Abstract
Evidence indicates that diet, nutrition, lifestyle, the environment, the microbiome, and other exogenous factors have pathogenic roles and also influence the genome, epigenome, transcriptome, proteome, and metabolome of tumor and nonneoplastic cells, including immune cells. With the need for big-data research, pathology must transform to integrate data science fields, including epidemiology, biostatistics, and bioinformatics. The research framework of molecular pathological epidemiology (MPE) demonstrates the strengths of such an interdisciplinary integration, having been used to study breast, lung, prostate, and colorectal cancers. The MPE research paradigm not only can provide novel insights into interactions among environment, tumor, and host but also opens new research frontiers. New developments—such as computational digital pathology, systems biology, artificial intelligence, and in vivo pathology technologies—will further transform pathology and MPE. Although it is necessary to address the rarity of trans-disciplinary education and training programs, MPE provides an exemplary model of integrative scientific approaches and contributes to advancements in precision medicine, therapy, and prevention.
Keywords: causal inference, exposome, genomics, immunity, public health, translational research
INTRODUCTION: PATHOLOGY AS A FUNDAMENTAL BIOMEDICAL SCIENCE MUST BE TRANSFORMED
Pathology is a fundamental field of biomedical science. Pathology develops methods to elucidate the etiologies and pathogenesis of diseases, thereby serving as the basis for all other medical sciences. In addition, pathology has had a critical role in medicine because clinical decision-making heavily depends on data from laboratory tests. As molecularly targeted interventions increasingly become available, molecular pathology has become a major part of pathology practice. Sophisticated laboratory technologies, including massively parallel sequencing (or next-generation sequencing) techniques, can now produce multi-omic data. Along with this, large amounts of pathological and biomedical data are accumulating in health-care systems worldwide. Advancements in molecular pathology are improving the disease classification system, which has been used in clinical and epidemiological studies, with attempts being made to translate findings into optimized therapeutic and preventive strategies. Hence, molecular pathology and diagnostics play important parts in the precision medicine initiative (1–4).
However, considerable challenges exist in pathology in the era of big-data omics science. First, generally, formal education and training in data science (including statistics, epidemiology, informatics, bioinformatics, and computational biology) are relatively scarce in pathology residency and fellowship programs. Second, because of this relative lack of data science training, there are shortages of pathologists and pathological scientists who are well versed in data science and able to utilize multi-omic data and interpret findings from their own studies and other groups. To maximally leverage molecular pathological technologies and data, pathology needs to transform into a field of pathobiological data science in research, education, and clinical practice. In this review, we introduce molecular pathological epidemiology (MPE) as a successful example of the integration of pathology and data science. We further discuss the multifaceted role of MPE not only in improving our understanding of pathogenesis but also in transforming pathology into a new field of pathobiological data science. Throughout this review, we use standard symbols for genes (see the sidebar, Use of Standardized Official Symbols for Genes and Gene Products).
MOLECULAR PATHOLOGICAL EPIDEMIOLOGY: A HYBRID OF PATHOLOGY AND DATA SCIENCE
Molecular pathology had been utilized in and incorporated into epidemiological research since the 1990s, and this combined approach started to show a substantial potential for expanding both pathology and epidemiology. The merging of molecular pathology and epidemiology culminated in the paradigm of MPE as an integrative field (5, 6). MPE is a hybrid field of pathology and data science because epidemiology is precisely a field of data science. Hence, MPE can address the need to transform pathology into data science.
Epidemiology and pathology have a common goal of elucidating the etiologies and pathogenesis of diseases. In addition, epidemiology is useful in pathology. Epidemiology concerns the development and standardization of methods to analyze populations, their health, and biomedical data. Virtually all biomedical investigations implicitly or explicitly use epidemiological principles to establish the internal and external validities of findings, which is often called reproducibility. The inappropriate use of statistical methods (due to a lack of understanding of epidemiological and statistical principles) is a major cause of nonreproducible findings in the literature. Therefore, epidemiological and statistical principles must be taught systematically in all biomedical disciplines.
Furthermore, epidemiology is useful for pathology in additional aspects. In the causal inference subfield of epidemiology, investigators have been developing methods—such as inverse probability weighting (7), marginal structural models, and Mendelian randomization—that can make a variable of interest (e.g., cigarette smoking) in observational data similar to a randomized exposure in a clinical trial. These data analysis methods likely have substantial potential for use in pathology. In addition to the use of model systems in experimental pathology, a large part of pathology research involves observational analyses of human tissues, body fluids, and cells. In these observational analyses, it is a considerable challenge to establish causal relationships. In addition, selection bias in terms of research participants, which often limits the generalizability of study findings, is not adequately addressed in most pathology investigations. To overcome these challenges, causal inference methods can be utilized in pathology research.
We are witnessing rapid growth in the use of molecular pathology as part of mainstream pathology research and practice. The widespread application of clinical molecular diagnostics with an improved disease classification system enables better predictions of clinical outcomes and better patient management (8, 9). The integrated MPE approach enables us to test specific etiological hypotheses that connect exogenous or endogenous factors to molecular pathology and a specific disease subtype, thereby augmenting causal inference (6, 10). In addition, the MPE approach can uncover potential risk factors that are not detectable in conventional epidemiological research without using molecular pathology methods (6). New research frameworks and analytical methodologies need to be developed to undertake integrative MPE research (11). MPE research can be used to assess not only well-defined clinical outcomes, such as disease incidence and mortality, but also intermediary biomarkers that can predict full-blown disease in the future (12, 13). Statistical methodologies have been developed to decipher heterogeneity in the relationships between a risk factor and different disease subtypes while addressing confounding, selection bias, and missing data (7, 14–18). The methods of network science and network medicine (19) have substantial potential to decipher exposures and phenotypes to advance MPE research (20). While the integrative approach of MPE has several strengths, pitfalls must also be noted (6). Because MPE research deals with multiple disease subtypes and biomarkers, it typically faces the issue of multiple hypothesis testing. Hence, investigators should appropriately use statistical methods and analyses. Relatively few cross-disciplinary experts and education programs exist (21, 22), which (as in any new field) is both a challenge and opportunity. MPE and its role and relevance have been among the topics of meetings of established international societies (23–25), as well as the focus of the evolving International MPE Meeting Series (26, 27). The MPE paradigm has been increasingly widely applied (28–43). While the MPE concept is most commonly used in cancer research, its methods can also be utilized in nonneoplastic diseases (11, 44).
UNDERSTANDING NEOPLASMS AS DYNAMIC INTERACTIONS AMONG ENVIRONMENT, HOST, AND TUMOR
Neoplasia is a complex, multifactorial disease. Each tumorigenic process is uniquely influenced by endogenous and exogenous factors and their interactions with both neoplastic and nonneoplastic cells (45–47). Each risk factor influences specific carcinogenic mechanisms, and different risk factors influence different carcinogenic mechanisms to different degrees. Because the exposome (the totality of exposures, including risk factors) differs from individual to individual, neoplasms have unique phenotypes in each individual (i.e., the unique tumor principle, or more broadly, the unique disease principle) (45, 46). Even within one individual, local microenvironments differ from place to place. Thus, each tumor area within one tumor has unique phenotypes (i.e., intratumor heterogeneity).
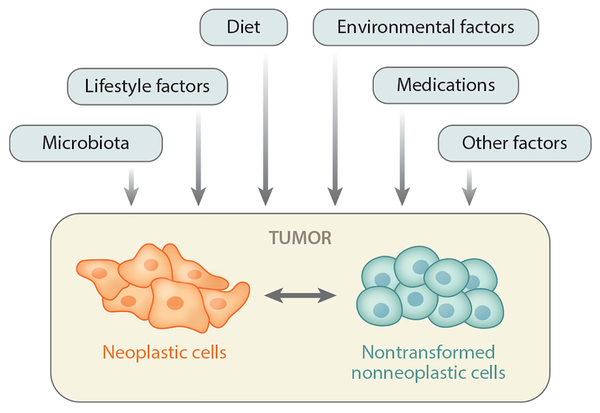
Despite the uniqueness of each disease process, the overarching premise of precision medicine and MPE is that persons who share similar molecular pathological characteristics likely share similar etiological mechanisms and similar responses to drugs and other interventions (45). According to this premise, pathologists and clinicians use molecular tests to make decisions about patient management, and researchers subtype neoplasms using molecular and pathological biomarkers to link putative risk factors to specific molecular pathological alterations. Endogenous and exogenous factors can influence and modify the phenotype of a neoplasm that possesses intrinsically dynamic and interacting components of transformed neoplastic cells and nontransformed cells (Figure 1). Hence, an investigation that takes into account the environment (i.e., in a broad sense, including exogenous and endogenous factors) is essential for us to better understand carcinogenic processes and to achieve the promise of precision medicine. Although much of the focus of oncological pathology research has been devoted to neoplastic cells and the surrounding tumor microenvironment, the influences of other exogenous and endogenous factors (including but not limited to environmental, microbial, nutritional, lifestyle, and socioeconomic) on pathogenic processes should be investigated to improve our understanding of neoplasia.
