Abstract
Objectives
Preclinical studies demonstrated antitumor activity of dovitinib in pancreatic cancer models. This phase Ib study aimed to determine the maximum tolerated dose of dovitinib in combination with gemcitabine and capecitabine and to characterize the safety and pharmacokinetic profile in patients with advanced pancreatic and biliary tract cancers (aPB) and solid malignancies.
Methods
Patients received gemcitabine 1000 mg/m2 intravenously on days 1 and 8, capecitabine 1300 mg/m2 oral daily from day 1 to 14, and dovitinib oral daily five days on and two days off, every 21-day cycle. The standard 3+3 dose escalation design was utilized and the study expanded to treat an additional 20 aPB patients at maximum tolerated dose.
Results
A total of 29 patients were enrolled. One patient experienced dose limiting grade 3 colitis. Two patients developed clinically significant neuropathy after the first cycle requiring dose reduction. The maximum tolerated dose was not reached and dovitinib 300 mg was declared the recommended dose for expansion. The most frequent grade 2 or worse adverse events were fatigue (45%), neutropenia (41%), thrombocytopenia (34%), anemia (24%), nausea (24%), and palmer-plantar erythrodysaesthesia syndrome (21%). Partial responses were observed in 5 patients. Pharmacokinetic studies showed no drug-drug interaction between dovitinib, capecitabine and gemcitabine. Fibroblast growth factor 23 plasma level increased in 4 of 5 patients during the first cycle of treatment.
Conclusions
Dovitinib 300 mg daily is the recommended dose when combined with gemcitabine and capecitabine, achieving clinically relevant plasma concentrations. The study combination demonstrated encouraging efficacy signals in advanced pancreatic cancer.
Keywords: dovitinib, pancreatic adenocarcinoma, FGFR/VEGFR inhibitor
INTRODUCTION
Pancreatic adenocarcinoma is the fourth leading cause of cancer death in the United States.1 The 5-year overall survival (OS) remains low for patients with advanced pancreatic cancer despite significant advances in chemotherapy strategies. Combination chemotherapy regimens such as gemcitabine plus nab-paclitaxel2 and FOLFIRINOX3 demonstrated moderate OS benefit compared to gemcitabine alone in the setting of locally advanced or metastatic pancreatic adenocarcinoma. Targeted therapy has also been explored. Large randomized trial of gemcitabine plus erlotinib or placebo showed statistically significant OS benefit, but insignificant clinical benefit.4
Fibroblast growth factor (FGF) signaling had been implicated in the oncogenesis of many cancer types including pancreatic cancer in the form of activating mutation, gene fusions, and gene amplification, which lead to tumor migration, proliferation, and survival.5 Activation of FGF signaling has also been implicated in the resistance to VEGF pathway inhibition.6 Dovitinib is a highly potent multi-kinase inhibitor of fibroblast growth factor receptors (FGFRs) with IC50<10 nmol/L, vascular endothelial growth factor receptor 2 (VEGFR2), and platelet-derived growth factor receptor β (PDGFRβ).7 Preclinical studies showed that inhibiting FGFR signaling using anti-FGFR substrate 2 shRNA achieved pro-apoptotic effect in pancreatic cancer, which could also be achieved pharmacologically using dovitinib in both cell line and primary patient tumor models.8 In addition, dovitinib as a single agent or in combination with chemotherapy has demonstrated tolerability and activity in patients with various types of cancers such as advanced renal cell carcinoma,9 urothelial carcinoma,10 hepatocellular carcinoma,11 and melanoma.12 To our knowledge, the feasibility of combining dovitinib with cytotoxic anti-cancer agents has not been evaluated prior to this study.
The primary objectives of this phase Ib trial were to determine the maximum tolerated dose (MTD) and recommended phase II dose (RP2D) of dovitinib when administered concurrently with gemcitabine and capecitabine in patients with advanced pancreatico-biliary cancers and solid malignancies, and also to characterize the safety profile of this regimen. The secondary objectives were to characterize the pharmacokinetic profile and pharmacodynamics effects of concurrent dovitinib, capecitabine, gemcitabine and their metabolites, and to determine the preliminary activity of the study combination in this patient population.
