SUMMARY
Haired skin is a defining characteristic of mammals. However, some specialized skin regions, such as human palms, soles and ventral wrist, and mouse plantar foot, are entirely hairless. Using mouse plantar skin as a model system, we show that the endogenous secreted Wnt inhibitor DKK2 suppresses plantar hair follicle development and permits the formation of hairless skin. Plantar skin retains all of the mechanistic components needed for hair follicle development, as genetic deletion of Dkk2 permits formation of fully functional plantar hair follicles that give rise to external hair, contain sebaceous glands and a stem cell compartment, and undergo regenerative growth. In the absence of Dkk2, Wnt/β-catenin signaling activity is initially broadly elevated in embryonic plantar skin and gradually becomes patterned, mimicking follicular development in normally haired areas. These data provide a paradigm in which regionally restricted expression of a Wnt inhibitor underlies specification of hairless versus hairy skin.
In Brief
What controls formation of hairless versus hairy skin? Song et al. find the secreted Wnt inhibitor Dkk2 is specifically expressed in embryonic mouse hairless, but not in rabbit hair-bearing, plantar skin. Mouse Dkk2 deletion permits plantar hair formation. Thus, evolutionary changes in Dkk2 expression contribute to species-specific hair patterns.
Graphical Abstract
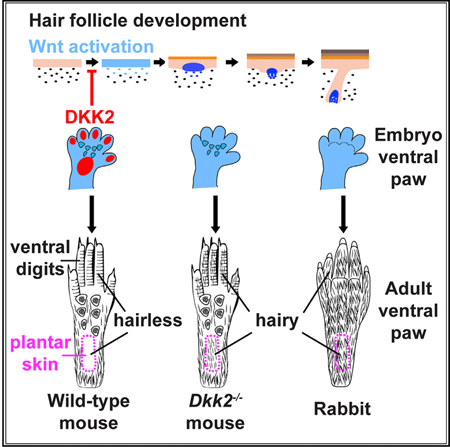
INTRODUCTION
While most body regions in mammals are covered in hair or fur of varying density, length, and thickness, certain areas remain completely hairless. Striking examples of this in humans are the palms of the hands, the ventral wrist, and the soles of the feet. In laboratory mice, a tractable model system for investigating the genetics underlying hair follicle development and patterning, plantar epidermis, analogous to human ventral wrist, and the eccrine sweat gland-bearing footpads, are hairless. By contrast, the skin in between the footpads displays a mixture of hair follicles and eccrine sweat glands whose relative numbers vary among mouse strains (Kamberov et al., 2015). Interestingly, plantar epidermis in rabbits and polar bears is furred, suggesting evolutionary adaptation of the mechanisms that regulate formation of hairless versus hair-bearing skin. In humans, hairy cutaneous malformations of the palms and soles (OMIM: 139650) are extremely rare, but have been reported in two families as autosomal dominant traits (Jackson et al., 1975;Schnitzler, 1973) and in two additional unrelated cases (Mehregan and Cos-key, 1972).
Wnt/β-catenin signaling plays key roles in the initiation, spacing, and postnatal growth of hair follicles. Interaction of Wnt ligands with Frizzled receptors and LRP5/6 co-receptors inactivates a complex of proteins that otherwise degrades cyto plasmic β-catenin, allowing it to accumulate and translocate to the nucleus where it complexes with members of the LEF/TCF family of DNA binding factors to activate transcription of target genes (Clevers and Nusse, 2012). Activation of Wnt/β-catenin signaling in the upper dermis in response to epithelial Wnt ligands precedes hair follicle placode formation and is required for this process (Chen et al., 2012). Additionally, Wnt/β-catenin signaling within surface ectodermal cells is necessary for their adoption of hair follicle fate (Andl et al., 2002; Huelsken et al.,2001) Conversely, mutation of epithelial β-catenin to a constitutively active form causes premature and ectopic formation of hair follicle placodes (Närhi et al., 2008; Zhang et al., 2008). Mature hair follicle structures fail to form in embryonic epidermis expressing activated β-catenin (Närhi et al., 2008; Zhang et al., 2008), indicating that Wnt/β-catenin signaling must be tightly controlled for normal hair follicle development to occur.
Several families of endogenous secreted inhibitors function to limit activity of the Wnt/β-catenin signaling pathway. These include members of the Dickkopf (DKK) family of secreted proteins, which antagonize Wnt/β-catenin signaling by associating with the extracellular domain of Wnt co-receptors LRP5/6 (Bafico et al., 2001; Semënov et al., 2001) and the transmembrane proteins Kremen1/2 (Niehrs, 2006). The DKK/LRP/KRM complex is internalized, removing LRP from the cell surface and preventing Wnt ligands from activating β-catenin signaling (Niehrs, 2006). An unrelated Wnt inhibitor, SOSTDC1, similarly functions by binding the LRP extracellular domain (Lintern et al., 2009). SOSTDC1 acts to limit the size of primary hair follicle placodes and prevents hair follicle formation in specialized nipple skin (Ahn et al., 2010; Närhi et al., 2012) but is not reported to suppress hair follicle development in larger hairless regions such as plantar skin (Närhi et al., 2012).
Deletion of the Dkk2 gene results in partial transformation of the cornea to a stratified epithelium that develops hair follicles (Mukhopadhyay et al., 2006). Forced expression of high levels of Dkk2 after primary hair follicle induction completely suppresses the formation of secondary hair follicle placodes and causes decreased primary hair follicle placode density and loss of the normal regular spacing of placode formation (Sick et al., 2006), suggesting a possible role for WNT/DKK mutual antagonism in setting up the pattern of hair follicle placode spacing in hairy skin (Sick et al., 2006). However, definitive loss of function experiments supporting a requirement for Dkk2 in placode induction or spacing have not been described.
To address this question, we analyzed skin phenotypes in Dkk2 null mice. These analyses revealed that patterning and development of hair follicles in hairy skin was unaffected by loss of Dkk2. Remarkably, however, normally hairless plantar skin displayed fully formed, mature hair follicles that gave rise to external hair, were associated with sebaceous glands, contained a stem cell compartment, and were capable of regenerative growth after hair plucking. In line with this striking observation, we found that while Dkk2 is expressed at low levels in hairy skin of wild-type mouse embryos and neonates, high levels of Dkk2 transcript localize specifically to plantar dermis. In the absence Dkk2, Wnt reporter gene expression is increased in embryonic plantar dermis and epidermis and subsequently becomes patterned, mimicking the gradual restriction of Wnt/β-catenin signaling that occurs during normal development of hair-bearing skin. Interestingly, we found that Dkk2 levels are not elevated in plantar versus dorsal paw skin in rabbit embryos, which develop hair follicles in their plantar region. These data identify Dkk2 as a Wnt inhibitor that is differentially expressed in mouse plantar versus hairy skin dermis and controls regional development of hairless skin. Our results further suggest that altered specification of hairless versus hairy skin in different mammalian species may be partially controlled by evolutionary changes in the regulation of Dkk2 expression.
RESULTS
Dkk2 Null Mice Have Normally Spaced Hair Follicles but Develop Ectopic Plantar Hair
To determine the requirements for Dkk2 in hair follicle development and patterning, we analyzed mice carrying a null mutation in Dkk2 (Li et al., 2005). In adult Dkk2−/− mutant mice, the appearance of the hair coat was similar to that of wild-type litter-mate controls (Figure 1A). Histological analysis of dorsal skin revealed no differences in hair follicle development, orientation, or density between mutants and controls at postnatal day (P) 17 (Figures 1B and 1C). To determine whether such differences might be apparent at an earlier developmental stage, we imaged whole mounted dissected dorsal skin at P2. This revealed patterns and densities of primary and secondary hair follicles that were indistinguishable between Dkk2−/− and control skin (Figures 1D and1E). In line with this, the sizes and spacing of primary hair follicle placodes, marked by whole mount in situ hybridization for Ctnnbl (β-catenin) mRNA at embryonic day (E) 14.5, were similar in mutant and control embryos (Figures 1F and1G).
Figure 1. Ectopic Hair Follicles Form In Plantar Skin of Dkk2 Null Mice.
