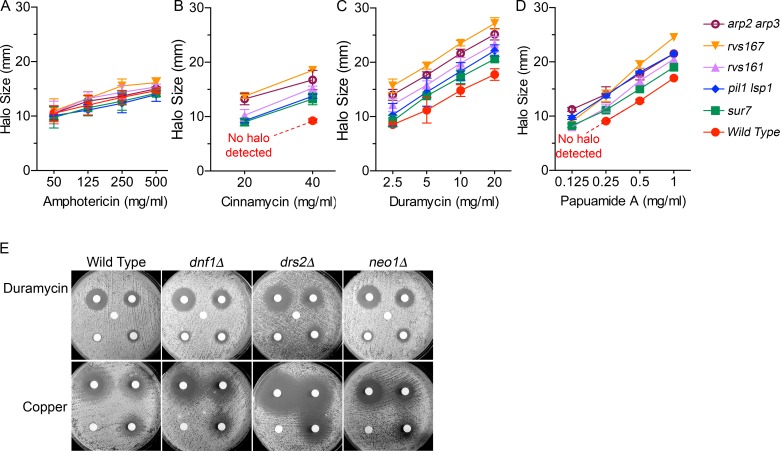
The increased ability of copper to permeabilize the mutants suggested that their cell surfaces may be altered, so plasma membrane organization was probed by assaying sensitivity to compounds that target specific plasma membrane lipids. The sur7Δ, pil1Δ lsp1Δ, rvs161Δ, rvs167Δ, and arp2Δ arp3Δ mutants all showed essentially the same sensitivity as wild type cells to the polyene antibiotic amphotericin B (Fig 7A), which binds ergosterol in the plasma membrane [54]. In contrast, the mutant cells were more sensitive to the lanthionine antibiotics cinnamycin and duramycin (Fig 7B and 7C), which bind phosphatidylethanolamine (PE) if it is in the outer leaflet of the plasma membrane [55]. Similarly, all of the mutants were more susceptible to the depsipeptide antibiotic papuamide A (Fig 7D), which binds phosphatidylserine (PS) in the outer leaflet [56, 57]. PE and PS are normally enriched in the inner leaflet of the plasma membrane by proper membrane trafficking and the action of flippase proteins that promote inward translocation of these lipids [58]. The increased sensitivity to these drugs therefore indicates an altered surface of the plasma membrane. Interestingly, the relative increased susceptibility of the sur7Δ, pil1Δ lsp1Δ, rvs161Δ, rvs167Δ, and arp2Δ arp3Δ mutants to cinnamycin, duramycin, and papuamide A correlated with their relative sensitivity to permeabilization by copper, consistent with an altered presentation of lipids on the cell surface contributing to the ability of copper to permeabilize a membrane.
Fig 7. Altered responses of copper sensitive morphogenesis mutants and flippase mutants to membrane-targeted drugs.
The susceptibility of copper-sensitive mutants to (A) amphotericin B, (B) cinnamycin, (C) duramycin and (D) papuamide A was determined by halo assays in which 2.5 x 105 cells of the indicated strain were spread onto the surface of minimal medium plates, filter discs containing different amounts of the drugs were placed on the surface of each plate, and then the zone of growth inhibition was quantified. (E) Susceptibility of C. albicans flippase mutants to duramycin and copper. The filter disks on one plate contained 10 μl of 0, 2.5, 5, 10, or 20 mg/ml duramycin. The other plate contained 0, 75, 150, or 300 mM CuSO4. The zone of growth inhibition (halo) surrounding each filter disc was recorded after incubation for 48 hr at 30°C. Note that there were no significant differences in halo sizes between the copper-sensitive mutants and the wild type for amphotericin B which targets ergosterol, but significant differences were detected for cinnamycin and duramycin that target phosphatidylethanolamine (PE) and for papuamide A that targets phosphatidylserine (PS). Graphs represent averages of three independent experiments performed on different days. A representative set of halo assays is shown in the supporting information (S5 Fig). None of the mutants were significantly different from the wild type for amphotericin B, but all of the mutants were significantly different from the wild type for the other drugs by two-way ANOVA (P < 0.05 or lower). Strains in panels A-D were wild type control DIC185, sur7Δ (YJA11), pil1Δ lsp1Δ (YHXW21-1), rvs161Δ (YLD14-3), rvs167Δ (YLD16), and arp2Δ arp3Δ (CaEE27). Strains in Panel E were wild type control DIC185, and the flippase mutants included neo1Δ (C1_04630CΔ; YLD224-9), dnf1Δ (C5_00570WΔ, YLD219-2-9-1) and drs2Δ (C3_07230WΔ; YLD220-14-18-1).
To confirm these results, we mutated members of the phospholipid flippase gene family in C. albicans, which encode P4-ATPases that promote inward translocation of lipids. Flippases are responsible for maintaining phospholipid asymmetry in the plasma membrane by translocating PS and PE from the outer leaflet to the inner leaflet of the plasma membrane [58]. Little or no analysis of flippases has been carried out in C. albicans, but in S. cerevisiae NEO1 is essential and the other four have redundant functions (DNF1, DNF2, DNF3 and DRS2). Surprisingly, although the apparent C. albicans ortholog of NEO1 (C1_04630C) is not essential, a neo1Δ mutant showed a detectable increase in sensitivity to both duramycin and copper (Fig 7E). Similar results were obtained for the dnf1Δ (lacking C5_00570W). Interestingly, the drs2Δ mutant (lacking C3_07230W) showed the strongest increase in sensitivity to both duramycin, and copper. These results provide additional evidence that altered plasma membrane organization contributes to the increased sensitivity to copper.
To test whether the sur7Δ strain was altered in response to other agents that compromise plasma membrane integrity, we treated cells with the polycations DEAE dextran hydrochloride (500 kDa), and poly-L-lysine hydrobromide (30 kD). Poly-L-lysine with molecular weight > 25 kDa was reported to permeabilize yeast cells, as were DEAE dextrans with masses from 5 to 380 kDa [59]. The sur7Δ mutant showed slightly increased viability after exposure to either 0.1 μg/ml poly-L-lysine or 0.5 μg/ml DEAE dextran compared to the wild type control, indicating it was more resistant to this type of membrane disruption (S4 Fig) These results further confirm that copper has a specific effect on the sur7Δ plasma membrane.
Previous studies indicated that introducing polyunsaturated fatty acids (PUFAs) into the S. cerevisiae plasma membrane, which normally lacks PUFAs, increased the susceptibility to permeabilization by copper [60]. However, this did not seem likely to be a factor for C. albicans, as fatty acid analysis indicated that there were no significant differences in PUFA content in C. albicans strains between the wild type control, sur7Δ, and the pil1Δ lsp1Δ mutant (S1 Table). Furthermore, in S. cerevisiae the susceptibility to copper permeabilization plateaued at 20% PUFAs, and C. albicans naturally contains about 30% PUFAs [61].
Phosphatidylserine (PS) promotes copper permeabilization of sur7Δ plasma membrane
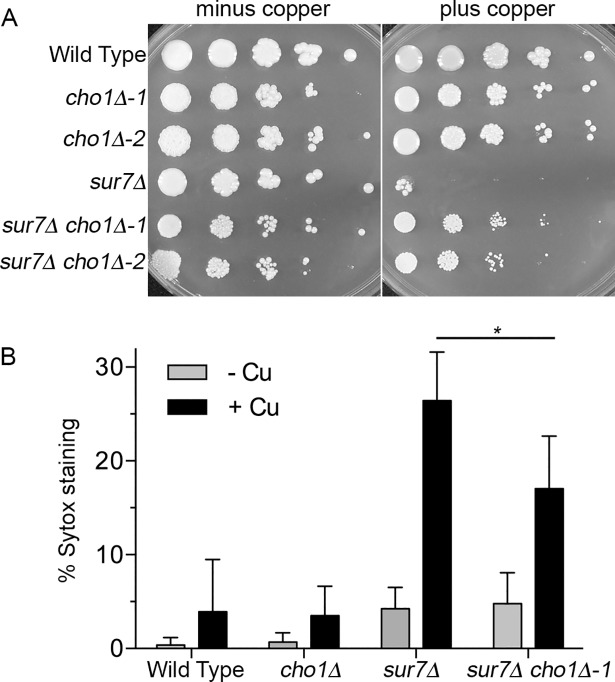
The increased sensitivity to papuamide indicated that PS was inappropriately exposed on the plasma membrane surface. Previous studies demonstrated that PS binds copper with very high affinity, and that this interaction can promote membrane damage that could lead to permeability [43, 44, 62]. To test the role of PS, we deleted the CHO1 gene that encodes PS synthase [63]. However, the sur7Δ cho1Δ cells that lack PS were more resistant to copper than were the parental sur7Δ cells (Fig 8A). The sur7Δ cho1Δ double mutant consistently showed decreased permeabilization by copper relative to the sur7Δ single mutant (Fig 8B). Although there was some day to day variability in the total number of cells stained with Sytox Green, the percent of stained sur7Δ cho1Δ cells subtracted from the sur7Δ single mutant value averaged 9.9 + 2.3%. Altogether, these results indicate that abnormal plasma membrane organization leads to exposure of PS on the plasma membrane where it can be bound and attacked by copper.