Figure 1:
The relationship between exogenous and endogenous factors and a tumor with its intrinsic and interacting components of neoplastic cells and nontransformed nonneoplastic cells in the tumor microenvironment. For simplicity, only a limited number of factors are shown, and complex interactions between the exogenous and endogenous factors are not shown. Various factors can influence the interactions between neoplastic and nonneoplastic cells and modify the biological nature of a given tumor. Thus, there are ample research opportunities for deciphering these complex relationships. The schematic shows the basic concept of an integrative analysis of various exogenous and endogenous factors, neoplastic cells, and nonneoplastic cells.
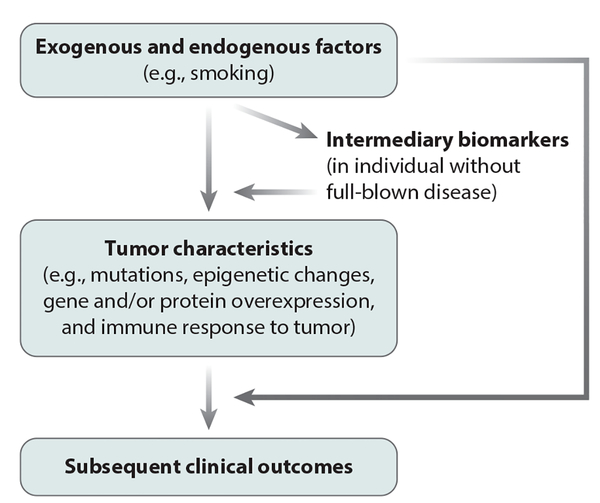
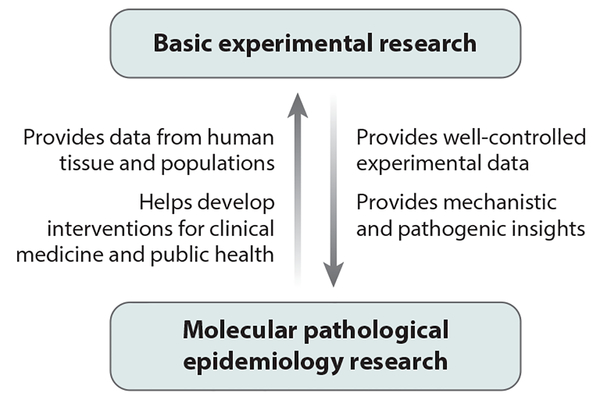
Figure 2 illustrates the multilevel research framework of MPE through which intermediary biomarkers (or phenotypes) and tumor pathological phenotypes can be analyzed in relation to exogenous and endogenous factors. MPE can generate data regarding how environmental, dietary, lifestyle, and microbial exposures influence host–tumor interactions: These data cannot be gained readily using conventional research approaches, especially human studies. The importance of MPE analyses of complex human diseases, such as neoplasms, conducted in human populations cannot be overemphasized because even the best in vivo experimental models cannot fully recapitulate human tumor–immune system interactions (48), not to mention the interactions of tumor and immune cells with exogenous and other endogenous factors. Therefore, the insights derived from population-based data can inform experimental research. Basic experimental studies and MPE studies have complementary strengths and augment each other (Figure 3). The synergism of MPE and basic experimental research can have an important role in pathology and other areas of biomedical science.
Figure 2:
Overview of a molecular pathological epidemiology data set and multilevel research. Intermediary biomarkers are measurements of molecules in biospecimens that are thought to precede full-blown disease (e.g., a malignant tumor). These biomarkers can be examined in radiological images, biopsy tissue, or in peripheral blood, sputum, saliva, urine, or other body fluids. Depending on the design of the data analysis, these biomarkers may be utilized as either exposure or outcome variables in epidemiological terms. The term exposures is broadly used for variables that can causally influence disease incidence, phenotype, or outcome, or a combination of these.
Figure 3:
Collaborative relationship between basic experimental research and molecular pathological epidemiology research. Strengths of both types of research can complement each other and work synergistically to advance biomedical sciences.
ASPIRIN AND COLORECTAL CARCINOMA: INSIGHTS INTO PATHOGENESIS AND CLINICAL IMPLICATIONS
The MPE approach can be merged with pharmacoepidemiology, potentially to uncover new indications for and to repurpose common drugs, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and statins (49). In this section, we focus on studies of aspirin and colorectal carcinoma to illustrate how MPE research can provide new insights into pathological mechanisms, synergize with laboratory experimental studies, and help to customize pathology and clinical practice.
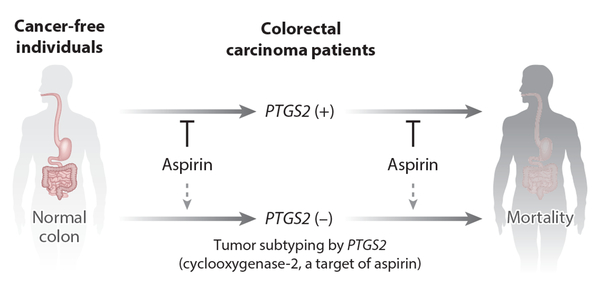
Although aspirin has been shown to exhibit anticancer effects (50, 51), a question remained about whether its effect might differ by tumor subtype—that is, according to the tumor’s molecular and immune status. To address this, MPE studies were performed that showed aspirin reduced the risk of a subtype of colorectal carcinoma with overexpression of PTGS2 (cyclooxygenase-2, a major target of aspirin) but not the PTGS2-negative subtype (52) and that aspirin reduced patient mortality in the PTGS2-positive colorectal carcinoma subtype but not the PTGS2-negative subtype (53) (Figure 4). These data suggest the dependency of the PTGS2-positive subtype of colorectal tumors on PTGS2 enzymatic activity for their growth both before and after the diagnosis of colorectal carcinoma. Consistent findings of antitumor effects of aspirin on the PTGS2-positive tumor subtype in both pre- and postdiagnosis phases of disease increase confidence in the validity of these findings. The survival association of aspirin use in PTGS2-positive colorectal cancer, but not in PTGS2-negative cancer, has been replicated in an independent study (54).
Figure 4:
Analytical framework of molecular pathological epidemiology research using. Studies of aspirin use and colorectal carcinoma PTGS2 (cyclooxygenase-2) expression are shown as examples. PTGS2 expression can be measured by RNA analysis or immunohistochemistry. One can test a hypothesis that aspirin use reduces the incidence of the PTGS2-positive colorectal carcinoma subtype but not that of the PTGS2-negative subtype (52) and another hypothesis that aspirin use reduces mortality in patients with the PTGS2-positive colorectal carcinoma subtype but not that of patients with the PTGS2-negative subtype (53). The study findings support both of these hypotheses, indicating the dependency of the PTGS2-positive subtype tumor cells on PTGS2 activity, leading to sensitivity to aspirin chemoprevention and treatment. The consistency of findings both before and after the diagnosis of colorectal cancer provides further evidence supporting the hypotheses.
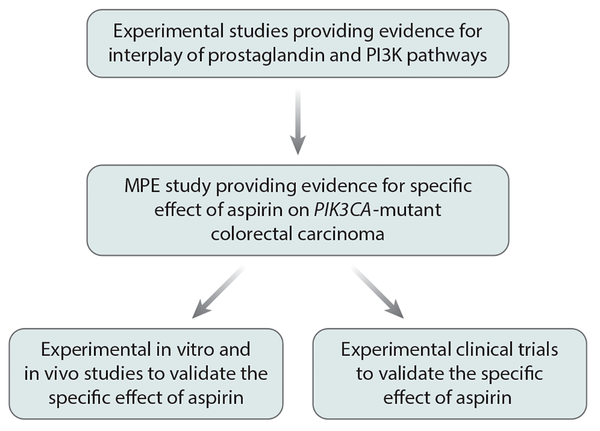
Colorectal cancers develop through driver mutations in genes such as APC, TP53, KRAS, PIK3CA, and BRAF. The sequencing of these genes can be readily implemented in clinical practice (55, 56). Considering experimental evidence for the interplay between the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and PTGS2 pathways (57, 58), the hypothesis was tested that a PIK3CA mutation might be a predictive biomarker for a response to aspirin (59). Regular aspirin use after a diagnosis of colorectal cancer was associated with longer survival in patients with PIK3CA-mutated tumors, but not in patients with PIK3CA wild-type tumors (59). A number of studies have been conducted to replicate these findings. Although each individual study might not have adequate statistical power, particularly for PIK3CA-mutant cases, most of these studies (but not all; see 54, 60) had similar findings (61–64). Moreover, in vitro experimental studies showed stronger antitumor effects of aspirin against PIK3CA-mutant colon and breast cancer cells compared with PIK3CA wild-type cells (65–67). These lines of evidence are consistent with aspirin having specific effects on PIK3CA-mutant colorectal carcinomas (Figure 5), and they provide a rationale for designing clinical trials that assess the therapeutic effects of aspirin and the predictive values of tumor biomarkers (33, 50, 68, 69).
