Abstract
Current inotropic therapies improve systolic function in heart failure patients but they also elicit undesirable side effects such as arrhythmias and increased intracellular Ca2+ transients. In order to maintain myocyte Ca2+ homeostasis, the increased cytosolic Ca2+ needs to be actively transported back to sarcoplasmic reticulum leading to depleted ATP reserves. Thus, an emerging approach is to design sarcomere-based treatments to correct impaired contractility via a direct and allosteric modulation of myosin’s intrinsic force-generating behavior –a concept that potentially avoids the “off-target” effects. To achieve this goal, various biophysical approaches are utilized to investigate the mechanistic impact of sarcomeric modulators but information derived from diverse approaches is not fully integrated into therapeutic applications. This is in part due to the lack of information that provides a coherent connecting link between biophysical data to in vivo function. Hence, our ability to clearly discern the drug-mediated impact on whole-heart function is diminished. Reducing this translational barrier can significantly accelerate clinical progress related to sarcomere-based therapies by optimizing drug-dosing and treatment duration protocols based on information obtained from biophysical studies. Therefore, we attempt to link biophysical mechanical measurements obtained in isolated cardiac muscle and in vivo contractile function.
Keywords: failing myocardium, myosin modulators, sarcomere-based therapies, biophysical measurements, cross-bridge behavior, in vivo function
Introduction:
Despite years of intense research on cardiovascular disease and therapies, most clinically practiced drug-therapies use decades-old symptom-targeting approaches that manipulate downstream neurohormonal and Ca2+ signaling pathways [1, 2]. As a result, these therapies induce several disruptive side effects and the prognosis for cardiac patients remains poor [3]. For conditions such as heart failure (HF), hypertrophic cardiomyopathy (HCM), and dilated cardiomyopathy (DCM) where abnormal contractility manifests from myofilament dysfunction [4], advances in molecular development may provide mechanistic-based drug therapies that directly target the cardiac sarcomere [2, 5]. To help identify these new drug targets and their impact on cardiac contractility, several biophysical approaches are used that include single molecule [6], in vitro motility assays [7, 8], solution-based assays [9], and detergent-skinned multicellular cardiac preparations [10–12]. Although these biophysical approaches have been instrumental in elucidating the actions of potential drug molecules on cardiac myocytes, implementation of the information derived from these experiments into therapeutic application has been slow. Part of the reason for this delay is that many of the cardiac muscle parameters measured by biophysical studies lack a clear translation to whole heart function, hindering the ability to better design and guide human clinical trials. Thus, the aim of this review is to relate biophysical parameters of cardiac muscle function with important contractile indices of in vivo heart performance, and bridge the translational gap between basic preclinical studies, clinical trials, and practice. Due to space limitations, we will focus mainly on the translational relevance of mechanical experiments using detergent-skinned multicellular myocardial preparations because they provide unique mechanistic insights that other biophysical assays cannot. Three such measurements we highlight are the rate of tension redevelopment (ktr), stretch activation (SA), and force-velocity (FV) measurements as they are commonly performed by many laboratories including our own.
A key advantage of skinned cardiac preparations is that they allow researchers to probe drug effects on cross-bridge (XB) behavior in a fully assembled sarcomeric lattice. In general, XBs produce force by cycling through weakly- and strongly-bound states with the thin filament (for an in-depth review, please see [13–15]). While molecular assays can identify drug effects on specific states of the myosin XB cycle [6, 7, 16–18], potential drugs may also function by targeting other sarcomere proteins that mediate XB behavior [19]. These may include both thin and thick filament proteins such as the troponin complex, tropomyosin, titin, and cardiac myosin binding protein C (cMyBPC) [19]. Additionally, experiments on skinned cardiac preparations allow researchers to characterize important cooperative effects that mediate XB behavior and cardiac muscle force generation [20]. For example, one measurement used to assess drug effects on global XB behavior is ktr [10] where a slack-restretch protocol mechanically disrupts XBs causing a force redevelopment as they reenter force-bearing states by repeatedly traversing the XB cycle [21]. However, ktr incorporates multiple processes of XB cycling, and is an index of the sum of all forward and reverse rate constants of XB cycling following a large stretch maneuver [21]. Thus, it is often useful to further dissect the elements of XB behavior as drugs commonly impact a particular state of myosin XB cycle. For this, researchers frequently use SA to record force responses of cardiac preparations to small step-length perturbations (for details, refer to [22, 23]). Specifically, SA transients permit measurement of the rates of XB detachment (krel) and recruitment (kdf) (Figure 1), reflecting XB transitions from their attached to detached and detached to attached states [23]. It is likely that both XB detachment and recruitment occur simultaneously during krel and kdf phase, however XB detachment predominates during krel phase leading to a net force decay and XB recruitment predominates during kdf phase leading to a net force development [22, 24]. SA also enables researchers to estimate XB detachment and recruitment magnitudes, permitting a more detailed understanding of drug-induced modulations of XB behavior [10–12]. Besides ktr and SA, FV relationships, which assess myocardial power output, can be determined from measuring loaded shortening velocity at various force clamps [25]. Since the rate of loaded muscle shortening is most dependent on the rate of XB detachment [26], it can be used to characterize length-mediated cooperative deactivation mechanisms [25] which play important roles during in vivo ventricular relaxation [15].
