Abstract
A variety of human and animal studies support the hypothesis that serotonin (5-hydroxytryptamine or 5-HT) system dysfunction is a contributing factor to the development of autism in some patients. However, many questions remain about how developmental manipulation of various components that influence 5-HT signaling (5-HT synthesis, transport, metabolism) persistently impair social behaviors. This review will summarize key aspects of central 5-HT function important for normal brain development, and review evidence implicating perinatal disruptions in 5-HT signaling in the pathophysiology of autism spectrum disorder. We discuss the importance, and relative dearth, of studies that explore the possible correlation to autism in the interactions between important intrinsic and extrinsic factors that may disrupt 5-HT homeostasis during development. In particular, we focus on exposure to 5-HT transport altering mechanisms such as selective serotonin-reuptake inhibitors or genetic polymorphisms in primary or auxiliary transporters of 5-HT, and how they relate to neurological stores of serotonin and its precursors. A deeper understanding of the many mechanisms by which 5-HT signaling can be disrupted, alone and in concert, may contribute to an improved understanding of the etiologies and heterogeneous nature of this disorder. We postulate that extreme bidirectional perturbations of these factors during development likely compound or synergize to facilitate enduring neurochemical changes resulting in insufficient or excessive 5-HT signaling, that could underlie the persistent behavioral characteristics of autism spectrum disorder.
Keywords: autism spectrum disorder, neurodevelopment, serotonin homeostasis, selective serotonin reuptake inhibitors, serotonin transporter, tryptophan
Graphical abstract
Abstract Summary Figure: Multiple factors in serotonin (5-hydroxytryptamine, 5-HT) signaling are hypothesized to influence autism spectrum disorder (ASD) etiologies by cumulatively producing excessive increases or decreases in 5-HT levels during critical developmental stages. In adults, 5-HT availability and demand are influenced by availability of the 5-HT precursor L-tryptophan (TRP), as well as by use of medications and/or functional polymorphisms that influence 5-HT signaling and metabolism (e.g., the 5-HT transporter-linked polymorphic region, 5-HTTLPR). During fetal development, maternal TRP is a rate-limiting factor in fetal 5-HT synthesis. Adulthood dietary TRP restriction or supplementation can produce measurable, but transient, behavioral consequences. Contrastingly, such changes in the fetal environment can disturb fetal growth and neurodevelopment, resulting in enduring impairments for the lifetime of the individual. We hypothesize that some of the behavioral heterogeneity observed in the autistic population is attributable to combined effects of several neurodevelopmental factors affecting 5-HT system function, rather than aberration of a single component.
1. Introduction
Autism spectrum disorder (ASD) is the broad term used to encompass a condition characterized by social behavior deficits and restrictive repetitive behaviors of heterogeneous severity. Though social deficits and repetitive behaviors are the core clinical features of this disorder, and both are necessary for an ASD diagnosis, the etiologies of this disorder remain largely unknown and are undoubtedly complex. ASD is prevalent among childhood disorders, with current Centers for Disease Control (CDC) estimates indicating that among 8-year-old children, 1 in 59 are diagnosed [1] (for context, childhood cancer affects 16 in 100,000 children [2], and attention deficit hyperactivity disorder (ADHD) affects 1 in 20 children [3]). Moreover, boys are disproportionally affected, exhibiting an incidence 4.5 times higher than girls [4]. Symptomatic and behavioral profiles of affected individuals are variable in complexity and severity, which has led to ASD being classified as a “spectrum” disorder. Symptoms can range from mild difficulty in social engagements (e.g. failure to use non-verbal behaviors to regulate social interaction), to severe (e.g. lack of verbal communication and non-functional routines that significantly impairs the individual’s ability to cope in social scenarios or those which deviate from their normal daily schedule) [5]. Individuals with ASD may also suffer from any of the following additional symptoms beyond the fundamental scope of an ASD diagnosis including: communication impairments [6], seizures [7,8], anxiety [9,10], mood disorders [11], hyperactivity [12], sleep deficits [13], and attention deficit disorders [14]. Furthermore, an ASD diagnosis is associated with a significantly increased risk for other major psychiatric disorders (ADHD, OCD), as well as for immune, gastrointestinal, cardiovascular, and endocrine disorders (e.g., hypertension, diabetes) [15]. The shared behaviors and biological markers of these comorbidities may exacerbate or convolute diagnosis, especially since there is overlap in the symptomology and neurochemistry of ASD and some of these other (esp. psychiatric and gastrointestinal) disorders, while the underlying mechanisms that result in comorbidity remain undefined. However, these comorbid phenomena may provide additional evidence for the serotonin (5-hydroxytryptamine, 5-HT) hypothesis of ASD, which posits that ASD symptoms result from perinatal exposure to either sub- or supra-optimal levels of 5-HT. Consequently, diseases that likewise may stem from transient or persistent 5-HT disruption, and are present in individuals with ASD, might suggest 5-HT involvement in the etiologies of both conditions [16–19].
