Abstract
INTRODUCTION:
Exercise is often prescribed as a therapy for chronic pain. Short-term exercise briefly increases the production of endogenous analgesics, leading to transient antinociception. In limited studies, exercise produced sustained increases in endogenous opioids,sustained analgesia, or diminished measures of chronic pain. This studytests the hypothesis that regular aerobic exercise leads to sustained reversal of neuropathic pain by activating endogenous opioid-mediated pain modulatory systems.
METHODS:
After baseline measurements, the L5 and L6 spinal nerves of male Sprague-Dawley rats were tightly ligated. Animals were randomized to sedentary or 5-week treadmill exercise-trained groups. Thermal and tactile sensitivities were assessed 23 hours after exercise, using paw withdrawal thresholds to von Frey filaments and withdrawal latenciesto noxious heat.Opioid receptor antagonists were administered by subcutaneous,intrathecal, or intracerebroventricularinjection. Opioid peptides were quantified usingimmunohistochemistry with densitometry.
RESULTS:
Exercise training ameliorated thermal and tactile hypersensitivity in spinal nerve-ligated animals within 3 weeks. Sensory hypersensitivity returned 5 days after discontinuation of exercise training. The effects of exercise were reversed bysystemically or intracerebroventricularly administered opioid receptor antagonists and prevented by continuous infusion of naltrexone. Exercise increased β-endorphin and met-enkephalin content in the rostral ventromedial medulla and the midbrain periaqeductal gray area.
CONCLUSIONS:
Regular moderate aerobic exercise reversed signs of neuropathic pain and increased endogenous opioid content in brainstem regions important in pain modulation. Exercise effectswere reversed by opioid receptor antagonists. These results suggest that exercise-induced reversal of neuropathic pain results from an upregulation of endogenous opioids.
Summary Statement:
Regular exercise training reverses sensory hypersensitivity in the spinal nerve ligation model of neuropathic pain by an opioid-mediated mechanism, including increased expression of endogenous opioids in the rostral ventromedial medulla and midbrain periaqueductal gray area.
INTRODUCTION
Clinical studies and clinical experience suggest that exercise decreases pain symptoms and improves functionin patients with chronic pain, including those with pain syndromes thought to have a neuropathic component.1–7 However, there are few scientific studies of the mechanisms underlying the pain-relieving effects of exercise.
Extensive research has shown that a single episode of exercise increases the production of endogenous opioids, leading to transient antinociceptionin both animals and humans.8 More recent studies in experimental animals have shown that repeated exercise produces long-lasting antinociception in otherwise untreated animals,9–12 and increases plasma and cerebrospinal fluid opioid concentrations.13-14Further, in animal modelsexercise diminishes measures of inflammatory pain,15chronic muscle pain,16 and chronic neuropathic pain.15,17,18 The mechanisms underlying exercise-induced reversal of chronic pain are incompletely understood. One study in a model of muscle pain showed exercise effects to be reversed by naloxone, suggesting the participation of endogenous opioids.16The present study tests the hypothesis that regular, repeated aerobic exercise will reverse neuropathic pain by enhancing endogenous opioid-mediated pain modulatory systems.
MATERIALS AND METHODS
Animals
Approval was obtained from the University of Arizona Animal Care and Use Committee (Tucson, Arizona). Male Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN) were 250–380 g throughout testing. They were allowed water and food ad libitum andhoused in a climate-controlled room.They weremaintained under reverse light/dark conditions,with the room litfrom 10 pm to 10 am. They were handled twice daily in a stress-free environment and were allowed to equilibrate to the surroundings for 7 days before experimental manipulations were begun. All procedures conformed to the ethical guidelines for the care and use of laboratory animals published by the International Association for the Study of Pain (IASP) and the National Institutes of Health (NIH).
Spinal Nerve Ligation
L5/L6 spinal nerve ligation (SNL) was performed as describedby Kim and Chung.19Underisoflurane anesthesia, an incision was made lateral to the lumbar spine. The paraspinal muscles were separated from the vertebral processes at the L4 to the S2 levels. The left L6 transverse process was completely removed with a small rongeur, and the L4 to L6 spinal nervesexposed. The leftL5 and L6 spinal nerves were isolated and tightly ligated with 4–0 silk sutures distalto the dorsal root ganglion. The incision was sutured closed, and each animalwas allowed to recover for 7 days prior to exercise training. Sham-operated animalswere prepared in an identical fashion, but the spinalnerves were not ligated. Non-operated animals underwent no surgery, but were otherwise treated identically to SNL or sham-operated animals. Animals demonstrating signs of sciatic nerve injury (an inverted foot with markedly ventroflexedtoes)19or of damage to the L4 spinal nerve (hindpawparalysis)19were removed from the study.
Exercise Training
Forced treadmill running was used for exercise training because it is an established model in which exercise intensity and duration can be readily controlled.20Prior to surgery, animals underwent exercise pre-testing on a rodent treadmill (18m/min for 10 minutes, 2 days per week for 2 weeks). Animals unable to complete the pretest, whether from inability to run or from signs of stress (e.g. porphoryn rings around the eyes, red nose), were removed from the study.Following surgery, animals were randomized to a sedentary group or an exercise-trained group. Exercisetraining consisted ofrunning on a 10-lane motor-driven rodent treadmill (in late afternoon,5 days per week, for 5 weeksat a speed of 14–16 m/min and an 8% grade). The duration of exercise was incrementally increased to 30 min/day over 14 days. An electric grid behind the treadmill was activated with a weak current. To minimize stress, animals were allowed contact with the grid for a maximum of 2 min up to 3 times per session before being removed from the treadmill apparatus. Sedentary animals were treated identically to exercise-trained animals, except that they were placed in the treadmill lanes in plexi-glass boxes that did not allow contact with the moving treadmill. This exposed them to the same handling and environmental conditions as exercised animals. Animals displaying signs of stress were removed from the study.
Assessment of tactile sensitivity
Tactile sensitivity was assessed by measuring the threshold for withdrawal of the hindpawfrom normally non-noxious tactile stimuli. Rats were allowed to acclimate for 30 min within plexiglass enclosures with wire mesh bottoms. Paw withdrawal thresholds were determined in response to probing of the lefthind paw with a series of calibrated von Frey filaments, in logarithmically spaced increments, applied perpendicularly to the plantar surface of the paw. A maximal cutoff of 15 g was used because larger filaments lift the paw,preventing the assessment of active paw withdrawal. Data were analyzed by the up-and-down method of Dixon, as described by Chaplan et al.21Tactile sensitivity was measured at baseline, 1 week after surgery, and at weekly increments during exercise training. Each time point consisted of one day of testing. Where applicable, measurements were performed 23 hours after exercise. Measurements were confirmed by an observer blinded to the treatment received.
