Abstract
In those who use nicotine, the likelihood of dependence, negative health consequences, and failed treatment outcomes differ as a function of gender. Women may be more sensitive to learning processes driven by repeated nicotine exposure that influence conditioned approach and craving. Sex differences in nicotine’s influence over overt behaviors (i.e. hypoactivity or behavioral sensitization) can be examined using passive drug administration models in male and female rats. Following repeated intravenous (IV) nicotine injections, behavioral sensitization is enhanced in female rats compared to males. Nonetheless, characteristics of the testing environment also mediate rodent behavior following drug administration. The current experiment used a within-subjects design to determine if nicotine-induced changes in horizontal activity, center entries, and rearing displayed by male and female rats is detected when behavior was recorded in round vs. square chambers. Behaviors were recorded from each group (males-round: n = 19; males-square: n = 18; females-square: n = 19; and females-round: n = 19) immediately following IV injection of saline, acute nicotine, and repeated nicotine (0.05 mg/kg/injection). Prior to nicotine treatment, sex differences were apparent only in round chambers. Following nicotine administration, the order of magnitude for the chamber that provided enhanced detection of hypoactivity or sensitization was contingent upon both the dependent measure under examination and the animal’s biological sex. As such, round and square testing chambers provide different, and sometimes contradictory, accounts of how male and female rats respond to nicotine treatment. It is possible that a central mechanism such as stress or cue sensitivity is impacted by both drug exposure and environment to drive the sex differences observed in the current experiment. Until these complex relations are better understood, experiments considering sex differences in drug responses should balance characteristics of the testing environment to provide a complete interpretation of drug-induced changes to behavior.
Keywords: Nicotine, Behavioral Sensitization, Locomotor Activity, Intravenous, Rats, Biological Sex, Thigmotaxis
1. Introduction
Over the 50-year period since the original Surgeon General’s report, the disease risk for tobacco smoking by women has increased despite reported decreases in rates of smoking by both genders (United States Department of Health and Human Services, 2014). Although more men report smoking, women smokers are now equal to men in the risk for lung cancers, cardiovascular disease, and chronic pulmonary obstructive disorder (Syamlal et al., 2014). Women now surpass men in the number of COPD deaths, and continue to experience greater mortality from lung cancer compared to breast cancer (USDHHS, 2014).
In individuals who abuse nicotine and other drugs, gender is an important predictor of dependence (Perkins, 1999; Lynch et al., 2002; Becker & Koob, 2016; Torres & O’Dell, 2016). Self-report studies indicate that women progress toward nicotine dependence at a faster rate (Westermeyer & Boedicker, 2000) and are less likely to benefit from treatment, resulting in lower rates of smoking cessation than men (Perkins, 1999). In the laboratory, women exhibited a greater attenuation of responding for cigarette smoke upon removal of conditional cues, indicating that tobacco smoking is maintained by non-nicotine cues to a greater extent in women relative to men (as reviewed in Perkins, 1999). Stress is also an important mediator of tobacco dependence and relapse following abstinence. Torres and O’Dell (2016) posit that the greater vulnerability to tobacco dependence for women is in part mediated by gender differences in the response to stress, and particularly stress that occurs during withdrawal.
Animal models of nicotine exposure have played an important role in demonstrating differences in responses to nicotine as a function of biological sex. Models of reinforcement which allow the animal to self-administer drug reveal that female rats acquire nicotine-reinforced responding at a lower dose (Donny et al., 2000; Chaudhri et al., 2005; Lynch, 2009) than age-matched males (also see Swalve et al., 2016) and exhibit higher break points on progressive ratio schedules of reinforcement (Donny et al., 2000). Compared to males, female rats also show a greater magnitude of responding for intravenous (IV) nicotine in the presence of a weakly reinforcing visual stimulus (Chaudrhi et al., 2005); supporting enhanced cue-dependent responding in females. Furthermore, estradiol mediates dopamine function in the nucleus accumbens (Peterson et al., 2015; Becker, 2016; Calipari et al., 2017) and may drive sex differences observed following nicotine administration and likewise, under nicotine withdrawal in conjunction with stress responses (O’Dell & Torres, 2014).
Models that passively administer nicotine to male and female rats have also been important in demonstrating sex differences in nicotine-induced behavioral responses. In an early study, daily subcutaneous infusions of nicotine (6.0 or 12.0 mg/kg/day) decreased body weight in female and male rats during the drug exposure period. For four months after nicotine cessation (12.0 mg/kg/day), the male’s body weights were reduced below that of vehicle controls, but females showed no differences from controls during the cessation period (Grunberg et al., 1987). Rats chronically treated with nicotine for seven days (3.16 mg/kg/day) also exhibited sex differences in nicotine withdrawal-related behaviors, and interestingly, detection of this sex difference was modulated by the testing environment. Females showed more withdrawal than males in a dimly-lit environment, but not in a brightly-lit environment (Hamilton et al., 2009).
Systemic injection of nicotine has also produced sex differences in rodent locomotor activity following acute and repeated administration (Booze et al., 1999; Kanyt et al., 1999; Elliot et al., 2004; Harrod et al., 2004; Ericson et al., 2010; Hamilton et al., 2014). First, studies that investigated the stimulus properties of nicotine on locomotor activity in a single sex showed that it modulates overt behavior in a complex manner (Stolerman et al., 1995). Acute injection induces a transient locomotor depressant effect (Morrison & Stephenson, 1972) and repeated administration produces a progressive increase in activity, termed behavioral sensitization (Morrison & Stephenson, 1972; Post, 1980; Clarke & Kumar, 1983; Ksir, 1994). Behavioral sensitization is descriptive of a learning process that occurs following repeated presentation of a single stimulus (Groves & Thompson, 1970), but is not a measure of reinforcement, as drug is administered non-contingently to the animal (DiFranza & Wellman, 2007). Instead, drug-induced sensitization is theorized to play an important role in addiction by increasing the intensity of a signal that is associated with reward which, in turn, is suggested to influence drug craving and approach behavior (Robinson & Berridge, 1993; DiFranza & Wellman, 2005). Several studies report that female rats exhibit a greater magnitude of nicotine-induced behavioral sensitization than males (Booze et al., 1999; Harrod et al., 2004; Hamilton et al., 2014). Sex differences in behavioral sensitization are thus suggested to indicate differential vulnerabilities to the conditional aspects of repeated nicotine exposure, e.g. conditional approach and drug craving (Robinson & Berridge, 1993).
Our laboratory has used the behavioral sensitization model to investigate sex differences in the stimulus properties of nicotine by administering it through the IV route of administration. The IV route mimics the absorption characteristics of inhaled nicotine (Benowitz, 1988) and the dose of nicotine used in the current model, 0.05 mg/kg/injection, produces peak arterial levels of ~ 25 ng/ml of nicotine with distribution and elimination half-lives of five and 50 minutes, respectively. Additionally, the 0.05 mg/kg/injection dose is within the range of doses that function as a reinforcer in operant studies of rodent self-administration (Donny et al., 2000; Chaudhri et al., 2005). Repeated, once/daily IV injections of this dose (e.g., 14 or 21 consecutive days) have been shown to produce behavioral sensitization in male and female rats without altering vaginal cytology, estrous cyclicity, or body weights (Booze et al., 1999). Booze et al., (1999) and Harrod et al., (2004), respectively, describe the influence of gonadal hormones as well as the association of dopamine transporter and D3 receptor levels with sex differences in nicotine-induced behaviors. Importantly, both studies describe enhanced sensitization in female rats compared to males when treated with repeated, IV nicotine. Booze et al., (1999) showed that females exhibited a greater sensitization of horizontal activity, rearing, and grooming incidence. The Harrod et al., (2004) study extended these findings, reporting that females exhibited greater sensitization of centrally directed behavior (i.e., entries and distance travelled), rearing duration, and rearing incidence. No locomotor depressant effects following acute injection were reported in either study, although, there was a sex difference in horizontal activity on this day, with females showing more activity than males (Booze et al., 1999). Interestingly, both experiments employed similar experimental designs and the same IV nicotine injection procedure, with the major difference being that the earlier study tested animals in square activity chambers and the latter study used round activity chambers. Although the sex difference in behavioral sensitization is reported in both studies, it is of interest in the current experiment to determine if one style of chamber is more or less sensitive to measure IV nicotine-induced locomotor depression and sensitization. Indeed, evidence from Hamilton et al., (2009), using passive nicotine administration in rats, showed that the testing environment influences expression of nicotine-induced changes in withdrawal behavior. The current experiment determined if round and square chambers differentially modulate the response to acute and repeated IV injection in adult female and male rats in an effort to increase rigor and reproducibility in studies of nicotine-induced behavioral sensitization. We reasoned that because square chambers provide corners as potential places of rest, round chambers promote a general increase in activity therefore increasing sensitivity to measure nicotine-induced changes in behavior. To our knowledge, no systematic comparison of activity produced by two different chamber shapes has been reported. Based on the previous literature, it was predicted that males and females would exhibit different amounts of activity, with females generally more active than males. Additionally, it was hypothesized that acute IV nicotine would produce locomotor depression, which will be more prominent in square chambers, and that sensitization elicited by repeated IV nicotine would therefore be greater in round chambers.
