Abstract
Objective:
Dysregulated proliferation of vascular smooth muscle cells (VSMC) plays an essential role in neointimal hyperplasia. CD36 functions critically in atherogenesis and thrombosis. We hypothesize that CD36 regulates VSMC proliferation and contributes to the development of obstructive vascular diseases.
Approach and Results:
We found by immunofluorescent staining that CD36 was highly expressed in human vessels with obstructive diseases. Using guide wire-induced carotid artery injury and shear stress-induced intima thickening models, we compared neointimal hyperplasia in Apoe−/−, Cd36−/−/Apoe−/−, and CD36 specifically deleted in VSMC (VSMCcd36−/−) mice. CD36 deficiency, either global or VSMC-specific, dramatically reduced injury-induced neointimal thickening. Correspondingly, carotid artery blood flow was significantly increased in Cd36−/−/Apoe−/− compared to Apoe−/− mice. In cultured VSMCs from thoracic aorta of WT and Cd36−/− mice, we found that loss of CD36 significantly decreased serum-stimulated proliferation and increased cell populations in S-phase, suggesting that CD36 is necessary for VSMC S/G2-M phase transition. Treatment of VSMCs with a thrombospondin 1 structural homology region (TSR) peptide significantly increased WT, but not Cd36−/− VSMC proliferation. TSR or serum treatment significantly increased cyclin A expression in WT but not in Cd36−/− VSMCs. STAT3, which reportedly enhances both VSMC differentiation and maturation, was higher in Cd36−/− VSMCs. CD36 deficiency significantly decreased expression of Col1A1 and TGFβ1, and increased expression of contractile proteins including calponin 1 and smooth muscle α actin, and dramatically increased cell contraction.
Conclusion:
CD36 promotes VSMC proliferation via upregulation of cyclin A expression that contributes to the development of neointimal hyperplasia, collagen deposition, and obstructive vascular diseases.
Keywords: CD36, vascular smooth muscle cell, neointimal hyperplasia, Animal Models of Human Disease, Basic Science Research, Smooth Muscle Proliferation and Differentiation, Vascular biology
Introduction
Obstructive and thrombotic cardiovascular diseases are the most common causes of death and disability in the developed world and comprise the largest category of disease burden.1 The etiology of these diseases includes genetic and environmental factors.2 An initiating step is often vascular injury induced by cellular oxidative stress, precipitated by the production of damaging reactive oxygen species (ROS) by many cell types, including vascular smooth muscle cells (VSMCs) and monocytes/macrophages.2 ROS contribute to injury by triggering intracellular signaling pathways and modifying extracellular molecules. For example, ROS-mediated oxidation of lipoproteins, such as oxidized low density lipoprotein (oxLDL) within the vessel wall, creates ligands for a family of cell surface receptors called “scavenger receptors”, which play a critical role in uptake of modified LDL by macrophages.3,4 Under conditions in which uptake cannot be balanced by natural efflux pathways, lipids accumulate in macrophages, leading to formation of foam cells: a hallmark for early atherosclerotic lesions.5-7 In addition to macrophages, VSMC phenotypic modulation and proliferation are other critical cellular events in the development of atherosclerosis and restenosis after angioplasty. Recent studies have suggested that a significant number of foam cells are derived from VSMCs.8 In fact, up to 70% of the mass of an atherosclerotic lesion has been estimated to be VSMCs or VSMC-derived,9 especially in the case of advanced lesions.
Our recent studies revealed that macrophage and platelet CD36 signaling pathways play central roles in mediating a pro-inflammatory response, and link the pathogenesis of atherosclerosis to these cellular populations.10,11 Although VSMCs in culture express CD3612-17 and this expression can be up regulated by peroxisome proliferator-activated receptor gamma,14 the function of CD36 on VSMCs in vivo is under-studied. We found that Cd36-deficient mice show attenuated vessel wall oxidative stress via upregulation of the antioxidant transcription factor, NF-E2-related factor 2 (Nrf2), and subsequent upregulation of specific antioxidant enzymes, including peroxiredoxin 2 (Prdx2) and heme oxygenase-1 (HO-1).18 Both Prdx2 and HO-1 have been reported to inhibit VSMC growth.19-22 Moreover, down-regulation of Nrf2/HO-1 expression has been reported to enhance neointima formation, which is associated with increased ROS-mediated VSMC proliferation.23 We thus hypothesized that CD36 contributes to the development of neointimal hyperplasia via regulation of VSMC function.
In this study, we report that CD36-mediated signaling enhances VSMC proliferation via upregulating expression of a cell cycle related protein, cyclin A. This effect is mediated, at least in part, by the CD36 ligand, thromobospondin 1 (TSP1). CD36 also promotes a de-differentiated VSMC phenotype via inhibition of signal transducer and activator of transcription (STAT) 3 activity, and increases expression of type 1 collagen A1 chain (Col1A1) and transforming growth factor beta 1 (TGF-β1). This study thus identifies CD36 as a novel target for the development of therapeutic strategies in prevention of obstructive vascular diseases.
Materials and Methods
The authors declare that all supporting data are available within the article and online supplementary files.
Mice
Many large population-based cohort studies report differences in atherosclerotic diseases in different sexes.24 To limit the potential effects of female hormones, only male mice ranging from 12 to 16 weeks of age were studied. Apoe knockout (Apoe−/−, Jackson Laboratory) and Cd36/Apoe double knockout (Cd36−/−/Apoe−/−) mice25 fed with chow diet were used for the guide wire-induced carotid artery injury model. These mice are C57Bl/6 congenic, and homozygous breeding was conducted on both strains.26 To examine the specific effect of VSMC CD36, a CD36 VSMC-specific deletion (VSMCcd36−/−) mouse strain was generated by crossing a CD36flox/flox mouse27 with a smooth muscle-specific Cre recombinase transgenic mouse, driven by the mouse transgelin (smooth muscle protein 22-alpha) promoter (B6.Cg-Tg(Tagln-cre)1Her/J, Stock # 017491, Jackson Laboratory). Both the CD36flox/flox and the Cre strain mice are C57Bl/6 congenic. The targeted deletion of CD36 in VSMC was verified by PCR assay of arterial segments (data not shown) as well as immunofluorescent staining. Mice were housed under specific pathogen free conditions and had ad libitum access to water and food. Handling and care of animals were approved and in compliance with the guidelines established by the Institutional Animal Care and Use Committee of the Cleveland Clinic.
Guide wire-induced carotid artery injury
Apoe−/− and Cd36−/−/Apoe−/− mice were anesthetized with ketamine/xylazine (100/10 mg/kg, IP) and the hair on the neck was shaved. Puralube Opthalmic Ointment (Pharmaderm, NDC 0462−0211−38) was applied to prevent corneal desiccation. Mice were secured in the supine position on the lid of a 15 cm tissue culture plate. The neck was sterilized using 70% isopropyl alcohol (Alcohol Preps, Kendall) and covered with a 3M™ Steri-Drape with a ~3 × 3 cm hole in the center to expose the surgical field. A middle cervical incision was made and the distal left common carotid artery and bifurcation were exposed. The proximal common and internal carotid arteries were temperately clamped with microvessel clamps. The external carotid artery was ligated with a 6−0 suture at the distal site. A 0.35 mm guide wire with coil (diameter 0.35 mm with coil, RADI) was introduced through the external carotid artery incision to the common carotid artery. The guide wire was pulled back and forth 3 times to remove the endothelium and to produce an injury on the medial smooth muscle layer throughout the common carotid artery. After removal of the guide wire, the external carotid artery was ligated proximal to the site of guide wire insertion. Carotid artery blood flow was confirmed; animals that did not reestablish carotid artery blood flow were excluded from further analysis. The wound was rinsed with saline and the incision closed with running 5−0 sutures (Ethicon J571). The mice were placed in a 37°C warming chamber and returned to the colony after retaining sternal recumbency.