Assessment of thermal sensitivity
Thermal sensitivity was assessed measuring latency to paw withdrawal from a noxious heat source, as described by Hargreaves et al.22Rats were allowed to acclimate within plexiglass enclosures on a clear glass plate maintained at 30°C. A radiant heat source was focused through the glass plate onto the plantar surface of the lefthind paw. Withdrawal latency was measured using a motion detector that shut off the heat stimulus and a timer upon withdrawal of the paw. A maximal cutoff of 40 s was used to prevent tissue damage. Thermal sensitivity was measured at baseline, 1 week after surgery, and at weekly increments during exercise training. Each time point consisted of one day of testing. Where applicable, measurements were conducted 23 hours after exercise. Measurements were confirmed by an observer blinded to the treatment received.
Osmotic minipumps
Osmotic minipumps were implanted subcutaneously under isoflurane anesthesia, one day before initiation of exercise training. After shaving of the surgical site, the skin was swabbed withpovidone-iodine and 70% alcohol. A small incision was made through the skin between the scapulae. A small pocked was formed using a hemostat to spread the subcutaneous connective tissue and an osmotic minipump(Alzetmodel 2006; Alza, Mountain View, CA) was inserted into the pocket with the flow moderator pointing away from the incision. Each pump wasincubated in 0.9% saline at 37°C for 60 hours prior to implantation. Theincision was closed with wound clips or sutures.
Drug Administration
All drugs were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in saline. For systemic administration, naloxone (1 mg/kg), naloxone methiode (0.1 mg/kg), or vehicle (saline) were administered subcutaneously in the neck in a volume of 1 ml/kg. Single doses of opioid receptor antagonists were administered 23 hours after completion of the final session of exercise training. For continuous administration of opioid receptor antagonist, subcutaneous minipumps delivered naltrexone (70 μg/hr) or vehicle (saline) in a volume of 0.15 μl/hr for one day before and during 35 consecutive days of exercise training.
IntracerebroventricularCannulation
Intracerebroventricular cannulas were inserted 5 days prior to the start of exercise training. Under isoflurane anesthesia, the scalp was shaved and the skin swabbed with povidone-iodine and 70% alcohol.The head wasplacedin a stereotaxic holder and the skull was exposed.A 22-gauge guide cannula was directed to the right lateral ventricle (1.3 mm caudal to bregma, 1.5 mm lateral to the sagittal suture, 3.5 mm ventral to the dural surface). The cannula was secured in place by small stainless steel screws and dental cement. Drug was administered though a 28-guage injection cannula inserted through the guide cannula. Naloxone methiodide (2 μg) was slowly injected in a total volume of 2.5 μl, 23 hours after completion of the final session of exercise training. Backflow was prevented by using an injection cannula 1mm longer than the guide cannula.
Intrathecal Injection
Intrathecal injections were performed under isoflurane anesthesia. The lumbar region was shaved and prepared with 70% ethanol. A 0.5 inch, 30-guage needle connected to a 25 μl Hamilton syringe was passed through the L5/L6 spinal interspace. Subarachnoid positioning of the tip of the needle was verified by a flicking motion of the tail or hindpaw. Lidocaine (4 %, 10–20 μl) was administered to a group of test animals, using temporary paralysis of the hindlimbsas an endpointto confirm the effectiveness of the injection technique. Naloxone methiodide (10 μg) was administered in a volume of 10 μl, 23 hours after completion of the final session of exercise training.
Immunohistochemistry
Rats were anesthetized with ketamine HCl/xylazine. The heart was surgically exposed and the animal was perfused transcardially with 0.01 M sodium phosphate-buffered saline (PBS; pH 7.4) until the exudate ran clear, and for approximately 15 additional min with 10% PBS-buffered formalin. All harvested tissues were post-fixed in 10% PBS-buffered formalin, and transferred to 20% sucrose in 0.1 M PBS. Slide-mounted serial frozen sections of the periaqueductal gray area(PAG) and the rostral ventromedial medulla (RVM) were pre-blocked with 10% normal goat serum in PBS (1 h, room temperature), followed by incubation with 2% normal goat serum/0.3% Triton X-100/PBS/primary antibody for 24 h at 4 °C. Secondary antibody was added for 2 h at room temperature.Primary antisera were rabbit anti-β-endorphin (1:5,000; ImmunoStar Incorporated, Hudson, WI), and rabbit anti-met-enkephalin (1:10,000; Chemicon International, Temecula, CA).Secondary antiserum was Alexa Fluor 568 goat anti-rabbit IgG (1:1000; Invitrogen, Carlsbad, CA).Following PBS washes, sections were dried and sealed with fluorescent mounting medium (Vector Laboratories, Burlingame, CA).
Image analysis and quantification
Fluorescence images of PAG and RVM brain sections were acquired with a Nikon E800 fluorescence microscope (Nikon, Tokyo, Japan) outfitted with 4X NA 0.2, 10X NA 0.45, 20X NA 0.75 and 40X NA 0.75 objectives, a filter set for Cy3 (excitation 540–580 nm/emission 560–620 nm) and a Hamamatsu C5810 color CCD camera and its proprietary Image Processor software (Hamamatsu Photonic Systems, Bridgewater, NJ). Digital images were produced using Adobe Photoshop 6.0 (Adobe Systems Inc., San Jose, CA). Quantitative measurements in PAG were performed on a total of 10 sectionstaken from 5 animals per group. Quantitative measurements in RVM were performed on a total of 26–32sectionstaken from 5 animals per group. Sections were selected for analysis based on the quality of the section (lack of tears, bubbles, etc.) Images were of identical dimensionsand were analyzed using the densitometry program Scion Image 4.0.3.2 (Scion Corp., Frederick, MD). Control and experimental tissues were processed and analyzed simultaneously.
Statistical analysis
The data were assumed to meet the assumptions of parametric tests, because the sample sizes used were not large enough to allow formal testing of these assumptions. When two groups were compared, a two sample, two-sided, independent Student’s t-test was used. Multiple groups were compared using one-way ANOVA. When the same group of animals was studied over time, one-way repeated measures ANOVA was applied. When multiple groups of animals were studied over time, two-way repeated measures ANOVA (group x time) was applied. Post-hoc testing consisted of pairwise comparison using Bonferroni’s Method for Multiple Comparisons. Where noted, only selected comparisons were performed, based upon pre-analysis planning, in order to minimize the statistical impact of multiple comparisons. All P–values were calculated using two-tailed tests. Significance was defined as P < 0.05. All data are reported as mean with 95% two-tailed confidence intervals (in parentheses). Statistical analysis and preparation of figures was performed with GraphPad Prism, Version 5 (GraphPad Software, La Jolla, CA) and Stata, Version 11 (Stata Corp, College Station, TX).
