Abstract
Objective
examined if horizontal head turns while seated or while walking, when instrumented with inertial sensors, were sensitive to the acute effects of concussion and whether horizontal head turns had utility for concussion management.
Setting
Applied field setting, athletic training room.
Participants
Twenty-four collegiate athletes with sport-related concussion and 25 healthy control athletes.
Design
Case-control; Longitudinal
Main Measures
Peak head angular velocity, peak head angle (range of motion) when performing head turns towards an auditory cue while seated or walking. Gait speed when walking with and without head turns.
Results
Athletes with acute sport-related concussion turned their head slower than healthy control subjects initially (group β = -49.47; SE = 16.33; p = 0.003), and gradually recovered to healthy control levels within 10 days post-concussion (group*time β = 4.80; SE = 1.41; p < 0.001). Peak head velocity had fair diagnostic accuracy in differentiating subjects with acute concussion compared to controls (AUC = 0.71 to 0.73). Peak head angle(p = 0.17) and gait speed (p = 0.64) were not different between groups and showed poor diagnostic utility (AUC = 0.57 to 0.62).
Conclusion
Inertial sensors can improve traditional clinical assessments by quantifying subtle, non-observable deficits in people following sport-related concussion.
Introduction
Concussion and mild traumatic brain injury (mTBI) may be often associated with subtle motor abnormalities that can be difficult to discern through clinical observation. These subtle motor signs, including balance and gait dysfunction, have traditionally been assessed with sophisticated force platforms 1,2, complex analyses 3-6, or expensive motion capture systems. Recent technological advances have enabled objective and robust measurements in clinical settings using wearable inertial sensors7,8. Compared to clinical ratings of balance and motor function, outcomes from inertial sensors can provide superior discriminative capability 9,10 and have shown utility identifying subtle motor impairments after concussion 11-14.
Inertial sensors have predominantly been used to quantify static balance 9,10 and gait in a straight line after concussion 12-14, but few, if any, studies have used inertial sensors to measure head movement during gait, despite common complaints of visual and vestibular deficits after concussion15. To address the potential degradation of visual and vestibular function during gait, clinical assessments of balance and gait commonly involve the patient walking while simultaneously rotating their head from side to side16-18. These forms of assessment, however, are subjectively based on whether the subject slows their gait or experiences imbalance while walking16-18; the quality or performance of the head turns, such as the speed of turning or the range of motion of the head, is not assessed.
Objective assessment of walking with horizontal head turns may be useful in concussion diagnosis and management. Although walking with head turns can be challenging to assess, it is frequently used in physical therapy and rehabilitation clinics to both assess and treat individuals with persistent symptoms after concussion 19,20. Despite its use in rehabilitation settings, however, walking with head turns is not typically assessed in typical cases of acute concussion that resolve without the need for rehabilitation. We suspect the lack of use of walking with head turns in standard concussion management is, at least partly, because the observational scoring scale may not be sensitive enough to detect subtle dysfunction. Clinical assessments that rely on observational scoring often have ceiling effects21 that limit the sensitivity to non-obvious motor dysfunction. Therefore, standard clinical assessments of walking with head turns may not be accurate or sensitive enough to identify subtle motor dysfunction in standard, resolving, non-complex concussion22.
Quantifying the quality of the head movement during horizontal head turns may yield additional information not detected in the standard clinical scoring. Using inertial sensors, Paul et al. 23 quantified head motion during walking with horizontal head turns and found that people with vestibular hypo-function (no history of concussion) had reduced head velocities and lower total angular displacements (i.e., range of motion). Yet, it remains unclear whether head motion during horizontal head turns is impaired after typical, non-complex concussion, and whether head motion during horizontal head turns offers added value over clinical scores for diagnostic decisions. Therefore, the purpose of this study was to examine whether head motion during horizontal head turns, performed while sitting or walking and quantified using inertial sensors, was sensitive to the acute effects of concussion and whether head motion had diagnostic utility when identifying concussion. We hypothesized that recently individuals with sport-related concussion (SRC) would turn their head slower, and have smaller angular displacements than healthy controls, but that function would improve gradually to a similar level as the healthy controls.