Fig 8. Deletion of the phosphatidylserine synthase gene CHO1 rescues copper sensitivity of the sur7Δ mutant.
(A) Dilutions of cells were spotted onto minimal synthetic medium in the absence or presence of 3 mM CuSO4 and incubated for 48 hr at 30°C. (B) The indicated cell types were incubated in water (- Cu) or 10 μM CuSO4 (+ Cu) for 2 hr at 37°C and then stained with SYTOX Green. The bars represent the average of at least 4 independent assays and error bars indicate SD. * indicates P < 0.02 by t test comparison. Strains used were wild type control DIC185, cho1Δ (YLD222-74 and YLD222-75), sur7Δ (YJA11), and sur7Δ cho1Δ (YLD221-5 and YLD221-6).
Discussion
Sur7 carries out a novel role in copper resistance
Copper resistance is important for a wide range of bacterial and fungal pathogens [8, 11, 22, 64, 65]. Previous studies indicated that the increased sensitivity of sur7Δ mutant cells to copper-mediated killing was not due to a defect in the Crp1 copper exporter, which carries out the major known copper resistance mechanism in C. albicans [18, 19, 26]. Therefore, this study was initiated to better understand the role of the MCC/eisosome protein Sur7 in promoting resistance to copper. Analysis of a set of mutants lacking different MCC/eisosome proteins revealed that a pil1Δ lsp1Δ mutant, which is defective in forming the furrow-like membrane invaginations corresponding to MCC/eisosomes, was also more sensitive to copper (Fig 1). Sur7 is not stably maintained in the plasma membrane of pil1Δ lsp1Δ mutants [31], so we overexpressed SUR7 and found that this strongly rescued the copper defect of the pil1Δ lsp1Δ cells, indicating that Sur7 plays the key role in copper resistance, rather than eisosome furrows that can form in the absence of Sur7. Further analysis of the sur7Δ cells showed that they were not highly sensitive to other metals (Fig 2), which indicates that the defect is specific to copper and not due to alteration in vacuolar function that is associated with increased sensitivity to multiple metals [20, 66, 67]. The effect of copper on the sur7Δ cells was not due to a general ability of copper to catalyze creation of reactive oxygen species, since the sur7Δ mutant did not show a correspondingly large increase in susceptibility to oxidative stress (Fig 3). These results indicate that Sur7 carries out a novel role in copper resistance.
Genetic approaches link copper sensitivity to altered plasma membrane organization and phosphatidylserine
Genetic screening of C. albicans mutants was used to gain further insight into the mechanisms underlying the increased copper sensitivity of sur7Δ cells. Interestingly, most of the copper-sensitive mutants that were identified have defects in membrane organization or trafficking (Fig 2). Targeted analysis of three mutants known to have strong defects in plasma membrane function and cortical actin (rvs161Δ, rvs167Δ, and arp2Δ arp3Δ mutants) revealed that they all showed increased susceptibility to killing by copper that was similar to or greater than the sur7Δ mutant (Fig 4). In support of a role for the actin cytoskeleton, wild type cells treated with the actin inhibitor cytochalasin A also displayed increased sensitivity to copper (Fig 4C). Furthermore, overexpression of SUR7, which rescued the copper defect of pil1Δ lsp1Δ cells (Fig 1B), was shown in a previous study to improve plasma membrane organization of cortical actin [31]. Altogether, these results indicate that altered plasma membrane organization caused by defects in the MCC/eisosome protein Sur7 or disruption of the actin cytoskeleton increases the susceptibility to killing by copper.
Copper binds strongly to membranes, which suggested it could have a direct effect on lipids. In particular, copper binds to PS with picomolar affinity and to PE with micromolar affinity [43, 44]. Copper is thought to bind PS with such high affinity relative to other divalent metals because it can interact in a special configuration with two molecules of PS [42–44]. Usually PS and PE are sequestered on the inner leaflet of the plasma membrane by the action of phospholipid flippases [68]. To determine whether these phospholipids were exposed on the cell surface of the copper-sensitive mutants, the mutant cells were tested for increased sensitivity to cinnamycin and duramycin, which bind to PE, and to papuamide A, which binds to PS (Fig 7). There was a good correlation between the relative increases in sensitivity to copper and to these drugs that target PS and PE, consistent with copper sensitivity reflecting the degree to which the surface exposure of PS and PE is altered in the mutants. The mutants that were most susceptible to being permeabilized by copper (sur7Δ, pil1Δ lsp1Δ, rvs161Δ, rvs167Δ, and arp2Δ arp3Δ) all had broad defects in plasma membrane structure that could result in the cell surface exposure of PS and PE [33, 52, 53, 69]. Furthermore, three different phospholipid flippase deletion mutants displayed increased sensitivity to copper, especially the drs2Δ mutant (Fig 7). We therefore examined the effects of deleting the gene encoding PS synthase (CHO1) to deplete cells of PS. Remarkably, the sur7Δ cho1Δ double mutant showed greatly improved resistance to copper (Fig 8). These results strongly support the conclusion that copper has a direct effect on the plasma membrane, and implicate interaction with PS as part of the underlying mechanism for copper-mediated killing of the mutant cells.
Mechanisms of plasma membrane permeabilization and killing by copper
Although plasma membrane permeabilization can be a consequence of cell death, several lines of evidence indicate that copper kills sur7Δ cells and the other new copper-sensitive mutants we identified by promoting membrane permeabilization. Previous studies have shown that copper is distinct from other divalent metal ions in its ability to bind and damage model membranes containing PS or PE [42–44]. Atomic force microscopy studies showed copper promotes lipid reorganization and affects membrane fluidity that could weaken the membrane barrier function [70, 71]. The ability of excess copper to permeabilize red blood cell membranes was correlated with a loss of membrane fluidity and decreased ability of the cells to tolerate deformation [72]. Copper has been known to permeabilize the plasma membranes of fungi and other cell types from plants to animals for 30 years, and has been used as a tool to form pores in the plasma membrane to selectively release cytoplasmic pools of amino acids [36, 38–41], but the mechanism by which copper permeabilizes the plasma membrane is not known. It is therefore significant that the sur7Δ, pil1Δ lsp1Δ, rvs161Δ, rvs167Δ, and arp2Δ arp3Δ mutants represent the first mutants reported for being more susceptible to copper permeabilization (Fig 5), as they provide new insights into the mechanisms underlying this process. Once cells become permeabilized, copper would be expected to enter cells and cause further lethal damage. In contrast, control studies showed that copper did not cause a significant increase in plasma membrane permeability for the crp1Δ, cup1Δ crp1Δ, and cup2Δ mutants in spite of their greatly increased sensitivity to this metal (Fig 5D). This supports the conclusion that there are independent pathways for killing by copper.