Flow-dependent neointimal hyperplasia model by partial carotid artery ligation
Because VSMCCd36−/− mice are in the C57BL6 background, which is resistant to wire injury-induced neointimal hyperplasia,28 we performed a second model. Partial carotid artery ligation has been shown to reduce flow in the ligated artery and induce shear stress-dependent vascular remodeling in mice.29-32 Therefore, this model was used to examine the role of VSMC-specific CD36 deletion in vascular remodeling. In brief, after exposing the left carotid artery as outlined above, the internal carotid artery, the occipital artery, and the external carotid artery after the branching of the superior thyroid artery, were ligated.31 Vascular remodeling was assessed 4 weeks later.
Carotid artery blood flow measurement and sampling
Neointimal thickening was evaluated at 2 weeks and 4 weeks after guide wire injury as well as 4 weeks after partial carotid artery ligation. Mice were anesthetized with ketamine/xylazine (100/10 mg/kg, IP), and the carotid arteries were carefully isolated from surrounding tissue and blood flow was measured using an ultrasonic blood flow probe (Ultrasonic Inc.). After blood flow measurements, mice were euthanized with a lethal dose of ketamine/xylazine (200/20 mg/kg, IP). The chest cavity was opened and the mouse was perfused with 10 ml of 4% formalin in PBS via the left ventricle to fix the vessels. The carotid arteries were then harvested for histological examination. Cold PBS was used for perfusion to remove intravascular blood if samples were used for RNA or protein isolation.
Histological examination
Hematoxylin and eosin staining and Van Gieson Staining of the elastic lamina were performed to assess morphological changes and to identify the neointimal thickness (represented as ratio of intima/media), neointimal area, and media area.19,33 Double immunofluorescent staining was performed for proliferating cell nuclear antigen (PCNA, SC-25280, Santa Cruz) and alpha smooth muscle actin (αSMA, Sigma, A2547, and Abcam, ab7817) to assess the proliferative VSMCs in the neointima. Alexa Fluor 488, 555, 568, and 633-conjugated secondary antibodies (all from Thermo Fisher Scientific, Grand Island, NY) were used to detect signals with proper combinations as stated in “Results”. PCNA index was assessed by determining the ratio of PCNA positive VSMCs to total VSMCs in the neointima.
In order to determine the localization, as well as the role of CD36 in both proliferative and non-proliferative settings in diseased human vessels, double immunofluorescence staining for CD36 (CD36/SR-B3 antibody, NB400-144, Novus Biologicals, Littleton, CO) and αSMA were performed by two independent groups. We examined CD36 expression in temporal arteritis vessels that were obtained from anonymized pathology specimens in an Institutional Review Board-approved biospecimen registry at the University of Toledo Medical Center (IRB#:202972, PI: Dr. David Kennedy). We also examined CD36 expression in aorta and coronary arteries harvested from patients with severe atherosclerotic coronary artery diseases that received heart transplantations in an Institutional Review Board-approved protocol at Guangdong General Hospital (IRB#: GDREC2016255H, PI: Dr. Qiuxiong Lin). Aorta from donor hearts were stained as controls.
Primary VSMC culture
Mouse VSMC cultures were established from thoracic aorta explants from 8 week old male mice as previously described.19,34 Cells were identified as VSMCs by immunocytochemistry using a monoclonal antibody to αSMA.35 Cells from 4 to 12 passages were used for experiments.
Cell cycle analysis
VSMCs were cultured overnight in Dulbecco’s Modified Eagle’s Medium (DMEM, ThermoFisher Scientific) containing 10% fetal calf serum (FCS), fixed with 70% ethanol and then the cell cycle was analyzed by flow cytometry using propidium iodide (ThermoFisher Scientific) staining.19
Real-time (RT) PCR based mRNA quantitation assay
Total RNA was extracted from VSMCs using the RNeasy Mini kit (Qiagen, Hilden, Germany). One microgram of total RNA was treated with DNase I, and cDNA was generated using the AMV First Strand cDNA Synthesis Kit for RT-PCR (Roche, Indianapolis, IN). Real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) with an iCycler iQ real-time PCR detection system36 and validated commercially available probes.
Cell proliferation and survival assays
Cell proliferation and survival were assessed by MTT (3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide) as described previously,19,37 or by Trypan blue exclusion staining and cell counting with a hemocytometer.
In some experiments, VSMCs were seeded in 12 well plates (104 cells/well), cultured overnight and then subjected to serum starvation for 24 hours. The cells were then stimulated with DMEM containing 1% FCS, thrombospondin type 1 repeat (TSR) peptide38,39 or platelet-derived growth factor (PDGF, P8147-1VL, Sigma). The cells were cultured for 24 hours and then cell proliferation was assessed using the MTT assay. To test if TSP1-containing serum affects VSMC proliferation, WT VSMCs synchronized with serum-starvation were treated with serum prepared from WT or TSP1 null mice for an additional 24 hours and cells proliferation was assessed using the MTT assay.
Collagen-gel contraction assay
Collagen-gel contraction assay was performed as described previously with some modifications.40 Briefly, 12-well plates were pre-coated with 1% agarose to promote gel detachment. Type 1 collagen (PureCol® EZ Gel, Bovine Collagen, DMEM/F-12 Solution 5 mg/ml, pH 6.9-7.4, Advanced BioMatrix, San Diego, CA) and cell suspensions were mixed at a ratio of 1:2, and 0.5 ml of this mixture was then added into each well. The final mixture contained approximately 1.6 mg/ml collagen and 2 ×105 cells. Gelation was induced at 37 °C for 2 hours in a cell culture incubator. After gelatinization, 0.5 ml DMEM supplemented with 10% FCS was added into each well, and the gels were lifted off the bottom of the wells and allowed to float freely. Gels were photographed 24 hours later and the gel size (area) was measured using Image J (NIH; Bethesda, MD).
Western blot assays
VSMCs (2 × 106) were seeded in 10 cm culture dishes and cultured overnight. The cells were synchronized for 24 hours in serum-free DMEM and then they were stimulated with DMEM containing 10% FCS or TSR as stated in “Results”. The cells were washed with cold PBS three times and then lysed in 1x radioimmunoprecipitation assay buffer (ThermoFisher Scientific) containing both proteinase and phosphatase inhibitor cocktails (Roche, Indianapolis, IN) on ice. Protein concentration was measured using Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Fifty micrograms of total protein was used for Western blot assay based on standard procedures.
Statistical analyses
Data are presented as mean ± standard error. GraphPad Prism 7 for Windows was used for statistical analysis. Unpaired t test or Mann-Whitney test was used to determine the difference between groups, and p≤0.05 was considered statistically significant. Each in vitro experiment was repeated at least three times with a different passage of cells.
Results
CD36 is expressed in VSMC of vessels with obstructive disease
As introduced in the Introduction section, although CD36 was detectable in VSMC in vitro, there is no direct evidence of CD36 expression in diseased human vessels. By double immunofluorescenct staining for CD36 and αSMA, we examined CD36 expression in temporal arteries with arteritis that is characterized by intimal hyperplasia and luminal occlusion.41 We found that CD36 was expressed in VSMCs in the thickened intima (Fig. 1A). CD36 was also expressed in aortic and coronary arteries harvested from patients who had severe diffused coronary obstruction and required heart transplantation (Fig. 1B). CD36 was not detectable in the aortas from healthy donors (Fig. 1B, bottom panel). These data suggest that elevated CD36 expression may correlate with the development of obstructive vascular diseases.