Figure 5:
Flow diagram of basic experimental research leading to molecular pathological epidemiology (MPE) research and back again to basic experimental research for the validation of findings. Another branch represents a flow from MPE observational research to experimental clinical trial research. In this example, research assessing the effect of aspirin on PIK3CA-mutant colorectal carcinomas is shown.
Recently, immunotherapies targeting immune checkpoint pathways, such as the CTLA4 (cytotoxic T lymphocyte antigen 4) and PDCD1 (programmed cell death 1) pathways, have come under intense investigation. To date, however, the possible influence of immunomodulators—including environmental, dietary, lifestyle, microbial, and pharmacological factors—on the response of tumors to immunotherapy has been relatively overlooked. Consistent with experimental evidence for a synergism of immune checkpoint blockade and aspirin (70, 71), an MPE study showed a favorable association of aspirin use after the diagnosis of colorectal cancer with survival in patients with CD274 (PD-L1)-low tumors but not in patients with CD274 (PD-L1)-high tumors (72). Therefore, clinical trials are needed to assess the combination of immune checkpoint blockade with aspirin and to assess the predictive values of tumor markers.
For precision cancer prevention, it is ideal to identify individuals free of cancer who can reduce their cancer risk by regularly using aspirin and to avoid the potential side effects of aspirin in those who would not benefit from it. The cancer-preventive effects of aspirin appear to be more prominent for gastrointestinal cancers, especially colorectal cancer, compared with other cancer types (73), which attests to the potential importance of the gut microbiota and inflammation in carcinogenesis. Hence, investigations have been conducted to identify biomarkers that can predict the ability of aspirin to prevent colorectal cancer. In a gene by environment interaction study (74), the rs2965667 single nucleotide polymorphism (SNP) at chromosome 12p12.3 near the MGST1 gene showed a statistically significant interaction with NSAIDs, including aspirin; regular NSAID use was associated with a lower risk of colorectal cancer among individuals with the rs2965667 TT genotype, but it was associated with a higher risk of colorectal cancer among those with the uncommon TA or AA genotypes (observed in 4% of the population). In addition, the rs16973225 SNP at chromosome 15q25.2 near the IL16 gene also showed a statistically significant interaction with NSAID use; regular NSAID use was associated with a lower risk of colorectal cancer among individuals with the rs16973225 AA genotype but not among those with the less common AC or CC genotypes (observed in 9% of the population) (74). Thus, these SNPs may serve as markers for identifying cancer-free individuals who will benefit from NSAID use to prevent colorectal cancer. Another study evaluated the statistical interactions of aspirin use with SNPs previously identified as risk alleles through genome-wide association studies, and it showed not only an interaction of aspirin with the rs6983267 SNP at chromosome 8q24 but also among individuals with the protective T allele, specificity of the association between aspirin use and a lower risk for nuclear CTNNB1 (β-catenin)-positive colorectal cancer (75).
Biomarkers detectable in normal or nonneoplastic tissue may provide information on the cellular and molecular statuses of the local microenvironment, which cannot be obtained through germline genetic analyses. As a metabolizing enzyme of the prostaglandin pathway, the HPGD (15-hydroxyprostaglandin dehydrogenase) protein antagonizes PTGS2 activity and is thought to function as a tumor suppressor and to augment the activity of aspirin (76). To assess the ability of biomarkers to predict the efficacy of aspirin chemoprevention, a study was conducted examining aspirin use in relation to the risk of colorectal cancer according to HPGD mRNA expression levels in adjacent normal colon mucosa (77). Aspirin use was associated with a lower risk of colorectal cancer that was accompanied by high levels of HPGD mRNA in adjacent normal colon tissue (77). Hence, an assessment of the efficacy of aspirin in relation to HPGD expression levels in normal colon tissue in cancer-free individuals may be warranted.
INSIGHTS INTO PATHOGENIC INTERACTIONS AMONG ENVIRONMENT, TUMOR, AND IMMUNE SYSTEM
In addition to well-established carcinogens (such as ultraviolet light) that can directly damage DNA, a causal relationship between a given risk factor and specific tumor DNA changes may not be easily demonstrated or proven. As depicted in Figure 1, nearly all exogenous and endogenous cancer risk factors may influence the tumor microenvironment. Tumor immunity and immunology recently have drawn much attention due to rapid advances being made in the field of cancer immunotherapy and the increasing need for the development of clinical biomarkers of the immune response (78–80). By combining an assessment of tumor immunology with the MPE approach, one can assess the influence of exogenous and endogenous factors on carcinogenic processes by gauging the immune response to a tumor (81). This integrative field of immunology–MPE (immuno-MPE) can fill a research gap between tumor immunology and epidemiology (81, 82), and it represents a future direction for cancer research (1, 83). Analyses of the immune status in the tumor microenvironment are increasingly being incorporated into large-scale epidemiological cohort studies (84–89). This relatively new approach has started to shed light on the carcinogenic mechanisms of certain exposures. Herein, we discuss some notable findings from immuno-MPE studies.
A controversy has persisted about the potential of fish oil or omega-3 polyunsaturated fatty acids (PUFAs) to prevent cancer (90, 91). In light of experimental evidence for the immunomodulatory effects of omega-3 PUFAs (92), an immuno-MPE study demonstrated that a higher intake of omega-3 PUFAs was associated with a lower risk of colorectal carcinomas with high FOXP3+ regulatory T cell (Treg) counts, but it did not lower the risk of carcinomas with low FOXP3+ Treg counts (87). The study also provided in vitro evidence that docosahexaenoic acid (an omega-3 PUFA) suppresses the function of FOXP3+ cells, leading to a proliferation of CD4+IL2RA− effector T cells (87). These findings support the idea that omega-3 PUFAs can suppress the function of Tregs, thereby stimulating an immune response to a tumor (87). Hence, integrative immuno-MPE research can provide new insights into the combined role of exposures and immune cells, and these findings can corroborate and be corroborated by experimental investigations.
Vitamin D is another immunomodulator of interest. Dietary and supplemental vitamin D intake and systemic vitamin D levels have been associated with lower risks of cancer incidence and mortality (93). To consider the dual roles of vitamin D in immune modulation and cancer prevention, an immuno-MPE study was conducted to analyze plasma vitamin D levels (an indicator of systemic vitamin D status) in relation to the incidence of colorectal carcinoma subtypes classified by immune response status (94). Interestingly, higher plasma vitamin D levels were associated with a lower incidence of colorectal carcinomas that had higher stromal lymphocyte counts but not with a lower incidence of those having lower lymphocyte counts (94). Therefore, the anticarcinogenic effects of vitamin D appear to be more prominent for carcinomas with a higher lymphocytic response (94). Because some immune cells have the ability to enzymatically convert 25-hydroxyvitamin D (a common circulating form of vitamin D) to an active form of vitamin D—that is, 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] (95, 96)—a subset of lymphocytes or other immune cells in the tumor microenvironment may activate vitamin D, which in turn may influence both neoplastic and nonneoplastic cells in a paracrine or autocrine fashion, thus hindering tumor growth and survival (94).
Aspirin has been discussed in the preceding section. Here, we describe a study that assessed aspirin use in relation to the immune response status of tumor tissue. Evidence indicates that aspirin has effects on both cancer and the immune system (31, 50). Hence, it is possible to hypothesize that aspirin may reduce cancer incidence through its effects on the immune system. A study was conducted to assess aspirin use in relation to the incidence of colorectal carcinoma subclassified on the basis of immune response (88). Regular aspirin use was associated with a lower incidence of colorectal carcinoma that had no or only low numbers of tumor-infiltrating lymphocytes (TILs), but not with a lower incidence of carcinoma having higher TIL counts (88). Consistent with these observations, another study showed that the incidence of colorectal carcinoma with lower levels of immune response appeared to be increased by proinflammatory diets (i.e., diets associated with higher levels of circulating inflammatory biomarkers) (97). Proinflammatory diets are rich in red and processed meat, sugar, and refined grains, while anti-inflammatory diets are rich in green leafy vegetables, dark yellow vegetables, coffee, and tea (97). These results suggest that evolving tumors that have lower levels of immune response may be more sensitive to the antitumor effects of aspirin and anti-inflammatory diets.
