Abstract
The purpose of this paper is to develop and validate a miniature system that can wirelessly acquire gastric electrical activity called slow waves, and deliver high energy electrical pulses to modulate its activity. The system is composed of a front-end unit, and an external stationary back-end unit that is connected to a computer. The front-end unit contains a recording module with three channels, and a single-channel stimulation module. Commercial off-the-shelf components were used to develop front- and back-end units. A graphical user interface was designed in LabVIEW to process and display the recorded data in real-time, and store the data for off-line analysis. The system was successfully validated on bench top and in vivo in porcine models. The bench-top studies showed an appropriate frequency response for analog conditioning and digitization resolution to acquire gastric slow waves. The system was able to deliver electrical pulses at amplitudes up to 10 mA to a load smaller than 880 Ω. Simultaneous acquisition of the slow waves from all three channels was demonstrated in vivo. The system was able to modulate –by either suppressing or entraining– the slow wave activity. This study reports the first high-energy stimulator that can be controlled wirelessly and integrated into a gastric bioelectrical activity monitoring system. The system can be used for treating functional gastrointestinal disorders.
Keywords: Gastric electrical activity, wireless monitoring, gastric entrainment
I. Introduction
Gastrointestinal (GI) motility is coordinated by underlying bio-electrical events known as slow waves. Abnormalities in slow waves have been implicated in major functional motility disorders such as gastroparesis, chronic nausea and unexplained vomiting, and functional dyspepsia [1]. Current management options are mainly limited to symptomatic and prokinetic therapies, with many patients incompletely treated. Gastric electrical stimulation (GES) has been proposed as an alternative therapeutic strategy, with potential to revert dysrhythmias to control symptoms [2].
As in cardiac electrophysiology, implantable pulse generators (IPGs) have been used for stimulating the stomach. Conventionally, two types of pulses have been applied for potential therapeutic effects: short pulses (high frequency/low energy) and long pulses (low frequency/high energy) [3]. Low-energy stimulation is typically delivered with a pulse-width in the order of a few hundred microseconds, at frequencies ranging from 5 to 100 Hz, and may improve symptoms such as nausea, vomiting, and bloating [4, 5]. High-energy stimulation (or pacing) is typically delivered with a pulse-width in the order of milliseconds (10–600 ms), at frequencies akin to the natural gastric frequency (i.e., 3 cpm). High-energy stimulation has demonstrated potential to pace slow waves and improve motility [6].
Conventionally, GES is delivered and adjusted through trial and error, without active monitoring of gastric slow wave responses. It is partly for this reason that conflicting results have been reported by various researchers and the role of GES, although investigated for over 50 years, remains controversial. A novel method called synchronized GES that includes slow wave data acquisition as an indicator for the efficacy of GES has been proposed in literature [6–8]. Results of using synchronized GES on canine models of postprandial antral hypomotility significantly increased the amplitude of gastric contractions and enhanced gastric motility [9]. However, the use of this method was limited by the large size of the recording and stimulating systems, and the fact that they were not implantable.
Advances in microelectronics and wireless system-on-chip (SoC) technology provide the opportunity to offer a better solution for integrated and implantable systems for in vivo monitoring of slow waves and GES. A capsule telemetry system has been developed for acquiring recordings from the luminal surface of the small intestine, during transit through the GI tract [10], and a telemetry system for cutaneous electrogastrography (EGG) has previously been presented [11]. However, the resolution of cutaneous EGG is low and it is prone to motion artifacts. Recently, we developed and validated wireless systems that can record slow waves from multiple channels in real time [12–16]; but, none of these systems can deliver GES.
We have previously advocated a closed-loop neuromodulation therapy for controlling nociception [17, 18]. Analogous to this study, feedback control of the gastric peristalsis has been proposed in the literature [19]; however, no long-term study has been conducted due to the lack of an implantable wireless device. We are planning to use slow waves as the feedback signal to improve the precision of GES. To this end, we have designed a bidirectional wireless system for acquiring in-vivo gastric slow wave signals and delivering electrical stimulation. The system can acquire signals from three distinct channels and can deliver a programmable electrical pulse through one channel. The system was tested in bench-top studies and in-vivo in an established pig model.
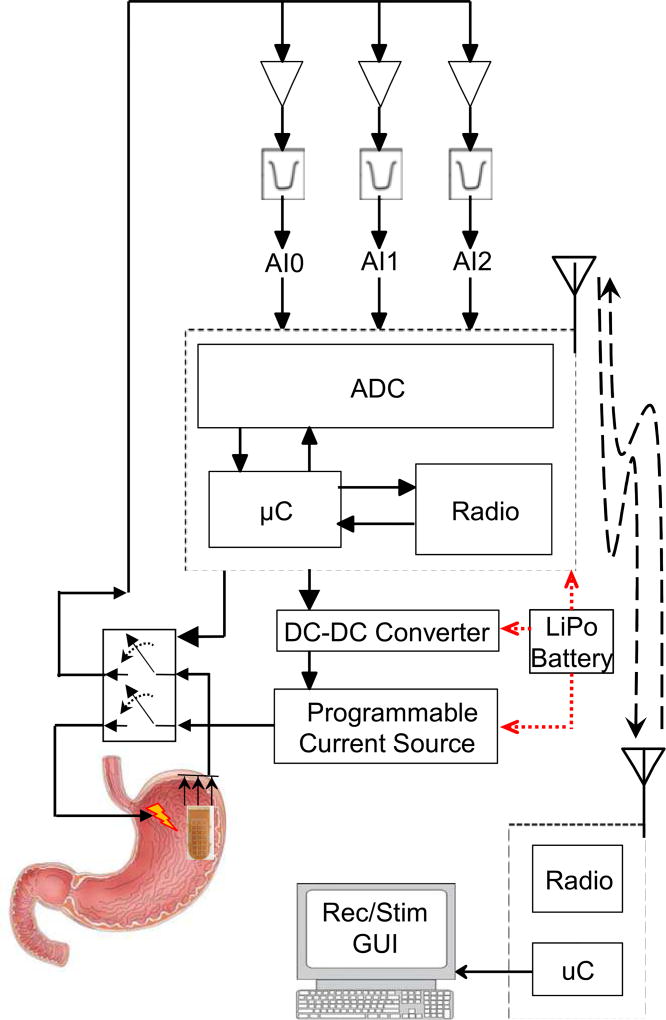
II. System Architecture
The system includes an implantable miniature front-end unit and an external stationary back-end unit that is connected to a computer. The front-end unit contains a recording module and a stimulation module. The recording module consists of an analog conditioning circuitry, and the stimulation module consists of a DC-to-DC converter, and a programmable current source. The two modules share a system-on-chip (CC430F5137, by Texas Instruments (TI)), which includes an analog-to-digital converter (ADC), a microcontroller, and a sub-1-GHz transceiver. The front-end also includes a 433 MHz chip antenna, an RF matching circuit with a balun, a magnetic switch, and a connector (FFC-FPC SMT, by Molex) to interface with the recording electrodes. The stationary back-end module is made up of the same SoC that is connected to the computer via a universal asynchronous receiver/transmitter (UART; 115,200 bps) communication link. Fig. 1 shows the block diagram of the system. A graphical user interface (GUI) was designed in LabVIEW to process and display the recorded data in real-time, and store the data for off-line analysis.
