Abstract
Chlamydia pneumoniae, an obligate intracellular bacterial pathogen, has long been investigated as a potential developmental or exacerbating factor in various pathologies. Its unique lifestyle and ability to disseminate throughout the host while persisting in relative safety from the immune response has placed this obligate intracellular pathogen in the crosshairs as a potentially mitigating factor in chronic inflammatory diseases. Many animal model and human correlative studies have been performed to confirm or deny a role for C. pneumoniae infection in these disorders. In some cases, antibiotic clinical trials were conducted to prove a link between bacterial infections and atherosclerosis. In this review, we detail the latest information regarding the potential role that C. pneumoniae infection may have in chronic inflammatory diseases.
Keywords: Alzheimer’s, arthritis, asthma, atherosclerosis, cancer, Chlamydia pneumoniae
I. INTRODUCTION
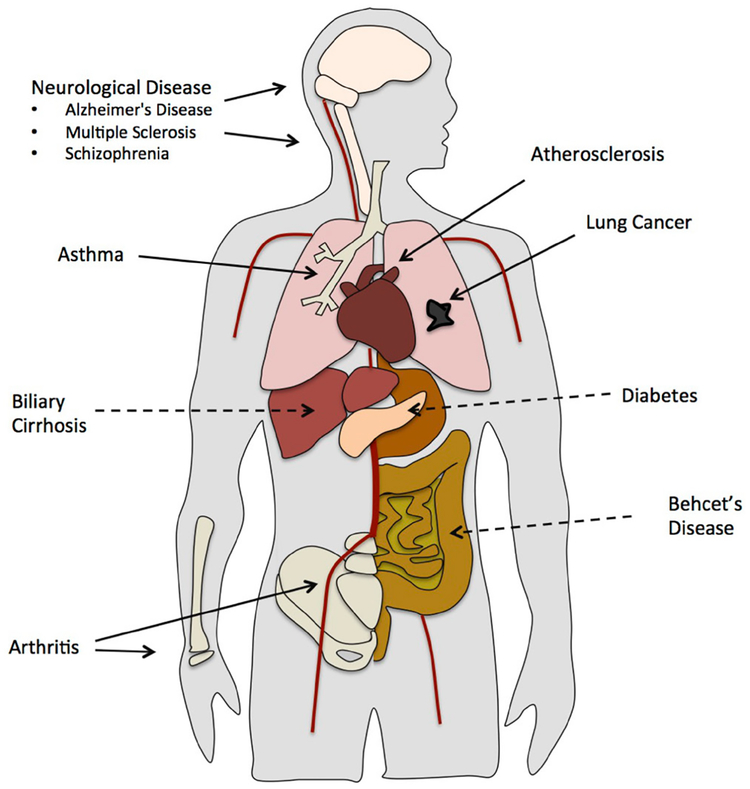
Chlamydia pneumoniae is an obligate intracellular bacterial pathogen that infects the respiratory tract. The majority of individuals are exposed to C. pneumoniae throughout their lifetimes with an antibody prevalence of 50% by age 20 and 80% by 60–70 years old.1 Although C. pneumoniae infection is predominantly asymptomatic or mild, it can result in the development of acute upper and lower respiratory illness including bronchitis, pharyngitis, sinusitis, and pneumonia.1 C. pneumoniae infection and its relationship to chronic inflammatory diseases remains a controversial topic. A mounting body of evidence shows that not only is C. pneumoniae involved in respiratory infection, it also contributes to the pathogenesis of a range of inflammatory diseases including, but not limited to, atherosclerosis, arthritis, asthma, lung cancer, and chronic obstructory pulmonary disease as well as neurological disorders, namely, Alzheimer’s disease, multiple sclerosis, and schizophrenia (Fig. 1). In this review, we investigate the latest findings regarding the role of C. pneumoniae in the development and/or exacerbation of chronic inflammatory diseases.
FIG. 1:
C. pneumoniae infection and inflammatory disease. In addition to pneumonia, C. pneumoniae infection may contribute to a range of inflammatory diseases including asthma and lung cancer. Dissemination of C. pneumoniae from the lung throughout the body can possibly lead to atherosclerosis, arthritis, and neurological diseases. Some evidence suggests that C. pneumoniae may also be associated with biliary cirrhosis, diabetes, and Bechet’s disease.
II. C. PNEUMONIAE INFECTION AND IMMUNE RESPONSE
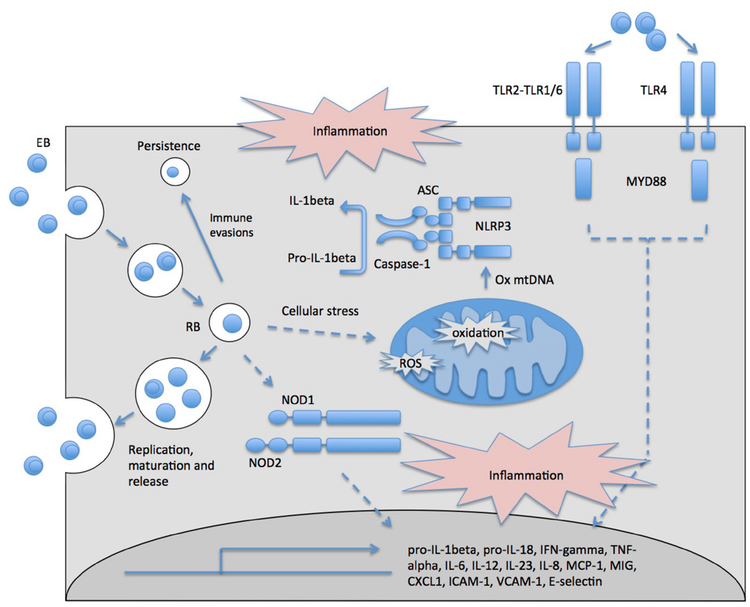
C. pneumoniae undergoes a biphasic life cycle alternating between morphologically distinct and functional forms (Fig. 2). The infectious and metabolically inert elementary body (EB) attaches to and enters cells via endocytosis, whereby it inhibits phagolysosome fusion. The EB then matures into a noninfectious metabolically active reticulate body (RB) that is separated from the cytosol within nonlysosomal inclusions. Inside these inclusion bodies, C. pneumoniae creates an intracellular niche, whereby it can modify host cell pathways, replicate, and form new infectious EBs that are released from the cell.
FIG. 2:
Innate immune response directed against C. pneumoniae. C. pneumoniae EBs are endocytosed, escaping lysosome fusion to create an intracellular niche (inclusion), in which they form RBs and replicate. C. pneumoniae EBs are detected by TLRs on the cell surface, and C. pneumoniae RBs are detected intracellularly by NOD1 and NOD2. Furthermore, cellular stress induced by C. pneumoniae infection such as enhanced ROS production results in activation of the NLRP3 inflammasome. Together, activation of these innate pathways results in the induction of inflammatory cytokines, chemokines, and cell adhesion molecules that act downstream to clear infection. Immune evasion by C. pneumoniae can result in persistent infection.
C. pneumoniae can infect a range of different cells types. In regard to respiratory infection, C. pneumoniae initially infects lung epithelial cells and alveolar macrophages. Infection can then spread to infiltrating immune cells such as monocytes, macrophages, monocyte-derived dendritic cells (DCs), lymphocytes, and neutrophils. Failure to eradicate C. pneumoniae can lead to chronic infection, during which C. pneumoniae enters a state of quiescence with intermittent periods of replication. Persistence of the RB within cells may occur during long periods of time due to its ability to hide from the host immune system within inclusion bodies. Through the lower respiratory tract, chronically infected monocytes may disseminate systemically throughout the body,2 leading to infection at distal sites including blood vessels, joints, and the central nervous system (CNS). At these distal sites, C. pneumoniae can infect endothelial cells, smooth muscle cells (SMCs), microglial cells, astrocytes, and neurons.
The innate immune response to C. pneumoniae is initiated through the detection of C. pneumoniae antigens by receptors specialized in bacterial sensing, such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptor (NOD) 1 and NOD2, and by triggering the NOD-like receptor family pyrin-domain-containing 3 (NLRP3) inflammasome pathway (Fig. 2). Activation of these pathways results in the up-regulation of proinflammatory cytokines and chemokines including interferon-γ (IFN-γ); tumor necrosis factor (TNF)-α; interleukin (IL)-6, −1β, −8, −12, and −23; monocyte chemoattractant protein-1 (MCP-1); macrophage-induced gene (MIG); regulated-on-activation normal T-cell expressed and secreted (RANTES); intracellular adhesion molecule (ICAM)-1; vascular cell adhesion molecule (VCAM)-1; and E selectin. Cells of the innate immune response, including macrophages, monocyte-derived DCs, plasmacytoid DCs, and neutrophils, are important for the eradication of infection.3–6 Furthermore, subsequent activation of the adaptive immune response, including CD8+ and CD4+ T cells, is also critical to resolve infection.5
A number of C. pneumoniae antigens have been implicated in the activation of the innate immune response. The outer membrane of the C. pneumoniae EB consists of lipopolysaccharides (LPS), the highly expressed chlamydial heat-shock protein 60 (hsp60), major outer-membrane protein, cytoseine-rich protein (CRP), and outer-membrane protein A. The immune response elicited by LPS and hsp60 are the best characterized. The detection of these antigens by TLR4 (LPS, hsp60) and TLR2 (hsp60) results in the activation of epithelial, endothelial, and antigen-presenting cells and consequently triggers the inflammatory immune response. In regard to the TLR family, TLR2 appears to be the most important for resolution of infection; however, although TLR4 also contributes to the innate immune response, it is not essential for the clearance of bacteria.7 The myeloid differentiation primary response 88 (MyD88)-dependent pathway is crucial for mediating the TLR response to C. pneumoniae, resulting in the production of inflammatory cytokines that in turn activate the cell-mediated immune response, predominantly T-helper (Th)1, that is required for clearance of infection.8
Intracellular receptors NOD1 and NOD2, which recognize peptidoglycan components, are also important players in the immune response to C. pneumoniae. This pathway, mediated by receptor interacting protein 2, is important for inducible nitric oxide synthase (iNOS) expression and nitric oxide (NO) production, IL-6, IFN-γ, chemokine C-X-C motif ligand 1 (CXCL1), and macrophage inflammatory protein 1 production, resulting in neutrophil recruitment and bacterial clearance.6
The NLRP3 inflammasome can detect cellular stress induced by C. pneumoniae infection, resulting in caspase-1 activation and production of IL-1β and IL-18 following priming by the TLR2/MyD88 pathway.4,9 IL-1β secretion, through the NLRP3 pathway, is essential for bacterial clearance during early stages of infection.4 Activation of the NLRP3 inflammasome may occur via C. pneumoniae reactive oxygen species (ROS) induction, K+ efflux, and lysosomal damage.10 Indeed, our laboratory has shown that K+ efflux and ROS can result in cytosolic oxidized mitochondrial DNA release that in turn can activate NLRP3.11 This pathway may also occur in response to C. pneumoniae infection, because it has been reported that C. pneumoniae can induce mitochondrial damage in alveolar macrophages.4 The exact mechanism of C. pneumoniae-induced mitochondrial damage, however, is not well characterized.