Figure 1. CD36 expression in human diseased vessels.
A. Immunofluorescence staining for CD36 (green, Alexa Fluor 488) and smooth muscle α actin (SM-α-actin, red, Alexa Fluor 568) on temporal arteritis vessel. B. Immunofluorescence staining for CD36 (red, Alexa Fluor 555) and SMC-α-actin (green, Alexa Fluor 633) on aorta (top panel) and coronary artery (CA, middle panel) isolated from patients with severe coronary artery disese and received heart transplantation. Aorta form healthy donor hearts was also stained as control.
CD36 enhances neointimal hyperplasia after arterial injury
We previously demonstrated the expression of CD36 in murine VSMCs,18 a type of cell which plays an important role in vessel obstruction. In combination with findings that expression of CD36 is higher in human VSMCs with obstructive diseases, we suspected that CD36 would affect VSMC function and so studied the role of CD36 in obstructive vascular diseases using murine models. Since chow fed mice in the C57Bl/6 background are resistant to endothelial denudation-induced neointimal hyperplasia42,43, we examined the role of CD36 on intimal thickening with the added insult of hyperlipidemia/oxidative stress, using the Apoe deficient model commonly used in atherosclerosis studies.26 Carotid artery blood flow, measured 2 weeks after guide wire injury, was increased over 20% in Cd36−/−/Apoe−/− mice when compared with Apoe−/− mice (Fig. 2A), suggesting that Cd36 deficiency attenuated vascular injury-induced vessel stenosis. Since less neointimal thickening was observed in the Cd36−/−/Apoe−/− mice at 2 weeks, we further assessed intimal thickness at 4 weeks after guide wire injury. H&E (Supplementary Figure I) and Van Gieson’s staining (Fig. 2B) of the carotid arteries revealed that neointimal hyperplasia developed in both Apoe−/− and Cd36−/−/Apoe−/− mice. The level of intimal thickness was significantly decreased in the Cd36−/−/Apoe−/− mice, as these mice showed a smaller intima area as well as ratio of intima to media (I/M ratio) (Fig. 2C), and the intima thickness was reduced more than 75% when compared to the Apoe−/− mice. Interestingly, the vessel wall of Cd36−/−/Apoe−/− mice showed denser and better-organized elastic lamellae (Fig. 2B).
Figure 2. Absence of CD36 in Apoe null mice increases carotid artery blood flow and inhibits neointimal hyperplasia after guide wire-induced arterial injury.
A. Carotid artery blood flow was measured 2 weeks after guide wire injury using a transonic flowmeter. B. Van Gieson staining (VGS) of carotid artery cross sections 4 weeks after guide wire injury. Upper panel is shown at 100x, and low panel is shown at 400x magnification. Bar = 50 μm. C. Intima (I) and media (M) areas were measured in carotid arteries after VGS with Image J and ratio of intima to media (I/M) was calculated, n=8/group. D and E. Partial carotid artery ligation was performed on VSMCCd36−/− and VSMCCd36+/+ mice and vascular remodeling was assessed 4 weeks later by H&E and VGS (D), and intima, media area as well as the I/M ration was determined (E, n=9/group). Bar = 50 μm. F. Double immunofluorescence staining was performed for proliferating cell nuclear antigen (PCNA, green) and smooth muscle α-actin (SM α-actin) (red) using partial ligated carotid arteries harvested from the VSMCCd36−/− and VSMCCd36+/+ mice. Nuclei were stained with DAPI. Yellow dotted lines indicate the border between neointima and media. Bar graph shows PCNA index, which was calculated as percentage of PCNA positive VSMCs in the media and intima to the total nuclei (n=4/group). Images are shown at 200x magnification. Bar = 50 μm. G. Immunohistochemical staining for CD36 in the WT and VSMCCd36−/− vessels, Images were shown at 400x magnifications. Bar = 50 μm.
To further explore a specific role of CD36 in VSMC-mediated neointimal hyperplasia, we assessed neointimal thickness in CD36 VSMC-specific knockout mice using a partial carotid artery ligation model. As shown in Fig. 2D and E, similar to the global CD36 knockout, VSMC-specific deletion of CD36 also dramatically decreased vessel wall remodeling and significantly reduced I/M ratio, which was only about 30% of the I/M ratio found in the VSMC-CD36 positive vessels. Double immunofluorescent staining for PCNA and αSMA confirmed a large number of proliferating VSMCs in the injured VSMCCd36+/+ vessel wall compared to VSMCCd36−/− vessels, which had a lower PCNA index that was only one third of the VSMCCd36+/+ vessels (Fig. 2F). Immunohistochemical staining confirmed the expression of CD36 in the intima of the VSMCCd36+/+ vessel walls, but not in that of the VSMCCd36−/− vessels (Fig. 2G). In combination with the data presented in Fig. 1, these data suggested that CD36 plays an important role in regulating VSMC function in response to vascular damage, and that CD36 may contribute to the development of obstructive vascular diseases.
CD36 enhances VSMC proliferation in vitro
Having shown that CD36 enhances VSMC proliferation in vivo, which plays a critical role in neointimal hyperplasia,19 we aimed to explore how CD36 affects VSMC function in vitro. An equal number of primary cultured WT and Cd36−/− VSMCs were seeded in 6 cm plates and cells were counted daily using a hemocytometer following trypan blue exclusion staining to differentiate live and dead cells. No difference was found in the number of suspended dead cells during cell culture (Data not shown). The number of live WT cells was significantly greater compared with Cd36−/− from day 2, and by day 4, was ~1.7 fold greater (Fig. 3A), indicating that the presence of CD36 promotes VSMC proliferation. This phenotype was observed in randomly paired VSMCs cultured from 5 different mice of each strain. The decreased proliferation of Cd36−/− cells was not due to enhanced cell death. In line with the direct cell counting data, MTT assay further confirmed that the growth rate of WT cells was significantly higher than Cd36−/− VSMCs (Fig. 3B).
Figure 3. CD36 promotes VSMC proliferation in vitro.
A. Assessment by cell counting. Equal number of VSMCs were seeded in 6 cm plates, cultured in DMEM containing 10% FCS and counted daily for 4 days (n=5/group). * p<0.05 WT vs. Cd36-/−. B. MTT cell proliferation assay. 105 cells/well were seeded onto a 6 well plate. For the basal control, the cell culture media was changed to serum-free media 6 hrs after plating (to allow adhesion) and cultured overnight. MTT assay was performed the next day (= day 0 control). For other time points, the MTT assay was performed exactly 24, 48 and 72 hrs after plating cells. Data are expressed as fold change over Day 0, n=9/group. C. CD36 is necessary for PDGF induced VSMC proliferation. Cells were seeded into a 96-well plate in a density of 103 cells/well and cultured for onvernight. The cells were then synchronized by incubation in serum-free DMEM for 24 hours, and then stimulated with indicated conditions. Cell proliferation were assessed by MTT assay. D. Cell cycle analysis. 5 × 105 cells were seeded into a 10 cm plate and cultured for 24 hrs. Cell cycle was then analyzed by flow cytometry using propidium iodide staining. n=3/group, * p<0.01 WT vs. Cd36-/−.