Fig. 1.
The block diagram of the system displays the front-end, back-end, and a computer which includes the graphic user interface. The system can record gastric slow waves from three channels and stimulate the stomach through one channel.
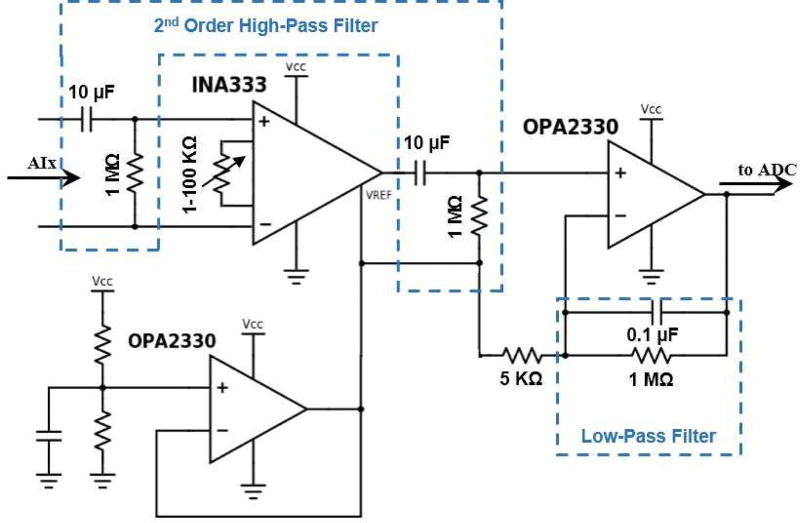
A. Recording Module
The analog conditioning circuitry acquires slow wave signals from three channels. Signals pass through a high-pass filter with the cut off frequency of 0.016 Hz to an instrumentation amplifier (INA333, by TI) with adjustable gain (from 1 to 100). The amplified signals were filtered through a band-pass filter (0.016–1.6 Hz) to eliminate noise prior to the next amplification with the gain of 200. Fig. 2 shows the analog conditioning circuitry for one of the channels. We have successfully validated and used a similar circuit in our previous works [12, 13, 20]. The amplified signals were sampled at 90 Hz per channel, digitized into 12 bits, and separated into two bytes. The data were loaded into packets (8 bytes) to be wirelessly transmitted by the front-end transceiver. The packets were received by the back-end transceiver and the microcontroller unloaded the data and sent them to the computer.
Fig. 2.
The analog conditioning circuitry for recording gastric slow waves is shown. This circuit amplifies the signals in two stages, and applies a band pass filter (0.016–1.6 Hz).
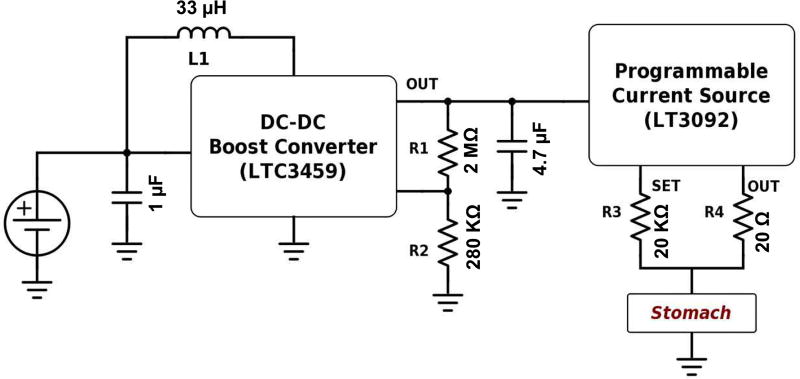
B. Simulation Module
The front-end can deliver monophasic electrical stimulation pulses to the stomach through one channel. Fig. 3 shows the analog circuitry necessary to convert the voltage pulse commands generated by the microcontroller into monophasic current pulses deliverable to the stomach. A boost converter (LTC3459, by Linear Technology) converts the input voltage level of 3.3–5 V to up to 10 V in the output. The ratio of the R1 and R2, and L1 are the major determinants for the output voltage of the boost converter. The 10 V pulse is delivered to a programmable current source (LT3092, by Linear Technology) that delivers current pulses with amplitudes of up to 10 mA. The output current depends on the ratio of R3 and R4, and is limited by the load impedance. The pulse width, frequency, and on-off periods can be tuned through the GUI, which is explained later.
Fig. 3.
The stimulation circuitry to deliver electrical pulses is shown. The DC-DC converter (LTC3549) boosts the input voltage to 10 V level; hence, the voltage controlled current source (LT3092) can deliver 10 mA current pulses to a load less than 880 Ω.
C. Firmware
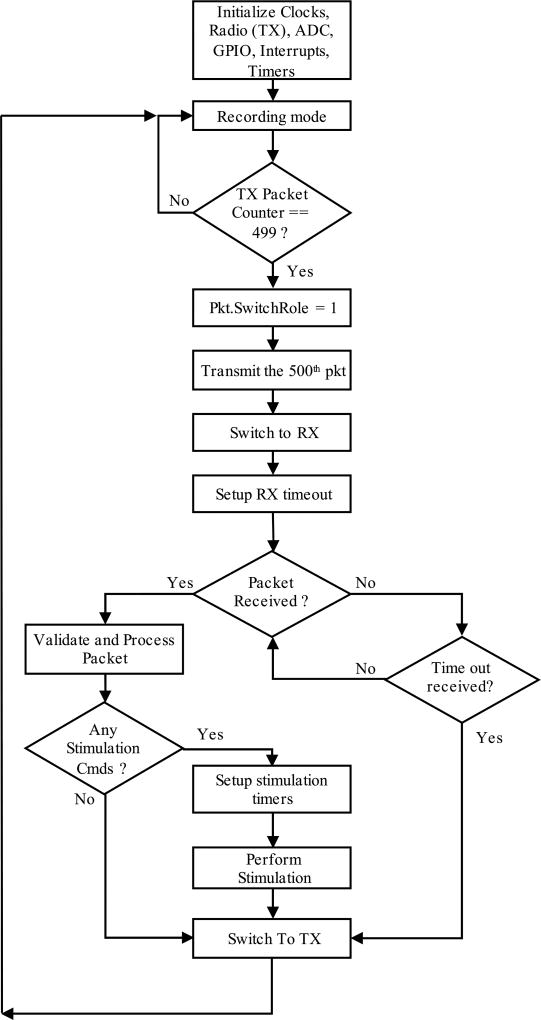
1) Front-end unit
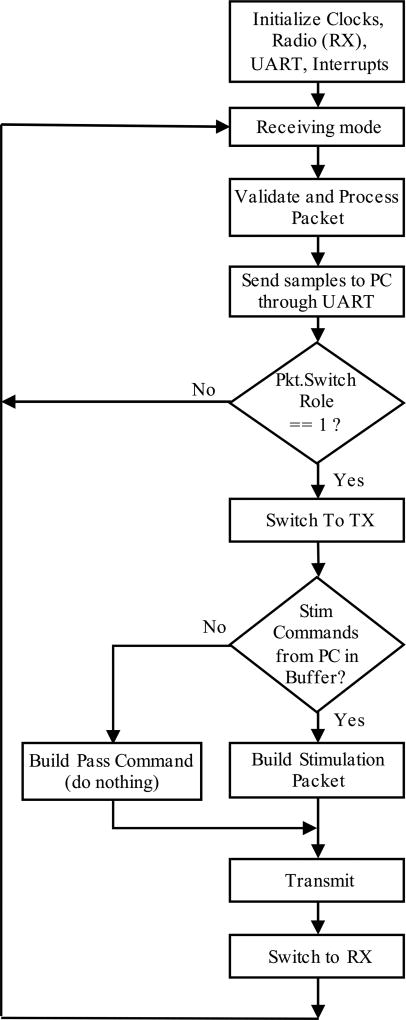
In the front-end unit, the clocks, ADC, GPIOs, interrupts, timers and radio (into transmitting mode) are initialized upon powering up the unit. The front-end unit starts from the recording mode, where it receives the conditioned signals from the three channels of the recording module, samples the signals at 90 Hz per channel, and digitizes each data point into 12 bits; hence, each data point is divided into two bytes. These bytes are placed into the wireless packet, and will be sent to the back-end. Each packet is composed of 4 bytes for header and 6 bytes of data (2 bytes per channel for 3 channels), so the total packet length is 10 bytes. The radio controller automatically adds