Such serotonergic disruptions could result from factors that influence 5-HT neurotransmission from the beginning of development, such as genetic polymorphisms, maternal use of antidepressant medications, or precursor deficiencies during pregnancy [20,21]. This review will provide an overview of 5-HT neurotransmission and how core ASD symptoms bear hallmarks of developmental 5-HT system disruptions. Then, we focus on the role that 5-HT influencing factors (particularly genetic or environmental causes of functional changes in 5-HT transport, precursor availability, or metabolism), may play on the developing nervous system. Finally, we encourage future studies to implement a multifactorial approach in evaluating the integrative risk of each of these factors, with the end goal of being able to uncover the sources of ASD symptom heterogeneity.
1.1. Serotonin Neurotransmission
Serotonin (5-HT) is synthesized from its precursor, the dietary acquired essential amino acid L-tryptophan (TRP), by the enzyme tryptophan hydroxylase 1 (TPH1) in the periphery and pineal gland, and by TPH2 in the central nervous system (CNS). Though the enteric nervous system of the gut contains the majority (> 90%) of the body’s 5-HT, it is within the CNS that this neurotransmitter mediates mood, appetite, and behaviors including aggression and sociability. The role of 5-HT in ASD is widely supported by findings that the hallmark behavioral symptoms of autism, social behavior deficits [22] and restrictive repetitive behaviors [23,24], are modulated by 5-HT signaling. Neuronal firing promotes release of 5-HT from vesicles within 5-HT neurons into synapses and the extracellular space, where 5-HT can bind to 5-HT hetero- or auto-receptors [25]. Following release, the major regulatory mechanism controlling extracellular 5-HT levels is reuptake by transporters. The transporter with the highest affinity for 5-HT is the 5-HT transporter (SERT), expressed by serotoninergic neurons and glia [26,27], in adults, as well as transiently by non-serotonergic neurons during neurodevelopment [28]. In the periphery, SERT is also found in platelets [29], blood vessels [30] placenta, bone marrow, lung, heart, kidney, liver, thyroid gland, small intestine, and pancreas [31]. Uptake of 5-HT enables maintenance of serotonergic tone via termination of extracellular 5-HT signaling, simultaneously facilitating its recycling or degradation (see [25] for details). Though an entire hypothesis for ASD development is based on developmental exposure to inappropriately high or low 5-HT levels, there is still uncertainty associated with how these aberrations may occur and the role that 5-HT plays during pre- and early post-natal development, adolescence, and adulthood.
