Summary
Background:
Full-length tissue factor (flTF) and alternatively spliced TF (asTF) contribute to growth and spread of pancreatic ductal adenocarcinoma. It is unknown, however, if flTF and/or asTF contribute to pathobiology of pancreatic neuroendocrine tumors (pNET).
Objective:
To assess TF expression in pNET and the effects of mTOR complex 1/2 (mTORC1/2) inhibition on pNET growth.
Methods:
Human pNET specimens were immunostained for TF. Human pNET cell lines QGP1 and BON were evaluated for TF expression and responsiveness to mTOR inhibition. shRNA were used to knock down TF in BON. TF cofactor activity was assessed using a two-step FXa generation assay. TF promoter activity was assessed using transient transfection of human TF promoter-driven reporter constructs into cells. Mice bearing orthotopic BON tumors were treated with the mTORC1/2 ATP-site competitive inhibitor sapanisertib / MLN0128 (3 mg/kg, oral gavage) for 34 days.
Results:
Immunostaining of pNET tissue revealed flTF and asTF expression. BON and QGP1 expressed both TF isoforms, with BON exhibiting higher levels. shRNA directed against TF suppressed BON proliferation in vitro. Treatment of BON with sapanisertib inhibited mTOR signaling and suppressed TF levels. BON tumors grown in mice treated with sapanisertib had significantly less TF protein and co-factor activity, and were smaller compared to tumors grown in control mice.
Conclusions:
TF isoforms are expressed in pNET. Sapanisertib suppresses TF mRNA and protein expression as well as TF co-factor activity in vitro and in vivo. Thus, further studies are warranted to evaluate the clinical utility of TF-suppressing mTORC1/2 inhibitor sapanisertib in pNET management.
Keywords: Carcinoma, Neuroendocrine, MLN0128, mTOR protein, human, Pancreatic Neoplasms, Tissue Factor
Introduction
Pancreatic neuroendocrine tumors (pNETs) arise from endocrine cells in the islets of Langerhans, characterized by expression of secretory proteins such as chromogranin A, synaptophysin, and enolase [1]. ≥80% of pNETs produce active hormone(s) characteristic of their cell type of origin [2]. FDA-approved treatment options for pNETs include everolimus (RAD001) targeting the mammalian target of rapamycin (mTOR) signaling pathway. Depending on its binding partners, the kinase mTOR is present in two complexes: mTORC1 and mTORC2. Everolimus is an allosteric inhibitor of mTORC1 which extends progression-free survival in gastrointestinal and lung NET patients [3]. However, everolimus treatment results in the activation of Akt and it incompletely inhibits a number of mTORC1 substrates, including p-4E-BP1, which can lead to increased cell survival and proliferation in some settings [4,5]. To address this problem, a second generation of mTOR kinase inhibitors (mTORKi) have been developed that bind to the ATP-site of mTOR. Among these, the drug sapanisertib (MLN0128) has been shown to inhibit the growth of numerous malignancies, including those of the gastrointestinal tract, and is currently being evaluated in clinical trials (clinicaltrials.gov identifiers NCT02893930 and NCT02465060) [6,7].
Full-length tissue factor (flTF) is a 47 kDa integral membrane protein that serves as the obligatory co-factor for the serine protease factor (F)VII(a). The flTF/FVIIa complex initiates coagulation. In cancer, flTF/FVIIa promotes tumor growth in part via activation of protease activated receptors (PARs) on cell surfaces [8]. The minimally coagulant, alternatively spliced TF isoform (asTF) is formed via skipping of exon 5 during TF (F3) pre-mRNA processing; a unique C-terminus in the asTF protein renders it soluble. asTF can promote growth and spread of cancer cells via binding and conformational activation of β1 integrins [9,10]. Expression of both TF isoforms is upregulated in pancreatic ductal adenocarcinoma (PDAC) [11–13]. flTF present on microvesicles released into the circulation from tumor cells is associated with thrombosis in PDAC [14]. Elevated plasma asTF is also associated with a more aggressive disease phenotype in PDAC [15]. It is not known if either TF isoform is expressed in pNETs.
Here, we report the expression of both TF isoforms in pNET. We demonstrate that inhibition of mTOR using sapanisertib causes reduction in TF expression, decrease in TF co-factor activity, and suppression of pNET tumor growth in vivo.
Materials and methods
Cell lines
BON [16], a gift from Dr. Courtney Townsend (UTMB Texas), and QGP1 are human pNET cell lines that have been characterized and possess mutations in ATRX, TP53, and various MUC genes; BON contain mutations in both TSC2 and NRAS, while QGP1 harbors a mutation in KRAS [17]. BON recapitulate the features of human pNET when implanted orthotopically in nude mice, including metastatic spread and cardiac carcinoid syndrome [18,19]. BON were maintained in DMEM:F12 (50:50) and QGP1 were maintained in RPMI. BON-pRetroX and BON-asTF are stably transfected cell lines generated by transducing BON with a lentivirus carrying either an empty pRetroX-TetOne™ vector (Clontech), or pRetroX-TetOne™ vector with a doxycycline (DOX)-inducible asTF expression cassette, respectively. Lentiviral production and shRNA studies are detailed in Data S1.
Reagents
Sapanisertib (MLN0128, INK128) and everolimus (RAD001) were purchased from LC Laboratories. Anti- human integrin β1/CD29 monoclonal antibody was purchased from R&D Systems (Cat. No. MAB1778, Clone 4B7R). The anti-TF monoclonal rabbit antibody, RabMab95, and anti-asTF monoclonal rabbit antibody RabMab1 have been characterized previously [9]. Unless otherwise mentioned, all other antibodies were purchased from Cell Signaling Technologies. Our custom asTF sandwich ELISA utilizes RabMab95 as the capture antibody, and RabMab1 as the detection antibody, as described [11].
Immunohistochemistry
Serial sections of formalin-fixed, paraffin embedded tissue specimens were obtained from BioMax (Cat No. PA806). Orthotopically grown BON tumors were harvested and processed as described [10]. Previously characterized polyclonal antibodies directed against flTF- and asTF-unique epitopes [20] were used as primary antibodies. Please see Data S1 regarding section processing.
qRT-PCR
Primers and probe sets for flTF, asTF, and GAPDH have been described elsewhere [21]. Total TF mRNA levels were assessed using a custom probe/primer set that produces an amplicon spanning exons 3 and 4; qRT-PCR was performed using Bio-Rad CFX96 under the following conditions: 50˚C, 2 min; 95˚C, 10 min; 41 cycles 95˚C, 15 sec, 57˚C, 1 min. Sequences are as follows: forward primer, 5’-CCAGAGTTCACACCTTACCTG-3’; reverse primer, 5’- AGACAAACCTCGGACAGCCAACA-3’; probe, 5’- CATTCACTTTTGTTCCCACCTG-3’.
Western blotting
Please see Data S1 for a list of western blotting reagents and the description of experimental procedures.
Transient transfection assay
F3 promoter-driven luciferase reporter plasmids pGL2–2106hTF-luc, pGL2–111hTF-luc, and pGL2–111hTFMt2-luc [22] were labelled with Cy5 using Mirus Label IT nucleic acid labeling kit (MIR 3725) according to the manufacturer’s recommendations. Following 24 hours of drug treatment, BON cells were transfected with unlabeled and labeled plasmid at a 10:1 ratio using FuGENE HD (Promega). The following day, cells were trypsinized and counted. For each treatment group, 6 × 105 cells/well were seeded into 3 wells of a 96-well plate. Bright-glo was then added and luminescence measured; remaining cells were assessed by flow cytometry whereby Cy5 intensity served as a measure of transfection efficiency.