preamble, address bytes, and cyclic redundancy check bytes to the packet. The frequency of the wireless communication is tuned at 433 MHz. A counter keeps the record of the sent packets. The 500th packet, in which the switching role flag is set, causes the back-end unit to turn into the transmitting mode. The front-end unit turns into the receiving mode, and sets a timer. Transmission of 500 packets is equal to 5.55 s of data. During the receiving mode, which takes 50 ms, the front-end looks for a stimulation packet transmitted from the back-end. If a packet is received, the front-end processes it, extracts the stimulation commands, including on-time (pulse width), off-time, and number of repetitions. The received payload includes the packet length (1 byte), command type (1 byte), and the stimulation parameters (2 bytes for each of the three parameters), which totals 8 bytes. After delivering the stimulation, the front-end unit switches back to the transmission and recording mode.
2) Back-end unit
Upon powering up the back-end unit, it initializes clocks, UART, interrupts, and radio (into receiving mode). The back-end receives and processes the packets from the front-end unit, and sends them to the computer through the UART communication, until it receives a packet with the “set” switching role flag byte. At this moment, the back-end switches to the transmission mode, and if there is a stimulation command —sent from the computer— in its buffer, it builds a stimulation packet, clears that command from the buffer, and transmits the command to the front-end unit. If there is no stimulation command in the buffer, the back-end builds a pass command packet and transmits it to the front-end, which translates into “do nothing”. After the transmission of these packets, the back-end switches back to receiving mode. Figs. 4 and 5 show the functional flow chart for the front- and back-end units, respectively.
Fig. 4.
The flow chart shows how the front-end module works in the recording mode (transmitting 500 packets), switches to the stimulation mode (receiving stimulation instructions) and restarts the loop.
Fig. 5.
The flow chart presents the back-end module processes receiving packets from the front-end in recording mode, switches to transmitting to send stimulation instructions and restarts the loop.
D. Graphical User Interface
A graphical user interface was developed in LabVIEW (National Instruments). The user can monitor the three recorded signals, and store signals for off-line analysis. The user may also enter the stimulation parameters, including pulse-width, off-time, and number of stimulation repetitions. The pulse width can vary from 2.5 ms to 33.5525 s, with incremental steps of 512 µs, and pulses can be repeated up to 65535 times. The user also has the option to choose the repetition to occur continuously for an infinite number of times.
III. Results
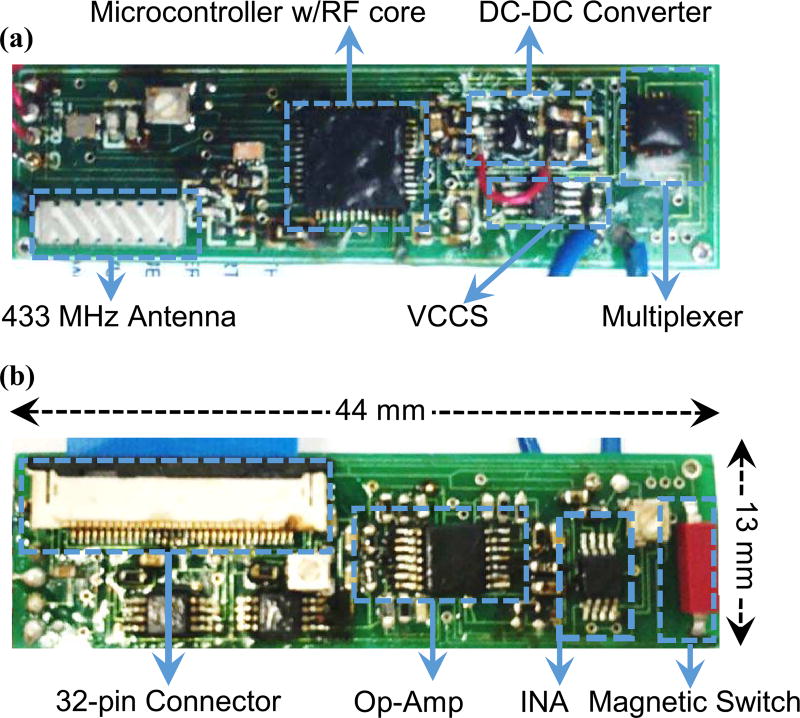
The front-end module was fabricated on a two-layer printed circuit board. The final product measures 44 mm × 13 mm × 5 mm and is shown in Fig. 6. The front-end can be activated through a magnetic switch, and it connects to the electrode through a 32-pin connector. Only three of the pins are connected in the front-end unit.
Fig. 6.
The fabricated front-end unit showing the components on (a) top and (b) bottom sides.