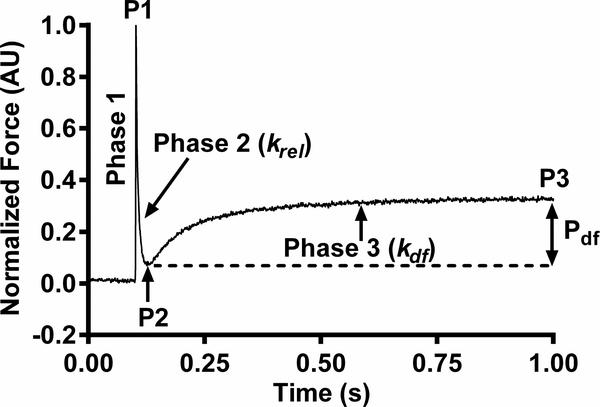
Figure 1. Representative stretch activation response in wild-type (WT) mouse myocardium.
The important phases of the force transient and various stretch activation parameters are highlighted. In an isometrically contracting mouse myocardial preparation, a sudden 2% stretch in muscle length (Phase 1) induces an abrupt spike in force (P1) due to strained elastic elements within the strongly bound XBs. Force then rapidly decays (Phase 2), with a rate constant krel and magnitude P2, due to the detachment of the strained XBs into a non-force bearing state. Next, there is a delayed force redevelopment (Phase 3), with a rate constant kdf, as XBs reenter force-bearing states via stretch-induced cooperative recruitment to reach a higher steady state force level (P3). Pdf denotes the magnitude of XB recruitment and is the difference between P3 and P2. For a detailed description of various phases, refer to [22, 23].
Finally, it should be noted that there are inherent caveats to translating results from skinned cardiac preparations to whole-heart function. For one, they cannot fully simulate the dynamic interplay between other regulatory mechanisms that prevail in vivo [27, 28]. Secondly, experiments on skinned cardiac preparations are generally performed in constant Ca2+ environments as the Ca2+ transients present in vivo are difficult to replicate. Instead, the contractile behavior of cardiac fibers is often studied at several different steady state Ca2+ concentrations ranging from pCa 9.0 to pCa 4.5. Doing so allows experimenters to build a more complete picture of how XB behavior changes as a function of Ca2+ activation level than would otherwise be possible. This approach has proven useful for studying XB kinetics and drug effects at submaximal Ca2+ levels, which have important physiological implications concerning in vivo force development and relaxation [11, 12]. It is also notable that much of skinned or intact fiber experiments are conducted at sub-physiologic temperatures (~15–25°C) to preserve myofilament integrity and minimize force rundown [29, 30]. While the absolute values of XB kinetics decrease as temperature decreases, their responsiveness to post-translational modifications and other regulatory mechanisms remain intact and correlate well with measurements made in vivo [31, 32]. This extrapolation allows for the study of critical phases of XB cycling and the underlying myofilament regulatory mechanisms. Thus, these experiments provide a flexible way to generate crucial preclinical data on drugs that could accelerate the translation of basic science research findings to clinical studies.
Relating contractile measurements in isolated cardiac muscle preparations to in vivo whole organ function:
There is an apparent gap between basic and clinical science methods and measurements investigating cardiac contractile function, making it difficult to directly relate specific parameters of a XB cycle to the events in a cardiac cycle. But it is clear that ventricular contractile function is heavily dependent on XB kinetics [15]. Thus, understanding the pathophysiologic link between sarcomeric processes and whole heart function can provide useful prognostic information and predict response to therapies.
Invasive left ventricular (LV) catheterization was among the earliest and most direct way of assessing in vivo LV pathophysiology, providing important indices of pressure and volume (PV) hemodynamic function. Though it is now rarely used in the diagnosis or clinical evaluation of cardiomyopathies, PV loop analysis still provides a basic physiological framework in relating XB mechanics to ventricular contractility. The effect of ventricular contractility on in vivo function has been well studied in models of familial HCM, as it is predominantly caused by autosomal dominant mutations in sarcomeric proteins [33]. Although experimental models using these mutations are not a direct representation of clinical function, they do recapitulate key phenotypic characteristics and fundamental effects of myofilament abnormalities on in vivo contractility. In particular, mutations in the gene encoding cardiac myosin binding protein-C (cMyBPC), a regulatory protein found within the C zone of thick filaments, are among the most common causes of familial HCM [34, 35]. While the complex regulatory effects of cMyBPC on XB kinetics is beyond the scope of this review (reviewed in [20]), there are numerous cMyBPC studies that can provide fundamental insights regarding changes in XB kinetics in relation to whole heart function [36–39].
Briefly, cMyBPC mediates acto-myosin interactions in response to neurohormonal adrenergic drive, a mechanism that phosphorylates cMyBPC. cMyBPC phosphorylation has been shown to promote acto-myosin interactions [20] and contribute to thin filament cooperative activation, thereby accelerating XB kinetics [39, 40]. The effects of cMyBPC on rates of XB recruitment (kdf) and detachment (krel) are a measure of the intrinsic myocardial contractility and can be related to specific events of the cardiac cycle including ventricular filling, isovolumic contraction (IVC), ejection, and isovolumic relaxation (IVR) (Figure 2). In skinned fiber stretch activation (SA) experiments, SA elicits a characteristic XB detachment and recruitment kinetics. At the whole-heart level, SA is analogous to the ventricular wall stretch during ventricular filling and IVC phases. Following the closure of mitral valve, myofilaments develop force during IVC at a constant muscle length as a result of XB recruitment (kdf). In addition, the timing of myocardial activation during IVC varies across different regions of the heart, first in the endocardium and later in the epicardium, such that at a given time there are some regions of the heart that undergo a stretch activation response [41]. Studies have demonstrated that cMyBPC-mediated XB recruitment is important during low [Ca2+] when thin filament cooperative activation is enhanced, in part, by cMyBPC binding to actin [42]. Thus, the kinetic measurements in fiber SA experiments are expected to play a role throughout IVC pressure development. Fiber and in vivo experiments in mice have demonstrated that the rate (kdf) and magnitude (Pdf) of XB recruitment of force generating states show a good correlation to the rate (i.e., dP/dtmax) and magnitude (i.e. maximal pressure) of pressure development during IVC [15, 43] (Figure 2). Overall, the presence of cMyBPC in the C zone serves to tune the timing of myofilament activation and slow the rate of pressure development. This slowing or braking role is thought to normalize the timing of contraction as well as prolong the force generation [44].