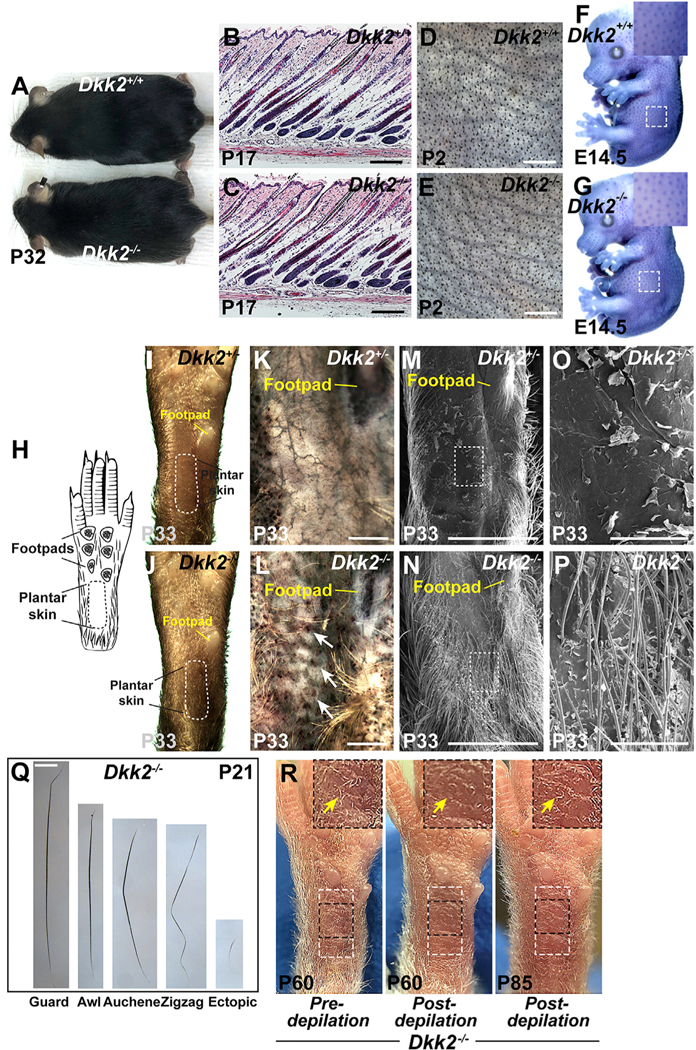
(A)Normal appearance of the hair coat of Dkk2−/− mouse at P32 compared with a wild-type littermate control (n > 40 mutants and littermate controls). (B and C) Indistinguishable histology of Dkk2−/− (C) and Dkk2+/+control littermate (B) dorsal skin at P17 (n = 3 mutants and n = 3 littermate controls). (D and E) Light microscopy of dissected dorsal skin at P2 reveals similar density and patterning of primary and secondary hair follicles in Dkk2−/− (E) and Dkk2+/+ control (D) littermate mice (n = 3 mutants and n = 3 littermate controls). (F and G) Whole mount in situ hybridization for Ctnnb1 (purple-blue signal) reveals similar sizes and patterning of primary hair follicle placodes in Dkk2−/− (G) and Dkk2+/+control (F) littermate embryos at E14.5 (n = 3 mutants and n = 3 littermate controls). Insets: higher magnification views of the boxed areas. (H) Schematic indicating the locations of footpads and plantar skin on the ventral side of a right hind foot. A dashed black line marks the border of plantar skin. (I-P) Light microscopy (I and J), alkaline phosphatase staining (purple/brown) of dissected plantar dermis (K and L), and scanning EM micrographs (M-P) of the ventral side of right hind feet from Dkk2+/−control (I, K, M, and O) and Dkk2−/− (J, L, N, and P) mice at P33 (n > 40 mutants and n > 40 controls for I and J; n = 3 mutants and n = 3 littermate controls for each experiment in K-P). The plantar region is delineated by a dashed white line in (I) and (J). White arrows in (L) indicate ectopic alkaline phosphatase-positive hair follicle dermal papillae. (O) and (P) are higher magnification views of the boxed regions in (M) and (N), respectively. Locations of the footpads are indicated in (I)-(N). Note formation of fully formed ectopic hair in Dkk2−/− plantar skin. (Q) Morphology of ectopic hair shaft compared with normal guard, awl, auchene, and zigzag hairs plucked from ventral limb skin at P21. Ectopic hair is shorter and finer than normal hair (n = 3 mutant mice analyzed). (R) Photographs of right hind foot of a Dkk2−/− mouse prior to depilation of the plantar region at P60 (left), immediately following depilation (middle), and 25 days later (P85) (right). Areas outlined with a dashed white box indicate the plantar region. Insets: higher magnification views of areas outlined by dashed black boxes. Arrows indicate ectopic plantar hair (left); lack of hair after depilation (middle); and re-growth of ectopic hair at P85 (right) (n = 3 Dkk2−/− mice depilated). Scale bars, 150 μm (B and C); 1.5 mm (D and E); 359 μm (K and L); 2 mm (M and N); 300 μm (O and P); 700 μm (Q). See also Figure S1.
By contrast with the lack of abnormalities in hairy skin, Dkk2−/− mutants developed a striking phenotype of ectopic hair growth in normally hairless plantar skin (Figures 1H and1J). This phenotype was present in all of >40 adult Dkk2 null mice observed grossly and in no wild-type Dkk2+/+ or heterozygous Dkk2+/−control littermates. The presence of external plantar hair was associated with hair follicle dermal papillae, revealed by staining for alkaline phosphatase, in Dkk2−/− mutant but not control plantar skin (Figures 1K and 1L). Closer inspection of the plantar region by scanning electron microscopy revealed that ectopic plantar hair in Dkk2−/− mutants extended as far as the most proximal footpad (Figures 1M-1P). We compared the morphology of plucked ectopic plantar hair with that of hair plucked from hairy regions of the ventral limb by light microscopy. Normal mouse hair falls into four classes: large guard over-hairs; shorter, straight awl hairs; auchene hairs that display one bend; and zigzag hairs that show several kinks. By contrast, ectopic mutant hair was straight and much shorter and finer than normal hair (Figure 1Q). Despite its small size, ectopic plantar hair re-grew following depilation at P60 (Figure 1R).
As the mutant and control mice were maintained on a mixed strain background, and hair growth varies among different mouse strains in inter-footpad regions of the ventral paw (Kamberov et al., 2015), we checked whether lack of plantar hair shows strain variability. We found that wild-type inbred C57BL/6, FVB/N, or CD-1 mice all lacked plantar hair (Figure S1). Thus, ectopic formation of plantar hair is specifically associated with Dkk2 deletion.
Dkk2 Is Specifically Upregulated In Plantar Dermis of Mouse but Not Rabbit Embryos
Given the lack of phenotypes in hairy skin and the striking formation of ectopic hair in plantar skin of Dkk2−/− mice, we examined expression of Dkk2 during mouse hairy and plantar skin development. Whole mount in situ hybridization of wild-type embryos at E14.5, when primary hair follicle placodes develop, showed intense expression of Dkk2 in the developing cornea and in limb digits and low expression in the inter-placode regions of hairy skin (Figure 2A). In situ hybridization of sectioned wild-type dorsal skin at P0 similarly revealed absence of signal in hair follicles, and low levels of expression in the upper (papillary) dermis in inter-follicular skin (Figures 2B and 2C). By contrast with low level expression in hair-bearing embryonic and neonatal skin, whole mount in situ hybridization showed striking and specific expression of Dkk2 in the plantar region of ventral hind paws at E15.5 (Figures 2D and 2E). Cryosectioning of whole mounted hind paws revealed specific, high level expression in plantar papillary dermis compared with low or undetectable levels in the adjacent footpad and in dorsal limb dermis (Figures 2F-2H). Dkk2 transcripts were also detected in ventral but not dorsal dermis in limb digits, as well as in developing joint regions and nail epithelium (Figure 2I). In line with expression of Dkk2 in wild-type ventral digit dermis, ventral digit skin is hairless in control mice and formed ectopic hairs in Dkk2−/− mutants (Figures S2A and S2B). Dkk2−/− mutants did not display obvious defects in nail growth, suggesting that Dkk2 may act redundantly with other Wnt inhibitors in the nail.
Figure 2. Dkk2 Is Specifically Upregulated In Plantar Dermis of Mouse but Not Rabbit Embryos.