MATERIALS AND METHODS
Patient eligibility
The eligibility criteria included patients with histologically or cytologically confirmed advanced or metastatic solid malignancies where the gemcitabine combination was considered standard therapy or a rational option; patients eligible for the expansion cohort would have locally advanced or metastatic pancreas or biliary tract adenocarcinoma; age ≥ 18 years; Eastern Cooperative Oncology Group (ECOG) performance status ≤ 1; adequate bone marrow, renal and liver function defined by absolute neutrophil count (ANC) ≥ 1,500 cells/mm3, platelets ≥ 100,000 cells/mm3, hemoglobin ≥ 9.0 g/dL, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤ 1.5 × upper limit of normal (ULN), total bilirubin ≤ 1.5 × ULN, serum creatinine ≤ 1.5 × ULN, international normalized ratio (INR) ≤ 1.5 (anti-coagulation is allowed if target INR ≤ 1.5 on a stable dose of warfarin or on a stable dose of low molecular weight heparin for > 2 weeks at the first dose of study agent), 24-hour urine protein < 2 grams if urinalysis showed proteinuria. Patients should have completed any anti-cancer therapy ≥ 4 weeks (≥ 2 weeks for targeted therapy; e.g., sunitinib, sorafenib, pazopanib) prior to initiation of study drug and without residual toxicity. Patients with active, clinically significant and/or uncontrolled medical conditions were excluded. The study protocol was reviewed and approved by the institutional review board, and written informed consent was mandatory.
Treatment plan
Dovitinib was supplied by Novartis Pharmaceuticals, in 100 mg hard gelatin capsule. Dovitinib may be taken with or without food. Gemcitabine was obtained commercially as a lyophilized powder and was reconstituted with normal saline per institutional standard. Capecitabine was obtained commercially as oral tablets in 150 mg and 500 mg strengths, and swallowed with water within 30 minutes after a meal. On days when dovitinib was to be administered with capecitabine, dovitinib was taken with water prior to capecitabine and capecitabine was taken within 30 minutes after a meal with water. Treatment consisted of gemcitabine 1000 mg/m2 administered intravenously as a 30-minute infusion on days 1 and 8, capecitabine 1300 mg/m2 total daily oral dose divided twice a day from day 1 to 14 and dovitinib oral daily day 1 to 5, 8 to 12 and 15 to 19 (5 days on/2 days off). Each cycle was 21 days. The sequence was dovitinib, capecitabine and gemcitabine on days when >1 drugs were administered. The planned dovitinib dose levels to be explored were 300 mg (starting), 400 mg and 500 mg daily. Prophylactic anti-emetics were administered per institutional standard. Treatment was administered until disease progression, intercurrent illness that prevented further treatment, unacceptable adverse event(s), withdrawal of consent or study closure. Adequate laboratory parameters at every evaluation were required prior to retreatment.
Dose modifications for toxicities related to gemcitabine and capecitabine were per guidelines in respective package inserts. Adverse events were categorized and graded according to National Cancer Institute, Common Toxicity Criteria Version 4.0 (NCI-CTCAE v 4.0). Dovitinib was withheld for ANC < 500/μL or platelets < 50,000/μL, and resumed ANC ≥ 500/μL and platelets ≥ 100,000/μL. Dovitinib was discontinued permanently for grade 4 creatinine elevation, ≥ grade 3 total bilirubin elevation, total bilirubin > 2 × ULN and ALT or AST > 3 × ULN, grade 4 cardiac event, ≥ grade 3 neurotoxicity, symptomatic ≥ grade 3 amylase or lipase elevation, and ≥ grade 2 pancreatitis. Dovitinib was withheld for grade 3 creatinine elevation, ≥ grade 3 AST or ALT, ≥ grade 3 hypertension, grade 3 cardiac event, ≥ grade 2 diarrhea, nausea or vomiting, grade 2 neurotoxicity, symptomatic grade 3 and grade 4 hypertriglyceridemia or hypercholesterolemia, ≥ grade 3 asymptomatic amylase or lipase elevation, and other ≥ grade 3 non-hematologic toxicities, until resolution.