A. Recording and Stimulation Functions Were Validated on Benchtop Experiments
1) Recording
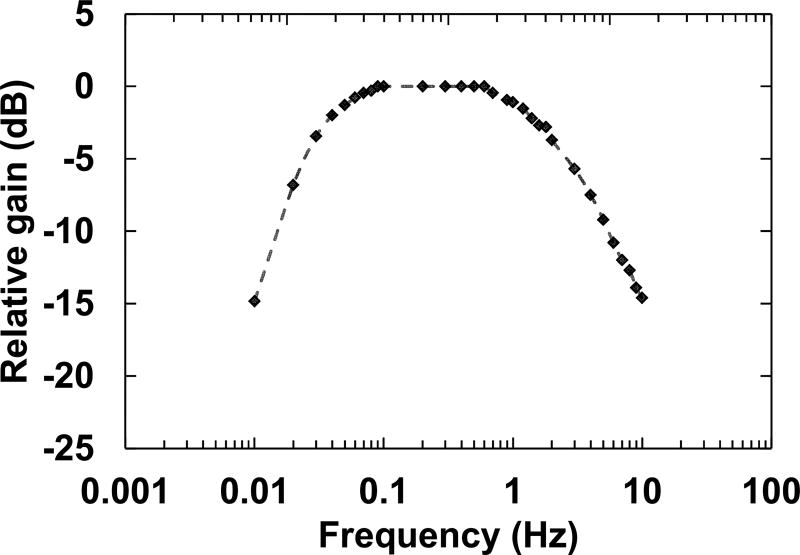
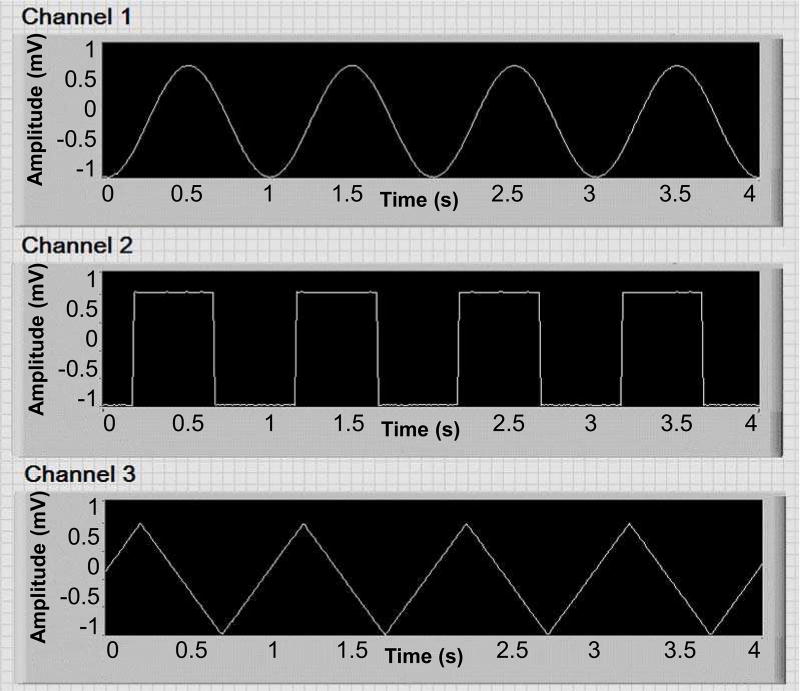
We created various sine waves at different amplitudes (ranging from 100 µV to 1 mV) and frequencies (10 mHz to 10 Hz) and streamed the signals to different channels directly, and in a saline solution. The frequency response of the system is shown in Fig. 7, and is consistent with our previous work [13]. Furthermore, signals with different waveforms were generated and streamed into the front-end unit to identify any possible cross-talking between the channels. Fig. 8 shows a snapshot of the GUI during one of these experiments, where no cross-talking was observed.
Fig. 7.
The frequency response of the system when the amplitude was set at 500 µV.
Fig. 8.
Signals with different waveforms were generated and streamed to the system to investigate any possible cross-talk.
2) Stimulation
We successfully simulated this circuit in LTSpice software (by Linear Technology). It takes 2 ms for the output to reach 10 mA, which can be ignored because the stimulation pulse widths are often greater than 100 ms in high energy stimulation. In one set of experiments, stimulation pulses were applied to different resistors ranging from 100 Ω to 3 kΩ. The front-end unit was able to deliver current pulses at 10 mA to the resistors up to 880 Ω.
In another set of experiments, we placed the source and sink leads of the simulator at different distances from each other within saline solution. The distance between the source and sink was measured at one inch, and then increased in one inch steps to 4 inches. An oscilloscope was connected to the stimulator leads and the voltage change was measured. The front-end successfully injected current into the saline solution at different distances. However, the measured pulses were curved due to the capacitive property of the impedance that is typical in stimulation of the excitable tissue [21]. An example of the measurements for this experiment is available in our previous publication [22].
B. Power Consumption of the Front-end Unit in Different Modes
The power consumption of the front-end was characterized in data transmitting and stimulation modes. The front-end module consumed 68.4 mW and 216 mW while it was in recording and stimulating modes, respectively. The power consumption during the stimulation mode was measured in the worst case scenario when the device was delivering pulses at 10 mA. The power consumption temporary rose to 216 mW during the stimulation and lasted for the period of the pulse.
Considering a Li-polymer battery that is 260 mAh, and is approximately the size of the implant (12 mm × 40 mm × 6.4 mm), the front-end can continuously acquire slow waves and transmit them wirelessly to the back-end for more than fourteen hours. During the stimulation mode (without any recording), considering the stimulation pulses with 500 ms pulse width that are applied every 20 seconds (translating to 3 cpm), the front-end can continuously operate for more than 170 hours.
C. Recording and Simulation Validation in Animal Experiments
In-vivo validation studies were performed in an established weaner pig model of gastric dysrhythmia [23], under approval from the University of Auckland Animal Ethics Committee. Full details of our animal preparation are provided elsewhere [24]; in brief, fasted animals (n=5) were subject to general anesthesia with Zoletil and isoflurane, followed by midline laparotomy under continuous physiological monitoring.
Stimulation electrodes were implanted at the greater curvature of the proximal gastric corpus and connected to the stimulator (either conventional or the developed system). To validate the GES capability of the novel system, the stimulator electrode was connected to the front-end unit of the developed system, and slow wave responses were acquired using validated flexible printed arrays [25], placed as a high-density grid (256 electrodes in a 16 × 16 array, with 0.3 mm gold contacts at 4 mm spacing (FlexiMap, New Zealand). Three of these electrodes were connected to the acquisition channels of the novel device; the remainder were connected to an ActiveTwo acquisition system (BioSemi, The Netherlands). To validate the signal acquisition capability of the developed system, the stimulation electrodes were connected to a conventional electrical stimulation device (DS8000, World Precision Instruments, Saratosa, FL). Data analysis was performed off line, in MATLAB (Mathworks, Natick, MA), and using the Gastric Electrical Mapping Suite (GEMS v1.6, FlexiMap, New Zealand) [26], including slow wave event detection, cycle clustering, activation mapping, and calculations of frequency, velocity and amplitude.