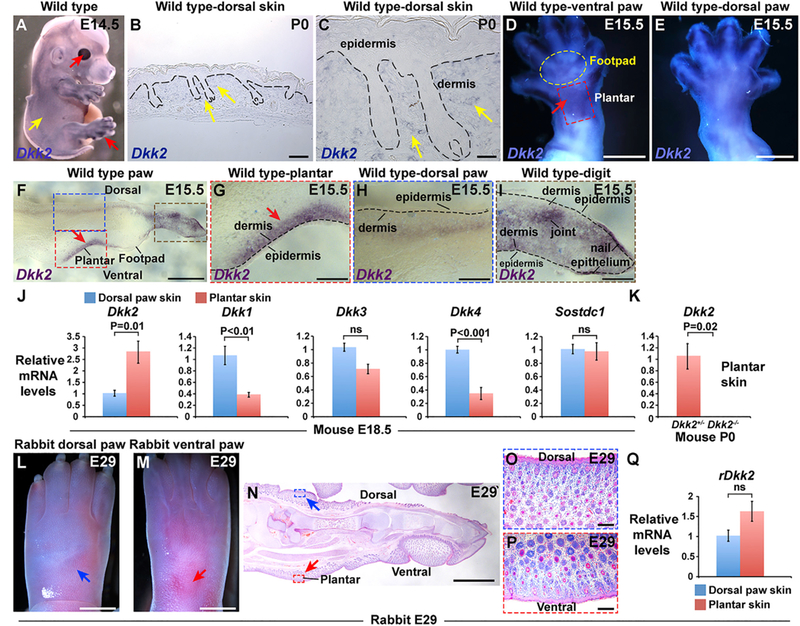
(A) Whole mount in situ hybridization for Dkk2 in wild-type mouse embryo at E14.5 (purple signal) reveals high expression levels in cornea and digits (red arrows) and low levels of expression in trunk and head skin between, but not within, hair follicle placodes (yellow arrow) (n = 6 biological replicates). (B) In situ hybridization for Dkk2 in sectioned wild-type mouse P0 dorsal skin (dark blue signal). (C) Higher magnification view of the area indicated by arrows in (B). Note low level expression in inter-follicular dermis (yellow arrows) (n = 3 biological replicates in B and C). (D and E) Ventral (D) and dorsal (E) views of an E15.5 mouse hind limb subjected to whole mount in situ hybridization for Dkk2 (purple-blue signal). Note high expression in the plantar region of the mouse ventral hind limb at E15.5 (arrow in D) (n = 4). (F) Cryosectioned mouse E15.5 hind limb following whole mount in situ hybridization for Dkk2 (purple signal) (n = 5 biological replicates). (G-I) higher magnification views of areas of (F) outlined with a dashed red box (G), a dashed blue box (H), and a dashed brown box (I). Note specific expression of Dkk2 in plantar dermis (arrows in F and G) compared with adjacent footpad skin and dorsal limb dermis at E15.5; low to undetectable levels of Dkk2 in dorsal limb dermis (H); and stronger signal in tissue located at a deeper level in the limb (H). In the limb digit, Dkk2 expression localizes to ventral but not dorsal dermis; relatively high levels of Dkk2 expression are also observed in nail epithelium and in the developing joint region (I). Dashed black lines in (B), (C), and (G)-(I) represent the dermal-epidermal border. (J) Relative levels of Dkk2, Dkk1, Dkk3, Dkk4, and Sostdc1 mRNA in full thickness dorsal paw skin (blue boxes) and plantar skin (pink boxes) from E18.5 mouse embryos, analyzed by qPCR (n = 5 plantar skin samples and n = 5 dorsal paw skin samples in each experiment). Note that only Dkk2 is specifically enriched in plantar skin. (K) Relative levels of Dkk2 mRNA in full thickness mouse Dkk2+/− control (pink box) and Dkk2−/−mutant (black box) plantar skin at P0, analyzed by qPCR (n = 3 littermate controls and n = 3 mutants). Dkk2 transcript levels are undetectable in Dkk2−/− skin. (L and M) Light microscopy of dorsal (L) and ventral (M) left hind paw of a rabbit embryo at E29. Developing hair follicles are present in the ventral plantar region (red arrow, M), as well as in dorsal skin (blue arrow, L). (N) Histology of embryonic rabbit left hind paw at E29 showing the presence of developing hair follicles in ventral plantar skin (red arrow) as well as dorsal paw skin (blue arrow). (O and P) Higher magnification views of the areas indicated in (N) by blue (O) and red (P) dashed boxes, respectively (5 biological replicates for L-P). (Q) Relative levels of rDkk2 mRNA in full thickness dorsal paw skin (blue box) and plantar skin (pink box)from E29 rabbit embryos, analyzed by qPCR (n = 5 plantar skin samples and n = 5 dorsal paw skin samples). Levels of rDkk2 mRNA are not significantly different between embryonic rabbit dorsal and plantar paw skin. All qPCR assays were performed in triplicate for each sample and data were normalized to Gapdh; mean values ± SD are shown. Statistical significance was calculated using Student’s t test. Scale bars, 100 μm (B); 30 μm (C); 750 μm (D and E); 470 μm (F); 150 μm (G-I, O, and P); 3 mm (L and M); 2 mm (N). See also Figure S2.
Consistent with the pattern of Dkk2 expression identified by in situ hybridization, quantitation of Dkk2 mRNA levels revealed significantly elevated expression in wild-type plantar skin compared with dorsal paw skin at E18.5. By contrast, transcripts for the related Wnt inhibitors Dkk1 and Dkk4, which are specifically expressed in hair follicles (Andl et al., 2002; Bazzi et al., 2007), were more abundant in dorsal paw skin than in plantar skin at this stage, and Dkk3 and Sostdc1 showed no significant differences in their levels in dorsal paw versus plantar skin (Figure 2J).
Quantitation of Dkk2 mRNA levels in neonatal Dkk2+/− control heterozygous and Dkk2−/− homozygous mutant plantar skin showed that Dkk2 levels were essentially undetectable in homozygous mutant plantar skin (Figure 2K). In line with this, whole mount in situ hybridization for Dkk2 revealed lack of a specific signal in limb digits or plantar skin of Dkk2−/− embryos (Figures S2C-S2F).
Unlike in mice and humans, plantar skin in rabbits is hair bearing. Developing hair follicle structures were visible in both dorsal and plantar regions of whole mounted rabbit paw at E29 (roughly equivalent to E18.5 of mouse embryogenesis) (Figures 2Land 2M) and by histological analysis (Figures 2N-2P). Interestingly, qPCR revealed no significant differences in the abundance of rDkk2 mRNA between rabbit plantar and dorsal paw skin at E29 (Figure 2Q). Thus, lack of specific upregulation of Dkk2 may permit the development of haired plantar skin in rabbits.
Formation of Ectopic Plantar Follicles Initiates in Late Embryogenesis
The first morphological sign of hair follicle development is the formation of a localized thickening of the epidermis, known as a placode. Placodal cells invaginate into the dermis and subsequently proliferate, forming a hair germ, which then elongates into the dermis. In parallel, signals from the placode induce condensation of underlying dermal cells, which eventually become the hair follicle dermal papilla and are almost completely enveloped by hair follicle epithelial cells in late embryogenesis and during the subsequent anagen growth phase (Millar, 2002). To determine the timing of ectopic hair follicle development, we analyzed plantar skin histology in Dkk2+/+ or Dkk2+/− control littermate and Dkk2−/− mutant mice between E16.5 and P15. Placode structures were first apparent in mutant, but not control, plantar epidermis at E18.5 and hair germ structures with adjacent dermal condensates formed by P1 (Figures 3A-3FꞋ; arrows in 3DꞋ and 3FꞋ). Postnatally, these structures continued to develop, forming associated sebaceous glands and keratinized hair shafts (Figures 3G–3JꞋ; arrows in 3HꞋ and 3JꞋ). Stratification of plantar epidermis was unperturbed by loss of Dkk2, indicated by similar expression of the stratification markers KRT14, KRT10, involucrin, and loricrin in control and Dkk2−/− mutant plantar epidermis at P7 (Figures S3A-S3H‘). Immunofluorescence for the proliferation marker Ki67 revealed similar levels of proliferation in plantar epidermal basal cells of Dkk2−/− mutants and Dkk2+/+ littermate controls, as well as the presence of proliferating cells in ectopic follicular structures in the mutants (Figures S3I-S3JꞋ).
Figure 3. Ectopic Hair Follicle Placodes Form in Dkk2−/− Plantar Skin by E18.5 and Mature Postnatally.
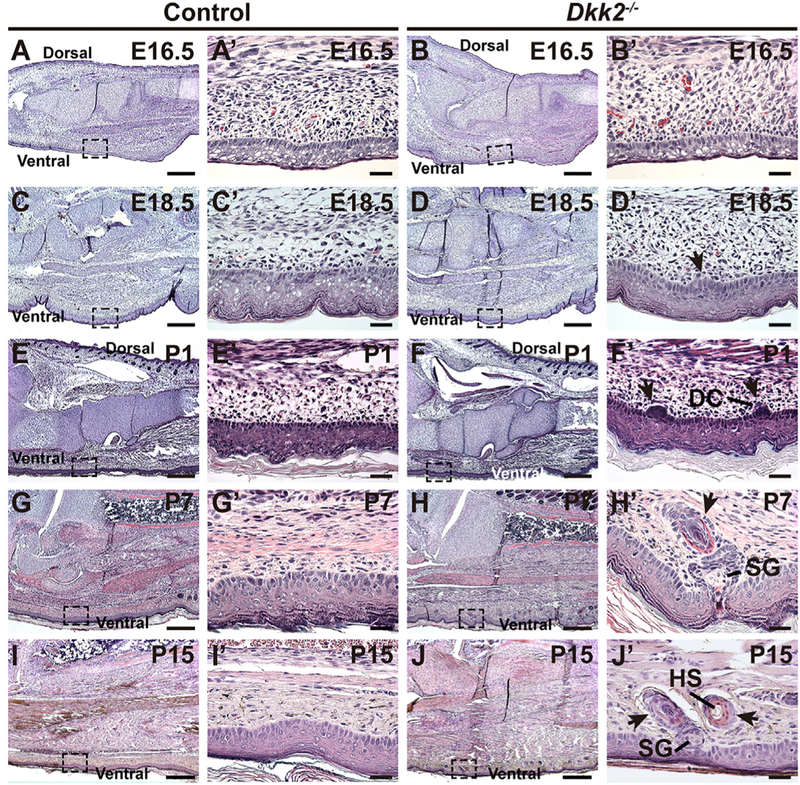
(A-JꞋ) Histology of hind foot skin from control littermate and Dkk2−/− mice as indicated, at E16.5 (A-BꞋ), E18.5 (C-DꞋ), P1 (E-FꞋ), P7 (G-HꞋ), and P15 (I-JꞋ). Ectopic placodes are present in Dkk2−/− plantar skin by E18.5 (DꞋ, arrow), form hair germs by P1 (FꞋ, arrows), and display differentiated structures at P7 and P15 (HꞋ and JꞋ, arrows). Boxed areas of ventral skin in (A)-(J) are shown at higher magnification in (AꞋ)-(JꞋ). Control genotypes were Dkk2+/− (A, AꞋ, E, and EꞋ) or Dkk2+/+ (C, CꞋ, G, GꞋ, I, and IꞋ). n = 3 mutants and n = 3 littermate controls for each analysis. DC, dermal condensate; SG, sebaceous gland; HS, hair shaft. Scale bars, 300 mm (A-J); 30 mm (AꞋ-JꞋ). See also Figure S3.