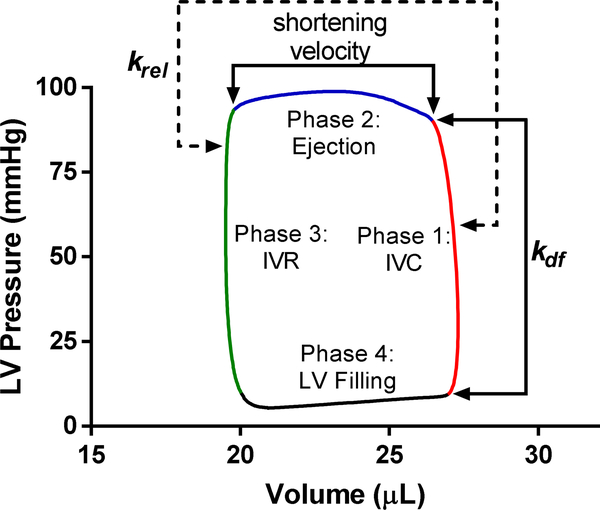
Figure 2. Representative pressure-volume trace (PV loop) with the phases of cardiac cycle and correlated parameters measured in skinned myocardial preparations.
Early isovolumic pressure development, dP/dtmax, and td during phase 1 is affected by the magnitude (Pdf) as well as the rate (kdf) of XB recruitment. kdf is Ca2+ sensitive due to the influx of Ca2+ during IVC. krel is a measure of XB turnover, so it may also contribute to the isovolumic pressure development during phase 1. ET during phase 2 is influenced by krel and shortening velocity. Since Ca2+ is absent at the end of the ejection phase, krel determines ET by altering the timing of cooperative deactivation of thin filaments. Early isovolumic relaxation during phase 3 can be correlated with krel as well because XB detachment rates influence early diastolic relaxation. dP/dtmax, maximum rate of pressure development; ET, ejection time; IVC, isovolumic contraction; IVR, isovolumic relaxation; kdf, rate of XB recruitment; krel, rate of XB detachment or XB turnover; LV, left ventricular; td, time to dp/dtmax; and XB, cross bridge.
The rate of XB detachment, krel, is a measure of XB turnover and contributes to XB on-time and duty ratio. The effect of krel on in vivo contractility is dependent on the number of times XBs turnover during a cardiac cycle [15]. Given what is currently known of sarcomere structure, XB turnover per heart beat is most likely between 1–3 cycles [15, 45]. Thus, as the number of times XBs turnover during one cardiac cycle is more than once per cardiac cycle, acceleration of XB detachment (krel) increases the overall rate of XB turnover and therefore also increase the rate of pressure development (dP/dt) [46]. Furthermore, XB turnover also likely determines the ejection duration and early IVR because the rates of cooperative XB deactivation will determine how long XBs remain attached in late systole [43, 46, 47] (Figure 2). Factors that accelerate krel (e.g. increased cMyBPC phosphorylation) also accelerate myocyte loaded shortening velocity and shortens in vivo ejection time [46, 48]. The acceleration of ejection is an important response during periods of increase adrenergic stimulus but is also a hallmark of dysfunction in HCM, where shortened basal ejection is indicative of hypercontractile myofilament function. XB detachment rate has also been shown to impact rate of early relaxation in early diastole [27, 46, 47, 49], which is thought to involve XB-mediated processes (i.e. cooperative thin filament deactivation) (Figure 2) [15, 26]. The observed blunted diastolic thin filament deactivation in HCM models is consistent with clinical findings that diastolic dysfunction is an important pathogenic component of pre-clinical HCM [49–51].
Transthoracic 2D and Doppler echocardiography have long been the gold standard for the assessment of ventricular function and diagnosis of cardiomyopathies. Yet a positive diagnosis can encompass a wide spectrum of clinical phenotypes depending on disease progression and primary disease mechanism [52, 53]. Furthermore, many cardiomyopathy-associated mutations show incomplete penetrance, and hence there is a need to distinguish genotype positive but phenotype negative individuals who have normal contractile function or delayed-onset age-dependent disease progression [51, 54, 55]. Conventional functional parameters such as ejection fraction (EF) and fractional shortening (FS) are load dependent and are subject to factors independent of myocardium contractility. However, recent advancements in strain analysis by tissue Doppler imaging (TDI), speckle-tracking echocardiography (STE), and cardiac magnetic resonance (CMR) provide an effective method to examine contractility that is loading independent [56–58]. These techniques offer informative and noninvasive assessment of contractile indices such as regional strain rate (SR) and time-to-peak SR (Figure 3). They have been shown to correlate well with invasive PV measurements such as time-to-peak pressure development [58], and in vitro physiological kinetic measurements (e.g. loaded shortening velocity) [59]. In addition, XB kinetic parameters would also be expected to contribute to SR, similar to effects observed with PV indices. Physiologic and pathologic acceleration of force development, kdf, would also be expected to increase SR similar to the observed changes in dP/dtmax in PV analysis. The relationship between the timing for force generation (e.g. early truncation of ejection) observed in PV analysis of the cMyBPC−/− mouse model [60] is also recapitulated in STE measurements (unpublished data), correlating well to both time-to-peak strain and time-to-peak SR measurements (Figure 3). Thus, capturing regional strain can provide a potential means of translating contractility changes at the fiber level to observed localized contractile dysfunction at the whole-heart level, bridging the translational disconnect between basic and clinical indices. Notably, in conditions such as HCM, early detection of subclinical contractility changes within specific cardiac regions (i.e. septum) can be of great prognostic importance [51].