Two-step FXa generation assay
1.8 × 104 intact, non-permeabilized cells were washed and incubated with 15 nM FVIIa and 150 nM FX (Enzyme Research Laboratories) at 37˚C for 15 minutes. Subsequently, CaCl2 was added to initiate FXa generation. The samples were incubated for another 15 minutes at 37˚C after which time the reaction was halted by the addition of EDTA-Bicine. Pefachrome FXa (5-Diagnostics P085–06-25) was then added and the OD405 recorded every 5 minutes for 1 hour using a VersaMax microplate reader (Molecular Devices). To assess TF activity in tumor specimens, frozen tissue was crushed in liquid nitrogen and then added to a buffer (10 mM HEPES, pH 7.4, 140 mM NaCl) containing 2 µM of E-64. Lysates were prepared via freeze-thaw on dry ice, and homogenized by passing through a 28G needle. Protein concentration was determined using BCA. 30 µg total protein per sample were used to perform two-step FXa assay as above.
Animal studies
All in vivo studies were approved by the Institutional Animal Care and Utilization Committee, University of Cincinnati. Athymic nude mice were purchased from Jackson Laboratories (007850). Mice were anesthetized with isoflurane; buprenorphine (0.1 mg/kg) was administered prior to surgery and once every twelve hours following surgery for 3 days. 1 × 106 BON cells were injected into the tails of pancreases via a midline laparotomy incision. Treatment with either sapanisertib (3 mg/kg) or vehicle (15% polyvinyl pyrrolidone; 5% 1-methyl-2-pyrrolidone) began 15 days post-surgery. Mice were treated every other day for 35 days and then euthanized. Tumors were excised and assessed for weight and volume. A portion of the tumor was snap-frozen in liquid nitrogen and the remainder fixed in 10% neutral buffered formalin. At euthanasia, whole blood was collected via cardiac puncture into 1.6 mg/mL EDTA, centrifuged to obtain platelet poor plasma, and snap frozen. Mice were fed Rodent Diet 2018 (with or without 635 mg/kg doxycycline, Envigo).
Enzyme-Linked Immunosorbent Assay (ELISA)
Plasma samples were assayed for human asTF as described [23]. Briefly, plasma was added to 96-well plates coated with a rabbit monoclonal anti-TF antibody, incubated for 2 hours, and probed with asTF-specific antibody.
Statistics
When comparing two groups, Student’s t-test was used. When comparing >2 groups, mean values were analyzed via multiple group comparisons using either one-way or two-way ANOVA where appropriate. p ≤ 0.05 was considered significant. All statistical analyses were performed using GraphPad Prism v6.03. Data are presented as mean ± standard deviation.
Results
TF isoforms are expressed in pNET tumors and cultured cells
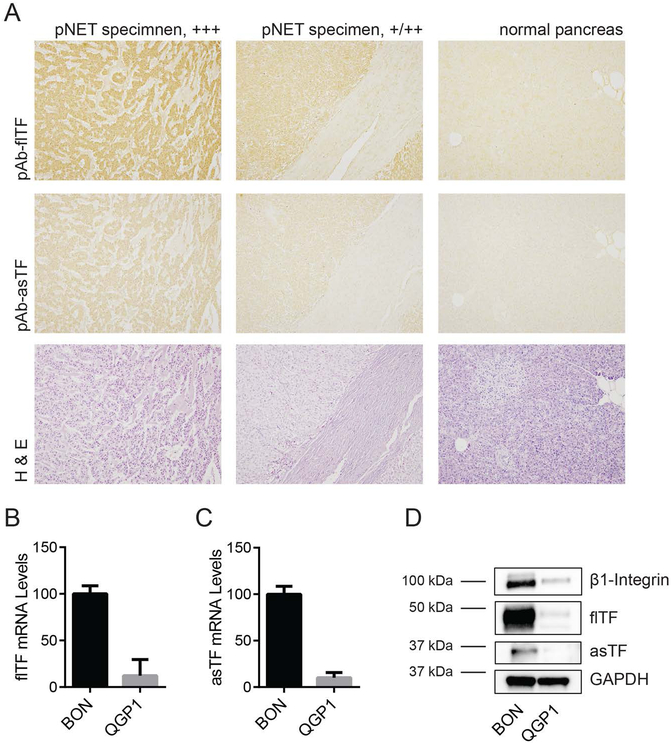
Previously, our laboratory has demonstrated an increase in the expression of asTF in PDAC compared with normal pancreas tissue [11]; high levels of flTF in PDAC tissue have been reported by other groups [12,13]. Here, we examined the expression of both TF isoforms in pNET. A tissue microarray containing 6 human pNET specimens along with 15 normal pancreas tissue specimens was stained for flTF and asTF. 4 pNET specimens scored highly positive for flTF as well as asTF, and the remaining 2 scored moderately positive; normal pancreas tissue exhibited only minimal staining for flTF and asTF (Fig. 1A).
Figure 1:
Pancreatic neuroendocrine tumors express TF isoforms. (A) Tissue microarray containing 6 human pNET specimens was immunostained for flTF and asTF. Shown are representative images of tumors staining highly positive (+++) and moderately positive (+/++) for flTF and asTF. Corresponding H&E stain is shown below. (B and C) Relative mRNA expression of flTF (B) and asTF (C) in BON and QGP1. Expression is shown as % of expression in BON. Combined results of 3 independent experiments. (D) Western blots of BON and QGP1 lysates. Representative of 3 independent experiments.
Two human pNET cell lines, BON and QGP1, were assessed for the expression of TF isoforms. qRT-PCR revealed that compared to QGP1, BON express ~10 times higher levels of flTF and asTF mRNA (Figs. 1B and C). Protein levels were in agreement with the mRNA data (Fig. 1D). Compared to the human PDAC cell line Pt45.P1, BON express significantly fewer copies of mRNA encoding both TF isoforms (Figs. S1A and B); protein levels were in agreement with the mRNA data (Fig. S1C). We also found that BON and QGP1 express β1-integrin chain that, in PDAC cells, facilitates asTF-mediated migration [24]; however, the level of α6-integrin chain, also critical for asTF-mediated PDAC cell migration, was low in BON (Fig. S1C). Given that BON express TF as well as β1-integrin at robust levels, we elected to focus on this cell line in our studies.