Dose escalation plan, definition of DLT and MTD
The study used the standard 3+3 dose escalation design. The study drug dose was escalated to the next higher level if 0 of the first 3 patients developed dose-limiting toxicity (DLT). If 1 of the first 3 patients developed DLTs, 3 additional patients were to be enrolled for treatment at the same dose level. If no further DLT was encountered, dose-escalation resumed. If > 1 patient developed DLT, the next cohort of 3 patents was to be treated at the next lower dose level. Once the maximum tolerated dose (MTD) was determined or escalation finalized, an expanded cohort enriched with patients with pancreatic and biliary tract cancers was enrolled and treated at the MTD to further determine the safety and tolerability of the treatment regimen to declare the recommended phase II dose (RP2D).
DLT was defined as any adverse event related to dovitinib occurring during cycle 1 that corresponds to any of the following criteria: grade 4 neutropenia lasting > 7 days, not reversible to ≤ grade 3 within 7 days without the use of growth factors; febrile neutropenia or neutropenia associated with bacteremia or sepsis or ≥ grade 3 neutropenia associated with treatment related fever or infection; grade 4 thrombocytopenia or grade 3 thrombocytopenia accompanied by clinically significant bleeding; ≥ grade 3 non-hematologic toxicity despite maximal supportive care except alopecia or grade 3 ALT or AST elevations that resolve in < 7 days; ≥ grade 3 nausea, vomiting or diarrhea that persists despite optimal supportive care. Patients were not evaluable for DLT and replaced, if any of the following occurred during cycle 1 due to reasons unrelated to toxicity: treatment delay for > 7 days after the last scheduled dose of any of the study drugs; received < 80% of planned dovitinib; received < 75% of planned capecitabine and failed to receive all 2 doses of gemcitabine. The MTD was defined as the highest dose level at which ≤ 1 of 6 patients experienced DLT during cycle 1.
Monitoring, assessment of safety and activity
Upon signing the consent form, patients underwent screening including a history and physical examination, ECOG performance status assessment, complete blood count (CBC), chemistries, amylase, lipase, coagulations, urinalysis, pregnancy test, stool occult blood, electrocardiogram (EKG), cardiac enzymes, tumor markers, echocardiogram and disease assessment by computer tomography (CT). CBC and chemistries were conducted weekly on day 1, 8 and 15 during cycle 1, and day 1 and 8 from cycle 2 onwards. Amylase, lipases, EKG and cardiac enzymes were evaluated weekly during cycle 1 and on day 1 from cycle 2 onwards. CT was repeated after every 2 cycles, and disease response was assessed by RECIST (version 1.0). Patients were considered evaluable for toxicity once therapy started and for efficacy if at least one cycle was administered.
Pharmacokinetic and biomarker studies
Plasma samples were collected from patients enrolled to the dose escalation part for pharmacokinetic and biomarker studies. Venous blood samples were collected into heparinized vacutainer tube preloaded with tetrahydrouridine at time-points below. For gemcitabine analysis, blood samples were collected at pre-dose, 10 min, 20 min, 30 min, 1 to 2 hours, and 4 to 5 hours post-infusion on day 1. For capecitabine and dovitinib analysis, blood samples were collected on day 1 cycle 1 at pre-dose, 0.5 hour, 1 to 2 hours, 4 to 5 hours, 6 to 8 hours post-dosing and Day 2 prior to next dose. Collections for dovitinib were repeated on day 19 cycle 1 at pre-dose, 0.5 hour, 1 to 2 hours, 4 to 5 hours, 6 to 8 hours, 24 hours and 48 hours post-dosing, and day 8 cycle 2 prior to next dose. The plasma concentration of dovitinib, gemcitabine, capecitabine and metabolites were measured by two high-performance liquid chromatography/tandem mass spectrometry (LC/MS/MS) assays.13,14 Lower limits of quantitation were 1.63 ng/mL for dovitinib, 1.00 ng/mL for gemcitabine, capecitabine and dFdU, and 5.00 ng/mL for 5-FU, DFUR and DFCR.