Activation of the cell-mediated immune response is important to control C. pneumoniae infection.5 CD8+ T cells regulate bacterial growth during early infection (at d 14 and 28), likely through the production of IFN-γ and TNF-α. At early time points, CD4+ T cells are not required for control of infection, but they do have an important role at later time points (60 d after infection) and upon reinfection.5 Interestingly, at early time points, CD4+ T cells actually promote bacterial growth in the absence of CD8+ T cells. This is thought to be the result of CD4+ T cells favoring a Th2 (not a Th1) response, in the absence of CD8+ T cells.5 In line with this, IFN-γ is essential for the resolution of infection, as evidenced by a significant bacterial load increased in IFN-γ receptor (Ifngr)−/− mice.5 This effect is mediated in part through IFN-γ–induced iNOS expression and NO production, which is important for killing and inhibiting bacterial growth.5 Because exposure to C. pneumoniae results predominantly in asymptomatic or mild infection, the development of chronic cases of C. pneumoniae infection and inflammatory disease likely requires additional host genetic or environmental factors that result in failure to eliminate infection or predispose to the relevant disease.
III. DETECTION OF C. PNEUMONIAE
C. pneumonia–specific antibody detection by immunofluorescence is the standard method for serological diagnosis of C. pneumoniae infection.12 The kinetics of immunoglobulin (Ig) M, G, and A secretion are taken into account when determining acute or chronic infection. IgM levels peak at 2–3 wk after initial infection, become undetectable at 2–3 mo, and are not induced following subsequent infection. In contrast, IgG typically peaks at 6–8 wk after initial infection and are rapidly induced following subsequent infections (1–2 wk). Chronic infection is somewhat more difficult to determine and requires the detection of persistent IgG levels, which is complicated by the fact that IgG has a half-life of weeks to months and may therefore be present for some time following acute infection. It has been proposed that IgA levels may provide a better indication of chronic infection, but according to Dowell et al.,12 the use of IgG and A serological markers alone should not be used. Polymerase chain reaction (PCR) methods are also used for the detection of C. pneumoniae infection within tissues and cell types.12,13 Identification of C. pneumoniae messenger RNA (mRNA) by real-time (RT)-PCR can also be used to determine whether C. pneumoniae is in a metabolically activated state.14 Other methods include bacterial culture, immunocytochemistry, and electron microscopy.15
IV. DISEASES
A. Atherosclerosis
Atherosclerosis, a chronic inflammatory disease characterized by the accumulation of lipids and fibrosis within large arteries, is the leading cause of heart disease and stroke. Increasing evidence shows a relationship between the pathogenesis of atherosclerosis and infectious agents including C. pneumoniae, Porphyromonas gingivitis, Helicobacter pylori, influenza A virus, hepatitis C virus, cytomegalovirus, and human immunodeficiency virus.16,17
Initiation of atherosclerosis involves the accumulation of low-density lipoproteins (LDLs) in the intima, the innermost layer of the artery that consists of an elastic membrane covered by a monolayer of endothelial cells. Oxidation and other modifications of LDLs within this subendothelial matrix initiate an inflammatory response. Stimulation of endothelial cells results in the expression of adhesion molecules, growth factors, and cytokines. Monocytes migrate through the endothelium, proliferate, differentiate into macrophages, and take up oxidized LDLs (oxLDLs) to form foam cells. Foam cell necrotic death enhances inflammation, resulting in the recruitment and proliferation of T cells, B cells, and DCs. Furthermore, inflammatory cytokines and mediators act on vascular SMCs, causing them to migrate and proliferate within the subendothelial matrix. SMC production of the extracellular matrix within this region forms a fibrotic plaque together with extracellular lipids covered by a fibrous cap. Disease progression can lead to rupture of this fibrous cap, heart disease, and stroke. Human studies, mouse models, and in vitro data point to an important role of C. pneumoniae infection at all stages of disease, from initiation through plaque rupture by acting through a range of cell types.
A large number of human studies have determined this association of C. pneumonia with atherosclerosis. A case-control meta-analysis using standardized serological criteria from 16 studies showed a significant increase in seroprevalence (C. pneumoniae-specific IgA and G) in atherosclerotic patients. Association of seropositivity to inflammatory makers in atherosclerotic patients was also evidenced using meta-analyses demonstrating that seropositive patients had significantly greater high-sensitivity C-reactive protein, IL-6, and fibrinogen levels.18 In addition to serological data, PCR, immunohistochemistry, and electron microscopy techniques have found evidence of C. pneumoniae within atherosclerotic tissues.13,14,19 Furthermore, when detected, C. pneumoniae and human hsp60 are localized to plaque macrophages in the majority of atherosclerotic tissue isolated from patients.20 Finally, C. pneumoniae isolated from atherosclerotic lesions has been cultured in vitro.21,22 These data therefore provide strong evidence that C. pneumoniae infection may play a part in the prevalence and/or severity of atherosclerosis.
Animal models provide further evidence that C. pneumoniae is involved in the pathogenesis of atherosclerosis. New Zealand white rabbits infected with C. pneumoniae can develop atherosclerosis with23 and without24 a cholesterol-enriched diet. Apolipoprotein-E knockout mice (Apoe−/−) and LDL receptor (LDLR) knockout mice (Ldlr−/−) are prone to the development of atherosclerosis and are commonly used to study this disease. Murine studies of C. pneumonia–induced atherosclerosis performed in wild-type (WT), Ldlr−/−, or Apoe−/− mice indicate that persistent C. pneumoniae infection of the aorta and C. pneumoniae-mediated enhancement of atherosclerosis in mice require high serum lipid levels. It was shown that Ldlr−/− mice present with enhanced atherosclerotic lesion areas following 9 mo of once-monthly intranasal inoculations or 6 mo of twice-monthly intranasal inoculations only when fed a high-fat diet.25,26 Furthermore, direct infection of the aorta of Ldlr−/− mice fed a high-fat diet resulted in enhanced collar-induced atherosclerosis accompanied by increased expression of MCP-1 and ICAM-1 within the arterial wall.27 Apoe−/− mice fed a high-fat diet present with enhanced atherosclerosis following single28 or multiple29,30 intranasal inoculations of C. pneumoniae before being fed a high-fat diet, accompanied by enhanced inflammation. This includes increased expression of cytokines MCP-1, IL-12p40, IL-6, IFN-γ, and TNF-α.31,32 Furthermore, enhanced infiltration of monocytes, macrophages, monocyte-derived DCs, plasmacytoid DCs (pDCs), and T cells are observed in the atherosclerotic lesion.28,30,31
Some murine studies did not find an association of C. pneumoniae infection with atherosclerosis.33 However, this discrepancy may be due to study design or the use of different C. pneumoniae strains. Studies in which no association was found used the Kajaani 7 strain33 or a Finnish C. pneumoniae isolate,34 as compared to the AR39,29,35 TWAR 2043 (American Type Culture Collection [ATCC] VR-1355),30,36 IOL-207,37 or CM-1 strains28 that were used in studies that found significant associations.
The first step involved in C. pneumonia–associated enhancement of atherosclerosis requires dissemination from the infected lung to the aorta. This occurs through chronically infected monocytes and macrophages that migrate from the lung and take residence within the arterial wall.2 Replication and release of C. pneumoniae within the arterial wall spreads infection to proximal endothelial cells and SMCs. As shown in carotid atherosclerotic lesions resected from patients, C. pneumoniae infects endothelial cells of the intima as well as macrophages and SMCs within the atherosclerotic plaque but is absent from surrounding tissues.14,15,21 C. pneumoniae can also be detected within the medial smooth muscle layer underlying the atherosclerotic plaque.38 In vitro studies also confirm direct C. pneumoniae infection of these cell types.39–45 Two virulence factors associated with C. pneumoniae (LPS and hsp60) that activate TLRs contribute to many of the pathogenic effects associated with C. pneumonia–mediated atherosclerosis.20,42,44,46–51 In line with this, C. pneumoniae inflammatory responses are dependent on TLRs and the Myd88-dependent pathway. This is observed in the Apoe−/− models wherein C. pneumoniae infection of Apoe/Tlr2, Apoe/Tlr4, and Apoe/Myd88 double knockout mice resulted in reduced inflammation and development of atherosclerosis, when compared to C. pneumonia–infected Apoe−/− mice.31 Interestingly, acute infection and enhanced inflammation can still be detected within the aorta of normocholesterolemic mice following intranasal C. pneumoniae inoculation, indicating that C. pneumoniae infection of the artery does not necessarily require a high-fat diet.35,52
The expression of inflammatory cytokines induced by C. pneumoniae likely contributes to C. pneumonia–mediated atherosclerosis. IFN-γ, which is expressed by Th1 cells and macrophages following C. pneumoniae infection, is found at high levels within atherosclerotic tissues and has long been implicated in the pathogenesis of atherosclerosis. This is evident within atherosclerosis mice models, in which knockout of the IFN-γ receptor reduces disease, and increased expression of IFN-γ enhances disease.53 Regarding the effects on macrophages, it can induce the expression of TNF-α, IL-6, and MMPs; increase lipid uptake; and enhance ROS.53 It can also act on endothelial cells to induce cell adhesion molecule expression and increase immune cell accumulation within the atherosclerotic plaque.53 TNF-α also has an important role, as evidenced by knockout of TNF-α receptor 1 (TNF-R1) p55, which results in reduced numbers and size of C. pneumonia–mediated atherosclerotic lesions in mice fed a high-fat diet.54 Recently, a role for Th17 cells in atherosclerosis was identified. IL-17A knockout in Apoe−/− mice attenuates C. pneumonia–mediated atherosclerosis as measured by plaque lipid content and lesion size.32 Other cytokines induced by C. pneumoniae, including MCP-1 and IL-1, −18, −6, and −12, have all been shown to enhance disease in murine models of atherosclerosis.55 However, experiments showing the knockout of these cytokines, or their receptors, in a C. pneumonia–mediated model of atherosclerosis are unavailable.