INSIGHTS INTO PATHOGENIC EFFECTS OF DIET AND MICROBIOTA
Undoubtedly, microorganisms play major parts not only in classic infectious diseases but also in neoplastic disorders. Many microorganisms have been implicated in the pathogenesis of neoplasms, including Epstein–Barr virus, hepatitis B and C viruses, HIV, human papillomavirus, human T cell lymphotropic virus, polyomaviruses, Helicobacter pylori, and Schistosoma haematobium. The field of microbiology has been closely related to both immunology and pathology, and it can be readily integrated into the framework of MPE (98). Our improved knowledge of microorganisms and their effects on diseases and the immune system can advance broad areas of biomedical and public health sciences.
The human body has many more microorganisms than it has human cells. The intestines are estimated to contain at least 100 trillion microorganisms, and the composition of these has been shown to be influenced by diet (99, 100). The gut microbiota appears to have an important role in the pathogenesis of tumors not only in the gastrointestinal tract but also at other body sites (101–103). Evidence suggests that diet, alcohol, medications, and other lifestyle factors influence the intestinal microbiota, and the gut microbiota can modulate and interact with the immune system, which influences the evolution of tumors. Some microbes produce metabolites that can promote tumor growth, whereas others produce short-chain fatty acids, such as butyrate, that can inhibit inflammatory reactions and tumor development in the intestine (104, 105).
Although a pathogenic link between the intestinal microbiota and colorectal carcinoma has long been speculated about, comprehensive investigations of the microorganisms that might be related to carcinogenesis were difficult before the development of next-generation sequencing technologies. Metagenomic analyses of colorectal carcinoma tissue and normal colon tissue identified enrichment for Fusobacterium nucleatum in cancers (106, 107). Subsequent studies have provided evidence for pathogenic roles of F. nucleatum in colorectal cancer (108–113). Colorectal tumors containing higher amounts of F. nucleatum have been associated with the serrated neoplasia pathway (114, 115) and with a number of clinicopathological and molecular characteristics, including proximal tumor location, especially with the cecum (116), and higher disease stage (117–119), worse survival (119, 120), lower levels of T cell infiltration (121, 122), higher levels of macrophage infiltration (123), high levels of microsatellite instability (MSI) (119, 124), and a high CpG island methylator phenotype (CIMP) (119, 124). Evidence also suggests that intratumor F. nucleatum influences the tumor response to immunotherapy and chemotherapy (125–127), implying that the analysis of the microbiota may be relevant in treatment decision-making.
Although diet has been associated with the risk of colorectal cancer, the exact mechanisms remain to be elucidated (128–130). Since diet influences the gut microbiota, it is conceivable that the effects of diet on tumor development may be at least partly mediated by the microbiota. Therefore, a study was conducted to test the association of diet with the incidence of colorectal carcinoma subtypes classified by intratumor amounts of F. nucleatum (131). Interestingly, a so-called prudent diet that is rich in whole grains and fiber was associated with a lower risk of colorectal carcinoma with detectable levels of F. nucleatum but not with a lower risk of carcinoma without the species (131). These findings suggest that certain diets may decrease the risk of colorectal cancer through suppression of F. nucleatum.
Considering the fact that even the best in vivo models (let alone in vitro models) cannot exactly recapitulate the human diet, microbiota, immune system, and tumor (let alone their interactions), robust data from human populations are valuable in gaining insights into the pathogenic interactions between environment, host, and tumor.
RESEARCH MODELS GENERATED FROM THE MOLECULAR PATHOLOGICAL EPIDEMIOLOGY PARADIGM
The integrative viewpoints of MPE have generated new research models. One is the etiological field effect model (13). The term field effect—also referred to as field defect, field carcinogenesis, or field cancerization—traditionally implied a field of premalignant cells with somatic genetic or epigenetic changes, or both, in a tissue or an organ (132). These premalignant cells may have altered histopathological (morphological) features. Moreover, cellular molecular alterations in such a field may be detected by molecular pathological analyses. The presence of the field effect has been associated with the subsequent risk of developing a malignant neoplasm or multiple synchronous or metachronous neoplasms. Thus, detecting the field effect, along with predicting the subsequent cancer risk, is of clinical significance (13, 132).
In MPE research, various exogenous or endogenous putative cancer risk factors have been shown to be associated with specific tumor subtypes. It has been suggested that many etiological factors (except DNA mutagens) do not cause genetic alterations directly; rather, they may cause changes in the tissue microenvironment that favor the growth of cells with a particular molecular alteration (13). According to this view, changes in the tissue microenvironment can be regarded as a field of cancer susceptibility (13). The term etiological field effect has been coined to describe this expanded field effect model that encompasses exogenous and endogenous etiological factors and their influences on the tissue microenvironment (13). The concept of the etiological field effect has stimulated new research that focuses on cancer prevention and treatment (132–136).
Another concept related to MPE is the colorectal continuum model (137). Because the colorectum is a long organ and colorectal carcinomas most often occur in the cecum, ascending colon, sigmoid colon, and rectum, researchers have used the splenic flexure to divide colorectal carcinomas into proximal colon carcinomas and distal colorectal carcinomas. The proximal colon originates from the midgut and is supplied by the superior mesenteric artery, while the distal colorectum originates from the hindgut and is supplied by the inferior mesenteric artery. These distinctions led to the conception of dichotomous molecular features in proximal and distal colorectal carcinomas (i.e., proximal tumors are associated with high-level MSI, while distal colorectal tumors are inversely associated with high-level MSI). However, unless researchers examine in detail the locations of carcinomas, the dichotomous model can never be challenged (137). As already mentioned, accumulating evidence implicates the gut microbiota in colorectal carcinogenesis, and the gut microbiota does not abruptly change at the splenic flexure, but gradually changes along the length of the colorectum (137). Yamauchi et al. (138) tested the hypothesis that a fraction of carcinomas with a specific molecular feature (such as high-level MSI) might gradually change along bowel subsites, rather than abruptly change at the splenic flexure. They compared carcinomas from different locations (including the cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, rectosigmoid junction, and rectum), and showed that the fractions of MSI-high carcinomas, CIMP-high carcinomas, and BRAF-mutant carcinomas gradually increased from the rectum to the ascending colon (138). These data challenge the dichotomous model and instead support a continuum model. Subsequent studies of independent data sets have supported the continuum model (122, 139–143), which is consistent with important roles for exogenous and endogenous factors in tumorigenesis, such as the microbiota and immunity.
CHALLENGES
Challenges exist in MPE, and importantly, these also are challenges for pathology more broadly. There is a relative lack of scientists with interdisciplinary expertise, knowledge, and skill sets. There also is a dearth of interdisciplinary educational programs that can provide adequate professional-level training in pathology, statistics, epidemiology, and bioinformatics. The rarity of interdisciplinary experts and the rarity of educational programs are interrelated and make it difficult to conduct integrative transdisciplinary research, which requires researchers who are competent in each of these diverse fields. Just as no untrained scientist should sign out a pathology or laboratory report, no scientist who lacks competence in statistical analyses should conduct such studies (144). Often, multidisciplinary projects are conducted by groups of scientists, each with expertise in a specific field. In fact, most MPE-type research projects in the 1990s and 2000s were conducted in this manner (6). However, until relatively recently, the power of the integrative viewpoint of the MPE approach had not adequately been demonstrated. Ideally, such studies would be led by one scientist with transdisciplinary expertise, which is optimal for developing novel concepts, paradigms, and models.
Another challenge is obtaining adequate support for interdisciplinary science. Despite data indicating the generally stronger impacts of interdisciplinary projects, there is evidence that trans-disciplinary proposals have a lower rate of funding success (145). One reason may be the paucity of transdisciplinary experts who can fairly evaluate the true value of interdisciplinary research. It is hoped that this will change as the value of transdisciplinary approaches becomes more evident.
OPPORTUNITIES, INCLUDING THE INTEGRATION OF NEW TECHNOLOGIES TO ASSESS PATHOLOGY
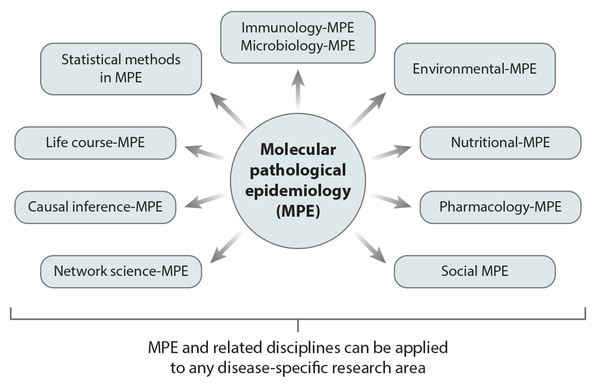
MPE offers ample opportunities. Figure 6 illustrates the new frontiers that have arisen or are arising from MPE. In addition to the emerging disciplines shown, there are, and will be, many hybrid disciplines, each of which comprises MPE combined with (or applied to) any of the numerous disease-based and medical practice–based disciplines. The most commonly studied hybrid area is cancer MPE, an integration of MPE and cancer research. Many of these emerging disciplines have not been extensively explored, thereby providing ample opportunity for the investigation and development of new paradigms, frameworks, and methodologies.
