Abstract
The double- or triple-decker 3D metallo-hexagons were obtained by self-assembly of multitopic tris-terpyridines with Cd2+ ions in near-quantitative yield. Comprising up to 72 ionic pairs, the multiple spoked wheels display characteristic reversible gelation properties under thermodynamic conditions. The supramolecular metallo-nanoarchitectures were characterized by 1H NMR, 2D NMR (COSY and NOESY), and diffusion-ordered spectroscopy (DOSY) and HR-ESI-MS, traveling-wave ion mobility mass spectrometry (TWIM-MS), TEM, and AFM. For the first time, the self-assembly of 45 units at once was demonstrated to yield exceptional giant triple-decker hexagons of up to circa 42000 Da.
Keywords: coordination, gelation, molecular spoked wheels, self-assembly, terpyridine
As an important part of supramolecular chemistry, coordination-driven self-assembly has attracted considerable attention owing to its promising applications in diverse fields, such as host–guest chemistry,[1] catalysis,[2,3] sensing,[4,5] biology,[6] and optical materials.[7] A series of high molecular-weight supramolecular architectures with precise shapes and sizes, from two-dimensional (2D) metallo-macrocycles[2,4,8] to three dimensional (3D) cages,[2,8a,9] polyhedra[10] and intricate molecular knots,[11] have been extensively reported. For the purpose of mimicking the complexity and functionality of biological systems, more elegant and complex molecules based on coordination-driven assembly have long been pursued by chemists. Among these, the self-assembly of huge discrete highly ordered supramolecular structures (MW > 30000 Da) is full of challenges in terms of their ligand design and architectural construction, which need precise control over the geometric angles and connectivity of multiple ligands and metal ions. Moreover, such motifs were desired for their potential applications in diverse fields.[12]
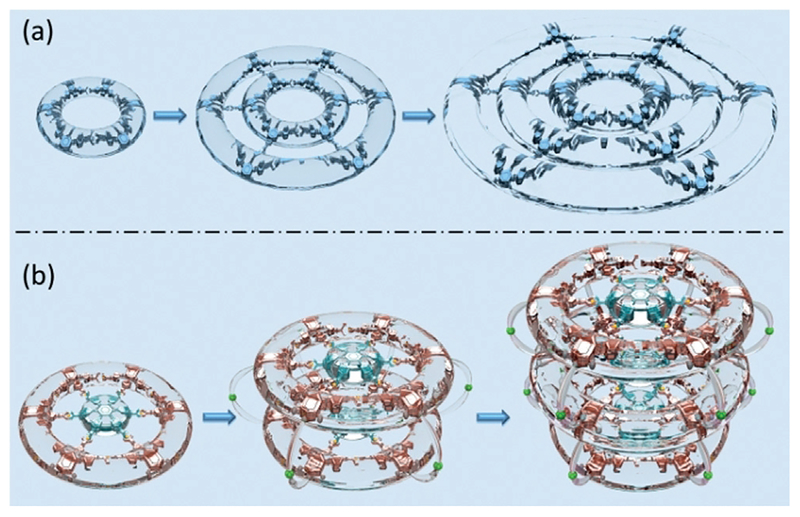
Tridentate 2,2′:6′,2″-terpyridine (tpy) and its analogues are common building blocks for constructing coordination-driven metallo-structures owing to their intrinsic ability to coordinate with different transition metal ions and excellent photoelectrical properties.[13] Much effort has also been devoted to construct complicated higher-order motifs through terpyridinyl ligand–metal coordination interactions. For example, Newkome and co-workers reported the self-assembly of the first generation Sierpiński triangle fractal and hexagonal gasket.[14] Li and co-workers prepared two species of concentric hexagons using tetratopic pyridine and terpyridine ligands coordinating with metal ions.[15] Furthermore, a well-defined spiderweb-like ring-in-ring-in-ring superstructure was successfully synthesized through self-assembly of the multivalent terpyridine ligands with metal ions by Chan et al.[16] It is worth noting that the above-mentioned super-structures were constructed by extending the structure in a 2D plane, just like the concentric ring system (Figure 1a). Herein, we introduce the 3D double- or triple-decker hexagon system by vertically extending the plane spoked wheels[17] into 3D space (Figure 1b).
Figure 1.
a) Cartoon representation of horizontal assembly of 2D metallo-macrocycles to extending ring-in-ring planar structures and b) vertical assembly of 3D double- or triple-decker hexagonal architectures.