The special redox properties of copper suggest that it damages membranes by promoting oxidation. Studies with copper-treated model membranes demonstrate that this metal can promote oxidation of both PS and PE [42, 44]. In our studies, the oxidation-sensitive C. albicans mutant pst1Δ pst2Δ pst3Δ ycp4Δ mutant (Fig 1A) and a cat1Δ catalase mutant (Fig 3) did not show a significant increase in susceptibility to copper. However, it may be that these intracellular proteins do not have a direct effect on oxidation events in the outer leaflet of the plasma membrane. Furthermore, other data showed that the oxidative effects of copper on PS are differentially affected by different subsets of anti-oxidant molecules, indicating that not all antioxidant pathways are likely to be equally effective [62]. Thus, copper may be acting in a specialized microenvironment at the cell surface to promote oxidative damage to membranes leading to permeabilization.
Potential roles of copper in antifungal therapy
The plasma membrane is an effective target for therapy, as the commonly used antifungal drugs alter the plasma membrane directly or indirectly (i.e. fluconazole, amphotericin, caspofungin) and many antimicrobial peptides act by permeabilizing the plasma membrane [54, 73]. One reason the antifungal drugs are effective is that even at sublethal doses they can broadly impact plasma membrane organization [74]. Proper architecture of proteins and lipids is required to form a strong protective barrier around the cell, and it plays key roles in virulence by coordinating a wide range of dynamic functions including secretion of virulence factors, cell wall synthesis, invasive hyphal morphogenesis, endocytosis, and nutrient uptake [74]. The identification in this study of new copper-sensitive mutants and the role of phosphatidylserine provides insights for new modes of antifungal therapy. Copper is amenable to incorporation into therapeutic strategies [75, 76]. Drugs that alter the plasma membrane surface may be used to increase the susceptibility of cells to copper in the host [6–10], and conversely copper could be used to enhance the membrane perturbing effects of currently used antifungal drugs [77]. In addition, our results have important implications for understanding microbial killing by metallic copper surfaces, which have been explored for preventing the spread of nosocomial infections [45, 46]. Thus, copper could be used in a synergistic fashion with other therapeutic strategies to more effectively kill fungal pathogens.
Materials and methods
Strains and media
The C. albicans strains used in this study appear in Table 1. Cultures were grown either in rich YPD medium (2% dextrose, 1% peptone, 2% yeast extract, 80 mg/L uridine), or synthetic medium (yeast nitrogen base, 2% dextrose) [78]. Synthetic medium was supplemented with amino acids and uridine, if required. The pbs2Δ, ssk2Δ, hog1Δ, cup2Δ, and neo1Δ strains [47] were made prototrophic by transformation with a PCR-amplified ARG4 DNA fragment that was integrated into the native locus. Primers used to amplify the cassette included 18 base pairs of homology approximately 500 base pairs upstream and downstream of the ARG4 open reading frame. The sla2Δ, vps28Δ, vps16Δ, cka2Δ, ckb2Δ, and ssn8Δ strains [48] were transformed with a similarly constructed HIS1 cassette to correct the remaining auxotrophy. PMA1-GFP strains were constructed with the photostable GFPγ variant, as described previously [79]. Homozygous dnf1Δ (orf19.932/C5_00570W) and drs2Δ (19.6778/ C3_07230W) mutants were constructed from C. albicans parental strain BWP17 by the sequential deletion of both copies of the targeted gene [80]. Deletion cassettes were generated by PCR amplification of the ARG4 or HIS1 selectable marker genes, using primers that included 70 to 80 base pairs of DNA sequence homologous to the upstream or downstream regions of the targeted open reading frame. Cells which had undergone homologous recombination to delete the targeted gene were identified by PCR analysis using a combination of primers flanking sites of cassette integration and internal primers. Homozygous cho1Δ mutants were constructed in C. albicans parental strain DIC185 and the previously described sur7Δ deletion (33), using the transient CRISPR-Cas9 system [81]. The gene-deletion construct was synthesized using the SAT1 flipper as a template and primers that included 80 base pairs upstream and downstream of the targeted gene [82]. Homozygous deletion clones were identified by rescue of the ethanolamine auxotrophy phenotype on synthetic medium with 1mM ethanolamine [63], and by PCR analysis using primers flanking the CHO1 gene, as well as SAT1 internal primers.
Table 1. C. albicans strains used in this study.
| Strain | Short genotype | Full genotype |
|---|---|---|
| BWP17 | parental strain | his1::hisG/his1::hisG arg4::hisG/arg4::hisG ura3::λimm434/ ura3::λimm434 |
| DIC185 | prototrophic WT control | ura3::λimm434/URA3 his1::hisG/ HIS1 arg4::hisG/ ARG4 |
| YJA11 | sur7Δ | sur7Δ::ARG4/sur7Δ::HIS1 URA3/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YJA12 | sur7Δ SUR7 | sur7Δ::ARG4/sur7Δ::HIS1 SUR7::URA3 ura3::λimm434/ ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YHXW21-1 | pil1Δ lsp1Δ | pil1Δ::ARG4/ pil1Δ::FRT lsp1Δ::HIS1/lsp1Δ::SAT1 flipper URA3/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YHXW23-1 | pil1Δ lsp1Δ LSP1 | pil1Δ::ARG4/pil1Δ::FRT lsp1Δ::HIS1/lsp1Δ::SAT1 flipper ura3::λimm434/ ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG LSP1::URA3 |
| YLD182-9-10-2 | seg1Δ | seg1Δ::ARG4/seg1Δ::HIS1 URA3/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YHXW36-1 | slm2Δ | orf19.3505Δ::ARG4/ orf19.3505Δ::HIS1 URA3/ ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YLD146-11-2-5 | pkh1Δ pkh3Δ PKH3 | pkh1Δ::ARG4/ pkh1Δ::HIS1 pkh3Δ::FRT/ PKH3 URA3/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| LLF60A | pst1Δ pst2Δ pst3Δ ycp4Δ | pst3-ycp4Δ::LEU2/pst3-ycp4Δ::HIS1pst2Δ::FRT/pst2Δ::FRT pst1Δ::FRT/pst1Δ::FRT ARG4/arg4Δ |
| YHXW14 | nce102Δ | nce102Δ::ARG4/ nce102Δ::HIS1 URA3/ ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YLD204-2 | pun1Δ | pun1Δ::HIS1/pun1Δ::LEU2 his1Δ/his1Δ leu2Δ/leu2Δ ARG4/arg4Δ URA3/ura3::imm IRO1/iro1Δ::imm |
| YHXW3 | fmp45Δ | fmp45Δ::ARG4/fmp45Δ::HIS1 URA3/ ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| KC25 | cup1Δ crp1Δ | cup1Δ::hisG/cup1Δ::hisG crp1Δ::hisG/crp1Δ::hisG-URA3-hisG ura3::imm434/ura3::imm434 |
| YHXW45-1 | pil1Δ lsp1Δ 1xSUR7 | pil1Δ::ARG4/pil1Δ::FRT lsp1Δ::HIS1/lsp1Δ::FRT ura3::λimm434/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG SUR7::URA3 |
| YHX46-1 | pil1Δ lsp1Δ 2xSUR7 | pil1Δ::ARG4/pil1Δ::FRT lsp1Δ::HIS1/lsp1Δ::FRT ura3::λimm434/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG SUR7::URA3 SUR7::NAT1 |