Initiation of disease through the accumulation, modification, and uptake of lipids by monocytes or macrophages to produce inflammatory foam cells is enhanced in the presence of C. pneumoniae. Murine models show that although C. pneumoniae has no effect on serum lipid levels, it enhances the accumulation of lipids within the aorta even in normocholesterolemic mice after repeated C. pneumoniae infections.56 C. pneumonia–infected monocytes oxidize LDL in a dose-dependent manner that is mediated by hsp60.57 Furthermore, C. pneumoniae stimulates platelet production of ROS, likely through an LPS-induced pathway and induction of iNOS and lipoxygenase. This is associated with enhanced oxidization of LDLs.46 oxLDL is highly inflammatory, causing injury to endothelial cells, formation of foam cells, and the migration and proliferation of immune cells and SMCs within the intima.58 Overall, these events create a highly inflammatory environment that can lead to the development and progression of disease.
C. pneumoniae infection of monocytes and macrophages also enhances foam cell formation directly in the presence of high-LDL concentrations through an LPS-induced pathway.42,47 Studies found that perturbed intracellular macrophage cholesterol homeostasis arises from C. pneumonia–induced expression of acetyl-CoA acetyltransferase 1 and reduced expression of adenosine triphosphate (ATP)-binding cassette subfamily A member 1 and subfamily G member 1 via a Jun amino-terminal kinase/peroxisome proliferator-activated receptor-γ pathway that facilitates cholesterol accumulation and foam cell formation.59 The contribution of perturbed macrophage lipid metabolism for C. pneumonia–mediated atherosclerosis is also evident with knockout of liver X receptor α in Apoe−/− mice, which results in enhanced foam cell formation and atherosclerosis when compared to Apoe−/− mice.31 In addition to altered metabolism, C. pneumoniae infection can induce macrophage uptake of nonoxidized LDL. Because macrophages typically only take up oxLDL, this mechanism leads to enhanced foam cell formation independent of LDL oxidization and the classical LDLR pathway.41
The endothelial layer is critical for maintenance of vascular function. C. pneumoniae can modify endothelial cell function and layer integrity by indirect mechanisms, such as through enhanced oxLDL or inflammatory molecules, as well as by C. pneumoniae antigen stimulation and direct infection of endothelial cells.40 Destabilized vascular integrity is evident by reduced endothelial relaxation in C. pneumonia–infected mice dependent on NO.37,60 C. pneumoniae also activates mitogen-activated protein kinase (MAPK) and nuclear factor–κB pathways within endothelial cells,39,61 consequently up-regulating expression of chemokines and adhesion molecules that enhance leukocyte rolling, adhesion, and transendothelial migration.39,61 Chemokines expressed by infected endothelial cells include IL-8, MIG, IFN-γ inducible protein (IP)-10, RANTES, and MCP-1,40,62,63 and adhesion molecules include ICAM-1, VCAM-1, and E selectin.39,61,62 C. pneumoniae infection of primary aortic endothelial cells also results in enhanced production of granulocyte macrophage colony stimulating factor (GM-CSF) in a TLR2-, TLR4-, and MyD88-dependent manner that may promote enhanced DC proliferation within the atherosclerotic lesions.31 Furthermore, C. pneumonia–infected endothelial cells display enhanced apoptosis and necrosis64 that may also exacerbate inflammation through danger-associated molecular pattern signaling pathways.
C. pneumoniae can also stimulate platelet activation, which has proatherogenic properties,48,65 likely through an LPS-dependent pathway and resulting in up-regulation of P selectin, platelet aggregation, and release of ATP.48 In addition, C. pneumoniae triggers platelets to release chemokine ligands (CCLs) including CCL3, 5, and 7 and CXCL8. This may recruit inflammatory cells to the lesions, thus exacerbating disease.66
C. pneumonia–mediated endothelial dysfunction also has an important role in promoting SMC migration and proliferation through the expression of soluble factors.43 These may include heparin-binding epidermal growth factor and platelet-derived growth factor subunit B, which increase in response to endothelial C. pneumoniae infection and have previously been shown to modify SMC proliferation.63 Direct infection of SMCs also promotes their migration and proliferation within the lesion. In vitro studies demonstrate that C. pneumonia–infected rat vascular SMCs enhanced migration in a TLR2-dependent manner.67 Rabbit-infected vascular SMCs show enhanced proliferation in response to C. pneumoniae through hsp60 and the extracellular regulated kinase-1 and −2 pathways; this is further enhanced in the presence of oxLDL.44,51 Furthermore, in vitro studies with human vascular SMCs show that C. pneumoniae EBs induce hsp60, and this activates TLR4-mediated p44/p42 MAPK activation and SMC proliferation.49
C. pneumoniae may also play a part in destabilization of atherosclerotic plaque. Ldlr−/−Apoe−/− mice present with a reduced fibrous cap area relative to total lesion size, a characteristic of rupture-prone plaques. This is accompanied by increased matrix metalloproteinase (MMP)-2 and MMP-9 expressions, likely mediators of fibrous cap thinning through breakdown of the extracellular matrix.36 C. pneumoniae enhancement of MMP production (including MMP-7 and −9) can occur through direct infection of monocytes and macrophages and is regulated by hsp60.20 SMCs may also express MMPs to destabilize the plaque, because culture of SMCs with soluble factors taken from supernatants of C. pneumonia–infected monocytes can induce MMP-2 expression.20,50,68 SMCs and endothelial cells may also contribute to plaque instability following direct infection, because in vitro studies show increased tissue factor, plasminogen activator inhibitor-1, and IL-6 expression following infection, all of which are implicated in thrombosis and reduced plaque instability.45 Finally, the T-cell response generated against C. pneumoniae may be involved in enhanced plaque instability. One study analyzed the T-cell population of unstable atherosclerotic plaques and found that T cells with antigen specificity for C. pneumoniae predominantly exhibited a Th1 response (94%).69 Indeed, IFN-γ, produced by Th1 cells, can inhibit SMCs proliferation and collagen synthesis and enhance MMP expression, thus contributing to plaque instability.70
A number of large-scale trials investigating the effects of antichlamydial antibiotics, including azithromycin, gatifloxacin, or clarithromycin, for the treatment of atherosclerosis have been performed.71–74 These studies failed to provide conclusive evidence that antibiotics are an effective treatment and have led to controversy as to whether C. pneumoniae actually has a role in the initiation/exacerbation of atherosclerosis. However, a number of factors have been hypothesized to explain why these trials failed, such as the timing of antibiotic treatment in the context of disease, antibiotic treatment regime, effectiveness of antibiotics in eliminating persistent C. pneumoniae infection, complications of other pathogenic factors, and use of other approved clinical treatment regimes masking the effectiveness of antibiotics.75 For example, these studies were performed on patients with advanced atherosclerosis, which would not take into account C. pneumonia–mediated initiation and early progression of disease. The trials also used a single antibiotic, which may not be as effective in clearing bacteria as combination treatment, particularly in regard to persistent infection.76–78 Therefore, the results of these trials cannot rule out a role for C. pneumoniae in the initiation/exacerbation of disease. Additional trials are needed to address these complicating factors and conclusively determine the effectiveness of antibiotic treatment for atherosclerosis.
Investigations into the effectiveness of antibiotic treatment in animal models of C. pneumonia–mediated atherosclerosis have yielded mixed results. Antibiotic trials of C. pneumonia–infected rabbits on a high-fat diet show reduction in atherosclerosis development following azithromycin treatment during a 7-wk time course started after the final infection.23 In another study, azithromycin treatment of C. pneumonia–infected rabbits begun at an early time point (5 d after initial infection) showed reduced incidence of atherosclerosis, but the same treatment regime begun at a later time point (2 wk after final infection) did not.79 The analysis of antibiotic treatment in the Apoe−/− mouse model of C. pneumonia–induced atherosclerosis showed that azithromycin begun 2 wk after infection did not have an effect.80 Additional investigation using combination antibiotic treatment is needed. Furthermore, the timing of antibiotic treatment in the context of disease must be further explored to enhance our understanding of the effectiveness of antibiotic treatment for atherosclerosis.