To definitively identify ectopic structures in Dkk2−/− mutant plantar skin as hair follicles, we used immunofluorescence for the transcription factor LHX2, which is expressed in hair follicles but not in sweat glands (Lu et al., 2016). As expected, LHX2 expression was detected in dorsal paw hair follicles in control littermate and mutant Dkk2−/− mice at E18.5, P1, and P7 (Figures 4A, 4B, 4E, 4F, 4I, and 4J). In plantar skin, specific signal for LHX2 was absent in controls at all stages analyzed; however ectopic structures in Dkk2−/− mutant plantar skin expressed LHX2 from P1, identifying these as hair follicles (Figures 4A, 4B, 4E, 4F, 4I, and 4J; yellow arrows). Upregulated expression of SOX9, a transcription factor essential for formation of the hair follicle outer root sheath and bulge stem cell compartment (Vidal et al., 2005), was detected in dorsal paw hair follicles in control littermate and mutant Dkk2−/− mice at E18.5 and P1 and in ectopic hair follicles in Dkk2−/− plantar skin (Figures 4C, 4D, 4G, and 4H, yellow arrows). Consistent with the production of external hair shafts by ectopic plantar follicles in Dkk2−/− mutants (Figures 1I-1R) and histological detection of hair shaft structures (Figures 3HꞋ and 3JꞋ), immunofluorescence with antibody AE13, which binds hair shaft keratins, was observed in ectopic plantar hair follicles at P7 (Figure 4J, white arrow), as well as in dorsal paw hair follicles of Dkk2+/+ control and Dkk2−/− mutant mice, but was absent in control plantar skin (Figures 4I and4J). Hair follicle epithelial stem cells can be identified by the marker KRT15 (Morris et al., 2004). Immunofluorescence for KRT15 at P15 revealed its expression in ectopic plantar hair follicles in Dkk2−/− mutants (Figure 4L, yellow arrow), while KRT15 signal was absent in littermate control plantar skin (Figure 4K). Together with our observation that ectopic plantar hair was capable of regeneration following depilation at P60 (Figure 1R), these results indicate that the ectopic hair follicles contained a functional stem cell compartment.
Figure 4. Ectopic Hair Follicles Express the Same Differentiation and Stem Cell Markers as Normal Hair Follicles.
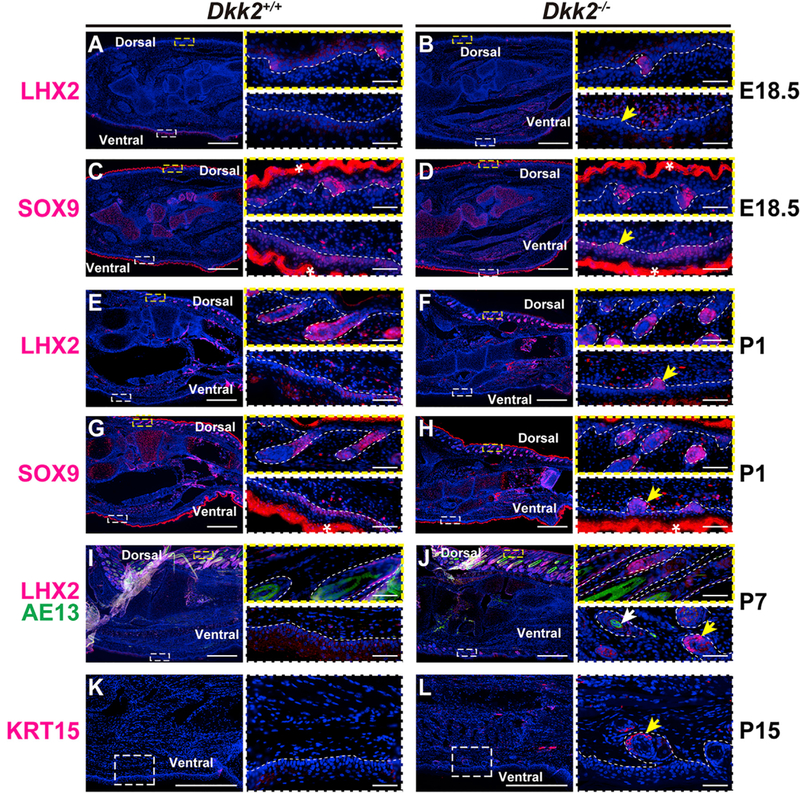
(A-L) Immunofluorescence staining of littermate Dkk2+/+ control (A, C, E, G, I, and K) and Dkk2−/− mutant (B, D, F, H, J, and L) sectioned mouse hind paws at E18.5 (A-D), P1 (E-H), P7 (I and J), and P15 (K and L) using antibodies to the hair follicle marker LHX2 (A, B, E, F, I, and J) (red), the outer root sheath and stem cell marker SOX9 (C, D, G, and H) (red), the hair shaft differentiation marker AE13 (I and J) (green), and the bulge stem cell marker KRT15 (K and L) (red). Dorsal skin regions outlined by dashed yellow boxes in (A)-(L) are shown at higher magnification to the upper right of each panel. Plantar skin regions outlined by dashed white boxes in (A)-(L) are shown at higher magnification to the lower right of each panel. n = 3 mutants and n = 3 littermate controls for each analysis. Ectopic plantar structures express the hair follicle marker SOX9 from E18.5 (D and H), and LHX2 from P1 (F and J) (yellow arrows), indicating they are hair follicles rather than sweat glands, which do not express LHX2. Expression of AE13 indicates production of keratinized hair shaft structures in ectopic follicles (J) (white arrow), and expression of KRT15 indicates that the ectopic follicles contain a bulge stem cell population (L) (yellow arrow). These markers are expressed in dorsal but not plantar skin of wild-type controls (A, C, E, G, I, and K). Dashed white lines indicate epidermal/dermal borders. Nuclei were counterstained with DAPI (blue). ‘Non-specific fluorescence. Scale bars, 500 μm (low-magnification images) and 50 μm (boxed areas photographed at higher magnification).
Dkk2 Suppresses Wnt/β-Catenin Signaling In Plantar Skin
Wnt/β-catenin signaling is required in both dermal and epithelial cells for embryonic hair follicle placode formation and promotes this process when forcibly activated (Andl et al., 2002; Huelsken et al., 2001; Närhi et al., 2008; Zhang et al., 2008; Chen et al., 2012). As Dkk2 can function as a Wnt inhibitor, we asked whether Wnt/β-catenin signaling is enhanced in plantar skin of Dkk2−/− mice. Dkk2 mutants were crossed to Axin2lacZ Wnt reporter mice, in which the lacZ gene is inserted into the Axin2 locus, a ubiquitous target of Wnt/β-catenin signaling (Lustig et al., 2002). Whole mount staining for β-galactosidase at E17.5, 1 day before the morphological appearance of ectopic placodes in the mutants, revealed patches of highly increased Wnt reporter expression in the plantar region of whole mounted Dkk2−/− Axin2lacZ homozygous mutant hind paws compared with Dkk2+/− Axin2lacZ littermate controls (Figures 5A and 5B, arrows). Cryosectioning of ventral hind paw skin showed that Wnt reporter expression localized to footpad structures in both Dkk2−/− Axin2lacZ mutants and Dkk2+/− Axin2lacZ controls (Figures 5C and 5D, red arrows), but was strongly elevated in the dermis and epidermis of mutant plantar skin compared with control plantar skin (Figures 5C and 5D, yellow arrows). These data indicate that, in wild-type mouse embryos, DKK2 secreted from upper dermal cells inhibits Wnt/β-catenin signaling both in the papillary dermis and in adjacent epidermal cells.
Figure 5. Wnt/β-Catenin Signaling Is Elevated in Embryonic and Neonatal Dkk2−/− Plantar Skin.