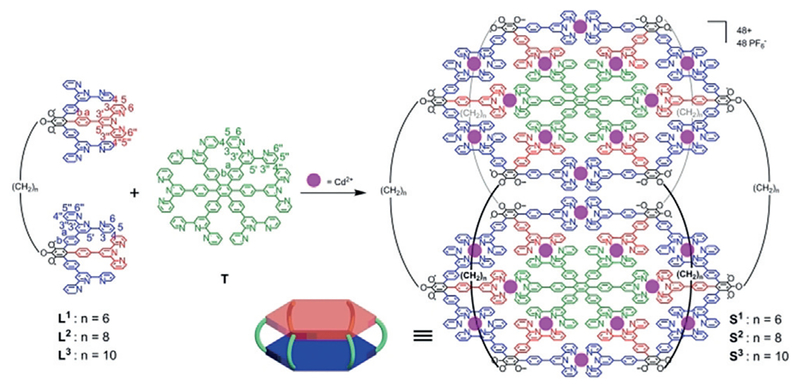
Initially, double tris-terpyridine compounds L1–3 were synthesized through a multi-fold Suzuki cross-coupling reaction of hexakis-Br-substituted 1a–1c with 4′-boronatophenyl- 2,2′:6′,2″-terpyridine using [Pd(PPh3)4] as the catalyst. Isolation was achieved by column chromatography (Al2O3) and recrystallization with CHCl3 and CH3OH. The structure of L1–3 was characterized by NMR spectroscopy and ESI-MS (Figure 2 and the Supporting Information).
Figure 2.
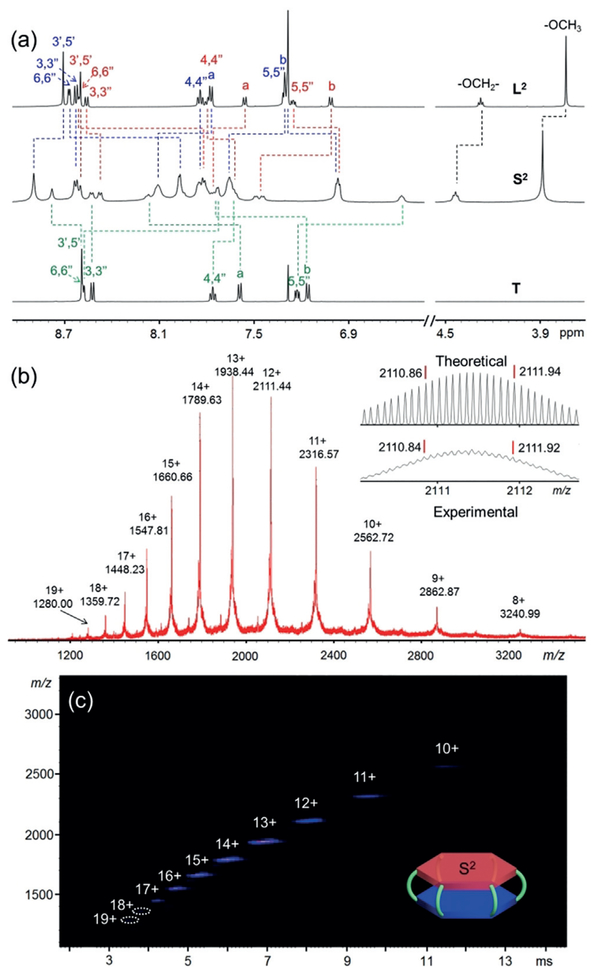
a) 1H NMR spectra (500 MHz) of ligands L2, T in CDCl3 and double-decker hexagon S2 in CD3CN. b) ESI-MS spectrum (Inset: theoretical and experimental isotopic patterns for 12 + moiety) and c) 2D ESI-TWIM-MS plot (m/z vs. drift time) of S2.