RESULTS
Exercise training reversedSNL-induced thermal and tactile hypersensitivity
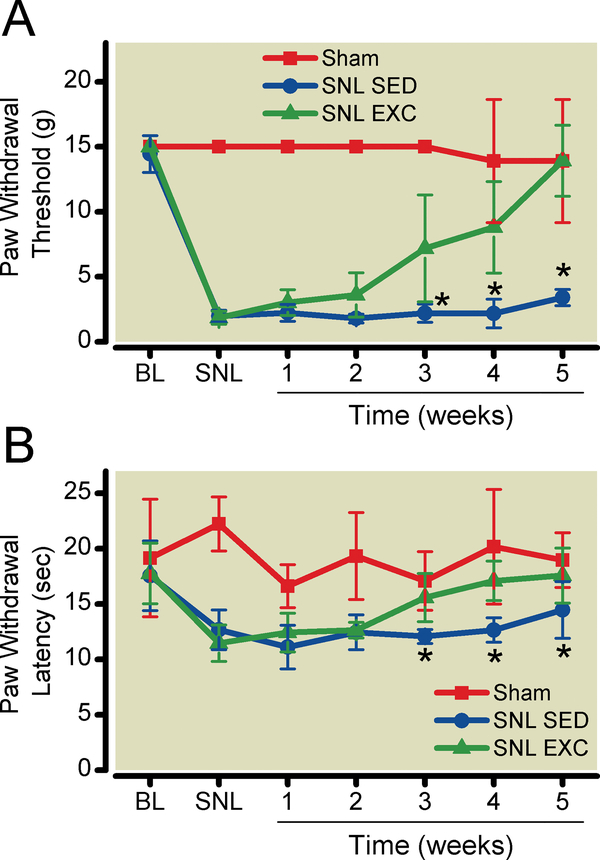
There were no differences between animals assigned to the SNL and sham-operated groups in pre-operative withdrawal thresholds or latencies. By one week after surgery, SNL animals developed sensory hypersensitivity (lower thermal withdrawal latencies and tactile withdrawal thresholds) compared to presurgical values (Fig. 1A,B), while sham- or non-operated animals did not. Statistical analysis using repeated measures two-way ANOVA (group x time) demonstrated a significant time-treatment interaction effect for both thermal withdrawal latencies (P <0.0001) and paw withdrawal thresholds (P<0.0001). Post-hoc testing was performed using Bonferroni’s Method for Multiple Comparisons, specifically making the following comparisons. Spinal nerve ligation reduced thermal withdrawal latency from 17.7 (16.0 to-19.4) to 12.1 (11.0 to 13.2) sec (P<0.0001) and tactile withdrawal thresholdfrom 14.7 (14.0 to15.4) to 1.8 (1.6 to 2.1) sec (P<0.0001). Regular exercise training ameliorated thermal and tactile hypersensitivity within 3 weeks. Thermal withdrawal latency increased after 3 weeks of exercise compared to sedentary animals (P = 0.0064), and after 5 weeks withdrawal latency had returned to 17.6 (15.1 to 20.1) sec (P = 0.0135 vs. SNL sedentary animals), compared to 19.0 (16.5 to 21.4) sec in sham-operated animals (Fig. 1A). Tactile withdrawal thresholds increased after 3 weeks of exercise compared to sedentary animals (P = 0.0001), and after 5 weeks withdrawal thresholdspaw withdrawal thresholds had returned to 13.9 (11.2 to 16.7) sec (P < 0.0001 vs. SNL sedentary animals), compared to 13.9 (9.2 to 18.6 sec) in sham-operated animals.Exercise did not alter withdrawal thresholds or latenciesin sham-operated or non-operated animals. Statistical analysis used two-way repeated-measures ANOVA. For tactile sensitivity P = 0.26 for group x time interaction and P = 0.33 for the effects of treatment. For thermal sensitivity, P = 0.51 for group x time interaction and 0.027 for the effects of treatment. However, post-hoc testing using Bonferroni’s method for multiple comparisons did not show an effect of exercise at any time point in either non-operated or sham-operated animals.
Figure 1:
Exercise training reverses nerve injury-induced sensory hypersensitivity. A. Tactile paw withdrawal threshold in exercise-trained vs. sedentary SNL animals. Data from sham-operated animals are included for comparison. B. Thermal paw withdrawal latency to radiant heat in exercise-trained vs. sedentary SNL animals. Data from shamoperated animals are included for comparison. Baseline measurements were taken one day before surgery. Further measurements were performed one day before and weekly after initiation of exercise training. Measurements were made 23 hrs after exercise. Data shown as mean with 95% two-tailed confidence intervals. *P < 0.05 compared to exercise-trained, spinal nerve-ligated rats. N = 6 animals per group. BL = pre-surgical baseline; SNL = after spinal nerve ligation surgery; SED = sedentary animals; EXC = exercise-trained animals.
Thermal sensitivity could not be studied for more than 5 weeks after surgery, because it returns to baseline after several weeks in the SNL model (MohabM. Ibrahim, Ph.D., M.D.; T. Philip Malan, Ph.D., M.D.; unpublished observations; Tucson, Arizona, 2000). In contrast, tactile hypersensitivity appears to last indefinitely. Because thermal hypersensitivity could not be studied in longer-term experiments, only tactile hypersensitivity was studied in the following experiments.
Exercise effects were dependent on exercise intensity but not frequency.