B. Asthma
Asthma is an allergic disease of the lung characterized by a Th2 response, high IgE titers, and increased eosinophil infiltration. The pathogenesis of allergic asthma has been associated with bacterial and viral infections. As evidenced through case-control and cohort studies, C. pneumoniae has been associated with initiation and exacerbation of asthma. Early studies indicated the association of C. pneumoniae Ig levels with wheezing and adult-onset asthma.81 Succeeding studies found that this was due to chronic C. pneumoniae infection, and wheezing induced by C. pneumoniae commenced before the development of asthma.82,83 The majority of subsequent studies support these findings. Serological studies found that increased C. pneumoniae IgG levels were associated with incidence of chronic allergic asthma, recent asthma, and the severity of chronic asthma.84,85 C. pneumoniae IgA levels were also found to be associated with asthma.86 Furthermore, the titer of C. pneumonia–specific IgE levels in asthmatic patients significantly correlated with asthma severity.87 C. pneumoniae was also found to be associated with chronic asthma, and patients that tested positive for mycoplasma and/or C. pneumoniae have increased mast-cell infiltration of the lung.88 There is also evidence for an association of C. pneumoniae with the development, incidence, and exacerbation of acute and chronic asthma in children. C. pneumoniae was associated with increased wheezing in pediatric asthma, which improved with antibiotic treatment.89 In addition, C. pneumonia–specific IgE levels were found to be greater in asthmatic children with C. pneumoniae infection.90 Another study found that chronic infection, as measured by PCR, as well as increased IgA levels, were associated with increased incidence of asthmatic exacerbations in children.91 Compared with asthmatic patients treated with low-dose steroids, increased titers of C. pneumonia–specific antibodies, 74.1% more IgG, and 70.1% more IgA were observed in asthmatic patients receiving a high-dose steroid treatment.92
C. pneumoniae is thought to promote the development of asthma by inducing a Th2 immune response; however, the specific mechanisms involved in this process are still unknown. This effect is evidenced by high IgE titers and enhanced mast-cell infiltration in asthmatic patients with C. pneumoniae infection.87,88,90 Murine models also shed light on the mechanisms by which C. pneumoniae induces asthma. Mice sensitized with the human serum albumin (HSA) allergen 5 d after low-dose C. pneumoniae infection, and challenged with HSA 2 wk later, developed lung inflammation characterized by enhanced eosinophil infiltration as well as increased goblet cells and HSA-specific IgE levels.93,94 This allergic response is dependent on DC activation through the MyD88 pathway.93 TLR4, but not TLR2, deficiency prevented the development of the allergic response.94 In fact, TLR2 deficiency allowed allergen sensitization but at a later time point, which was not observed in WT mice.94 Those disparities were due to a differential regulatory T (Treg)–cell response, with TLR4 deficiency enhancing and TLR2 deficiency reducing Treg-cell amounts in the lung after infection.94 The allergic response, however, was only observed with low-dose C. pneumoniae infection; high-dose infection increased Treg cells and pDCs in the lung, which in turn act to suppress sensitization.94 Other studies investigating chlamydia family member Chlamydia muridarum indicate that infection induces airway hyper-responsiveness in mice through reduced expression of the IL-13 decoy receptor IL-13Rα2, thus enhancing IL-13 and signal transducer and activator of transcription–6 signaling.95 Further studies indicate that reduced expression of IL-13Rα2 is a consequence of programmed death ligand (PDL)-1, expressed on lung DCs and monocytes, and its receptor PD-1 expressed on T cells following infection.96 These proteins have previously been shown to induce a Th2 response and enhance airway hyper-responsiveness and lung inflammation.97 Whether these pathways are also important for C. pneumoniae induction of allergic airway disease remains to be elucidated.
C. Cancer
The development of cancer has been long associated with a number of pathogens, both causative and opportunistic.98 A number of case-control and cohort studies have looked for association of C. pneumoniae with lung cancer, although with somewhat varied results. A meta-analysis99 of 12 studies was performed based on detection of IgA or IgG within patient or control blood according to standardized serological criteria,12, with adjustment for confounding effects of lung cancer risk factors and timing of blood sampling (before or after disease onset). This identified a significant association of C. pneumoniae infection with lung cancer risk, although sampling of blood before lung cancer diagnosis resulted in a weaker association than sampling after diagnosis. Overall association of C. pneumoniae with lung cancer was found to increase with stricter IgA cutoff levels (IgA ≥ 16, odds ratio [OR] = 1.22, and 95% confidence interval [CI] = 1.06–1.41 compared to IgA ≥ 64, OR = 2.35, and 95% 1.88–2.93). These findings are also in line with a meta-analysis conducted by Littman et al.100 Enhanced infection after disease onset indicates that C. pneumoniae infection during cancer is opportunistic. However, given that a significant association was found for infection before disease onset, this also indicates a causal relationship.
The mechanism of C. pneumonia–mediated lung cancer development is not well defined, but lessons from other bacterial species associated with cancer development provide insight as to how this may occur. Bacterial infection of the epithelium leads to the production of chemokines, which promotes immune cell activation and tissue infiltration that result in the production of inflammatory mediators such as TNF- α, IL-1β, IFN-γ, IL-6, and IL-8. Expression of these inflammatory mediators can enhance ROS production, resulting in tissue damage, DNA damage, increased cell turnover, and DNA mutations that promote uncontrolled cell proliferation.101 Indeed, C. pneumoniae infection can induce expression of these inflammatory mediators and enhance ROS production, as discussed previously.
D. Arthritis
Persistent bacterial infection within the joint can lead to the development of reactive arthritis. Many studies have found that both C. trachomatis and C. pneumoniae are associated with reactive and other forms of arthritis, although a stronger association exists for C. trachomatis. In regard to C. pneumoniae, disease arises following dissemination of infected lung monocytes and macrophages that take residence within the joint and induce inflammation. The association of C. pneumoniae with arthritis has been through detection of C. pneumoniae serologically as well as within the synovial membrane and synovial fluid, using immunofluorescence, PCR, electron microscopy, and bacterial culture. In one study, serological evidence of C. pneumoniae was found in five of 70 patients, and synovial lymphocytes from these patients demonstrated C. pneumonia–mediated proliferation in vitro.102 Furthermore, three of these patients presented with upper respiratory tract infection before disease onset, indicating causality. Another study found serological evidence of C. pneumoniae infection in four of 35 patients with arthritis, and three of these patients experienced lower respiratory illness before disease onset.103 C. trachomatis was not identified in these patients, suggesting that C. pneumoniae was the causative agent.103 In addition, three of these patients were positive for human leukocyte antigen (HLA)-B27, a risk factor for arthritis, indicating that C. pneumoniae may work synergistically with host genetic factors to promote disease. This is further supported by findings that the APOe4 allele, a risk factor for late-onset arthritis, is associated with enhanced C. pneumoniae within the joint, as measured by PCR.104 Additional studies using PCR demonstrated that C. pneumoniae was present within 12.7% and 7.9% of joint tissue and synovial fluid from arthritic patients, respectively.105 Furthermore, a study using RT-PCR to detect C. pneumoniae mRNA, the presence of which indicates a metabolically active state, determined positive results for ten patients who were also found to be positive by PCR. These patients had various forms of arthritis including Reiter’s syndrome, undifferentiated oligoarthritis, reactive arthritis, rheumatoid arthritis, and osteoarthritis.106 Another study also found, using PCR, RT-PCR, or bacterial culture, that C. pneumoniae was present within the synovial fluid or peripheral blood mononuclear cells (PBMCs) in synovitis–acne–pustulosis–hyperostosis–osteitis syndrome, psoriatic arthritis, undifferentiated oligoarthritis, and ankylosing spondylitis.107
E. Neurological Diseases
C. pneumoniae infection has also been associated with a number of neurological diseases including Alzheimer’s disease, multiple sclerosis, and schizophrenia. These arise following transmigration of infected monocytes, disseminated from the lung, across the blood–brain barrier.108,109 Mediated by infection of the human brain microvascular endothelial cells, this results in up-regulation of VCAM-1 and ICAM-1.108 Within the CNS, C. pneumoniae infection is likely to spread, thus inducing an inflammatory response and mediating disease. This is supported by studies that show C. pneumoniae can also infect brain microglial cells, astrocytes, and neuronal cells.109–111
1. Alzheimer’s Disease
Alzheimer’s disease is a neurodegenerative disorder leading to dementia, usually occurring in later life. Alzheimer’s is characterized by intracellular neurofibrillary tangles and extracellular amyloid deposits that induce inflammation, leading to neurite and brain atrophy and impaired synaptic signaling.112 Many studies now support a role for pathogens in promoting Alzheimer’s disease, which include herpes simplex virus 1, hepatitis C virus, cytomegalovirus, toxoplasma, and C. pneumoniae.113,114 Studies investigating a role for C. pneumoniae in Alzheimer’s patients have been variable. However, a meta-analysis of case-control studies indeed indicates a positive association (OR = 5.66, 95% CI = 1.83–17.51).115 Furthermore, C. pneumoniae has been cultured from the brains of Alzheimer’s disease patients.116
Murine models demonstrate that respiratory inoculation with a C. pneumoniae isolate from Alzheimer’s diseased brain can result in amyloid-β deposits in the neuronal cells of the murine brain for up to 3 mo after infection, progressively increasing over time.117 It is likely that C. pneumoniae initiates or exacerbates inflammatory events within the brain, thus contributing to disease. Such mechanisms may occur via induction of oxidative stress or increased production of inflammatory cytokines by activated microglial cells, which can influence amyloid-β expression or directly contribute to neurodegeneration.118
2. Multiple Sclerosis
Multiple sclerosis is a chronic autoimmune disease of the CNS that is characterized by demyelination of axons, resulting in progressive neurological deterioration.119 A number of pathogens are associated with the development of multiple sclerosis including Epstein-Barr virus, human herpes virus 6, human endogenous retrovirus, Staphylococcus aureus, Mycoplasma pneumoniae, and C. pneumoniae.120 Studies investigating the association of C. pneumoniae and multiple sclerosis have also generated varying results, likely due to study design and detection methods. However, a meta-analysis of 26 studies found that multiple sclerosis patients have increased C. pneumoniae levels, as measured by PCR, within their cerebrospinal fluid (OR = 3.216, 95% CI = 1.204–8.585).121 In contrast, no significant association was found for C. pneumoniae Ig levels within the serum or cerebrospinal fluid.121
Animal models suggest that C. pneumoniae may have a role in initiating multiple sclerosis. Studies in a rat model demonstrate that a C. pneumoniae peptide that shares a seven–amino acid motif with a myelin basic protein epitope can induce a Th1 response, resulting in severe experimental autoimmune encephalomyelitis (EAE).122 Furthermore, mild symptoms of EAE could be induced following immunization of rats with sonicated C. pneumoniae together with complete Freund’s adjuvant.122 Another study using the EAE model in mice showed that the disease was exacerbated with intraperitoneal administration of C. pneumonia; this was attenuated with antibiotic treatment.123
3. Schizophrenia
Schizophrenia is a mental illness characterized by hallucination, delusions, paranoia, and depressive symptoms.124 In the pathogenesis of disease, evidence exists for a role of pathogen infection, particularly during brain development.125 Pathogens associated with schizophrenia include herpes virus, influenza, cytomegalovirus, Borna disease virus, Toxoplasma gondii, cytomegalovirus, Toxoplasma gondii, Chlamydia psittaci, and C. pneumoniae.125 A number of studies by Fellerhoff et al. have found an association of C. pneumoniae with schizophrenia.126–128 A meta-analysis of the two earlier studies by Fellerhoff et al. identified a significant positive association of C. pneumoniae with schizophrenia (OR = 6.34, CI 95% = 2.83–14.19, p < 0.001).125 These studies measured C. pneumoniae within PBMCs by PCR.126 The latter study by Fellerhoff et al. identified four times as much chlamydial DNA within the frontal cortex in schizophrenia patients as compared to controls.127 A study by another group failed to find an association, but this was hypothesized to be due to the high prevalence of C. pneumoniae within healthy controls of the Korean population studied.129
F. Other Diseases
C. pneumoniae has also been implicated in other inflammatory diseases including Behcet’s disease, primary biliary cirrhosis, and diabetes. Evidence, however, is scarce, as outlined below, and additional studies are required to provide further support for a role of C. pneumoniae in these diseases.