PDGF is a potent SMC mitogen and has been used widely in VSMC studies.34 We thus examined the response of WT and Cd36−/− VSMCs to PDGF. As reported elsewhere, PDGF dramatically increased proliferation of WT VSMCs when compared to serum-starved cells (Fig. 3C). However, surprisingly, in the absence of CD36, PDGF significantly inhibited VSMC proliferation rather than enhanced cell growth. These data further suggest that deficiency of CD36 in VSMC generates a phenotype that is unfavorable for VSMC proliferation.
As descrived in the Instroduction, CD36 deficiency induced both Prdx2 and HO-1 expression, and HO-1 is known to inhibit VSMCs in the G1 phase.19 To understand how CD36 enhances VSMC proliferation, we analyzed the role of CD36 in cell cycle progression using a propidium iodide staining based flow cytometric assay. In comparison to WT VSMCs, Cd36−/− VSMCs had a significant increase (~60%) in the percentage of cells in S-phase, and a decrease of cells in G0/1 and G2/M (Fig. 3D). These data suggest that CD36 deficiency induces cell cycle arrest in the S-phase and reduces S-G2/M transition.
CD36 enhances VSMC proliferation by upregulating expression of cyclin A
Since CD36 deficiency induced S-phase arrest in VSMCs, we hypothesized that CD36-mediated signaling pathways must regulate expression of cell cycle related proteins in VSMCs. First, we examined the expression of cyclin proteins by Western blot assay. As shown in Fig. 4A, serum-stimulated induction of cyclins A and B expression was dramatically decreased in Cd36−/− VSMCs when compared to WT. This phenomenon was found up to 72 hours after serum stimulation (Fig. 4B), highlighting a slower cell cycle progression of the CD36 null VSMCs. However, the level of p21Cip1 (Fig. 4A), a cyclin dependent kinase inhibitor, was higher in WT VSMCs than in the Cd36−/− cells. This finding is in line with a previous study in which increased p21Cip1 expression was found when quiescent cells were stimulated to proliferate.44 Expression of p27Kip1 (Fig. 4C), another cyclin dependent kinase inhibitor, was also lower in serum stimulated Cd36−/− cells, suggesting that cyclin dependent kinase inhibitors were not responsible for the decreased proliferation of Cd36−/− VSMCs. The ubiquitin ligase protein, p19sky1, which contributes to cyclin A degradation, was expressed similarly in WT and Cd36−/− cells (Fig. 4C), ruling this out as a potential mechanism.
Figure 4. CD36 on expression of cyclin proteins and cyclin-dependent kinase inhibitors.
A to C. Expression of cyclin proteins and p21cip1, p27kip1 and p19skp1. WT and Cd36−/− cells were cultured in 10 cm plates (106 cells) for 24 hrs and then serum starved for 24 hrs. The cells were then treated with DMEM containing 10% FCS for the indicated times and expression of cyclins A and B, as well as cyclin-dependent kinase inhibitors p21cip1, p27kip1 and ubiquitin ligase protein, p19skp1, were assessed by Western blot. Actin or GAPDH served as loading controls. D and E. Expression of cyclin proteins using cell cycle inhibitors. Cells were plated as in A, serum starved for 24 hrs, and then treated with DMEM containing 10% FCS in the presence or absence of cell cycle progression inhibitors L-mimosine (G1), aphidicoline (S) or nocodazole (M) for 24 hrs (D) or 48 hours (E). Expression of cyclins A, B and D were determined by Western blot.
Since cyclin A is involved in both S phase DNA replication and S-G2/M phase transition,45,46 we considered that cyclin A may be responsible for the decreased cell cycle progression in Cd36−/− cells. To this end, we synchronized WT and Cd36−/− cells by serum starvation and then stimulated the cells with DMEM containing 10% FCS in the presence of L-mimosine (400 nM, Sigma), aphidicoline (10 μg/ml, Sigma) or nocodazole (400 nM, Sigma), which inhibits cell cycle in G1, early S or M phase, respectively, for 24 hours. We measured the levels of cyclins A, B, and D. Cells stimulated with DMEM containing 10% FCS alone served as control. As shown in Fig. 4D, there was no detectable cyclin A in serum-starved cells or in cells stimulated with DMEM containing 10% FCS in the presence of L-mimosine (G1 inhibition), as expected. Cyclin D is necessary for G1/S transition,47 which was unaffected in Cd36−/− cells (Fig. 4D). Levels of cyclin D were higher in Cd36−/− cells treated with L-mimosine, but were similar when the cells were treated with aphidicoline (S phase arrest). Cyclin B, a mitotic cyclin, which drives cells into and out of M phase, was comparable between WT and Cd36−/− cells after nicodazole-induced M-phase arrest. The level of cyclin A was less in Cd36−/− VSMCs than in the WT cells when the cells were inhibited in early S phase (aphidicoline), and after serum stimulation for 24 hours (Fig. 4D). However, in the presence of these cell cycle inhibitors, serum stimulation for 48 hours induced cyclin A expression reached to the same levels in WT and Cd36−/− cells at G1 and S-phases. Weak cyclin A expression was confirmed during M phase inhibition in the Cd36−/− cells, further suggesting slowed cell cycle progression in these cells. These data suggest that CD36-mediated signaling pathways promote cyclin A expression, and that the blockade of cyclin A expression may be responsible for the reduced proliferation of Cd36−/− VSMCs upon serum stimulation.
A TSP1/CD36 signaling pathway regulates cyclin A expression
Serum contains TSP1, which binds to CD36 through a specific site known as TSR,5,38 that can be mimicked with a TSR peptide. Strong upregulation of TSP1 expression has been reported in rat carotid arteries after balloon-angioplasty, as well as in the mouse vessel wall after carotid artery ligation.48,49 To determine whether TSP1 was involved in serum-stimulated VSMC proliferation, WT and Cd36−/− cells were synchronized by starvation in serum free media for 24 hours and then stimulated with the TSR peptide for another 24 hours. The TSR peptide significantly induced cyclin A expression in WT VSMCs, but had no effect in Cd36−/− cells (Fig. 5A). Using the MTT assay, we found that the TSR peptide also significantly increased WT VSMC proliferation, again having no effect on Cd36−/− cells (Fig. 5B). Compared to WT cells treated with 0.5% serum isolated from WT mice, cells treated with 0.5% serum isolated from TSP1 null mice showed significantly less proliferation (Fig. 5C). These data strongly suggest that TSP1 ligand interaction with CD36 promotes VSMC proliferation through upregulation of cyclin A expression.
Figure 5. A TSP1/CD36 signaling pathway increases cyclin A expression and promotes VSMC proliferation.
A. A TSP1/CD36 signaling pathway increases cyclin A expression. Serum starvation synchronized VSMCs were treated with media containing different concentrations of a TSR peptide for 24 hrs and cyclin A expression was assessed by Western blot. Actin served as loading control. B. A TSP1/CD36 signaling pathway promotes VSMC proliferation. VSMCs were seeded into a 12 well plate (104 cells/well), cultured overnight and then serum starved for 24 hrs. The cells were then stimulated with DMEM containing 1% FCS (control) or the TSR peptide, at the indicated concentrations. The cells were cultured for another 24 hrs and cell proliferation was assessed by MTT assay (n=3/group). * p<0.05 WT vs. Cd36-/−. C. TSP1 deficient plasma has less effect on WT VSMC proliferation. WT VSMCs were seeded into 96-well plate, serum-starvation for 24 hours and then stimulated with serum prepared from WT or TSP1 null mice for additonal 24 hours. Cell proliferation was assessed with MTT assay.