TF suppression inhibits growth in BON
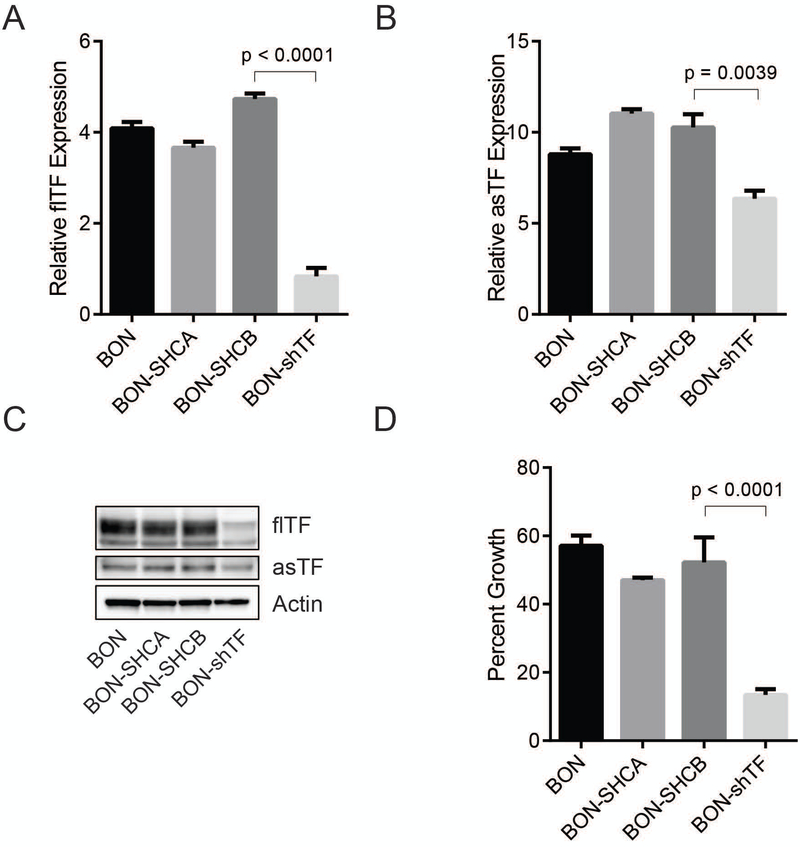
Little is known about the levels of and functional significance of TF in pNET. We made use of publicly available RNA-Seq datasets of human pNET [25] and determined that F3 belongs to the top 200 genes overexpressed in pNET tissue compared to normal endo pancreas; notably, clustering for F3 expression revealed a strong association with genes involved in cell cycle control, as well as cell migration (Fig. S2, Table S1). Thus, we sought to determine whether a reduction in TF expression impacts BON phenotype. To do so, BON cells were transduced with either a lentiviral vector encoding a short-hairpin construct directed against F3, or one of two non-targeting short-hairpin controls. qPCR analysis of these cells showed a significant reduction of both TF mRNA isoforms in BON-shTF compared to the parental control and non-targeting control cells (Figure 2A and B). The reduction of TF protein expression in BON-shTF was confirmed by western blot (Figure 2C). A proliferation assay was then performed, revealing a 74.3% ±3.2% reduction in growth of BON-shTF cells after 48 hours compared to control cells (Figure 2D).
Figure 2:
shRNA knockdown of F3 mRNA suppresses BON proliferation. BON were stably transduced with shTF or non-targeting short-hairpin control vectors (SHCA and SHCB). (A) Relative flTF mRNA levels of transduced cells and BON parental cells. (B) Relative asTF mRNA levels and BON parental cells. (C) Western blots of cell lysates. (D) 48 hour proliferation assay. Representative of 3 independent experiments.
mTOR inhibitors reduce TF expression and pro-coagulant activity in BON
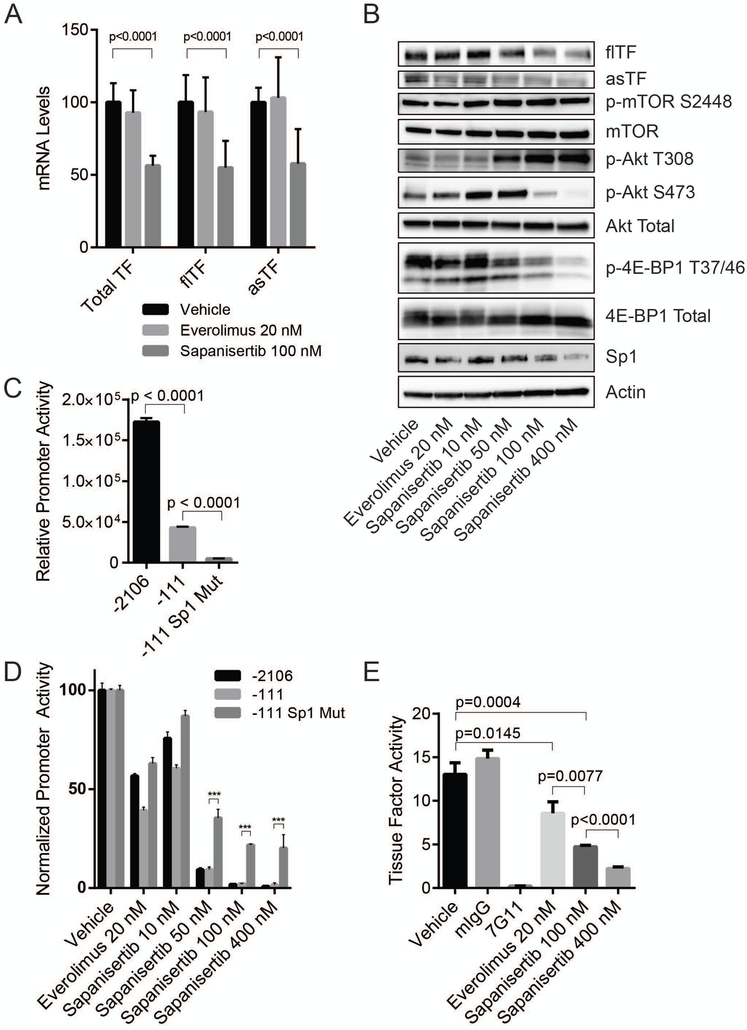
Both TF isoforms take part in extracellular signaling processes which promote proliferation, migration, and angiogenesis through activation of mitogen-activated protein kinases (MAPKs) and Akt. Cleavage of PAR2 by the flTF-FVIIa complex can lead to the activation of all three major MAPKs as well as the PI3K-Akt pathway [8], while asTF-mediated signaling occurs through its interaction with β1 integrins leading to the activation of Erk1/2, p38-MAPK, and Akt [9,11]. Given that mTOR inhibitors were recently demonstrated to synergize with MAPK/ERK inhibitors [26,27] and MAPK/ERK inhibitors are known to suppress TF protein expression [28], we sought to determine if everolimus and/or sapanisertib impact the expression levels of TF. qRT-PCR analysis of BON treated for 48 hours with either 20 nM everolimus or 100 nM sapanisertib revealed that sapanisertib, but not everolimus, decreased TF mRNA expression (Fig 3A). Western blotting revealed a decrease in the protein levels of flTF and asTF in response to sapanisertib (Fig.3B). Sapanisertib caused a concentration-dependent suppression of 4E-BP1 and Akt activation indicative of a suppression of mTORC1/2 activity. (Fig. 3B). Sapanisertib also caused in increase in Akt phosphorylation at T308 due to relief of mTORC1-mediated feedback inhibition of receptor tyrosine kinase activity.