1.2. Critical Role of Serotonin in Neurodevelopment
5-HT is essential for proper brain development, acting not only as a neurotransmitter but also as a neurotrophic factor (see [28] reviewed by [32–35]). Indeed, structural organization of both serotonergic and non-serotonergic neuronal projections in the brain is significantly influenced by 5-HT. Therefore, early life disruptions in 5-HT levels can have lifelong consequences on brain development and function [35–39], 5-HT immunoreactive cells and fibers develop early in embryogenesis (embryonic day (ED) 12 in rodents and by 5 weeks gestation in humans) [38,40]. SERT is a major regulator of this essential 5-HT signaling during neurodevelopment [41], and is transiently expressed on non-serotonergic neurons in early life, facilitating organization of neuronal projections by regulating the duration of 5-HT signaling [28]. Indeed, fetal 5-HT receptors are functional and assist in the morphogenic function of 5-HT on developing neurons [42–44]. In rodents, SERT and the 5-HT2B receptor are measurable at ED 8, TPH1 at ED 14.5 and 15.5 in the pineal gland and enteric nervous system of the gut respectively, and TPH2 in developing neurons at ED 11 [42,43,45]. With the necessary enzymes, receptors, and transporters in place at these early time points, 5-HT is able to orchestrate critical neuronal cell processes including division, differentiation, migration, and synaptogenesis, and also plays a critical role in the proper patterning and organization of neurons in the sensory cortex [37,38,46]. Given the critical role of 5-HT in brain development, studies have investigated how perturbed 5-HT signaling durations during critical developmental time points may be etiologically linked to ASD [47–49].
Both human and preclinical rodent evidence indicates that significant increases or decreases in 5-HT signaling, due to shifts in 5-HT synthesis, release, signaling, uptake, or metabolism, may increase ASD risk or symptom severity [50–55]. Understanding how multiple genetic and environmental factors can interact to promote imbalances in 5-HT signaling during fetal development is critical for determining how persistent neurochemical and behavioral changes in ASD may arise (Figure 1). Further, this basic mechanistic information is essential to advancing understanding of ASD etiologies, and to revealing targets for novel therapeutic intervention or prevention.
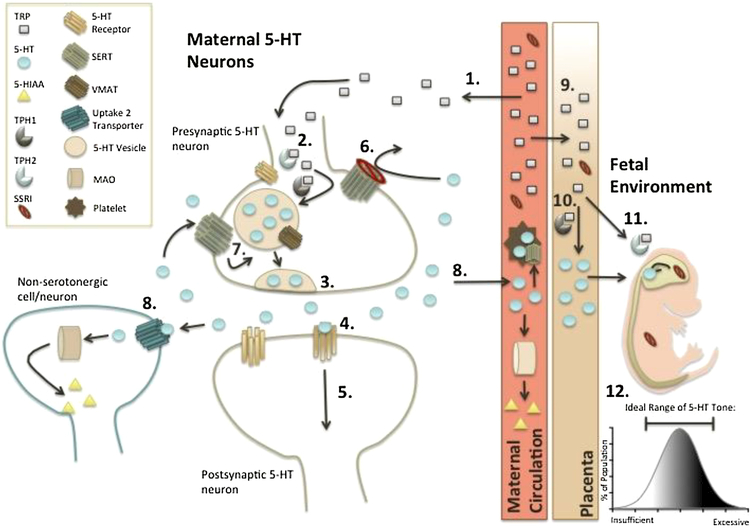
Figure 1.