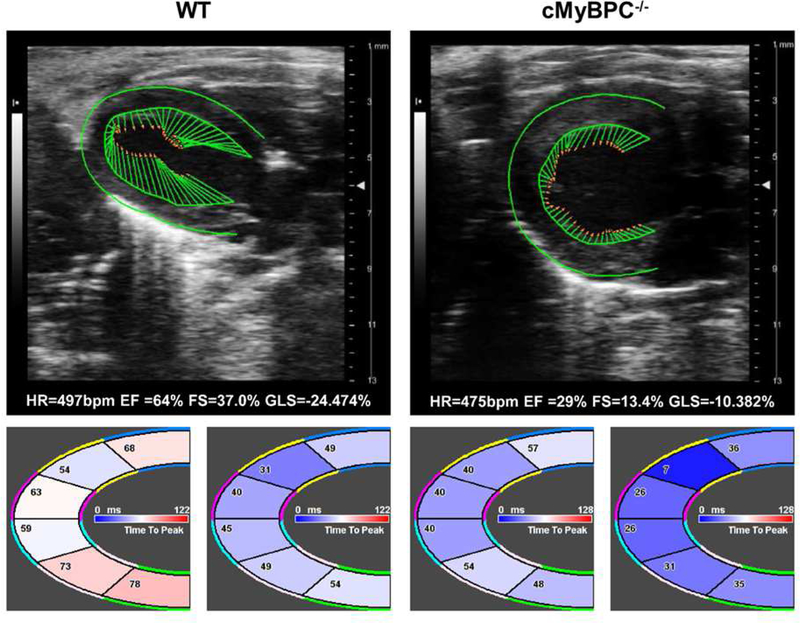
Figure 3. Representative speckle-tracking echocardiography (STE) analysis of WT and cMyBPC−/− mouse myocardium.
The top panel shows representative STE images along with the heart rate (HR), ejection fraction (EF), fractional shortening (FS), and global longitudinal strain (GLS) of WT (left) and cMyBPC−/− (right) mouse myocardium. The regional time-to-peak strain (left) and time-to-peak strain rate (right) are shown below WT and cMyBPC−/− STE images. Notably, cMyBPC−/− STE shows reduced GLS, shortened time-to-peak strain, and shortened time-to-peak strain rate.
Skinned cardiac muscle fiber measurements provide mechanistic insights into effects of drugs in clinical trials:
It is now well established that current inotropic therapies enhance cardiac contractility but concurrently elicit undesirable increases in intracellular [Ca2+], myocardial oxygen and ATP needs –factors that ultimately cause arrhythmias and mortality [61]. Thus, a novel approach to improve cardiac performance is to develop a new class of sarcomere-based drugs that evade cellular signaling pathways and instead directly target the cardiac sarcomere [61, 62]. The importance of novel sarcomere-based therapies is highlighted by clinical trials showing promising results using drugs that correct in vivo myocardial function by directly modulating force-generating properties of myosin [63–66]. Specifically, omecamtiv mecarbil (OM) and mavacamten (Myk461) are two myosin modulators in clinical and preclinical trials for improving systolic function or suppressing hyperdynamic ventricular function, respectively [16, 65]. Because of their selective affinity to cardiac myosin, any in vivo functional changes induced by OM or Myk461 can be linked to their impact on sarcomeric force generation and myosin XB properties. In this context, biophysical measurements using chemically-skinned cardiac muscle fiber preparations can provide insights into the sarcomeric mechanisms governing in vivo drug-induced functional changes and as such, predictions from skinned fiber experiments may also aid in developing novel therapies for other sarcomere targets.
Of clinical importance, OM infusions improve in vivo systolic function in healthy and HF populations [63–66]. Specifically, OM increases stroke volume, FS, and EF along with prolonged systolic ejection time (SET) and slowed heart rate [63, 64]. A better understanding of OM’s in vivo effects can be aided by muscle fiber experiments. For example, systolic enhancement can be explained by OM-induced force enhancement at submaximal Ca2+ activations in skinned myocardial preparations [10, 12] –suggesting that OM recruits additional XBs to actin during early stages of IVC when sarcomeric Ca2+ levels are not at their peak. Interestingly, despite enhancing force generation, OM significantly slows both the rates of XB recruitment (kdf) and force redevelopment (ktr), indicating that OM promotes cooperative XB-mediated XB recruitment to actin, a time-consuming process that slows and also sustains the overall force development [10, 12]. This sarcomeric mechanism supports OM-induced in vivo SET prolongations. SET prolongation is further supported by the finding that OM extends the overall time to achieve the peak of force development (Tpk) in myocardial preparations [12]. Additionally, OM also slowed XB detachment (krel) suggesting a net increase in XB duty time [10, 12], a finding supported by FV (loaded shortening) experiments (Figure 4) and other biophysical approaches [8, 67, 68]. This OM-induced FV slowing suggests that in vivo, OM maintains myocardial power output and contractility for a longer duration. Interestingly, despite a prolonged SET, diastolic duration was only slightly reduced which is likely attributed to a decreased heart rate following OM infusion [63], –thus plasma OM infusions (200–500ng/mL) caused only non-significant decreases in end diastolic volumes (EDVs) [63]. However, in the same study it was observed that EDVs were significantly decreased when [OM] exceeded 500ng/mL. This observation can be explained by results from skinned myocardial experiments which showed that high OM doses not only excessively prolong the Tpk but also slow krel in HF myocardium [12], an additive effect that potentially impairs ventricular relaxation and end-diastolic filling as observed clinically [63] –thus, indicating a need for dose optimization such that only the beneficial effects are elicited. Another aspect to be considered for sarcomere-based therapies is that HF myocardium likely undergoes some degree of remodeling and as a result, myosin modulators may behave differently in HF myocardium. For example, skinned fiber studies revealed a substantial time delay in XB recruitment (Trec) which subsequently delayed the Tpk in OM-treated HF myocardial preparations [12] –suggesting that OM-induced force enhancements may be delayed in remodeled myocardium.