Figure 3:
Sapanisertib decreases expression of TF isoforms and TF activity in BON. (A) Relative total TF mRNA expression as well as flTF and asTF isoform specific relative mRNA levels in response to 48 hour treatment with either 20 nM everolimus or 100 nM sapanisertib. Expression is shown as % of vehicle control expression levels. Combined results of 3 independent experiments. (B) Western blots of BON lysates from cells treated with vehicle control, 20 nM everolimus, or sapanisertib (10, 50 100, or 400 nM) for 48 hours. (C) Relative luminescence produced using three F3 promoter reporter constructs: pGL2–2106hTF, pGL2–111hTF, and pGL2–111hTF-Sp1-Mut (Denoted as −2106, −111, and −111 Sp1 Mut, respectively). Representative of 3 independent experiments. ***p<0.0001. (D) Relative luminescence of cells transfected with TF promoter luciferase reporter constructs following 24 hours of pretreatment with vehicle control, 20 nM everolimus, or sapanisertib (10, 50, 100, or 400 nM). Luminescence measured 24 hours post-transfection. Representative of 3 independent experiments. (E) TF activity of 2×104 intact cells pretreated with 20 nM everolimus, 100 nM sapanisertib, 400 nM sapanisertib, or vehicle control for 48 hours. Vehicle control cells were incubated for 30 min with either 7G11 or murine isotype control antibody. Representative of 3 independent experiments.
Given that human F3 promoter contains Sp1, AP1, and NF-κB cis-acting elements [29], we sought next to determine if changes in expression and/or functional status of these transcription factors may contribute to TF suppression when mTORC is inhibited. No changes in c-jun or JunB were observed, and there was no decrease in the activation of NF-κB (data not shown); however, sapanisertib did reduce levels of Sp1 protein (Fig. 3B).
To further investigate if inhibition of F3 transcription may contribute to a decrease in TF expression, we employed a panel of well–characterized human F3 promoter luciferase reporter constructs: pGL2–2106hTF-luc which contains 2106 bp of the F3 promoter, and pGL2–111hTF-luc contains only the core promoter; in the construct termed pGL2–111hTF-Sp1mut, the three Sp1 sites are mutagenized [22]. BON cells were transfected with all three constructs. Compared to pGL2–2106hTF-luc, the construct pGL2–111hTF-luc produced ~25% of luciferase activity whereas pGL2–111hTF-Sp1mut produced ~3% (Fig 3C). BON were treated with either vehicle control, everolimus 20 nM or sapanisertib (10, 50, 100, or 400 nM) and 24 hours later, transfected with TF promoter constructs. The following day, luminescence was measured in an equal number of treated cells. Increasing sapanisertib concentrations yielded a dose-dependent decrease in luciferase levels for all constructs (Fig. 3D). Notably, at concentrations of 50 nM and higher of sapanisertib, pGL2–111hTF-Sp1mut was significantly less sensitive to the drug’s effects.
As mentioned, cofactor activity of flTF contributes to cancer pathobiology and its co-morbidities yet indiscriminate targeting of flTF’s function is risky due to a high potential of bleeding complications; hence, there is considerable interest in decreasing flTF levels and activity specifically in tumor tissue but not in normal tissue [30]. Our findings that mTORKi decrease flTF levels are potentially valuable in this regard, because flTF is the pro-coagulant TF form. Using a two-step FXa generation assay, we determined that pre-treating BON for 48 hrs. with either everolimus or sapanisertib significantly decreased the levels of TF co-factor activity on intact cell surfaces (Fig. 3E). Pre-incubation with 7G11, an inhibitory antibody which prevents flTF complexation with FVII(a) [31], resulted in complete abrogation of FXa generation, indicating that FX to FXa conversion was TF specific. While everolimus reduced TF activity by 34%, treatment with sapanisertib produced a more robust dose-dependent suppression of TF activity by 64% and 83% at concentrations of 100 nM and 400 nM, respectively.
Overexpression of asTF in BON does not impact the effects of sapanisertib
To investigate the potential significance of tumor-derived asTF in the pNET setting, we generated a cell line termed BON-asTF, capable of DOX-inducible asTF overexpression. qRT-PCR analysis revealed that when induced with DOX, BON-asTF expressed ~20 times more asTF than un-induced BON-asTF and BON-pRetroX control cells (Fig. S3A). Overexpression of asTF did not influence the levels of flTF (Figs. S3B and C). asTF overexpression yielded no increase in pro-migratory ability in a transwell migration assay (Fig. S3D). To validate the pRetroX expression system, Pt45.P1 cells, a well-characterized PDAC cell line [11], were transduced with either pRetroX-asTF or empty vector control. As expected, Pt45.P1-asTF induced with 20 ng/mL DOX demonstrated a significant increase in migration compared to non-induced Pt45.P1 cells (Fig S3D). Lack of asTF effect on BON migration could be due to relatively low levels of α6-integrin expression in these cells (Fig. S1C). When grown in vivo in an orthotopic setting, BON-pRetroX cells yielded tumors with comparable volume irrespective of DOX in the chow whereas BON-asTF cells, somewhat unexpectedly, yielded ~40% smaller tumors in mice fed DOX chow (Figs. S4A and B). While BON-asTF +DOX tumors were smaller, immunohistochemical analysis of tumor sections (CD31 staining) revealed BON-asTF +DOX tumors were significantly more vascular than BON-pRetroX ± DOX and BON-asTF mice which received control diet (Figs. S4C and D).
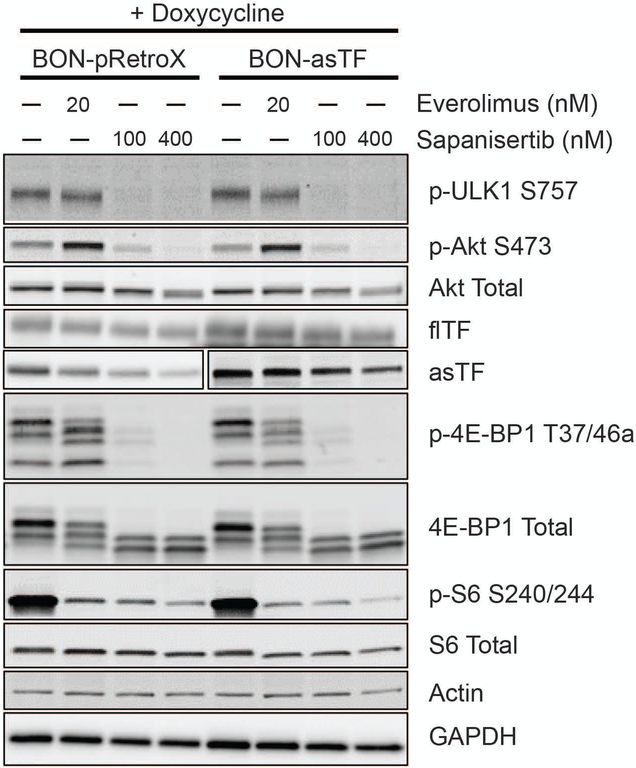
Given that asTF:α6β1 can activate Akt, we wished to ascertain if the impact of mTORKi on the downstream targets is altered in the context of asTF overexpression. asTF overexpression did not lead to detectable changes in basal mTOR signaling and/or response to mTORKi, as measured by phosphorylation levels of downstream substrates including p-4E-BP1 or p-ULK1, or by S6K1-mediated phosphorylation of ribosomal protein S6 (p-S6), an indicator of mTORC1 activation (Fig. 4). In BON-asTF, mTORKi led to a significant decrease in the levels of asTF and flTF. Consistent with the mTOR signaling data, there was no significant change in the proliferation rate of BON-asTF irrespective of DOX treatment (Fig. S5). Moreover, as previously reported [5], the decrease in proliferation due to mTORKi appeared to correlate with p-4EBP1 levels (Fig. 4). We note that overexpression of asTF in our system is driven by CMV promoter, which is Sp1-dependent [32].