CD36 deficiency activates signaling pathways that enhance vessel maturation and distensibility
While STAT3 has been previously reported to mediate growth factor- or cytokine-induced VSMC proliferation, recent studies indicate that activated STAT3 is necessary to drive VSMCs into a more mature, non-proliferative phenotype.50 We previously found that increased activation and expression of STAT3 inversely correlates with serum-stimulated proliferation of thymidine phosphorylase overexpressing rat VSMCs, and that this depends on the Src family kinase Lyn.51 Lyn, as well as Fyn, another Src family kinase, mediates CD36 signaling pathways,10,18 suggesting a potential link between CD36 and STAT3. Using a Western blot assay, we found that the serum-stimulated induction of STAT3 phosphorylation at tyrosine 705 (p-Y705-STAT3) was significantly higher in Cd36−/− VSMCs compared to WT cells (Fig. 6A). This phenomenon was sustained for up to 24 hours (Fig. 6B). CD36 deficiency did not affect STAT3 phosphorylation at serine727 (p-S727-STAT3). Total–STAT3 (T-STAT3) showed no difference at early time points after serum stimulation (Fig. 6A), but was significantly increased in Cd36−/− VSMCs after 24 hours (Fig. 6C).
Figure 6. CD36 deficiency increases STAT3 activation.
A and B. WT and Cd36−/− VSMCs were cultured in 10 cm plates (106 cells/plate) for 24 hrs and serum starved for 24 hrs. Cells were then treated with DMEM containing 10% FCS for the indicated times and expression of active forms of STAT3 and total STAT3 (T-STAT3) were analyzed by Western blot. Actin served as loading control. C. Synchronized cells were treated with DMEM containing 10% serum for 24 hrs and p-Y705-STAT3 and T-STAT3 were assessed by Western blot. D. WT VSMCs were trasnsfeted with empty pcDNA6B, pcDNA6B/WT STAT3, or pCDNA6B/Y705F STAT3 plasmid vectors, and positived transfected cells were selected with blasticidin treatment (7 mg/ml). These gene transfected cells were then divided into 3 plates, serum starved for 24 hrs, and then stimulated with DMEM containing 10% FCS for 24 and 48 hours. The cells were lysed in radioimmunoprecipitation assay buffer containing proteinase and phosphotase inhibitors and then Western blot assays were performed using indicated antibodies.
OxLDL, a CD36 ligand, upregulates cyclin A expression in fibroblasts.52 STAT3 reportedly down-regulates cyclin D expression during liver regeneration.53 We thus determined whether STAT3 down-regulates expression of cyclin A. In contrast to our prediction, overexpression of mouse WT STAT3 dramatically increased cyclin A expression in WT VSMCs in response to serum stimulation. Overexpression of Y705F mutant STAT3 significantly inhibited serum stimulated cyclin A expression (Fig. 6D). These data suggest that the decreased cyclin A in the CD36 null cells is not related to the high activity of STAT3.
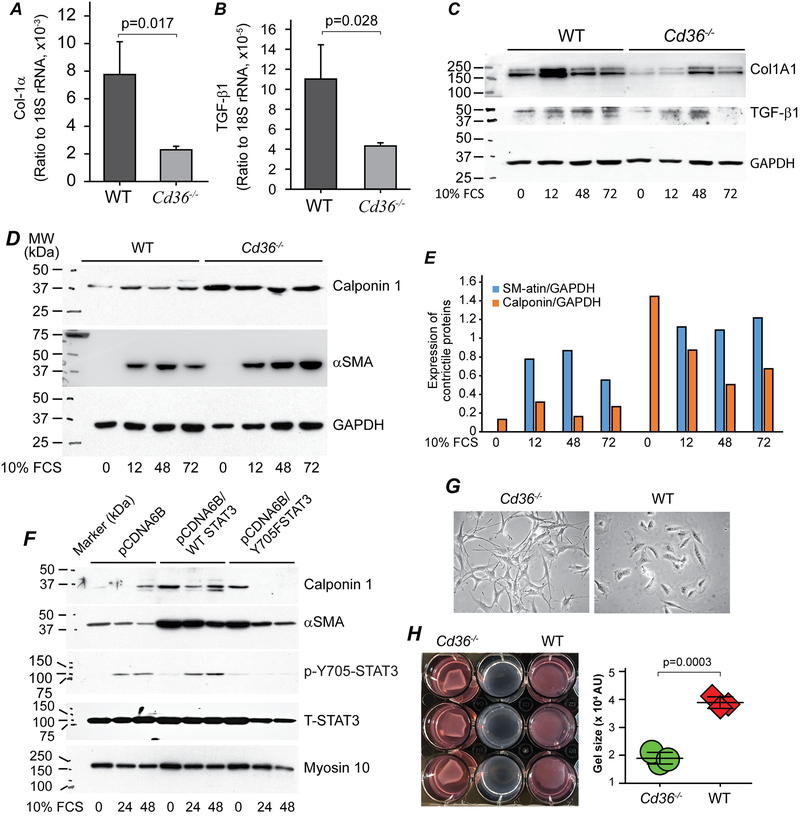
The greater density of elastic lamellae in the vessel wall of Cd36−/− mice post injury (Fig. 2B) suggested that inhibition of CD36 signaling might promote a more mature vessel wall and underlie a faster and greater functional recovery. To test this hypothesis, we examined the expression of Col1A1 and the pro-fibrotic growth factor, TGF-β154 by quantitative RT-PCR in WT and Cd36−/− cells after serum stimulation for 24 hours. We found CD36 deficiency dramatically decreased expression of both Col1A1, the major component of an atherosclerotic vessel, and TGF-β1, which is necessary for upregulation of Col1A1 expression (Fig. 7A & B). Western blot confirmed the decreased expression of Col1A1 in Cd36−/− cells, which is paralleled with the decreased expression of precursor TGF beta 1 (Fig. 7C). These data suggest that CD36 deficiency may create an environment that drives VSMCs to a more mature phenotype that may enhance vessel distensibility.
Figure 7. CD36 deficiency switches VSMCs to a contraction phenotype.
A & B. WT and Cd36−/− cells were cultured in 10 cm plates (106 cells) for 24 hrs and serum starved for 24 hrs. Cells were then stimulated with DMEM containing 10% FCS for an additional 24 hours. Total RNAs were isolated, converted to cDNA and qPCRs was performed for Col1A1 (A) and TGF-β (B), and data were normalized to 18s rRNA, n=3/group. C, D and E. Serum-starvation synchronized WT and Cd36−/− cells were stimulated with 10% FCS for the indicated times, and then Col1A1, TGF-b1 precursor (C), as well as Calponin 1 and aSMA (D & E) were assessed by Western blot assay. GAPDH served as loading control in both blots. F. Cell lysate prepared as mentioned in Fig. 6D were used for examination of Calponin 1, aSMA and myosin 10. Blots for T-STAT3 and p-Y705-STAT3 were same to that shown in Fig. 6D. G. Phase-contract images of WT and Cd36−/− cells. H. Collagen-gel based cell contraction assay.