2. Materials and Methods
2.1. Animals
Eighty-two adult male (n = 41) and female (n = 41) Sprague-Dawley rats were purchased from Harlan Laboratories, Inc. (Indianapolis, IN) and arrived to the animals care facilities at 90 days of age. Rats were quarantined for seven days and then transferred to the colony room. Rodent food (Pro-Lab Rat, Mouse Hamster Chow #3000) and water were available ad libitum through the experiment and conditions of the colony room were maintained at 21 ± 2°C, 50% ± 10% relative humidity with a 12-h light: 12-h dark cycle with lights on at 0700h (EST) daily. Once transferred to the colony room located in the Department of Psychology, each animal was weighed and received an IV saline injection. Surgical implantation of intracath IV catheters was conducted at Harlan Laboratories, Inc. according to the procedures described in Mactutus et al., (1994) prior to arrival. Briefly, subcutaneous ports for IV catheters were implanted dorsally and the distal end of the catheter was secured into each animal’s jugular vein to ease daily administration procedures. The completely internalized nature of the IV catheter prevents cage-mates in the pair-housed environment from damaging the catheter. Seven animals were excluded from the final analyses due to insufficient catheter patency during the course of the experiment. The Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina approved the animal protocol for this research.
2.2. IV Nicotine Administration Procedure
The current experiment used the IV route of administration because it produces a rapid influx of arterial nicotine that is characteristic of the pharmacokinetic profile of nicotine absorption from cigarette smoking (Benowitz, 1988). Nicotine bitartrate was dissolved in saline in an injection volume of 1.0 ml/kg (pH = 7.2). The IV nicotine dose, 0.05 mg/kg/injection, was chosen from Booze et al. (1999), which showed that it produces peak behavioral activity, and plasma nicotine levels comparable to that demonstrated in tobacco smokers. IV nicotine and saline were administered over a 15-s duration. To maintain catheter patency, all IV injections were followed by an injection of 0.2 ml heparinized (2.5%) saline, also delivered over a 15-s duration.
2.3. Apparatus
Behavioral testing occurred under dim lighting conditions (< 10 lx) in Hamilton-Kinder photocell activity chambers equipped with 32 emitter/detector beam pairs ~ 4 cm above the chamber floor. All chambers were constructed of clear Plexiglas and were initially square in shape (~ 40 × 40 cm). A Plexiglas ring (~ 40 cm diameter) was placed into half of the chambers to create the round field design. The manufacturer tuned photocell emitter/detector pairs to account for the width of the Plexiglas inserts. The absence of the corners in the round chambers does not preclude animals from breaking photobeams in the round chamber, which also extend into the corner regions of the square chambers. Thus, there are no photobeams intersecting the corner areas of square chambers that do not also intersect a non-corner area in the round chambers. Locomotor activity was automatically recorded by computer. Horizontal activity was defined by a new beam break in either the X- or Y-plane, and in a direction parallel to the chamber floor. Center entries were counted when the animal’s body crossed into the center area: the center 25% of the chamber as designated by the computer software. Rearing, or vertical activity, was recorded by a second set of beams located ~ 16.5 cm above, and parallel to, the chamber floor. The conversion of square to round chambers resulted in a difference of testing areas between the two contexts. The addition of the Plexiglas insert removes the corners thereby reducing the testing area relative to square chambers. Expanding the diameter of round chambers would be necessary to equate the testing areas of the round and square chambers; however, expanding the diameter produces a longer distance from the wall to the center of the round chamber relative to square. Given that the measure of center entry is a dependent measure in the current experiment, the diameter of the round chambers were not expanded to equate for general testing area.
2.4. Experimental Design
Males and females were matched and randomly assigned to one of four groups used for analysis: males and females tested in round chambers (MR; n = 19; FR; n = 19) and males and females tested in square chambers (MS; n = 18; FS; n = 19). Figure 1 shows the timeline of the current experimental design. Each session in the testing chamber was 60-minutes in duration.
Figure 1. Timeline of experimental design.
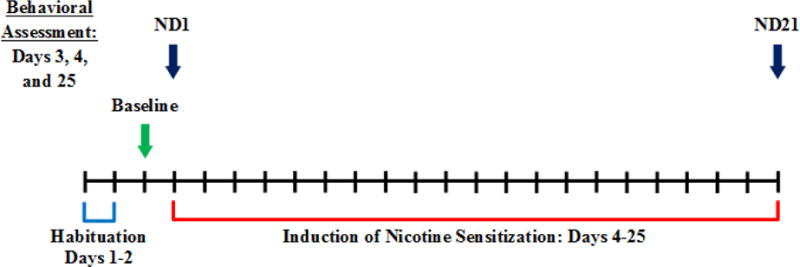
Animals underwent two 60-minute habituation sessions (one/day) within round or square chambers. On Day 3, animals received a 0.05 mg/kg IV saline injection before horizontal activity, center entries, and rearing behavior was recorded for 60-minutes. For the next 21 days (Days 4–25), animals received daily IV nicotine injection. On Days 5–24, animals were returned to their home cage post-injection to prevent Pavlovian conditioning following context-nicotine pairings. Behavior was tested only on days indicated by an arrow: Days 3, 4, and 25.
First, animals were habituated to their respective round or square chambers for two sessions (one/day). On day three, an IV saline injection was administered to all rats prior to immediate placement into testing chambers to measure baseline activity levels (Baseline). The following day, behavior was measured again immediately following acute IV nicotine injection (ND1). For the next 19 days, nicotine was administered once daily in the home cage. No additional testing was conducted until day 25 of the experiment, immediately following the 21st IV nicotine injection (ND21). IV nicotine exposure was largely conducted away from the testing chambers to limit context-drug (conditional stimulus-unconditional stimulus) pairings, and ultimately, context-elicited changes in activity. Lacy et al., (2016) described conditioned hyperactivity in adult male and female rats when saline was substituted for once daily methamphetamine injection, creating a context-only test of activity and demonstrating context-elicited changes in activity. Although associations of contextual cues with nicotine exposure are implicated in the etiology of nicotine dependence (Perkins, 1999; Chaudrhi et al., 2005), the possibility of nicotine-induced contextual conditioning was limited in order to assess the influence of nicotine alone on sensitized behavior. Similarly, no observational tests of behavior were conducted (Fray et al., 1980) as in our previous experiments (Booze et al., 1999; Harrod et al., 2004) because this would require the observer to stand directly in front of each chamber to time-sample an array of potential behaviors, and the repeated presence of the experimenter may have affected the animals behavior differently as a function of chamber shape.
2.5. Statistical Analysis
Baseline sex differences in activity are commonly reported, and females generally exhibit more baseline activity than males (Booze et al., 1999; Kanyt et al., 1999; Zakharova et al., 2012; Lacy et al., 2016; also see Hamilton et al., 2009; Hamilton et al., 2014). Because the primary research question of the current experiment was in regards to the unique influence of chamber shape on nicotine-induced changes in behavior, we first determined if biological sex was a justified covariate by testing for sex differences in baseline activity. Data representing horizontal activity, center entries, and rearing during the 60-minute test following saline injection were analyzed using a 2 × 2 × 12 (sex × chamber shape × time) mixed-factors ANOVA. Here, biological sex and chamber shape were the between-subjects’ factors, and time (12, five-minute intervals) was the within-subjects’ factor. Baseline sex differences were apparent in two of three measures. A Bonferroni correction was used to determine in which chamber shape(s) sex differences exist when there was a significant chamber shape × biological sex interaction.
Next, to define the variance uniquely attributable to chamber shape, behavioral data following saline, acute, and repeated nicotine injections for horizontal activity, center entries, and rearing was adjusted to control for baseline sex differences with an analysis of covariance (ANCOVA). In the mixed-factors 2 × 3 × 12 ANCOVA, shape was the between-subject’s factor and day and time were the within-subject’s factors. Orthogonal decompositions were used to determine if activity across time or days followed a linear or quadratic trend. When both linear and quadratic components were significant at an alpha level of 0.05, the function that corresponded to the highest F-value is presented. Pairwise comparisons with Bonferroni corrections were made when there was a significant interaction to determine under which within-subject conditions one chamber shape elicited more activity as well as in which chamber shape significant hypoactivity and sensitization occurred.
