Abstract
The growing interest in designing functionalized, RNA-based nanoparticles (NPs) for applications such as cancer therapeutics requires simple, efficient assembly assays. Common methods for tracking RNA assemblies such as native polyacrylamide gels and atomic force microscopy are often time-intensive and, therefore, undesirable. Here we describe a technique for rapid analysis of RNA NP assembly stages using the formation of fluorescent silver nanoclusters (Ag NCs). This method exploits the single-stranded specificity and sequence dependence of Ag NC formation to produce unique optical readouts for each stage of RNA NP assembly, obtained readily after synthesis.
Keywords: Silver nanoclusters, Fluorescence, RNA nanotechnology, RNA nanoparticles, RNA self-assembly, RNA cubes
1. Introduction
Chemotherapy is the main technique currently used for cancer treatment. Most of the existing chemotherapeutic agents demonstrate meager specificity in targeting tumor tissues and are frequently limited by dose-limiting toxicity. As an alternative, numerous anticancer therapeutic nanoparticles (NPs) have been developed [1, 2]. To date, NP therapy is still in its infancy, and much greater progress needs to be achieved in this area for clinical applications.
Recently, RNA interference (RNAi) [3] revealed a significant potential as a therapeutic agent to downregulate specific gene expression in cancerous [4, 5] or viral-infected cells [6, 7]. Moreover, modified RNA aptamer conjugates demonstrated promising therapeutic or diagnostic properties [8, 9]. Therefore, RNA can also serve as a prospective scaffolding material in engineering functional NPs [10-14]. In previous works, we constructed three-dimensional (3D) RNA-based NPs with the geometry of a cube [15]. These NPs can be easily functionalized by extension of the RNA strands entering the scaffold composition with the RNA-based cargo of interest [15-18]. This allows a precise positioning in 3D space of various functional therapeutic agents like siRNAs or aptamers.
Tracking the assembly of various RNA-based NPs in vitro is often accomplished by using different conventional techniques: native polyacrylamide gel electrophoresis (native-PAGE) [19], cryo-electron microscopy (cryo-EM) [15, 20], atomic force microscopy (AFM) [21-24], crystallography [25], and nuclear magnetic resonance (NMR) [26]. The possibilities of tracking the assembly in real time via the fluorescent response were also investigated [15, 27, 28]. These label-free fluorescent techniques however, are not widely used, and the development of other fluorescent-based reporting techniques confirming the assemblies of RNA NPs is highly desirable.
Oligonucleotide-stabilized, fluorescent silver nanoclusters (Ag:RNAs [29] and Ag:DNAs [30]) are an emerging class of fluorophore that is rapidly gaining interest in the fields of RNA and DNA nanotechnology. Ag:DNAs display highly sequence dependent optical properties [31], with colors ranging from the blue to the near-IR, making them excellent sensors for detecting specific miRNAs in solution [32], hybridization of target strands [33], and single base mutations [34, 35]. While Ag:RNAs are less studied, recent work has shown them to have analogous properties to Ag:DNAs formed on homopolymer strands of the canonical bases [29], albeit with substantial shifts in wavelength in some cases. Ag:DNAs additionally display photostabilities rivaling the best organic dyes [36] and quantum yields reaching up to 90 % [37] making them useful in fields of biological imaging [38] and single molecule studies [39]. While there have been no corresponding studies of Ag:RNAs, similar properties may be expected.
Here, we exploit the fact that fluorescent clusters form exclusively on single stranded oligonucleotides containing cytosine and guanine bases [31, 29] to demonstrate that the synthesis of Ag:RNAs may be used as an efficient assay to confirm complete and partial assembles of RNA NPs.
2. Materials
All solutions should be prepared using double-deionized ultrapure water (18 MΩ at 25 °C) or in Ultra Pure Water (Quality Biological, Inc.) and biological grade reagents. All reagents should be freshly prepared and filtered and can be stored at room temperature (unless indicated otherwise). Please carefully follow all waste disposal regulations when disposing waste materials.
2.1. Transcription and RNA Nanoparticle Assembly Components
Transcription buffer, final concentrations (1×): 15 mM MgCl2, 2 mM spermidine, 50 mM Tris buffer (pH 7.5), 2.5 mM NTPs, 10 mM DTT, 0.1 μg/μL IPP, and 0.8 U/μL RNasin.