This hypothesis was supported by examining the expression of contractile proteins including αSMA and calponin 1.55,56 As shown in Fig. 7D & E, although αSMA was not detectable in serum-starved synchronized cells, calponin 1 expression was significantly higher in the Cd36−/− VSMCs when compared with the WT cells. Serum-stimulation increased the expression of αSMA in both cells, however, the levels were higher in the Cd36−/− VSMCs. Interestingly, a recent study indicated that αSMA inhibits VSMC proliferation,55 which is in line with our current findings. Serum stimulation increased calponin 1 expression in WT cells, but reduced its expression in Cd36−/− cells; however, the levels of calponin 1 were still higher in the Cd36−/− VSMCs than in the WT cells. The increased expression of calponin 1 and αSMA maybe due to the increased T-STAT3 expression in the Cd36−/− cells. Because both WT STAT3 and Y705F-STAT3 overexpression increased calponin 1 and αSMA expression in resting cells (Fig 7F), in which there was neglectable p-STAT3. These data suggest that CD36 deficiency leads to a mature and contractive VSMC phenotype, which matches their morphology as shown in Fig. 7F. To examine this speculation more directly, we performed a gel contraction assay. As shown in Fig. 7G, the size of the collagen gels containing Cd36−/− cells were only half the size of the gels containing WT cells. These data suggest that CD36 deficiency increases VSMCs contraction.
Discussion
VSMCs are the major cell population in the arterial vessel wall. They are highly specialized and differentiated. Changes in VSMC behavior, function, and antioxidant status contribute to the development of obstructive vascular diseases, such as atherosclerosis and restenosis after percutaneous transluminal coronary angioplasty. However, signaling pathways that contribute to VSMC differentiation and proliferation are still not well understood. CD36, a widely expressed “pattern recognition” scavenger receptor, plays an important role in the development of atherosclerosis and thrombosis.26,57 VSMCs also express CD36. Our previous work demonstrated that CD36 inhibits Nrf2 activation, resulting in downregulation of phase II antioxidant enzymes and subsequently, enhances ROS generation in VSMCs.18 In the current study, we demonstrate that VSMC proliferation is dramatically increased by the CD36 ligand, TSP1, which is upregulated in injured vessels. We found that ablation of CD36 in VSMCs significantly decreases cell proliferation and neointimal hyperplasia in injured carotid arteries. These studies suggest that targeting VSMC CD36 could be beneficial in preventing atherosclerosis and restenosis.
CD36 is a multifunctional receptor with an independent capacity to bind at least 3 major classes of ligands: modified phospholipids, long-chain fatty acids, and proteins containing a TSR.5 TSP1 expression has been reported to strongly increase in the matrix of the neointima and adventitia after vascular injury, and TSP1 deficiency delays activation, proliferation, and migration of media VSMCs.49 Our findings corroborate these previous studies and further suggest that the role of TSP1 on VSMCs is mediated, at least in part, by CD36. Interestingly, both HO-1 and Prdx2, which are upregulated in the absence of CD36, inhibit VSMC proliferation.19,22,58 HO-1 induces cell cycle arrest in G1 phase by inducing the expression of cyclin dependent kinase inhibitors p27kip1 and p21Cip1.19,58 Prdx2 down-regulates a platelet-derived growth factor receptor-mediated signaling pathway.22 The current data suggest that TSP1 enhances VSMC proliferation through CD36 by an unrelated mechanism. Our findings are in line with a previous study in which TSP1 deficiency dramatically decreased SMC area in atherosclerotic lesions.59
Modulation of the mitotic cell cycle is controlled by a large number of proteins including cyclin dependent kinase and cyclins. In this study, we showed that CD36-induced VSMC proliferation is dependent upon enhanced cyclin A expression and thus, CD36 deficiency delays cell cycle S/G2-M transition. Both TSP1 and oxLDL are high affinity CD36 ligands that bind to CD36 at neighboring sites.60 TSP1 binds to the CD36, LIMP-2, Emp sequence homology domain (AA 93-155) via its TSR, and oxLDL binds to the site (AA 155-183) next to the CD36, LIMP-2, Emp sequence homology domain.38,61 OxLDL has been shown to enhance insulin-growth factor-I induced VSMC proliferation and anti-CD36 antibody diminished this effect and inhibited phosphorylation of mitogen-activated protein kinases JNK1 and JNK2.62 A recent study demonstrated that oxLDL induces cyclin A expression in fibroblasts in a JNK dependent manner.52 Our previous work revealed that engagement of CD36 ligands in platelets and macrophages induced JNK activation via Fyn and Lyn.10,11 We thus postulate that the engagement of CD36 ligands such as oxLDL and TSP1, generated during oxidative stress and/or pathological vascular injury, promotes cyclin A expression through activation of JNK signaling, or other mitogen-activated protein kinases, such as ERK, in VSMCs. However, despite our current endeavors, the exact mechanisms and signaling pathways that mediate TSP1/CD36 driven cyclin A expression are unclear. Additional detailed studies are needed to clarify this issue.
One interesting finding of our study is that CD36 deficiency induced higher STAT3 phosphorylation at Y705 in response to serum stimulation, but showed no obvious effect on S727. Phosphorylation at S727 has been considered a secondary event following Y705 phosporylation, but may not be essential to STAT3 downstream events.63 The STAT3 signaling pathway is considered to play a critical role in growth factor- or cytokine-induced VSMC proliferation and migration. We also found that overexpression of STAT3 upregulates cyclin A expression, and Y705F mutant STAT3 diminished serum-stimulated cyclin A expression, suggesting that the increased STAT3 phosphorylation in the Cd36−/− cells may be a compensatory effect. However, a recent study showed that high levels of STAT3 drive VSMCs into a more mature/differentiated phenotype.50 We also found that in addition to p-Y705-STAT3, T-STAT3 was higher in thymidine phosphorylase overexpressing VSMCs, which have a decreased proliferation rate when compared to control cells,51 and increased cell contraction as well (Li, unpublished data). In addition, non-canonical STAT3 activation regulates excess TGF-β1 and collagen I expression in muscles with stricturing Crohn’s disease.64 Taken together, our studies combined with recently published data suggest that activated STAT3 may not only contribute to a switch in VSMCs to a more mature, differentiated phenotype, but also may be necessary in enhancing VSMC proliferation by regulating cyclin A expression. Additional studies are necessary to clarify this consequence.
In summary, our study demonstrates that CD36 enhances VSMC proliferation in vitro and promotes neointimal hyperplasia in vivo. In addition to its role on macrophages and platelets, this newly identified role for CD36 on VSMCs may further contribute to the development of atherosclerosis and/or thrombosis. Targeting CD36 may attenuate vascular injury associated diseases.
Supplementary Material
Highlights.
CD36 is highly expressed in human vessels with obstructive diseases
Thrombospondin 1, through CD36 mediated signaling, enhances proliferation of vascular smooth muscle cells via upregulated cyclin A expression
CD36 deficiency in vivo reduces vascular injury induced intimal hyperplasia
CD36 deficiency enhances vascular smooth muscle cell maturation and increases cell contraction.
Acknowledgements
b) Sources of Funding
This study is supported by Marshall University Institute Fund (To Dr. Wei Li), Marshall University, School of Medicine and College of Pharmacy Collaborative Grant (PI: Dr. Wei Li), NIH grants HL081011 (PI: Roy Silverstein), HL137004 (PI: David Kennedy), as well as NIAAA 017748 and HL092747 (PI: Thomas M McIntyre). Dr. Wei Li is partially supported by NIH grants: NIH R01HL129179 (PI: Anirban Sen Gupta) and R01HL130090 (PI: Thomas M McIntyre), as well as WV-INBRE grant P20GM103434 (PI: Gary Rankin). Dr. Yueheng Wu is supported by Natural Science Foundation of Guangdong Province, China (project No.2015A030313659) and The Science and Technology Program of Guangzhou, China (project No. 201510010190). Dr. Kennedy is supported by American Heart Association Scientist Development Grant (14SDG18650010), as well as the David and Helen Boone Foundation Research Fund, and the University of Toledo Women and Philanthropy Genetic Analysis Instrumentation Center.