Behcet’s disease is an autoimmune systemic vasculitis characterized by recurrent episodes of oral aphtous ulcers, genital ulcers, skin lesions, ocular lesions, and other manifestations involving vascular, gastrointestinal, and neurological systems. Evidence suggests that C. pneumoniae may be involved in the pathogenesis of Behcet’s disease, because patients have increased C. pneumoniae IgA and IgG levels compared to controls,130 and hsp60 has been found in gastrointestinal lesions associated with this disease.131
Primary biliary cirrhosis is an autoimmune disease of the liver. Pathogens are hypothesized to induce inflammation within the liver and trigger disease. A number of studies have yielded mixed results regarding the involvement of C. pneumoniae in the pathogenesis of disease. One study found the C. pneumoniae antigen and RNA in the liver of patients.132 Another study found that C. pneumoniae IgM levels were increased in patients compared to controls; however, there was no difference compared to posthepatitis cirrhosis, thus generating inconclusive results.133 Another study found no association of C. pneumoniae seropositivity with disease, although there was a positive correlation with disease severity.134 Murine studies of infection have found the presence of C. pneumoniae within the liver.2,135 Additionally, C. pneumoniae has been shown to replicate in murine Kupffer cells, resulting in TNF-α production.135
The pathogenesis of type-2 diabetes, characterized by insulin resistance, has been linked to bacterial infections including C. pneumoniae. However, association studies are complicated because patients with diabetes are more prone to infection. Nevertheless, one study found that C. pneumoniae seropositivity was linked to insulin resistance in healthy middle-aged men, and this correlation increased with increased C. pneumoniae Ig levels.136 Murine studies show that C. pneumoniae can enhance insulin resistance and inflammation in obese mice, which is dependent on TNF-α.137 Murine studies also indicate that C. pneumoniae infection can increase IL-1β levels in the pancreas, a pathogenic factor associated with type-2 diabetes.138 Furthermore, C. pneumoniae infection of mast cells promotes reduced β-cell ATP and insulin production and enhanced β-cell destruction.138
V. CONCLUSION
Given the complexities regarding disease development and/or exacerbation, it is not surprising that it is extremely difficult to definitively correlate or establish a causal relationship between C. pneumoniae infection and the various diseases described here. Whereas mouse studies have generally established that C. pneumoniae infection can exacerbate or accelerate most of these pathologies, human studies can only infer on the basis of circumstantial evidence. Additionally, negative results from poorly designed clinical trials have led to a reduction in enthusiasm for these types of studies. However, a better understanding of the possible mechanisms by which a persistent bacterial infection might affect chronic inflammatory conditions has reopened the idea linking C. pneumoniae infection and the development of various diseases. Although there has been no “smoking gun” evidence for this association, it is clear from the sheer number of positive studies, and the multitude of diseases affected, that where there is smoke there may be fire, and further investigations are warranted to understand the role of C. pneumoniae infection in disease development and the potential interventions that may be designed to reduce and/or eliminate this possible risk.
ACKNOWLEDGMENTS
We thank Dr. James Miller for his many tireless years of Treponema studies and his impact on the development of countless researchers. This work was supported by grant no. AI112826 from the National Institutes of Health to T.R.C.
ABBREVIATIONS:
- Apoe
Apoplipoprotein
- CCL
chemokine ligands
- DC
dendritic cells
- hsp60
heat-shock protein 60
- IFN-γ
interferon γ
- ICAM-1
intracellular adhesion molecule-1
- Ig
immunoglobulin
- LDL
low-density lipoprotein
- LPS
lipopolysaccharides
- MCP-1
monocyte chemoattractant protein
- MMP
matrix metalloproteinase
- MyD88
myeloid differentiation primary response 88
- NLRP3
NOD-like receptor family pyrin-domain-containing 3
- NOD
nucleotide-binding oligomerization domain-like receptor
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- RT-PCR
real-time PCR
- SMC
smooth muscle cell
- Th
T helper
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor α
- VCAM-1
vascular cell adhesion molecule 1
REFERENCES
- 1.Kuo CC, Jackson LA, Campbell LA, Grayston JT. Chlamydia pneumoniae (TWAR). Clin Microbiol Rev. 1995;8(4):451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang ZP, Kuo CC, Grayston JT. Systemic dissemination of Chlamydia pneumoniae following intranasal inoculation in mice. J Infect Dis. 1995;171(3):736–8. [DOI] [PubMed] [Google Scholar]
- 3.Crother TR, Ma J, Jupelli M, Chiba N, Chen S, Slepenkin A, Alsabeh R, Peterson E, Shimada K, Arditi M. Plasmacytoid dendritic cells play a role for effective innate immune responses during Chlamydia pneumoniae infection in mice. PLoS ONE. 2012;7(10):e48655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimada K, Crother TR, Karlin J, Chen S, Chiba N, Ramanujan VK, Vergnes L, Ojcius DM, Arditi M. Caspase-1 dependent IL-1β secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS ONE. 2011;6(6):e21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rottenberg ME, Gigliotti Rothfuchs AC, Gigliotti D, Svanholm C, Bandholtz L, Wigzell H. Role of innate and adaptive immunity in the outcome of primary infection with Chlamydia pneumoniae, as analyzed in genetically modified mice. J Immunol. 1999;162(5):2829–36. [PubMed] [Google Scholar]
- 6.Shimada K, Chen S, Dempsey PW, Sorrentino R, Alsabeh R, Slepenkin AV, Peterson E, Doherty TM, Underhill D, Crother TR, Arditi M. The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. PLoS Pathog. 2009;5(4):e1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez N, Wantia N, Fend F, Dürr S, Wagner H, Miethke T. Differential involvement of TLR2 and TLR4 in host survival during pulmonary infection with Chlamydia pneumoniae. Eur J Immunol. 2006;36(5):1145–55. [DOI] [PubMed] [Google Scholar]
- 8.Naiki Y, Michelsen KS, Schröder NW, Alsabeh R, Slepenkin A, Zhang W, Chen S, Wei B, Bulut Y, Wong MH, Peterson EM, Arditi M. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. J Biol Chem. 2005;280(32):29242–9. [DOI] [PubMed] [Google Scholar]
- 9.He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2010;184(10):5743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada K, Crother TR, Arditi M. Innate immune responses to Chlamydia pneumoniae infection: role of TLRs, NLRs, and the inflammasome. Microbes Infect. 2012;14(14):1301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowell SF, Peeling RW, Boman J, Carlone GM, Fields BS, Guarner J, Hammerschlag MR, Jackson LA, Kuo CC, Maass M, Messmer TO, Talkington DF, Tondella ML, Zaki SR. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clin Infect Dis. 2001;33(4):492–503. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CC, Grayston JT, Campbell LA, Goo YA, Wissler RW, Benditt EP. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15–34 years old). Proc Natl Acad Sci USA. 1995;92(15):6911–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shor A, Kuo CC, Patton DL. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S Afr Med J. 1992;82(3):158–61. [PubMed] [Google Scholar]
- 15.Kuo CC, Shor A, Campbell LA, Fukushi H, Patton DL, Grayston JT. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167(4):841–9. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011;106(5):858–67. [DOI] [PubMed] [Google Scholar]
- 17.Sessa R, Pietro MD, Filardo S, Turriziani O. Infectious burden and atherosclerosis: a clinical issue. World J Clin Cases. 2014;2(7):240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filardo S, Di Pietro M, Farcomeni A, Schiavoni G, Sessa R. Chlamydia pneumoniae-mediated inflammation in atherosclerosis: a meta-analysis. Mediators Inflamm. 2015;2015:378658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo CC, Gown AM, Benditt EP, Grayston JT. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb. 1993;13(10):1501–4. [DOI] [PubMed] [Google Scholar]
- 20.Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-α and matrix metalloproteinase expression. Circulation. 1998;98(4):300–7. [DOI] [PubMed] [Google Scholar]
- 21.Jackson LA, Campbell LA, Kuo CC, Rodriguez DI, Lee A, Grayston JT. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176(1):292–5. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez JA. The Chlamydia pneumoniae/Atherosclerosis Study Group. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125(12):979–82. [DOI] [PubMed] [Google Scholar]