Buffer for eluting the RNA, final concentrations (1×): 300 mM NaCl, 10 mM Tris pH 7.5, 0.5 mM EDTA.
Enzymes: T7 RNA Polymerase, DNase.
Tris-borate buffer, final concentrations (1×): 89 mM Tris, 80 mM Boric Acid, pH 8.3.
Ammonium acetate buffer (assembly buffer), final concentrations (1×): 10 mM NH4OAc, 1 mM Mg(OAc)2 (see Note 1).
2.2. Ag:RNA Components
All RNA assemblies should be performed in ammonium acetate buffer.
100× AgNO3 (5.5 mM): In a 14 mL centrifuge tube, weigh out 9.34 mg AgNO3. Add 10 mL water and invert solution multiple times to mix. Store at 4 °C in the dark.
10× RNA solutions (50 μM): If RNA is ordered from a manufacturer, multiply the specified nmol quantity by 20. Add this much water to dehydrated RNA to hydrate strands. Store at −20 °C.
1,000× NaBH4 solution (2.75 mM): In an eppendorf tube, weigh out ~0.104 mg NaBH4. Add 1 mL of water and mix vigorously (see Note 2).
3. Methods
3.1. RNA Synthesis
For 100 μL transcription mixture: add 25 μL of DNA template (encoding RNA strand), 75 μL of 1× transcription buffer, and 0.8 U/μL T7 RNA polymerase.
Incubate at 37 °C for 4 h.
Stop the transcription by adding DNase (1 U/μL) and incubating for additional 30 min at 37 °C.
Purify transcription mixture on a denaturing urea gel (8 % acrylamide, 8 M urea, 1× Tris-borate buffer) and recover the RNA strands by eluting from the gel pieces (overnight) and further RNA precipitation [16].
Measure all concentrations of RNAs using UV spectrometer. Extinction coefficients for all RNAs are calculated using nearest neighbor method [16].
3.2. Assembly of RNA Nanocubes
Mix RNA strands at equimolar concentrations.
Incubate the mixture in a heat block at 95 °C for 2 min to melt all hydrogen bonds.
Snap cool the mixture by rapid transferring to the heating block set at 45 °C and incubate for 20 min.
Carry out quality control experiments using native polyacrylamide gel electrophoresis (native-PAGE) technique (see Note 3). For visualization, total staining with ethidium bromide or SYBR Gold nucleic acid gel stains can be used [15]. As an alternative, assemblies containing one body-labeled [17] RNA strand together with non-labeled RNAs can be visualized.
3.3. Native-PAGE Experiments for RNA Assemblies
Prepare a sequencing gel for vertical electrophoresis for high resolution PAGE, that is 10 % (37.5:1) acrylamide, 1× Tris-borate buffer (pH 8.3), 1 mM Mg(OAc)2.
For vertical gel of dimensions 31 cm × 38.5 cm with the spacer thickness of 0.75 mm, load samples in individual lanes of a gel (5 μL per lane), perform electrophoresis for 3 h at 20 W at 4 °C.
For body-labeled RNAs, transfer the gel to the Whatman chromatography paper and dry the gel on the gel drier and expose the dried gel overnight to a phosphorimaging screen then scan it using phosphorimaging instrument (Storm, Typhoon, or similar). Assembled RNA cubes are expected to migrate as a single band on native-PAGE.
For SYBR Gold stained gels, a Hitachi FMBIO II Multi-View Imager can be used. For staining, please follow the manufacturer’s protocol.
3.4. Ag:RNA Synthesis
The following procedure is for a final volume of 100 μL.
Make a 10× dilution of 100× AgNO3 stock.
In an eppendorf tube, combine 10 μL of 10× RNA, 10 μL of 10× AgNO3 and 10 μL of 10× buffer (optimal concentrations of both RNA and AgNO3 depend on the strand composition). Mix thoroughly.
Store the tube containing RNA, AgNO3, and buffer at 4 °C for 20 min.
Make 1,000× NaBH4 solution and subsequently dilute to 10×.
Add 60 μL of water to RNA, AgNO3, buffer mixture.
Lastly, add 10 μL of 10× NaBH4 solution. Mix thoroughly.
Store the solution at 4 °C (yields of Ag:RNA species in solution change with time. For rapid analyses, fluorescent products may be detected within 1 h of reduction. For more reproducible spectra, however, waiting for 24 h to measure fluorescence is recommended).