Figure 4:
Everolimus and sapanisertib decrease TF expression in BON-asTF. BON-pRetroX and BON-asTF were induced with DOX for 48 hours prior to treatment with 20 nM everolimus, 100 nM sapanisertib, 400 nM sapanisertib, or vehicle control. asTF blot is split to show different exposures due to the overexpression of asTF in induced BON-asTF. Representative of 3 independent experiments.
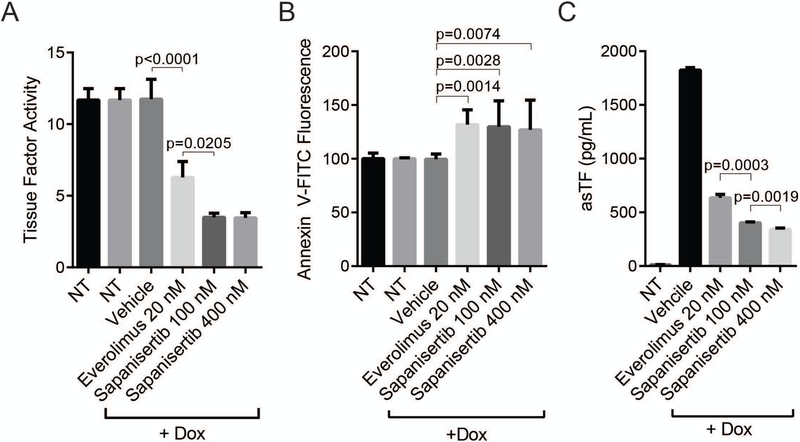
Overexpression of asTF did not impact the ability of sapanisertib to decrease cell surface TF activity in BON; however, dose response to sapanisertib was no longer observed (Fig. 5A). Because flTF requires surface phosphatidylserine (PS) to exert its co-factor activity [33], we measured surface PS levels (Fig. 5B). While both everolimus and sapanisertib caused a slight but significant increase in surface PS levels, the difference in the magnitude to which TF activity was suppressed by everolimus and sapanisertib (Fig. 5A) did not follow surface PS levels, suggesting that surface PS levels were not rate-limiting and that the decrease in TF activity post-mTORKi is mediated by transcriptional and translational regulation of TF. mTORKi were also able to significantly reduce the secretion of asTF in DOX induced BON-asTF cells compared to untreated cells (Fig. 5C).
Figure 5:
Everolimus and sapanisertib decrease TF activity and asTF secretion in BON-asTF. (A) Tissue factor activity of DOX-induced BON-asTF following 48 hrs. treatment with 20 nM everolimus, 100 nM sapanisertib, 400 nM sapanisertib, or vehicle control. Representative of 3 independent experiments. (B) Annexin V staining of DOX-induced BON-asTF cells following 48 hrs. treatment with 20 nM everolimus, 100 nM sapanisertib, 400 nM sapanisertib, or vehicle control. Combined results of 4 independent experiments. (C) ELISA of asTF using media collected from DOX-induced BON-asTF treated with 20 nM everolimus, 100 nM sapanisertib, 400 nM sapanisertib, or vehicle control.
Sapanisertib suppresses BON tumor growth and TF activity in an orthotopic setting.
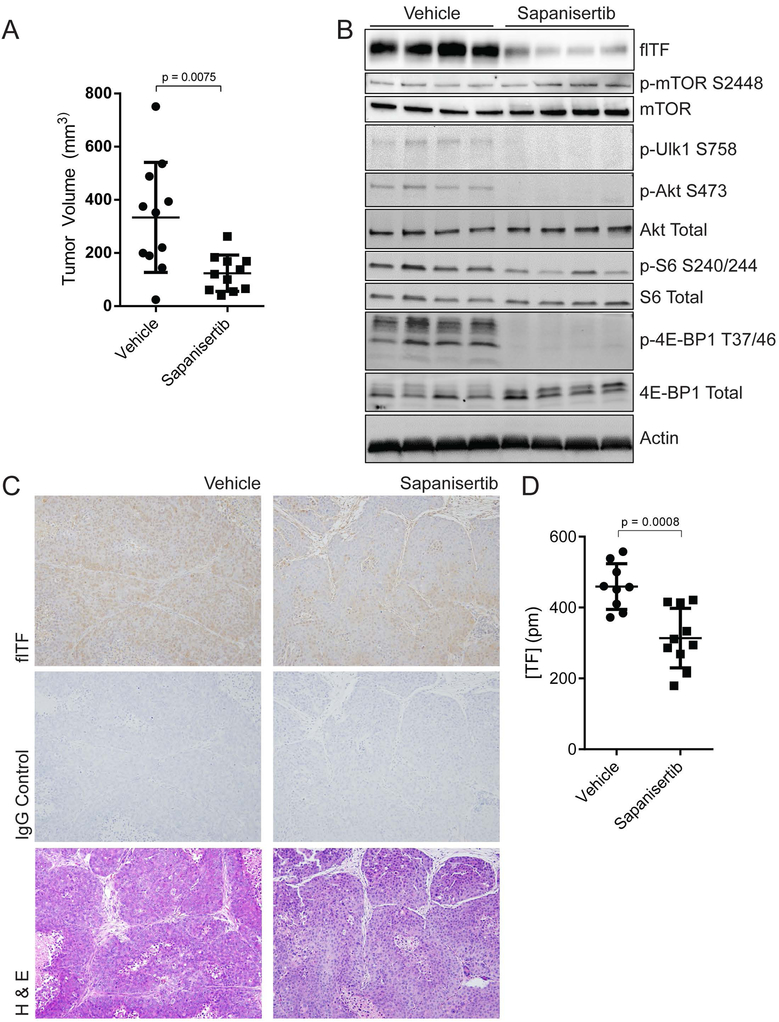
To investigate the anti-tumor activity of sapanisertib in vivo, we orthotopically implanted 1 × 106 BON cells into the pancreases of nude mice. Mice treated with sapanisertib bore smaller tumors than those receiving vehicle control (Fig. 6A). In agreement with in vitro data, western blot analyses of tumor lysates demonstrated that sapanisertib potently reduced mTORC1/2 activation (Fig. 6B). TF protein and activity levels were both significantly decreased by sapanisertib (Figs. 6C and D). We also observed a trend toward a decrease in circulating asTF levels in the plasma collected at sacrifice (Fig. S6). Plasma levels of thrombin-antithrombin and MV-TF activity were not different between the treatment groups (data not shown); in this regard, we note that BON cells form tumors that are much less vascular compared to those formed by PDAC cell line Pt45.P1 as assessed by CD31 staining (0.41±0.20% vs 0.93±0.31% staining per field of view, p = 0.0011).
Figure 6:
Sapanisertib suppresses pNET tumor growth in vivo. BON cells were grown in the pancreases of nude mice for 15 days, followed by 34 days of treatment with 3mg/kg sapanisertib or vehicle. (A) End-of-study volumes of excised tumors. (B) Western blots of representative tumor lysates. (C) Representative images, immunohistochemical analysis of tumor sections probed with polyclonal antibody directed against flTF. (D) Two-step Factor Xa generation assay with tumor lysates normalized to protein content.
Discussion
In this study, we describe the expression and co-factor activity of TF in human pNET tissue and cultured cells, raising the possibility that TF contributes to pathobiology of pNET as discussed below.