To determine the pharmacodynamic effects, blood samples were obtained prior to dovitinib dosing on day 1, 12 and 19 of cycle 1 in EDTA tubes to assess the following plasma biomarkers: FGF23, basic FGF (bFGF), soluble VEGFR1 (sVEGFR1), and VEGF. Total FGF23 was determined in human plasma using a sandwich ELISA kit (Millipore, Billerica, MA) with the lower limit of detection of 3.5 pg/mL. The ELISA kit is specific to human FGF23 and does not significantly cross-react to human EGF, FGF-2, FGF-19, or FGF-21. All other biomarkers were analyzed using specific Quantikine® ELISA kit obtained from R&D Systems (Minneapolis, MN). The lower limit of detection of bFGF, sVEGFR1, and VEGF is 3 pg/mL, 3.5 pg/mL, and 9 pg/mL, respectively. The bFGF ELISA kit is specific to human bFGF and does not significant cross-react to human EGF or other FGF members. The sVEGFR1 ELISA kit is specific to human VEGFR1 and does not cross-react to VEGFR2 or VEGFR3. The VEGF ELISA kit is specific to human VEGF and does not recognize VEGF-C or VEGF-D. All assays were performed according to the manufacturer’s manual.
RESULTS
Patient characteristics
A total of 29 patients with advanced solid tumors were consented and received treatment from 2012 to 2014, including 9 patients in the dose escalation and 20 in the expansion cohort. Twenty-four patients had pancreatic cancer; 4 patients had biliary tract cancer, and 1 patient has small intestine cancer. Eighteen patients were evaluable for response by RECIST. Fifteen of the 24 pancreatic cancer patients were chemo-naïve and received study treatment in first-line setting, and 18 were gemcitabine-naïve (including 3 who received FOLFIRINOX previously). The median follow-up time for all pancreatic cancer patients was 14.3 months. The demographic and clinical characteristics of the enrolled patients are listed in Table 1.
Table 1.
Patient Characteristics (n=29)
| Sex | |
| Male | 16 |
| Female | 13 |
| Age, years | |
| Median | 65.5 |
| Range | 42 to 81 |
| ECOG performance status | |
| 0 | 13 |
| 1 | 16 |
| Stage | |
| III | 7 |
| IV | 22 |
| Primary Tumor Site | |
| Pancreas | 24 |
| Biliary | 4 |
| Small intestine | 1 |
Dose-escalation process
Nine patients were treated during dose escalation including 6 who were evaluable for DLT and 3 were replaced as they were not evaluable for DLT. Three patients were enrolled to the starting dose level (dovitinib 300 mg) at the beginning of the study and 1 patient experienced dose limiting grade 3 colitis. Three more patients were enrolled and treated at the same dose level and none experienced DLT. However, 2 patients from the first cohort developed clinically significant grade 2 sensory neuropathy after 4 cycles that resolved following interruption of dovitinib dosing. Both patients had pancreatic cancer. One patient was chemo-naïve and the other one previously received oxaliplatin as part of the FOLFIRINOX regimen. The neuropathy did not recur in both patients following the resumption of dovitinib at 200 mg, the next lower dose level. The toxicity and tolerability of the study treatment were reviewed with the Novartis medical team. The starting dose level was declared as the MTD, and the study proceeded to enroll an additional 20 patients to the expansion cohort.
Safety and activity
All 29 patients received at least 1 dose of study treatment and were evaluable for toxicities. Grade 2 or higher treatment-related adverse events from all cycles are summarized in Table 2. Per above, there was 1 dose limiting grade 3 colitis. Other grade 3 or worse non-dose limiting treatment-related toxicities from all cycles that were reported were neutropenia (41%), thrombocytopenia (24%), anemia (14%), leukopenia (14%), hypertension (10%), nausea (7%), palmer-plantar erythrodysaesthesia syndrome (7%), increased lipase (7%), febrile neutropenia (3% for the rest), diarrhea, gastrointestinal hemorrhage, vomiting, acute coronary syndrome, pyrexia, dehydration, skin rash, and increased level of AST, ALP, GGT, total bilirubin and amylase. Common (>10%) ≥ grade 2 treatment-related adverse events from all cycles included fatigue (45%), neutropenia (41%), thrombocytopenia (34%), anemia (24%), nausea (24%), palmer-plantar erythrodysaesthesia syndrome (21%), diarrhea (17%), increased GGT (13%) and leukopenia (14%). There was no grade 5 adverse event.