| YLD197-1 | pbs2 | pbs2Δ::HIS1/pbs2Δ::LEU2 his1Δ/his1Δ leu2Δ/leu2Δ ARG4/arg4Δ URA3/ura3::imm IRO1/iro1Δ::imm |
| YLD185-7 | ssk2Δ | ssk2Δ::HIS1/ssk2Δ::LEU2 his1Δ/his1Δ leu2Δ/leu2Δ ARG4/arg4Δ URA3/ura3::imm IRO1/iro1Δ::imm |
| YLD184-3 | hog1Δ | hog1Δ::HIS1/hog1Δ::LEU2 his1Δ/his1Δ leu2Δ/leu2Δ ARG4/arg4Δ URA3/ura3::imm IRO1/iro1Δ::imm |
| YLD14-3 | rvs161Δ | rvs161Δ::ARG4/rvs161Δ::HIS1 URA3/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YLD16-11 | rvs167Δ | rvs167Δ::ARG4/rvs167Δ::HIS1 URA3/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YLD193-10 | sla2Δ | sla2::URA3/sla2::URA3-ARG4-URA3 HIS1/his1::hisG arg4::hisG/arg4::hisG ura3::λimm434/ ura3::λimm434 |
| YLD187-11 | pep7 | pep7Δ::HIS1/pep71Δ::LEU2 his1Δ/his1Δ leu2Δ/leu2Δ ARG4/arg4Δ URA3/ura3Δ::imm IRO1/iro1Δ::imm |
| YLD188-1 | och1 | och1Δ::HIS1/och1Δ::LEU2 his1Δ/his1Δ leu2Δ/leu2Δ ARG4/arg4Δ URA3/ura3::imm IRO1/iro1Δ::imm |
| YLD200-2 | vps28Δ | vps28::URA3/vps28::URA3-ARG4-URA3 HIS1/his1::hisG arg4::hisG/arg4::hisG ura3::λimm434/ura3::λimm434 |
| YLD186-1 | cne1 | cne1Δ::HIS1/cne1Δ::LEU2 his1Δ/his1Δ leu2Δ/leu2Δ ARG4/arg4Δ URA3/ura3::imm IRO1/iro1Δ::imm |
| YLD191-4 | stt4 | stt4Δ::HIS1/stt4Δ::LEU2 his1Δ/his1Δ leu2Δ/leu2Δ ARG4/arg4Δ URA3/ura3::imm IRO1/iro1Δ::imm |
| YLD202-1 | vps16Δ | vps16::URA3/vps16::URA3-ARG4-URA3 HIS1/his1::hisG arg4::hisG/arg4::hisG ura3::λimm434/ura3::λimm434 |
| YLD194-2 | cka2 | cka2::URA3/cka2::URA3-ARG4-URA3 HIS1/his1::hisG arg4::hisG/arg4::hisG ura3::λimm434/ura3::λimm434 |
| YLD195-1 | ckb2 | ckb2Δ::URA3/ckb2::URA3-ARG4-URA3 HIS1/his1::hisG arg4::hisG/arg4::hisG ura3::λimm434/ura3::λimm434 |
| YLD196-7 | ssn8 | ssn8::URA3/ssn8::URA3-ARG4-URA3 HIS1/his1::hisG arg4::hisG/arg4::hisG ura3::λimm434/ ura3::λimm434 |
| YLD115-1 | crp1 | URA3::λimm434/ura3Δ::λimm434 crp1::hisG/crp1::hisG |
| YLD117-1 | cup2 | cup2Δ::HIS1/cup2Δ::LEU2 his1Δ/his1Δ leu2Δ/leu2Δ ARG4/arg4Δ URA3/ura3Δ::imm IRO1/iro1Δ::imm |
| YLD116-7 | cup1Δ | URA3/ura3Δ::λimm434 cup1Δ::hisG/cup1Δ::hisG |
| MT505-A | cat1Δ | cat1Δ::FRT/cat1Δ::FRT |
| CaEE227 | arp2Δ arp3Δ | arp2::LEU2/arp2::HIS1 arp3::URA3/arp3::ARG4 |
| KC16 | crp1Δ | ura3::imm434 /ura3::imm434 crp1Δ::hisG/crp1Δ::hisG |
| YHXW11 | PMA1-GFPγ | ura3::λimm434/ ura3::λimm434 PMA1-GFPγ::URA3 |
| YHXW61 | sur7Δ PMA1-GFPγ | sur7Δ::ARG4/ sur7Δ::HIS1 ura3::λimm434 /ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG PMA1-GFPγ::URA3 |
| YLD219-2-9-1 | dnf1Δ | dnf1Δ::ARG4/dnf1Δ::HIS1 URA3/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YLD220-14-18-1 | drs2Δ | drs2Δ::ARG4/drs2Δ::HIS1 URA3/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| YLD224-9 | neo1Δ | neo1Δ::HIS1/dnf1Δ::LEU2 his1Δ/his1Δ leu2Δ/leu2Δ ARG4/arg4 URA3/ura3:: imm IRO1/iro1Δ::imm |
| YLD221-5 | sur7Δ cho1Δ | sur7Δ::ARG4/sur7Δ::HIS1 URA3/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG cho1Δ::FRT/cho1Δ:: SAT1 flipper |
| YLD221-6 | sur7Δ cho1Δ | sur7Δ::ARG4/sur7Δ::HIS1 URA3/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG cho1Δ::FRT/cho1Δ:: SAT1 flipper |
| YLD222-74 | cho1Δ | ura3::λimm434/URA3 his1::hisG/ HIS1 arg4::hisG/ ARG4 cho1Δ::FRT/cho1Δ:: SAT1 flipper |
| YLD222-75 | cho1Δ | ura3::λimm434/URA3 his1::hisG/ HIS1 arg4::hisG/ ARG4 cho1Δ::FRT/cho1Δ:: SAT1 flipper |
Screening of mutant strains for sensitivity to copper and other metals
Mutant strain libraries of S. Noble [47], A. Mitchell [48], and O. Homann [23], along with strains in our own collection were replica-plated onto synthetic agar medium containing 500 μM, 250 μM, 100 μM, 20 μM, or 0 μM CuSO4, plus added arginine, histidine, or uridine, if necessary. Other metals were tested similarly by replica-plating onto solid agar medium containing 1.5 M NaCl, 1.5 M KCl, 150 mM LiCl, 800 mM RbCl, 300 mM CsCl, 3 mM CoCl2, 300 μM CdCl2, 100 mM MnCl2, 20 mM ZnCl2, 4 mM NiCl2, 2 mM FeSO4, 200 μM CrO3, and 500 mM CaCl2. The concentrations of these metals were determined by identifying a dose at which the growth of wild type cells was reduced, but not lethal. Plates were incubated at 30°C for 48-72 hr, and then the extent of growth inhibition was recorded daily on a scale of 0 to 5, with 0 indicating the level of growth of the wild type strain and 5 indicating no growth. The results represent the average of at least two assays for screening each of the different metals, and then the susceptible mutants were confirmed in additional assays. The mutant strains were clustered using the program Cluster, and then a heat map was generated. The mutants that were not detectably sensitive to copper are listed in the Supplementary Information (S2 Table).
Assays for viability and sensitivity to copper
Strains were grown overnight at 30°C in synthetic medium. Cells were then diluted to 5 x 103 cells/ml in synthetic medium and 300 μl was added to the first row of wells of a 96-well plate (Costar, Corning, Inc., Kennebunk, ME). 240 μl of cells was applied to subsequent rows of the plate. CuSO4 was then added to wells of the first row to a final concentration of 10 mM and serial dilutions were made by transferring 60 μl from one row to the next. Plates were then covered with AeraSeal oxygen permeable sealing film and incubated at 37°C for 48 hr. The highest concentration of copper that supported growth was recorded. Similar methods were used to quantify the sensitivity of C. albicans strains to other metals. Viability following exposure to copper was assayed by growing strains overnight in synthetic medium to log phase at 30°C. The cells were then washed with sterile H2O and diluted to 3.0 x 103 cells/ml in sterile 1 mM MES buffer pH 6 in the presence or absence of 0.5 μM CuSO4. Cells were then incubated at 37°C for the indicated time, 100 μl of each sample was then spread onto YPD agar plates, incubated at 30°C for 48 hr, and then CFUs were quantified. The effects of the actin polymerization inhibitor cytochalasin A (Sigma-Aldrich, St. Louis, MO) were examined with cells that were grown overnight in synthetic medium at 30°C to log phase, diluted to 1 x 106 cells/ml in synthetic medium, and then cytochalasin A was added to a final concentration of 10 μg/ml every 30 min for 2 hr, with incubation at 30°C. Cytochalasin was added every 30 min to maintain a high level of this drug as it is not stable under these conditions [83]. Cells were then washed with sterile H2O, diluted to 3 x 103 cells/ml in sterile H2O, a final aliquot of cytochalasin A was added, and cells were incubated with the indicated concentrations of CuSO4 for 2 hr at 37°C. 100 μl of each sample was then spread onto YPD agar plates and incubated at 30°C for 48 hr to determine the viable CFUs. Statistical analysis of the data was carried out using Prism software (GraphPad Inc.).