1) Recording
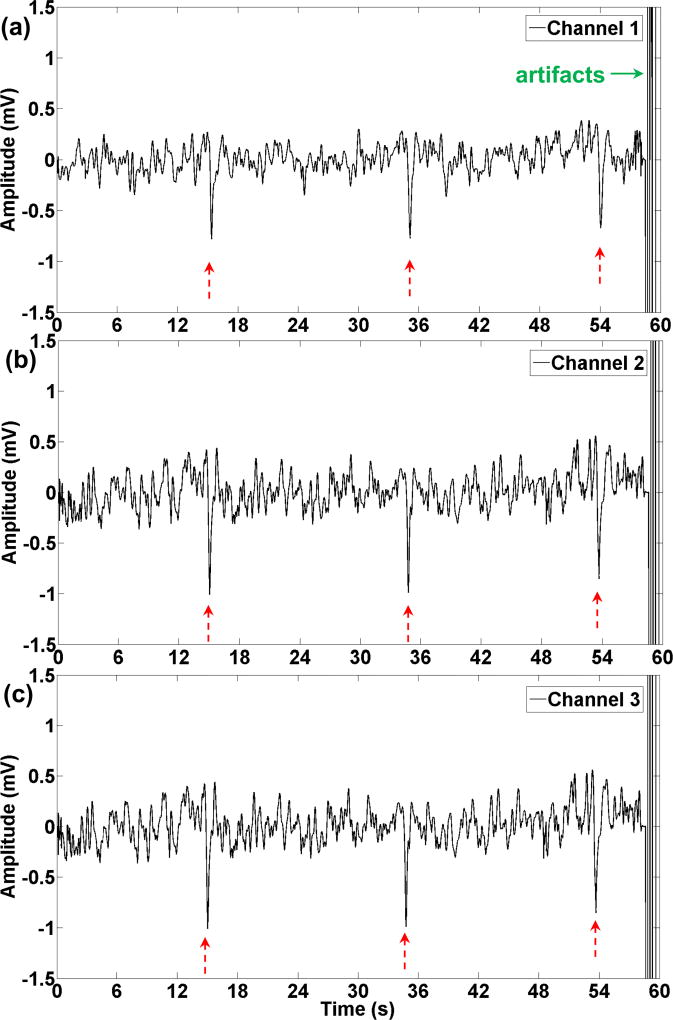
Recording of gastric slow waves was conducted successfully on two (out of 5) animals. The remaining three animals were not studied because we had already demonstrated recordings from the stomach in our previous publications [12, 13]. One minute of recording gastric electrical activity is shown in Fig. 9. In this experiment the three channels were located circumferentially on the greater curvature, and the distance between the electrodes was 4 mm. Three distinguished peaks can be detected on all three channels, which translate into three cycles per minute. The magnitude of the greatest peak (calculated from zero) was 0.8 mV, 1 mV, and 1 mV in channels 1, 2, and 3, respectively. The signal to noise ratio (SNR) for channels 1–3 was calculated as 8.1 dB, 4.3 dB, and 9.1 dB, respectively. Although the magnitude of the peaks in channel 1 were smaller than channel 2, the signal to noise ratio was better in channel 1. The gain of the amplifiers for all the channels was set at 66 dB in this experiment. The artifact at the end of the signal is due to delivering electrical stimulation to the stomach that saturated the recorded signal. The duration of the saturation varied in different experiments depending on the stimulation parameters; however, the complete saturation and complete stabilization of the signal typically took less than 60s, and 90s, respectively.
Fig. 9.
One minute of slow waves recorded with the wireless device. (a)–(c) corresponds to channels 1–3. Three peaks (shown with red arrows) can be distinguished in this segment that matches with the regular frequency of the stomach (3cpm). Electrical stimulation was delivered to the stomach that caused the artifacts (green arrow) at the end of the one minute signal.
2) Stimulation
Experiments were conducted in vivo with various electrical stimulation parameters (different amplitudes, pulse widths, and intervals). During these experiments electrical stimulation was applied with the developed wireless module, and signals were recorded using the ActiveTwo acquisition system (Biosemi, the Netherlands). In one experiment discussed for demonstration purposes, the stimulation parameters were 8 mA with 10 s intervals. In this experiment the pulse width (PW) increased from 500 ms to 900 ms and back to 500 ms with following steps.
First slow waves were recorded for 2 minutes, while the stimulator was off. Then stimulation was applied for 2 minutes with the 500ms PW. After 190 s of baseline recording the stimulation was delivered for 2 minutes. Stimulation was turned off for 280 seconds, and then it was applied with the 500 ms PW for another two minutes. Finally, the stimulator was turned off and slow waves were recorded during this recovery period. Table I shows how the frequency of the slow waves varied during this experiment.
TABLE I.
Effect of Electrical Stimulation with 10s Intervals on Slow Waves
| Experiment time (s) |
Slow waves frequency (cpm) |
Electrical stimulation pulse width (ms) |
|---|---|---|
| 0–120 | 3 | Off (baseline) |
| 120–240 | 3 | 500 |
| 430–550 | 1.5 | 900 |
| 830–950 | 1.5 | 500 |
| 1060–1260 | 2.5 | Off (recovery) |
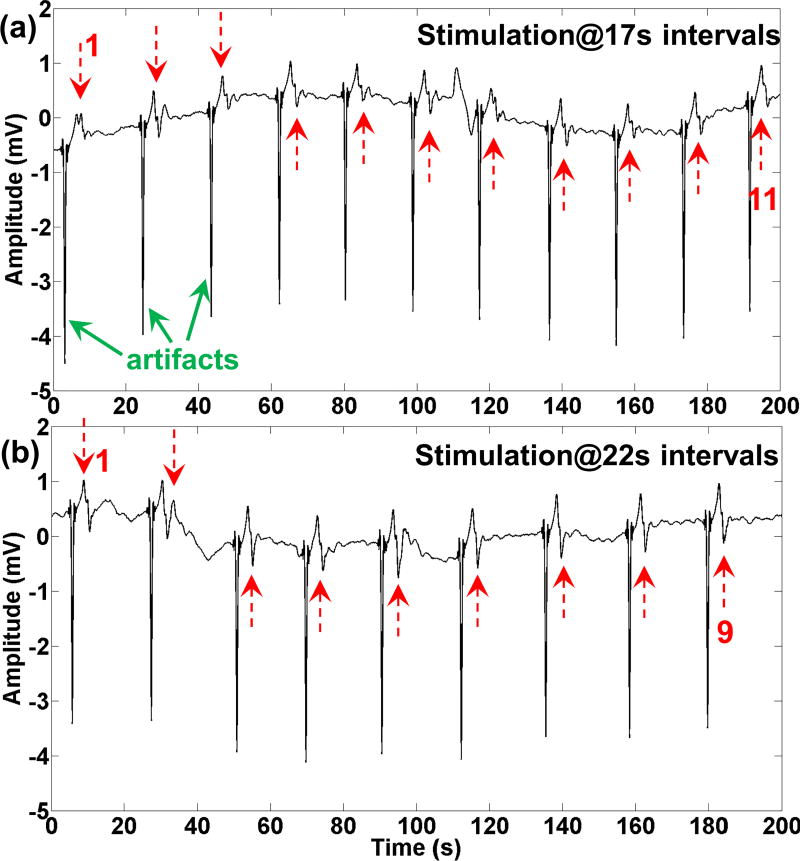
In another demonstration experiment, we used the front-end module to deliver electrical stimulations with intervals that are close to the natural frequency of the stomach. In this experiment the amplitude and the pulse width were fixed, and set at 5 mA and 500 ms, respectively. The interval of the stimulation was increased from 16 s to 22 s. Each stimulation interval was recorded for approximately two minutes. The frequency of the slow waves at baseline was 2.3 cpm. Fig. 10 shows the entrainment of the slow waves according to the stimulation intervals of 17 s and 22 s. Stimulation artifacts (green arrows) are followed by slow wave events (red arrows).