Methods
Participants
Participant recruitment, inclusion, and exclusion criteria was previously reported by Parrington et al. 24. Briefly, twenty-four collegiate athletes who suffered a sports-related concussion (SRC) and 25 healthy control athletes were recruited from colleges and universities in the greater Portland, Oregon area. All participants were referred to the study by their athletic trainer, and athletes who sustained a SRC were referred to the study within 48 hours of a concussion. Concussions were identified by the team athletic trainer and confirmed by the team physician or an Oregon Health & Science University (OHSU) sports physician; concussion diagnosis following the definition provided by the 4th International Consensus Statement on Concussion in Sport25. Control subjects were collegiate athletes recruited from the same university and matched, where possible, for age, gender, height and mass. Exclusion criteria included any history of injury or diagnosis that would impair cognition or mobility, or any significant injury or surgery within the last 6 months that could influence balance. Demographic characteristics for each group are provided in Table 1. Recruitment procedures and experimental protocols were approved by the OHSU Institutional Review Board.
Table 1.
Participant demographic data for both sport-related concussion (SRC) and control groups. Data are presented as mean (SD) unless otherwise stated.
| SRC n = 24 | Control n =25 | |
|---|---|---|
| Gender (n, % female) | 6(25%) | 6 (24%) |
| Age (years) | 20.3 (1.3) | 20.9 (1.4) |
| Height (cm) | 180.3 (9.1) | 178.1 (10.3) |
| Mass (kg) | 91.0 (21.6) | 83.0 (21.0) |
| Previous lifetime concussions* | 1 (0-5) | 1 (0-3) |
| Days from concussion to return-to-play* | 13 (8-20) | - |
presented as median (range)
Procedures
Gait and balance was longitudinally tested in people with SRC over 9 times over 8 weeks at a mean (SD) timeline of 2 (0.6), 5 (1), 10 (0.7), 17 (3), 23 (3), 30 (3), 37 (2), 44 (2), and 51 (2) days post-concussion, as described by Parrington et al.24. Control subjects were tested on a similar schedule of 2 (0), 5 (0.7), 10 (1), 17 (1), 25 (1), 31 (1), 38 (2), 45 (1), and 52 (1). For all control subjects, day 2 was defined as the date of the first testing session and used as a reference for the remaining session. To account for the influence of return-to-play on longitudinal data in athletes with SRC 24,26, only measurements taken prior to return-to-play were analyzed here (Days 2, 5, 10 post-concussion). Due to scheduling conflicts, not all participants completed the testing protocol at every session. The location of testing varied; participants were tested at their college or university in a quiet well-lit hallway within, or in close proximity to, the athletic training facility. At each session, subjects completed the Sport Concussion Assessment Tool 3 (SCAT3) before completing three tasks that involved rotating their head according to an auditory tone delivered to either the left or right ear through dual-channel headphones. Trials were not randomized, and were presented in the following order: 1) a self-paced walking trial without head rotations (Walk), 2) rotating their head to the same side as the audio cue while seated (seated head turns [Seated-HT]), and 3) rotating the head to the same side as the cue while walking (walking head turns [Walk-HT]). The audio cue and timing was randomized across the conditions, but fixed across participants and time. Cues were delivered randomly with an interval ranging from 1.65 – 4 seconds. Each task lasted two minutes. For Seated-HT and Walk-HT conditions, participants were instructed to turn their head towards the cue; head turns away from the cue were recorded as incorrect responses to understand task accuracy. No instruction regarding speed, angle, or range of motion was given. During the walking trials, participants were instructed to walk at their normal walking pace back and forth between two lines spaced approximately 25 meters apart. During all tasks, participants were instrumented with four inertial sensors (Opal v1, APDM, Inc. Portland, OR USA), worn on the forehead, over the lumbar spine, and bilaterally on the anterior distal region of each shank. Each sensor collected tri-axial accelerations and angular velocities at a sampling frequency of 128 Hz.
Analysis
Angular velocities corresponding to yaw rotation in the transverse plane were extracted from the head and lumbar sensors for each trial and filtered using a low-pass 6 Hz, 4th order recursive Butterworth filter. For each cue, a window spanning -0.75 seconds to +1.5 seconds was extracted, with the cue occurring at time 0. The peak head angular velocity towards the intended direction (i.e., towards the audio cue) of the head sensor was identified for each window. The peak head angular displacement (i.e. head angle) was obtained by integrating the angular velocities of the head across each window to get the peak angular displacement of the head and extracting the maximum angle in the instructed direction. To exclude motion that was associated with turning at the end of the walkway, the raw lumbar gyroscope was low-pass filtered using a 1 Hz, 4th order recursive Butterworth filter, and whole-body turns were identified when the filtered lumbar angular velocity exceeded 2 standard deviations away from the mean over the whole trial. Cue windows that contained any part of a whole-body turn were excluded from all analyses (i.e., the lumbar sensor exceeded 2 standard deviations at any time in the 2.25 window). Rotations of the head that did not exceed 10° in the intended direction were deemed incorrect responses, and the corresponding head velocity and angle excluded from the statistical analyses (Figure 1). The peak head velocity and angle were averaged over all correct responses for each participant at each time point for comparisons. Gait speed was extracted from MobilityLab (Analysis Version 1, Software version 1, APDM, Inc. Portland, OR, USA) using commercial software for both walking trials.