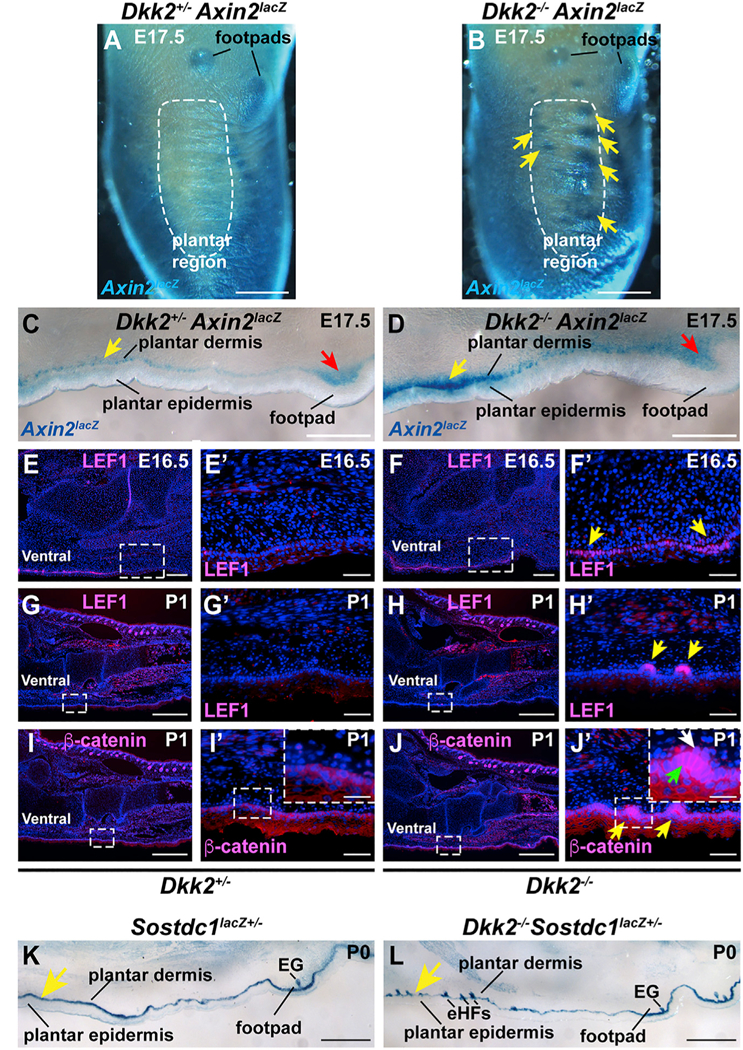
(A and B) X-gal-stained whole mounted control Dkk2+/− Axin2lacZ (A) and Dkk2−/− Axin2laaZ (B) hind paws at E17.5. Note elevated Axin2lacZ Wnt reporter activity (blue signal) in the developing plantar region of the Dkk2−/− mutant (B, arrows). The plantar region is outlined by a dashed white line in (A) and (B) and footpad locations are indicated. (C and D) Cryosections of ventral skin from X-gal stained Dkk2+/− Axin2lacZcontrol littermate (C) and Dkk2−/− Axin2lacZ (D) hind paw whole mounts at E17.5. Wnt reporter expression is elevated in plantar papillary dermis and plantar epidermis in the Dkk2−/− mutant compared with the control (yellow arrows). Signaling levels are similar in mutant and control footpads (red arrows). (E-JꞋ) Hind paw sections from Dkk2+/− control littermate (E, EꞋ, G, GꞋ, I, and IꞋ) and Dkk2−/− (F, FꞋ, H, HꞋ, J, and JꞋ) mice at E16.5 (E-FꞋ) or P1 (G-JꞋ) subjected to immunofluorescence (pink signal) for LEF1 (E-HꞋ) or p-catenin (I-JꞋ). Boxed areas in (E)-(J) are shown at higher magnification in (EꞋ)-(JꞋ). Boxed areas in (IꞋ) and (JꞋ) are shown at further increased magnification in the insets. Yellow arrows indicate ectopic expression of LEF1 or β-catenin in Dkk2−/− plantar skin. Green arrow in (JꞋ) inset indicates nuclear and cytoplasmic localization of β-catenin in epithelial cells and white arrow in (JꞋ) inset indicates nuclear localized β-catenin in the dermal condensate of an ectopic hair germ. (K and L) Expression of the Wnt inhibitor Sostdc1 in cryosectioned X-gal stained littermate control (K) and Dkk2−/− mutant (L) ventral paw skin at P1, indicated by expression of a lacZ reporter (blue staining) knocked into the Sostdc1 locus. Note expression of Sostdc1 in plantar and footpad basal epidermis in both control and littermate samples; Sostdc1 also localizes to sweat glands in the footpad of both control and mutant and to ectopic hair follicles in the mutant plantar region. n = 3 mutants and n = 3 littermate controls for each analysis in (A)-(L). EG, eccrine gland; eHFs, ectopic hair follicles. Scale bars, 500 μm (A and B); 300 μm (C and D); 500 μm (E-J); 50 μm (EꞋ-JꞋ); 20 μm (insets in IꞋ and JꞋ); 400 μm (K and L).
LEF1 is a β-catenin transcriptional partner that is positively regulated by Wnt signaling and localizes to developing hair follicles (Huber et al., 1996; Liu et al., 2004; Zhou et al., 1995). Immunofluorescence staining revealed broadly enhanced LEF1 expression in Dkk2−/− compared with Dkk2+/− control plantar epidermis from as early as E16.5 (Figures 5E-5F’, arrows). By P1, strong signal for LEF1 localized to ectopic hair germ structures (Figures 5G-5HꞋ, arrows). As shown in Figures 5I-5JꞋ, nuclear β-catenin localized to ectopic hair germ epithelium (yellow and green arrows in Figure 5JꞋ) and dermal condensates (white arrow in Figure 5JꞋ) in Dkk2−/− plantar skin at P1 but did not show specific upregulation in control plantar skin. Taken together, these data indicate that endogenous DKK2 functions to suppress Wnt/β-catenin signaling in plantar dermis and epidermis and allow the formation of hairless skin (Figures 5I and5IꞋ).
The initial broad but patchy pattern of upregulation of Wnt/ β-catenin signaling in Dkk2−/− plantar skin and the small size of ectopic hairs could result from non-uniform expression of Wnt ligands and/or from the activity of additional Wnt inhibitors in the plantar region. In line with the latter hypothesis, we found that, while it is not differentially regulated in plantar versus dorsal paw skin (Figure 2J), expression of the secreted Wnt inhibitor Sostdc1, indicated by X-gal staining for a Sostdc1lacZ reporter allele (Yanagita et al., 2006), localized to basal plantar epidermis in Sostdc1lacZ control and Sostdc1lacZDkk2−/− mutant mice and to ectopic hair follicles in the Sostdc1lacZ Dkk2−/− mutants (Figures 5K and 5L). Sostdc1 could thus potentially act to limit Wnt signaling in the absence of Dkk2.
DISCUSSION
Wnt/β-catenin signaling plays key roles in initiating hair follicle development during embryogenesis (Andl et al., 2002; Huelsken et al., 2001; Chen et al., 2012). However, uncontrolled activity of the Wnt/β-catenin pathway prevents the morphogenesis of normally functioning, regularly spaced hair follicles (Närhi et al., 2008; Zhang et al., 2008). These observations suggest that endogenous Wnt inhibitory factors must control Wnt activity to permit normal patterning and development of hair follicles. Here, we investigated the functions of the secreted Wnt inhibitor DKK2 in these processes. Our analyses revealed a key and unexpected role for DKK2 in specifying hairless versus hairy skin.
While hair follicles developed normally in dorsal skin of mice lacking Dkk2, these mutants displayed a highly unusual phenotype of hair growth in the normally hairless plantar region of the mouse paw. Hair growth in this region was not observed in wild-type or heterozygous mutant littermate mice, or in wild-type mice of several common inbred strains. In situ hybridization and qPCR studies revealed that Dkk2 is expressed at higher levels in plantar than in adjacent footpad skin or haired dorsal paw skin and localizes to the upper (papillary) dermis. Consistent with DKK2’s function as a Wnt inhibitor in most in vivo contexts (Gage et al., 2008; Li et al., 2005; Li et al., 2012; Mukhopadhyay et al., 2006), Wnt signaling was elevated in plantar skin of Dkk2−/− mutant embryos, and this correlated with formation of ectopic hair follicle placodes in late embryogenesis. Although ectopic plantar hair was shorter and finer than any of the normal hair types in dorsal skin, the ectopic hair follicles expressed the stem cell marker KRT15 and regenerated external hair following depilation, indicating that they were fully functional and contained a stem cell compartment. Thus, Dkk2 functions non-redundantly to suppress Wnt signaling and permit hair follicle development in the plantar region. This finding provides a paradigm to explain the formation of regions of hairless versus hairy skin.