Self-assembly of S1 was performed by mixing a stoichiometric ratio (3:1:12) of L1, T, and Cd(NO3)2·4H2O in CHCl3/CH3OH at 65°C for 12 h. After adding excess NH4PF6, a pale-yellow precipitate was obtained followed by a thorough washing with CH3OH. Then S1 was generated as a faint yellow solid in 96% yield. S2 and S3 were synthesized in 95% and 98% yields, respectively, by a similar procedure as S1 (Scheme 1). 1H NMR spectra of ligands L2 and T and complex S2 are shown in Figure 2a. In the aromatic region of the NMR spectrum of ligand L2, two distinguishable sets of tpy resonances represented by two characteristic singlets at 8.71 and 8.60 ppm are observed, which were assigned to tpyH3′,5′ peaks; in the non-aromatic region, one triplet and one singlet appeared at 4.28 and 3.74 ppm in a 1:3 integration ratio, which were attributed to methylene and methoxy groups. For the complex S2, the 1H NMR spectrum exhibited broad resonance peaks presumably owing to the low tumbling motion on the NMR time scale for the large supramolecular architecture.[18] Three characteristic singlets corresponding to tpyH3′,5′ protons were observed at 8.89, 8.78, and 8.60 ppm with downfield shifts, along with the upfield shifts of tpyH6,6″ protons compared to L2 and T. Additionally, the methylene and methoxy protons at 4.44 and 3.88 ppm also exhibited down-field shifts, agreeing with the expected structure. Assignments of other protons were confirmed by 2D COSY and NOESY NMR experiments. In contrast to the slight downfield shifts in most of tpy-based supramolecular structures, the tpyH4,4″ and tpyH5,5″ protons in S2 exhibited distinct upfield shifts, especially for the tpy5,5″ protons of hexakis-terpyridine T (δ = 6.56 ppm, Δδ = 0. 67 ppm). This could be ascribed to the highly shielded environment from the proximity of two < tpy–Cd2+–tpy > connections and such a result strongly supported the stacking hexagon formation.[16,19] Similarly, the 1H NMR spectra of S1 and S3 showed distinct patterns and all of the peaks were fully assigned (see the Supporting Information).
Scheme 1.
Synthesis of double tris-terpyridines L1–3 and self-assembly of double hexagonal structures S1–3.
Electrospray ionization mass spectrometry (ESI-MS) spectrum of S2 showed a series of peaks with charge states from 8 + to 19 +, which correspond to the loss of a different number of PF6− anions (Figure 2b). The experimental isotope patterns were in accord with the corresponding simulated ones in exact agreement with a molecular weight of 27098.8 Da for S2. The traveling-wave ion mobility-mass spectrometry (TWIM-MS) spectrum (Figure 2c) exactly showed a set of narrow drift time distribution plots for the 10 + to 19 + species, indicating that no superimposed fragments, isomers, or conformers result from self-assembly. The compositions of S1 and S3 were also confirmed by ESI-MS (Supporting Information, Figures S61 and S62, respectively).
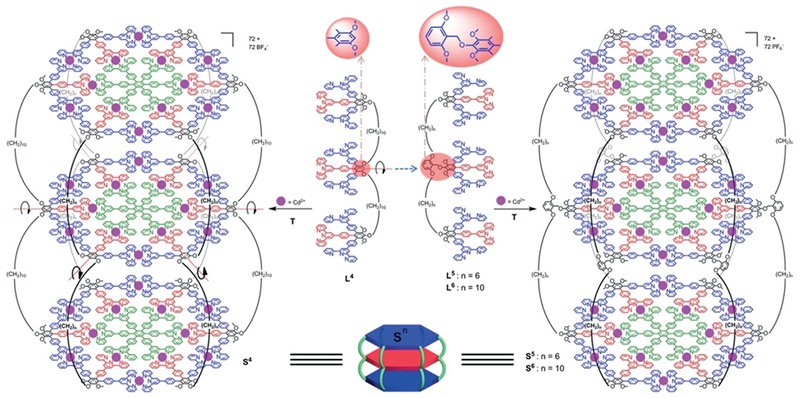
Encouraged by the successful self-assembly of the double-stacked hexagonal superstructures, L4 was synthesized by a multi-fold Suzuki coupling reaction and characterized by NMR spectroscopy and HR-ESI-MS (Figures S37, S38, and S48). The assembly experiment was conducted by mixing with L4, T, and Cd(NO3)2·4H2O in a precise 2:1:12 ratio following a similar procedure (Scheme 2, left). Unfortunately, the resultant assemblies were nearly insoluble in CH3CN. The NMR spectrum exhibited a complicated and broad pattern, demonstrating no discrete self-assembled structure formed (Figure S83). Likewise, the ESI mass spectrum (Figure S84) showed a big hump with no signals responding to the proposed triple-decker hexagon structure. In order to avoid an anion exchange process, Cd(BF4)2 was used for the self-assembly with L4 in CHCl3/CH3CN solution rather than Cd(NO3)2. ESI-MS measurements were directly performed for the assemblies, and the spectrum showed a series of successive peaks from 13 + to 23 +, which fitted well with the desired S4 (Figure S63), demonstrating the expected giant triple-decker spoked wheels S4 formed in solution.
Scheme 2.
Self-assembly of triple-decker hexagon S4–6.