Lastly, significant sex × chamber shape interactions were apparent at baseline. Because sex differences were controlled for in the main analysis, further comparisons were conducted separately for females (FR and FS) and males (MR and MS) to provide descriptive information as to whether acute and repeated nicotine injection produced locomotor depressant and sensitization effects, (e.g., baseline vs. acute nicotine; baseline vs. repeated nicotine) respectively, in round and square chambers.
Data presented in text represents the mean ± standard error (X ± SEM). Effect sizes are presented as partial eta squared (ηp2), which has a maximum value of 1.0, for significant findings in the initial baseline 2 × 2 × 12 ANOVA and the 2 × 3 × 12 ANCOVA that involve biological sex or chamber shape. On time course graphs, the raw data is plotted as individual points for each of the five-minute intervals and habituation curves representing the best fitting one- or two-phase decay curves ± 95% confidence intervals (CIs) were plotted to abridge presentation. Data analyses were performed using SPSS 24 (IBM Software) and Graph Pad Prism 5 (GraphPad Software, Inc.).
3. Results
3.1. Horizontal Activity
Horizontal activity data from Baseline, ND1, and ND21 for females and males in round and square chambers are shown in Figure 2A. The 2 × 2 × 12 (sex × chamber shape × time) mixed-factors ANOVA conducted on baseline data revealed a significant sex × chamber shape interaction [F(1,78) = 5.569, p ≤ 0.021, ηp2 = 0.067; left panel]. Females were more active than males if tested in round chambers [F(1,78) = 5.743, p ≤ 0.019], but males and females were not different if tested in square chambers [F(1,78) = 0.885, p ≥ 0.350]. During the time course of the 60-minute baseline test, rats exhibited within-session habituation of horizontal activity [linear, time: F(1,78) = 1083.895, p ≤ 0.001, ηp2 = 0.933; data not shown]. The pattern of habituation on Baseline was dependent on animals’ biological sex [quadratic, sex × time: F(1,78) = 8.946, p ≤ 0.004, ηp2 = 0.103] and was further influenced by the chamber shape in which testing occurred [linear, sex × chamber shape × time: F(1,78) = 7.025, p ≤ 0.010, ηp2 = 0.083]. Figure 2B shows that females tested in round chambers were more active during the first 15 minutes of sessions compared to males [minute 5: F(1,78) = 9.958, p ≤ 0.002; minute 10: F(1,78) = 7.801, p ≤ 0.007; minute 15: F(1,78) = 4.343, p ≤ 0.040], whereas when tested in square chambers both sexes showed similar habituation curves throughout the entire session. Thus, sex differences in baseline horizontal activity were observed, and females were significantly more active than males when testing occurred in round, but not square chambers.
Figure 2. Unadjusted Horizontal Activity.
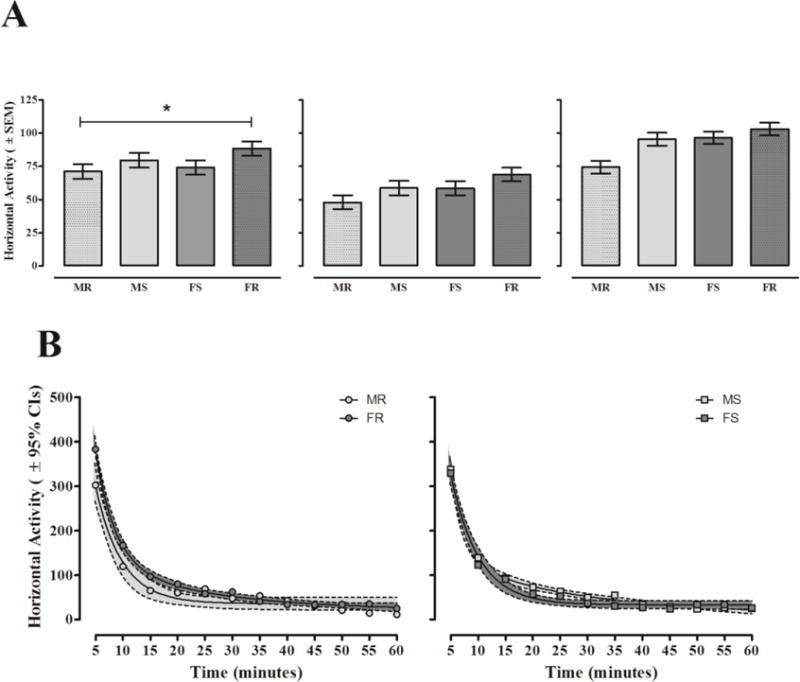
Mean horizontal activity levels (± SEM) following IV saline (Baseline: left), acute IV nicotine (ND1: middle), and repeated IV nicotine (ND21: right). *A sex difference at baseline was apparent in female and male rats tested in round chambers [F(1,78) = 5.743, p ≤ 0.019]. Note: unadjusted data from ND1 and ND21 were not analyzed using ANOVA due to sex differences at baseline. Thus, no asterisks are provided in the middle and right panels. B: Average habituation of horizontal activity (± 95% CIs) in round (left panel) and square (right panel) chambers across all testing sessions. Females tested in round chambers (FR) displayed higher levels of horizontal activity than males (MR) during the first 15 minutes of sessions while females and males tested in square chambers (FS and MS) displayed statistically similar habituation curves.
In the 2 × 3 × 12 mixed-factors ANCOVA the findings for the covariate, biological sex, were consistent with what was found at baseline. Biological sex was significantly related to animals’ overall horizontal activity [F(1,72) = 5.840, p ≤ 0.018, ηp2 = 0.075] and within-session habituation of horizontal activity [quadratic, sex × time: F = 8.701, p ≤ 0.004, ηp2 = 0.108], with females displaying more activity than males. However, the sex × day interaction was not significant [linear, sex × day: F = 3.068, p ≤ 0.084] suggesting that the unique influence of biological sex on horizontal activity may be consistent across the three testing occasions. Generally, both acute and repeated nicotine exposure altered horizontal activity relative to saline [quadratic, day: F(1,72) = 13.140, p ≤ 0.001, ηp2 = 0.154; data not shown]. Horizontal activity was significantly reduced following acute nicotine [p ≤ 0.001; Baseline: X = 78.203 ± 2.780; ND1: X = 58.379 ± 2.653]. Additionally, animals displayed behavioral sensitization as indicated by a significant increase in horizontal activity following repeated IV nicotine relative to levels of activity following saline [p ≤ 0.001; ND21: X = 92.271 ± 2.525].
Chamber shape interacted with day of testing [linear, chamber shape × day: F(1,72) = 3.978, p ≤ 0.050, ηp2 = 0.052] and the interaction is shown in Figure 3A. Behavioral sensitization of horizontal activity was modulated by the shape of testing chambers alone, whereas the locomotor depressant effects produced by acute IV nicotine were not. Acute nicotine produced significant locomotor depression in rats tested in both chamber shapes (square chambers: p ≤ 0.001; round chambers: p ≤ 0.001), and the percent decrease was 23.8% and 26.8%, respectively. Animals tested in square and round chambers significantly increased horizontal activity levels from Baseline to ND21 after repeated IV nicotine injection, i.e. behavioral sensitization (square chambers: p ≤ 0.001; round chambers: p ≤ 0.045). The percent increase in activity of animals was 25.0% and 11.2% in square and round chambers, respectively.
Figure 3. Influence of chamber shape on nicotine-induced changes to horizontal activity. A.