In line with the restriction of high levels of endogenous Dkk2 expression to the plantar region of ventral foot skin, formation of ectopic hair follicles in Dkk2−/− mice did not extend to the footpads, which are characterized by development of sweat glands but not hair follicles. Thus, the Dkk2−/− phenotype is distinct from that caused by mutation of epithelial β-catenin to a constitutively active form, which results in ectopic formation of hair follicles in the footpads and other normally hairless regions of ventral paw skin (Zhang et al., 2008). Forced expression of the BMP inhibitor Noggin also promotes hair follicle development in the footpads and suppresses sweat gland formation (Lu et al., 2016; Plikus and Chuong, 2004). Wnt/β-catenin signaling is normally elevated in footpad compared with dorsal skin dermis but is reduced in developing sweat gland compared with hair follicle epithelial cells (Lu et al., 2016). These effects correlate with elevated mesenchymal BMP signaling in footpad skin (Lu et al., 2016), but the underlying mechanisms connecting these two pathways in sweat gland development have not been fully delineated. The Wnt inhibitor Dkk4 localizes to developing sweat gland buds and suppresses sweat gland formation when overexpressed (Cui et al., 2014); however, its requirement for sweat gland development has not been determined through genetic loss of function assays. Thus, the interesting question of the roles of secreted Wnt inhibitors in suppressing hair follicle development in the footpad, and in determining hair follicle versus sweat gland fate, has yet to be elucidated.
Despite its ability to alter hair follicle placode patterning in hairy skin when overexpressed (Sick et al., 2006), loss of endogenous Dkk2 did not affect this process or other aspects of embryonic hair follicle development in hairy skin. Dkk2 displays general, low level expression in embryonic and neonatal hairy skin dermis and is excluded from hair follicles at these stages. Its expression partially overlaps with that of transcripts for the related gene Dkk1, which localize to dermal cells adjacent to hair follicle placodes and germs (Andl et al., 2002; Narhi et al., 2008) and contrasts with that of Dkk4, a direct Wnt/β-catenin target that is specifically induced in placodes (Bazzi et al., 2007). Expression of the unrelated secreted Wnt inhibitor Sostdc1 is excluded from hair follicle placodes at E14.5, but localizes to epidermal rather than dermal cells at this stage (Närhi et al., 2008). Primary hair follicle placodes are enlarged in Sostd1l null mice but do not display altered spacing, although patterning of certain other ectodermal appendages including mammary glands and vibrissae is altered (Närhi et al., 2012), and analyses of hair follicle patterning have not been described in mice lacking Dkk1 or Dkk4. Thus, the possibility remains open that Dkk2 contributes to placode patterning in hairy skin but performs functions that are redundant with those of other Dkk family members and/or Sostdc1.
Interestingly, our data revealed that the process of ectopic hair follicle formation in plantar skin of Dkk2 mutants involves broad activation of Wnt/β-catenin signaling in the dermis and epidermis, followed by its gradual refinement to defined locations. These events mimic the gradual patterning of Wnt/β-catenin activity observed during normal hair follicle development in dorsal skin (Chen et al., 2012; Zhang et al., 2009), a process thought to require reaction-diffusion mechanisms that involve competition between Wnt ligands and secreted Wnt inhibitors (Sick et al., 2006). Thus, while DKK2 is necessary to dictate absence of hair follicles in plantar skin, it is not required for the patterning of either normal or ectopic follicles, which must therefore involve other inhibitory factors. These could include the related genes Dkk1 and Dkk4 that are specifically expressed in hair follicles after initiation of hair follicle development (Andl et al., 2002; Bazzi et al., 2007) and are activated in response to elevated Wnt/β-catenin signaling (Bazzi et al., 2007; Lieven et al., 2014). While we find that Dkk1 and Dkk4 are expressed at low levels in hairless wild-type plantar skin relative to their expression in wild-type hairy skin, their expression is likely induced in Dkk2−/− ectopic plantar placodes and could contribute to their patterning. In addition, we find that expression of Sostdc1 localizes to plantar basal epidermis and ectopic hair follicles in Dkk2−/− mutants, suggesting its potential to limit Wnt signaling in the plantar region in the absence of Dkk2.
The data outlined here indicate that plantar skin retains all of the mechanisms needed to form hair follicles and that relief from DKK2-mediated Wnt inhibition is sufficient to initiate this process. Given evolutionary conservation of the roles of Wnt/β-catenin signaling in mammalian hair follicle biology (Marvin et al., 2011; Xu et al., 2017), it is likely that our findings are relevant to understanding how hairless skin forms in humans. It is also interesting to consider whether spatially restricted expression of Wnt inhibitors could contribute to other regional variations in hair follicle development that have long intrigued skin biologists. Examples include the formation of long, thick hairs in the human scalp, versus shorter thick hairs in the eyebrows and fine vellus hairs in many other body regions. The extreme rarity of hairy cutaneous malformations of human palms and soles complicates analysis of their genetic origin; however, it is possible that such mutations bypass extracellular Wnt inhibitory mechanisms by activating signaling downstream of Wnt receptors.
It will be exciting in the future to probe whether similar Wnt inhibitory mechanisms operate to prevent formation of fully functional skin containing hair follicles in regenerative scenarios such as healing of severe burns and to determine whether small molecule Wnt inhibitor antagonists might be used to combat such mechanisms.
We find that in rabbit embryos, which, unlike mouse embryos, normally develop plantar hair, expression levels of Dkk2 are similar in embryonic plantar and dorsal paw skin. Our data therefore suggest that evolutionary forces have altered expression of Dkk2 in some species to allow hair development in plantar skin. While the mechanisms underlying this difference require further investigation, these could include mutation of Dkk2 enhancer elements. In addition, the Dkk2 gene is silenced via DNA hyper-methylation in several contexts (Mu et al., 2017; Zhu et al., 2012), and Dkk2 mRNA is de-stabilized by multiple microRNAs (e.g., Hassan et al., 2012; Li et al., 2013; Lu et al., 2017). Thus, differential expression, activity, or targeting of chromatin modifying factors, altered expression of miRNAs, or evolutionary changes in miRNA target sites in the Dkk2 3’UTR could contribute to upregulation or repression of Dkk2 mRNA levels in plantar skin of different species. Future studies examining species differences in the expression patterns of Dkk2 and other secreted Wnt inhibitors are likely to shed additional light on the mechanisms by which specific hair follicle distributions and characteristics have evolved in mammals.
STAR⋆METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Sarah E. Millar (millars@pennmedicine.upenn.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse strains and genotyping
Mice (Mus musculus) were maintained on a mixed C57BL/6 / FVB/N / SJL background in a Specific Pathogen Free (SPF) barrier facility on a standard light-dark cycle. Cages were changed twice per week, and mice received water and chow ad libitum. A sentinel program was used to monitor animal health. Littermates of the same sex were randomly assigned to experimental groups. Mice were housed at no more than five per cage; males from different litters were not co-housed. Dkk2 null (RRID:IMSR_JAX:030130), Axin2LacZ (RRID:IMSR_JAX:009120) and Sostdc1lacZ (RRID:MGI:4360517) mice have been described previously (Li et al., 2005; Lustig et al., 2002; Yanagita et al., 2006). Dkk2 null mice were crossed to Axin2lacZ mice or Sostdc1lacZ mice for several generations to obtain Dkk2−/− Axin2lacZ mice, Dkk2−/− Sostdc1lacZ mice, and littermate control mice. Embryonic ages were determined from the time of appearance of a vaginal plug (E0.5). Male and female animals were analyzed at embryonic and neonatal stages, and no sex-related differences were observed. Male mice were used for analysis of postnatal hair growth, as the timing of hair follicle cycling is variable in female mice. Mice were genotyped by PCR of tail biopsy DNA. Primer sequences are provided in Table S1. All animal experiments were conducted under protocols approved by the University of Pennsylvania IACUC Committee.
Rabbit tissue
Freshly harvested or 4% paraformaldehyde-fixed New Zealand White (NZW) rabbit embryo tissues were purchased from Covance Research Products, Denver, PA (Cat#NZ-CTM).
METHOD DETAILS
Histology and immunostaining
Tissues were fixed in 4% paraformaldehyde at 4°C overnight, paraffin embedded and sectioned at 5 mm for hematoxylin and eosin staining and immunofluorescence. For frozen sections, tissues were fixed in 4% paraformaldehyde at 4°C overnight, followed by overnight incubation at 4°C in 30% DEPC-treated sucrose, embedding in O.C.T. compound (ThermoFisher Scientific, Cambridge, MA, Cat#23–730-571), and cryosectioning at 8 mm. For immunofluorescence, sections were dehydrated, blocked with 5% goat serum, and then incubated in blocking reagent. Details of the antibodies used are provided in the Key Resources Table.