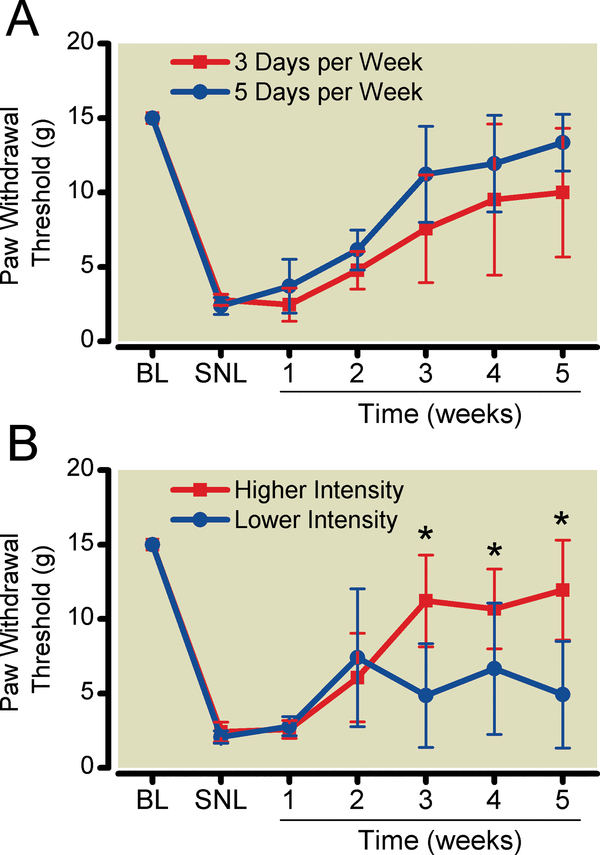
Exercise training was conducted in SNL animals for either 3days per week or 5 days per week. Statistical analysis using repeated measures two-way ANOVA demonstrated that the effects of time-frequency interaction (P= 0.112) or frequency (P= 0.091) were not significant (Fig. 2A).
Figure 2:
More intense exercise, but not more frequent exercise training resulted in more complete reversal of sensory hypersensitivity. A. Tactile paw withdrawal threshold after exercise training 3 or 5 days per week. B. Tactile paw withdrawal threshold after exercise training at 10 m/min (lower-intensity) or 16 m/min (higher-intensity) 5 days per week. Data shown as mean with 95% two-tailed confidence intervals. *P < 0.05 compared to lower-intensity exercise. N = 6 animals per group. BL = pre-surgical baseline; SNL = after spinal nerve ligation surgery.
Exercise training was conducted in SNL animals at speeds of either 10 m/min (lower intensity) or 16m/min (higher intensity). Walking was required at the lower intensity and running at the higher intensity. Animals could not be trained at speeds of more than 16 m/min, because animals exercised at higher speeds exhibited signs of stress. The higher intensity group had a more complete reversal of tactile hypersensitivity than did the lower intensity group (Fig. 2B). Statistical analysis using repeated measures two-way ANOVA demonstrated a significant intensity-time interaction effect (P <0.0001) andintensity effect (P = 0.0064). Post-hoc testing was performed using Bonferroni’s Method for Multiple Comparisons, comparing paw withdrawal thresholds between higher- and lower-intensity training at each time point. Tactile threshold was 12.3(9.3 to 15.3) g after 5 weeks of higher-intensity exercise, compared to 4.9(1.3 to 8.5) g (P = 0.0003) after 5 weeks of lower-intensity exercise. Post hoc analysis with repeated measures one-way ANOVA also demonstrated that lower-intensity exercise did not significantly increase paw withdrawal threshold compared to pre-exercise values (P=0.068). In contrast, post hoc analysis with repeated measures one-way ANOVA demonstrated that higher-intensity exercise significantly increased paw withdrawal threshold compared to pre-exercise values (P< 0.0001).
The onset of exercise effects was determined by the number of weeks of exercise training
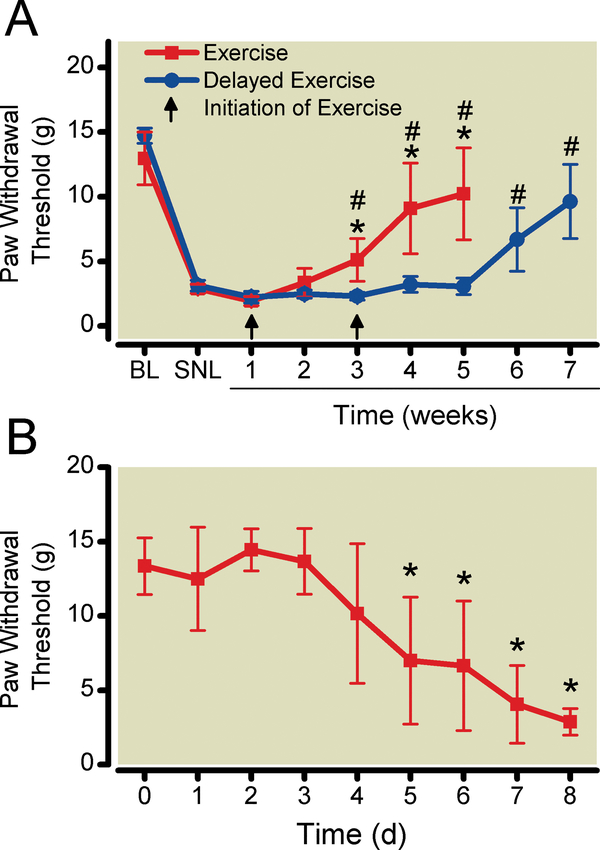
The observed timing of exercise effects might be due to the number of weeks of exercise training required to reverse sensory hypersensitivity. Alternatively, the timing of exercise effects might be due to a maturation of the SNL model in which sensory hypersensitivity was not susceptible to exercise reversal until 3 weeks after surgery. To distinguish between these possibilities, in one group of animals exercise was initiated 4 weeks after SNL surgery as opposed to the 1-week recovery period used in other experiments. Reduction of sensory hypersensitivity occurred 3 weeks after initiation of exercise, regardless of the interval after surgery (Fig. 3A).Statistical analysis using repeated measures two-way ANOVA (group X time) demonstrated a significant group-time interaction effect (P<0.0001) and group effect (P = 0.0009).Post-hoc testing was performed using Bonferroni’s Method for Multiple Comparisons, specifically comparing paw withdrawal threshold at each time point after initiation of exercise to paw withdrawal latency after surgery and before exercise testing. The results of this analysis are presented in Fig. 3A.
Figure 3:
A. Onset of exercise-induced reversal of sensory hypersensitivity occurred 3 weeks after initiation of exercise training. Tactile sensitivity was measured in sedentary SNL, exercise-trained SNL, and delayed-exercise SNL animals. Data shown as mean # with 95% two-tailed confidence intervals. P < 0.05 compared to pre-exercise, spinal nerve-ligated animals. *P < 0.05 compared to delayed exercise group. N = 6 animals per group. B. Tactile hypersensitivity returned 5 days after cessation of exercise training. Data shown as mean with 95% two-tailed confidence intervals. N = 6 animals per group. BL = pre-surgical baseline; SNL = after spinal nerve ligation surgery.
Sensory hypersensitivity returned within 1 week of cessation of exercise
Tactile hypersensitivity began to return5 days after cessation of exercise training and returned to pre-exercise levels 8 days after discontinuing exercise (Fig. 3B). Statistical analysis with one-way repeated-measures ANOVA demonstrated a significant time effect(P<0.0001). Post hoc analysis was performed using Bonferroni’s Method for Multiple comparisons, specifically comparing paw withdrawal threshold at each time point with paw withdrawal threshold before discontinuation of exercise training. The first statistically significant decrease in withdrawal threshold occurred on day 5 (P = 0.0006).