Figure 6:
Molecular pathological epidemiology (MPE) is developing new scientific frontiers. Because MPE is a method-based discipline, its analytical frameworks can be applied to other scientific disciplines, such as environmental science, nutritional science, and pharmacology. MPE also drives the development of new analytical methods, creating subfields such as causal inference-MPE and life course-MPE. Finally, MPE and its related disciplines can be applied to any disease-specific research area.
Why does the MPE framework generate such a wide range of new opportunities? Both pathology and epidemiology are genuinely method-based scientific fields in contrast to other fields that focus narrowly on, for example, certain environmental factors (e.g., nutritional science focusing on questions of nutrition), diseases (e.g., oncology focusing on cancer), or a particular type of medical practice (e.g., perinatology focusing on perinatal medicine). Hence, both pathology and epidemiology are applicable to research on any disease or any other biomedical or health science field. And as an integration of pathology and epidemiology, MPE is also a genuinely method-based discipline that can be applied to any research area (21). Because of its versatile nature, MPE can enhance not only research on virtually all human diseases but also a wide range of scientific fields (11). For instance, the MPE framework and its methods can be integrated into immunology (81), life course epidemiology (146), microbiology (98, 131), pharmacology (49), and the social sciences (147).
Computational pathology is a discipline emerging in parallel with the evolution of big data science and precision medicine (148). The rapid increases in computational capacity (including artificial intelligence) and the availability of large amounts of digitized pathological and other clinical data have created an opportunity to apply mathematical models and machine-learning algorithms to big data. In clinical medicine, artificial intelligence can be used to support health-care providers in making objective and comprehensive diagnoses by using various data sources (e.g., patient information, laboratory data, radiological images, and pathology results), and computational algorithms can also be used to aid decision-making. Digital computational pathology may potentially solve the problem of inter- and intraobserver variations and measurement errors, which have been nearly intractable in conventional anatomical pathology practice. Hence, computational pathology can contribute to developing an accurate, standardized diagnosis system to improve health outcomes as well as to accelerating the use of MPE in population science. For example, quantitative digital pathology imaging systems can help us to implement tissue immune-cell analysis, which has not been adequately integrated into routine clinical practice due to difficulty in standardizing assays. In the near future, computational pathology will have a central role in integrating pathological, clinical, and epidemiological data, and further, it will drive precision medicine and public health.
While current pathological analyses are usually applied to cells and tissues that are not alive, pathological states may be better evaluated using living cells and tissues. Endomicroscopy (or endocytoscopy) is one such in vivo pathology technology that allows real-time histopathological examination in vivo without the need for biopsy or tissue resection (149, 150), and it has the potential to transform pathology, medical practice, and epidemiology. In MPE research, in vivo pathology technologies have the potential to provide novel insights into the influence of host factors on the occurrence and progression of diseases. Gastrointestinal neoplasms serve as a practical model for MPE research using in vivo pathology because malignant and premalignant lesions are accessible endoscopically.
HOW CAN MOLECULAR PATHOLOGICAL EPIDEMIOLOGY BE USED IN PATHOLOGY TRAINING PROGRAMS?
Much beyond its roles in research, learning the principles and methods that underlie MPE can enhance education and training in pathology and ensure rigor in research and clinical practice. There has been a growing problem of nonreproducibility in research (144, 151). While there are multiple interrelated causes, one major cause is inadequate training in statistics and epidemiology. This issue is becoming even more substantial as we increasingly deal with big data (144). To solve this problem, scientists in the field of MPE (who have ties to pathology) can provide educational programs in which trainees can learn the principles of data science, statistics, and epidemiology, including important methods for analyzing and interpreting pathology data (21). By implementing MPE programs in pathology, departments and divisions can ameliorate current weaknesses in the field of pathology and help ensure rigor in pathology science and practice.
CONCLUSIONS
In the era of precision medicine and big-data research, the field of pathology needs to transform to integrate data science. The evolving field of MPE represents a successful model of the interdisciplinary integration of pathology and data science. The field of MPE can contribute to advancements in the field of pathology. In addition, consistent with the unquestionable importance of exogenous and endogenous factors (i.e., the exposome or envirome) in cancer and other diseases, MPE research has provided novel insights into the pathogenic roles of these factors. These insights could not be gained through traditional research approaches. Because of the complexities of human diseases, MPE research must work with both in vivo and in vitro experimental research to improve our understanding of disease pathogenesis. To foster transdisciplinary science, we must address current inadequacies in integrative transdisciplinary education and improve funding mechanisms for unconventional paradigm-shifting approaches. Only by doing so will the promise of integrative scientific approaches such as MPE be fully realized.
SUMMARY POINTS.
In the era of precision medicine and multi-omic big data, pathology needs to transform by adopting pathobiological data science approaches.
All biomedical researchers need to be competent in study design, statistical analysis, and data interpretation.
The transdisciplinary field of molecular pathological epidemiology (MPE) is a successful model for integrating pathology and data science.
MPE can develop the conceptual and analytical frameworks needed to examine the interactive pathogenic roles of exogenous and endogenous factors (including the microbiome), the host (including germline genetics and immunity), and tumor molecular pathology.
MPE will not only enhance our understanding of the pathophysiological roles of interactions among environment, host, and tumor but also help develop preventive and therapeutic strategies for precision medicine.
USE OF STANDARDIZED OFFICIAL SYMBOLS FOR GENES AND GENE PRODUCTS.
We use the symbols for genes and gene products approved by the HUGO Gene Nomenclature Committee, including BRAF, CD4, CD274, CTLA4, CTNNB1, FOXP3, HPGD, IL2RA, IL16, KRAS, MGST1, PDCD1, PIK3CA, PTGS2, and TP53, all of which are described at https://www.genenames.org. The official symbols are italicized to differentiate them from the colloquial names that are used along with the official symbols. This format enables readers to familiarize themselves with the official symbols for genes and gene products together with the colloquial names.
ACKNOWLEDGMENTS
This work was supported in part by grants from the US National Institutes of Health (R35 CA197735 to S.O., K07 CA190673 to R.N.) and a Nodal Award from the Dana-Farber Harvard Cancer Center (to S.O.).