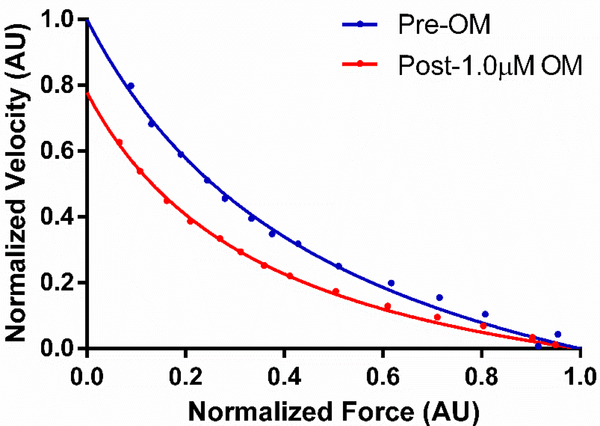
Figure 4. Representative force velocity (FV) curves demonstrating the effect of OM on loaded shortening velocity in donor human myocardium.
Representative FV curves collected at ~30% of maximal force generation and at ~25°C are shown and demonstrate a slowed loaded shortening velocity following incubation of skinned myocardial fibers with 1.0μM OM.
Another class of myosin modulators are myosin inhibitors (e.g., Myk461), in development to correct hyperdynamic systolic function in HCM patients. Preclinical studies using mouse and feline HCM models show that Myk461 effectively reduces hyperdynamic systolic function, and also reverses pathological remodeling [16, 69]. Supporting evidence from kinetic assays showed that Myk461-mediated decreases in in vivo contractility are perhaps related to its ability to inhibit phosphate release from myosin heads, and thereby prevent XB transitions to their strongly-bound states [16, 17]. Using skinned fiber experiments, we recently showed that Myk461-mediated reductions in hypercontractility are primarily due to decreased force development by slowing the net transition of cooperatively recruited XBs to their force-bearing state [11]. Interestingly it appears that Myk461 may affect XB function differently depending on whether cMyBPC is present or absent in the sarcomere. Specifically, we observed that the Myk461-mediated force reductions are attenuated when myocardium lacks cMyBPC, which may be due to a preferential Myk461-induced slowing of krel in myocardium lacking cMyBPC [11], although more studies will be required to understand the precise contributions of cMyBPC expression on Myk461-mediated modulation of XB behavior. Moreover, this finding is clinically relevant because cMyBPC-related HCM is very common (~40% of all hereditary HCM cases [34, 35]) and largely leads to reduced cMyBPC expression via haploinsufficiency [70–73]. Collectively, in addition to providing key mechanistic insights into in vivo clinical data, skinned fiber studies may also be valuable for elucidating mechanisms that are otherwise hard to isolate using in vivo techniques, and thus help explain unexpected consequences of therapeutic drugs or dose-dependent effects in a complex whole organ system.
Summary:
Advancement of cardiovascular medicine requires that clinical trials leverage the molecular insights derived from biophysical measurements in isolated cardiac muscle. Integration of unique findings at the sarcomeric level will lead to a better understanding of drug function in vivo and optimization of clinical trials to achieve improved patient outcomes.
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health (HL-114770 to Stelzer; T32-HL007567 and GM007250 to Li), American Heart Association (16POST30730000 to Mamidi), and National Institutes of Health (P30 EY011373) grants.