There are many facets to serotonin (5-hydroxytryptamine, 5-HT) signaling, including synthesis, storage, uptake, and degradation. When certain components are genetically or pharmacologically altered, significant effects on neurodevelopment are hypothesized to promote autism-relevant outcomes. This schematic begins with (1) the dietary acquired L-tryptophan (TRP) being (2) converted by the rate-limiting enzyme tryptophan hydroxylase (TPH1 or 2 in the peripheral or central nervous system, respectively) then amino acid decarboxylase into 5-HT. Within 5-HT neurons, 5-HT is stored in vesicles before (3) release from the presynaptic neuron due to neuronal activation. Once in the synapse, (4) binding to 5-HT hetero- and autoreceptors (5-HTRs) occurs to (5) propagate 5-HT signaling. During this signaling process, extracellular 5-HT is also cleared for maintenance of synaptic tone. Primarily, extracellular levels of 5-HT are maintained by uptake via the serotonin transporter (SERT), the target of selective serotonin reuptake inhibitor (SSRI) antidepressants. SERT uptake function can be constitutively altered by polymorphisms, such as the SERT-linked polymorphic region (5-HTTLPR). (6) SSRIs, or loss of function polymorphisms, attenuate reuptake of 5-HT back into presynaptic neurons, thereby reducing (7) 5-HT recycling back into vesicles through vesicular monoamine transporters (VMAT). Instead, 5-HT is predominantly removed from the extracellular space by alternative mechanisms, including uptake through secondary transporters such as (8) organic cation transporters and the plasma membrane monoamine transporter (i.e., “uptake 2” transporters). Because these transporters are expressed on non-serotonergic neurons and astrocytes in addition to serotonergic neurons, most transported 5-HT is degraded by monoamine oxidase (MAO) rather than recycled. Consequently, (1) TRP demand increases for (2) de novo 5-HT synthesis to replenish intracellular 5-HT stores. During pregnancy, (9) fetal 5-HT synthesis requires TRP from maternal circulation throughout gestation. Early in gestation (10), TPH1 converts TRP to 5-HT in the placenta, whereas late in gestation (11), TPH2 converts TRP to 5-HT in fetal hindbrain. Thus, numerous factors that influence this pathway have the potential, both alone and in combination, to influence 5-HT system function and development. For example, pharmacologic inactivation (e.g., SSRI) and/or genetic (functional polymorphisms) modulation of SERT function, within the mother, fetus, or both, can have cascading effects on TRP demand and 5-HT signaling capabilities. These disruptions, during the critical neurodevelopmental period, can cause permanent deficits in serotonergic system tone. Because 5-HT serves as a neurotrophic factor during brain development, such perturbations can also profoundly affect non-serotonergic neurons and pathways. As a result, excessive or insufficient 5-HT tone (12) can facilitate neurophysiological and behavioral outcomes relevant to autism, such as social behavior deficits and repetitive stereotypies. For this diagram, an important consideration is that all processes occurring to the left of the maternal circulation representation are also simultaneously occurring in the fetal environment (i.e., SSRIs and TRP cross the placenta).
2. Evidence for Bidirectional Aberrations in Serotonin Signaling and Transport in Autism Spectrum Disorder and Relevant Behaviors in Animal Models
2.1. Clinical Evidence for Bimodal Serotonergic Disruption in Autism Spectrum Disorder: Hypo- and Hyperserotonemia
The 5-HT hypothesis of ASD emerged from a large, though still contentious body of evidence observing 5-HT abnormalities in individuals with an ASD diagnosis (reviewed in more detail in [56]). Perhaps though, the most convincing evidence emerged in 1961, when it was discovered that 6% of a group of autistic patients demonstrated hyperserotonemia [57], a condition of high platelet 5-HT content, putatively a consequence of increased 5-HT uptake through SERT. Platelet hyperserotonemia is now considered a biomarker for ASD risk, as it is found in approximately one third of autistic patients [44,57–61], and low free 5-HT levels in the blood have been correlated with increased severity of symptoms [62]. Through a large meta-analysis study, statistically significant increases in 5-HT levels were found in ASD patients in both whole blood and platelet-rich plasma, but not in platelet-poor plasma (free), suggesting that 5-HT levels have the potential to be a biomarker at least in some cases of ASD[63]. Recently, it was demonstrated that hyperserotonemia may be more prevalent in prepubescent ASD populations, and more common in males than females [64].
Clearly, however, hyperserotonemia is not a ubiquitous condition among individuals with autism, as hyposerotonemia (or low platelet 5-HT) has also been implicated in ASD [65–67]. Further, a study from 2006 demonstrated that plasma serotonin levels were lower in mothers who had children with autism than mothers who had typically developing children, suggesting that low maternal plasma serotonin may negatively influence fetal brain development [68]. Regardless of the bidirectionality seen in 5-HT as a biomarker, there is clear evidence for a persistent 5-HT abnormality related to this disorder.