3.5. Ag:RNA Optical Measurements
All Ag:RNA solutions may be excited with either UV or species-dependent visible light. Thus, for rapid analyses, fluorescence may be directly observed on top of a UV box. For quantitative analyses, however, measuring fluorescence with a fluorometer is recommended (see Note 3).
To monitor assembly of an RNA nanostructure:
Perform Ag:RNA synthesis on all individual strands.
Using 280 nm excitation, collect emission spectra of individual strands.
Repeat steps 1 and 2 along different stages of assembly (see Note 3 and Fig. 1).
Fig. 1.
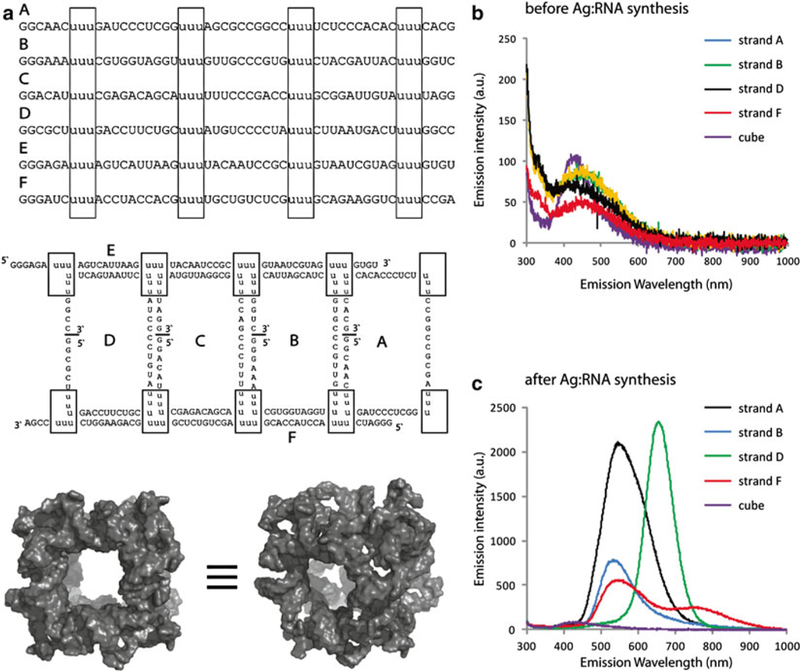
Ag:RNA synthesis may be used to differentiate between different RNA sequences and assembled nanostructures. (a) RNA sequences designed to form a cube, their 2D connectivities and a 3D model of the resulting assembly (boxes indicate cube corners) and fluorescence emission spectra taken (b) before and (c) after performing Ag:RNA synthesis on several individual strands and full cube assembly. For (b-c), all solutions were excited using a 280 nm LED source
Fig. 2.
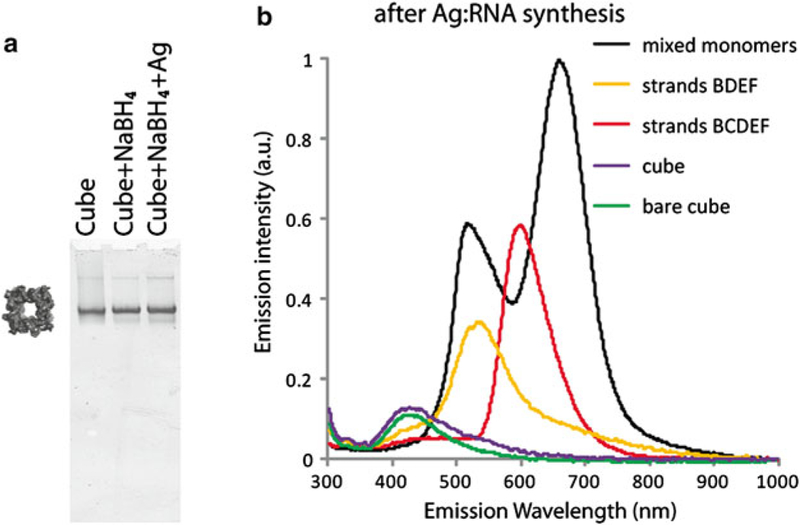
Native-PAGE experiments visualizing RNA cube assemblies before and after Ag:RNA synthesis (a) and some examples of fluorescence emission spectra taken after performing Ag:RNA synthesis at different stages of cube assembly (b). All solutions were excited using a 280 nm LED source
Acknowledgements
This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (to BAS). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This research was also supported by NIH grant no. R01GM-079604 (to LJ) and by NSF grants CHE-1213895 and CHE-0848375 (to EG).