Table 2.
Grade 2 and above treatment-related adverse events over all cycles (n=29)
| Adverse Events | G2 | G3/4 | Adverse Events | G2 | G3/4 |
|---|---|---|---|---|---|
| Hematologic | Cardiovascular | ||||
| Anemia | 3 | 4 | Acute coronary syndrome | 0 | 1 |
| Leukopenia | 0 | 4 | Deep vein thrombosis | 1 | 0 |
| Neutropenia | 0 | 12 | Embolism | 1 | 0 |
| Febrile neutropenia | 0 | 1 | Hypertension | 0 | 3 |
| Thrombocytopenia | 3 | 7 | |||
| Others | |||||
| Gastrointestinal | Fatigue | 13 | 0 | ||
| Diarrhea | 4 | 1 | Pyrexia | 0 | 1 |
| Enterocolitis | 0 | 1* | Weight loss | 2 | 0 |
| Hemorrhage, GI | 0 | 1 | Dehydration | 0 | 1 |
| Nausea | 5 | 2 | Gout | 1 | 0 |
| Stomatitis | 2 | 0 | Pain in extremity | 1 | 0 |
| Vomiting | 2 | 1 | Peripheral neuropathy | 2 | 0 |
| Anorexia | 2 | 0 | Alopecia | 1 | 0 |
| Hiccups | 1 | 0 | Palmar-plantar erythrodysaesthesia syndrome | 4 | 2 |
| Rash | 1 | 1 | |||
| Hepatic | Amylase increased | 1 | 1 | ||
| ALT increased | 1 | 0 | Lipase increased | 0 | 2 |
| AST increased | 1 | 1 | |||
| ALP increased | 1 | 1 | |||
| Bilirubin, Total | 0 | 1 | |||
| GGT increased | 3 | 1 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; GGT: Gamma-glutamyltransferase
DLT
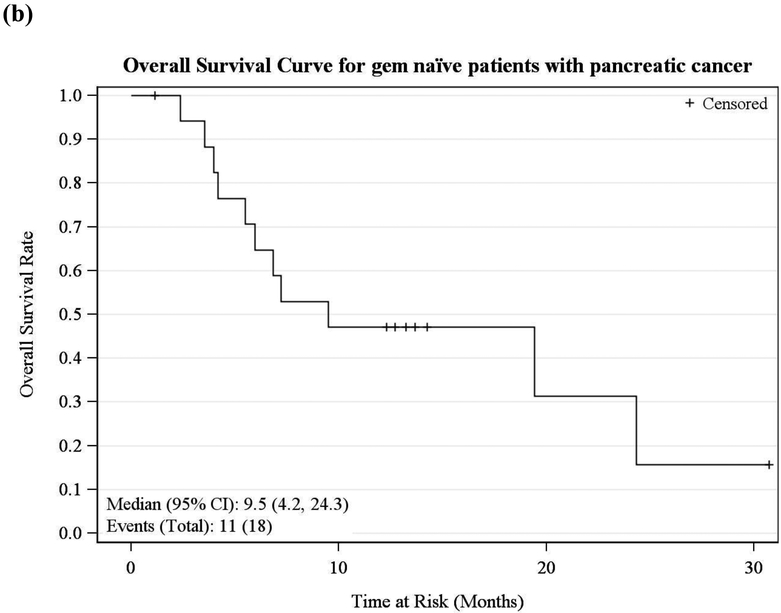
Among evaluable patients, partial responses by RECIST were observed in a total of 5 (28%) patients. Three of them had pancreatic cancer and 2 had biliary tract cancer. Ten (55%) patients had stable disease. Three (17%) patients had progressive disease. Fifteen pancreatic cancer patients received the study treatment as first-line therapy (9 stage IV, 6 stage III). As in Figure 1, the median OS and 6-month survival were 13.3 months and 64%, respectively. Eighteen pancreatic cancer patients enrolled were naïve to gemcitabine (11 stage IV, 7 stage III) and the median OS and 6-month survival were 9.5 months and 65%, respectively.
Figure 1.