There are several factors that could contribute to hyper- or hyposerotonemia phenotypes, a few of which will be discussed throughout the remainder of this review. Changes in SERT function have perhaps been the most comprehensively studied of these factors thus far. Hypo- or hyperserotonemia detected in platelets could correspondingly indicate increased or decreased extracellular brain 5-HT, attributable to decreased or increased SERT-mediated uptake, respectively [69]. This will be discussed in more detail beginning in Section 3.
Additional factors, which have been relatively neglected to this point, also implicate 5-HT disruption in some forms of ASD. Several studies have implicated polymorphisms in genes coding for enzymes responsible for synthesizing or metabolizing 5-HT in the etiologies of ASD. There is mixed evidence for whether [52,70] or not [71,72] THP2 genetic polymorphisms are associated with ASD. Polymorphisms in the metabolizing enzymes monoamine oxidase A or B (MAOA/B) have also been associated with ASD especially in male children. [73–76]. A noteworthy recent study reported that MAOA polymorphisms were only associated with ASD when examined as an interacting factor with forkhead box protein P2 (FOXP2) polymorphisms [77]. This highlights the need to study ASD risk factors not only in isolation, but together with other factors which in combination may reveal important causative mechanisms.
2.2. Autism Spectrum Disorder Relevant Perinatal Experimental Manipulation of the Serotonin System
In order to study these implicated factors and the role they may play in combination with each other, when they coexist within a single system, animal models of these human polymorphisms have become a useful tool to understand the role these factors may play in ASD etiologies. There is growing awareness of critical times during pre- and post-natal development wherein exposure to either low or high 5-HT levels, influenced by its metabolism and its precursor availability, can have long-lasting detrimental effects that may contribute to ASD related symptoms, as illustrated in a review by Daws and Gould [49].
To further explore the importance of 5-HT in ASD-related behaviors, multiple groups have utilized animal models to perturb 5-HT signaling through both genetic [78–80] and pharmacologic manipulations [81], to model and study the associations seen in human populations (Table 1). Preclinical studies involving genetic deletion of the peripherally expressed TPH1 enzyme in mice revealed that normal maternal 5-HT levels are crucial for normal fetal development. Embryos from TPH1 knock-out (KO) dams were 15-30% smaller, had abnormal brain structure, and decreases in mitotic capacity in the ventricular zone (30%) and the future cerebral cortex (24%) [45]. Regardless of fetal enzyme function, maternal genotypes resulting in reduced 5-HT synthesis led to autismlike phenotypes in offspring [45]. Further, ablation of the TPH2 gene in mice produces 5-HT deficiency in animals that then exhibit social behavior deficits and repetitive behaviors [78,82,83]. Similarly, inducing lesions of 5-HT neurons in the bilateral medial forebrain bundle of newborn mice with the neurotoxin 5,7-dihydroxytryptamine (5,7-DHT) produced adulthood impairments in social and sensory behaviors, and increased repetitive behaviors [81].
Table 1. 5-HT Relevant Factors in Humans Associated with ASD, and Analogous Mouse Models of Genetic or Environmental Conditions.
All factors listed in the table have an association with ASD in humans. Genetic or environmental manipulation of these factors in mouse models either demonstrate social behavior deficits, repetitive behaviors, both, or in the case of NSF has yet to be tested in a mouse model.