Opioid receptor antagonists reversed exercise effects
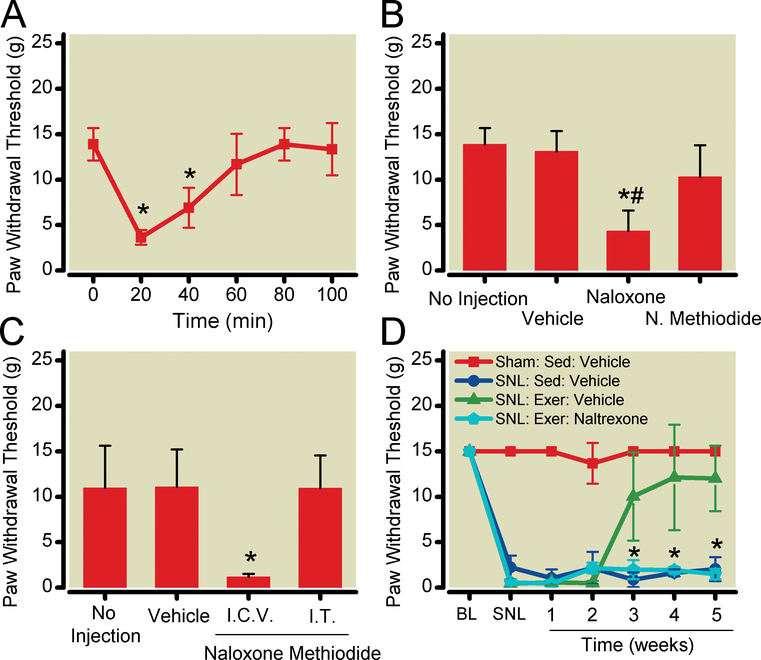
Subcutaneous naloxone (1 mg/kg)decreased tactile withdrawal threshold in exercise-trained SNL animals from 13.9 (12.1 to 15.7) g to 3.6 (2.8 to 4.4)g 20 min after injection (Fig. 4A). Statistical analysis using one-way repeated-measures ANOVA demonstrated a significant time effect(P< 0.0001). Post-hoc testing using Bonferroni’s Method for Multiple Comparisons was used to specifically compare paw withdrawal threshold at each time point to the pre-naloxone value. P<0.0001 for paw withdrawal threshold 20 min after injection vs. the pre-injection value.
Figure 4:
Opioid receptor antagonists reverse the effects of exercise on sensory hypersensitivity. A. Naloxone reversibly inhibited exercise effects. Exercise-trained SNL animals were administered naloxone (1 mg/kg) subcutaneously.*P < 0.05 compared to pre-naloxone values. B. Subcutaneous naloxone methiodide (0.1 mg/kg) had no effect on tactile hypersensitivity in spinal nerve-ligated, exercise-trained animals. *P < 0.05 compared to spinal nerve-ligated, exercise-trained animals receiving vehicle. #P < 0.05 compared to spinal nerve-ligated, exercise-trained animals receiving naloxone methiodide. C. Reversal of nerve injury-induced sensory hypersensitivity by exercise is mediated by endogenous opioids in the central nervous system. Intracerebroventricular injection of naloxone methiodide (2 μg) reversed exercise-training mediated reversal of sensory hypersensitivity, while intrathecal injection of naloxone methiodide (10 μg) had no effect. *P < 0.05 compared to vehicle-treated, exercise-trained, spinal nerve-ligated animals. D. Continuous naltrexone infusion (70 μg/hr) prevented exercise-induced reversal of tactile hypersensitivity. *P < 0.05 vs. vehicle-infused animals. Data shown as mean with 95% two-tailed confidence intervals. N = 6 animals per group. N. Methiodide = naloxone methiodide; i.c.v. = intracerebroventricular; i.t. = intrathecal; BL = presurgical baseline; SNL = after spinal nerve ligation surgery; SED = sedentary animals; EXER = exercise-trained animals.
Subcutaneous naloxone methiodide (0.1 mg/kg) had no effect (Fig 4B). Statistical analysis using one-way ANOVA demonstrated a significant between group effect (P<0.0001). Post hoc analysis using Bonferroni’sMethod for Multiple Comparisonscompared paw withdrawal thresholds in drug-treated animals to thresholds in vehicle-treated animals and withdrawal thresholds in naloxone treated animals to thresholds in naloxone methiodide-treated animals Systemic naloxone (1 mg/kg) reduced paw withdrawal threshold in SNL exercised animals compared to vehicle (P = 0.0001), while systemic naloxone methiodide did not (P = 0.061). Withdrawal thresholds were lower after systemic naloxone administration than after systemic naloxone methiodideinjection (P = 0.002). Neither naloxone or naloxone methiodide altered withdrawal thresholds in sham-operated or SNL sedentary animals (P = 0.886; drug treatment effect from two-way ANOVA).
Intracerebroventricular injection of naloxone reversed exercise effects
When naloxone methiodide (2 μg) was administered intracerebroventricularly after 5 weeks of exercise training, paw withdrawal threshold compared to vehicle decreased from 11.1 (6.98 to 15.22) g to 1.2 (0.9 to 1.52) g (Fig. 4C). Intrathecaladminitration of naloxone methiodide (10 μg) had no effect. Statistical analysis performed using one-way ANOVA yielded P<0.0001 for between group difference. Post hoc analysis using Bonferroni’sMethod for Multiple Comparisons to compare naloxone methiodide-treated to vehicle-treated animals demonstrated that intracerebroventricular naloxone methiodide reduced paw withdrawal threshold in SNL exercised animals compared to vehicle (P = 0.0004), while intrathecal naloxone methiodide did not (P = 0.923). Because intrathecal naloxone methiodide had no effect, we verified the effectiveness of this dose of intrathecalnaloxone methiodide in reversing the analgesic effects of intrathecally administered morphine. Statistical analysis performed using one-way ANOVA yielded P = 0.0034 for a between group difference. Post hoc analysis was performed using Bonferroni’sMethod for Multiple Comparisons.In otherwise untreated animals, morphine (30 μg intrathecal) increased paw withdrawal latency to radiant heat from 20.8 (18.2 to 23.4) sec at baseline to 32.2(25.4 to 39.0) sec 30 min after drug administration (P = 0.0024). Pre-administration of naloxone methiodide 15 min before morphine injection prevented morphine-induced analgesia, with a resulting paw withdrawal latency of 24.4(19.2 to 29.6)s (P = 0.002vs morphinetreated animals). Intracerebroventricular naloxone methiodide-treated, exercise-trained SNL animals showed signs of opioid withdrawal within 20 min of naloxone methiodide injection (e.g. aggressive behavior, diarrhea). Naloxone methiodide did not alter tactile sensitivity or produce withdrawal in exercise-trained, sham-operated animals.
