Abstract
Plasma concentrations of thalidomide and primary 5-hydroxylated metabolites including 5,6-dihydroxythalidomide and glutathione (GSH) conjugate(s) were investigated in chimeric mice with highly “humanized” liver cells harboring cytochrome P450 3A5*1. Following oral administration of thalidomide (100 mg/kg), plasma concentrations of GSH conjugate(s) of 5-hydroxythalidomide were higher in humanized mice than in controls. Simulation of human plasma concentrations of thalidomide were achieved with a simplified physiologically-based pharmacokinetic model in accordance with reported thalidomide concentrations. The results indicate that the pharmacokinetics in humans of GSH conjugate and/or catechol primary 5-hydroxylated thalidomide contributing in vivo activation can be estimated for the first time.
Graphical Abstract

The chemotherapeutic drug thalidomide is metabolized by cytochrome P450 (P450) 3A4/5 in human livers to 5-hydroxythalidomide and further oxidation, leading to non-enzymatic glutathione (GSH) conjugation,1,2 which may be relevant to the pharmacological and toxicological actions. However, the disposition of these human proportionate phenyl ring-based 5-hydroxythalidomide-GSH conjugates in human in vivo situations is not known.3 “Humanized” TK-NOG mice4,5 were developed by transplantation of human liver cells. In vivo kinetic cooperativity of human P450 3A enzymes was reported, with a higher area-under-the-curve value for 1´-hydroxymidazolam following co-treatment with thalidomide in the humanized mice.6 Although in vivo formation of glutathione conjugate and 5,6-dihydroxylated metabolites derived from thalidomide and primary 5-hydroxythalidomide were recently determined in humanized TK-NOG mice,5 those metabolic profiles were strongly affected by the predominant mouse P450 enzymes in the TK-NOG mice, as judged by a relatively high concentration of 5´-hydroxythalidomide than that of parent substrate, a major product formed in rodents.
The purpose of this study was to re-investigate the oxidative metabolism of thalidomide by human liver in vivo to better understand the activation of thalidomide in TK-NOG mice containing highly substituted (>95%) human liver cells harboring P450 3A5*1. Human hepatocytes transplanted in this study were used to overcome the species differences. Concentrations of human secondary oxidized thalidomide metabolites (involving conjugation of 5-hydroxythalidomide with GSH in vivo were simulated here using a physiologically-based pharmacokinetic (PBPK) model, indicating that activation of thalidomide could occur to produce an electrophilic product in human liver after single or repeated doses.
Thalidomide and its primary (5´- and 5-hydroxythalidomide) and secondary metabolites (5-hydroxythalidomide-GSH conjugate) were measured (Figures 1 and 2) by LC-MS/MS analysis in plasma samples obtained from mice following oral administration (100 mg/kg). Thalidomide was detected 30 min following oral administration (Figure 2A). Plasma concentrations of 5´-hydroxythalidomide were significantly higher in control TK-NOG mice than in humanized TK-NOG mice (Figure 2B). Plasma concentrations of the primary metabolite 5-hydroxythalidomide were similar in two systems, but the secondary metabolite 5-hydroxythalidomide-GSH conjugate(s) (Figure 2D) formed in vivo was preferentially produced in humanized TK-NOG mice. Roughly equivalent levels of 5´-hydroxythalidomide and 5-hydroxythalidomide plus 5-hydroxythalidomide-GSH conjugates were observed in plasma samples from humanized TK-NOG mice administered thalidomide under these conditions. One-tenth the level of 5,6-dihydroxythalidomide compared with 5-hydroxythalidomide-GSH conjugates was seen in humanized TK-NOG mice (results not shown). The possible production of reactive metabolites of thalidomide formed by intestinal mouse P450s has not yet been considered in the case where the drug is administered orally to chimeric mice with humanized livers.
Figure 1.
Chemical structures of thalidomide and its metabolites.
Figure 2.
Plasma concentrations of thalidomide (A), 5´-hydroxythalidomide (B), 5-hydroxythalidomide (C), and 5-hydroxythalidomide-GSH conjugate (D) in control TK-NOG mice (open circles) and chimeric TK-NOG mice with humanized liver cells (solid triangles) after oral administration of thalidomide (100 mg/kg). Results are expressed as mean values (± SD) obtained with four mice (***p < 0.001, **p < 0.01, and *p < 0.05, two-way ANOVA with Bonferroni post tests).
Limited information is available regarding thalidomide primary metabolism in vivo, especially in humans. Only the 5’-hydroxy metabolite was reportedly found in low concentrations in plasma samples from eight healthy male volunteers who received thalidomide orally; other metabolites could not be found (HPLC, detection limit 1–2 ng/mL).7 No peaks corresponding to hydroxylated thalidomide metabolites have been also noted in the chromatograms for the other seven patient plasma fractions.8
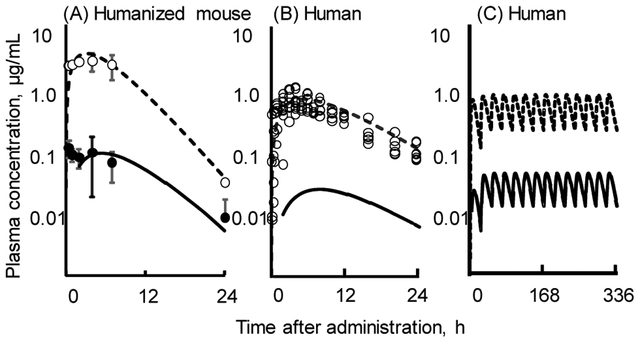
Based on the present in vivo experiments, the kinetic parameters in chimeric TK-NOG mice were calculated and are shown in Table S2 (Supporting Information). By solving the equations that make up the simplified PBPK models, plasma concentration curves were created; the resulting estimated in silico concentration curves are shown (Figure 3A). Using an allometric scaling method and derived values (Table S2, Supporting Information), human PBPK models for thalidomide and its combined primary metabolites were set up based on the human PBPK models (Figure 3B). The available reported human plasma data,7 obtained after administration of a single or multiple low doses of 100 mg thalidomide to human subjects, could be reasonably estimated by the present simplified human PBPK model, with a linear assumption (Figures 3B, 3C).