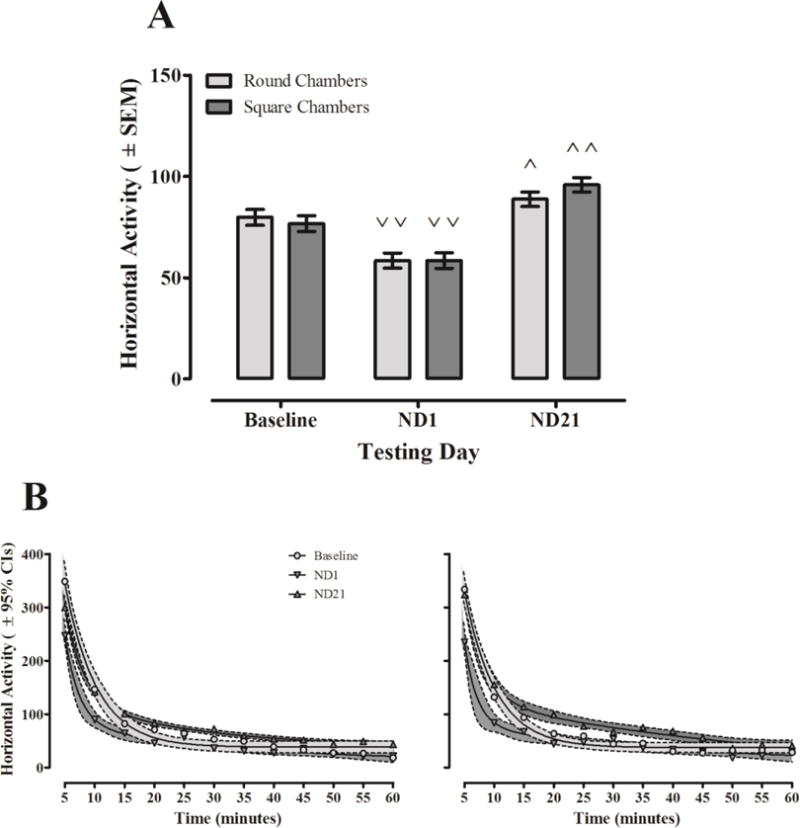
Mean horizontal activity (± SEM) displayed by animals tested in round and square chambers after controlling for biological sex differences at baseline. Compared to Baseline, animals tested in both chamber shapes displayed nicotine-induced hypoactivity on ND1 (˅˅: p ≤ 0.001) and sensitization on ND21 (˄: p ≤ 0.045; ˄˄: p ≤ 0.001). B: Habituation of horizontal activity (± 95% CIs) in round (left) and square (right) chambers on Baseline, ND1, and ND21. While hypoactivity is evident in both chamber shapes for the first 15 minutes on ND1, animals in round chambers displayed hypoactivity at more of the 5-minute “bins” than animals tested in square chambers. Behavioral sensitization is more prominent on ND21 when animals are tested in square chambers compared to round (linear-linear function of day × time × chamber shape: F(1,72) = 8.365, p ≤ 0.005).
The difference in the magnitude of behavioral sensitization between round and square chambers was also observed in the time course of horizontal activity: the within-session habituation curves were modulated by chamber shape and day [linear, time: F(1,72) = 103.344, p ≤ 0.001, ηp2 = 0.589; quadratic-linear, day × time: F(1,72) = 9.193, p ≤ 0.003, ηp2 = 0.113; linear-linear, chamber shape × day × time: F(1,72) = 8.365, p ≤ 0.005, ηp2 p = 0.104]. Figure 3B shows the time course data for the three testing days in animals tested in round and square chambers after adjusting mean horizontal activity levels to control for biological sex differences. First, the locomotor depressant effects of nicotine can be seen as a leftward and downward shift in the habituation curve. The lack of overlap in CIs between the saline and the acute nicotine data illustrates nicotine-induced hypoactivity during the first 10 minutes of the session for animals tested in both chamber types [Round chambers: minute 5: p ≤ 0.001, minute 10: p ≤ 0.001; Square chambers: minute 5: p ≤ 0.001, minute 10: p ≤ 0.001]. Behavioral sensitization by animals in both chamber shapes was observed as an upward and rightward shift in the habituation curve relative to baseline. Figure 3B shows that animals in square chambers exhibit an enhanced sensitization response at more of the five-minute “bins” than animals tested in round chambers throughout the 60-minute session. Animals in round chambers only displayed significant sensitization at minutes 55 and 60 [minute 55: p ≤ 0.021, minute 60: p ≤ 0.006] and actually display significantly lower levels of horizontal activity than baseline at minute 5 [p ≤ 0.001].
3.2. Center Entries
Figure 4 shows the unadjusted center entry data for males and females tested in round and square chambers following saline, acute nicotine, and repeated nicotine injections. The 2 × 2 × 12 (sex × chamber shape × time) mixed-factors ANOVA conducted on baseline data indicate that biological sex interacted with chamber shape [sex × chamber shape: F(1,78) = 4.771, p ≤ 0.032, ηp2 = 0.058; left panel]. Males and females displayed similar amounts of centrally directed behavior when tested in square chambers [F(1,78) = 0.800, p ≤ 0.374; MS: X = 5.108 ± 1.257; FS: X = 6.679 ± 1.226] and exhibited relatively more center entries when tested in round chambers compared to square chambers [chamber shape: F(1,78) = 35.124, p ≤ 0.001, ηp2 = 0.310]. However, males showed more center entries in round chambers relative to females also tested in round chambers [F(1,78) = 4.817, p ≤ 0.031; MR: X = 15.179 ± 1.226; FR: X = 11.325 ± 1.257]. Thus, sex differences in centrally directed behavior were observed, and males showed more centrally directed behavior than females if testing occurred in round chambers.
Figure 4. Unadjusted Center Entries.
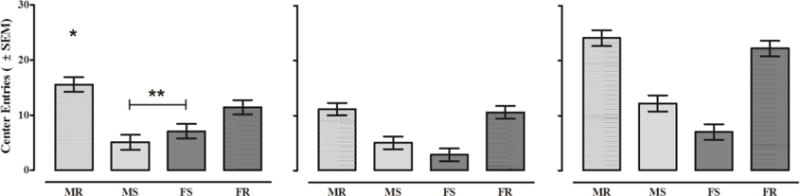
Mean center entries (± SEM) displayed by male and female rats in round and square chambers on Baseline (left), ND1 (middle), and ND21 (right). **Male and female rats in square chambers (MS and FS) display lower levels of center entry than those in round chambers [F(1,78) = 35.124, p ≤ 0.001]. *Animals in round chambers (MR and FR) exhibit a sex difference with the MR group entering the center of the chamber more often than the FR group [F(1,78) = 4.817, p ≤ 0.031]. Note: unadjusted data from ND1 and ND21 were not analyzed using ANOVA due to sex differences at baseline. Thus, no asterisks are provided in the middle and right panels.
Biological sex was not a significant covariate in the 2 × 3 × 12 (chamber shape × day × time) mixed-factors ANCOVA, suggesting that, alone, sex did not significantly contribute to overall center entry behavior [F(1,72) = 3.834, p ≤ 0.054]. However, the ANCOVA revealed a finding that was not apparent from the analysis on Baseline data; sex was significantly related to animals’ within-session habituation of center entries [linear, sex × time: F(1,72) = 5.115, p ≤ 0.027, ηp2 = 0.066] with males showing more center entry activity than females. A significant main effect of day [linear, day: F(1,72) = 17.638, p ≤ 0.001, ηp2 = 0.197; adjusted data not shown] indicated that relative to saline, acute nicotine induced a significant decrease in center entries [p ≤ 0.001; Baseline: X = 9.835± 0.687; ND1: X = 7.416± 0.569] and repeated nicotine produced behavioral sensitization of this centrally directed behavior [p ≤ 0.001; ND21: X = 16.351 ± 0.716].
Additionally, there was a main effect of chamber shape, with greater center entries in round compared to square chambers [F(1,72) = 82.558, p ≤ 0.001, ηp2 = 0.534; round: 15.847 ± 0.718; square: 6.554 ± 0.728]. Animals habituated center entry behaviors over the course of 60-minute testing sessions and the pattern of habituation was modified by pre-session treatment [linear, time: F(1,72) = 66.201, p ≤ 0.001, ηp2 = 0.479; quadratic-linear, day × time: F(1,72) = 6.656, p ≤ 0.012, ηp2 = 0.085].
The time course within-session habituation curves were also altered as a function of chamber shape [linear, chamber shape × time: F(1,72) = 75.395, p ≤ 0.001, ηp2 = 0.512]. Figure 5A shows that rats tested in square chambers exhibited a downward and leftward shift in the habituation curve relative rats tested in round chambers. The non-overlapping CIs between the two lines indicate that round chambers elicited more centrally directed behavior than square chambers throughout the entire 60-minute session. The chamber shape × day interaction [linear, nicotine produced significant locomotor depressant effects relative to saline for rats tested in round, but not square chambers [round chambers: p ≤ 0.009; square chambers: p ≤ 0.080]. Rats tested in round and square chambers decreased center entries by 19.5% and 35.8%, respectively. Comparisons of Baseline and ND21 data indicate that rats tested in both round and square chambers exhibited sensitization [round: p ≤ 0.001; square: p ≤ 0.001] and the magnitude of behavioral sensitization was greatest in animals tested in round chambers. The percent increases of behavior that occurred with repeated nicotine in round and square chambers are 71.1% and 55.6%, respectively. The chamber shape × day × time interaction was not significant. Figure 5C shows that animals in round chambers showed locomotor depression and sensitization, exhibited as shifts in the habituation curves relative to baseline (left panel). Similar patterns of habituation are evident in rats tested in square chambers (right panel); however, the amount of activity is greater on each day of testing in round chambers, and the enhanced sensitization effect in round chambers was evident for a longer duration of the test session compared to that produced by square chambers.