Fig. 10.
Electrical stimulation pulses were applied at 17 seconds and 22 seconds intervals, in (a) and (b), respectively. The slow waves were entrained according to the stimulation pulses intervals. Red arrows show the slow waves, and green arrows show the stimulation artifacts. The baseline frequency of the stomach was 2.3 cpm.
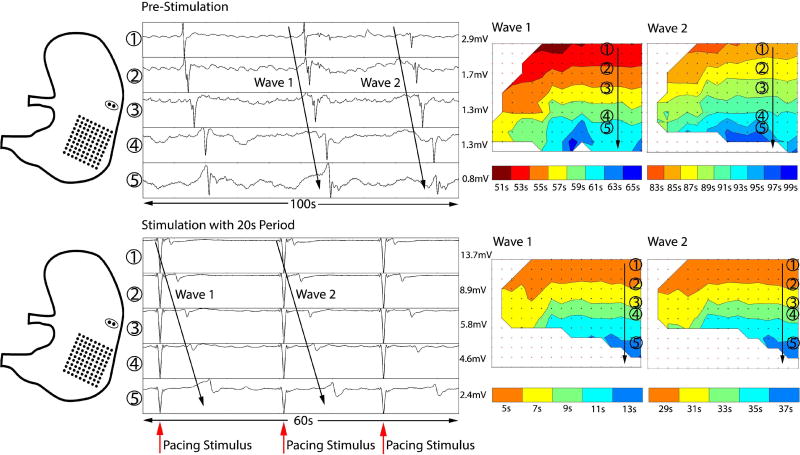
These studies demonstrated that slow waves can be successfully modulated by applying high-energy stimulation pulses using the developed system. The propagation of slow waves prior to and during high-energy pacing is shown in Fig. 11. In this experiment the baseline frequency of the slow waves was 1.8 cpm, showing bradygastria. With application of high-energy pulses at an interval of 20 s, the system was able to revert slow waves to a normal interval of 20 s, with consistent antegrade propagation.
Fig. 11.
Validation of gastric pacing for modulating bradygastria, by simultaneous HR electrical slow wave mapping. Left: Diagram showing position of the stimulation leads and HR electrode mapping array for validation. Middle: Electrograms of slow wave activity before and after gastric pacing. Prior to stimulation, the slow wave frequency was bradygastric (period 30–40s). During simulation at period 20s, the frequency was increased to 3cpm. Right: HR propagation maps showing antegrade slow wave propagation over the anterior stomach before and after gastric pacing.
IV. Discussion
A novel miniature wireless electrical pacing system that can record gastric slow waves from three channels, and deliver high-energy electrical pulses to the stomach has been developed and validated in bench-top and in-vivo experiments. The in vivo pilot studies are conducted to validate the functionality of the system, and not to prove any hypothesis.
The number of recording channels is limited by the size of the front-end and its power consumption. The maximum number of channels that can satisfy the size constraint in our design is three channels. On the other hand, if the three channels are placed on the stomach in triangular arrangement, one can deduce direction and velocity of the slow wave propagation in addition to the basic frequency.
Compared to our previous works [12, 13], we have increased the resolution of the ADC from 8 to 12 bits, and have considered variable gain on the first stage of the analog conditioning circuitry. This modification was inevitable because the amplitude of the slow waves can reach 4 or 5 mV. To eliminate saturation in the analog conditioning circuitry, the gain was decreased, whilst the higher resolution of the ADC guarantees preserved signal quality.
Our preliminary studies showed that the stimulation artifacts can saturate the amplifiers which typically last less than 90 seconds. The multiplexer switch, which determines the recording or stimulation pathways, disconnects the analog conditioning circuity from the recording electrodes while stimulation pulses are delivered to prevent the saturation of the amplifiers, and protect the circuitry from electrical shocks. However, due to the large time constant of the analog conditioning circuity (approximately 15 seconds to observe the signal and 75 seconds for a stabilized signal), disconnection of the circuitry results into a delay to acquire stable slow waves.
The front-end unit can generate monophasic electrical pulses with the amplitude of up to 10 mA. The value of the amplitude is determined by R3 and R4 (see Fig. 3), and once set, cannot be modified. We will utilize digital potentiometers in the future designs, to be able to modify the amplitude through the GUI. The pulse width of the system can vary from 2.5 ms to 33.5525 s with incremental steps of 512 µs. The limiting factor to generate narrower pulses with microsecond pulse width is the time that it takes to charge the DC-DC converter. It takes 2 ms for the DC-DC converter to change the voltage level. One possible solution is to turn on the DC-DC converter while initializing the front-end, and keep it on for the rest of the time. However, this may increase the power consumption of the system, while the system is not delivering electrical stimulations. The stimulation parameters can be modified wirelessly based on the recorded signal, which is of critical importance once the system is used in clinical setting.
Monophasic pulses were used to demonstrate the modulation of the gastric slow waves, but these have been shown to potentially cause erosion of the stimulation electrodes in the long term; hence, formation of toxic products inside the tissue [21, 27]. Monophasic pulses can also result into muscle fatigue if applied for an extended period [21, 27]. Further improvements to the system will include the ability to deliver biphasic pulses.
The front-end is designed in rectangular shape with a width of 13 mm for possible endoscopic implantation in the future. We have already demonstrated such procedures in our previous work [28]. In the future, we will add wireless power transfer capability to the system, and encapsulate the front-end module with a biocompatible material for long-term studies. Furthermore, to improve the system for better clinical practice, we will consider running the GUI on a miniature handheld device in lieu of the computer in the back-end. The integration of the recording and stimulation modules takes us one step closer to realization of a fully-functional closed-loop system for treating gastric disorders. To that end, criteria to automatically detect abnormal slow wave activity and optimize stimulation parameters need to be further explored.
V. Conclusion
A novel miniature system that can wirelessly record gastric electrical activity from three channels and deliver high-energy electrical pulses to the stomach was developed and validated on bench-top and animal experiments. It was demonstrated that the system can successfully modulate gastric slow waves. To our knowledge this is the first time a high-energy stimulator that can be controlled wirelessly has been developed and integrated into a gastric bioelectrical activity monitoring system.
Acknowledgments
We thank Linley Nisbet for her expert technical assistance. SS, PD, TRA, GO, LKC and NP are shareholders in FlexiMap and/or hold intellectual property in the field of gastric electrophysiology. No commercial or industry financial support was provided for this study.