Nonstandard Abbreviations and Acronyms
- ROS
reactive oxygen species
- VSMCs
vascular smooth muscle cells
- oxLDL
oxidized low density lipoprotein
- Nrf2
NF-E2-related factor 2
- HO-1
heme oxygenase-1
- Prdx2
peroxiredoxin 2
- TSP1
thromobospondin 1
- STAT
signal transducer and activator of transcription
- Col1A1
type 1 collagen A1 chain
- TGF-β1
transforming growth factor beta 1
- αSMA
alpha smooth muscle actin
- PCNA
proliferating cell nuclear antigen
- DMEM
Dulbecco’s Modified Eagle’s Medium
- FCS
fetal calf serum
- TSR
thrombospondin type 1 repeat
- MTT
3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide
- PDGF
platelet-derived growth factor
- WT
wild type
Footnotes
c) Disclosure
None
Literature Cited
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (dalys) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2197–2223 [DOI] [PubMed] [Google Scholar]
- 2.Fearon IM, Faux SP. Oxidative stress and cardiovascular disease: Novel tools give (free) radical insight. J Mol Cell Cardiol 2009;47:372–381 [DOI] [PubMed] [Google Scholar]
- 3.Stephen SL, Freestone K, Dunn S, Twigg MW, Homer-Vanniasinkam S, Walker JH, Wheatcroft SB, Ponnambalam S. Scavenger receptors and their potential as therapeutic targets in the treatment of cardiovascular disease. Int J Hypertens 2010;2010:646929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: From pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverstein RL, Febbraio M. Cd36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat Med 2002;8:1211–1217 [DOI] [PubMed] [Google Scholar]
- 7.Blum A. The possible role of red blood cell microvesicles in atherosclerosis. Eur J Intern Med 2009;20:101–105 [DOI] [PubMed] [Google Scholar]
- 8.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–1559 [DOI] [PubMed] [Google Scholar]
- 9.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 2012;74:13–40 [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Febbraio M, Li W, Silverstein RL. A specific cd36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circulation research. 2008;102:1512–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A cd36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab 2006;4:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto K, Hirano K, Nozaki S, Takamoto A, Nishida M, Nakagawa-Toyama Y, Janabi MY, Ohya T, Yamashita S, Matsuzawa Y. Expression of macrophage (mphi) scavenger receptor, cd36, in cultured human aortic smooth muscle cells in association with expression of peroxisome proliferator activated receptor-gamma, which regulates gain of mphi-like phenotype in vitro, and its implication in atherogenesis. Arterioscler Thromb Vasc Biol 2000;20:1027–1032 [DOI] [PubMed] [Google Scholar]
- 13.Ricciarelli R, Zingg JM, Azzi A. Vitamin e reduces the uptake of oxidized ldl by inhibiting cd36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation. 2000;102:82–87 [DOI] [PubMed] [Google Scholar]
- 14.Lim HJ, Lee S, Lee KS, Park JH, Jang Y, Lee EJ, Park HY. Ppargamma activation induces cd36 expression and stimulates foam cell like changes in rvsmcs. Prostaglandins Other Lipid Mediat 2006;80:165–174 [DOI] [PubMed] [Google Scholar]
- 15.Kwok CF, Juan CC, Ho LT. Endothelin-1 decreases cd36 protein expression in vascular smooth muscle cells. Am J Physiol Endocrinol Metab 2007;292:E648–652 [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira Silva C, Delbosc S, Arais C, Monnier L, Cristol JP, Pares-Herbute N. Modulation of cd36 protein expression by ages and insulin in aortic vsmcs from diabetic and non-diabetic rats. Nutr Metab Cardiovasc Dis 2008;18:23–30 [DOI] [PubMed] [Google Scholar]
- 17.Xue JH, Yuan Z, Wu Y, Liu Y, Zhao Y, Zhang WP, Tian YL, Liu WM, Kishimoto C. High glucose promotes intracellular lipid accumulation in vascular smooth muscle cells by impairing cholesterol influx and efflux balance. Cardiovasc Res 2009 [DOI] [PubMed] [Google Scholar]
- 18.Li W, Febbraio M, Reddy SP, Yu DY, Yamamoto M, Silverstein RL. Cd36 participates in a signaling pathway that regulates ros formation in murine vsmcs. J Clin Invest 2010;120:3996–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Tanaka K, Morioka K, Uesaka T, Yamada N, Takamori A, Handa M, Tanabe S, Ihaya A. Thymidine phosphorylase gene transfer inhibits vascular smooth muscle cell proliferation by upregulating heme oxygenase-1 and p27kip1. Arterioscler Thromb Vasc Biol 2005;25:1370–1375 [DOI] [PubMed] [Google Scholar]
- 20.Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2001;104:1519–1525 [DOI] [PubMed] [Google Scholar]
- 21.Schillinger M, Exner M, Minar E, Mlekusch W, Mullner M, Mannhalter C, Bach FH, Wagner O. Heme oxygenase-1 genotype and restenosis after balloon angioplasty: A novel vascular protective factor. J Am Coll Cardiol 2004;43:950–957 [DOI] [PubMed] [Google Scholar]
- 22.Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of pdgf signalling and vascular remodelling by peroxiredoxin ii. Nature. 2005;435:347–353 [DOI] [PubMed] [Google Scholar]
- 23.Kim SE, Lee MY, Lim SC, Hien TT, Kim JW, Ahn SG, Yoon JH, Kim SK, Choi HS, Kang KW. Role of pin1 in neointima formation: Down-regulation of nrf2-dependent heme oxygenase-1 expression by pin1. Free Radic Biol Med 2010;48:1644–1653 [DOI] [PubMed] [Google Scholar]
- 24.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies-statement from atvb council. Arterioscler Thromb Vasc Biol 2018;38:292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of cd36 protects against atherosclerosis in apoe knock-out mice with no additional protection provided by absence of scavenger receptor a i/ii. Cardiovasc Res 2008;78:185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class b scavenger receptor cd36 protects against atherosclerotic lesion development in mice. J Clin Invest 2000;105:1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagendran J, Pulinilkunnil T, Kienesberger PC, Sung MM, Fung D, Febbraio M, Dyck JR. Cardiomyocyte-specific ablation of cd36 improves post-ischemic functional recovery. J Mol Cell Cardiol 2013;63:180–188 [DOI] [PubMed] [Google Scholar]
- 28.Hui DY. Intimal hyperplasia in murine models. Curr Drug Targets. 2008;9:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rezvan A, Ni CW, Alberts-Grill N, Jo H. Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: Role of oxidative stress. Antioxidants & redox signaling. 2011;15:1433–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: A model for carotid intima-media thickening. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:2185–2191 [DOI] [PubMed] [Google Scholar]
- 31.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. American journal of physiology. Heart and circulatory physiology. 2009;297:H1535–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan CJ, Hoying JB. Flow-dependent remodeling in the carotid artery of fibroblast growth factor-2 knockout mice. Arteriosclerosis, thrombosis, and vascular biology. 2002;22:1100–1105 [DOI] [PubMed] [Google Scholar]