Figure 1.
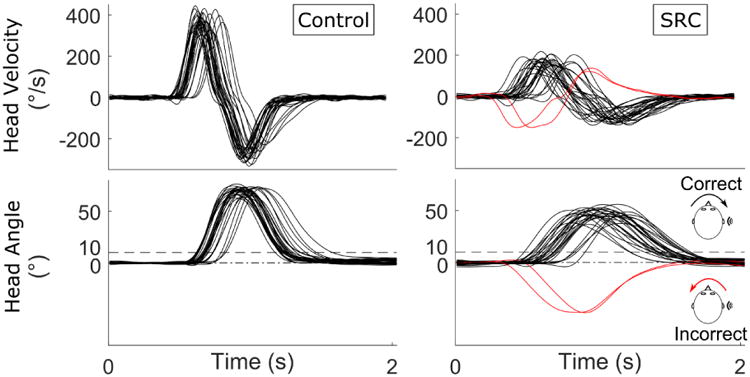
Example of head angular velocity and angular displacement obtained during walking with head turns (Walk-HT) in a healthy control (left) and a subject with a SRC (right). Each turn of the head is represented with a different trace. Responses were required to exceed 10 degrees of rotation towards the cue. Responses away from the cue (red) were classified as incorrect and used to characterize the task accuracy, but excluded from the analyses on head motion.
Statistical Analysis
To evaluate whether the peak head angular velocity or head angle during each condition varied by group, time, or condition, linear mixed models (LMMs) were constructed for each outcome. One LMM was fit per outcome. Each model was adjusted for group (SRC versus control), time, condition (seated versus walking), group*time, and group*condition interactions. Time was modeled continuously using the exact day of testing for each participant. Similarly, LMMs were fit for gait speed and adjusted for group (SRC versus control), time, condition (Walk versus Walk-HT), and group*time and group*condition interactions. If a group*condition interaction was detected at a 0.05 significant level, the models were stratified by condition and run in different LMMs. If no group*condition effect was found, the group*condition interaction was removed from the final models. Time was modeled continuously in all models and random slopes and random intercepts over time were fit for each subject to account for within-subject correlations. A two-tailed corrected significance level of 0.0125 (0.05 / 4 final LMMs) was used for all comparisons from the LMMs. If significant group and group*time effects were present in the same model, post-hoc pairwise comparisons using independent sample t tests were performed at each cross-sectional point in time.
To test if peak head angular velocities or peak head angles during the Walk-HT condition were associated with neck pain or other self-reported symptoms in the concussed group, we compared the initial self-reported severity of neck pain and total symptom severity on the SCAT3 at Day 2 to peak head angular velocity and peak head angle using Spearman's correlation coefficients. Spearman's correlation coefficients also compared whether the changes in peak head angular velocity or peak head angle between Day 2 and Day 10 were associated with initial reported symptoms at Day 2. A two-tailed corrected significance level of 0.0125 (0.05 / 4 correlations) was used for all correlation comparisons.
A post-hoc analysis compared the diagnostic utility of gait speed, peak head angular velocity, peak head angle, response accuracy (1- # of incorrect responses / # of total responses), and clinical balance from the Balance Error Scoring System (BESS) when differentiating SRC from healthy control subjects within 48 hours of SRC. For each outcome, receiver-operator characteristic (ROC) curves were constructed based on the differentiation of SRC versus healthy control. The diagnostic accuracies were compared using the areas under the ROC curves (AUC). Confidence bounds of each AUC were calculated using a bias corrected and accelerated percentile bootstrap procedure with 10,000 replicas.
Finally, reliability of peak head angular velocity and peak head angle were assessed using two-way Intra-class Correlation Coefficients (ICCs) for absolute agreement of average measurements, ICC(A,k)27 using healthy control data on Days 2 and 5. All analysis was performed in MATLAB and the Statistics and Machine Learning Toolbox (r2017a, The MathWorks, Inc., Natick, MA, USA).
Results
Descriptive statistics of neck pain, number of symptoms, symptom severity, and BESS errors for the SRC group are presented in Table 2. A total of 21 athletes with SRC completed the testing at Day 2, 20 completed Day 5, and 20 completed Day 10. A total of 25 control subjects completed Days 2 and 10, with 23 subjects completing Day 5. All subjects completed at least 2 sessions.
Table 2.