Kaplan-Meier Survival Curve for (a) first-line and (b) gemcitabine-naïve advanced pancreatic cancer patients
Pharmacokinetic studies
The pharmacokinetic parameters of dovitinib, capecitabine and gemcitabine on day 1, and dovitinib on day 19 are summarized in Table 3. The plasma concentration versus time are shown in Figure 2. The median Cmax and Area Under the Curve (AUC) of dovitinib were similar following 3 weeks of dosing: Day 1, 181 ng/mL and 3419 hr·ng/mL and Day 19, 221 ng/mL and 3854 hr·ng/mL, respectively, indicating slight accumulation of dovitinib at 300 mg dose. The median half-life (T1/2) of dovitinib was approximately 15 hours on Day 1 and decreased to approximately 13 hours on Day 19. The metabolites of gemcitabine and capecitabine including dFdU, 5’-FU, 5’-DFCR and 5’-DFUR were analyzed and consistent with the literature.15,16
Table 3.
Pharmacokinetic parameters of dovitinib, gemcitabine, and capecitabine administered concurrently. Gemcitabine AUC 0–7.5 hours, capecitabine AUC 0–8 hours, dovitinib AUC 0–24 hours.
| Day | Drug | T1/2 (hr) | Cmax (ng/mL) | AUC (hr·ng/mL) | |
|---|---|---|---|---|---|
| Day 1 | Gemcitabine | N | 7 | 7 | 7 |
| Min | 0.7 | 5640 | 6370 | ||
| Median | 4.55 | 14000 | 9237 | ||
| Max | 11.89 | 25000 | 12222 | ||
| Capecitabine | N | 5 | 7 | 7 | |
| Min | 0.68 | 98.8 | 488 | ||
| Median | 1.05 | 5150 | 4229 | ||
| Max | 4.07 | 30300 | 14439 | ||
| Dovitinib | N | 1 | 9 | 9 | |
| Min | 15.34 | 99.4 | 1529 | ||
| Median | 15.34 | 181 | 3419 | ||
| Max | 15.34 | 279 | 5394 | ||
| Day 19 | Dovitinib | N | 5 | 5 | 5 |
| Min | 9.38 | 80.1 | 1198 | ||
| Median | 13.17 | 221 | 3854 | ||
| Max | 19.84 | 313 | 4261 |
Figure 2.
Plasma concentration-time profiles of gemcitabine and capecitabine on day 1 and dovitinib on day 1 and day 19 (mean±SD).
Pharmacodynamic studies
A total of 40 plasma samples from 9 patients treated with dovitinib 300 mg daily 5 days on and 2 days off were evaluable by ELISA for the level of FGF23, bFGF, sVEGFR1 and VEGF. Due to small sample size, we could not detect relationship between baseline plasma level of these markers and clinical outcome. Paired samples from 5 patients were evaluable for changes in the biomarker levels between baseline and after 19 days of dovitinib. Plasma FGF23 level increased in 4 out of 5 patients during the first cycle of treatment. There was no observable trend in the changes of bFGF, sVEGFR1 and VEGF plasma levels. (Figure 3)
Figure 3.
Changes in pre-dovitinib plasma FGF23, bFGF, sVEGFR1 and VEGF levels in 5 patients during the first cycle of treatment.
DISCUSSION
In this phase Ib study, we determined that dovitinib could be safely co-administered with gemcitabine and capecitabine at the RP2D. We further determined that there was no significant drug-drug interaction of dovitinib by the other 2 cytotoxic drugs including oral capecitabine during co-administration. When this study was planned in 2009, gemcitabine plus capecitabine was a standard in the treatment of advanced pancreatic cancer patients and was selected as the chemotherapy backbone17 Subsequently, gemcitabine plus nab-paclitaxel became a standard in 20132 and the study was amended to include cholangiocarcinoma in the expansion cohort. In addition, the FOLFIRINOX regimen, and its modified versions, became a standard first-line therapy for advanced or metastatic pancreatic adenocarcinoma in patients with good performance status since 2011 and the study regimen was tolerable in patients previously treated with FOLFIRINOX.3 Dovitinib was one of the most potent multi-kinase inhibitors against FGFR signaling in clinical development when the study was first planned. We observed FGFR signaling modulation at the RP2D, albeit a small sample size, and together with the encouraging efficacy signal observed, the role of dovitinib in the treatment of pancreatico-biliary cancers should be investigated further.