Fluorescence microscopy
SYTOX Green (Invitrogen, Molecular Probes, Eugene, OR) is a membrane impermeable nucleic acid stain that can be used to assay plasma membrane integrity [84]. For the analysis, strains were grown in synthetic medium overnight at 30°C to log phase, washed in sterile H2O, and diluted to 1 x 107 cells/ml. Following incubation in CuSO4 with 1 mM MES buffer pH 6 at 37°C, cells were washed in sterile H2O, SYTOX Green was added to a final concentration of 2.5 nM, and cells were incubated at room temperature for 5 min. Cells were washed again in sterile H2O and then analyzed by fluorescence microscopy. Propidium iodide (Invitrogen, Molecular Probes, Eugene, OR) staining was carried out in a similar manner, except the stain was added to a final concentration of 4.2 μg/ml, and incubation was carried out for 15 min at room temperature. Cells were stained with the lipophilic dye FM4-64 (Invitrogen, Molecular Probes, Eugene, OR) by adding it to a final concentration of 20 μM [85]. Cells were incubated on ice for 20 min, washed in cold sterile H2O, and viewed immediately by fluorescence microscopy. Images were obtained using an Olympus BH2 microscope (Melville, NY) equipped with a Zeiss AxioCam digital camera (Thornwood, NY).
Antifungal drug growth inhibition assays
Cells were grown overnight in synthetic medium at 30°C, diluted to 1.0 x 106 cells/ml in synthetic medium, and 250 μl was spread onto the surface of a synthetic medium agar plate. Paper filter disks (Becton Dickinson and Company, Sparks, MD) containing 10 μl of the concentration of drug to be assayed were applied onto the surface of plates containing the indicated strains. After incubation for 48 hr at 30°C, the diameters of the zones of growth inhibition were measured. Drugs tested included duramycin (Sigma Aldrich, St. Louis, MO), cinnamycin (Santa Cruz Biotechnology, Santa Cruz, CA), papuamide A (Flintbox, British Columbia, Canada), and amphotericin B (Sigma-Aldrich, St. Louis, MO). Determination of sensitivity to the oxidizing agent H2O2 (Fisher Scientific, Fair Lawn, NJ), was performed by spot assay. Cells grown overnight in synthetic medium at 30°C were diluted to 1.0 x 107 cells/ml in synthetic medium and a 10-fold dilution series was made. 2 μl of each dilution was spotted onto YPD containing the indicated concentrations of H2O2 and plates were incubated at 30°C for 48 hr and then photographed. Strains tested for H2O2 sensitivity were also spotted onto SD medium containing CuSO4 for confirmation of copper sensitivity. To assay cells for reaction to polycations, strains were grown overnight in synthetic medium, washed in H2O, diluted to 3 x 103 cells/ml in H2O, and then incubated with DEAE dextran hydrochloride (500 KDa, Sigma-Aldrich, St. Louis, MO) or poly-L-lysine hydrobromide (30 kDa, Sigma-Aldrich, St. Louis, MO) for 2 hr at 37°C. 100 μl of each sample was then spread onto YPD medium, incubated at 30°C for 48 hr, and then CFUs were quantified to assess viability.
Fatty acid analysis
Cells were grown overnight in synthetic medium at 30°C, diluted to 1.0 x 106 cells/ml in 50 ml synthetic medium, and then incubated with shaking at 37°C until a concentration of approximately 1.0 x 107 cells/ml. Cells were then harvested by centrifugation, washed in sterile H2O, and then frozen cell pellets were shipped to Microbial ID, Inc. (Newark, DE) for fatty acid analysis. Sample preparation involved saponification, methylation, and extraction of fatty acid methyl esters. Gas chromatography was used to create a cellular fatty acid profile identifying the percent of each fatty acid species and localization of unsaturated bonds. For a second analysis, cells were streaked onto YPD medium, incubated at 30°C, and then submitted to Microbial ID, Inc. for testing.
Supporting information
C. albicans strains were incubated in a solution of CuSO4 for the indicated time, and then the viability of the cells was determined by plating cells on YPD medium and counting the CFUs. This assay was similar to that shown in Fig 1D, except that HCl was used to lower the pH. The results represent the average of three independent experiments performed on different days. Error bars indicate SD. Strains used were: WT, wild-type DIC185; sur7Δ (YJA11); sur7Δ comp. (YJA12); pil1Δ lsp1Δ (YHXW21-1); pil1Δ lsp1Δ comp (+ LSP1, YHXW23-1); and cup1Δ crp1Δ (KC25).
(TIF)
Representative samples of cells stained with the membrane impermeable dye SYTOX Green. Wild type and sur7Δ cells were incubated in the presence of 10 μM CuSO4 with 1 mM MES buffer at pH 6 for two hr. Cells were then washed, stained with SYTOX Green for 5 min, and then viewed by fluorescence microscopy. WT, wild-type DIC185; sur7Δ (YJA11).
(TIF)
(A) Log phase sur7Δ cells engineered to produce a fusion between the plasma membrane H+ ATPase Pma1 and GFP were incubated in water or 10 μM CuSO4 for 2 hr at 37°C. Cells were then stained with the membrane-impermeable dye propidium iodide (PI). The graph depicts how copper treatment causes a loss in GFP fluorescence and an increase in membrane permeability, indicated by PI staining.
(B) Photographs showing that sur7Δ PMA1-GFP cells that lost the GFP signal with CuSO4 treatment stained with PI. The graph represents averages of three independent experiments performed on different days. Strains used were the wild type control PMA1- GFPγ (YHXW11) and sur7Δ PMA1-GFPγ (YHXW61).
(C) The sur7Δ strain PMA1-GFP (YHXW61) was incubated in the presence or absence of 10 μM CuSO4 with 1 mM MES buffer at pH 6 for two hr, stained with FM4-64, and then imaged by fluorescence microscopy. Note that loss of Pma1-GFP correlated with intense staining by FM4-64.
(TIF)
The indicated strains were incubated with DEAE dextran hydrochloride (500 kDa) or poly-L-lysine hydrobromide (30 kDa) for 2 hr at 37°C. Samples were then plated onto YPD medium, incubated at 30°C for 48 hr, and then CFUs were counted to assess viability. WT, wild-type DIC185; sur7Δ (YJA11).