Footnotes
Disclosures
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].MacRae CA, Roden DM, Loscalzo J, The Future of Cardiovascular Therapeutics, Circulation 133(25) (2016) 2610–7. [DOI] [PubMed] [Google Scholar]
- [2].Tardiff JC, Carrier L, Bers DM, Poggesi C, Ferrantini C, Coppini R, Maier LS, Ashrafian H, Huke S, van der Velden J, Targets for therapy in sarcomeric cardiomyopathies, Cardiovasc Res 105(4) (2015) 457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burchfield JS, Xie M, Hill JA, Pathological ventricular remodeling: mechanisms: part 1 of 2, Circulation 128(4) (2013) 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].van der Velden J, Ho CY, Tardiff JC, Olivotto I, Knollmann BC, Carrier L, Research priorities in sarcomeric cardiomyopathies, Cardiovasc Res 105(4) (2015) 449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mamidi R, Li J, Gresham KS, Stelzer JE, Cardiac myosin binding protein-C: a novel sarcomeric target for gene therapy, Pflugers Arch 466(2) (2014) 225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rohde AJ, Thomas DD, Muretta MJ, Mavacamten stabilizes the auto-inhibited state of twoheaded cardiac myosin, 2018. [DOI] [PMC free article] [PubMed]
- [7].Liu C, Kawana M, Song D, Ruppel KM, Spudich JA, Controlling load-dependent kinetics of beta-cardiac myosin at the single-molecule level, Nat Struct Mol Biol 25(6) (2018) 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang Y, Ajtai K, Burghardt TP, Analytical comparison of natural and pharmaceutical ventricular myosin activators, Biochemistry 53(32) (2014) 5298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, Rogers CS, Gorham JM, Wong FL, Morck MM, Seidman JG, Ruppel KM, Irving TC, Cooke R, Green EM, Spudich JA, Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers, Proc Natl Acad Sci U S A 115(35) (2018) E8143–E8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mamidi R, Gresham KS, Li A, Dos Remedios CG, Stelzer JE, Molecular effects of the myosin activator omecamtiv mecarbil on contractile properties of skinned myocardium lacking cardiac myosin binding protein-C, J Mol Cell Cardiol 85 (2015) 262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mamidi R, Li J, Doh CY, Verma S, Stelzer JE, Impact of the Myosin Modulator Mavacamten on Force Generation and Cross-Bridge Behavior in a Murine Model of Hypercontractility, Journal of the American Heart Association 7:e009627 (2018) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mamidi R, Li J, Gresham KS, Verma S, Doh CY, Li A, Lal S, Dos Remedios CG, Stelzer JE, Dose-Dependent Effects of the Myosin Activator Omecamtiv Mecarbil on Cross-Bridge Behavior and Force Generation in Failing Human Myocardium, Circ Heart Fail 10(10) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gordon AM, Homsher E, Regnier M, Regulation of contraction in striated muscle, Physiol Rev 80(2) (2000) 853–924. [DOI] [PubMed] [Google Scholar]
- [14].Maughan DW, Kinetics and energetics of the crossbridge cycle, Heart Fail Rev 10(3) (2005) 175–85. [DOI] [PubMed] [Google Scholar]
- [15].Stehle R, Iorga B, Kinetics of cardiac sarcomeric processes and rate-limiting steps in contraction and relaxation, J Mol Cell Cardiol 48(5) (2010) 843–50. [DOI] [PubMed] [Google Scholar]
- [16].Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, Henze M, Kawas R, Oslob JD, Rodriguez HM, Song Y, Wan W, Leinwand LA, Spudich JA, McDowell RS, Seidman JG, Seidman CE, A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice, Science 351(6273) (2016) 617–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kawas RF, Anderson RL, Ingle SRB, Song Y, Sran AS, Rodriguez HM, A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle, J Biol Chem 292(40) (2017) 16571–16577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R, Baliga R, Cox DR, Garard M, Godinez G, Kawas R, Kraynack E, Lenzi D, Lu PP, Muci A, Niu C, Qian X, Pierce DW, Pokrovskii M, Suehiro I, Sylvester S, Tochimoto T, Valdez C, Wang W, Katori T, Kass DA, Shen YT, Vatner SF, Morgans DJ, Cardiac myosin activation: a potential therapeutic approach for systolic heart failure, Science 331(6023) (2011) 1439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hwang PM, Sykes BD, Targeting the sarcomere to correct muscle function, Nat Rev Drug Discov 14(5) (2015) 313–28. [DOI] [PubMed] [Google Scholar]
- [20].Moss RL, Fitzsimons DP, Ralphe JC, Cardiac MyBP-C regulates the rate and force of contraction in mammalian myocardium, Circ Res 116(1) (2015) 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Campbell K, Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics, Biophys J 72(1) (1997) 254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ford SJ, Chandra M, Mamidi R, Dong W, Campbell KB, Model representation of the nonlinear step response in cardiac muscle, J Gen Physiol 136(2) (2010) 159–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stelzer JE, Larsson L, Fitzsimons DP, Moss RL, Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function, J Gen Physiol 127(2) (2006) 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Piazzesi G, Linari M, Reconditi M, Vanzi F, Lombardi V, Cross-bridge detachment and attachment following a step stretch imposed on active single frog muscle fibres, J Physiol 498 ( Pt 1) (1997) 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McDonald KS, Ca2+ dependence of loaded shortening in rat skinned cardiac myocytes and skeletal muscle fibres, J Physiol 525 Pt 1 (2000) 169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hanft LM, Korte FS, McDonald KS, Cardiac function and modulation of sarcomeric function by length, Cardiovasc Res 77(4) (2008) 627–36. [DOI] [PubMed] [Google Scholar]