Median and range of symptom scores and balance error scoring system (BESS) errors for the sport-related concussion (SRC) group at each point in time. Data are presented as median, range unless otherwise stated.
| Neck Pain* | Neck Pain Severity | Number of Symptoms | Symptom Severity | BESS | |||||
|---|---|---|---|---|---|---|---|---|---|
| Percent | Median | Range | Median | Range | Median | Range | Median | Range | |
| Day 2 | 62% | 1 | 0-5 | 11 | 5-20 | 30 | 5-67 | 12 | 5-25 |
| Day 5 | 14% | 0 | 0-1 | 4 | 0-17 | 4 | 0-41 | 12 | 4-23 |
| Day 10 | 0% | 0 | 0-0 | 0 | 0-14 | 0 | 0-20 | 13 | 0-33 |
presented as percentage of participants with SRC reporting >0 neck pain on the SCAT 3 symptom scale.
A significant group*condition interaction was found for peak head angular velocity in preliminary analysis (p = 0.023), leading to two LMM stratified by condition. Stratified models for peak head angular velocity found significant effects of group and group*time interactions during the Walk-HT condition (Table 3), with slower peak angular velocities in people with SRC at time 0 and increasing peak head angular velocities over time relative to controls (Figure 2). The SRC group had significantly slower peak head angular velocities at Day 2 (t = -4.793, p< 0.001) and Day 5 (t = -2.201, p = 0.029), but not Day 10 (t = 0.304, p = 0.761). No main effects of group or group*time interaction were found during the Seated-HT condition. No group*condition interactions were found for peak head angle (p = 0.348) or gait speed (p = 0.291). No main effects of group or group*time interactions were found for peak head angle or gait speed.
Table 3.
Results from the linear mixed models for peak head angular velocity with beta coefficients, standard error (SE), p value, lower and upper 95% confidence intervals (CI) of the beta coefficient. A significant group*condition effect was found for peak head velocity, resulting in two final models stratified by condition. A significant main effect of group and a significant group*time interaction was found for peak head velocity during Walk-HT, but not Seated-HT, where athletes with SRC turned their heads slower initially, but gradually increased over time, compared to controls. No significant group*condition effects were found in preliminary models for head angle or gait speed. No significant group*time effect was found for head angle or gait speed.
| Beta | SE | PValue | Lower CI | Upper CI | |
|---|---|---|---|---|---|
| Peak Head Angular Velocity | |||||
|
| |||||
| Seated-HT | |||||
| Intercept | 196.47 | 9.19 | < 0.001 | 178.29 | 214.65 |
| Group | -26.71 | 13.58 | 0.051 | -53.58 | 0.16 |
| Time | 1.25 | 1.13 | 0.269 | -0.98 | 3.48 |
| Group*Time | 3.06 | 1.74 | 0.080 | -0.38 | 6.50 |
| Walk-HT | |||||
| Intercept | 275.44 | 11.12 | <0.001 | 253.22 | 297.67 |
| Group | -49.47 | 16.33 | 0.003 | -81.78 | -17.16 |
| Time | -0.23 | 0.91 | 0.802 | -2.03 | 1.57 |
| Group*Time | 4.80 | 1.41 | <0.001 | 2.01 | 7.60 |
|
| |||||
| Peak Head Angle | |||||
|
| |||||
| Intercept | 62.65 | 1.57 | <0.001 | 59.57 | 65.74 |
| Group | -3.13 | 2.26 | 0.166 | -7.58 | 1.31 |
| Time | -0.33 | 0.19 | 0.076 | -0.70 | 0.03 |
| Walk (ref Seated) | 6.03 | 0.64 | < 0.001 | 4.77 | 7.28 |
| Group*Time | 0.28 | 0.28 | 0.322 | -0.28 | 0.84 |
|
| |||||
| Gait Speed | |||||
|
| |||||
| Intercept | 1.39 | 0.02 | <0.001 | 1.35 | 1.43 |
| Group | -0.01 | 0.03 | 0.644 | -0.07 | 0.04 |
| Time | 0.01 | 0.00 | 0.442 | 0.00 | 0.01 |
| Walk-HT (ref Walk) | -0.06 | 0.01 | < 0.001 | -0.08 | -0.03 |
| Group*Time | 0.01 | 0.00 | 0.099 | -0.00 | 0.01 |
Figure 2.