| Genetic Polymorphisms |
Functional Outcome |
Human Reference |
Mouse Model |
Social Deficits |
Repetitive Behaviors |
Model Reference |
|---|---|---|---|---|---|---|
| 5-HTTLPR | ||||||
| SLC6A4 S/S | hypofunctional SERT uptake | [93] | KO | ✓ | [88,94] | |
| SLC6A4 S/L | hypofunctional SERT uptake | [21,95] | HET | ✓ | [96] | |
| SLC6A4 Gly56Ala | hyperfunctional SERT uptake | [24] | Ala56 | ✓ | ✓ | [79,97] |
| TPH | ||||||
| TPH2: introns | decreased 5-HT synthesis | [52,70] | KO | ✓ | ✓ | [82,78,98] |
| MAO | ||||||
| MAOA | decreased 5-HT metabolism | [73,74,76] | KO | ✓ | ✓ | [84,86] |
| MAOB | decreased 5-HT metabolism | [76,99] | KO | ✓ | ✓ | [85,86] |
| Proteins | ||||||
| αIIbβ3 | decreased SERT expression | [100,101] | KO | ✓ | ✓ | [102] |
| NSF | decreased SERT expression | [101,103] | KD | NE | NE | [103] |
|
Environmental Factors |
||||||
| TRP Reduction | decreased 5-HT synthesis | [104,105] | dietary reduction | [106] |
Knock-out (KO), knock-down (KD), heterozygote (HET), not examined (NE) (behavioral tests have not been conducted with the N-ethylmaleimide-sensitive factor (NSF) KD mouse), integrin alphaIIbbeta3 transmembrane complex (αIIbβ).
Conversely, mice with elevated 5-HT levels due to genetic deletion of MAOA/B), major enzymes responsible for degradation of 5-HT and other monoamines, also exhibit social and repetitive behaviors relevant to ASD [84–86].
Likewise, reduced social interaction and increased repetitive behaviors have been reported in mice with constitutive SERT reduction or ablation, wherein extracellular levels of 5-HT, 5-HT synthesis, and turnover are all elevated 2- to 10-fold compared to wild-type littermates [21,87–92]. In sum, this preclinical evidence suggests that bidirectional 5-HT disruptions outside of an optimal range – either too low or too high – might contribute to the development and/or maintenance of autism-associated behaviors. Taken together, these types of preclinical serotonergic manipulations in rodent models, where such things as environment, diet, etc., are all controlled for, provide the strongest support for the serotonin hypothesis. However, there is still a long way to go in order to understand the etiologies underlying these phenotypes and the interactions between factors that may be necessary to result in ASD. Preclinical studies should be taken a step further in the future to dissect out the pathways that are developmentally disrupted to lead to social deficits vs. those necessary to produce repetitive behaviors, as they may or may not be present together based on the mechanisms of the serotonergic manipulation.
3. Bidirectional Perturbations of Serotonin Transporter Function in the Etiologies of Autism Spectrum Disorder
SERT is the major (high affinity, low capacity) gateway through which 5-HT accumulates in platelets and neurons, and its functionality significantly affects both the duration and concentration of 5-HT available for receptor-mediated signaling [107]. As a consequence, SERT is one of the most widely studied monoamine transporters [108], with its numerous genetic variants implicated in several diseases including alcoholism [109], and Alzheimer’s [110], as well as a primary target for studying the potential causes of the heterogeneous nature of 5-HT in ASD. Directly relevant to the present review, numerous studies have explored the contributions of both common and rare SERT gene polymorphisms that confer either hyper- or hypo-functional SERT variants that have been associated with an ASD diagnosis [93,111–114].
3.1. Hypofunctional Perturbations of Serotonin Transporter
By far the most widely studied gene polymorphism governing the level of SERT expression is the SERT-linked polymorphic region (5-HTTLPR). This is an insertion/deletion polymorphism that occurs within the promoter region of the SERT gene (SLC6A4). The deletion (short or “s”) allelic variant is associated with reduced SERT density and 5-HT uptake relative to the insertion (“l” or long) variant [50,115,116]. Compared to individuals homozygous for the long form, those with the short form have significantly less (1.4-2 fold) SERT in the dorsal raphe, a locus of 5-HT neuronal cell bodies that project extensively to the forebrain [117]. Given the importance of SERT and 5-HT in brain organization, both serotonergic and non-serotonergic neuron projections are likely compromised during development when 5-HT uptake is impaired [31,35,93,96]. Though both the “s” and “l” variants have been associated with ASD symptoms, findings have been contradictory (possibly related to a number of interacting single nucleotide polymorphisms in the SERT gene) and remain a source of contention that is beyond the scope of the present review [95,117–120]. Recent studies have indicated that genotypes with at least one short allele are more prevalent in children with ASD as compared to healthy controls [21,95,121]. Interestingly, and not necessarily contradicting the former finding, is evidence from parent-of-origin studies suggesting an increased risk of ASD in offspring of mothers who are homozygous for the long form of the allele [122]. This was hypothesized to be attributable to elevated 5-HT transport into the placenta, given the increased SERT function associated with this genotype [40]. However, this may actually result from exacerbation of 5-HT precursor availability, as will be discussed later in section 6.2. Taken together, findings like these may hint that further consideration should be given to both maternal and fetal 5-HT demands, and how these might additively or synergistically influence 5-HT availability to the extent that fetal development can be adversely and permanently disrupted.