In situ hybridization
In situ hybridization probe templates were synthesized by PCR of E14.5 mouse cDNA, using primers containing T7 RNA polymerase binding sites to amplify Dkk2 (GenBank: NM_020265.4, nt 1141–1970 or GenBank: NM_020265.4, nt 1125–1242) and Ctnnb1 (GenBank: NM_007614, nt 150 – 540). Antisense digoxigenin-labeled RNA probes were prepared with MAXIscript T7 in vitro transcription kit (ThermoFisher Scientific, Cat#AN1312). The RNA probes were purified with MEGAclear Transcription Clean-Up Kit (ThermoFisher Scientific, Cat#AM1908) to remove free nucleotides.
For whole-mount RNA in situ hybridization, embryos were fixed in 4% paraformaldehyde overnight at 4°C and stored in methanol at −20°C. Rehydrated samples were treated with proteinase K and incubated with 1 μg digoxigenin-labeled RNA probe in 1 ml hybridization buffer at 70°C. After overnight incubation, embryos were washed in 2X saline sodium citrate buffer at 70°C and then treated with RNAase A solution (10 μg/ml) at 37°C to remove non-specific binding. After washing, embryos were incubated with anti-digoxigenin alkaline phosphatase in blocking buffer (Roche, Branchburg, NJ, DIG Nuclei Acid Detection Kit, Cat#11175041910) at 4°C overnight. After 72 hours of washing with 1X washing buffer (Roche, Cat#11175041910), embryos were incubated with detection buffer (Roche, Cat#11175041910) for 10 minutes. Signals were detected by immersing embryos in BM-Purple solution (Roche, Cat#11442074001) for 2–4 hours. After color development, tissues were fixed overnight in 4% paraformaldehyde, incubated in 30% sucrose, embedded in O.C.T and cryosectioned at 12 μm.
For RNA in situ hybridization with tissue sections, tissues were dissected in DEPC treated 1X PBS and fixed in 4% paraformaldehyde overnight. Fixed tissues were embedded in paraffin and sectioned at 6 μm. In situ hybridization assays were performed with antisense digoxigenin-labeled RNA probes. After overnight hybridization at 65°C, the slides were washed with 2X saline sodium citrate buffer and maleic acid buffer and incubated with anti-digoxigenin alkaline phosphatase (Roche, DIG Nuclei Acid Detection Kit, Cat#11175041910) at 4°C overnight. The signals were developed with BCIP-NBT (Roche, DIG Nuclei Acid Detection Kit, Cat#11175041910).
Whole mounted embryos were photographed using a Leica MZ16F microscope and Leica DFC7000T digital camera (Leica Microsystems, Buffalo Grove, IL). Sections were photographed using a Leica DM5000B microscope and Leica digital cameras DFC 360 FX and DFC 420.
Alkaline Phosphatase Staining
Plantar skin was dissected and digested at 4°C overnight with 2 mg/ml Dispase II (ThermoFisher Scientific, Cat#17105041). Dermis was separated from epidermis using forceps, fixed in 4% paraformaldehyde at room temperature for 20 minutes, washed with DPBS (ThermoFisher Scientific, Cat#14190–136), rinsed once in AP buffer (100mM Tris-HCl, 5mM MgCl2,100 mM NaCl, pH9.5), and incubated at 37°C for up to 4 hours in NBT/BCIP staining solution (Roche, Cat#11681451001). The reaction was stopped by rinsing with DPBS. Samples were photographed using a Leica MZ16F microscope and Leica DFC7000T digital camera (Leica Microsystems, Buffalo Grove, IL).
Scanning electron microscopy
Samples were fixed overnight in 4% PFA, dehydrated in graded ethanols, incubated for20 minutes in 50% HMDS in ethanol followed by three changes of 100% HMDS (Sigma-Aldrich, C7207, Cat#440911), air-dried overnight, mounted on stubs and sputter coated with gold palladium. Specimens were observed and photographed using a Quanta 250 scanning electron microscope (FEI, Hillsboro, OR) at 10 kV accelerating voltage.
Wax depilation
Sally Hansen Extra Strength Brazilian Bikini Wax (Amazon.com, Seattle, WA) was melted at 60°F in a Makartt Hair Removal Hot Wax Warmer (Amazon.com). Dkk2−/− mice were anesthetized with 1%−3% isofluorane using a nose cone prior to and during the procedure. Warm wax was applied and was peeled off after 3–5 minutes. Wax was re-applied after 10 minutes and peeling was repeated once to ensure removal of all ectopic plantar hair.
Quantitative PCR
Full thickness dorsal paw skin and plantar skin was dissected from mouse embryos at E18 and from rabbit embryos at E29. Full thickness plantar skin samples were dissected from Dkk2+/− and Dkk2−/− mouse pups at P0. Total RNA was extracted using RNeasy Plus Mini Kit (QIAGEN, Valencia, CA, Cat#74134) and cDNA synthesized using SuperScript III First-Strand Synthesis System (ThermoFisher Scientific, Cat#18080051). Reactions were set up using Fast SYBR Green Master Mix (ThermoFisher Scientific, Cat#4385612) following the manufacturer’s instructions. The sequences of qPCR primers are provided in Table S1. Relative expression levels were calculated using the 2-∆∆CT method and normalized to Gapdh. At least three biological replicates were assayed, and three technical replicates were performed for each sample. Statistical significance was calculated using two-tailed Student’s t test.
X-gal staining
Whole mounted dissected mouse embryonic hind paws were fixed in 2% PFA for 1 hour on ice. After fixation, tissues were transferred to washing buffer containing 0.02% IGEPAL CA-630 (Sigma-Aldrich, Cat#18896), 0.01% sodium deoxycholate and 2mM MgCl2 in 1x PBS for 3 hours and then incubated at 37°C for 5 hours in the dark in 1mg/ml X-gal (EMD Millipore, Cat#BG-3-G) in Base Solution (EMD Millipore, Cat#BG-8-C). After staining, tissues were fixed in 4% PFA, incubated in 30% sucrose, embedded in O.C.T and cryosectioned at 12 μm.
QUANTIFICATION AND STATISTICAL ANALYSIS
We utilized n = 5 control and n = 5 experimental mice wherever possible, providing 80% power at a two-sided significance level of 0.05 to detect a difference (effect size) of 2.0 s where s is the standard deviation; at a minimum we utilized n = 3 control and n = 3 experimental mice, providing 80% power at a two-sided significance level of 0.05 to detect a difference of 2.8 s. For quantitation of rDkk2 levels, n = 5 plantar and n = 5 dorsal paw skin samples from rabbit embryos were analyzed. Statistical significance was calculated using Student’s t test. Statistical parameters are reported in the relevant Figure Legends.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Ki67 (1:200) | Abcam | Cat#ab16667; RRID AB_302459 |
| Rabbit monoclonal anti-LEF1 (1:100) | Cell Signaling | Cat#2286s; RRID AB_10706166 |
| Mouse monoclonal anti-b-catenin (1:1000) | Sigma-Aldrich | Cat# C7207; RRID AB_476865 |
| Rabbit polyclonal anti-LHX2 (1:200) | Millipore | Cat# ABE1402; RRID AB_2722523 |
| Rabbit polyclonal anti-Keratin10 (1:1000) | Covance | Cat# PRB-159P; RRID AB_291580 |
| Rabbit polyclonal anti-Keratin14 (1:1000) | BioLegend | Cat#905301; RRID AB_2565048 |
| Rabbit polyclonal anti-Loricrin (1:1000) | BioLegend | Cat# PRB-145P; RRID AB_10064155 |
| Rabbit polyclonal anti-Involucrin (1:1000) | BioLegend | Cat# PRB-140C; RRID AB_291569 |
| Rabbit polyclonal anti-SOX9 (1:200) | EMD-Millipore | Cat#AB5535, RRID:AB_2239761 |
| Mouse monoclonal anti-hair cortex keratin (AE13) (1:100) | Abcam | Cat#ab16113, RRID:AB_302268 |
| Mouse monoclonal anti-Keratin15 (1:2000) | Abnova Corporation | Cat#MAB10723, RRID:AB_11189389 |
| Goat anti-Mouse IgG, Alexa Fluor 555 (1:1000) | ThermoFisher | Cat# A21425; RRID AB_1500751 |
| Goat anti-Rabbit IgG, Alexa Fluor 555 (1:1000) | ThermoFisher | Cat# A21428; RRID AB_141784 |
| Critical Commercial Assays | ||
| RNeasy Fibrous Tissue Mini Kit | QIAGEN | Cat#74704 |
| SuperScript III First-Strand Synthesis System | ThermoFisher | Cat#18080–051 |
| Fast SYBR Green Master Mix | ThermoFisher | Cat#4385612 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Dkk2tm1Dwu, mixed C57BL/6 / FVB/N / SJL strain background | The Jackson Laboratory | RRID:IMSR_JAX:030130 |
| Mouse: Axin2tm1Wbm, mixed C57BL/6 / FVB/N / SJL strain background | The Jackson Laboratory | RRID:IMSR_JAX:009120 |
| Mouse: Sostdc1lacZ, mixed C57BL/6 / FVB/N / SJL strain background | Dr. Gabriela Loots, Lawrence Livermore National Laboratory | RRID:MGI:4360517 |
| Rabbit: New Zealand white (NZW) | Covance Research Products | NZ-CTM |
| Oligonucleotides | ||
| Please see Table S1 | N/A | N/A |
| Recombinant DNA | ||
| In situ hybridization probe for Dkk2 GenBank: NM_020265.4 nt 1141–1970 | This paper | N/A |
| In situ hybridization probe for Dkk2 GenBank: NM_020265.4, nt 1125–1242 | This paper | N/A |
| In situ hybridization probe for Ctnnb1 (β-catenin) GenBank: NM 007614, nt 150–540 | Zhang et al., 2009 | N/A |
Highlights.