Figure 5. Influence of chamber shape on nicotine-induced changes to center entries. A.
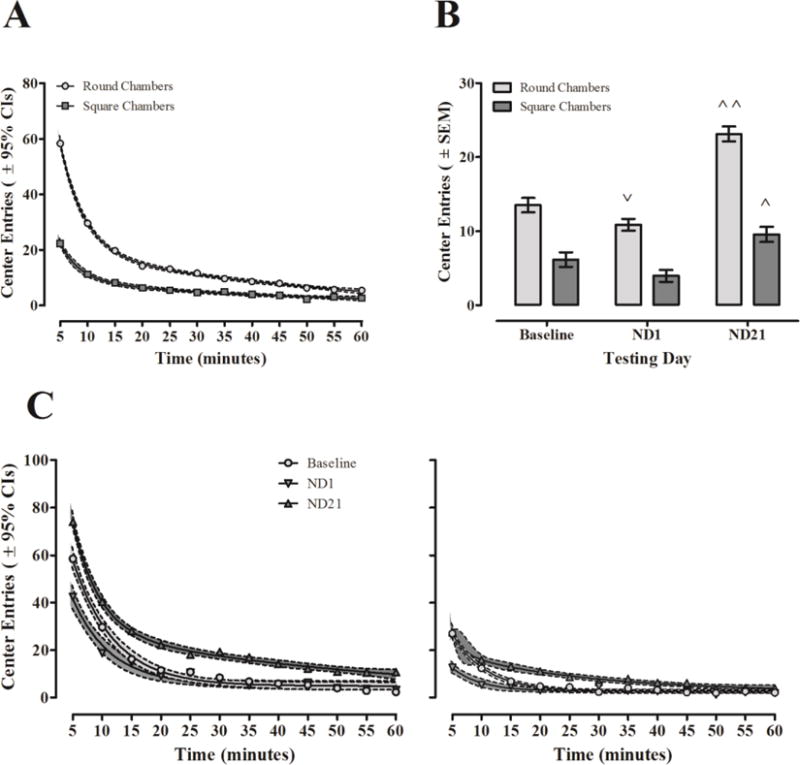
Habituation of center entries (± 95% CIs) by rats tested in round and square chambers across all testing days. At each 5-minute interval, animals in round chambers displayed greater center entry activity than those in square chambers. B: Mean center entries (± SEM) of animals tested in round and square chambers Baseline, ND1, and ND21 after adjusting for sex differences at baseline. Only animals tested in round chambers displayed significant hypoactivity on ND1 (˅: p ≤ 0.022) but, animals displayed significant behavioral sensitization on ND21 regardless of camber shape (˄: p ≤ 0.007; ˄˄: p ≤ 0.001). C: Average habituation of center entries (± 95% CIs) on Baseline, ND1, and ND21 in round (left) and square (right) chambers. While animals in square chambers displayed fewer center entries than those in round chambers the pattern of habituation did not change as a function of chamber shape.
3.3. Rearing
The rearing data for males and females tested in round and square chambers following saline, acute nicotine, and repeated nicotine injection is shown in Figure 6. The 2 × 2 × 12 (sex × chamber shape × time) mixed-factors ANOVA conducted on baseline data revealed no main effects of sex. The only significant main effect was of time [linear, time: F(1,78) = 442.567, p ≤ 0.001, ηp2 = 0.850; data not shown] and there were no significant interactions at Baseline.
Figure 6. Unadjusted Rearing.
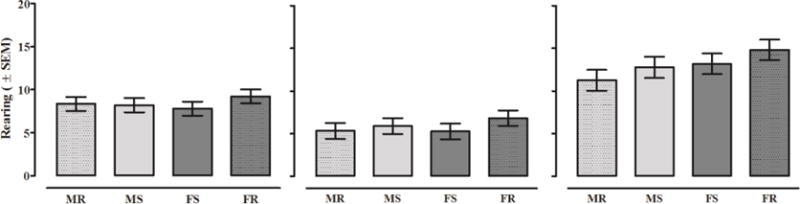
Mean rearing (± SEM) displayed by animals tested in square and round chambers on Baseline (left), ND1(middle), and ND21(right). There were no significant effects of biological sex or chamber shape on rearing behavior on baseline activity.
Although there were no sex differences at baseline, a 2 × 3 × 12 (chamber shape × day × time) mixed-factors ANCOVA was conducted on Baseline, ND1, and ND21 to be consistent with the strategy used to analyze horizontal activity and center entry data. Sex was not a significant covariate for rearing behavior [F(1,72) = 1.347, p ≤ 0.250]. The significant main effect of day [quadratic, day: F(1,72) = 9.380, p ≤ 0.003, ηp2 = 0.115] indicates that acute IV nicotine produced depression of rearing [p ≤ 0.001; Baseline: X = 8.395 ± 0.401; ND1: X = 5.826 ± 0.459] and repeated exposure resulted in increased rearing behavior relative to Saline Base [p ≤ 0.001; ND21: X = 13.013 ± 0.617]. Rats exhibited habituation of rearing behavior within 60-minute testing sessions [quadratic, time: F(1,72) = 59.377, p ≤ 0.001, ηp2 = 0.452] that was modified by nicotine exposure [quadratic-linear, day × time: F(1,72) = 12.138, p ≤ 0.001, ηp2 = 0.144; data not shown]. The chamber shape × time interaction [quadratic, chamber shape × time: F(1,72) = 4.401, p ≤ 0.039, ηp2 = 0.058; data not shown] indicates that rats tested in round chambers exhibited increased rearing relative to those in square chambers; however, this effect was transient, occurring only during the first five minutes of the test session [minute 5: F(1,72) = 4.468, p ≤ 0.038]. There was no effect of chamber shape for rearing behavior.
3.4. Occurrence of IV nicotine-induced locomotor depression and behavioral sensitization in the MR, MS, FS, and FR groups: Nominal and percent-change data
The results presented thus far indicate that testing chamber shape altered the ability to detect sex differences in horizontal activity and center entry behavior prior to nicotine exposure. When the variance in activity due to biological sex was adjusted for, chamber shape significantly influenced rats’ expression of nicotine-induced hypoactivity and behavioral sensitization of horizontal activity and center entries. However, using ANCOVA to determine the variance in activity due uniquely to chamber shape precluded the analysis of sex and chamber shape interactions. Thus, to know if the MR, MS, FS, and FR groups exhibited nicotine-mediated locomotor hypoactivity and sensitization, respectively; horizontal, center entry, and rearing data from each group was analyzed by comparing data from Baseline to ND1 and ND21 using simple within-subjects comparisons (ANOVA) on the unadjusted data, presented in Figures 2A, 4, and 6. The percent change from Baseline was also calculated to indicate the qualitative likenesses or differences between groups in the magnitude of the hypoactive and sensitizing effects of nicotine. The occurrence of hypoactivity and sensitization, the percent-change data, and the F-statistics for males and females are shown in Table 1, respectively. These analyses are justified by the sex × chamber shape interactions found prior to nicotine exposure to determine if the expression of drug-induced hypoactivity and sensitization in round and square chambers are consistent across sex. Furthermore, investigating potential differences in the occurrence of sensitization in each group remains important for novel hypotheses regarding sex differences in the stimulus properties of acute and repeated nicotine exposure.
Table 1.