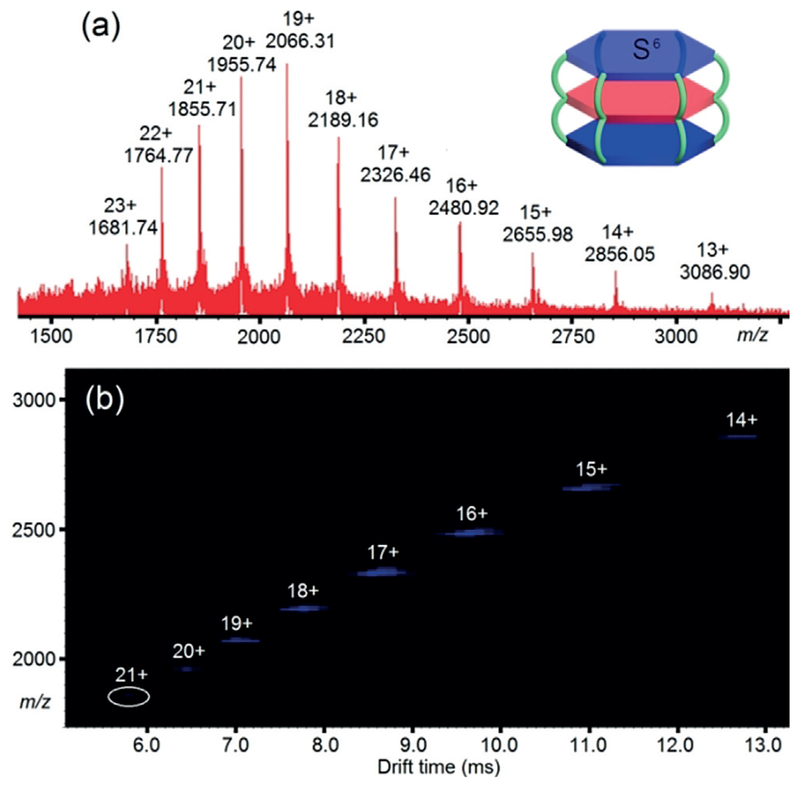
Based on above results, two modified flexible ligands L5 and L6 were further synthesized and fully characterized by NMR spectroscopy and HR-ESI (see Supporting Information). Self-assemblies of S5–6 were achieved by treating L5–6 and T with Cd(NO3)2·4H2O in a precise 2:1:12 ratio (Scheme 2, right). 1H NMR spectrum of S5 displayed a complex and broad resonance in the aromatic region, which was attributed to the conformational heterogeneity of the giant supramolecular structure.[18] Nevertheless, there were two diagnostic triplets at 4.46 and 4.25 ppm (methylene groups) and two sharp singlets at 3.86 and 3.92 ppm (methoxy groups) in the non-aromatic region, suggesting the formation of the expected discrete structure (Figure S59). The ESI mass spectrum of S6 (Figure 3a) showed eleven distinct peaks with continuous charge states (from 13 + to 23 +) confirming the molecular composition of 6 L6 units, 3 T units, 36 Cd2+ ions, and 72 PF6− units. Just like most high molecular-weight supramolecules, the high-resolution isotope pattern for each charge peak of S6 could not be obtained owing to the resolution limitation of the mass spectrometer.[15a] The TWIM-MS spectrum (Figure 3b) clearly showed a set of narrow drift time distribution plots for the 14 + to 21 + species, demonstrating that no superimposed fragments, isomers, or conformers are present in the resultant self-assemblies. After deconvolution, the molecule weight of S6 was measured to be 42014.3 Da, which is among one of the largest metallo-supramolecules in the field of coordination-driven supramolecular self-assembly.[10a,20]
Figure 3.
a) ESI-MS and b) 2D ESI-TWIM-MS plot (m/z vs. drift time) of complex S6. The charge states of intact assemblies are marked.