DKK2 localizes to embryonic mouse hairless, but not rabbit hairy, plantar skin
Deletion of mouse Dkk2 causes formation of mature, regenerative plantar hair
Dkk2 specifies development of hairless versus hairy skin
Evolutionary changes in Dkk2 regulation underlie speciesspecific hair patterns
ACKNOWLEDGMENTS
We thank Xiaoling Zhang for statistical analysis support and Donna Brennan-Crispi for X-gal staining protocols. This work was supported by a scholarship from China Scholarship Council, China (to Y.S.); National Institutes of Health (NIH), USA (R37AR047709 to S.E.M.); NIH, USA (Penn Skin Diseases Research Center P30AR057217); NIH, USA (Penn Skin Biology and Diseases Resource-based Center P30AR069589); and a Career Development Award from the Dermatology Foundation, USA (to M.X.). G.G.L. works under the auspices of the United States Department of Energy, USA (DE-AC52–07NA27344) at Lawrence Livermore National Laboratory.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes three figures and one table and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.11.017.
REFERENCES
- Ahn Y, Sanderson BW, Klein OD, and Krumlauf R (2010). Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development 137, 3221–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, and Millar SE (2002). WNT signals are required for the initiation of hair follicle development. Dev. Cell 2, 643–653. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, Gazit A, and Aaronson SA (2001). Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3, 683–686. [DOI] [PubMed] [Google Scholar]
- Bazzi H, Fantauzzo KA, Richardson GD, Jahoda CA, and Christiano AM (2007). The Wnt inhibitor, Dickkopf 4, is induced by canonical Wnt signaling during ectodermal appendage morphogenesis. Dev. Biol. 305, 498–507. [DOI] [PubMed] [Google Scholar]
- Chen D, Jarrell A, Guo C, Lang R, and Atit R (2012). Dermal p-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 139, 1522–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, and Nusse R (2012). Wnt/β-catenin signaling and disease. Cell 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- Cui CY, Yin M, Sima J, Childress V, Michel M, Piao Y, and Schles- singer D (2014). Involvement of Wnt, Eda and Shh at defined stages of sweat gland development. Development 141, 3752–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Qian M, Wu D, and Rosenberg KI (2008). The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev. Biol. 317, 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL, et al. (2012). miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J. Biol. Chem. 287 42084–42092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, and Kemler R (1996). Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech. Dev. 59, 3–10. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, and Birchmeier W (2001). beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105, 533–545. [DOI] [PubMed] [Google Scholar]
- Jackson CE, Callies QC, Krull EA, and Mehregan A (1975). Hairy cutaneous malformations of palms and soles. A hereditary condition. Arch. Dermatol. 111, 1146–1149. [PubMed] [Google Scholar]
- Kamberov YG, Karlsson EK, Kamberova GL, Lieberman DE, Sabeti PC, Morgan BA, and Tabin CJ (2015). A genetic basis of variation in eccrine sweat gland and hair follicle density. Proc. Natl. Acad. Sci. USA 112, 9932–9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Bos-key A, Sun L, et al. (2005). Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat. Genet. 37, 945–952. [DOI] [PubMed] [Google Scholar]
- Li X, Shan J, Chang W, Kim I, Bao J, Lee HJ, Zhang X, Samuel VT, Shulman GI, Liu D, et al. (2012). Chemical and genetic evidence for the involvement of Wnt antagonist Dickkopf2 in regulation of glucose metabolism. Proc. Natl. Acad. Sci. USA 109, 11402–11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Shen K, Zhao Y, He X, Ma C, Wang L, Wang B, Liu J, and Ma J (2013). MicroRNA-222 promotes tumorigenesis via targeting DKK2 and activating the Wnt/β-catenin signaling pathway. FEBS Lett. 587, 1742–1748. [DOI] [PubMed] [Google Scholar]
- Lieven O, Dronka J, Burmuhl S, and Ruther U (2014). Differential binding of Lef1 and Msx1/2 transcription factors to Dkk1 CNEs correlates with reporter gene expression in vivo. PLoS ONE 9, e115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintern KB, Guidato S, Rowe A, Saldanha JW, and Itasaki N (2009). Characterization of wise protein and its molecular mechanism to interact with both Wnt and BMP signals. J. Biol. Chem. 284, 23159–23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BY, McDermott SP, Khwaja SS, and Alexander CM (2004). The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc. Natl. Acad. Sci. USA 101, 4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CP, Polak L, Keyes BE, and Fuchs E (2016). Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science 354, aah6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wei JH, Feng ZH, Chen ZH, Wang YQ, Huang Y, Fang Y, Liang YP, Cen JJ, Pan YH, et al. (2017). miR-106b-5p promotes renal cell carcinoma aggressiveness and stem-cell-like phenotype by activating Wnt/β-catenin signalling. Oncotarget 8, 21461–21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, and Behrens J (2002). Negative feedback loop of Wnt signaling through upregulation of con- ductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin ML, Mazzoni SM, Herron CM, Edwards S, Gruber SB, and Petty EM (2011). AXIN2-associated autosomal dominant ectodermal dysplasia and neoplastic syndrome. Am. J. Med. Genet. A 155A, 898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehregan AH, and Coskey RJ (1972). Pigmented nevi of sole. A report of two cases with histologic evidence of hair follicle formation. Arch. Dermatol. 106, 886–887. [DOI] [PubMed] [Google Scholar]
- Millar SE (2002). Molecular mechanisms regulating hairfollicle development. J. Invest. Dermatol. 118, 216–225. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, and Cotsarelis G (2004). Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22, 411–417. [DOI] [PubMed] [Google Scholar]
- Mu J, Hui T, Shao B, Li L, Du Z, Lu L, Ye L, Li S, Li Q, Xiao Q, et al. (2017). Dickkopf-related protein 2 induces G0/G1 arrest and apoptosis through suppressing Wnt/β-catenin signaling and is frequently methylated in breast cancer. Oncotarget 8, 39443–39459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay M, Gorivodsky M, Shtrom S, Grinberg A, Niehrs C, Mo- rasso MI, and Westphal H (2006). Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development 133, 2149–2154. [DOI] [PubMed] [Google Scholar]
- Närhi K, Jarvinen E, Birchmeier W, Taketo MM, Mikkola ML, and The-sleff I (2008). Sustained epithelial beta-catenin activity induces precocious hair development but disrupts hair follicle down-growth and hair shaft formation. Development 135, 1019–1028. [DOI] [PubMed] [Google Scholar]
- Närhi K, Tummers M, Ahtiainen L, Itoh N, Thesleff I, and Mikkola ML (2012). Sostdc1 defines the size and number of skin appendage placodes. Dev. Biol. 364, 149–161. [DOI] [PubMed] [Google Scholar]
- Niehrs C (2006). Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25, 7469–7481. [DOI] [PubMed] [Google Scholar]
- Plikus M, and Chuong CM (2004). Making waves with hairs. J. Invest. Dermatol. 122, vii–ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler ML (1973). Dysembryoplasie pilaire circonscrite des paumes: un cas familial. Bull. Soc. Franc. Derm. Syph. 80, 323–324. [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kühl M, Sokol S, and He X (2001). Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11, 951–961. [DOI] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, and Schlake T (2006). WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 314, 1447–1450. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lützkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, and Schedl A (2005). Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 15, 1340–1351. [DOI] [PubMed] [Google Scholar]
- Xu M, Horrell J, Snitow M, Cui J, Gochnauer H, Syrett CM, Kallish S, Seykora JT, Liu F, Gaillard D, et al. (2017). WNT10A mutation causes ectodermal dysplasia by impairing progenitorcell proliferation and KLF4-mediated differentiation. Nat. Commun. 8, 15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita M, Okuda T, Endo S, Tanaka M, Takahashi K, Sugiyama F, Kunita S, Takahashi S, Fukatsu A, Yanagisawa M, et al. (2006). Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. J. Clin. Invest. 116, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A, et al. (2008). Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development 135, 2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tomann P, Andl T, Gallant NM, Huelsken J, Jerchow B, Birchmeier W, Paus R, Piccolo S, Mikkola ML, et al. (2009). Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev. Cell 17, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Byrne C, Jacobs J, and Fuchs E (1995). Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 9, 700–713. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang S, Gu L, and Di W (2012). Epigenetic silencing of DKK2 and Wnt signal pathway components in human ovarian carcinoma. Carcinogenesis 33, 2334–2343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