Occurrences of hypoactivity and behavioral sensitization.
| MALES | Round | Square | ||||
|---|---|---|---|---|---|---|
| Incidence1 | % change | F-statistic | Incidence | % change | F-statistic | |
| Horizontal Activity | ||||||
| Hypoactivity | Yes | 32.5% ↓ | F(1,18) = 27.561, p ≤ 0.001 | Yes | 26.3% ↓ | F(1,18) = 14.550, p ≤ 0.001 |
| Sensitization | No | 4.6% | F(1,18) = 0.480, p ≤ 0.497 | Yes | 19.9% ↑ | F(1,18) = 11.556, p ≤ 0.003 |
| Center Entries | ||||||
| Hypoactivity | Yes | 28.4% ↓ | F(1,18) = 9.452, p ≤ 0.007 | No | 1.0% | F(1,18) = 0.004, p ≤ 0.948 |
| Sensitization | Yes | 54.7% ↑ | F(1,18) = 66.308, p ≤ 0.001 | Yes | 139.2% ↑ | F(1,18) = 24.606, p ≤ 0.001 |
| Rearing | ||||||
| Hypoactivity | Yes | 36.2% ↓ | F(1,18) = 22.851, p ≤ 0.001 | Yes | 28.0% ↓ | F(1,18) = 9.036, p ≤ 0.008 |
| Sensitization | Yes | 34.7% ↑ | F(1,18) = 13.989, p ≤ 0.001 | Yes | 55.8% ↑ | F(1,18) = 18.707, p ≤ 0.001 |
| FEMALES | Round | Square | ||||
| Incidence | % change | F-statistic | Incidence | % change | F-statistic | |
| Horizontal Activity | ||||||
| Hypoactivity | Yes | 22.2% ↓ | F(1,18) = 14.478, p ≤ 0.001 | Yes | 21.2% ↓ | F(1,18) = 8.463, p ≤ 0.009 |
| Sensitization | Yes | 16.4% ↑ | F(1,18) = 6.044, p ≤ 0.024 | Yes | 30.3% ↑ | F(1,18) = 18.933, p ≤ 0.001 |
| Center Entries | ||||||
| ypoactivity | No | 7.5% | F(1,18) = 0.538, p ≤ 0.473 | Yes | 59.5% ↓ | F(1,18) = 7.245, p ≤ 0.015 |
| Sensitization | Yes | 93.5% ↑ | F(1,18) = 43.393, p ≤ 0.001 | No | 1.6% | F(1,18) = 0.005, p ≤ 0.943 |
| Rearing | ||||||
| Hypoactivity | Yes | 26.1% ↓ | F(1,18) = 4.918, p ≤ 0.040 | Yes | 32.5% ↓ | F(1,18) = 8.202, p ≤ 0.010 |
| Sensitization | Yes | 60.4% ↑ | F(1,18) = 17.271, p ≤ 0.001 | Yes | 69.2% ↑ | F(1,18) = 10.672, p ≤ 0.004 |
Yes or no refers to whether significant hypoactivity or motor sensitization was found by within-subjects tests on the data presented in Figures 2A, 4, and 6. The ↓ or ↑ symbols denote the direction of change, relative to baseline.
3.4.1. Hypoactivity following acute IV nicotine
If the outcome measure was either horizontal activity or rearing both sexes exhibited significant locomotor depression following acute injection of IV nicotine, regardless of chamber type. The occurrence of nicotine-induced hypoactivity of center entries in females and males, however, depended on the shape of the chamber. Acute nicotine elicited hypoactivity in females tested in square (p ≤ 0.015; FS = 59.1% ↓), but not round chambers (F < 1.0; FR = 7.8% ↓). Males exhibited hypoactivity if tested in round (p ≤ 0.001; MR = 28.2% ↓), but not square chambers (F < 1.0; MS = 0% change). The lack of locomotor depression in these groups is not likely due to floor effects because there were no instances of individual animals in either the MS or FR groups that showed raw scores of zero entries into the center when the data are represented as collapsed over the entire hour (see Figure 4).
For the males, round chambers yielded the higher overall percent decrease in activity according to all three measures (i.e., MR > MS). For the females, the percent decrease in horizontal activity was within one percent difference between the FS and FR groups (i.e., FR = FS). Otherwise, the FS group showed the greatest percent decreases in center entries (59.1%) and rearing (31.2%; Table 1). These data indicate that both round and square chambers are sensitive to measure locomotor depression induced by acute IV nicotine (0.05 mg/kg/injection); however, the sensitivity of detecting decreases in centrally directed behavior following acute nicotine may depend on both biological sex and the type of chamber used for testing.
3.4.2. Locomotor sensitization following repeated IV nicotine
The upper panel of Table 1 shows that the males tested in either round or square chambers developed behavioral sensitization according to all three measures, with the exception of the MR group, which did not show a significant increase in horizontal activity. The percent increase in horizontal activity shown by the MR group, from Baseline to ND21 was 5%, and the within-subjects comparison of these two test days was non-significant (F < 1.0). The percent increase for the MS group was 20% and it was a significant increase from Baseline (p ≤ 0.003). Lastly, the square chambers yielded the greatest percent increase for all three measures compared to round chambers (horizontal activity: 20% vs. 5%; center entry: 139% vs. 54%; rearing: 56.1% vs. 34.5%).
The lower panel of Table 1 shows that aside from the FS group, females exhibited behavioral sensitization according to all three-outcome measures. The exception was with square chambers and the center entry measure. The percent-change of center entries from Baseline to ND21 in the FS group was 0% and the comparison was non-significant (F < 1.0), whereas the FR group showed a 93% increase, and the comparison was also significant (p ≤ 0.001). Thus, for the measure of center entry, the round chambers are more sensitive to detect nicotine-induced behavioral sensitization in females when the testing chambers are round. Otherwise, the highest percent increases were in square chambers. The FS vs. FR percent-changes for horizontal activity were 30% and 16.5% and 69.9% and 60.1% for rearing, respectively.
4. Discussion
The current experiment determined if the testing chamber shape, round vs. square, altered the activity of male and female rats to significantly influence the detection of shifts in behavior driven by acute and repeated IV nicotine exposure.
First, sex differences in activity during baseline testing were observed for two of the three outcome measures, but only in round chambers. Second, acute IV nicotine produced depression of activity and repeated IV nicotine produced behavioral sensitization; however, the shape of the chamber in which testing occurred modulated the magnitude of these shifts in horizontal activity and center entries. Third, the influence of chamber shape over the magnitude of nicotine-induced hypoactivity or sensitization is dependent on an animal’s biological sex, as seen most clearly with completely opposing consequences on center entries in male and female rats. Our findings indicate that the ability to detect nicotine-induced changes to horizontal activity and center entries changes as a function of chamber shape; however these shifts in behavior are also impacted by the biological sex of an animal. By examining the four experimental groups (MR, MS, FS, and FR), the current findings demonstrate that males and female rats display complex, and sometimes opposing, responses to testing in round and square chambers following nicotine treatment.
4.1. Sex differences at baseline
Men and women display different rates of drug use and other drug-related behaviors. It has been suggested that women develop nicotine dependence at a faster rate (Westermeyer & Boedicker, 2000) and exhibit lower rates of smoking cessation compared to males (Perkins, 1999). Our findings indicate that there were biological sex differences in activity at baseline, which is consistent with previous results showing sex differences in locomotor activity prior to drug administration (Booze et al., 1999; Kanyt et al., 1999; Hamilton et al., 2009; Zakharova, et al., 2012; Hamilton et al., 2014; Lacy et al., 2016). In most of those experiments, females are generally reported to be more active than males at baseline or saline control. The pattern of sex differences reported at baseline in the current study is novel and of interest in that it shows that the order of magnitudes representing the sex difference between animals tested in round chambers was the opposite (i.e., F > M; M > F) depending on the measure. For horizontal activity, FR > MR (FS = MS), whereas for the center entry measure, MR > FR (and MS = FS). It is important to note that there were no sex differences for either measure during baseline when testing occurred in square chambers; however, in round chambers the order of magnitude for males or females changed depending on the measure. Additionally, there were no sex differences for the measure of rearing. It is clear that reported sex differences in nicotine-induced changes to locomotor behavior can also change as a function of the dependent variable of interest. Harrod et al., (2004) reported that center entry behavior, when male and female rats were tested in round chambers, significantly predicted differential levels of dopamine transporter and D3 receptors in the nucleus accumbens of male and female rats. It is of interest to examine if a correlational relationship exists between horizontal or rearing activity and levels of the same or other dopaminergic markers in the nucleus accumbens of male and female rats.
The assessment of baseline activity revealed sex differences in horizontal and centrally directed activity that were dependent on the round-shaped testing chamber. There were no sex differences on any measure if animals were tested in square chambers. On one hand, the findings from the present experiment support previous research showing that females are more active than males during initial testing, e.g., horizontal activity. The same experiment also shows, however, that males exhibited more activity than females if center entry was the outcome. These findings indicate that sex differences in baseline activity are more likely to occur in round testing chambers.