- 23.Muhlestein JB, Anderson JL, Hammond EH, Zhao L, Trehan S, Schwobe EP, Carlquist JF. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation. 1998;97(7):633–6. [DOI] [PubMed] [Google Scholar]
- 24.Fong IW, Chiu B, Viira E, Jang D, Mahony JB. De novo induction of atherosclerosis by Chlamydia pneumoniae in a rabbit model. Infect Immun. 1999;67(11):6048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Hu H, Ji H, Murdin AD, Pierce GN, Zhong G. Chlamydia pneumoniae infection significantly exacerbates aortic atherosclerosis in an LDLR−/− mouse model within six months. Mol Cell Biochem. 2000;215(12):123–8. [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Pierce GN, Zhong G. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J Clin Invest. 1999;103(5):747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauer AD, de Vos P, Peterse N, ten Cate H, van Berkel TJ Stassen FR, Kuiper J. Delivery of Chlamydia pneumoniae to the vessel wall aggravates atherosclerosis in LDLr−/− mice. Cardiovasc Res. 2006;69(1):280–8. [DOI] [PubMed] [Google Scholar]
- 28.Sorrentino R, Yilmaz A, Schubert K, Crother TR, Pinto A, Shimada K, Arditi M, Chen S. A single infection with Chlamydia pneumoniae is sufficient to exacerbate atherosclerosis in ApoE deficient mice. Cell Immunol. 2015;294(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moazed TC, Campbell LA, Rosenfeld ME, Grayston JT, Kuo CC. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J Infect Dis. 1999;180(1):238–41. [DOI] [PubMed] [Google Scholar]
- 30.Ezzahiri R, Nelissen-Vrancken HJ, Kurvers HA, Stassen FR, Vliegen I, Grauls GE, van Pul MM, Kitslaar PJ, Bruggeman CA. Chlamydophila pneumoniae (Chlamydia pneumoniae) accelerates the formation of complex atherosclerotic lesions in Apo E3-leiden mice. Cardiovasc Res. 2002;56(2):269–76. [DOI] [PubMed] [Google Scholar]
- 31.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schröder NW, Crother TR, Bulut Y, Doherty TM, Bradley M, Shaposhnik Z, Peterson EM, Tontonoz P, Shah PK, Arditi M.TLR/MyD88 and liver X receptor α signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008;181(10):7176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Shimada K, Zhang W, Huang G, Crother TR, Arditi M. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185(9):5619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aalto-Setälä K, Laitinen K, Erkkilä L, Leinonen M, Jauhiainen M, Ehnholm C, Tamminen M, Puolakkainen M, Penttilä I, Saikku P. Chlamydia pneumoniae does not increase atherosclerosis in the aortic root of apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21(4):578–84. [DOI] [PubMed] [Google Scholar]
- 34.Caligiuri G, Rottenberg M, Nicoletti A, Wigzell H, Hansson GK. Chlamydia pneumoniae infection does not induce or modify atherosclerosis in mice. Circulation. 2001;103(23):2834–8. [DOI] [PubMed] [Google Scholar]
- 35.Campbell LA, Blessing E, Rosenfeld M, Lin Tm, Kuo C. Mouse models of C. pneumoniae infection and atherosclerosis. J Infect Dis. 2000;181 Suppl 3:S508–13. [DOI] [PubMed] [Google Scholar]
- 36.Ezzahiri R, Stassen FR, Kurvers HA, van Pul MM, Kitslaar PJ, Bruggeman CA. Chlamydia pneumoniae infection induces an unstable atherosclerotic plaque phenotype in LDL-receptor, ApoE double knockout mice. Eur J Vasc Endovasc Surg. 2003;26(1):88–95. [DOI] [PubMed] [Google Scholar]
- 37.Liuba P, Karnani P, Pesonen E, Paakkari I, Forslid A, Johansson L, Persson K, Wadström T, Laurini R. Endothelial dysfunction after repeated Chlamydia pneumoniae infection in apolipoprotein E-knockout mice. Circulation. 2000;102(9):1039–44. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita K, Ouchi K, Shirai M, Gondo T, Nakazawa T, Ito H. Distribution of Chlamydia pneumoniae infection in the athersclerotic carotid artery. Stroke. 1998;29(4):773–8. [DOI] [PubMed] [Google Scholar]
- 39.Krüll M, Klucken AC, Wuppermann FN, Fuhrmann O, Magerl C, Seybold J, Hippenstiel S, Hegemann JH, Jantos CA, Suttorp N. Signal transduction pathways activated in endothelial cells following infection with Chlamydia pneumoniae. J Immunol. 1999;162(8):4834–41. [PubMed] [Google Scholar]
- 40.Molestina RE, Miller RD, Ramirez JA, Summersgill JT. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect Immun. 1999;67(3):1323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalayoglu MV, Miranpuri GS, Golenbock DT, Byrne GI. Characterization of low-density lipoprotein uptake by murine macrophages exposed to Chlamydia pneumoniae. Microbes Infect. 1999;1(6):409–18. [DOI] [PubMed] [Google Scholar]
- 42.Kalayoglu MV, Byrne GI. A Chlamydia pneumoniae component that induces macrophage foam cell formation is chlamydial lipopolysaccharide. Infect Immun. 1998;66(11):5067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coombes BK, Mahony JB. Chlamydia pneumoniae infection of human endothelial cells induces proliferation of smooth muscle cells via an endothelial cell-derived soluble factor(s). Infect Immun. 1999;67(6):2909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirono S, Dibrov E, Hurtado C, Kostenuk A, Ducas R, Pierce GN. Chlamydia pneumoniae stimulates proliferation of vascular smooth muscle cells through induction of endogenous heat shock protein 60. Circ Res. 2003;93(8):710–6. [DOI] [PubMed] [Google Scholar]
- 45.Dechend R, Maass M, Gieffers J, Dietz R, Scheidereit C, Leutz A, Gulba DC. Chlamydia pneumoniae infection of vascular smooth muscle and endothelial cells activates NF-κB and induces tissue factor and PAI-1 expression: a potential link to accelerated arteriosclerosis. Circulation. 1999;100(13):1369–73. [DOI] [PubMed] [Google Scholar]
- 46.Kälvegren H, Bylin H, Leanderson P, Richter A, Grenegård M, Bengtsson T. Chlamydia pneumoniae induces nitric oxide synthase and lipoxygenase-dependent production of reactive oxygen species in platelets. Effects on oxidation of low density lipoproteins. Thromb Haemost. 2005;94(2):327–35. [DOI] [PubMed] [Google Scholar]
- 47.Kalayoglu MV, Byrne GI. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis. 1998;177(3):725–9. [DOI] [PubMed] [Google Scholar]
- 48.Kälvegren H, Majeed M, Bengtsson T. Chlamydia pneumoniae binds to platelets and triggers P-selectin expression and aggregation: a causal role in cardiovascular disease? Arterioscler Thromb Vasc Biol. 2003;23(9):1677–83. [DOI] [PubMed] [Google Scholar]
- 49.Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via Toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ Res. 2001;89(3):244–50. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt R, Redecke V, Breitfeld Y, Wantia N, Miethke T, Massberg S, Fischel S, Neumann FJ, Schömig A, May AE. EMMPRIN (CD 147) is a central activator of extracellular matrix degradation by Chlamydia pneumoniae-infected monocytes. Implications for plaque rupture. Thromb Haemost. 2006;95(1):151–8. [PubMed] [Google Scholar]
- 51.Chahine MN, Deniset J, Dibrov E, Hirono S, Blackwood DP, Austria JA, Pierce GN. Oxidized LDL promotes the mitogenic actions of Chlamydia pneumoniae in vascular smooth muscle cells. Cardiovasc Res. 2011;92(3):476–83. [DOI] [PubMed] [Google Scholar]
- 52.Blessing E, Lin TM, Campbell LA, Rosenfeld ME, Lloyd D, Kuo C. Chlamydia pneumoniae induces inflammatory changes in the heart and aorta of normocholesterolemic C57BL/6J mice. Infect Immun. 2000;68(8):4765–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voloshyna I, Littlefield MJ, Reiss AB. Atherosclerosis and interferon-γ: new insights and therapeutic targets. Trends Cardiovasc Med. 2014;24(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell LA, Nosaka T, Rosenfeld ME, Yaraei K, Kuo CC. Tumor necrosis factor α plays a role in the acceleration of atherosclerosis by Chlamydia pneumoniae in mice. Infect Immun. 2005;73(5):3164–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(5):969–79. [DOI] [PubMed] [Google Scholar]
- 56.Törmäkangas L, Erkkilä L, Korhonen T, Tiirola T, Bloigu A, Saikku P, Leinonen M. Effects of repeated Chlamydia pneumoniae inoculations on aortic lipid accumulation and inflammatory response in C57BL/6J mice. Infect Immun. 2005;73(10):6458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalayoglu MV, Hoerneman B, LaVerda D, Morrison SG, Morrison RP, Byrne GI. Cellular oxidation of low-density lipoprotein by Chlamydia pneumoniae. J Infect Dis. 1999;180(3):780–90. [DOI] [PubMed] [Google Scholar]
- 58.Chatterjee S, Ghosh N. Oxidized low density lipoprotein stimulates aortic smooth muscle cell proliferation. Glycobiology. 1996;6(3):303–11. [DOI] [PubMed] [Google Scholar]
- 59.Liu W, He P, Cheng B, Mei CL, Wang YF, Wan JJ. Chlamydia pneumoniae disturbs cholesterol homeostasis in human THP-1 macrophages via JNK-PPARγ dependent signal transduction pathways. Microbes Infect. 2010;12(14–15):1226–35. [DOI] [PubMed] [Google Scholar]
- 60.Törmäkangas L, Ketonen J, Leinonen M, Saikku P, Paakkari I. Increased prostanoid dependency of arterial relaxation in Chlamydia pneumoniae-infected mice. J Med Microbiol. 2006;55(Pt 8):1017–21. [DOI] [PubMed] [Google Scholar]