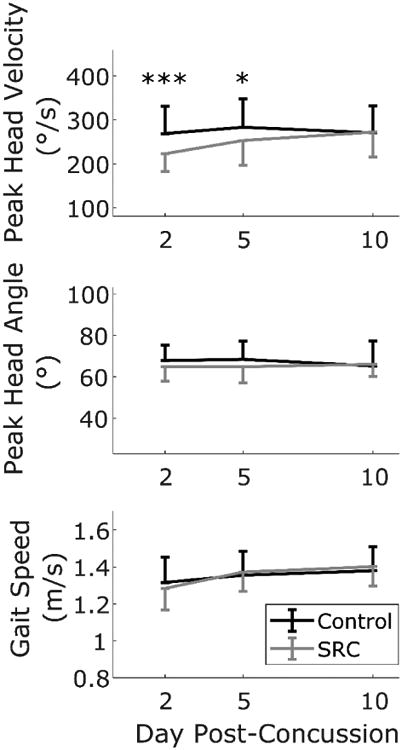
Mean peak head angular velocity (top), angle (middle), and gait speed (bottom) of athletes during the Walk-HT condition. Athletes with SRC (gray) had slower peak head angular velocities, and increased velocity over time, compared to healthy controls. Error bars indicate standard deviations. For clarity, only one side of the error bar is displayed. Peak head angular velocity was the only outcome significantly different between groups (*** post-hoc independent t-test p< 0.001, * post-hoc independent t-test p = 0.029).
During the Walk-HT condition, larger increases in peak head velocity between Days 2 and 10 in the concussed group were associated with greater total symptom severity at Day 2 (n = 17, Spearman's rho= 0.76, p < 0.001), but head velocity was not specifically associated with initial neck pain (n = 17, Spearman's rho= 0.28, p = 0.272). Changes in peak head angle were not associated with total symptom severity (n = 17, Spearman's rho= -0.20, p = 0.442) or neck pain (n = 17, Spearman's rho= 0.05, p = 0.851). At initial assessment within 48 hours, neither peak head velocity (n = 21, Spearman's rho= 0.22, p = 0.239) nor angle (n = 21, Spearman's rho= -0.45, p = 0.043) were associated with total symptom severity. Similarly, initial neck pain was not associated with peak head velocity (n = 21, Spearman's rho= 0.07, p = 0.767) or peak head angle (n = 21, Spearman's rho= 0.37, p = 0.102) when assessed within 48 hours after SRC.
Within 48 hours, peak head angular velocity had fair diagnostic accuracy (0.7< AUC < 0.8) regardless of the condition when differentiating athletes with a SRC from healthy athletes (Table 5). Conversely, the peak head angle had poor diagnostic accuracy (0.6< AUC < 0.7) during Walk-HT, and failed to correctly categorize subjects during Seated-HT (AUC < 0.6). Gait speed, response accuracy, and clinical BESS error count were unable to classify subjects in any condition (AUC < 0.6). Between Day 2 and Day 5, peak head angular velocity and peak head angle had excellent reliability within the control group (n = 23) with ICC(A,k) = 0.936 and 0.893, respectively.
Table 5.
Areas under the receiver-operator characteristic curve (AUC) when differentiating athletes with SRC (n = 21) from control subjects (n = 25) at day 2 post-concussion for peak head angular velocity, peak head angle, gait speed, response accuracy for each task, and BESS scores.
| AUC | Lower Bound | Upper Bound | |
|---|---|---|---|
| Seated-HT | |||
| Head Velocity | 0.71 | 0.53 | 0.84 |
| Head Angle | 0.57 | 0.39 | 0.73 |
| Accuracy | 0.54 | 0.46 | 0.62 |
| Walk-HT | |||
| Head Velocity | 0.73 | 0.56 | 0.85 |
| Head Angle | 0.62 | 0.44 | 0.77 |
| Accuracy | 0.55 | 0.42 | 0.68 |
| Gait Speed | 0.57 | 0.40 | 0.74 |
| Walk | |||
| Gait Speed | 0.57 | 0.39 | 0.73 |
| Clinical Balance | |||
| BESS | 0.59 | 0.42 | 0.75 |
Discussion
In this study we examined head motion, quantified using inertial sensors, when walking with horizontal head turns to determine if this test was sensitive to the acute effects of concussion, and whether head motion had diagnostic utility when identifying acute concussion. Our results indicated that peak head angular velocity, but not peak head angle, was able to discriminate between athletes with acute SRC and healthy controls. As peak head angular velocity is not easily observable in a clinical setting, these findings support the notion that inertial sensors can improve traditional clinical assessments by quantifying subtle, non-observable deficits in people with SRC.