Glossary
- MPE
molecular pathological epidemiology
- NSAID
nonsteroidal anti-inflammatory drug
- SNP
single nucleotide polymorphism
- PUFA
polyunsaturated fatty acid
- TILs
tumor-infiltrating lymphocytes
- MSI
microsatellite instability
- CIMP
CpG island methylator phenotype
Footnotes
DISCLOSURE STATEMENT
The authors do not have any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Jaffee EM, Dang CV, Agus DB, Alexander BM, Anderson KC, et al. 2017. Future cancer research priorities in the USA: a Lancet Oncology Commission. Lancet Oncol. 18:e653–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyskens FL Jr., Mukhtar H, Rock CL, Cuzick J, Kensler TW, et al. 2016. Cancer prevention: obstacles, challenges and the road ahead. J. Natl. Cancer Inst 108:djv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kensler TW, Spira A, Garber JE, Szabo E, Lee JJ, et al. 2016. Transforming cancer prevention through precision medicine and immune-oncology. Cancer Prev. Res 9:2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spira A, Yurgelun MB, Alexandrov L, Rao A, Bejar R, et al. 2017. Precancer Atlas to drive precision prevention trials. Cancer Res. 77:1510–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogino S, Stampfer M. 2010. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J. Natl. Cancer Inst. 102:365–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogino S, Chan AT, Fuchs CS, Giovannucci E. 2011. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 60:397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Nevo D, Nishihara R, Cao Y, Song M, et al. 2018. Utility of inverse probability weighting in molecular pathological epidemiology. Eur. J. Epidemiol 33:381–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colussi D, Brandi G, Bazzoli F, Ricciardiello L. 2013. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int. J. Mol. Sci 14:16365–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. 2017. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 17:79–92 [DOI] [PubMed] [Google Scholar]
- 10.Nishihara R, VanderWeele TJ, Shibuya K, Mittleman MA, Wang M, et al. 2015. Molecular pathological epidemiology gives clues to paradoxical findings. Eur. J. Epidemiol 30:1129–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogino S, Nishihara R, VanderWeele TJ, Wang M, Nishi A, et al. 2016. The role of molecular pathological epidemiology in the study of neoplastic and non-neoplastic diseases in the era of precision medicine. Epidemiology 27:602–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lochhead P, Chan AT, Giovannucci E, Fuchs CS, Wu K, et al. 2014. Progress and opportunities in molecular pathological epidemiology of colorectal premalignant lesions. Am. J. Gastroenterol 109:1205– 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lochhead P, Chan AT, Nishihara R, Fuchs CS, Beck AH, et al. 2015. Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression. Mod. Pathol 28:14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg CB, Orlow I, Zabor EC, Arora A, Sharma A, et al. 2015. Identifying etiologically distinct sub-types of cancer: a demonstration project involving breast cancer. Cancer Med. 4:1432–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Spiegelman D, Kuchiba A, Lochhead P, Kim S, et al. 2016. Statistical methods for studying disease subtype heterogeneity. Stat. Med 35:782–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Kuchiba A, Ogino S. 2015. A meta-regression method for studying etiologic heterogeneity across disease subtypes classified by multiple biomarkers. Am. J. Epidemiol 182:263–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richiardi L, Barone-Adesi F, Pearce N. 2017. Cancer subtypes in aetiological research. Eur. J. Epidemiol 32:353–61 [DOI] [PubMed] [Google Scholar]
- 18.Nevo D, Nishihara R, Ogino S, Wang M. 2018. The competing risks Cox model with auxiliary case covariates under weaker missing-at-random cause of failure. Lifetime Data Anal. 24:425–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene JA, Loscalzo J. 2017. Putting the patient back together—social medicine, network medicine, and the limits of reductionism. N. Engl. J. Med 377:2493–99 [DOI] [PubMed] [Google Scholar]
- 20.Nishihara R, Glass K, Mima K, Hamada T, Nowak JA, et al. 2017. Biomarker correlation network in colorectal carcinoma by tumor anatomic location. BMC Bioinform. 18:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogino S, King EE, Beck AH, Sherman ME, Milner DA, Giovannucci E. 2012. Interdisciplinary education to integrate pathology and epidemiology: towards molecular and population-level health science. Am. J. Epidemiol 176:659–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogino S, Lochhead P, Giovannucci E, Meyerhardt JA, Fuchs CS, Chan AT. 2014. Discovery of colorectal cancer PIK3CA mutation as potential predictive biomarker: power and promise of molecular pathological epidemiology. Oncogene 33:2949–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogino S 2013. Molecular pathological epidemiology (MPE): overview of its paradigm and wide applicability even without tumor tissue. Cancer Prev. Res 6(Suppl.):CN06–01 (Abstr.) [Google Scholar]
- 24.Kuller LH, Bracken MB, Ogino S, Prentice RL, Tracy RP. 2013. The role of epidemiology in the era of molecular epidemiology and genomics: summary of the 2013 AJE-sponsored Society of Epidemiologic Research Symposium. Am. J. Epidemiol 178:1350–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epplein M, Bostick RM, Mu L, Ogino S, Braithwaite D, Kanetsky PA. 2014. Challenges and opportunities in international molecular cancer prevention research: an ASPO Molecular Epidemiology and the Environment and International Cancer Prevention Interest Groups report. Cancer Epidemiol. Biomark. Prev 23:2613–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogino S, Campbell PT, Nishihara R, Phipps AI, Beck AH, et al. 2015. Proceedings of the Second International Molecular Pathological Epidemiology (MPE) Meeting. Cancer Causes Control 26:959–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell PT, Rebbeck TR, Nishihara R, Beck AH, Begg CB, et al. 2017. Proceedings of the Third International Molecular Pathological Epidemiology (MPE) Meeting. Cancer Causes Control 28:167–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rescigno T, Micolucci L, Tecce MF, Capasso A. 2017. Bioactive nutrients and nutrigenomics in age-related diseases. Molecules 22:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtin K, Slattery ML, Samowitz WS. 2011. CpG island methylation in colorectal cancer: past, present and future. Pathol. Res. Int 2011:902674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishehsari F, Mahdavinia M, Vacca M, Malekzadeh R, Mariani-Costantini R. 2014. Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J. Gastroenterol 20:6055–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang M, Dai J, Gu D, Huang Q, Tian L. 2017. Aspirin in pancreatic cancer: chemopreventive effects and therapeutic potentials. Biochim. Biophys. Acta 1866:163–76 [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Useros J, Garcia-Foncillas J. 2016. Obesity and colorectal cancer: molecular features of adipose tissue J. Transl. Med 14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chia WK, Ali R, Toh HC. 2012. Aspirin as adjuvant therapy for colorectal cancer—reinterpreting paradigms. Nat. Rev. Clin. Oncol 9:561–70 [DOI] [PubMed] [Google Scholar]
- 34.Campbell PT, Newton CC, Newcomb PA, Phipps AI, Ahnen DJ, et al. 2015. Association between body mass index and mortality for colorectal cancer survivors: overall and by tumor molecular phenotype. Cancer Epidemiol. Biomark. Prev 24:1229–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serafino A, Sferrazza G, Colini Baldeschi A, Nicotera G, Andreola F, et al. 2017. Developing drugs that target the Wnt pathway: recent approaches in cancer and neurodegenerative diseases. Expert Opin. Drug Discov. 12:169–86 [DOI] [PubMed] [Google Scholar]
- 36.Patil H, Saxena SG, Barrow CJ, Kanwar JR, Kapat A, Kanwar RK. 2017. Chasing the personalized medicine dream through biomarker validation in colorectal cancer. Drug Discov. Today 22:111–19 [DOI] [PubMed] [Google Scholar]
- 37.Kuroiwa-Trzmielina J, Wang F, Rapkins RW, Ward RL, Buchanan DD, et al. 2016. SNP rs16906252C>T is an expression and methylation quantitative trait locus associated with an increased risk of developing MGMT-methylated colorectal cancer. Clin. Cancer Res. 22:6266–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slattery ML, Lee FY, Pellatt AJ, Mullany LE, Stevens JR, et al. 2017. Infrequently expressed miRNAs in colorectal cancer tissue and tumor molecular phenotype. Mod. Pathol. 30:1152–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes LA, Simons CC, van den Brandt PA, van Engeland M, Weijenberg MP. 2017. Lifestyle, diet, and colorectal cancer risk according to (epi)genetic instability: current evidence and future direction of molecular pathological epidemiology. Curr. Colorectal Cancer Rep 13:455–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman HG, Xie SH, Lagergren J. 2018. The epidemiology of esophageal adenocarcinoma. Gastroenterology 154:390–405 [DOI] [PubMed] [Google Scholar]
- 41.Carr PR, Alwers E, Bienert S, Weberpals J, Kloor M, et al. 2018. Lifestyle factors and risk of sporadic colorectal cancer by microsatellite instability status: a systematic review and meta-analysis. Ann. Oncol. 29:825–34 [DOI] [PubMed] [Google Scholar]
- 42.Galipeau PC, Oman KM, Paulson TG, Sanchez CA, Zhang Q, et al. 2018. NSAID use and somatic exomic mutations in Barrett’s esophagus. Genome Med. 10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajpoot M, Sharma AK, Sharma A, Gupta GK. 2018. Understanding the microbiome: emerging biomarkers for exploiting the microbiota for personalized medicine against cancer. Semin. Cancer Biol. In press. 10.1016/j.semcancer.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 44.Field AE, Camargo CA, Ogino S. 2013. The merits of subtyping obesity: One size does not fit all. JAMA 310:2147–48 [DOI] [PubMed] [Google Scholar]