- [27].Biesiadecki BJ, Davis JP, Ziolo MT, Janssen PM, Tri-modal regulation of cardiac muscle relaxation; intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics, Biophys Rev 6(3-) (2014) 273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sadayappan S, The Myofilament Field Revisited in the Age of Cellular and Molecular Biology, Circ Res 121(6) (2017) 601–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Tombe PP, Stienen GJ, Impact of temperature on cross-bridge cycling kinetics in rat myocardium, J Physiol 584(Pt 2) (2007) 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Milani-Nejad N, Xu Y, Davis JP, Campbell KS, Janssen PM, Effect of muscle length on crossbridge kinetics in intact cardiac trabeculae at body temperature, J Gen Physiol 141(1) (2013) 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gresham KS, Mamidi R, Li J, Kwak H, Stelzer JE, Sarcomeric protein modification during adrenergic stress enhances cross-bridge kinetics and cardiac output, J Appl Physiol (1985) (2016) jap 00306 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mamidi R, Gresham KS, Verma S, Stelzer JE, Cardiac Myosin Binding Protein-C Phosphorylation Modulates Myofilament Length-Dependent Activation, Front Physiol 7 (2016) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tardiff JC, Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes, Heart Fail Rev 10(3) (2005) 237–48. [DOI] [PubMed] [Google Scholar]
- [34].Barefield D, Sadayappan S, Phosphorylation and function of cardiac myosin binding protein-C in health and disease, J Mol Cell Cardiol 48(5) (2010) 866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nag S, Trivedi DV, Sarkar SS, Adhikari AS, Sunitha MS, Sutton S, Ruppel KM, Spudich JA, The myosin mesa and the basis of hypercontractility caused by hypertrophic cardiomyopathy mutations, Nat Struct Mol Biol 24(6) (2017) 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Colson BA, Bekyarova T, Fitzsimons DP, Irving TC, Moss RL, Radial displacement of myosin cross-bridges in mouse myocardium due to ablation of myosin binding protein-C, J Mol Biol 367(1) (2007) 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nagayama T, Takimoto E, Sadayappan S, Mudd JO, Seidman JG, Robbins J, Kass DA, Control of in vivo left ventricular [correction] contraction/relaxation kinetics by myosin binding protein C: protein kinase A phosphorylation dependent and independent regulation, Circulation 116(21) (2007) 2399–408. [DOI] [PubMed] [Google Scholar]
- [38].Stelzer JE, Dunning SB, Moss RL, Ablation of cardiac myosin-binding protein-C accelerates stretch activation in murine skinned myocardium, Circ Res 98(9) (2006) 1212–8. [DOI] [PubMed] [Google Scholar]
- [39].Stelzer JE, Patel JR, Walker JW, Moss RL, Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation, Circ Res 101(5) (2007) 503–11. [DOI] [PubMed] [Google Scholar]
- [40].Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL, Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function, Circ Res 103(9) (2008) 974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stelzer JE, Moss RL, Contributions of stretch activation to length-dependent contraction in murine myocardium, J Gen Physiol 128(4) (2006) 461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kampourakis T, Yan Z, Gautel M, Sun YB, Irving M, Myosin binding protein-C activates thin filaments and inhibits thick filaments in heart muscle cells, Proc Natl Acad Sci U S A 111(52) (2014) 18763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mamidi R, Gresham KS, Li J, Stelzer JE, Cardiac myosin binding protein-C Ser(302) phosphorylation regulates cardiac beta-adrenergic reserve, Sci Adv 3(3) (2017) e1602445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Previs MJ, Prosser BL, Mun JY, Previs SB, Gulick J, Lee K, Robbins J, Craig R, Lederer WJ, Warshaw DM, Myosin-binding protein C corrects an intrinsic inhomogeneity in cardiac excitation-contraction coupling, Sci Adv 1(1) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Spudich JA, Hypertrophic and dilated cardiomyopathy: four decades of basic research on muscle lead to potential therapeutic approaches to these devastating genetic diseases, Biophys J 106(6) (2014) 1236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gresham KS, Stelzer JE, The contributions of cardiac myosin binding protein C and troponin I phosphorylation to beta-adrenergic enhancement of in vivo cardiac function, J Physiol 594(3) (2016) 669–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van Dijk SJ, Kooiker KB, Napierski NC, Touma KD, Mazzalupo S, Harris SP, Point mutations in the tri-helix bundle of the M-domain of cardiac myosin binding protein-C influence systolic duration and delay cardiac relaxation, J Mol Cell Cardiol 119 (2018) 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hanft LM, Emter CA, McDonald KS, Cardiac myofibrillar contractile properties during the progression from hypertension to decompensated heart failure, Am J Physiol Heart Circ Physiol 313(1) (2017) H103–H113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pohlmann L, Kroger I, Vignier N, Schlossarek S, Kramer E, Coirault C, Sultan KR, El-Armouche A, Winegrad S, Eschenhagen T, Carrier L, Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes, Circ Res 101(9) (2007) 928–38. [DOI] [PubMed] [Google Scholar]
- [50].Michels M, Soliman OI, Kofflard MJ, Hoedemaekers YM, Dooijes D, Majoor-Krakauer D, ten Cate FJ, Diastolic abnormalities as the first feature of hypertrophic cardiomyopathy in Dutch myosin-binding protein C founder mutations, JACC Cardiovasc Imaging 2(1) (2009) 58–64. [DOI] [PubMed] [Google Scholar]
- [51].Voigt C, Munch J, Avanesov M, Suling A, Witzel K, Lund G, Patten M, Early segmental relaxation abnormalities in hypertrophic cardiomyopathy for differential diagnostic of patients with left ventricular hypertrophy, Clin Cardiol 40(11) (2017) 1026–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A, Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases, Eur Heart J 29(2) (2008) 270–6. [DOI] [PubMed] [Google Scholar]