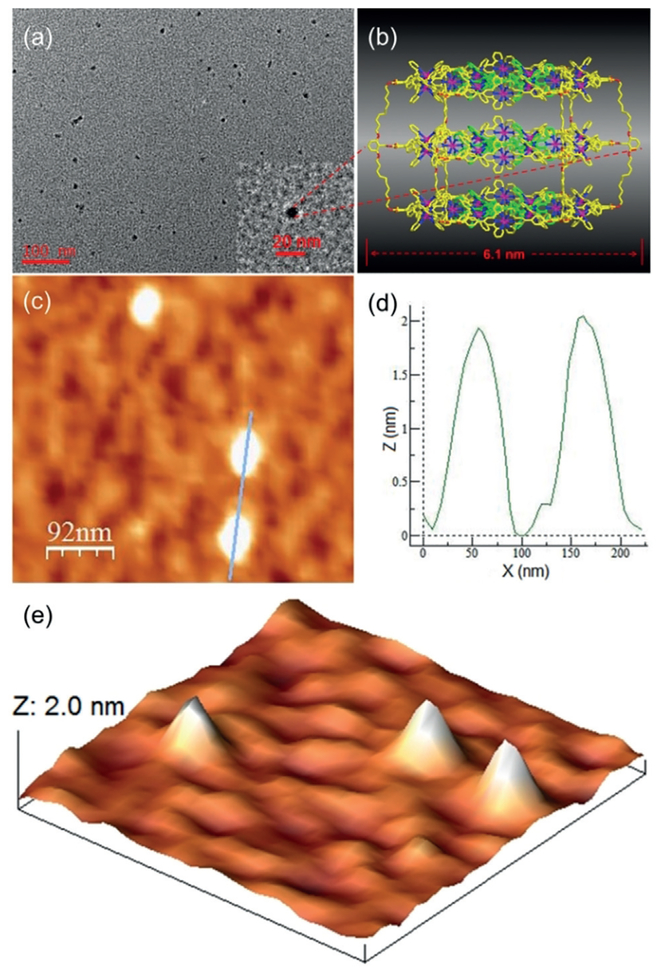
To acquire further size information, diffusion-ordered NMR spectroscopy (DOSY) experiments were performed. All spectra displayed a single narrow band, indicating the formation of the discrete structures (see Supporting Information). Using the Stokes–Einstein equation, the dynamic radii were calculated to be 5.9, 6.6, and 7.1 nm for S1–3 and 7.2 nm and 8.7 nm for S5 and S6, respectively, which agreed well with the sizes obtained by molecular modeling. Further, the TEM images were also obtained, in which widespread individual particles could be clearly observed (Figure 4a and the Supporting Information). The measured diameter from the higher magnification image was in good accordance with the theoretical 5.7 nm for S1–3 and 6.1 nm for S5–6 calculated by molecular modeling (Figure 4b and the Supporting Information). Furthermore, atomic force microscopy (AFM) images of triple-decker hexagon structure S6 displayed uniform dots with a larger width than the theoretically predicted diameter owing to the tip broadening effect[21] (Figure 4c,e). Whereas the height of circa 2.0 nm (Figure 4d), which is slightly less than three times the height of monolayer tpy-complexes,[14a,22] matched well with the expected structure. Similarly, S1–3 had nearly twice the height of single tpy-complexes (see the Supporting Information).
Figure 4.
a) TEM image, b) representative energy-minimized structure from molecular modeling, c) AFM image, d) height histogram from AFM, and e) 3D AFM image of triple-decker hexagon S6.
After the successful self-assemblies of triple-decker hexagon superstructures S4–6, we reasoned that triple-decker structure S4 was more labile than S6, because the two bilateral tris(tpy)s moieties of S4 directly linked to the phenyl group of the middle tris(tpy)s moiety by the aliphatic chain imposed intense strain on the middle tris(tpy)s connection, resulting in a labile stacked structure (Scheme 2, left). Therefore, their stabilities were evaluated by tandem mass spectrometry, in which the other parameters were identical but the voltage of trap cell was gradually changed to increase collision energies (Figures S81 and S82). When the voltage reached 50 V, all peaks of S4 completely disappeared. However, the peaks of S6 were present until 70 V, which is higher than S4, indicating that the triple-decker hexagon S6 was more stable than S4. These results will guide the design of more sophisticated 3D architectures in future.
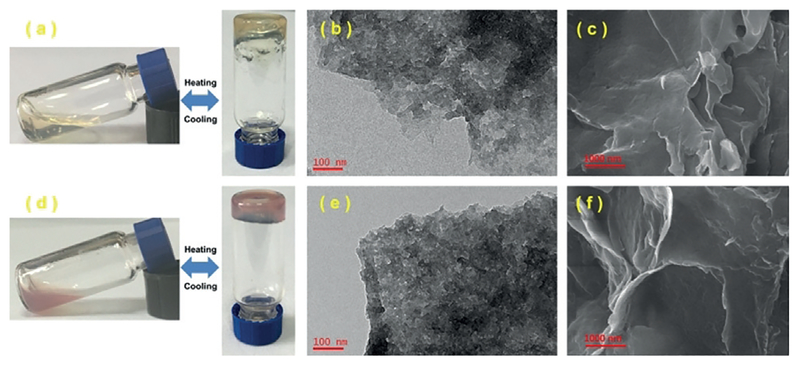
The giant self-assembled double- and triple-decker hexagons could form organometallic gels in DMF. The metallogelation displayed temperature-responsive properties; the formed gel gradually turning into solutions upon heating and subsequently reforming gels after cooling down to room temperature (Figure 5a,d). TEM and scanning electron microscopy (SEM) were conducted to determine the morphologies of the supramolecular gels of S1 and S5 (Figure 5 and the Supporting Information). All images of two gels showed the bulk networks, which were formed by the intermolecular interaction (i.e., π–π stacking and hydro-phobic interactions),[15b,23] and sol-gel phase transitions were rationalized by the ability of the organometallic gels based on metal-ligand bonds and noncovalent interactions to dynamically assemble or disassemble by varying temperature.[24]
Figure 5.