Continuousinfusion of naltrexone prevented exercise effects
One mechanism that would explain the observation that naloxone lowered withdrawal thresholds would be antagonist-precipitated withdrawal from the effects of endogenous opioidsupregulated by exercise training. To eliminate possible opioid withdrawal, and its confounding effects, we tested whether continuous infusion of naltrexone prevented exercise-associated reversal of sensory hypersensitivity. SNL animals were implanted with subcutaneous osmotic minipumps, which delivered either naltrexone (70 μg/h) or vehicle for 5 weeks. Naltrexone infusion prevented exercise training-induced reversal of SNL-induced sensory hypersensitivity at all time points tested (Fig. 4D).Statistical analysis performed using two-way repeated measures ANOVA (group x time) yielded P = 0.0001 for treatment-time interaction and P = 0.0001 for treatment effect. Post-hoc analysis was performed using Bonferroni’s Multiple Comparison Test to compare exercise-trained animals to exercise-trained animals receiving naltrexone infusion. Exercise trained SNL animals treated with naltrexone exhibited mean paw withdrawal thresholds of 1.5(0.9 to 2.1)g after 5 weeks of exercise training, lower than those of vehicle-treated animals [12.0 (8.4 to 15.6) g, (P < 0.0001)], and similar those of sedentary SNL animals [2.0 (0.7 to 3.3) g].
Exercise training increases endogenous opioid levels in the PAG and RVM
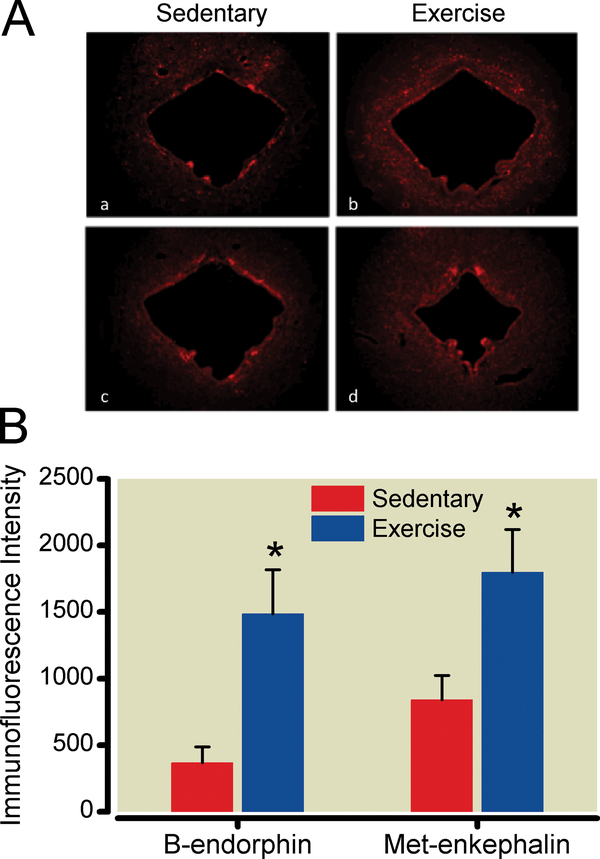
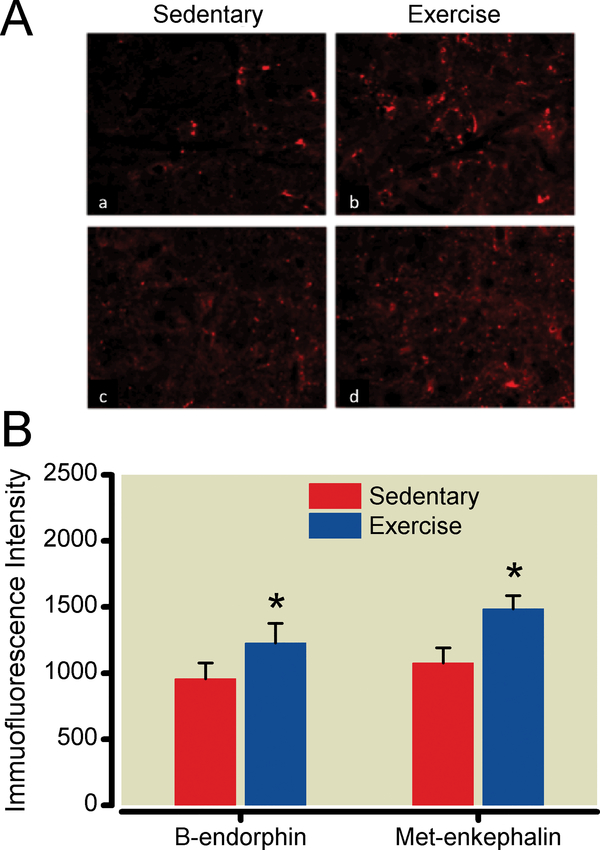
The PAG and RVM displayed increased immunoreactivity, detected using immunohistochemical techniques, for β-endorphin and met-enkephalin in exercise-trained compared to sedentary SNL animals. Measurements from sections from exercise-trained animals, obtained after 5 weeks of exercise training, were compared to those from sedentary animals using unpaired, two-tailed Student’s t-test. While additional time points may have allowed temporal correlation of brainstem opioid content with exerciseinduced reversal of sensory hypersensitivity, we limited this study to one measurement after the effects of exercise were established to minimize animal use. Exercise training increased PAG β-endorphin and met-enkephalinimmunoreactivity by 304% and 114%, respectively (P<0.0001, P<0.0001) (Fig. 5). Exercise training increased RVM β-endorphin and met-enkephalin levels by 28% and 38%, respectively (P = .0086, P = 0.0009) (Fig. 6).
Figure 5:
Exercise training increases endogenous opioid content in the midbrain periaqueductal gray area (PAG). A. After 5 weeks of exercise training or sedentary conditions, PAG sections from nerve-injured animals were labeled with antibodies to β-endorphin (a,b) or met-enkephalin (c,d). Images were magnified 20x. B. Densitometry was performed on 10 images from 5 animals per group. *P < 0.05 compared to spinal nerve-ligated, sedentary animals. Data shown as mean with 95% two-tailed confidence intervals.