Our findings also indicate that center entries was the only measure that yielded a main effect of shape, indicating that animals tested in round chambers exhibited more centrally-directed behavior than animals tested in square chambers at baseline. Certain behavioral metrics, such as center entries, appear to be more sensitive to changes in chamber shape than metrics such as horizontal activity or rearing. It is unclear if characteristics of the testing environment are able to instruct certain, more sensitive, behaviors directly or if such behaviors are mediated by a central process and are thus indirectly influenced by the environment. For example, center entry behavior in rats, similar to time spent in open arms of an elevated plus maze (Elliott et al., 2004; Caldarone et al., 2008) or time spent in the center of an open field apparatus (Cao, et al., 2010) may be mediated by an internal mechanism such as stress. As such, characteristics of the testing environment may induce anxiogenic or anxiolytic responses in rats that indirectly moderate center entry behavior making this metric more sensitive to environmental changes than behaviors that are not as heavily influenced by stress.
4.2. Chamber Shape modulates IV nicotine-induced changes in activity
Without considering biological sex differences, chamber shape significantly altered the magnitude of change from Baseline to nicotine treatment when the dependent measure was horizontal activity or center entries. Animals in round and square chambers both displayed significant hypoactivity and behavioral sensitization when measured by changes to horizontal activity levels. While hypoactivity induced by acute nicotine was not substantially influenced by chamber shape, the percent-change from Baseline to ND21 was reduced in round chambers compared to square. Additionally, animals tested in square chambers displayed sensitization of horizontal activity for more of the 60-minute session on ND21 than those tested in round chambers. The current results indicate that square chambers may provide a higher sensitivity than round chambers to detect nicotine-induced behavioral sensitization, but not hypoactivity, of horizontal activity.
When center entry was the dependent measure, round chambers, compared to square chambers, increased the number of center entries made by rats at baseline. This main effect of chamber shape clearly shows that prior to nicotine administration, round chambers profoundly increase center entries displayed by male and female rats (Figure 4). Given the differences at baseline, it is difficult to determine if there are differences in the magnitude of nicotine-induced hypoactivity or sensitization between square and round chambers. For example, the magnitude of sensitization in square chambers (55.6% increase) appears to be reduced compared to that in round chambers (71.1% increase) however the significant shift in center entry levels at baseline may be, in part, responsible for an increased ability to detect nicotine-induced hypoactivity and sensitization in round chambers.
4.3. Occurrences of hypoactivity and behavioral sensitization in the MR, MS, FS, and FR groups
Nicotine-induced hypoactivity and behavioral sensitization of horizontal activity expressed by male and female rats changed depending on whether testing took place in round or square chambers. The current finding supplements those of other experiments that establish sex differences in the influence of non-pharmacological components of testing conditions over behavior prior to drug administration (Zakharova et al., 2012), during chronic drug exposure (Hamilton et al., 2014), and in response to nicotine withdrawal (Hamilton et al., 2009). While hypoactivity was not substantially influenced by chamber shape in the current experiment, for both male and female rats, the percent increase induced by repeated nicotine is greater in square chambers. Interestingly, behavioral sensitization of horizontal activity was apparent in all groups except the MR group. While a similar decrease in the degree of sensitization was also seen in the FR group compared to FS, sensitization was still statistically significant in the FR group. Two important points can be made from these findings. First, nicotine-induced behavioral sensitization in males is severely blunted if tested in round, relative to square chambers. Second, females exhibited nicotine-induced behavioral sensitization in both environments, indicating that the females showed greater nicotine-induced behavioral sensitization, relative to males.
Similarly, the influence that chamber shape and biological sex have together, as displayed in Table 1, on our ability to detect hypoactivity or behavioral sensitization of center entries is complex. The MR group displayed significant nicotine-induced hypoactivity and behavioral sensitization of center entries. In comparison, the MS group did not display hypoactivity of center entries following acute nicotine, but displayed significant behavioral sensitization with chronic nicotine that was enhanced compared to the MR group. Thus, for male rats, it appears our ability to detect hypoactivity is greater in round chambers whereas our ability to detect behavioral sensitization is enhanced in square chambers, although also detectable in round. The influence of chamber shape over the ability to detect hypoactivity and behavioral sensitization of center entries was more prominent in females. While the FR group failed to show hypoactivity but showed significant behavioral sensitization, the FS group showed significant hypoactivity but failed to show sensitization of center entries with nicotine treatment. In sharp contrast to males, our ability to detect hypoactivity in female rats appears to be enhanced in square chambers compared to round, whereas our ability to detect sensitization is enhanced in round chambers compared to square.
4.4. Limitations & Future Directions
The limitations of the current study may provide directions for future analysis of nicotine-induced behavior in male and female rats. First, because our primary analysis did not reveal significant sex × day interactions and our follow-up analysis only compared male and female rats within a respective chamber shape, it is it is difficult to comment on if females were generally more active than males across chambers, i.e., sex differences in behavioral sensitization, as was shown in Booze et al., (1999) and Harrod et al., (2004). However, results from our analysis at Baseline indicated that there were no significant differences between groups when rearing activity was the dependent measure. Increases in rearing as a function of repeated nicotine can be seen in Figure 6 by comparing group activity levels at the left and right panels. With no significant group differences at Baseline, females appear to show enhanced sensitization of rearing behavior compared to males. If so, this would be consistent with the results of Harrod et al., (2004).
Second, behaviors other than horizontal activity, center entries, and rearing, that are not analyzed in the current experiment may provide a different interpretation of male and female rat’s differential responses to nicotine or chamber shape. Nicotine exerts a variety of complex effects at different levels of the nervous system and further knowledge can be gained from analyzing the influence of these factors on behaviors such as time spent resting or distance traveled throughout the session. However, horizontal activity, center entries, and rearing were chosen for analysis in the current experiment because they were thought to be orthogonal in nature. Whereas, for example, horizontal activity (also known as “ambulations”) and distance traveled both measure horizontal movement and would likely yield redundant results (Ksir, 1994). In addition, future research should aim to replicate the current experiment using observed measures of behavior rather than automatically recorded behaviors to confirm and extend the current results. Observational tests of behavior, such as those conducted in Fray et al., (1980), were not included in the current experimental procedure to prevent the experimenter’s presence from modifying animal behavior as a function of chamber shape.
Third, as a major consideration in the design of this study involved biological sex differences in responses to nicotine exposure, it may have been beneficial to track changes to hormone levels throughout the testing period. Gonadal hormones affect plasma levels of nicotine in rats following repeated IV nicotine injections (Harrod et al., 2007) and further, the menstrual cycle reportedly effects the subjective effects of smoking and withdrawal severity in human females (Lynch, 2009). As such, tracking of the estrous cycle in female rats may provide evidence for hormonal correlates with behavioral responses to nicotine. However, Booze et al., (1999) demonstrated that female rats maintain the normal pattern of estrous cyclicity with over 14 days of IV nicotine treatment under procedures similar to the current experiment. Additionally, variability between female rats was not distinctly greater than that between males in the current experiment, suggesting that hormones may not have a prominent influence over the measured motor behaviors in rats.
4.6. Conclusions
In combination with the existing literature, our results demonstrate that certain behavioral metrics used to characterize responses to nicotine treatment or withdrawal are sensitive to changes in the testing chamber’s shape. Inconsistent experimental results within or between laboratories may be a product of testing conditions that influence behavior patterns. What is most interesting is that there was a significant chamber shape × biological sex interaction for horizontal activity and center entry measures, suggesting that shifts in behavior elicited by chamber shape are different for male and female rats. The contrasting influence of chamber shape in male and female rats has significant implications for interpreting sex-dependent shifts in rats’ behavioral responses, and may be of considerable interest to investigators that emphasize rigor and reproducibility in studies of sex differences and drugs of abuse.
An unidentified mechanism may exist to promote sex differences and motor responses to nicotine when the corners of the testing chamber are removed. Mechanisms underlying cue sensitivity may be related to those underlying the influence of chamber shape over behavior. However, it is important to recognize that in the current experiment, rats were placed in testing chambers on only 3 occasions, limiting the opportunity for an association to form between the unconditional effects of nicotine and the conditional effects of the testing environment. Nonetheless, these limited opportunities may still be sufficient for an association to form between the context and the effects of nicotine and so some activity could have been elicited by the chamber alone. Thus, if females indeed acquire drug-context associations faster than males, this may also be a source of differential expression of behavior. As such, it is difficult to comment to what degree sensitivity to environmental cues influence the sex differences presented in the current results.