Footnotes
Notes
Avoid Tris buffers and buffers containing high concentrations (above 2 mM) of Mg2+ for Ag:RNA synthesis.
NaBH4 is very hygroscopic. Stock containers of powder should be kept in desiccators. This solution must be prepared freshly before reduction. Solutions of NaBH4 will not keep due to the evolution of hydrogen gas. For this same reason, be sure that waste containers of NaBH4 are not capped tightly.
Quality control experiments. Fluorescent silver clusters only form on single stranded regions containing a fraction of cytosine and/or guanine bases. Thus, Ag:RNA clusters may be used to identify different RNAs based on their specific sequence signatures (Fig. 1) as well as to differentiate between partial and complete assemblies of RNA nanostructures (Fig. 2). As an example, Fig. 2 shows that performing the Ag:RNA synthesis at different stages of an RNA cube assembly yields fluorescent products until the cube is fully assembled. Importantly, Ag:RNA synthesis does not disrupt cube formation (Fig. 2a). Without any single stranded cytosine and/or guanine containing regions required to stabilize clusters, the assembled cubes produce the same low intensity emission spectrum regardless of silver reduction (Fig. 2b).
References
- 1.Mukerjee A, Ranjan AP, Vishwanatha JK (2012) Combinatorial nanoparticles for cancer diagnosis and therapy. Curr Med Chem 19: 3714–3721 [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Gu FX, Chan JM et al. (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83:761–769 [DOI] [PubMed] [Google Scholar]
- 3.Fire A, Xu S, Montgomery MK et al. (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811 [DOI] [PubMed] [Google Scholar]
- 4.Davis ME, Zuckerman JE, Choi CH et al. (2010) Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464:1067–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devi GR (2006) siRNA-based approaches in cancer therapy. Cancer Gene Ther 13:819–829 [DOI] [PubMed] [Google Scholar]
- 6.Berkhout B, Sanders RW (2011) Molecular strategies to design an escape-proof antiviral therapy. Antiviral Res 92:7–14 [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Nandamuri KM (2004) Inhibition of hepatitis viral replication by siRNA. Expert Opin Biol Ther 4:1649–1659 [DOI] [PubMed] [Google Scholar]
- 8.Farokhzad OC, Jon S, Khademhosseini A et al. (2004) Nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer Res 64:7668–7672 [DOI] [PubMed] [Google Scholar]
- 9.McNamara JO 2nd, Andrechek ER, Wang Y et al. (2006) Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol 24:1005–1015 [DOI] [PubMed] [Google Scholar]
- 10.Yingling YG, Shapiro BA (2007) Computational design of an RNA hexagonal nanoring and an RNA nanotube. Nano Lett 7:2328–2334 [DOI] [PubMed] [Google Scholar]
- 11.Afonin KA, Viard M, Koyfman AY et al. (2014) Multifunctional RNA nanoparticles. Nano Lett 14:5662–5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afonin KA, Lindsay B, Shapiro BA (2013) Engineered RNA nanodesigns for applications in RNA nanotechnology. RNA Nanotechnol 1:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo P (2010) The emerging field of RNA nanotechnology. Nat Nanotechnol 5:833–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla GC, Haque F, Tor Y et al. (2011) A boost for the emerging field of RNA nanotechnology. ACS Nano 5:3405–3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afonin KA, Bindewald E, Yaghoubian AJ et al. (2010) In vitro assembly of cubic RNA-based scaffolds designed in silico. Nat Nanotechnol 5:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afonin KA, Grabow WW, Walker FM et al. (2011) Design and self-assembly of siRNA-functionalized RNA nanoparticles for use in automated nanomedicine. Nat Protoc 6: 2022–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afonin KA, Kireeva M, Grabow WW et al. (2012) Co-transcriptional assembly of chemically modified RNA nanoparticles functionalized with siRNAs. Nano Lett 12:5192–5195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afonin KA, Viard M, Kagiampakis I et