Figure 6:
Exercise training increases endogenous opioid content in the rostral ventomedial medulla (RVM). A. After 5 weeks of exercise training or sedentary conditions, RVM sections from nerve-injured animals were labeled with antibodies to β-endorphin (a,b) or met-enkephalin (c,d). Images were magnified 40x. B. Densitometry was performed on 26–32 images from 5 animals per group. *P < 0.05 compared to spinal nerve-ligated, sedentary animals. Data shown as mean with 95% two-tailed confidence intervals.
DISCUSSION
Regular moderate aerobic exercise reversed the sensory hypersensitivity observed in the SNL model of neuropathic pain. Naloxone reversed the effects of exercise, suggesting that the activity of endogenous opioids is necessary for exercise trainingmediated reversal of SNL-induced sensory hypersensitivity. The non-blood brain barrierpermeable opioid receptor antagonist naloxone methiodide had no effect when administered subcutaneously, suggesting that endogenous opioids mediate exercise effects by acting in the central nervous system. In this regard, intracerebroventricular injection of naloxone methiodide reversed exercise effects, while intrathecal injection of naloxone methiodide did not, suggesting that the effects of exercise may be due to an increase in endogenous opioid content or release in the brain. This hypothesis was confirmed by the observation that content of β-endorphin and met-enkephalin in the PAG and RVM were increased in exercise-trained SNL animals. Taken together with previous work demonstrating that supraspinal administration of morphine reverses sensory hypersensitivity in the SNL model,23,24these findings support the hypothesis that regular exercise training reverses the signs ofneuropathic pain by increasing expression of endogenous brainstem opioids.
Our results confirm and extend previous findings that exercise produces antinociceptive effects. An extensive body of research in both humans and animals demonstrates that a single episode of exercise increases nociceptive thresholds measured soon after exercise (reviewed in Koltyn8). Acute forms of exercise have been shown to increase the release of endogenous opioids, suggesting that these endogenous analgesics may be responsible for the antinociceptive effects observed in acute exercise models.
The antinociceptive effects of regular exercise have been less extensively studied. Shyu et al.9demonstrated that several weeks of voluntary wheel running increased nociceptive thresholds in rats, as assessed using the threshold to vocalization in response to subcutaneous electrical stimulation. This increase in vocalization threshold was reversed by the opioid receptor antagonist naloxone. The increase in nociceptive threshold persisted until the day after exercise, as opposed to the antinociceptive effects of acute exercise, which last only hours after cessation of exercise.Other, more recent, studies have also demonstrated antinociceptive effects of regular exercise that are reversed by naloxone.9-12These findings, together with observations that regular exercise produces tolerance to the effects of exogenously administered opioids, 11,12 suggested that regular exercise may increase endogenous opioid expression in the central nervous system.
Regular running exercise increases serum and cerebrospinal fluid content of endogenous opioids.13,14 In these studies, cerebrospinal fluid13 and serum14β-endorphin concentrations were elevated after 5–6 and 8 weeks of exercise training, respectively (earlier time points were not tested), similar to our findings of naloxone-mediated reversal of sensory hypersensitivity and increased brainstem opioid content 5 weeks after initiation of exercise training. Our finding that exercise effects on SNL-induced sensory hypersensitivity began to diminish 4 days after discontinuation of exercise is consistent with previous work demonstrating a rapid decrease in endogenous opioid expression after cessation of exercise. Hoffman et al.13found thatcerebrospinal fluid levels of β-endorphin remained elevated for only 48 hours after cessation of regular exercise training.
The effects of exercise training in animals with pre-existing pain conditions has not been thoroughly studied. Low intensity treadmill training reversed mechanical hypersensitivity in animals with chronic muscle pain.16Treadmill running completely reversed signs of neuropathic pain in animals with spinal cord injury.17Extended swimming was found to reduce inflammatory and peripheral neuropathic pain.15Finally, 5 days of treadmill training reversed sensory hypersensitivity produced by chronic constriction injury of the sciatic nerve.16Our study adds to this body of work by focusing on the role of endogenous opioids in exercise training-induced reversal ofneuropathic pain.
Both the PAG and RVM are important sites in the regulation of descending pain-modulating pathways. They are the two principal areas of the brainstem responsible for the analgesia that occurs after opioid administration, with interactions between both structures producing the most effective analgesia.25It is likely that these same sites are responsible for the reversal of nerve injury-induced sensory hypersensitivity by supraspinal actions of opioids. In this regard, microinjection of opioids into the PAG reversed sensory hypersensitivity in rats with injuryof the tibial and sural nerves.26
Previous studies in models of spinal cord or peripheral nerve injury-induced neuropathic pain have suggested that additional mechanisms may contribute to exercise training-induced reversal of sensory hypersensitivity. Hutchinson et al.17found that treadmill training restored spinal cord and soleus muscle levels of brain-derived neurotrophic factor (BDNF), which had been diminished by spinal cord injury, to normal levels. Interestingly, BDNF administration in brain or spinal cord produces naloxone-reversible analgesia,27–29raising the possibility that an exercise-induced increase in BDNF levels may lead to increased levels of endogenous opioids.While the effects of endogenous opioids reported here appear to be localized in the brain and Hutchinson et al. observed increased BDNF expression in spinal cord, it must be noted that that Hutchinson et al. utilized a model of direct spinal cord injury and focused exclusively on spinal cord BDNF content. More recently, Cobianchi et al.18demonstrated that 5 days of exercise training reduced sensory hypersensitivity in the chronic constriction injury model of neuropathic pain, but that this effect dissipated with additional training. They found reduced spinal microglia expression after both short-term and long-term treadmill running, reduced astrocyte expression after short-term treadmill running, and normalization of astrocyte expression after longer-term treadmill running. They also found that short-term treadmill running resulted in upregulation of Cdc2 and GAP-43 proteins in sciatic nerve. It is not clear to what extent these processes in spinal cord and peripheral nerve are responsible for the reversal of sensory hypersensitivity or how they may interact with the opioid-mediated mechanisms described here. Further, it is not clear why Cobianchi et al. observed a transient effect of exercise, while the changes in sensory sensitivity we observed persisted for at least 5 weeks, if exercise training was maintained.
In summary, regular moderate aerobic exercise reverses the sensory hypersensitivity observed in an animal model of neuropathic pain. Moderate aerobic exercise also increases endogenous opioid content in areas of the brainstem known to be important in pain modulation. Finally, the effects of exercise are reversed by opioid receptor antagonists. These results suggest that an increase in brainstem expression of endogenous opioids is necessary for exercise-induced reversal of neuropathic pain. Previous work has suggested that the activation of brain opioid receptors is sufficient to reverse nerve injury-induced sensory hypersensitivity, supporting the feasibility of an endogenous opioid-mediated mechanism.22,23Clinical studies in humans have shown that exercise reduces chronic pain, including pain likely to have a neuropathic component.1–7The present findings provide a mechanism that may contribute the beneficial effects of exercise in the treatment of neuropathic pain.