(TIF)
Representative halo assay for testing the sensitivity of cells to different drugs. A lawn of 2.5 x 105 cells was spread on the surface of a synthetic medium agar plate, and then paper filter disks containing 10 μl of different concentrations of the drug were applied to the surface of the plate. After incubation for 48 hr at 30°C, the plates were photographed. Concentrations used for amphotericin were 500, 250, 125, 50, and 0 μg/ml. Concentrations used for cinnamycin were 40, 20, and 0 μg/ml. Concentrations used for duramycin were 20, 10, 5, and 2.5 μg/ml and 0 μg/ml. Concentrations used for papuamide A were 1000, 500, 250, 125, and 0 μg/ml. Strains used were DIC185, sur7Δ (YJA11), rvs161Δ (YLD14-3), rvs167Δ (YLD16), and arp2Δ arp3Δ (CaEE27)
(TIF)
(DOCX)
(XLSX)
Acknowledgments
We thank the members of our lab for their helpful advice and comments on the manuscript. We also acknowledge the Fungal Genetics Stock Center for providing libraries of mutant C. albicans strains that were deposited by Drs. Suzanne Noble, Alexander Johnson, and Aaron Mitchell. We also thank Dr. Todd Reynolds for sending C. albicans strains and advice.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by a grant from the National Institutes of Health awarded to JBK. (RO1AI047837). https://www.nih.gov The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science translational medicine. 2012;4(165):165rv13 10.1126/scitranslmed.3004404 . [DOI] [PubMed] [Google Scholar]
- 2.Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015;373(15):1445–56. 10.1056/NEJMra1315399 . [DOI] [PubMed] [Google Scholar]
- 3.O'Meara TR, Robbins N, Cowen LE. The Hsp90 Chaperone Network Modulates Candida Virulence Traits. Trends Microbiol. 2017;25(10):809–19. Epub 2017/05/28. 10.1016/j.tim.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dantas Ada S, Day A, Ikeh M, Kos I, Achan B, Quinn J. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules. 2015;5(1):142–65. 10.3390/biom5010142 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown AJ, Haynes K, Quinn J. Nitrosative and oxidative stress responses in fungal pathogenicity. Curr Opin Microbiol. 2009;12(4):384–91. 10.1016/j.mib.2009.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besold AN, Culbertson EM, Culotta VC. The Yin and Yang of copper during infection. J Biol Inorg Chem. 2016;21(2):137–44. Epub 2016/01/23. 10.1007/s00775-016-1335-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Santamarina S, Thiele DJ. Copper at the Fungal Pathogen-Host Axis. J Biol Chem. 2015;290(31):18945–53. Epub 2015/06/10. 10.1074/jbc.R115.649129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgkinson V, Petris MJ. Copper homeostasis at the host-pathogen interface. J Biol Chem. 2012;287(17):13549–55. 10.1074/jbc.R111.316406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackie J, Szabo EK, Urgast DS, Ballou ER, Childers DS, MacCallum DM, et al. Host-Imposed Copper Poisoning Impacts Fungal Micronutrient Acquisition during Systemic Candida albicans Infections. PloS one. 2016;11(6):e0158683 10.1371/journal.pone.0158683 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A. 2015;112(38):E5336–42. 10.1073/pnas.1513447112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284(49):33949–56. Epub 2009/10/08. M109.070201 [pii] 10.1074/jbc.M109.070201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent M, Duval RE, Hartemann P, Engels-Deutsch M. Contact killing and antimicrobial properties of copper. J Appl Microbiol. 2017. Epub 2017/12/28. 10.1111/jam.13681 . [DOI] [PubMed] [Google Scholar]
- 13.Elzanowska H, Wolcott RG, Hannum DM, Hurst JK. Bactericidal properties of hydrogen peroxide and copper or iron-containing complex ions in relation to leukocyte function. Free radical biology & medicine. 1995;18(3):437–49. Epub 1995/03/01. 089158499400150I [pii]. . [DOI] [PubMed] [Google Scholar]
- 14.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284(5415):805–8. Epub 1999/04/30. . [DOI] [PubMed] [Google Scholar]
- 15.Smith AD, Logeman BL, Thiele DJ. Copper Acquisition and Utilization in Fungi. Annu Rev Microbiol. 2017;71:597–623. Epub 2017/09/10. 10.1146/annurev-micro-030117-020444 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marvin ME, Mason RP, Cashmore AM. The CaCTR1 gene is required for high-affinity iron uptake and is transcriptionally controlled by a copper-sensing transactivator encoded by CaMAC1. Microbiology. 2004;150(Pt 7):2197–208. Epub 2004/07/17. 10.1099/mic.0.27004-0 . [DOI] [PubMed] [Google Scholar]
- 17.Gleason JE, Li CX, Odeh HM, Culotta VC. Species-specific activation of Cu/Zn SOD by its CCS copper chaperone in the pathogenic yeast Candida albicans. J Biol Inorg Chem. 2014;19(4-5):595–603. Epub 2013/09/18. 10.1007/s00775-013-1045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weissman Z, Berdicevsky I, Cavari BZ, Kornitzer D. The high copper tolerance of Candida albicans is mediated by a P-type ATPase. Proc Natl Acad Sci U S A. 2000;97(7):3520–5. Epub 2000/03/29. 97/7/3520 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riggle PJ, Kumamoto CA. Role of a Candida albicans P1-type ATPase in resistance to copper and silver ion toxicity. J Bacteriol. 2000;182(17):4899–905. Epub 2000/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wysocki R, Tamas MJ. How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol Rev. 2010;34(6):925–51. Epub 2010/04/09. 10.1111/j.1574-6976.2010.00217.x . [DOI] [PubMed] [Google Scholar]
- 21.Jo WJ, Loguinov A, Chang M, Wintz H, Nislow C, Arkin AP, et al. Identification of genes involved in the toxic response of Saccharomyces cerevisiae against iron and copper overload by parallel analysis of deletion mutants. Toxicol Sci. 2008;101(1):140–51. Epub 2007/09/06. kfm226 [pii] 10.1093/toxsci/kfm226 . [DOI] [PubMed] [Google Scholar]
- 22.Ding C, Festa RA, Chen YL, Espart A, Palacios O, Espin J, et al. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell host & microbe. 2013;13(3):265–76. Epub 2013/03/19. 10.1016/j.chom.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Homann OR, Dea J, Noble SM, Johnson AD. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 2009;5(12):e1000783 Epub 2009/12/31. 10.1371/journal.pgen.1000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiele DJ. ACE1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol Cell Biol. 1988;8(7):2745–52. Epub 1988/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz JA, Olarte KT, Michalek JL, Jandu GS, Michel SL, Bruno VM. Regulation of copper toxicity by Candida albicans GPA2. Eukaryot Cell. 2013;12(7):954–61. Epub 2013/04/16. 10.1128/EC.00344-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douglas LM, Wang HX, Keppler-Ross S, Dean N, Konopka JB. Sur7 Promotes Plasma Membrane Organization and Is Needed for Resistance to Stressful Conditions and to the Invasive Growth and Virulence of Candida albicans. MBio. 2012;3(1):e00254–11. Epub 2011/12/29. 10.1128/mBio.00254-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas LM, Konopka JB. Fungal membrane organization: the eisosome concept. Annu Rev Microbiol. 2014;68:377–93. 10.1146/annurev-micro-091313-103507 . [DOI] [PubMed] [Google Scholar]
- 28.Malinsky J, Opekarova M, Tanner W. The lateral compartmentation of the yeast plasma membrane. Yeast. 2010;27(8):473–8. Epub 2010/07/20. 10.1002/yea.1772 . [DOI] [PubMed] [Google Scholar]
- 29.Ziolkowska NE, Christiano R, Walther TC. Organized living: formation mechanisms and functions of plasma membrane domains in yeast. Trends in cell biology. 2012;22(3):151–8. 10.1016/j.tcb.2011.12.002 . [DOI] [PubMed] [Google Scholar]
- 30.Stradalova V, Stahlschmidt W, Grossmann G, Blazikova M, Rachel R, Tanner W, et al. Furrow-like invaginations of the yeast plasma membrane correspond to membrane compartment of Can1. J Cell Sci. 2009;122(Pt 16):2887–94. Epub 2009/07/30. jcs.051227 [pii] 10.1242/jcs.051227 . [DOI] [PubMed] [Google Scholar]