Regardless of the population, clinical assessments typically rely on observable deficits 22 like loss of balance, decreases in gait speed measured with a stopwatch, or noticeably less range of motion. In our sample of athletes with acute SRC, however, clinically observable features of gait speed and head angle (i.e., range of motion of the head) were not impaired when walking with head turns. Only the subtle feature of peak head angular velocity was different between athletes with SRC and healthy controls, and it mirrored clinical recovery in subjects with concussion. Importantly, peak angular velocity is unlikely to be accurately and reliably evaluated with clinical observation; human visual perception is not well suited to differentiate between angular velocities or accelerations 28. However, our results suggest that horizontal head turns, when objectively quantified with inertial sensors, may be sensitive to acute SRC, responsive to clinical recovery, and reliably evaluated across time.
We found that peak head angular velocity had superior diagnostic performance (indicated by a higher AUC) than observable characteristics such as head angle, gait speed, or the BESS error count. Recently, the capacity to differentiate athletes with acute SRC from healthy controls was directly compared to clinical scores10. King and colleagues found that sensor-based measures of sway were superior to the clinical error count of the modified Balance Error Scoring System. In addition, a number of other studies have shown differences in motor performance between individuals with acute concussion and healthy controls through instrumented procedures, which have not been typically quantified through clinical observation 9,11-14,29,30. Taken together, these studies and our results suggest inertial sensors can provide valuable information over clinical observations. This study used a commercial sensor suite and custom analysis scripts, but inertial sensors are becoming increasingly affordable, ubiquitous (e.g., within smartphones), and easy to analyze and interpret without specialized personnel. While more research is needed to identify the feasibility of using inertial sensors for clinical decision making based on clinician and patient input, future work should enable the rapid utilization of sensor-based measures as a tool for concussion management by further minimizing the cost, time, and specialized software needed for analysis.
The significant group*condition effect for peak head velocity and the lack of significant effect for head angle or gait speed found in preliminary models suggests athletes with SRC may have been prioritizing the gait task over the head turning task during the Walk-HT condition. Walking with horizontal head turns disrupts the input to the vestibular canals in proportion to the angular velocity of the head turn 31 and can be destabilizing for people with vestibular dysfunction17. Additionally, head turns disrupt downstream visual information according to both the velocity and angle of rotation 32. As athletes with SRC had slower peak head angular velocities, but not smaller angles of rotation, compared to controls, the SRC group may have been unwilling or unable to walk steadily with larger disruptions to vestibular input when vision was simultaneously disrupted. While sensory integration during walking is only modestly related to sensory integration during standing 33, this interpretation is in line with problems integrating visual and vestibular sensory information during standing in people with SRC 34 or othermTBI2. Interestingly, both groups increased the peak head angular velocity when walking compared to sitting, speculatively suggesting that both groups may have prioritized restoring downstream visual information over maintaining accurate vestibular information.
While this study presents evidence for the utility of inertial sensors in clinical settings using a standard task of walking with horizontal head turns, important limitations should also be considered. The testing environments were not identical across all subjects. While locations were chosen to be similar with limited distractions, it is possible that the visual environment may have influenced some participants differently than others. Nevertheless, these environments are representative of typical clinical settings, and especially representative of applied field settings. Additionally, the order of testing was not randomized or counterbalanced across participants; comparisons between different conditions, such as between sitting and walking should therefore be interpreted cautiously as participants may have become more familiar with the task over time. Participants were excluded if they reported balance problems pre-dating their concussion, but we lacked data from their medical history about pre-existing vestibular or oculomotor problems that may have influenced horizontal head turning. It is possible that individuals may have had pre-existing vestibular or oculomotor problems that contributed to the impaired head movements. Nonetheless, as the peak head angular velocities in the SRC group recovered back to healthy control levels, it is unlikely that pre-existing problems were the main drivers of our results. Finally, participants were not specifically excluded if they had neck pain or neck injury. We are unaware of any reported neck injuries within our participants, and our analysis indicated that neck pain was not associated with slower head singular velocities or smaller head angles. However, neck pain was rated on a Likert scale from 0 to 6 that may have lacked sufficient resolution for a significant association. Therefore, while we cannot conclude neck pain did not influence our results, the association between total symptom score and improved peak head angular velocity suggests the slower speeds, and improvements over time, are indicative of more general dysfunction following SRC.
This study illustrates that walking with horizontal head turns, when instrumented with inertial sensors, can quantify subtle, non-observable motor deficits in people with acute SRC. The clinical utility of observational ratings when walking with head turns is still unclear in people with acute SRC, but inertial sensors provided an objective way to quantify subtle impairments and can easily be implemented into clinical practice. It is possible that objective quantification of head movements using inertial sensors could complement existing clinical tools (e.g., SCAT5) for the identification and management of concussion. While we examined people with acute SRC during a specific task of horizontal head turns, the utility of inertial sensors in clinical settings should continue to be investigated in across a variety of tasks.