This work was supported in part by the National Institutes of Health SPARC program under Grant NIBIB-U18EB021789, in part by National Institutes of Health Grant R01 DK64775, in part by the Health Research Council of New Zealand, and in part by the New York Institute of Technology through the Institutional Support for Research and Creativity (ISRC) Grant.
Biographies

Rui Wang received the B.Sc. degree in Control Engineering in 2013 from Beijing Jiaotong University, China. He received the M.Sc. degree in Electrical and Computer Engineering in 2015 from New York Institute of Technology, United States of America. His research interests are embedded systems design and wireless communication with applications in medical devices.

Zaid Abukhalaf received his B.Sc. degree in Computer Engineering in 2013 from Hashemite University, Jordan. He earned his M.Sc. degree in Electrical and Computer Engineering in 2016 from New York Institute of Technology. His research focus is high-performance and low-power hardware accelerators and digital systems. Zaid currently works in the embedded systems industry implementing low-level software and systems architectures.

Amir Javan-Khoshkholgh received the M.Sc. and Ph.D. degrees in Electronics engineering from the Polytechnic University of Turin, Italy, in 2011 and 2015, respectively. He is currently a Postdoctoral Researcher at the Integrated Medical Systems Laboratory and Adjunct Assistant Professor in the Electrical and Computer Engineering department at New York Institute of Technology (NYIT). Before joining NYIT, he was a Research Scholar at the Microwave Microsystems Laboratory at the University of California, Davis.
His research interests are low-power analog / mixed-signal / digital circuit design for wireless and RF applications and wireless power transfer for biomedical systems and implantable devices.

Tim Wang received a Bachelor of Medicine and Bachelor of Surgery (MBChB) at the University of Auckland in 2016. He is a current surgical registrar at Auckland City Hospital and is also completing a PhD in Surgery at the University of Auckland.

Shameer Sathar received a bachelor’s degree (B.Tech) with first class (Distinction) from Cochin University of Science and Technology, India in 2010 and received a PhD in Bioengineering from the University of Auckland in 2015. He is currently a Research Fellow at the Auckland Bioengineering Institute, University of Auckland.

Peng Du received a PhD in Bioengineering from the University of Auckland. He is a Senior Research Fellow at the Auckland Bioengineering Institute, University of Auckland.

Timothy R. Angeli received a BSE (Magna Cum Laude) and MSE in Biomedical Engineering from the University of Michigan in 2008 and 2009, respectively. He received a PhD in Bioengineering from the University of Auckland in 2014. Timothy is currently a Senior Research Fellow at the Auckland Bioengineering Institute, University of Auckland.

Leo K. Cheng received a BE (Hons) in Engineering Science and a PhD in Bioengineering from the University of Auckland in 1997 and 2002, respectively. He is currently a Professor at the Auckland Bioengineering Institute, University of Auckland.,

Greg O’Grady (MBChB, PhD, FRACS) is an Associate Professor of Surgery at the University of Auckland, New Zealand. He leads the Surgical Engineering Lab, and practices clinically as a gastrointestinal surgeon

Niranchan Paskaranandavadivel received a BE in Biomedical Engineering and ME (Hons) and PhD in Bioengineering at the University of Auckland in 2007, 2009 and 2014 respectively. He is a Research Fellow at the Auckland Bioengineering Institute at the University of Auckland.

Aydin Farajidavar (S’09-M’12) received the B.Sc. and M.Sc. degrees in biomedical engineering, in 2004 and 2007, respectively. He received the Ph.D. degree in biomedical engineering from the joint program of the University of Texas at Arlington and the University of Texas Southwestern Medical Center, Dallas in 2011. He is currently an Assistant Professor of Electrical and Computer Engineering and the Director of Integrated Medical Systems Laboratory at New York Institute of Technology (NYIT). Before joining NYIT, he was a Post-doctoral Fellow in the School of Electrical and Computer Engineering at the Georgia Institute of Technology.
His research experience and interests cover a broad range, from Medical Cyber Physical Systems (implantable, wearable and assistive technology) to modeling biological systems. Dr. Farajidavar has authored more than 50 peer-reviewed journal and conference papers/abstracts. He has served as technical committee member for several international IEEE conferences. He serves as a member of MTT-10, Biological Effect and Medical Applications of RF and Microwave committee.
Contributor Information
Rui Wang, Integrated Medical Systems (IMS) Laboratory at the School of Engineering and Computing Sciences, New York Institute of Technology, Old Westbury, NY 11568, USA.
Zaid Abukhalaf, Integrated Medical Systems (IMS) Laboratory at the School of Engineering and Computing Sciences, New York Institute of Technology, Old Westbury, NY 11568, USA.
Amir Javan-Khoshkholgh, Integrated Medical Systems (IMS) Laboratory at the School of Engineering and Computing Sciences, New York Institute of Technology, Old Westbury, NY 11568, USA.
Tim H.-H. Wang, Department of Surgery, University of Auckland, New Zealand
Shameer Sathar, Auckland Bioengineering Institute, University of Auckland, New Zealand.
Peng Du, Auckland Bioengineering Institute, University of Auckland, New Zealand.
Timothy R. Angeli, Auckland Bioengineering Institute, University of Auckland, New Zealand
Leo K. Cheng, Auckland Bioengineering Institute, University of Auckland, New Zealand; Department of Surgery, Vanderbilt University, Nashville, TN, USA.
Greg O’Grady, Auckland Bioengineering Institute, University of Auckland, New Zealand; Department of Surgery, University of Auckland, New Zealand.
Niranchan Paskaranandavadivel, Auckland Bioengineering Institute, University of Auckland, New Zealand; Department of Surgery, University of Auckland, New Zealand.
Aydin Farajidavar, Integrated Medical Systems (IMS) Laboratory at the School of Engineering and Computing Sciences, New York Institute of Technology, Old Westbury, NY 11568, USA.