- 33.Handa M, Li W, Morioka K, Takamori A, Yamada N, Ihaya A. Adventitial delivery of platelet-derived endothelial cell growth factor gene prevented intimal hyperplasia of vein graft. J Vasc Surg 2008;48:1566–1574 [DOI] [PubMed] [Google Scholar]
- 34.Yue H, Lee JD, Shimizu H, Uzui H, Mitsuke Y, Ueda T. Effects of magnesium on the production of extracellular matrix metalloproteinases in cultured rat vascular smooth muscle cells. Atherosclerosis. 2003;166:271–277 [DOI] [PubMed] [Google Scholar]
- 35.Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, Bussolati G, Ravazzola M, Orci L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem 1989;37:315–321 [DOI] [PubMed] [Google Scholar]
- 36.Yang M, Li K, Ng MH, Yuen PM, Fok TF, Li CK, Hogg PJ, Chong BH. Thrombospondin-1 inhibits in vitro megakaryocytopoiesis via cd36. Thromb Res 2003;109:47–54 [DOI] [PubMed] [Google Scholar]
- 37.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63 [DOI] [PubMed] [Google Scholar]
- 38.Klenotic PA, Page RC, Li W, Amick J, Misra S, Silverstein RL. Molecular basis of antiangiogenic thrombospondin-1 type 1 repeat domain interactions with cd36. Arterioscler Thromb Vasc Biol 2013;33:1655–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klenotic PA, Page RC, Misra S, Silverstein RL. Expression, purification and structural characterization of functionally replete thrombospondin-1 type 1 repeats in a bacterial expression system. Protein Expr Purif 2011;80:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mujumdar VS, Tummalapalli CM, Aru GM, Tyagi SC. Mechanism of constrictive vascular remodeling by homocysteine: Role of ppar. Am J Physiol Cell Physiol 2002;282:C1009–1015 [DOI] [PubMed] [Google Scholar]
- 41.Makkuni D, Bharadwaj A, Wolfe K, Payne S, Hutchings A, Dasgupta B. Is intimal hyperplasia a marker of neuro-ophthalmic complications of giant cell arteritis? Rheumatology (Oxford). 2008;47:488–490 [DOI] [PubMed] [Google Scholar]
- 42.Kuhel DG, Zhu B, Witte DP, Hui DY. Distinction in genetic determinants for injury-induced neointimal hyperplasia and diet-induced atherosclerosis in inbred mice. Arterioscler Thromb Vasc Biol 2002;22:955–960 [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Paigen B. Comparative genetics of atherosclerosis and restenosis: Exploration with mouse models. Arterioscler Thromb Vasc Biol 2002;22:884–886 [DOI] [PubMed] [Google Scholar]
- 44.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573 [DOI] [PubMed] [Google Scholar]
- 45.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci 2005;30:630–641 [DOI] [PubMed] [Google Scholar]
- 46.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin a is required at two points in the human cell cycle. EMBO J. 1992;11:961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimura T, Fukumoto M, Kunugita N. The role of cyclin d1 in response to long-term exposure to ionizing radiation. Cell Cycle. 2013;12:2738–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raugi GJ, Mullen JS, Bark DH, Okada T, Mayberg MR. Thrombospondin deposition in rat carotid artery injury. Am J Pathol 1990;137:179–185 [PMC free article] [PubMed] [Google Scholar]
- 49.Moura R, Tjwa M, Vandervoort P, Cludts K, Hoylaerts MF. Thrombospondin-1 activates medial smooth muscle cells and triggers neointima formation upon mouse carotid artery ligation. Arterioscler Thromb Vasc Biol 2007;27:2163–2169 [DOI] [PubMed] [Google Scholar]
- 50.Kirchmer MN, Franco A, Albasanz-Puig A, Murray J, Yagi M, Gao L, Dong ZM, Wijelath ES. Modulation of vascular smooth muscle cell phenotype by stat-1 and stat-3. Atherosclerosis. 2014;234:169–175 [DOI] [PubMed] [Google Scholar]
- 51.Yue H, Tanaka K, Furukawa T, Karnik SS, Li W. Thymidine phosphorylase inhibits vascular smooth muscle cell proliferation via upregulation of stat3. Biochimica et biophysica acta. 2012;1823:1316–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maziere C, Trecherel E, Ausseil J, Louandre C, Maziere JC. Oxidized low density lipoprotein induces cyclin a synthesis. Involvement of erk, jnk and nfkappab. Atherosclerosis. 2011;218:308–313 [DOI] [PubMed] [Google Scholar]
- 53.Matsui T, Kinoshita T, Hirano T, Yokota T, Miyajima A. Stat3 down-regulates the expression of cyclin d during liver development. J Biol Chem 2002;277:36167–36173 [DOI] [PubMed] [Google Scholar]
- 54.Tang R, Zhang G, Wang YC, Mei X, Chen SY. The long non-coding rna gas5 regulates transforming growth factor beta (tgf-beta)-induced smooth muscle cell differentiation via rna smad-binding elements. J Biol Chem 2017;292:14270–14278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L, DeWispelaere A, Dastvan F, Osborne WR, Blechner C, Windhorst S, Daum G. Smooth muscle-alpha actin inhibits vascular smooth muscle cell proliferation and migration by inhibiting rac1 activity. PLoS One. 2016;11:e0155726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu R, Jin JP. Calponin isoforms cnn1, cnn2 and cnn3: Regulators for actin cytoskeleton functions in smooth muscle and non-muscle cells. Gene. 2016;585:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh A, Li W, Febbraio M, Espinola RG, McCrae KR, Cockrell E, Silverstein RL. Platelet cd36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in mice. J Clin Invest 2008;118:1934–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JY, Cho HJ, Sir JJ, et al. Sulfasalazine induces haem oxygenase-1 via ros-dependent nrf2 signalling, leading to control of neointimal hyperplasia. Cardiovasc Res 2009;82:550–560 [DOI] [PubMed] [Google Scholar]
- 59.Moura R, Tjwa M, Vandervoort P, Van Kerckhoven S, Holvoet P, Hoylaerts MF. Thrombospondin-1 deficiency accelerates atherosclerotic plaque maturation in apoe−/− mice. Circulation research. 2008;103:1181–1189 [DOI] [PubMed] [Google Scholar]
- 60.Nergiz-Unal R, Rademakers T, Cosemans JM, Heemskerk JW. Cd36 as a multiple-ligand signaling receptor in atherothrombosis. Cardiovasc Hematol Agents Med Chem 2011;9:42–55 [DOI] [PubMed] [Google Scholar]
- 61.Silverstein RL, Li W, Park YM, Rahaman SO. Mechanisms of cell signaling by the scavenger receptor cd36: Implications in atherosclerosis and thrombosis. Trans Am Clin Climatol Assoc 2010;121:206–220 [PMC free article] [PubMed] [Google Scholar]
- 62.Allen LB, Capps BE, Miller EC, Clemmons DR, Maile LA. Glucose-oxidized low-density lipoproteins enhance insulin-like growth factor i-stimulated smooth muscle cell proliferation by inhibiting integrin-associated protein cleavage. Endocrinology. 2009;150:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakaguchi M, Oka M, Iwasaki T, Fukami Y, Nishigori C. Role and regulation of stat3 phosphorylation at ser727 in melanocytes and melanoma cells. J Invest Dermatol 2012;132:1877–1885 [DOI] [PubMed] [Google Scholar]
- 64.Li C, Iness A, Yoon J, Grider JR, Murthy KS, Kellum JM, Kuemmerle JF. Noncanonical stat3 activation regulates excess tgf-beta1 and collagen i expression in muscle of stricturing crohn’s disease. J Immunol 2015;194:3422–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.