Table 4.
Descriptive data of the percentage of participants that made 1-2 or 3+ errors in each task at each point in time.
| % of Participants with 1-2 errors | % of Participants with 3 or more errors | |||
|---|---|---|---|---|
|
| ||||
| Accuracy | SRC | Controls | SRC | Controls |
| Seated-HT | ||||
| Day 2 | 5 | 12 | 0 | 4 |
| Day 5 | 0 | 0 | 0 | 0 |
| Day 10 | 19 | 8 | 0 | 0 |
| Walk-HT | ||||
| Day 2 | 29 | 24 | 0 | 0 |
| Day 5 | 40 | 22 | 0 | 0 |
| Day 10 | 20 | 20 | 5 | 0 |
Contributor Information
Peter C. Fino, Oregon Health & Science University.
Jennifer Wilhelm, Oregon Health & Science University.
Lucy Parrington, Oregon Health & Science University.
Samuel Stuart, Oregon Health & Science University.
James C. Chesnutt, Oregon Health & Science University.
Laurie A. King, Oregon Health & Science University.
References
- 1.Guskiewicz KM. Balance assessment in the management of sport-related concussion. Clinics in Sports Medicine. 2011;30(1):89–102. doi: 10.1016/j.csm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Haran FJ, Slaboda JC, King LA, Wright WG, Houlihan D, Norris JN. Sensitivity of the Balance Error Scoring System and the Sensory Organization Test in the Combat Environment. J Neurotrauma. 2016;33(7):705–711. doi: 10.1089/neu.2015.4060. [DOI] [PubMed] [Google Scholar]
- 3.Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer VS, Stergiou N. Recovery of postural control after cerebral concussion: new insights using approximate entropy. Journal of Athletic Training. 2006;41(3):305. [PMC free article] [PubMed] [Google Scholar]
- 4.De Beaumont L, Mongeon D, Tremblay S, et al. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train. 2011;46(3):234–240. doi: 10.4085/1062-6050-46.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fino PC, Nussbaum MA, Brolinson PG. Decreased high-frequency center-of-pressure complexity in recently concussed asymptomatic athletes. Gait & Posture. 2016;50:69–74. doi: 10.1016/j.gaitpost.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Sosnoff JJ, Broglio SP, Shin S, Ferrara MS. Previous mild traumatic brain injury and postural-control dynamics. Journal of Athletic Training. 2011;46(1):85. doi: 10.4085/1062-6050-46.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horak F, King L, Mancini M. Role of body-worn movement monitor technology for balance and gait rehabilitation. Phys Ther. 2015;95(3):461–470. doi: 10.2522/ptj.20140253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss A, Herman T, Plotnik M, Brozgol M, Giladi N, Hausdorff JM. An instrumented timed up and go: the added value of an accelerometer for identifying fall risk in idiopathic fallers. Physiological measurement. 2011;32(12):2003–2018. doi: 10.1088/0967-3334/32/12/009. [DOI] [PubMed] [Google Scholar]
- 9.King LA, Horak FB, Mancini M, et al. Instrumenting the balance error scoring system for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch Phys Med Rehabil. 2014;95(2):353–359. doi: 10.1016/j.apmr.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King LA, Mancini M, Fino PC, et al. Sensor-Based Balance Measures Outperform Modified Balance Error Scoring System in Identifying Acute Concussion. Ann Biomed Eng. 2017 doi: 10.1007/s10439-017-1856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doherty C, Zhao L, Ryan J, Komaba Y, Inomata A, Caulfield B. Concussion is associated with altered preparatory postural adjustments during gait initiation. Hum Mov Sci. 2017;52:160–169. doi: 10.1016/j.humov.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Fino PC. A preliminary study of longitudinal differences in local dynamic stability between recently concussed and healthy athletes during single and dual-task gait. J Biomech. 2016;49(9):1983–1988. doi: 10.1016/j.jbiomech.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Howell DR, Stillman A, Buckley TA, Berkstresser B, Wang F, Meehan WP., 3rd The utility of instrumented dual-task gait and tablet-based neurocognitive measurements after concussion. Journal of science and medicine in sport / Sports Medicine Australia. 2017 doi: 10.1016/j.jsams.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Howell DR, Beasley M, Vopat L, Meehan WP., 3rd The Effect of Prior Concussion History on Dual-Task Gait following a Concussion. J Neurotrauma. 2017;34(4):838–844. doi: 10.1089/neu.2016.4609. [DOI] [PubMed] [Google Scholar]