- [53].Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB, American Heart A, H.F. Council on Clinical Cardiology, C. Transplantation, C. Quality of, R. Outcomes, G. Functional, G. Translational Biology Interdisciplinary Working, E. Council on, Prevention, Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention, Circulation 113(14) (2006) 1807–16. [DOI] [PubMed] [Google Scholar]
- [54].Bondue A, Arbustini E, Bianco A, Ciccarelli M, Dawson D, De Rosa M, Hamdani N, Hilfiker-Kleiner D, Meder B, Leite-Moreira AF, Thum T, Tocchetti CG, Varricchi G, Van der Velden J, Walsh R, Heymans S, Complex roads from genotype to phenotype in dilated cardiomyopathy: scientific update from the Working Group of Myocardial Function of the European Society of Cardiology, Cardiovasc Res 114(10) (2018) 1287–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lopes LR, Syrris P, Guttmann OP, O’Mahony C, Tang HC, Dalageorgou C, Jenkins S, Hubank M, Monserrat L, McKenna WJ, Plagnol V, Elliott PM, Novel genotype-phenotype associations demonstrated by high-throughput sequencing in patients with hypertrophic cardiomyopathy, Heart 101(4) (2015) 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, Drinko JK, Rodriguez LL, Thomas JD, Garcia MJ, Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility, Circulation 105(1) (2002) 99–105. [DOI] [PubMed] [Google Scholar]
- [57].Kinno M, Nagpal P, Horgan S, Waller AH, Comparison of Echocardiography, Cardiac Magnetic Resonance, and Computed Tomographic Imaging for the Evaluation of Left Ventricular Myocardial Function: Part 2 (Diastolic and Regional Assessment), Curr Cardiol Rep 19(1) (2017) 6. [DOI] [PubMed] [Google Scholar]
- [58].Weidemann F, Jamal F, Sutherland GR, Claus P, Kowalski M, Hatle L, De Scheerder I, Bijnens B, Rademakers FE, Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate, Am J Physiol Heart Circ Physiol 283(2) (2002) H792–9. [DOI] [PubMed] [Google Scholar]
- [59].Abraham TP, Laskowski C, Zhan WZ, Belohlavek M, Martin EA, Greenleaf JF, Sieck GC, Myocardial contractility by strain echocardiography: comparison with physiological measurements in an in vitro model, Am J Physiol Heart Circ Physiol 285(6) (2003) H2599–604. [DOI] [PubMed] [Google Scholar]
- [60].Brickson S, Fitzsimons DP, Pereira L, Hacker T, Valdivia H, Moss RL, In vivo left ventricular functional capacity is compromised in cMyBP-C null mice, Am J Physiol Heart Circ Physiol 292(4) (2007) H1747–54. [DOI] [PubMed] [Google Scholar]
- [61].Teerlink JR, A novel approach to improve cardiac performance: cardiac myosin activators, Heart Fail Rev 14(4) (2009) 289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Malik FI, Morgan BP, Cardiac myosin activation part 1: from concept to clinic, J Mol Cell Cardiol 51(4) (2011) 454–61. [DOI] [PubMed] [Google Scholar]
- [63].Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA, Malik FI, The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial, Lancet 378(9792) (2011) 676–83. [DOI] [PubMed] [Google Scholar]
- [64].Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, Elliott L, Bee R, Habibzadeh MR, Goldman JH, Schiller NB, Malik FI, Wolff AA, Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study, Lancet 378(9792) (2011) 667–75. [DOI] [PubMed] [Google Scholar]
- [65].Teerlink JR, Felker GM, McMurray JJ, Solomon SD, Adams KF Jr., Cleland JG, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsanyi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N, C.-H. Investigators, Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial, Lancet 388(10062) (2016) 2895–2903. [DOI] [PubMed] [Google Scholar]
- [66].Teerlink JR, Felker GM, McMurray JJV, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, Cleland JGF, Kim JB, Lei L, Knusel B, Wolff AA, Malik FI, Wasserman SM, A.-A. Investigators, Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure: The ATOMIC-AHF Study, J Am Coll Cardiol 67(12) (2016) 1444–1455. [DOI] [PubMed] [Google Scholar]
- [67].Liu Y, White HD, Belknap B, Winkelmann DA, Forgacs E, Omecamtiv Mecarbil modulates the kinetic and motile properties of porcine beta-cardiac myosin, Biochemistry 54(10) (2015) 1963–75. [DOI] [PubMed] [Google Scholar]
- [68].Swenson AM, Tang W, Blair CA, Fetrow CM, Unrath WC, Previs MJ, Campbell KS, Yengo CM, Omecamtiv Mecarbil Enhances the Duty Ratio of Human beta-Cardiac Myosin Resulting in Increased Calcium Sensitivity and Slowed Force Development in Cardiac Muscle, J Biol Chem 292(9) (2017) 3768–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Stern JA, Markova S, Ueda Y, Kim JB, Pascoe PJ, Evanchik MJ, Green EM, Harris SP, A Small Molecule Inhibitor of Sarcomere Contractility Acutely Relieves Left Ventricular Outflow Tract Obstruction in Feline Hypertrophic Cardiomyopathy, PLoS One 11(12) (2016) e0168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jacques AM, Copeland O, Messer AE, Gallon CE, King K, McKenna WJ, Tsang VT, Marston SB, Myosin binding protein C phosphorylation in normal, hypertrophic and failing human heart muscle, J Mol Cell Cardiol 45(2) (2008) 209–16. [DOI] [PubMed] [Google Scholar]
- [71].Marston S, Copeland O, Gehmlich K, Schlossarek S, Carrier L, How do MYBPC3 mutations cause hypertrophic cardiomyopathy?, J Muscle Res Cell Motil 33(1) (2012) 75–80. [DOI] [PubMed] [Google Scholar]
- [72].Marston S, Copeland O, Jacques A, Livesey K, Tsang V, McKenna WJ, Jalilzadeh S, Carballo S, Redwood C, Watkins H, Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency, Circ Res 105(3) (2009) 219–22. [DOI] [PubMed] [Google Scholar]
- [73].van Dijk SJ, Dooijes D, dos Remedios C, Michels M, Lamers JM, Winegrad S, Schlossarek S, Carrier L, ten Cate FJ, Stienen GJ, van der Velden J, Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction, Circulation 119(11) (2009) 1473–83. [DOI] [PubMed] [Google Scholar]