- 45.Ogino S, Lochhead P, Chan AT, Nishihara R, Cho E, et al. 2013. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease Mod. Pathol. 26:465–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogino S, Fuchs CS, Giovannucci E. 2012. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert Rev. Mol. Diagn. 12:621–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogino S, Galon J, Fuchs CS, Dranoff G. 2011. Cancer immunology—analysis of host and tumor factors for personalized medicine. Nat. Rev. Clin. Oncol. 8:711–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cramer DW, Finn OJ. 2011. Epidemiologic perspective on immune-surveillance in cancer. Curr. Opin. Immunol. 23:265–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogino S, Jhun I, Mata DA, Soong TR, Hamada T, et al. 2017. Integration of pharmacology, molecular pathology, and population data science to support precision gastrointestinal oncology. NPJ Precis. Oncol 1:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drew DA, Cao Y, Chan AT. 2016. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat. Rev. Cancer 16:173–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benelli R, Vene R, Ferrari N. 2018. Prostaglandin-endoperoxide synthase 2 (cyclooxygenase-2), a complex target for colorectal cancer prevention and therapy. Transl. Res. 196:42–61 [DOI] [PubMed] [Google Scholar]
- 52.Chan AT, Ogino S, Fuchs CS. 2007. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 356:2131–42 [DOI] [PubMed] [Google Scholar]
- 53.Chan AT, Ogino S, Fuchs CS. 2009. Aspirin use and survival after diagnosis of colorectal cancer. JAMA 302:649–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gray RT, Cantwell MM, Coleman HG, Loughrey MB, Bankhead P, et al. 2017. Evaluation of PTGS2 expression, PIK3CA mutation, aspirin use and colon cancer survival in a population-based cohort study. Clin. Transl. Gastroenterol. 8:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, et al. 2017. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol 35:1453–86 [DOI] [PubMed] [Google Scholar]
- 56.Inamura K 2018. Colorectal cancers: an update on their molecular pathology. Cancers 10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Umar A, Steele VE, Menter D, Hawk ET. 2016. Mechanisms of non-steroidal anti-inflammatory drugs (NSAIDs) in cancer prevention. Semin. Oncol. 43:67–77 [DOI] [PubMed] [Google Scholar]
- 58.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, et al. 2009. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30:377–86 [DOI] [PubMed] [Google Scholar]
- 59.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, et al. 2012. Aspirin use, tumor PIK3CA mutation status, and colorectal cancer survival. N. Engl. J. Med. 367:1596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reimers MS, Bastiaannet E, Langley RE, van Eijk R, van Vlierberghe RL, et al. 2014. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern. Med. 174:732–39 [DOI] [PubMed] [Google Scholar]
- 61.Domingo E, Church DN, Sieber O, Ramamoorthy R, Yanagisawa Y, et al. 2013. Evaluation of PIK3CA mutation as a predictor of benefit from NSAID therapy in colorectal cancer. J. Clin. Oncol. 31:4297–305 [DOI] [PubMed] [Google Scholar]
- 62.Kothari N, Kim R, Jorissen RN, Desai J, Tie J, et al. 2015. Impact of regular aspirin use on overall and cancer-specific survival in patients with colorectal cancer harboring a PIK3CA mutation. Acta Oncol. 54:487–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paleari L, Puntoni M, Clavarezza M, DeCensi M, Cuzick J, DeCensi A. 2016. PIK3CA mutation, aspirin use after diagnosis and survival of colorectal cancer: a systematic review and meta-analysis of epidemio-logical studies. Clin. Oncol. 28:317–26 [DOI] [PubMed] [Google Scholar]
- 64.Murphy C, Turner N, Wong HL, Sinnathamby M, Tie J, et al. 2017. Examining the Impact of regular aspirin use and PIK3CA mutations on survival in stage 2 colon cancer. Intern. Med. J. 47:88–98 [DOI] [PubMed] [Google Scholar]
- 65.Turturro SB, Najor MS, Ruby CE, Cobleigh MA, Abukhdeir AM. 2016. Mutations in PIK3CA sensitize breast cancer cells to physiologic levels of aspirin. Breast Cancer Res. Treat. 156:33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zumwalt TJ, Wodarz D, Komarova NL, Toden S, Turner J, et al. 2017. Aspirin-induced chemoprevention and response kinetics are enhanced by PIK3CA mutations in colorectal cancer cells. Cancer Prev. Res. 10:208–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu M, Nishihara R, Chen Y, Li W, Shi Y, et al. 2017. Aspirin exerts high anti-cancer activity in PIK3CA-mutant colon cancer cells. Oncotarget 8:87379–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reimers MS, Zeestraten EC, Kuppen PJ, Liefers GJ, van de Velde JH. 2013. Biomarkers in precision therapy in colorectal cancer. Gastroenterol. Rep. 1:166–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michel P, Boige V, Andre T, Aparicio T, Bachet JB, et al. 2018. Aspirin versus placebo in stage III or high-risk stage II colon cancer with PIK3CA mutation: a French randomised double-blind phase III trial (PRODIGE 50-ASPIK). Dig. Liver Dis 50:305–07 [DOI] [PubMed] [Google Scholar]
- 70.Zelenay S, van der Veen AG, Bottcher JP, Snelgrove KJ, Rogers N, et al. 2015. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162:1257–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang D, DuBois RN. 2016. The role of prostaglandin E2 in tumor-associated immunosuppression. Trends Mol. Med. 22:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamada T, Cao Y, Qian ZR, Masugi Y, Nowak JA, et al. 2017. Aspirin use and colorectal cancer survival according to tumor CD274 (Programmed Cell Death 1 Ligand 1) expression status. J. Clin. Oncol 35:1836–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao Y, Nishihara R, Wu K, Wang M, Ogino S, et al. 2016. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2:762–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nan H, Hutter CM, Lin Y, Jacobs EJ, Ulrich CM, et al. 2015. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA 313:1133–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nan H, Morikawa T, Suuriniemi M, Imamura Y, Werner L, et al. 2013. Aspirin use, 8q24 single nucleotide polymorphism rs6983267, and colorectal cancer according to CTNNB1 alterations. J. Natl. Cancer Inst. 105:1852–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, et al. 2006. 15-hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. PNAS 103:12098–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fink SP, Yamauchi M, Nishihara R, Jung S, Kuchiba A, et al. 2014. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD). Sci. Transl. Med 6:233re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, et al. 2014. Towards the introduction of the Immunoscore in the classification of malignant tumors. J. Pathol. 232:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Basile D, Garattini SK, Bonotto M, Ongaro E, Casagrande M, et al. 2017. Immunotherapy for colorectal cancer: Where are we heading? Expert Opin. Biol. Ther 17:709–21 [DOI] [PubMed] [Google Scholar]
- 80.Grizzi F, Basso G, Borroni EM, Cavalleri T, Bianchi P, et al. 2018. Evolving notions on immune response in colorectal cancer and their implications for biomarker development. Inflamm. Res. 67:375–89 [DOI] [PubMed] [Google Scholar]
- 81.Ogino S, Nowak JA, Hamada T, Phipps AI, Peters U, et al. 2018. Integrative analysis of exogenous, endogenous, tumour, and immune factors for precision medicine. Gut 67:1168–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ogino S, Giannakis M. 2018. Immunoscore for (colorectal) cancer precision medicine. Lancet 391:2084– 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fletcher R, Wang YJ, Schoen RE, Finn OJ, Yu J, Zhang L. 2018. Colorectal cancer prevention: immune modulation taking the stage. Biochim Biophys Acta. 1869:138–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanyuda A, Ogino S, Rong Qian Z, Nishihara R, Song M, et al. 2016. Body mass index and risk of colorectal cancer according to tumor lymphocytic infiltrate. Int. J. Cancer 139:854–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rozek LS, Schmit SL, Greenson JK, Tomsho LP, Rennert HS, et al. 2016. Tumor-infiltrating lymphocytes, Crohn’s-like lymphoid reaction, and survival from colorectal cancer. J. Natl. Cancer Inst. 108:djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, et al. 2016. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 15:857–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song M, Nishihara R, Cao Y, Chun E, Qian ZR, et al. 2016. Marine ω−3 polyunsaturated fatty acid intake and risk of colorectal cancer characterized by tumor-infiltrating T cells. JAMA Oncol. 2:1197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cao Y, Nishihara R, Qian ZR, Song M, Mima K, et al. 2016. Regular aspirin use associates with lower risk of colorectal cancers with low numbers of tumor-infiltrating lymphocytes. Gastroenterology 151:879–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prizment AE, Vierkant RA, Smyrk TC, Tillmans LS, Nelson HH, et al. 2017. Cytotoxic T-cells and granzyme B associated with improved colorectal cancer survival in a prospective cohort of older women. Cancer Epidemiol. Biomark. Prev. 26:622–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song M, Chan AT, Fuchs CS, Ogino S, Hu FB, et al. 2014. Dietary intake of fish, ω−3 and ω−6 fatty acids and risk of colorectal cancer: a prospective study in U.S. men and women. Int. J. Cancer 135:2413–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen XJ, Zhou JD, Dong JY, Ding WQ, Wu JC. 2012. Dietary intake of n-3 fatty acids and colorectal cancer risk: a meta-analysis of data from 489 000 individuals. Br. J. Nutr. 108:1550–56 [DOI] [PubMed] [Google Scholar]
- 92.Calder PC. 2015. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 1851:469–84 [DOI] [PubMed] [Google Scholar]
- 93.Dou R, Ng K, Giovannucci EL, Manson JE, Qian ZR, Ogino S. 2016. Vitamin D and colorectal cancer: molecular, epidemiological and clinical evidence. Br. J. Nutr. 115:1643–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song M, Nishihara R, Wang M, Chan AT, Qian ZR, et al. 2016. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut 65:296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, et al. 2007. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 8:285–93 [DOI] [PubMed] [Google Scholar]