Figure 3.
Plasma concentrations of thalidomide (open circles, broken lines) and the sum of 5-hydroxythalidomide metabolites containing 5-hydroxythalidomide-GSH conjugate and 5,6-dihyrdoxythalidomide (filled circles, solid lines) measured in chimeric TK-NOG mice with humanized liver cells (A) and estimated in humans (B,C) after oral administration of a single (A,B) or multiple doses (C) of thalidomide (100 mg/kg for mice and 100 mg per day for humans).
In the present study, we analyzed thalidomide metabolism in vivo using humanized TK-NOG mice, in which the liver was highly replaced with transplanted human liver cells harboring P450 3A5*1. Taken together, evidence is presented that 5-hydroxythalidomide can be further oxidized by human liver and is further oxidized to form highly reactive and unstable intermediate(s). Although Tao et al.9 have reported two cases of slow elimination of thalidomide in human plasma and/or semen from male patients administered thalidomide, relatively fast elimination of thalidomide and primary 5-hydroxylated metabolites from human blood were estimated in this study, based on the pharmacokinetics in the highly substituted humanized TK-NOG mice model.
In conclusion, the present study situationally demonstrates that human liver cells harboring P450 3A5 expressed in chimeric TK-NOG mice effectively mediate thalidomide 5-hydroxylation and further oxidation leading to a GSH conjugate and/or 5,6-dihydroxythalidomide in vivo, which may be relevant to its pharmacological and toxicological actions via adduct formation.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Drs. Miyuki Kuronuma, Makiko Shimizu, Shotaro Uehara, and Norie Murayama for their technical assistance.
Funding Sources
This work was supported in part by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (H.Y.) and United States National Institutes of Health grant R37 CA090426 (F.P.G.).
ABBREVIATIONS:
- P450
cytochrome P450
- PBPK
physiologically-based pharmacokinetic
- TK-NOG mice
a herpes simplex virus type 1 thymidine kinase transgene expressing highly immunodeficient non-obese diabetes- severe combined immunodeficiency- interleukin-2 receptor gamma chain-deficient mice
Footnotes
Supporting Information. Experimental Procedures, Table S1 (Chemical properties of thalidomide and the primary human metabolite), and Table S2 (Experimental and calculated parameters for PBPK models of thalidomide disposition in humanized mice and humans). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Chowdhury G, Murayama N, Okada Y, Uno Y, Shimizu M, Shibata N, Guengerich FP, and Yamazaki H (2010) Human liver microsomal cytochrome P450 3A enzymes involved in thalidomide 5-hydroxylation and formation of a glutathione conjugate. Chem. Res. Toxicol 23, 1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Chowdhury G, Shibata N, Yamazaki H, and Guengerich FP (2014) Human cytochrome P450 oxidation of 5-hydroxythalidomide and pomalidomide, an amino analog of thalidomide. Chem. Res. Toxicol 27, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Murayama N, van Beuningen R, Suemizu H, Guguen-Guillouzo C, Shibata N, Yajima K, Utoh M, Shimizu M, Chesne C, Nakamura M, Guengerich FP, Houtman R, and Yamazaki H (2014) Thalidomide increases human hepatic cytochrome P450 3A enzymes by direct activation of pregnane X receptor. Chem. Res. Toxicol 27, 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hasegawa M, Kawai K, Mitsui T, Taniguchi K, Monnai M, Wakui M, Ito M, Suematsu M, Peltz G, Nakamura M, and Suemizu H (2011) The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochem. Biophys. Res. Commun 405, 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Yamazaki H, Suemizu H, Shimizu M, Igaya S, Shibata N, Nakamura N, Chowdhury G, and Guengerich FP (2012) In vivo formation of dihydroxylated and glutathione conjugate metabolites derived from thalidomide and 5-hydroxythalidomide in humanized TK-NOG mice. Chem. Res. Toxicol 25, 274–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yamazaki H, Suemizu H, Murayama N, Utoh M, Shibata N, Nakamura M, and Guengerich FP (2013) In vivo drug interactions of the teratogen thalidomide with midazolam: Heterotropic cooperativity of human cytochrome P450 in humanized TK-NOG mice. Chem. Res. Toxicol 26, 486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Eriksson T, Bjorkman S, Roth B, Bjork H, and Hoglund P (1998) Hydroxylated metabolites of thalidomide: formation in-vitro and in-vivo in man. J. Pharm. Pharmacol 50, 1409–1416. [DOI] [PubMed] [Google Scholar]
- (8).Lu J, Palmer BD, Kestell P, Browett P, Baguley BC, Muller G, and Ching LM (2003) Thalidomide metabolites in mice and patients with multiple myeloma. Clin. Cancer Res 9, 1680–1688. [PubMed] [Google Scholar]
- (9).Teo SK, Chandula RS, Harden JL, Stirling DI, and Thomas SD (2002) Sensitive and rapid method for the determination of thalidomide in human plasma and semen using solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 767, 145–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.