The MTD of the study regimen was not reached by study definition though we observed clinically significant grade 2 neuropathy after multiple dosing requiring dose reduction in 2 patients. Following discussions with the Novartis medical team, the 300 mg daily dose level was declared the RP2D and to be used for the expansion cohort. The dose was lower than the 500 mg daily dose on a 5-days-on/2-days-off schedule used in phase II and III dovitinib monotherapy trials though we did observe plasma FGF23 changes indicative of FGFR modulation at the RP2D.11,18 Interestingly, there was no additional dose limiting sensory neuropathy observed in the dose expansion cohort. Sensory neuropathy was not a significant adverse event for dovitinib monotherapy in the phase III trial in metastatic renal cell carcinoma.18 However, Loriot et al. did report a patient who developed mild peripheral sensory neuropathy with numbness and pain following 2 months of dovitinib monotherapy on a phase I trial.19 An electromyogram evaluation showed a distal axonal neuropathy. FGFR signaling has been implicated in healthy nerve physiology and the anti-FGFR effects of dovitinib may have underlined the sensory changes.20–22
The most common grade 3 and 4 toxicities from dovitinib, gemcitabine, and capecitabine are hematologic. Although caution should be taken to compare across studies, the incidence of neutropenia and thrombocytopenia were numerically higher than that from single agent dovitinib used in advanced renal cell carcinoma18 or gemcitabine plus capecitabine used in advanced pancreatic adenocarcinoma.17 The increased toxicities including neutropenia and thrombocytopenia have been observed in previous studies on combining multi-kinase inhibitors and cytotoxic agents, which often led to dose reduction, delay, or discontinuation. The additive myelosuppression of dovitinib to chemotherapy was likely due to its inhibition of FLT3 and c-kit.23 Despite the increased hematologic toxicities observed in this study, most of the patients were able to tolerate multiple cycles of the treatment. The toxicity profile of the combination was largely consistent with the known side effect profiles of respective drugs. In contrast, another phase Ib study evaluating dovitinib in combination with gemcitabine plus cisplatin or carboplatin in patients with advanced solid tumors had to be closed due to severe dose-limiting hematological toxicities observed during the dose escalation phase.23
This phase Ib trial was not designed to evaluate activity/efficacy of dovitinib. However, the overall response rate and overall survival appeared to be equivalent or slightly better compared to gemcitabine plus capecitabine in the first-line setting for the treatment of advanced or metastatic pancreatic adenocarcinoma.17 The activity of dovitinib, gemcitabine, and capecitabine combination observed in this study can at least be partially explained by the fact that a significant number of the patients received this regimen as first-line therapy or were naïve to gemcitabine-based chemotherapy. In contrast, most of the previous early phase trials of dovitinib were in heavily treated patients with other types of malignancies.
Dovitinib is mainly metabolized by CYP 1A1/2 and flavin-containing monooxygenase.12 Previous pharmacokinetic studies showed that both dovitinib at lower daily dose (<400 mg) on a consecutive 28-day cycle12 and dovitinib at higher daily dose on a 5-day on and 2-day off schedule9 led to significant autoinduction of CYP1A1/2. At 300 mg daily dose on 5 days on and 2 days off schedule as used in our study, we did not observe significant difference in dovitinib level between Day1 and 19 to indicate significant autoinduction. This was likely due to different dose level and schedule of administration in the absence of drug-drug interaction between dovitinib, gemcitabine, and capecitabine. Pharmacodynamic studies using plasma samples from a small number of patients showed effect of dovitinib on FGFR signaling at the 300 mg dose though effects on the VEGF signaling pathway were unclear.
In summary, this phase I study successfully determined a tolerable dosing schedule for dovitinib in combination with gemcitabine plus capecitabine, and demonstrated encouraging efficacy signal in advanced pancreatic cancer patients compared to historical controls.2–4 Potential predictive biomarker for dovitinib in preclinical studies from our group8 may be relevant to the development of other FGFR inhibitors in pancreatic cancer. Future clinical trials evaluating FGFR-targeting agents in pancreatic cancer should be biomarker-driven using combinatorial approaches, which are more likely to be successful.