High TF expression is a well-documented feature of PDAC and correlates with increased risk for venous thromboembolism (VTE) [12,34] and poorer prognosis [13,35,36]. In contrast, little is known about TF expression in the context of pNET. It is well known, however, that TF is constitutively expressed in the islets of Langerhans. Previously, we have shown that islets, in a phenotypically normal tissue adjacent to PDAC, stain positive for TF isoforms [11]. Additionally, islet TF expression is the major contributor to instant blood-mediated inflammatory reaction (IBMIR) leading to the rejection of the majority of clinically transplanted islets [37,38]. Here, we report for the first time the expression of TF in pNETs.
Up to 90% of all PDAC tumors possess constitutively activating mutations in KRAS [39], while such mutations are rare in pNET [40,41]. This may be relevant to asTF mediated signaling in a cancer setting, as most of the cell lines that were responsive to asTF in vitro and in vivo contained gain-of-function KRAS mutations (MiaPaCa-2, Capan-1, Pt45.P1, MDA-MB-321) [9,11,42]. In colorectal cancer cells, constitutively active KRAS has been shown to promote TF expression through PI3K and MAPK pathways, which are believed to be among the main effectors of KRAS-driven oncogenesis [28]. However, the impact of TF overexpression in the context of wild-type KRAS remains unclear. Here, we show that overexpression of asTF does not promote proliferation and/or migration of BON cells, which contain wild type KRAS. It should be noted, however, that, asTF is able to promote vessel formation without causing an increase in the proliferation of endothelial cells [24], and pNETs are normally highly vascularized tumors [43]. While BON are able to recapitulate the carcinoid syndrome characteristic for pNET when grown in nude mice [18], these cells form hypovascular tumors which likely account for the lack of MV-TF activity in the plasma of tumor-bearing mice. Even though overexpression of asTF did, as expected, result in an increased vascularization of BON tumors, the tumors themselves were smaller which likely points to an autocrine effect of asTF distinct from what was observed thus far in breast cancer and/or pancreatic ductal adenocarcinoma cells [9–11]. Engagement of the same ligand by different integrin heterodimers can trigger distinct signaling in the cell and, conversely, the same integrin heterodimers can differentially control intracellular signaling pathways in distinct cell subtypes [44]. Further studies are required to elucidate the mechanistic underpinnings of asTF’s effects on BON cell growth in vivo.
Both TF isoforms contribute to angiogenesis in various settings [45]. While a causal role for TF in pNET vascularity has yet to be ascertained, here we show that shRNA suppression of TF causes severe suppression in cell proliferation. We also show that mTORKi using sapanisertib suppresses TF expression and activity in vitro. Additionally, we showed that both everolimus and sapanisertib suppress F3 promoter activity and that this effect is muted when the three Sp1 sites in the F3 promoter are mutagenized. Consistent with this data, we found that mice bearing orthotopic BON tumors treated with sapanisertib had smaller tumors that expressed less TF and thus harbored less TF cofactor activity. Given that mTORc1/2 control multiple aspects of cell growth and metabolism, TF suppression is not likely to be the sole contributor to sapanisertib’s anti-tumor effects in vivo. However, these findings further bolster sapanisertib’s potential utility in pNET treatment, especially with the currently ongoing phase II clinical trial with sapanisertib in patients with metastatic or refractory pNETs (NCT02893930). Additionally, these drugs may have potential utility in the suppression of IBMIR. Previously it has been shown that transfection of porcine islets with TF anti-sense RNA suppress IBMIR in an in vitro model [46]. In this light, pretreatment of donor islet cells prior to islet transplantation with sapanisertib may also suppress IBMIR and this approach should, therefore, be further investigated.
Little is known concerning the relationship between mTORC1/2 and TF. Previously it has been reported that the allosteric inhibitor of mTORC1, rapamycin, enhances TF expression by human umbilical vein endothelial cells in the presence of VEGF and mice bearing orthotopic PDAC tumors had increased intratumoral microthrombi when treated with rapamycin [47]. Rapamycin has also been reported to enhance TF expression in human aortic endothelial cells, but not human vascular smooth muscle cells. This is of particular concern in patients with rapamycin-eluting stents, which may contribute to the risk of in-stent thrombosis [48]. It was also shown that the TF-FVIIa-FXa complex induces a PAR1/2 mediated activation of the mTOR pathway in Adr-MCF-7 cells leading to enhanced cell migration, which is diminished by pretreatment with rapamycin [49]. While TF expression in response to rapamycin was not directly examined in the aforementioned study, these findings are in agreement with ours in that mTORKi suppresses TF levels and co-factor activity. Here we have shown a link between mTORC1/2 inhibition and a strong suppression of F3 promoter activity, suggesting a role for mTOR in the regulation of TF expression in endocrine cells of the pancreas.
While it has been repeatedly reported that Sp1 contributes to gene expression control of mTOR pathway components [50–52], little is known about how the mTOR pathway may in turn control expression levels of Sp1. The transcription of Sp1 is controlled by Sp1 itself along with Sp3, E2F and NF-Y[53]. Rapamycin has been shown to suppress E2F transcriptional activity in T lymphocytes due to the suppression of p70s6k [54]. Additionally, Akt activation has been shown to correlate with NF-Y DNA-binding and cell cycle progression in PC12 cells [51]. Therefore, it is likely mTORc1/2 inhibition leads to a suppression of Sp1 transcription. However, the exact mechanism behind sapanisertib mediated suppression of Sp1 requires further investigation.
In summary, here we report that human pNET express both TF isoforms which contribute to the growth of cells derived from human pNET; inhibition of mTORC1/2 suppresses F3 promoter activity and TF expression in these cells, leading to a reduction in TF co-factor activity. Further evaluation of sapanisertib for pNET management is thus highly warranted.
Supplementary Material
Essentials.
Tissue factor (TF) isoforms are expressed in pancreatic neuroendocrine tumors (pNET)
TF knockdown inhibits proliferation of human pNET cells in vitro
mTOR kinase inhibitor sapanisertib / MLN0128 suppresses TF expression in human pNET cells
Sapanisertib suppresses TF expression and activity and reduces the growth of pNET tumors in vivo
Acknowledgements
This work was partially supported by the NIH (grant 1R01-CA190717–01A0 to V.Y. Bogdanov) and the John C. Parker Professorship (N. Mackman). Y. Hisada is supported by T32-HL007149. The authors are grateful to Dr. Courtney Townsend (University of Texas Medical Branch) for providing the BON cell line, and Dr. Daniel Kirchhofer (Genentech) for providing anti-human TF monoclonal antibody 7G11.
Footnotes
Disclosure of Conflict of Interest
The authors state that they have no conflict of interest.
Addendum
C.S. Lewis designed and performed experiments, interpreted data, and wrote the manuscript. H.E. Thomas designed and performed experiments, interpreted data, and edited the manuscript. M. Orr-Asman, L.C. Green, R.E. Boody, K. Matiash, A. Karve, Y. Hisada and H.W. Davis performed experiments and interpreted data. F.V. Lucas interpreted data. B. Aronow designed and performed computational analysis. X. Qi and C. Mercer designed experiments. N. Mackman and H.H. Versteeg designed experiments, interpreted data, and edited the manuscript. V.Y. Bogdanov conceived the study, designed experiments, interpreted data, and edited the manuscript.