O’Dell and Torres (2014) have hypothesized that females display altered vulnerability to nicotine initiation and nicotine withdrawal as a result of estrogen-induced adjustments to levels of dopamine and corticotrophin-releasing factor. An interaction between nicotine and stress responses in females may drive some of the complicated interactions seen between sex and chamber shape, specifically for the measure of center entries. Nicotine has been reported to enhance anxiety-like behavior in female rodents at a lower dose than males when measured by elevated-plus maze (Elliot et al., 2004; Caldarone et al., 2008). Interestingly, using square automated activity chambers, similar in size to those used in the present experiment (i.e., 43.2 × 43.2 cm), Cao et al., (2010) report that adult male rats exhibited more center entries following acute IV nicotine injection relative to their baseline (IV Saline). Adult female rats displayed decreased center entry behavior following acute IV nicotine compared to baseline, together suggesting that nicotine injection produces an anxiogenic effect in females and an anxiolytic effect in males. In the present study, adult male rats did not exhibit significant decreases in center entries following acute IV nicotine; however, female rats displayed an attenuation of center entries, consistent with Cao et al., (2010). The present experiment did not use an elevated-plus maze and did not measure corticosterone following IV nicotine injection. Thus it is unclear if the decrease in centrally-directed behavior - as well as the sex difference on this measure - is mediated by nicotine’s anxiogenic effects per se (Elliot et al., 2004; Caldarone et al., 2008; Cao et al., 2010). It is noteworthy that acute IV nicotine produced the opposite effect when females and males were tested in round chambers: males showed significant locomotor depressant effects, relative to saline baseline, whereas there were no differences in hypoactivity with the female round group. The sex difference following acute IV nicotine cannot be attributed to possible anxiogenic effect alone because opposing behavioral responses were observed in square and round chambers.
It is unclear which chamber shape, round or square, elicits a pattern of behavior that most accurately represents the influence of nicotine over overt behaviors in male and female rats. The initial hypothesis, that round chambers would increase activity overall and therefore enhance detection of behavioral sensitization, was based on the premise that rodents exhibit thigmotaxis and may remain idle in the corners of square chambers. The current findings demonstrate that at baseline round chambers produce more center entries than square chambers. For investigators interested in centrally-directed behavior, we therefore suggest using round over square chambers. For studies examining sex differences in repeated nicotine administration, square chambers are suggested if horizontal activity is the preferred measure based on the current results showing that both male and female rats exhibited sensitization in square but not round chambers. Rearing behavior does not appear to depend on chamber shape as both female and male rats exhibited significant sensitization, regardless of shape. Given the interest in elucidating mechanisms which mediate biological sex differences in drug effects (e.g. sex hormones, corticosterone, or pharmacodynamics) it would be important for future experimenters to balance chamber shapes within studies to provide a complete interpretation of sex differences in responses to drug administration.
Highlights.
Male and female rats display different levels of horizontal activity and center entries prior to drug administration when tested in round, but not square chambers.
The testing environment modulates nicotine-induced depression of activity and behavioral sensitization.
A complete interpretation of nicotine-induced hypoactivity, behavioral sensitization, and sex differences may require that both round and square testing chambers are included in experimental procedures.
Acknowledgments
We thank Dr. Amanda Fairchild of the University of South Carolina for her assistence in our analysis procedure, as well as Dr. Sylva Fitting and Holly Jarrell for participation in the experiments. This work was supported by the National Institutes of Health (DA013712, R.M.B; DA013137, R.M.B) and in part by a NIH T32 training grant supported by the University of South Carolina Behavioral-Biomedical Interface Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker JB. Sex differences in addiction. Dialogues in Clinical Neuroscience. 2016;18:395–402. doi: 10.31887/DCNS.2016.18.4/jbecker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacological Reviews. 2016:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacologica aspects of cigarrent smoking and nicotine addiction. New England Journal of Medicine. 1988;319:1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Booze RM, Welch MA, Wood ML, Billings KA, Apple SR, Mactutus CF. Behavioral sensitization following repeated intravenous nicotine administration: gender differences and gonadal hormones. Pharmacology Biochemistry and Behavior. 1999;64:827–839. doi: 10.1016/s0091-3057(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neuroscience Letters. 2008;439:187–191. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Nestler EJ. Dopaminergic dynamics underlying sex-specific cocaine reward. Nature Communications. 2017;8 doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Dao JM, Chen Y, Leslie FM. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2010;96:82–90. doi: 10.1016/j.pbb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self- administration in rats. Psychopharmacology. 2005;180:258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. British Journal of Pharmacology. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: Integrating the clinical and basic science literature. Nicotine & Tobacco Research. 2005;7:9–26. doi: 10.1080/14622200412331328538. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2004;77:21–28. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Ericson M, Norrsjo G, Svensson AI. Behavioral sensitization to nicotine in female and male rats. Journal of Neural Transmission. 2010;117:1033–1039. doi: 10.1007/s00702-010-0449-9. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Sahakian BJ, Robbins TW, Koob GF, Iversen SD. An observational method for quantifying the behavioral effects of dopamine agonists: contrasting effects of d-amphetamine and apomorphine. Psychophamacology. 1980;69:253–259. doi: 10.1007/BF00433091. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Winders SE, Popp KA. Sex differences in nicotine’s effects on consummatory behavior and body weight in rats. Psychopharmacology. 1987;91:221–225. doi: 10.1007/BF00217067. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Berger SS, Perry ME, Grunberg NE. Behavioral effects of nicotine withdrawal in adult male and female rats. Pharmacology Biochemistry and Behavior. 2009;92:51–59. doi: 10.1016/j.pbb.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Elliott BM, Berger SS, Grunberg NE. Environmental enrichment attenuates nicotine behavioral sensitization in male and female rats. Experimental and Clinical Psychopharmacology. 2014;22:356–363. doi: 10.1037/a0037205. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Booze RM, Mactutus CF. Sex differences in nicotine levels following repeated intravenous injection in rats are attenuated by gonadectomy. Pharmacology Biochemistry and Behavior. 2007;86:32–36. doi: 10.1016/j.pbb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, Booze RM. Sex differences and repeated intravenous nicotine: behavioral sensitization and dopamine receptors. Pharmacology Biochemistry and Behavior. 2004;78:581–592. doi: 10.1016/j.pbb.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Kanyt L, Stolerman IP, Chandler CJ, Saigusa T, Pogun S. Influence of sex and female hormones on nicotine- induced changes in locomotor activity in rats. Pharmacology Bichemistry and Behavior. 1999;62:179–187. doi: 10.1016/s0091-3057(98)00140-3. [DOI] [PubMed] [Google Scholar]
- Ksir C. Acute and chronic nicotine effects on measures of activity in rats: a multivariate analysis. Psychopharmacology. 1994;115:105–109. doi: 10.1007/BF02244758. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Brown RW, Morgan AJ, Mactutus CF, Harrod SB. Intravenous prenatal nicotine exposure alters METH- induced hyperactivity, conditioned hyperactivity, and BDNF in adult rat offsrping. Developmental Neuroscience. 2016;38:171–185. doi: 10.1159/000446563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacology Biochemistry and Behavior. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Herman AS, Booze RM. Chronic intravenous model for studies of drug (ab)use in the pregnant and/or group-housed rat: an initial study with cocaine. Neurotixicology and Teratology. 1994;16:183–191. doi: 10.1016/0892-0362(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Morrison CF, Stephenson JA. The occurrance of tolerance to a central depressant effect of nicotine. British Journal of Pharmacology. 1972;45:151–156. doi: 10.1111/j.1476-5381.1972.tb06857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology. 2014;76:566–580. doi: 10.1016/j.neuropharm.2013.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Nicotine discrimination in men and women. Pharmacology Biochemistry and Behavior. 1999;64:295–299. doi: 10.1016/s0091-3057(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Structure and Function. 2015;220:2415–2422. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sciences. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addicition. Brain research reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Mirza NR. Dissociations between the locomotor stimulant and depressant effects of nicotinic agonists in rats. Psychopharmacology. 1995;117:430–437. doi: 10.1007/BF02246215. [DOI] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Carroll ME. Sex differences in the aquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology. 2016;233:1005–1013. doi: 10.1007/s00213-015-4183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syamlal G, Mazurek JM, Dube SR. Gender differences in smoking among U.S. working adults. American Journal of Preventive Medicine. 2014;47:467–475. doi: 10.1016/j.amepre.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking-50 Years of Progress. A Report of the Surgeon General: Executive Summary. 2014:1–36. [Google Scholar]
- Westermeyer J, Boedicker AE. Course, Severity, and Treatment of Substance Abuse Among Women Versus Men. American Journal on Drug and Alcohol Abuse. 2000;26:523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Starosciak A, Wade D, Izenwasser S. Sex differences in the effects of social and physical environment on novelty-induced exploratory behavior and cocaine-stimulated locomotor activity in adolescent rats. Behavioural Brain Research. 2012;230:92–99. doi: 10.1016/j.bbr.2012.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