a,d) Images showing of thermal reversibility of the supramolecular gel, b,e) TEM images and c,f) SEM images of S1 (up) and S5 (down) gel in DMF.
In summary, the giant double- (S1–3) and triple-decker (S4–6) hexagons have been designed and prepared in nearly quantitative yield through a novel multicomponent self-assembly procedure based on < tpy–Cd2+–tpy > connectivity. The composition, size, and shape of the self-assembled supramolecules were unambiguously verified by ESI-MS and TWIM-MS, NMR and DOSY NMR spectroscopy, and TEM and AFM imaging. By varying the connecting lengths of alkyl linkers, the portals between the molecular internal and the external space could be adjusted. Thus, these architectures have potential application in molecular recognition and encapsulation for special-sized and special-shaped molecules. Based on these results, the research into giant hollow triple-and quadruple-stacked hexagonal supramolecules and their encapsulation properties is ongoing. The strategy of using the flexible linkers as connections to vertically construct 3D structures provides a new sight for more sophisticated and functional supramolecular architectures.
Supplementary Material
Acknowledgements
We gratefully acknowledge support from the Distinguished Professor Research Fund from Guangzhou University of China, National Science Foundation of U.S.A (CHE-1151991 to G.R.N., CHE-1506722 to X.L.), National Institutes of Health (1R01GM128037 to X.L.) and the Modern Analysis and Testing Center of CSU.
Footnotes
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.201809819.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Die Liu, Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education Institute of Environmental Research at Greater Bay Guangzhou University, Guangzhou 510006 (China).
Mingzhao Chen, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083 (China).
Yiming Li, Department of Chemistry, University of South Florida, Tampa, FL 33640 (USA).
Yixian Shen, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083 (China).
Jian Huang, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083 (China).
Xiaoyu Yang, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083 (China).
Zhilong Jiang, Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education Institute of Environmental Research at Greater Bay Guangzhou University, Guangzhou 510006 (China); College of Chemistry and Chemical Engineering, Central South University, Changsha 410083 (China).
Xiaopeng Li, Department of Chemistry, University of South Florida, Tampa, FL 33640 (USA).
George R. Newkome, Departments of Polymer Science and Chemistry, University of Akron Akron, OH 44325-4717 (USA) Center for Molecular Biology and Biotechnology, Florida Atlantic University, 5353 Parkside Dr., Jupiter, FL 33458 (USA).
Pingshan Wang, Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education Institute of Environmental Research at Greater Bay Guangzhou University, Guangzhou 510006 (China); College of Chemistry and Chemical Engineering, Central South University, Changsha 410083 (China).