References
- 1.Ehehalt F, Saeger HD, Schmidt CM, Grutzmann R. Neuroendocrine Tumors of the Pancreas. Oncologist 2009; 14: 456–67. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. PDQ® Adult Treatment Editorial Board. PDQ Pancreatic Neuroendocrine Tumors (Islet Cell Tumors) Treatment. PDQ Cancer Information Summaries. 2015. [Google Scholar]
- 3.Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh D-Y, Strosberg J, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016; 387: 968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Reilly KE, Rojo F, She Q-B, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006; 66: 1500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowling RJO, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 2010; 328: 1172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Cui J-F, Chen M-B, Liu C-Y, Liu F, Zhang Q-D, Zou J, Lu P-H. The preclinical evaluation of the dual mTORC1/2 inhibitor INK-128 as a potential anti-colorectal cancer agent. Cancer Biol Ther 2015; 16: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lou H, Weng X, Pan H, Pan Q, Sun P, Liu L, Chen B. The novel mTORC1/2 dual inhibitor INK-128 suppresses survival and proliferation of primary and transformed human pancreatic cancer cells. Biochem Biophys Res Commun 2014; 450: 973–8. [DOI] [PubMed] [Google Scholar]
- 8.Versteeg HH, Ruf W. Emerging Insights in Tissue Factor-Dependent Signaling Events. Semin Thromb Hemost 2006; 32: 024–32. [DOI] [PubMed] [Google Scholar]
- 9.Kocatürk B, Van den Berg YW, Tieken C, Mieog JSD, de Kruijf EM, Engels CC, van der Ent M a, Kuppen PJ, Van de Velde CJ, Ruf W, Reitsma PH, Osanto S, Liefers G-J, Bogdanov VY, Versteeg HH. Alternatively spliced tissue factor promotes breast cancer growth in a β1 integrin-dependent manner. Proc Natl Acad Sci U S A 2013; 110: 11517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unruh D, Ünlü B, Lewis CS, Qi X, Chu Z, Sturm R, Keil R, Ahmad SA, Sovershaev T, Adam M, Dreden P Van, Woodhams BJ, Ramchandani D, Weber GF, Rak JW, Wolberg AS, Mackman N, Versteeg HH, Bogdanov VY, Unruh D, et al. Antibody-based targeting of alternatively spliced tissue factor: a new approach to impede the primary growth and spread of pancreatic ductal adenocarcinoma. Oncotarget 2016; 7: 25264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unruh D, Turner K, Srinivasan R, Kocatürk B, Qi X, Chu Z, Aronow BJ, Plas DR, Gallo CA, Kalthoff H, Kirchhofer D, Ruf W, Ahmad SA, Lucas F V, Versteeg HH, Bogdanov VY. Alternatively spliced tissue factor contributes to tumor spread and activation of coagulation in pancreatic ductal adenocarcinoma. Int J Cancer 2014; 134: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas SL, Jesnowski R, Steiner M, Hummel F, Ringel J, Burstein C, Nizze H, Liebe S, Löhr JM. Expression of tissue factor in pancreatic adenocarcinoma is associated with activation of coagulation. World J Gastroenterol 2006; 12: 4843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RC. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg 1995; 82: 1101–4. [DOI] [PubMed] [Google Scholar]
- 14.Khorana AA, Francis CW, Menzies KE, Wang J-G, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost 2008; 6: 1983–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unruh D, Sagin F, Adam M, Van Dreden P, Woodhams BJ, Hart K, Lindsell CJ, Ahmad SA, Bogdanov VY. Levels of Alternatively Spliced Tissue Factor in the Plasma of Patients with Pancreatic Cancer May Help Predict Aggressive Tumor Phenotype. Ann Surg Oncol 2015; 22: 1206–11. [DOI] [PubMed] [Google Scholar]
- 16.Evers BM, Townsend CM, Upp JR, Allen E, Hurlbut SC, Kim SW, Rajaraman S, Singh P, Reubi JC, Thompson JC. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology 1991; 101: 303–11. [DOI] [PubMed] [Google Scholar]
- 17.Vandamme T, Peeters M, Dogan F, Pauwels P, Van Assche E, Beyens M, Mortier G, Vandeweyer G, de Herder W, Van Camp G, Hofland LJ, Op de Beeck K. Whole-exome characterization of pancreatic neuroendocrine tumor cell lines BON-1 and QGP-1. J Mol Endocrinol 2015; 54: 137–47. [DOI] [PubMed] [Google Scholar]
- 18.Orr-Asman MA, Chu Z, Jiang M, Worley M, LaSance K, Koch SE, Carreira VS, Dahche HM, Plas DR, Komurov K, Qi X, Mercer CA, Anthony LB, Rubinstein J, Thomas HE. mTOR kinase inhibition effectively decreases progression of a subset of neuroendocrine tumors that progress on rapalog therapy and delays cardiac impairment. Mol Cancer Ther 2017; 16: 2432–41. [DOI] [PubMed] [Google Scholar]
- 19.Scholz A, Wagner K, Welzel M, Remlinger F, Wiedenmann B, Siemeister G, Rosewicz S, Detjen KM. The oral multitarget tumour growth inhibitor, ZK 304709, inhibits growth of pancreatic neuroendocrine tumours in an orthotopic mouse model. Gut 2009; 58: 261–70. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan R, Ozhegov E, Van Den Berg YW, Aronow BJ, Franco RS, Palascak MB, Fallon JT, Ruf W, Versteeg HH, Bogdanov VY. Splice variants of tissue factor promote monocyte-endothelial interactions by triggering the expression of cell adhesion molecules via integrin-mediated signaling. J Thromb Haemost 2011; 9: 2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szotowski B, Goldin-Lang P, Antoniak S, Bogdanov VY, Pathirana D, Pauschinger M, Dörner A, Kuehl U, Coupland S, Nemerson Y, Hummel M, Poller W, Hetzer R, Schultheiss HP, Rauch U. Alterations in myocardial tissue factor expression and cellular localization in dilated cardiomyopathy. J Am Coll Cardiol 2005; 45: 1081–9. [DOI] [PubMed] [Google Scholar]
- 22.Mackman N Regulation of the tissue factor gene. FASEB J 1995; 9: 883–9. [DOI] [PubMed] [Google Scholar]
- 23.Davila M, Robles-Carrillo L, Unruh D, Huo Q, Gardiner C, Sargent IL, Adam M, Woodhams BJ, Francis JL, Bogdanov VY, Amirkhosravi A. Microparticle association and heterogeneity of tumor-derived tissue factor in plasma: is it important for coagulation activation? J Thromb Haemost 2014; 12: 186–96. [DOI] [PubMed] [Google Scholar]
- 24.van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, Spek C a, Reitsma PH, Bogdanov VY, Versteeg HH. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A 2009; 106: 19497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadanandam A, Wullschleger S, Lyssiotis CA, Grötzinger C, Barbi S, Bersani S, Körner J, Wafy I, Mafficini A, Lawlor RT, Simbolo M, Asara JM, Bläker H, Cantley LC, Wiedenmann B, Scarpa A, Hanahan D. A Cross-Species Analysis in Pancreatic Neuroendocrine Tumors Reveals Molecular Subtypes with Distinctive Clinical, Metastatic, Developmental, and Metabolic Characteristics. Cancer Discov 2015; 5: 1296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espinoza JL, Elbadry MI, Taniwaki M, Harada K, Trung LQ, Nakagawa N, Takami A, Ishiyama K, Yamauchi T, Takenaka K, Nakao S. The simultaneous inhibition of the mTOR and MAPK pathways with Gnetin-C induces apoptosis in acute myeloid leukemia. Cancer Lett 2017; 400: 127–36. [DOI] [PubMed] [Google Scholar]