References
- 1.O’Grady G, Wang TH, Du P, Angeli T, Lammers WJ, Cheng LK. Recent progress in gastric arrhythmia: Pathophysiology, clinical significance and future horizons. Clin Exp Pharmacol Physiol. 2014 Oct.41(10):854–862. doi: 10.1111/1440-1681.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol. 1998 Jan.274(1):186–191. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- 3.Hasler WL. Methods of gastric electrical stimulation and pacing: a review of their benefits and mechanisms of action in gastroparesis and obesity. Neurogastroenterol Motil. 2009 Mar.21(3):229–243. doi: 10.1111/j.1365-2982.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- 4.O’Grady G, Egbuji JU, Du P, Cheng LK, Pullan AJ, Windsor JA. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg. 2009 Aug.33(8):1693–1701. doi: 10.1007/s00268-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deb S, Tang SJ, Abell TL, Rao S, Huang WD, To SD, Lahr C, Chiao JC. An Endoscopic Wireless Gastrostimulator (with video) Gastrointest Endosc. 2012 Feb.75(2):411–415. doi: 10.1016/j.gie.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Chen JD. Systematic review: applications and future of gastric electrical stimulation. Aliment Pharmacol Ther. 2006 Oct.24(7):991–1002. doi: 10.1111/j.1365-2036.2006.03087.x. [DOI] [PubMed] [Google Scholar]
- 7.Peles S, Petersen J, Aviv R, Policker S, Abu-Hatoum O, Ben-Haim SA, Gutterman DD, Sengupta JN. Enhancement of antral contractions and vagal afferent signaling with synchronized electrical stimulation. Am J Physiol Gastrointest Liver Physiol. 2003 Sep.285(3):577–585. doi: 10.1152/ajpgi.00109.2003. [DOI] [PubMed] [Google Scholar]
- 8.Jalilian E, Onen D, Neshev E, Mintchev MP. Implantable neural electrical stimulator for external control of gastrointestinal motility. Med. Eng. Phys. 2006 May;29(2):238–252. doi: 10.1016/j.medengphy.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Zhu H, Sallam H, Chen DD, Chen JD. Therapeutic potential of synchronized gastric electrical stimulation for gastroparesis: enhanced gastric motility in dogs. Am J Physiol Regul Integr Comp Physiol. 2007 Sep.293(5):1875–1881. doi: 10.1152/ajpregu.00821.2006. [DOI] [PubMed] [Google Scholar]
- 10.Woo SH, Cho JH. Telemetry system for slow wave measurement from the small bowel. Med. Biol. Eng. Comput. 2010 Mar.48(3):277–283. doi: 10.1007/s11517-009-0567-4. [DOI] [PubMed] [Google Scholar]
- 11.Haddab S, Laghrouche M. Microcontroller-based system for electrogastrography monitoring through wireless transmission. Measurement Science Review. 2009 Nov.9(5):122–126. [Google Scholar]
- 12.Farajidavar A, O’Grady G, Rao SM, Cheng LK, Abell T, Chiao JC. A Miniature Bidirectional Telemetry System for in-vivo Gastric Slow Wave Recordings. Physiol Meas. 2012 Jun.33(6):N29–N37. doi: 10.1088/0967-3334/33/6/N29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paskaranandavadivel N, Wang R, Sathar S, O’Grady G, Cheng LK, Farajidavar A. A wireless multichannel system for in-vivo monitoring of gastric activity propagation. Neurogastroenterol Motil. 2015 Apr.27(4):580–585. doi: 10.1111/nmo.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim A, Farajidavar A, Kiani M. Towards a Highly-Scalable Wireless System-on-Chip for Gastric Electrophysiology. Conf Proc IEEE Eng Med Biol Soc (EMBS) 2015 Aug.1:2689–2692. doi: 10.1109/EMBC.2015.7318946. [DOI] [PubMed] [Google Scholar]
- 15.Javan-Khoshkholgh A, Abukhalaf Z, Li J, Miller LS, Kiani M, Farajidavar A. An Inductive Narrow-Pulse RFID Telemetry System for Gastric Slow Waves Monitoring. Conf Proc IEEE Eng Med Biol Soc (EMBS) 2016 Aug.1:4820–4823. doi: 10.1109/EMBC.2016.7591806. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim A, Farajidavar A, Kiani M. A 64-Channel Wireless Implantable System-on-Chip for Gastric Electrical-Wave Recording. Conf Proc IEEE Sensors. 2016 Oct.1:1–3. [Google Scholar]
- 17.Farajidavar A, Hagains CE, Peng YB, Behbehani K, Chiao J-C. Recognition and Inhibition of Dorsal Horn Nociceptive Signals within a Closed-loop System. Conf Proc IEEE Eng Med Biol Soc (EMBS) 2010 Sept.1:1535–1538. doi: 10.1109/IEMBS.2010.5626830. [DOI] [PubMed] [Google Scholar]
- 18.Farajidavar A, Hagains CE, Peng YB, Chiao J-C. A Closed Loop Feedback System for Automatic Detection and Inhibition of Mechano-nociceptive Neural Activity. IEEE Trans. Neural Syst. Rehabil. Eng. 2012 Jul.20(4):478–487. doi: 10.1109/TNSRE.2012.2197220. [DOI] [PubMed] [Google Scholar]
- 19.Arriagada AJ, Jurkov AS, Neshev E, Muench G, Andrews CN, Mintchev MP. Design, implementation and testing of an implantable impedance-based feedback-controlled neural gastric stimulator. Physiol. Meas. 2011 Aug.32(8):1103–1115. doi: 10.1088/0967-3334/32/8/007. [DOI] [PubMed] [Google Scholar]
- 20.Farajidavar A, McCorkle PG, Wiggins TW, Rao SRM, Hagains CE, Peng YB, Seifert JL, Romero MI, O’Grady G, Cheng LK, Sparagana S, Delgado MR, Tang S, Abell T, Chiao J-C. A miniature power-efficient bidirectional telemetric platform for in-vivo acquisition of electrophysiological signals. Conf. Proc. IEEE MTT-S International Microwave Symposium. 2011 Jun.1:1–4. [Google Scholar]
- 21.Merril DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005 Feb.141(2):171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Lee A, Wang R, Farajidavar A. A Wireless system for Gastric Slow Wave Acquisition and Gastric Electrical Stimulation. Conf. Proc. IEEE Radio & Wireless Week (RWW) 2016 Jan.1:49–51. [Google Scholar]
- 23.O’Grady G, Egbuji JU, Du P, Lammers WJ, Cheng LK, Windsor JA, Pullan AJ. High-resolution spatial analysis of slow wave initiation and conduction in porcine gastric dysrhythmia. Neurogastroenterol Motil. 2011 Sep.23(9):345–355. doi: 10.1111/j.1365-2982.2011.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Grady G, Du P, Lammers WJ, Egbuji JU, Mithraratne P, Cheng LK, Windsor JA, Pullan AJ. High-resolution entrainment mapping for gastric pacing: a new analytic tool. Am J Physiol Gastrointest Liver Physiol. 2010 Feb.298(2):314–321. doi: 10.1152/ajpgi.00389.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du P, O’Grady G, Egbuji JU, Lammers WJ, Budgett D, Windsor JA, Pullan AJ, Cheng LK. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: methodology and validation. Ann Biomed Eng. 2009 Apr.37(4):839–846. doi: 10.1007/s10439-009-9654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yassi R, O’Grady G, Paskaranandavadivel N, Du P, Angeli TR, Pullan AJ, Cheng LK, Erickson JC. The gastrointestinal electrical mapping suite (GEMS): software for analyzing and visualizing high-resolution (multi-electrode) recordings in spatiotemporal detail. BMC Gastroenterol. 2012 Jun.12(60):1–14. doi: 10.1186/1471-230X-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soffer E, Abell T, Lin Z, Lorincz A, McCallum R, Parkman H, Policker S, Ordog T. Review article: gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009 Jul.30(7):681–694. doi: 10.1111/j.1365-2036.2009.04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vegesna A, Miller LS, Alshelleh M, Farajidavar A. Endoscopic Method for Device Implantation into the Gastrointestinal tract. Gastrointest Endosc. 2017 May;85(5, Supplement):AB502. [Google Scholar]