References
- [1].a) Zhou Z, Yan X, Cook TR, Saha ML, Stang PJ, J. Am. Chem. Soc 2016, 138, 806–809; [DOI] [PubMed] [Google Scholar]; b) Rizzuto FJ, Wu WY, Ronson TK, Nitschke JR, Angew. Chem. Int. Ed 2016, 55, 7958–7962; Angew. Chem. 2016, 128, 8090 – 8094; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Fiedler D, Bergman RG, Raymond KN, Angew. Chem. Int. Ed 2004, 43, 6748–6751; Angew. Chem. 2004, 116, 6916 – 6919; [DOI] [PubMed] [Google Scholar]; d) Sun L, Chen Y, Han X, Liu Y, Angew. Chem. Int. Ed 2017, 56, 7062–7065; [DOI] [PubMed] [Google Scholar]
- [2].Cook TR, Stang PJ, Chem. Rev 2015, 115, 7001–7045. [DOI] [PubMed] [Google Scholar]
- [3].a) Pluth MD, Bergman RG, Raymond KN, Science 2007, 316, 85; [DOI] [PubMed] [Google Scholar]; b) Bolliger JL, Belenguer AM, Nitschke JR, Angew. Chem. Int. Ed 2013, 52, 7958–7962; Angew. Chem. 2013, 125, 8116 – 8120; [DOI] [PubMed] [Google Scholar]; c) Marcos V, Stephens AJ, Jaramillo-Garcia J, Nussbaumer AL, Woltering SL, Valero A, Lemonnier J-F, Vitorica-Yrezabal IJ, Leigh DA, Science 2016, 352, 1555; [DOI] [PubMed] [Google Scholar]; d) Wang Q-Q, Gonell S, Leenders SHAM, Dürr M, Ivanović-Burmazović I, Reek JNH, Nat. Chem 2016, 8, 225–230. [DOI] [PubMed] [Google Scholar]
- [4].Jiang B, Zhang J, Ma J-Q, Zheng W, Chen L.-J. B. Sun, Li C, Hu B-W, Tan H, Li X, Yang H-B, J. Am. Chem. Soc 2016, 138, 738–741. [DOI] [PubMed] [Google Scholar]
- [5].a) Neelakandan PP, Jimenez A, Nitschke JR, Chem. Sci 2014, 5, 908–915; [Google Scholar]; b) Yan X, Wang H, Hauke CE, Cook TR, Wang M, Zhou L, Saha Z, Zhang MM, Li X, Huang F, Stang PJ, J. Am. Chem. Soc 2015, 137, 15276–15286. [DOI] [PubMed] [Google Scholar]
- [6].a) Cook TR, Vajpayee V, Lee MH, Stang PJ, Chi K-W, Acc. Chem. Res 2013, 46, 2464–2474; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fujita D, Suzuki K, Sato S, Yagi-Utsumi M, Yamaguchi Y, Mizuno N, Kumasaka T, Takata M, Noda M, Uchiyama S, Kato K, Fujita M, Nat. Commun 2012, 3, 1093; [DOI] [PubMed] [Google Scholar]; c) Andreas W, Michael G, George RN, Ulrich SS, Curr. Top. Med. Chem 2012, 12, 158–175;22236160 [Google Scholar]; d) Matsumura Y, Maeda H, Cancer Res 1986, 46, 6387. [PubMed] [Google Scholar]
- [7].a) Wang H, Ji X, Li Z, Huang F, Adv. Mater 2017, 29, 1606117; [DOI] [PubMed] [Google Scholar]; b) Yamashina M, Sartin MM, Sei Y, Akita M, Takeuchi S, Tahara T, Yoshizawa M, J. Am. Chem. Soc 2015, 137, 9266–9269; [DOI] [PubMed] [Google Scholar]; c) Yan X, Cook TR, Wang P, Huang F, Stang PJ, Nat. Chem 2015, 7, 342–348; [DOI] [PubMed] [Google Scholar]; d) Yam VW-W, Au VK-M, Leung SY-L, Chem. Rev 2015, 115, 7589–7728; [DOI] [PubMed] [Google Scholar]; e) Winter A, Hoeppener S, Newkome GR, Schubert US, Adv. Mater 2011, 23, 3484–3498. [DOI] [PubMed] [Google Scholar]
- [8].a) Chakrabarty R, Mukherjee PS, Stang PJ, Chem. Rev 2011, 111, 6810–6918; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Newkome GR, Moorefield CN, Chem. Soc. Rev 2015, 44, 3954–3967. [DOI] [PubMed] [Google Scholar]
- [9].a) Mosquera J, Ronson TK, Nitschke JR, J. Am. Chem. Soc 2016, 138, 1812–1815; [DOI] [PubMed] [Google Scholar]; b) Pluth MD, Bergman RG, Raymond KN, Acc. Chem. Res 2009, 42, 1650–1659. [DOI] [PubMed] [Google Scholar]
- [10].a) Fujita D, Ueda Y, Sato S, Mizuno N, Kumasaka T, Fujita M, Nature 2016, 540, 563–566; [DOI] [PubMed] [Google Scholar]; b) Fujita D, Ueda Y, Sato S, Yokoyama H, Mizuno N, Kumasaka T, Fujita M, Chem 2016, 1, 91–101. [Google Scholar]