- 27.Sun D, Liu H, Dai X, Zheng X, Yan J, Wei R, Fu X, Huang M, Shen A, Huang X, Ding J, Geng M. Aspirin disrupts the mTOR-Raptor complex and potentiates the anti-cancer activities of sorafenib via mTORC1 inhibition. Cancer Lett 2017; 406: 105–15. [DOI] [PubMed] [Google Scholar]
- 28.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 2005; 105: 1734–41. [DOI] [PubMed] [Google Scholar]
- 29.Mackman N Regulation of the tissue factor gene. Thromb Haemost 1997; 78: 747–54. [PubMed] [Google Scholar]
- 30.Milsom C, Rak J. Tissue factor and cancer. Pathophysiol Haemost Thromb 2009; 36: 160–76. [DOI] [PubMed] [Google Scholar]
- 31.Kirchhofer D, Moran P, Chiang N, Kim J, Riederer MA, Eigenbrot C, Kelley RF. Epitope Location on Tissue Factor Determines the Anticoagulant Potency of Monoclonal Anti-tissue Factor Antibodies. Thromb Haemost 2000; 84: 1072–81. [PubMed] [Google Scholar]
- 32.Thrower AR, Bullock GC, Bissell JE, Stinski MF, Nelson JA, Ghazal P, Fleckenstein B, Nelson JA, Ghazal P. Regulation of a human cytomegalovirus immediate-early gene (US3) by a silencer-enhancer combination. J Virol 1996; 70: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwaal RF, Comfurius P, Bevers EM. Lipid-protein interactions in blood coagulation. Biochim Biophys Acta 1998; 1376: 433–53. [DOI] [PubMed] [Google Scholar]
- 34.Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY, Bach RR, Rak J, Church FC, Wolberg AS, Pawlinski R, Key NS, Yeh JJ, Mackman N. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood 2012; 119: 5543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, Hostetter G, Harvey J, Taubman MB. Tissue Factor Expression, Angiogenesis, and Thrombosis in Pancreatic Cancer. Clin Cancer Res 2007; 13: 2870–5. [DOI] [PubMed] [Google Scholar]
- 36.Nitori N, Ino Y, Nakanishi Y, Yamada T, Honda K, Yanagihara K, Kosuge T, Kanai Y, Kitajima M, Hirohashi S. Prognostic Significance of Tissue Factor in Pancreatic Ductal Adenocarcinoma. Clin Cancer Res 2005; 11: 2531–9. [DOI] [PubMed] [Google Scholar]
- 37.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Källen R, Østraat Ø, Salmela K, Tibell A, Tufveson G, Elgue G, Ekdahl KN, Korsgren O, Nilsson B. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet 2002; 360: 2039–45. [DOI] [PubMed] [Google Scholar]
- 38.Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Källen R, Salmela K, Tibell A, Tufveson G, Ekdahl KN, Elgue G, Korsgren O, Nilsson B. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes 2005; 54: 1755–62. [DOI] [PubMed] [Google Scholar]
- 39.Cowan RW, Maitra A. Genetic progression of pancreatic cancer. Cancer J 2014; 20: 80–4. [DOI] [PubMed] [Google Scholar]
- 40.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N. DAXX/ATRX, MEN1, and mTOR Pathway Genes Are Frequently Altered in Pancreatic Neuroendocrine Tumors. Science 2011; 331: 1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan F, Shi M, Ji J, Shi H, Zhou C, Yu Y, Liu B, Zhu Z, Zhang J. KRAS and DAXX/ATRX Gene Mutations Are Correlated with the Clinicopathological Features, Advanced Diseases, and Poor Prognosis in Chinese Patients with Pancreatic Neuroendocrine Tumors. Int J Biol Sci 2014; 10: 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Signaevsky M, Hobbs J, Doll J, Liu N, Soff G. Role of Alternatively Spliced Tissue Factor in Pancreatic Cancer Growth and Angiogenesis. Semin Thromb Hemost 2008; 34: 161–9. [DOI] [PubMed] [Google Scholar]
- 43.Couvelard A, O’Toole D, Turley H, Leek R, Sauvanet A, Degott C, Ruszniewski P, Belghiti J, Harris AL, Gatter K, Pezzella F. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer 2005; 92: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 2018; 18: 533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Versteeg HH, Heemskerk JWM, Levi M, Reitsma PH. New Fundamentals in Hemostasis. Physiol Rev 2013; 93: 327–58. [DOI] [PubMed] [Google Scholar]
- 46.Ma X, Ye B, Gao F, Liang Q, Dong Q, Liu Y, Rong P, Wang W, Yi S. Tissue Factor Knockdown in Porcine Islets: An Effective Approach to Suppressing the Instant Blood-Mediated Inflammatory Reaction. Cell Transplant 2012; 21: 61–71. [DOI] [PubMed] [Google Scholar]
- 47.Guba M, Yezhelyev M, Eichhorn ME, Schmid G, Ischenko I, Papyan A, Graeb C, Seeliger H, Geissler EK, Jauch K-W, Bruns CJ. Rapamycin induces tumor-specific thrombosis via tissue factor in the presence of VEGF. Blood 2005; 105: 4463–9. [DOI] [PubMed] [Google Scholar]
- 48.Steffel J, Latini RA, Akhmedov A, Zimmermann D, Zimmerling P, Lüscher TF, Tanner FC. Rapamycin, but Not FK-506, Increases Endothelial Tissue Factor Expression: Implications for Drug-Eluting Stent Design. Circulation 2005; 112: 2002–11. [DOI] [PubMed] [Google Scholar]
- 49.Jiang X, Zhu S, Panetti TS, Bromberg ME. Formation of tissue factor-factor VIIa-factor Xa complex induces activation of the mTOR pathway which regulates migration of human breast cancer cells. Thromb Haemost 2008; 100: 127–33. [DOI] [PubMed] [Google Scholar]
- 50.Finotti A, Bianchi N, Fabbri E, Borgatti M, Breveglieri G, Gasparello J, Gambari R. Erythroid induction of K562 cells treated with mithramycin is associated with inhibition of raptor gene transcription and mammalian target of rapamycin complex 1 (mTORC1) functions. Pharmacol Res 2015; 91: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S-R, Park J-H, Park EK, Chung CH, Kang S-S, Bang O-S. Akt-induced promotion of cell-cycle progression at G2/M phase involves upregulation of NF-Y binding activity in PC12 cells. J Cell Physiol 2005; 205: 270–7. [DOI] [PubMed] [Google Scholar]
- 52.Jablonska A, Polouliakh N. In silico discovery of novel transcription factors regulated by mTOR-pathway activities. Front Cell Dev Biol 2014; 2: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicolás M, Noé V, Ciudad CJ. Transcriptional regulation of the human Sp1 gene promoter by the specificity protein (Sp) family members nuclear factor Y (NF-Y) and E2F. Biochem J 2003; 371: 265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brennan P, Babbage JW, Thomas G, Cantrell D. p70(s6k) integrates phosphatidylinositol 3-kinase and rapamycin-regulated signals for E2F regulation in T lymphocytes. Mol Cell Biol 1999; 19: 4729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.