- 61.Krüll M, Kramp J, Petrov T, Klucken AC, Hocke AC, Walter C, Schmeck B, Seybold J, Maass M, Ludwig S, Kuipers JG, Suttorp N, Hippenstiel S. Differences in cell activation by Chlamydophila pneumoniae and Chlamydia trachomatis infection in human endothelial cells. Infect Immun. 2004;72(11):6615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Högdahl M, Söderlund G, Kihlström E. Expression of chemokines and adhesion molecules in human coronary artery endothelial cells infected with Chlamydia (Chlamydophila) pneumoniae. APMIS. 2008;116(12):1082–8. [DOI] [PubMed] [Google Scholar]
- 63.Coombes BK, Mahony JB. cDNA array analysis of altered gene expression in human endothelial cells in response to Chlamydia pneumoniae infection. Infect Immun. 2001;69(3):1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schöier J, Högdahl M, Söderlund G, Kihlström E. Chlamydia (Chlamydophila) pneumoniae-induced cell death in human coronary artery endothelial cells is caspase-independent and accompanied by subcellular translocations of Bax and apoptosis-inducing factor. FEMS Immunol Med Microbiol. 2006;47(2):207–16. [DOI] [PubMed] [Google Scholar]
- 65.Badimon L, Padró T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care. 2012;1(1):60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Bannawi A, Al-Wesebai K, Taha S, Bakhiet M. Chlamydia pneumoniae induces chemokine expression by platelets in patients with atherosclerosis. Med Princ Pract. 2011;20(5):438–43. [DOI] [PubMed] [Google Scholar]
- 67.Wang B, Zhang L, Zhang T, Wang H, Zhang J, Wei J, Shen B, Liu X, Xu Z, Zhang L. Chlamydia pneumoniae infection promotes vascular smooth muscle cell migration through a Toll-like receptor 2-related signaling pathway. Infect Immun. 2013;81(12):4583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vehmaan-Kreula P, Puolakkainen M, Sarvas M, Welgus HG, Kovanen PT. Chlamydia pneumoniae proteins induce secretion of the 92-kDa gelatinase by human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol. 2001;21(1):E1–8. [DOI] [PubMed] [Google Scholar]
- 69.de Boer OJ, van der Wal AC, Houtkamp MA, Ossewaarde JM, Teeling P, Becker AE. Unstable atherosclerotic plaques contain T-cells that respond to Chlamydia pneumoniae. Cardiovasc Res. 2000;48(3):402–8. [DOI] [PubMed] [Google Scholar]
- 70.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11(5):1223–30. [DOI] [PubMed] [Google Scholar]
- 71.O’Connor CM, Dunne MW, Pfeffer MA, Muhlestein JB, Yao L, Gupta S, Benner RJ, Fisher MR, Cook TD. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. J Am Med Assoc. 2003;290(11):1459–66. [DOI] [PubMed] [Google Scholar]
- 72.Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, Cairns R, Skene AM. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352(16):1646–54. [DOI] [PubMed] [Google Scholar]
- 73.Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, Rogers WJ, Crouse JR, Borrowdale SL, Schron E, Knirsch C. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352(16):1637–45. [DOI] [PubMed] [Google Scholar]
- 74.Jespersen CM, Als-Nielsen B, Damgaard M, Hansen JF, Hansen S, Helø OH, Hildebrandt P, Hilden J, Jensen GB, Kastrup J, Kolmos HJ, Kjøller E, Lind I, Nielsen H, Petersen L, Gluud C. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. Br Med J. 2006;332(7532):22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campbell LA, Rosenfeld ME. Persistent C. pneumoniae infection in atherosclerotic lesions: rethinking the clinical trials. Front Cell Infect Microbiol. 2014;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kutlin A, Roblin PM, Hammerschlag MR. Effect of prolonged treatment with azithromycin, clarithromycin, or levofloxacin on Chlamydia pneumoniae in a continuous-infection model. Antimicrob Agents Chemother. 2002;46(2):409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freidank HM, Losch P, Vögele H, Wiedmann-Al-Ahmad M. In vitro susceptibilities of Chlamydia pneumoniae isolates from German patients and synergistic activity of antibiotic combinations. Antimicrob Agents Chemother. 1999;43(7):180810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malinverni R, Kuo CC, Campbell LA, Lee A, Grayston JT. Effects of two antibiotic regimens on course and persistence of experimental Chlamydia pneumoniae TWAR pneumonitis. Antimicrob Agents Chemother. 1995;39(1):45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fong IW, Chiu B, Viira E, Jang D, Fong MW, Peeling R, Mahony JB. Can an antibiotic (macrolide) prevent Chlamydia pneumoniae-induced atherosclerosis in a rabbit model? Clin Diagn Lab Immunol. 1999;6(6):891–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rothstein NM, Quinn TC, Madico G, Gaydos CA, Lowenstein CJ. Effect of azithromycin on murine arteriosclerosis exacerbated by Chlamydia pneumoniae. J Infect Dis. 2001;183(2):232–8. [DOI] [PubMed] [Google Scholar]
- 81.Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. J Am Med Assoc. 1991;266(2):225–30. [PubMed] [Google Scholar]
- 82.Hahn DL. Chlamydia pneumoniae and asthma. Thorax. 1998;53(12):1095–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hahn DL, Anttila T, Saikku P. Association of Chlamydia pneumoniae IgA antibodies with recently symptomatic asthma. Epidemiol Infect. 1996;117(3):513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Von Hertzen L, Töyrylä M, Gimishanov A, Bloigu A, Leinonen M, Saikku P, Haahtela T. Asthma, atopy and Chlamydia pneumoniae antibodies in adults. Clin Exp Allergy. 1999;29(4):522–8. [DOI] [PubMed] [Google Scholar]
- 85.Cook PJ, Davies P, Tunnicliffe W, Ayres JG, Honeybourne D, Wise R. Chlamydia pneumoniae and asthma. Thorax. 1998;53(4):254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Von Hertzen L, Vasankari T, Liippo K, Wahlström E, Puolakkainen M. Chlamydia pneumoniae and severity of asthma. Scand J Infect Dis. 2002;34(1):22–7. [DOI] [PubMed] [Google Scholar]
- 87.Hahn DL, Schure A, Patel K, Childs T, Drizik E, Webley W. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS ONE. 2012;7(4):e35945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107(4):595–601. [DOI] [PubMed] [Google Scholar]
- 89.Emre U, Roblin PM, Gelling M, Dumornay W, Rao M, Hammerschlag MR, Schachter J. The association of Chlamydia pneumoniae infection and reactive airway disease in children. Arch Pediatr Adolesc Med. 1994;148(7):727–32. [DOI] [PubMed] [Google Scholar]
- 90.Emre U, Sokolovskaya N, Roblin PM, Schachter J, Hammerschlag MR. Detection of anti-Chlamydia pneumoniae IgE in children with reactive airway disease. J Infect Dis. 1995;172(1):265–7. [DOI] [PubMed] [Google Scholar]
- 91.Cunningham AF, Johnston SL, Julious SA, Lampe FC, Ward ME. Chronic Chlamydia pneumoniae infection and asthma exacerbations in children. Eur Respir J. 1998;11(2):345–9. [DOI] [PubMed] [Google Scholar]
- 92.Black PN, Scicchitano R, Jenkins CR, Blasi F, Allegra L, Wlodarczyk J, Cooper BC. Serological evidence of infection with Chlamydia pneumoniae is related to the severity of asthma. Eur Respir J. 2000;15(2):254–9. [DOI] [PubMed] [Google Scholar]
- 93.Schröder NW, Crother TR, Naiki Y, Chen S, Wong MH, Yilmaz A, Slepenkin A, Schulte D, Alsabeh R, Doherty TM, Peterson E, Nel AE, Arditi M. Innate immune responses during respiratory tract infection with a bacterial pathogen induce allergic airway sensitization. J Allergy Clin Immunol. 2008;122(3):595–602.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crother TR, Schröder NW, Karlin J, Chen S, Shimada K, Slepenkin A, Alsabeh R, Peterson E, Arditi M. Chlamydia pneumoniae infection induced allergic airway sensitization is controlled by regulatory T-cells and plasmacytoid dendritic cells. PLoS ONE. 2011;6(6):e20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Starkey MR, Essilfie AT, Horvat JC, Kim RY, Nguyen DH, Beagley KW, Mattes J, Foster PS, Hansbro PM. Constitutive production of IL-13 promotes early-life chlamydia respiratory infection and allergic airway disease. Mucosal Immunol. 2013;6(3):569–79. [DOI] [PubMed] [Google Scholar]
- 96.Starkey MR, Nguyen DH, Brown AC, Essilfie AT, Kim RY, Yagita H, Horvat JC, Hansbro PM. Programmed death ligand 1 promotes early-life chlamydia respiratory infection-induced severe allergic airway disease. Am J Respir Cell Mol Biol. 2016;54:493–503. [DOI] [PubMed] [Google Scholar]
- 97.Singh AK, Stock P, Akbari O. Role of PD-L1 and PD-L2 in allergic diseases and asthma. Am J Respir Cell Mol Biol. 2016;54(4):493–503. [DOI] [PubMed] [Google Scholar]
- 98.Cummins J, Tangney M. Bacteria and tumours: causative agents or opportunistic inhabitants? Infect Agent Cancer. 2013. March 28;8(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhan P, Suo LJ, Qian Q, Shen XK, Qiu LX, Yu LK, Song Y. Chlamydia pneumoniae infection and lung cancer risk: a meta-analysis. Eur J Cancer. 2011;47(5):742–7. [DOI] [PubMed] [Google Scholar]