- 31.Wang HX, Douglas LM, Vesela P, Rachel R, Malinsky J, Konopka JB. Eisosomes promote the ability of Sur7 to regulate plasma membrane organization in Candida albicans. Mol Biol Cell. 2016;27(10):1663–75. 10.1091/mbc.E16-01-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karotki L, Huiskonen JT, Stefan CJ, Ziolkowska NE, Roth R, Surma MA, et al. Eisosome proteins assemble into a membrane scaffold. J Cell Biol. 2011;195(5):889–902. 10.1083/jcb.201104040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez FJ, Douglas LM, Rosebrock A, Konopka JB. The Sur7 protein regulates plasma membrane organization and prevents intracellular cell wall growth in Candida albicans. Mol Biol Cell. 2008;19:5214–25. 10.1091/mbc.E08-05-0479 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HX, Douglas LM, Aimanianda V, Latge JP, Konopka JB. The Candida albicans Sur7 protein is needed for proper synthesis of the fibrillar component of the cell wall that confers strength. Eukaryot Cell. 2011;10(1):72–80. Epub 2010/12/01. EC.00167-10 [pii] 10.1128/EC.00167-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernardo SM, Lee SA. Candida albicans SUR7 contributes to secretion, biofilm formation, and macrophage killing. BMC Microbiol. 2010;10(1): 10.1186/471-2180-10-133 Epub 2010/05/04. [pii] 10.1186/1471-2180-10-133. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dupont CL, Grass G, Rensing C. Copper toxicity and the origin of bacterial resistance--new insights and applications. Metallomics. 2011;3(11):1109–18. Epub 2011/10/11. 10.1039/c1mt00107h . [DOI] [PubMed] [Google Scholar]
- 37.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A. 2009;106(20):8344–9. Epub 2009/05/07. 10.1073/pnas.0812808106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohsumi Y, Kitamoto K, Anraku Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J Bacteriol. 1988;170(6):2676–82. Epub 1988/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chardwiriyapreecha S, Hondo K, Inada H, Chahomchuen T, Sekito T, Iwaki T, et al. A simple and specific procedure to permeabilize the plasma membrane of Schizosaccharomyces pombe. Biosci Biotechnol Biochem. 2009;73(9):2090–5. Epub 2009/09/08. 10.1271/bbb.90319 . [DOI] [PubMed] [Google Scholar]
- 40.Demidchik V, Sokolik A, Yurin V. Characteristics of non-specific permeability and H+-ATPase inhibition induced in the plasma membrane of Nitella flexilis by excessive Cu2+. Planta. 2001;212(4):583–90. Epub 2001/08/30. . [DOI] [PubMed] [Google Scholar]
- 41.Tang CH, Lin CY, Lee SH, Wang WH. Cellular membrane accommodation of copper-induced oxidative conditions in the coral Seriatopora caliendrum. Aquat Toxicol. 2014;148:1–8. Epub 2014/01/21. 10.1016/j.aquatox.2013.12.027 . [DOI] [PubMed] [Google Scholar]
- 42.Kusler K, Odoh SO, Silakov A, Poyton MF, Pullanchery S, Cremer PS, et al. What Is the Preferred Conformation of Phosphatidylserine-Copper(II) Complexes? A Combined Theoretical and Experimental Investigation. J Phys Chem B. 2016;120(50):12883–9. Epub 2016/12/14. 10.1021/acs.jpcb.6b10675 . [DOI] [PubMed] [Google Scholar]
- 43.Monson CF, Cong X, Robison AD, Pace HP, Liu C, Poyton MF, et al. Phosphatidylserine reversibly binds Cu2+ with extremely high affinity. J Am Chem Soc. 2012;134(18):7773–9. Epub 2012/05/03. 10.1021/ja212138e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poyton MF, Sendecki AM, Cong X, Cremer PS. Cu(2+) Binds to Phosphatidylethanolamine and Increases Oxidation in Lipid Membranes. J Am Chem Soc. 2016;138(5):1584–90. Epub 2016/01/29. 10.1021/jacs.5b11561 . [DOI] [PubMed] [Google Scholar]
- 45.Santo CE, Quaranta D, Grass G. Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. Microbiologyopen. 2012;1(1):46–52. Epub 2012/09/06. 10.1002/mbo3.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quaranta D, Krans T, Espirito Santo C, Elowsky CG, Domaille DW, Chang CJ, et al. Mechanisms of contact-mediated killing of yeast cells on dry metallic copper surfaces. Appl Environ Microbiol. 2011;77(2):416–26. Epub 2010/11/26. 10.1128/AEM.01704-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet. 2010;42(7):590–8. Epub 2010/06/15. ng.605 [pii] 10.1038/ng.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nobile CJ, Bruno VM, Richard ML, Davis DA, Mitchell AP. Genetic control of chlamydospore formation in Candida albicans. Microbiology. 2003;149(Pt 12):3629–37. 10.1099/mic.0.26640-0 . [DOI] [PubMed] [Google Scholar]
- 49.Pradhan A, Herrero-de-Dios C, Belmonte R, Budge S, Lopez Garcia A, Kolmogorova A, et al. Elevated catalase expression in a fungal pathogen is a double-edged sword of iron. PLoS Pathog. 2017;13(5):e1006405 Epub 2017/05/26. 10.1371/journal.ppat.1006405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith DA, Nicholls S, Morgan BA, Brown AJ, Quinn J. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol Biol Cell. 2004;15(9):4179–90. 10.1091/mbc.E04-03-0181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, Naseem S, Sharma S, Konopka JB. Flavodoxin-Like Proteins Protect Candida albicans from Oxidative Stress and Promote Virulence. PLoS Pathog. 2015;11(9):e1005147 10.1371/journal.ppat.1005147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Douglas LM, Martin SW, Konopka JB. BAR domain proteins Rvs161 and Rvs167 contribute to Candida albicans endocytosis, morphogenesis, and virulence. Infect Immun. 2009;77(9):4150–60. Epub 2009/07/15. IAI.00683-09 [pii] 10.1128/IAI.00683-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epp E, Walther A, Lepine G, Leon Z, Mullick A, Raymond M, et al. Forward genetics in Candida albicans that reveals the Arp2/3 complex is required for hyphal formation, but not endocytosis. Mol Microbiol. 2010;75(5):1182–98. Epub 2010/02/10. 10.1111/j.1365-2958.2009.07038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Odds FC, Brown AJ, Gow NA. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003;11(6):272–9. . [DOI] [PubMed] [Google Scholar]
- 55.Hullin-Matsuda F, Makino A, Murate M, Kobayashi T. Probing phosphoethanolamine-containing lipids in membranes with duramycin/cinnamycin and aegerolysin proteins. Biochimie. 2016;130:81–90. Epub 2016/10/30. 10.1016/j.biochi.2016.09.020 . [DOI] [PubMed] [Google Scholar]
- 56.Cassilly CD, Maddox MM, Cherian PT, Bowling JJ, Hamann MT, Lee RE, et al. SB-224289 Antagonizes the Antifungal Mechanism of the Marine Depsipeptide Papuamide A. PloS one. 2016;11(5):e0154932 Epub 2016/05/18. 10.1371/journal.pone.0154932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parsons AB, Lopez A, Givoni IE, Williams DE, Gray CA, Porter J, et al. Exploring the mode-of-action of bioactive compounds by chemical-genetic profiling in yeast. Cell. 2006;126(3):611–25. Epub 2006/08/12. S0092-8674(06)00906-8 [pii] 10.1016/j.cell.2006.06.040 . [DOI] [PubMed] [Google Scholar]
- 58.Pomorski TG, Menon AK. Lipid somersaults: Uncovering the mechanisms of protein-mediated lipid flipping. Prog Lipid Res. 2016;64:69–84. Epub 2016/11/05. 10.1016/j.plipres.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Nobel JG, Klis FM, Munnik T, Priem J, van den Ende H. An assay of relative cell wall porosity in Saccharomyces cerevisiae, Kluyveromyces lactis and Schizosaccharomyces pombe. Yeast. 1990;6(6):483–90. Epub 1990/11/01. 10.1002/yea.320060605 . [DOI] [PubMed] [Google Scholar]
- 60.Howlett NG, Avery SV. Induction of lipid peroxidation during heavy metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation. Appl Environ Microbiol. 1997;63(8):2971–6. Epub 1997/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peltroche-Llacsahuanga H, Schmidt S, Seibold M, Lutticken R, Haase G. Differentiation between Candida dubliniensis and Candida albicans by fatty acid methyl ester analysis using gas-liquid chromatography. J Clin Microbiol. 2000;38(10):3696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gal S, Lichtenberg D, Bor A, Pinchuk I. Copper-induced peroxidation of phosphatidylserine-containing liposomes is inhibited by nanomolar concentrations of specific antioxidants. Chem Phys Lipids. 2007;150(2):186–203. Epub 2007/09/29. 10.1016/j.chemphyslip.2007.08.001 . [DOI] [PubMed] [Google Scholar]