- 15.Wright WG, Tierney RT, McDevitt J. Visual-vestibular processing deficits in mild traumatic brain injury. J Vestib Res. 2017;27(1):27–37. doi: 10.3233/VES-170607. [DOI] [PubMed] [Google Scholar]
- 16.Horak FB, Wrisley DM, Frank J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89(5):484–498. doi: 10.2522/ptj.20080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitney SL, Hudak MT, Marchetti GF. The dynamic gait index relates to self-reported fall history in individuals with vestibular dysfunction. J Vestib Res. 2000;10(2):99–105. [PubMed] [Google Scholar]
- 18.Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84(10):906–918. [PubMed] [Google Scholar]
- 19.Alsalaheen BA, Mucha A, Morris LO, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. Journal of neurologic physical therapy : JNPT. 2010;34(2):87–93. doi: 10.1097/NPT.0b013e3181dde568. [DOI] [PubMed] [Google Scholar]
- 20.Alsalaheen BA, Whitney SL, Mucha A, Morris LO, Furman JM, Sparto PJ. Exercise prescription patterns in patients treated with vestibular rehabilitation after concussion. Physiother Res Int. 2013;18(2):100–108. doi: 10.1002/pri.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleffelgaard I, Roe C, Soberg HL, Bergland A. Associations among self-reported balance problems, post-concussion symptoms and performance-based tests: a longitudinal follow-up study. Disability and rehabilitation. 2012;34(9):788–794. doi: 10.3109/09638288.2011.619624. [DOI] [PubMed] [Google Scholar]
- 22.Toro B, Nester C, Farren P. A review of observational gait assessment in clinical practice. Physiotherapy Theory and Practice. 2003;19(3):137–149. [Google Scholar]
- 23.Paul SS, Dibble LE, Walther RG, Shelton C, Gurgel RK, Lester ME. Characterization of Head-Trunk Coordination Deficits After Unilateral Vestibular Hypofunction Using Wearable Sensors. JAMA otolaryngology-- head & neck surgery. 2017;143(10):1008–1014. doi: 10.1001/jamaoto.2017.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parrington L, Fino NF, Fino PC, Murchison CF, Chesnutt JC, King LA. Inflection points in longitudinal models: Tracking recovery and return-to-play following concussion. Scandinavian Journal of Medicine & Science in Sports. doi: 10.1111/sms.13239. In Review. [DOI] [PubMed] [Google Scholar]
- 25.McCrory P, Meeuwisse W, Johnston K, et al. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med. 2009;43(1):i76–90. doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- 26.Howell DR, Osternig LR, Chou LS. Return to activity after concussion affects dual-task gait balance control recovery. Medicine and science in sports and exercise. 2015;47(4):673–680. doi: 10.1249/MSS.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 27.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychological methods. 1996;1(1):30. [Google Scholar]
- 28.Hecht H. Judging rolling wheels: dynamic and kinematic aspects of rotation-translation coupling. Perception. 1993;22(8):917–928. doi: 10.1068/p220917. [DOI] [PubMed] [Google Scholar]
- 29.Doherty C, Zhao L, Ryan J, Komaba Y, Inomata A, Caulfield B. Clinical biomechanics. Vol. 42. Bristol, Avon: 2017. Quantification of postural control deficits in patients with recent concussion: An inertial-sensor based approach; pp. 79–84. [DOI] [PubMed] [Google Scholar]
- 30.Howell DR, Stracciolini A, Geminiani E, Meehan WP., 3rd Dual-task gait differences in female and male adolescents following sport-related concussion. Gait Posture. 2017;54:284–289. doi: 10.1016/j.gaitpost.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 31.Raphan T, Imai T, Moore ST, Cohen B. Vestibular compensation and orientation during locomotion. Annals of the New York Academy of Sciences. 2001;942:128–138. doi: 10.1111/j.1749-6632.2001.tb03740.x. [DOI] [PubMed] [Google Scholar]
- 32.Crane BT, Demer JL. Human gaze stabilization during natural activities: translation, rotation, magnification, and target distance effects. J Neurophysiol. 1997;78(4):2129–2144. doi: 10.1152/jn.1997.78.4.2129. [DOI] [PubMed] [Google Scholar]
- 33.Chien JH, Eikema DJ, Mukherjee M, Stergiou N. Locomotor sensory organization test: a novel paradigm for the assessment of sensory contributions in gait. Ann Biomed Eng. 2014;42(12):2512–2523. doi: 10.1007/s10439-014-1112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guskiewicz KM, Ross SE, Marshall SW. Postural Stability and Neuropsychological Deficits After Concussion in Collegiate Athletes. J Athl Train. 2001;36(3):263–273. [PMC free article] [PubMed] [Google Scholar]