- 100.Littman AJ, Jackson LA, Vaughan TL. Chlamydia pneumoniae and lung cancer: epidemiologic evidence. Cancer Epidemiol Biomark Prev. 2005;14(4):773–8. [DOI] [PubMed] [Google Scholar]
- 101.Chang AH, Parsonnet J. Role of bacteria in oncogenesis. Clin Microbiol Rev. 2010;23(4):837–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Braun J, Laitko S, Treharne J, Eggens U, Wu P, Distler A, Sieper J. Chlamydia pneumoniae—a new causative agent of reactive arthritis and undifferentiated oligoarthritis. Ann Rheum Dis. 1994;53(2):100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hannu T, Puolakkainen M, Leirisalo-Repo M. Chlamydia pneumoniae as a triggering infection in reactive arthritis. Rheumatology (Oxford). 1999;38(5):411–4. [DOI] [PubMed] [Google Scholar]
- 104.Gérard HC, Wang GF, Balin BJ, Schumacher HR, Hudson AP. Frequency of apolipoprotein E (APOE) allele types in patients with chlamydia-associated arthritis and other arthritides. Microb Pathog. 1999;26(1):35–43. [DOI] [PubMed] [Google Scholar]
- 105.Schumacher HR Jr, Gérard HC, Arayssi TK, Pando JA, Branigan PJ, Saaibi DL, Hudson AP. Lower prevalence of Chlamydia pneumoniae DNA compared with Chlamydia trachomatis DNA in synovial tissue of arthritis patients. Arthritis Rheum. 1999;42(9):1889–93. [DOI] [PubMed] [Google Scholar]
- 106.Gérard HC, Schumacher HR, El-Gabalawy H, Goldbach-Mansky R, Hudson AP. Chlamydia pneumoniae present in the human synovium are viable and metabolically active. Microb Pathog. 2000;29(1):17–24. [DOI] [PubMed] [Google Scholar]
- 107.Contini C, Grilli A, Badia L, Guardigni V, Govoni M, Seraceni S. Detection of Chlamydophila pneumoniae in patients with arthritis: significance and diagnostic value. Rheumatol Int. 2011;31(10):1307–13. [DOI] [PubMed] [Google Scholar]
- 108.MacIntyre A, Abramov R, Hammond CJ, Hudson AP, Arking EJ, Little CS, Appelt DM, Balin BJ. Chlamydia pneumoniae infection promotes the transmigration of monocytes through human brain endothelial cells. J Neurosci Res. 2003;71(5):740–50. [DOI] [PubMed] [Google Scholar]
- 109.MacIntyre A, Hammond CJ, Little CS, Appelt DM, Balin BJ. Chlamydia pneumoniae infection alters the junctional complex proteins of human brain microvascular endothelial cells. FEMS Microbiol Lett. 2002;217(2):167–72. [DOI] [PubMed] [Google Scholar]
- 110.Dreses-Werringloer U, Gérard HC, Whittum-Hudson JA, Hudson AP. Chlamydophila (Chlamydia) pneumoniae infection of human astrocytes and microglia in culture displays an active, rather than a persistent, phenotype. Am J Med Sci. 2006;332(4):168–74. [DOI] [PubMed] [Google Scholar]
- 111.Appelt DM, Roupas MR, Way DS, Bell MG, Albert EV, Hammond CJ, Balin BJ. Inhibition of apoptosis in neuronal cells infected with Chlamydophila (Chlamydia) pneumoniae. BMC Neurosci. 2008;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar A, Singh A, Ekavali E. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67(2):195–203. [DOI] [PubMed] [Google Scholar]
- 113.Hill JM, Clement C, Pogue AI, Bhattacharjee S, Zhao Y, Lukiw WJ. Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD). Front Aging Neurosci. 2014;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, Bullido MJ, Carter C, Clerici M, Cosby SL, Del Tredici K, Field H, Fulop T, Grassi C, Griffin WS, Haas J, Hudson AP, Kamer AR, Kell DB, Licastro F, Letenneur L, Lövheim H, Mancuso R, Miklossy J, Otth C, Palamara AT, Perry G, Preston C, Pretorius E, Strandberg T, Tabet N, Taylor-Robinson SD, Whittum-Hudson JA. Microbes and Alzheimer’s disease. J Alzheimers Dis. 2016;51(4):979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maheshwari P, Eslick GD. Bacterial infection and Alzheimer’s disease: a meta-analysis. J Alzheimers Dis. 2015;43(3):957–66. [DOI] [PubMed] [Google Scholar]
- 116.Dreses-Werringloer U, Bhuiyan M, Zhao Y, Gérard HC, Whittum-Hudson JA, Hudson AP. Initial characterization of Chlamydophila (Chlamydia) pneumoniae cultured from the late-onset Alzheimer brain. Int J Med Microbiol. 2009;299(3):187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Little CS, Hammond CJ, MacIntyre A, Balin BJ, Appelt DM. Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol Aging. 2004;25(4):419–29. [DOI] [PubMed] [Google Scholar]
- 118.Shima K, Kuhlenbäumer G, Rupp J. Chlamydia pneumoniae infection and Alzheimer’s disease: a connection to remember? Med Microbiol Immunol. 2010;199(4):283–9. [DOI] [PubMed] [Google Scholar]
- 119.Goldenberg MM. Multiple sclerosis review. P&T. 2012;37(3):175–84. [PMC free article] [PubMed] [Google Scholar]
- 120.Libbey JE, Cusick MF, Fujinami RS. Role of pathogens in multiple sclerosis. Int Rev Immunol. 2014;33(4):266–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bagos PG, Nikolopoulos G, Ioannidis A. Chlamydia pneumoniae infection and the risk of multiple sclerosis: a meta-analysis. Mult Scler. 2006;12(4):397–411. [DOI] [PubMed] [Google Scholar]
- 122.Lenz DC, Lu L, Conant SB, Wolf NA, Gérard HC, Whittum-Hudson JA, Hudson AP, Swanborg RH. A Chlamydia pneumoniae-specific peptide induces experimental autoimmune encephalomyelitis in rats. J Immunol. 2001;167(3):1803–8. [DOI] [PubMed] [Google Scholar]
- 123.Du C, Yao SY, Ljunggren-Rose A, Sriram S. Chlamydia pneumoniae infection of the central nervous system worsens experimental allergic encephalitis. J Exp Med. 2002;196(12):1639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schultz SH, North SW, Shields CG. Schizophrenia: a review. Am Fam Physician. 2007;75(12):1821–9. [PubMed] [Google Scholar]
- 125.Arias I, Sorlozano A, Villegas E, de Dios Luna J, McKenney K, Cervilla J, Gutierrez B, Gutierrez J. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res. 2012;136(1–3):128–36. [DOI] [PubMed] [Google Scholar]
- 126.Fellerhoff B, Laumbacher B, Wank R. High risk of schizophrenia and other mental disorders associated with chlamydial infections: hypothesis to combine drug treatment and adoptive immunotherapy. Med Hypotheses. 2005;65(2):243–52. [DOI] [PubMed] [Google Scholar]
- 127.Fellerhoff B, Wank R. Increased prevalence of Chlamydophila DNA in post-mortem brain frontal cortex from patients with schizophrenia. Schizophr Res. 2011;129(2–3):191–5. [DOI] [PubMed] [Google Scholar]
- 128.Fellerhoff B, Laumbacher B, Mueller N, Gu S, Wank R. Associations between Chlamydophila infections, schizophrenia and risk of HLA-A10. Mol Psychiatry. 2007;12(3):264–72. [DOI] [PubMed] [Google Scholar]
- 129.Park MH, Kwon YJ, Jeong HY, Lee HY, Hwangbo Y, Yoon HJ, Shim SH. Association between intracellular infectious agents and schizophrenia. Clin Clin Psychopharmacol Neurosci. 2012;10(2):117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ayaşlioğlu E, Düzgün N, Erkek E, Inal A. Evidence of chronic Chlamydia pneumoniae infection in patients with Behcet’s disease. Scand J Infect Dis. 2004;36(6–7):428–30. [DOI] [PubMed] [Google Scholar]
- 131.Imamura Y, Kurokawa MS, Yoshikawa H, Nara K, Takada E, Masuda C, Tsukikawa S, Ozaki S, Matsuda T, Suzuki N. Involvement of Th1 cells and heat shock protein 60 in the pathogenesis of intestinal Behcet’s disease. Clin Exp Immunol. 2005;139(2):371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abdulkarim AS, Petrovic LM, Kim WR, Angulo P, Lloyd RV, Lindor KD. Primary biliary cirrhosis: an infectious disease caused by Chlamydia pneumoniae? J Hepatol. 2004;40(3):380–4. [DOI] [PubMed] [Google Scholar]
- 133.Liu HY, Deng AM, Zhang J, Zhou Y, Yao DK, Tu XQ, Fan LY, Zhong RQ. Correlation of Chlamydia pneumoniae infection with primary biliary cirrhosis. World J Gastroenterol. 2005;11(26):4108–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Montaño-Loza A, Vázquez-Ballesteros E, Meza-Junco J, Villalobos-Zapata I, Olivera-Martínez M. Seropositivity for Chlamydia pneumoniae in patients with primary biliary cirrhosis. Gastroenterol Hepatol. 2006;29(3):113–7. [DOI] [PubMed] [Google Scholar]
- 135.Marangoni A, Donati M, Cavrini F, Aldini R, Accardo S, Sambri V, Montagnani M, Cevenini R. Chlamydia pneumoniae replicates in Kupffer cells in mouse model of liver infection. World J Gastroenterol. 2006;12(40):6453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fernández-Real JM, López-Bermejo A, Vendrell J, Ferri MJ, Recasens M, Ricart W. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29(5):1058–64. [DOI] [PubMed] [Google Scholar]
- 137.Wang C, Gao D, Kaltenboeck B. Acute Chlamydia pneumoniae reinfection accelerates the development of insulin resistance and diabetes in obese C57BL/6 mice. J Infect Dis. 2009;200(2):279–87. [DOI] [PubMed] [Google Scholar]
- 138.Rodriguez AR, Plascencia-Villa G, Witt CM, Yu JJ, José-Yacamán M, Chambers JP, Perry G, Guentzel MN, Arulanandam BP. Chlamydia pneumoniae promotes dysfunction of pancreatic β cells. Cell Immunol. 2015;295(2):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]