al. (2015) Triggering of RNA interference with RNA-RNA, RNA-DNA, and DNA-RNA nanoparticles. ACS Nano 9:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afonin KA, Lin YP, Calkins ER et al. (2012) Attenuation of loop-receptor interactions with pseudoknot formation. Nucleic Acids Res 40:2168–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Severcan I, Geary C, Chworos A et al. (2010) A polyhedron made of tRNAs. Nat Chem 2: 772–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chworos A, Severcan I, Koyfman AY et al. (2004) Building programmable jigsaw puzzles with RNA. Science 306:2068–2072 [DOI] [PubMed] [Google Scholar]
- 22.Grabow WW, Zakrevsky P, Afonin KA et al. (2011) Self-assembling RNA nanorings based on RNAI/II inverse kissing complexes. Nano Lett 11:878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shu D, Shu Y, Haque F et al. (2011) Thermodynamically stable RNA three-way junction for constructing multifunctional nanoparticles for delivery of therapeutics. Nat Nanotechnol 6:658–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu Y, Haque F, Shu D et al. (2013) Fabrication of 14 different RNA nanoparticles for specific tumor targeting without accumulation in normal organs. RNA 19(6):767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dibrov SM, McLean J, Parsons J et al. (2011) Self-assembling RNA square. Proc Natl Acad Sci U S A 108:6405–6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis JH, Tonelli M, Scott LG et al. (2005) RNA helical packing in solution: NMR structure of a 30 kDa GAAA tetraloop-receptor complex. J Mol Biol 351:371–382 [DOI] [PubMed] [Google Scholar]
- 27.Afonin KA, Danilov EO, Novikova IV et al. (2008) TokenRNA: a new type of sequence-specific, label-free fluorescent biosensor for folded RNA molecules. Chembiochem 9: 1902–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afonin KA, Viard M, Martins AN et al. (2013) Activation of different split functionalities on re-association of RNA-DNA hybrids. Nat Nanotechnol 8:296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz D, Gwinn E (2011) Stabilization of fluorescent silver clusters by RNA homopolymers and their DNA analogs: C, G versus A, T(U) dichotomy. Chem Commun (Camb) 47: 4715–4717 [DOI] [PubMed] [Google Scholar]
- 30.Petty JT, Zheng J, Hud NV et al. (2004) DNA-templated Ag nanocluster formation. J Am Chem Soc 126:5207–5212 [DOI] [PubMed] [Google Scholar]
- 31.Gwinn EG, O’Neill PR, Guerrero AJ et al. (2008) Sequence-dependent fluorescence of DNA-hosted silver nanoclusters. Adv Mater 20:279–283 [Google Scholar]
- 32.Yang SW, Vosch T (2011) Rapid detection of microRNA by a silver nanocluster DNA probe. Anal Chem 83:6935–6939 [DOI] [PubMed] [Google Scholar]
- 33.Yeh HC, Sharma J, Han JJ et al. (2010) A DNA-silver nanocluster probe that fluoresces upon hybridization. Nano Lett 10:3106–3110 [DOI] [PubMed] [Google Scholar]
- 34.Guo W, Yuan J, Dong Q et al. (2010) Highly sequence-dependent formation of fluorescent silver nanoclusters in hybridized DNA duplexes for single nucleotide mutation identification. J Am Chem Soc 132:932–934 [DOI] [PubMed] [Google Scholar]
- 35.Ma K, Cui Q, Liu G et al. (2011) DNA abasic site-directed formation of fluorescent silver nanoclusters for selective nucleobase recognition. Nanotechnology 22:305502. [DOI] [PubMed] [Google Scholar]
- 36.Richards CI, Choi S, Hsiang JC et al. (2008) Oligonucleotide-stabilized Ag nanocluster fluorophores. J Am Chem Soc 130:5038–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz D, Gardner K, Oemrawsingh SS et al. (2010) Evidence for rod-shaped DNA-stabilized silver nanocluster emitters. Adv Mater 25(20):2797–2803 [DOI] [PubMed] [Google Scholar]
- 38.Yu J, Choi S, Richards CI et al. (2008) Live cell surface labeling with fluorescent Ag nano-cluster conjugates. Photochem Photobiol 84: 1435–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oemrawsingh SSR, Markešević N, Gwinn EG et al. (2012) Spectral properties of individual DNA-hosted silver nanoclusters at low temperatures. Phys Chem C 116:25568–25575 [Google Scholar]