ACKNOWLEDGMENTS
This work was partially supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park’s Bioanalytics, Metabolomics and Pharmacokinetics (BMPK) Shared Resource.
Financial support:
Novartis Oncology provided research funding and dovitinib
REFERENCES
- 1.Hidalgo M Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. [DOI] [PubMed] [Google Scholar]
- 2.von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. [DOI] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. [DOI] [PubMed] [Google Scholar]
- 5.Turner N, Grose R. Fibroblast growth factor signalling: From development to cancer. Nat Rev Cancer. 2010;10(2):116–129. [DOI] [PubMed] [Google Scholar]
- 6.Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8(4):299–309. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Lopes de Menezes D, Vora J, et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin Cancer Res. 2005;11(10):3633–3641. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Hylander BL, LeVea C, et al. Enhanced FGFR signalling predisposes pancreatic cancer to the effect of a potent FGFR inhibitor in preclinical models. Br J Cancer. 2014;110(2):320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angevin E, Lopez-Martin JA, Lin C-C, et al. Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin Cancer Res. 2013;19(5):1257–1268. [DOI] [PubMed] [Google Scholar]
- 10.Milowsky MI, Dittrich C, Durán I, et al. Phase 2 trial of dovitinib in patients with progressive FGFR3-mutated or FGFR3 wild-type advanced urothelial carcinoma. Eur J Cancer. 2014;50(18):3145–3152. [DOI] [PubMed] [Google Scholar]
- 11.Cheng A-L, Thongprasert S, Lim HY, et al. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2016;64(3):774–784. [DOI] [PubMed] [Google Scholar]
- 12.Kim KB, Chesney J, Robinson D, et al. Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma. Clin Cancer Res. 2011;17(23):7451–7461. [DOI] [PubMed] [Google Scholar]
- 13.Vainchtein LD, Rosing H, Thijssen B, et al. Validated assay for the simultaneous determination of the anti-cancer agent gemcitabine and its metabolite 2’,2’-difluorodeoxyuridine in human plasma by high-performance liquid chromatography with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(14):2312–2322. [DOI] [PubMed] [Google Scholar]
- 14.Salvador A, Millerioux L, Renou A. Simultaneous LC-MS-MS Analysis of Capecitabine and its Metabolites (5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil) After Off-Line SPE from Human Plasma. Chroma. 2006;63(11–12):609–615. [Google Scholar]
- 15.Plunkett W, Huang P, Xu YZ, et al. Gemcitabine: Metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22(4 Suppl 11):3–10. [PubMed] [Google Scholar]
- 16.Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40(2):85–104. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25(16):2212–2217. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Porta C, Vogelzang NJ, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: An open-label, randomised phase 3 trial. The Lancet Oncology. 2014;15(3):286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loriot Y, Massard C, Angevin E, et al. FGFR inhibitor induced peripheral neuropathy in patients with advanced RCC. Ann Oncol. 2010;21(7):1559–1560. [DOI] [PubMed] [Google Scholar]
- 20.Jungnickel J, Gransalke K, Timmer M, et al. Fibroblast growth factor receptor 3 signaling regulates injury-related effects in the peripheral nervous system. Mol Cell Neurosci. 2004;25(1):21–29. [DOI] [PubMed] [Google Scholar]
- 21.Klimaschewski L, Nindl W, Feurle J, et al. Basic fibroblast growth factor isoforms promote axonal elongation and branching of adult sensory neurons in vitro. Neuroscience. 2004;126(2):347–353. [DOI] [PubMed] [Google Scholar]
- 22.Furusho M, Dupree JL, Bryant M, et al. Disruption of fibroblast growth factor receptor signaling in nonmyelinating Schwann cells causes sensory axonal neuropathy and impairment of thermal pain sensitivity. J Neurosci. 2009;29(6):1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galsky MD, Posner M, Holcombe RF, et al. Phase Ib study of dovitinib in combination with gemcitabine plus cisplatin or gemcitabine plus carboplatin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;74(3):465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]