- 63.Chen YL, Montedonico AE, Kauffman S, Dunlap JR, Menn FM, Reynolds TB. Phosphatidylserine synthase and phosphatidylserine decarboxylase are essential for cell wall integrity and virulence in Candida albicans. Mol Microbiol. 2010;75(5):1112–32. Epub 2010/02/06. 10.1111/j.1365-2958.2009.07018.x . [DOI] [PubMed] [Google Scholar]
- 64.Wolschendorf F, Ackart D, Shrestha TB, Hascall-Dove L, Nolan S, Lamichhane G, et al. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2011;108(4):1621–6. Epub 2011/01/06. 1009261108 [pii] 10.1073/pnas.1009261108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osman D, Waldron KJ, Denton H, Taylor CM, Grant AJ, Mastroeni P, et al. Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J Biol Chem. 2010;285(33):25259–68. Epub 2010/06/11. M110.145953 [pii] 10.1074/jbc.M110.145953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franke K, Nguyen M, Hartl A, Dahse HM, Vogl G, Wurzner R, et al. The vesicle transport protein Vac1p is required for virulence of Candida albicans. Microbiology. 2006;152(Pt 10):3111–21. Epub 2006/09/29. 10.1099/mic.0.29115-0 . [DOI] [PubMed] [Google Scholar]
- 67.Kitanovic A, Nguyen M, Vogl G, Hartmann A, Gunther J, Wurzner R, et al. Phosphatidylinositol 3-kinase VPS34 of Candida albicans is involved in filamentous growth, secretion of aspartic proteases, and intracellular detoxification. FEMS Yeast Res. 2005;5(4-5):431–9. Epub 2005/02/05. 10.1016/j.femsyr.2004.11.005 . [DOI] [PubMed] [Google Scholar]
- 68.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–24. 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Epp E, Nazarova E, Regan H, Douglas LM, Konopka JB, Vogel J, et al. Clathrin- and Arp2/3-Independent Endocytosis in the Fungal Pathogen Candida albicans. MBio. 2013;4(5): e00476–13. 10.1128/mBio.00476-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Last JA, Waggoner TA, Sasaki DY. Lipid membrane reorganization induced by chemical recognition. Biophysical journal. 2001;81(5):2737–42. Epub 2001/10/19. 10.1016/S0006-3495(01)75916-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suwalsky M, Ungerer B, Quevedo L, Aguilar F, Sotomayor CP. Cu2+ ions interact with cell membranes. J Inorg Biochem. 1998;70(3-4):233–8. Epub 1998/08/28. . [DOI] [PubMed] [Google Scholar]
- 72.Adams KF, Johnson G Jr., Hornowski KE, Lineberger TH. The effect of copper on erythrocyte deformability: a possible mechanism of hemolysis in acute copper intoxication. Biochim Biophys Acta. 1979;550(2):279–87. Epub 1979/01/19. . [DOI] [PubMed] [Google Scholar]
- 73.Xia X, Cheng L, Zhang S, Wang L, Hu J. The role of natural antimicrobial peptides during infection and chronic inflammation. Antonie Van Leeuwenhoek. 2018;111(1):5–26. Epub 2017/09/01. 10.1007/s10482-017-0929-0 . [DOI] [PubMed] [Google Scholar]
- 74.Douglas LM, Konopka JB. Plasma membrane organization promotes virulence of the human fungal pathogen Candida albicans. J Microbiol. 2016;54(3):178–91. 10.1007/s12275-016-5621-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szymanski P, Fraczek T, Markowicz M, Mikiciuk-Olasik E. Development of copper based drugs, radiopharmaceuticals and medical materials. Biometals. 2012;25(6):1089–112. Epub 2012/08/24. 10.1007/s10534-012-9578-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Festa RA, Helsel ME, Franz KJ, Thiele DJ. Exploiting innate immune cell activation of a copper-dependent antimicrobial agent during infection. Chem Biol. 2014;21(8):977–87. Epub 2014/08/05. 10.1016/j.chembiol.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chudzik B, Czernel G, Miaskowski A, Gagos M. Amphotericin B-copper(II) complex shows improved therapeutic index in vitro. Eur J Pharm Sci. 2017;97:9–21. Epub 2016/11/07. 10.1016/j.ejps.2016.10.040 . [DOI] [PubMed] [Google Scholar]
- 78.Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. [DOI] [PubMed] [Google Scholar]
- 79.Zhang C, Konopka JB. A photostable green fluorescent protein variant for analysis of protein localization in Candida albicans. Eukaryot Cell. 2010;9(1):224–6. Epub 2009/11/17. EC.00327-09 [pii] 10.1128/EC.00327-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181(6):1868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Min K, Ichikawa Y, Woolford CA, Mitchell AP. Candida albicans Gene Deletion with a Transient CRISPR-Cas9 System. mSphere. 2016;1(3):10.1128/mSphere.00130-16. 10.1128/mSphere.00130-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reuss O, Vik A, Kolter R, Morschhauser J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene. 2004;341:119–27. Epub 2004/10/12. S0378111904003555 [pii] 10.1016/j.gene.2004.06.021 . [DOI] [PubMed] [Google Scholar]
- 83.Rida PC, Nishikawa A, Won GY, Dean N. Yeast-to-hyphal transition triggers formin-dependent Golgi localization to the growing tip in Candida albicans. Mol Biol Cell. 2006;17(10):4364–78. Epub 2006/07/21. 10.1091/mbc.E06-02-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thevissen K, Terras FR, Broekaert WF. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol. 1999;65(12):5451–8. Epub 1999/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128(5):779–92. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
C. albicans strains were incubated in a solution of CuSO4 for the indicated time, and then the viability of the cells was determined by plating cells on YPD medium and counting the CFUs. This assay was similar to that shown in Fig 1D, except that HCl was used to lower the pH. The results represent the average of three independent experiments performed on different days. Error bars indicate SD. Strains used were: WT, wild-type DIC185; sur7Δ (YJA11); sur7Δ comp. (YJA12); pil1Δ lsp1Δ (YHXW21-1); pil1Δ lsp1Δ comp (+ LSP1, YHXW23-1); and cup1Δ crp1Δ (KC25).
(TIF)
Representative samples of cells stained with the membrane impermeable dye SYTOX Green. Wild type and sur7Δ cells were incubated in the presence of 10 μM CuSO4 with 1 mM MES buffer at pH 6 for two hr. Cells were then washed, stained with SYTOX Green for 5 min, and then viewed by fluorescence microscopy. WT, wild-type DIC185; sur7Δ (YJA11).
(TIF)
(A) Log phase sur7Δ cells engineered to produce a fusion between the plasma membrane H+ ATPase Pma1 and GFP were incubated in water or 10 μM CuSO4 for 2 hr at 37°C. Cells were then stained with the membrane-impermeable dye propidium iodide (PI). The graph depicts how copper treatment causes a loss in GFP fluorescence and an increase in membrane permeability, indicated by PI staining.
(B) Photographs showing that sur7Δ PMA1-GFP cells that lost the GFP signal with CuSO4 treatment stained with PI. The graph represents averages of three independent experiments performed on different days. Strains used were the wild type control PMA1- GFPγ (YHXW11) and sur7Δ PMA1-GFPγ (YHXW61).
(C) The sur7Δ strain PMA1-GFP (YHXW61) was incubated in the presence or absence of 10 μM CuSO4 with 1 mM MES buffer at pH 6 for two hr, stained with FM4-64, and then imaged by fluorescence microscopy. Note that loss of Pma1-GFP correlated with intense staining by FM4-64.
(TIF)
The indicated strains were incubated with DEAE dextran hydrochloride (500 kDa) or poly-L-lysine hydrobromide (30 kDa) for 2 hr at 37°C. Samples were then plated onto YPD medium, incubated at 30°C for 48 hr, and then CFUs were counted to assess viability. WT, wild-type DIC185; sur7Δ (YJA11).
(TIF)
Representative halo assay for testing the sensitivity of cells to different drugs. A lawn of 2.5 x 105 cells was spread on the surface of a synthetic medium agar plate, and then paper filter disks containing 10 μl of different concentrations of the drug were applied to the surface of the plate. After incubation for 48 hr at 30°C, the plates were photographed. Concentrations used for amphotericin were 500, 250, 125, 50, and 0 μg/ml. Concentrations used for cinnamycin were 40, 20, and 0 μg/ml. Concentrations used for duramycin were 20, 10, 5, and 2.5 μg/ml and 0 μg/ml. Concentrations used for papuamide A were 1000, 500, 250, 125, and 0 μg/ml. Strains used were DIC185, sur7Δ (YJA11), rvs161Δ (YLD14-3), rvs167Δ (YLD16), and arp2Δ arp3Δ (CaEE27)
(TIF)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.