- 96.Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, et al. 2010. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. PNAS 107:22593–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu L, Nishihara R, Qian ZR, Tabung FK, Nevo D, et al. 2017. Association between inflamma-tory diet pattern and risk of colorectal carcinoma subtypes classified by immune responses to tumor. Gastroenterology 153:1517–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamada T, Keum N, Nishihara R, Ogino S. 2017. Molecular pathological epidemiology: new developing frontiers of big data science to study etiologies and pathogenesis. J. Gastroenterol. 52:265–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, et al. 2015. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 6:6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Keefe SJ. 2016. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 13:691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mima K, Ogino S, Nakagawa S, Sawayama H, Kinoshita K, et al. 2017. The role of intestinal bacteria in the development and progression of gastrointestinal tract neoplasms. Surg. Oncol. 26:368–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J, Domingue JC, Sears CL. 2017. Microbiota dysbiosis in select human cancers: evidence of association and causality. Semin. Immunol. 32:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zitvogel L, Pietrocola F, Kroemer G. 2017. Nutrition, inflammation and cancer. Nat. Immunol.18:843–50 [DOI] [PubMed] [Google Scholar]
- 104.Drewes JL, Housseau F, Sears CL. 2016. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br. J. Cancer 115:273–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen J, Pitmon E, Wang K. 2017. Microbiome, inflammation and colorectal cancer. Semin. Immunol. 32:43–53 [DOI] [PubMed] [Google Scholar]
- 106.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, et al. 2012. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 22:292–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, et al. 2012. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 22:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. 2013. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, et al. 2013. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor–immune microenvironment. Cell Host Microbe 14:207–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, et al. 2014. Microbiota organization is a distinct feature of proximal colorectal cancers. PNAS 111:18321–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang Y, Weng W, Peng J, Hong L, Yang L, et al. 2017. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating Toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology 152:851–66.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ye X, Wang R, Bhattacharya R, Boulbes D, Fan F, et al. 2017. Fusobacterium nucleatum subspecies animalis influences pro-inflammatory cytokine expression and monocyte activation in human colorectal tumors. Cancer Prev. Res. 10:398–409 [DOI] [PubMed] [Google Scholar]
- 113.Hussan H, Clinton SK, Roberts K, Bailey MT. 2017. Fusobacterium’s link to colorectal neoplasia sequenced: a systematic review and future insights. World J. Gastroenterol. 23:8626–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ito M, Kanno S, Nosho K, Sukawa Y, Mitsuhashi K, et al. 2015. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int. J. Cancer 137:1258–68 [DOI] [PubMed] [Google Scholar]
- 115.Park CH, Han DS, Oh YH, Lee AR, Lee YR, Eun CS. 2016. Role of Fusobacteria in the serrated pathway of colorectal carcinogenesis. Sci. Rep. 6:25271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mima K, Cao Y, Chan AT, Qian ZR, Nowak JA, et al. 2016. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin. Transl. Gastroenterol. 7:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li YY, Ge QX, Cao J, Zhou YJ, Du YL, et al. 2016. Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J. Gastroenterol. 22:3227–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamaoka Y, Suehiro Y, Hashimoto S, Hoshida T, Fujimoto M, et al. 2018. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J. Gastroenterol. 53:517–52 [DOI] [PubMed] [Google Scholar]
- 119.Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, et al. 2016. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65:1973–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Flanagan L, Schmid J, Ebert M, Soucek P, Kunicka T, et al. 2014. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis 33:1381–90 [DOI] [PubMed] [Google Scholar]
- 121.Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, et al. 2015. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 1:653–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, et al. 2016. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J. Gastroenterol. 22:557–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park HE, Kim JH, Cho NY, Lee HS, Kang GH. 2017. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 471:329–36 [DOI] [PubMed] [Google Scholar]
- 124.Tahara T, Yamamoto E, Suzuki H, Maruyama R, Chung W, et al. 2014. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 74:1311–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu T, Guo F, Yu Y, Sun T, Ma D, et al. 2017. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170:548–63.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johnson CH, Spilker ME, Goetz L, Peterson SN, Siuzdak G. 2016. Metabolite and microbiome interplay in cancer immunotherapy. Cancer Res. 76:6146–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nagarsheth N, Wicha MS, Zou W. 2017. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 17:559–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin JH, Giovannucci E. 2014. Environmental exposure and tumor heterogeneity in colorectal cancer risk and outcomes. Curr. Colorectal Cancer Rep. 10:94–104 [Google Scholar]
- 129.Marley AR, Nan H. 2016. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 7:105–14 [PMC free article] [PubMed] [Google Scholar]
- 130.Song M, Garrett WS, Chan AT. 2015. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 148:1244–60.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mehta RS, Nishihara R, Cao Y, Song M, Mima K, et al. 2017. Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA Oncol. 3:921–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Curtius K, Wright NA, Graham TA. 2018. An evolutionary perspective on field cancerization. Nat. Rev.Cancer 18:19–32 [DOI] [PubMed] [Google Scholar]
- 133.Ericsson AC, Akter S, Hanson MM, Busi SB, Parker TW, et al. 2015. Differential susceptibility to colorectal cancer due to naturally occurring gut microbiota. Oncotarget 6:33689–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gupta S, Sun H, Yi S, Storm J, Xiao G, et al. 2014. Molecular markers of carcinogenesis for risk stratification of individuals with colorectal polyps: a case–control study. Cancer Prev. Res. 7:1023–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roy HK, Turzhitsky V, Wali R, Radosevich AJ, Jovanovic B, et al. 2017. Spectral biomarkers for chemo-prevention of colonic neoplasia: a placebo-controlled double-blinded trial with aspirin. Gut 66:285–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wennersten C, Andersson G, Boman K, Nodin B, Gaber A, Jirstrom K. 2014. Incident urothelial cancer in the Malmö Diet and Cancer Study: cohort characteristics and further validation of ezrin as a prognostic biomarker. Diagn. Pathol. 9:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, et al. 2012. Colorectal cancer: a tale of two sides or a continuum? Gut 61:794–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, et al. 2012. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 61:847–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rosty C, Young JP, Walsh MD, Clendenning M, Sanderson K, et al. 2013. PIK3CA activating mutation in colorectal carcinoma: associations with molecular features and survival. PLOS ONE 8:e65479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Phipps AI, Buchanan DD, Makar KW, Burnett-Hartman AN, Coghill AE, et al. 2012. BRAF mutation status and survival after colorectal cancer diagnosis according to patient and tumor characteristics. Cancer Epidemiol. Biomark. Prev. 21:1792–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Phipps AI, Chan AT, Ogino S. 2013. Anatomic subsite of primary colorectal cancer and subsequent risk and distribution of second cancers. Cancer 119:3140–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jess P, Hansen IO, Gamborg M, Jess T. 2013. A nationwide Danish cohort study challenging the categorisation into right-sided and left-sided colon cancer. BMJ Open 3:e002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Loree JM, Pereira AA, Lam M, Willauer AN, Raghav K, et al. 2018. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin. Cancer Res. 24:1062–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ogino S, Nishihara R. 2016. All biomedical and health science researchers, including laboratory physicians and scientists, need adequate education and training in study design and statistics. Clin. Chem. 62:1039–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bromham L, Dinnage R, Hua X. 2016. Interdisciplinary research has consistently lower funding success.Nature 534:684–87 [DOI] [PubMed] [Google Scholar]
- 146.Nishi A, Kawachi I, Koenen KC, Wu K, Nishihara R, Ogino S. 2015. Lifecourse epidemiology and molecular pathological epidemiology. Am. J. Prev. Med. 48:116–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nishi A, Milner DA, Giovannucci EL, Nishihara R, Tan AS, et al. 2016. Integration of molecular pathology, epidemiology, and social science for global precision medicine. Expert Rev. Mol. Diagn. 16:11– 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Louis DN, Feldman M, Carter AB, Dighe AS, Pfeifer JD, et al. 2016. Computational pathology: a path ahead. Arch. Pathol. Lab. Med. 140:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Takeda K, Kudo SE, Mori Y, Misawa M, Kudo T, et al. 2017. Accuracy of diagnosing invasive colorectal cancer using computer-aided endocytoscopy. Endoscopy 49:798–802 [DOI] [PubMed] [Google Scholar]
- 150.Tearney GJ, Kang D. 2017. Introduction to biomedical optical imaging. Lasers Surg. Med. 49:214. [DOI] [PubMed] [Google Scholar]
- 151.Ioannidis JP, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, et al. 2014. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 383:166–75 [DOI] [PMC free article] [PubMed] [Google Scholar]