- [11].a) Ayme J-F, Beves JE, Campbell CJ, Leigh DA, Chem. Soc. Rev 2013, 42, 1700–1712; [DOI] [PubMed] [Google Scholar]; b) Cao P-F, Mangadlao J, Advincula R, Angew. Chem. Int. Ed 2015, 54, 5127–5131; [DOI] [PubMed] [Google Scholar]
- [12].Schneider H-J, Applications of Supramolecular Chemistry, CRC Press, Boca Raton, FL, 2012. [Google Scholar]
- [13].a) Schubert US, Eschbaumer C, Angew. Chem. Int. Ed 2002, 41, 2892–2926; Angew. Chem. 2002, 114, 3016 – 3050; [DOI] [PubMed] [Google Scholar]; b) Hofmeier H, Schubert US, Chem. Soc. Rev 2004, 33, 373–399; [DOI] [PubMed] [Google Scholar]; c) Constable EC, Coord. Chem. Rev 2008, 252, 842–855; [Google Scholar]; d) De S, Mahata K, Schmittel M, Chem. Soc. Rev 2010, 39, 1555–1575. [DOI] [PubMed] [Google Scholar]
- [14].a) Newkome GR, Wang P, Moorefield CN, Cho TJ, Mohapatra PP, Li S, Hwang S-H, Lukoyanova O, Echegoyen L, Palagallo JA, Iancu V, Hla S-W, Science 2006, 312, 1782–1785; [DOI] [PubMed] [Google Scholar]; b) Sarkar R, Guo K, Moorefield CN, Saunders MJ, Wesdemiotis C, Newkome GR, Angew. Chem. Int. Ed 2014, 53, 12182–12185; [DOI] [PubMed] [Google Scholar]
- [15].a) Sun B, Wang M, Lou Z, Huang M, Xu C, Li X, Chen L-J, Yu Y, Davis GL, Xu B, Yang H-B, Li X, J. Am. Chem. Soc 2015, 137, 1556–1564; [DOI] [PubMed] [Google Scholar]; b) Wang M, Wang K, Wang C, Huang M, Hao X-Q, Shen M-Z, Shi G-Q, Zhang Z, Song B, Cisneros A, Song M-P, Xu B, Li X, J. Am. Chem. Soc 2016, 138, 9258–9268. [DOI] [PubMed] [Google Scholar]
- [16].Fu J-H, Lee Y-H, He Y-J, Chan Y-T, Angew. Chem. Int. Ed 2015, 54, 6231–6235; [DOI] [PubMed] [Google Scholar]
- [17].Wang J-L, Li X, Lu X, Hsieh IF, Cao Y, Moorefield CN, Wesdemiotis C, Cheng SZD, Newkome GR, J. Am. Chem. Soc 2011, 133, 11450–11453. [DOI] [PubMed] [Google Scholar]
- [18].a) Sun Q-F, Sato S, Fujita M, Nat. Chem 2012, 4, 330–333; [DOI] [PubMed] [Google Scholar]; b) Black SP, Stefankiewicz AR, Smulders MMJ, Sattler D, Schalley CA, Nitschke JR, Sanders JKM, Angew. Chem. Int. Ed 2013, 52, 5749–5752; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ludlow JM III, Tominaga M, Chujo Y, Schultz A, Lu X, Xie T, Guo K, Moorefield CN, Wesdemiotis C, Newkome GR, Dalton Trans 2014, 43, 9604–9611. [DOI] [PubMed] [Google Scholar]
- [20].Xie T-Z, Wu X, Endres KJ, Guo Z, Lu X, Li J, Manandhar E, Ludlow JM III, Moorefield CN, Saunders MJ, Wesdemiotis C, Newkome GR, J. Am. Chem. Soc 2017, 139, 15652–15655. [DOI] [PubMed] [Google Scholar]
- [21].a) Radmacher M, Fritz M, Hansma HG, Hansma PK, Science 1994, 265, 1577–1579; [DOI] [PubMed] [Google Scholar]; b) Josep C-F, Eugenio C, Alicia F-A, Elena P-C, Nat. Nanotech 2014, 25, 395703. [Google Scholar]
- [22].Bauer T, Zheng Z, Renn A, Enning R, Stemmer A, Sakamoto J, Schlüter AD, Angew. Chem. Int. Ed 2011, 50, 7879–7884; [DOI] [PubMed] [Google Scholar]
- [23].Zheng W, Yang G, Shao N, Chen L-J, Ou B, Jiang S-T, Chen G, Yang H-B, J. Am. Chem. Soc 2017, 139, 13811–13820. [DOI] [PubMed] [Google Scholar]
- [24].a) Cordier P, Tournilhac F, Soulie-Ziakovic C, Leibler L, Nature 2008, 451, 977–980; [DOI] [PubMed] [Google Scholar]; b) Freye S, Michel R, Stalke D, Pawliczek M, Frauendorf H, Clever GH, J. Am. Chem. Soc 2013, 135, 8476–8479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.