Acknowledgments
Funded by the National Institute on Drug Abuse, Bethesda, Maryland (R01 DA015866) and by the University of Arizona Department of Anesthesiology, Tucson, Arizona
REFERENCES
- 1.Malmros B, Mortensen L, Jensen MB, Charles P.Positive effects of physiotherapy on chronic pain and performance in osteoarthritis.OsteoporosInt 1998; 8: 215–21. [DOI] [PubMed] [Google Scholar]
- 2.McCain GA, Bell DA, Mai FM, Halliday PD. A controlled study of the effects of a supervised cardiovascular fitness training program on the manifestations of primary fibromyalgia. Arthritis Rheum 1988; 31:1135–41. [DOI] [PubMed] [Google Scholar]
- 3.Ferrell BA, Josephson KR, Pollan AM, Loy S, Ferrell BR. A randomized trial of walking versus physical methods for chronic pain management. Aging (Milano) 1997;9: 99–105. [DOI] [PubMed] [Google Scholar]
- 4.Gowans SE, Dehueck A, Voss S, Silaj A, Abbey SE. Six-month and one-year followup of 23 weeks of aerobic exercise for individuals with fibromyalgia. Arthritis Rheum 2004; 51: 890–8. [DOI] [PubMed] [Google Scholar]
- 5.Hayden JA, Van Tulder MW, Malmivaara AV, Koes BW. Meta-analysis: Exercise therapy for nonspecific low back pain. Ann Intern Med 2005; 142: 765–75. [DOI] [PubMed] [Google Scholar]
- 6.Robb KA, Williams JE, Duvivier V, Newham DJ. A pain management program for chronic cancer-treatment-related pain: A preliminary study. J Pain 2006; 7: 82–90. [DOI] [PubMed] [Google Scholar]
- 7.Chatzitheodorou D, Kabitsis C, Malliou P, Mougios V. A pilot study of the effects of high-intensity aerobic exercise versus passive interventions on pain, disability, psychological strain, and serum cotisol concentrations in people with chronic low back pain.Phys Ther 2007; 87: 304–12. [DOI] [PubMed] [Google Scholar]
- 8.Koltyn KF. Analgesia following exercise: A review. Sports Med 2000; 29: 85–98. [DOI] [PubMed] [Google Scholar]
- 9.Shyu B, Andersson S, Thoren P. Endorphin mediated increase in pain threshold induced by long-lasting exercise in rats. Life Sci 1982; 30: 833–40. [DOI] [PubMed] [Google Scholar]
- 10.Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: μ-opioid tolerance and physical dependence. Psychopharmacology 2003; 168: 426–34. [DOI] [PubMed] [Google Scholar]
- 11.Smith MA, Lyle MA. Chronic exercise decreases sensitivity to mu opioids in female rats: Correlation with exercise output. PharmacolBiochemBehav 2006; 85: 12–22. [DOI] [PubMed] [Google Scholar]
- 12.Mathes WF, Kanarek RB. Chronic running wheel activity attenuates the antinociception actions of morphine and morphine-6-glucoronide administration into the periaqueductal grey in rats. PharmacolBiochemBehav 2006; 83:578–84. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman P, Terenius L, Thoren P. Cerebrospinal fluid immunoreactive b-endorphin concentration is increased by voluntary exercise in the spontaneously hypertensive rat.RegulPept 1990; 28: 233–9. [DOI] [PubMed] [Google Scholar]
- 14.Su CF, Chang YY, Pai HH, Liu IM, Lo CY, Cheng JT. Mediation of beta-endorphin in exercise-induced improvement in insulin resistance in obese Zucker rats. Diabetes/Metab Res Rev 2005; 21:175–82. [DOI] [PubMed] [Google Scholar]
- 15.Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain 2007; 8: 989–97. [DOI] [PubMed] [Google Scholar]
- 16.Bement MK, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil 2005; 86: 1736–40. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson KJ, Gomez-Pinilla, Crowe MJ, Ying Zhe, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004; 127: 1403–14. [DOI] [PubMed] [Google Scholar]
- 18.Cobianchi S, Marinelli S, Florenzano F, Pavone F, Luvisetto S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience 2010; 168: 273–87 [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992; 50: 355–63. [DOI] [PubMed] [Google Scholar]
- 20.Kregel KC, Henriksen EJ, Toth L, Ra’anan A.Resource Book for the Design of Animal Exercise Protocols. Bethesda, American Physiological Society, 2006, pp. 23–30. [Google Scholar]
- 21.Chaplan SR, Bach FW, Pogel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 22.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia.Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 23.Bian D, Nichols ML, Ossipov MH, Lai J, Porreca F (1995). Characterization of the antiallodynic efficacy of morphine in a model of neuropathic pain in rats.NeuroReport 1995; 6: 1981–4. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y, Chaplan SR, Yaksh TL. Systemic and supraspinal, but not spinal, opiates suppress allodynia in a rat neuropathic pain model. Neurosci. Lett 1995; 186: 111–4. [DOI] [PubMed] [Google Scholar]
- 25.Holden JE, Jeong Y, Forrest J. The endogenous opioid system and clinical pain management.AACN Clinical Issues 2005; 16: 291–301. [DOI] [PubMed] [Google Scholar]
- 26.Sohn J, Lee BH, Park SH, Ryu J, Kim B, Park YG. Microinjection of opiates into the periaqueductal gray matter attenuates neuropathic pain symptoms in rats.Neuroreport 2000; 11: 1413–6. [DOI] [PubMed] [Google Scholar]
- 27.Nawa H, Pelleymounter MA, Carnahan J. Intraventricular administration of BDNF increases neuropeptide expression in newborn rat brain. J Neurosci 1994; 14: 3751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauer H, Campbell K, Wiegand SJ, Lindsay RM, Bjorklund. Brain-derived neurotrophic factor enhances striatal neuropeptide expression in both the intact and the dopamine-depleted rat striatum. Neuroreport 1994; 31: 609–12. [DOI] [PubMed] [Google Scholar]
- 29.Suiciak JA, Wong V, Pearsall D, Wiegand SJ, Lindsay RM. BDNF produces analgesia in the formalin test and modifies neuropeptide levels in rat brain and spinal cord areas associated with nociception. Eur J Neurosci 1995; 7: 663–70. [DOI] [PubMed] [Google Scholar]