Though there are significant differences in 5-HTTLPR allele frequency across ethnicities [123,124], this polymorphism is thought to account for only 25-30% of changes in platelet 5-HT uptake [50]. For a detailed table on allele frequency within control and ASD populations, please see please see [125]. Further, a recent review on PET and SPECT imaging of patients with ASD compared to controls, nicely describes the targets measured with this technology but highlights the confounds and limitations which currently exist in utilizing this data to draw ASD-relevant etiological or physiological conclusions [126]. This, in addition to the lack of complete penetrance of ASD in populations with a specific 5-HTTLPR genotype, exemplifies the importance of considering multiple contributing factors in the pathophysiology of autism, rather than a single genetic variation that alone does not confer ASD pathology.
3.2. Hyperfunctional Perturbations of Serotonin Transporter
Aside from the 5-HTTLPR, 5 rare coding variants in the SERT gene have been found in families with a history of autism. These variants produce a gain of SERT functionality, accelerating 5-HT uptake from extracellular fluid and, in the blood, concentrating 5-HT into platelets and producing hyperserotonemia [49,55,79,114,127]. The most prevalent gain-of-function variants (found in 0.5-1% of the population descending from European ancestry) [24,128] is Gly56Ala, or an alanine substitution for glycine at amino acid residue 56 in the SERT protein, due to a non-synonymous nucleotide polymorphisms in SLC6A4 [45].
Commonly referred to as Ala56, mice with this amino acid substitution genetically knocked-in exhibit higher 5-HT receptor sensitivity, increased 5-HT clearance, plasma hyperserotonemia, and demonstrate elevations in autism-related behaviors including social impairments and repetitive behaviors [79,129]. However among inbred mice, it has been demonstrated that the genetic background likely plays a major role in the susceptibility to social deficits, as the same KO in different strains of mice (C57BL/6 vs. 129) have different behavioral profiles (129 background may be protective) [130]. Again, rodent models provide a necessary tool for understanding how these SERT variants contribute to the pathophysiology of ASD. Not only do animal models provide the potential to evaluate this multifactorial pathology from a more comprehensive perspective, but also allow for more detailed explorations of the etiologies, for example, with development of cell type-specific knock-in of ALA56, or KO of SERT, to parse apart where in the brain accelerated or decelerated 5-HT uptake has the greatest impact on lifelong behavior.
3.3. Additional Effectors of Serotonin Transporter Function
In addition to genetically driven shifts in SERT expression or function, membrane expression of SERT has also been implicated in ASD etiologies. Recent studies demonstrate that binding or association of particular proteins with SERT can affect cell surface expression, and may contribute to serotonergic disturbances and autism-related behavioral disruptions.
For example, the enzyme N-ethylmaleimide-sensitive factor (NSF), a factor important in vesicle transport and fusion that co-localizes with SERT. A knock-down of NSF in a mouse model, showed reduced cell surface expression of SERT and 5-HT uptake when measured in vitro [103]. In post-mortem brain tissue from patients with ASD, levels of this enzyme trend towards decreases within the 5-HT cell body region, the dorsal raphe, even when the expression of SERT was not different compared to controls [103]. This highlights the possibility that additional factors outside of SERT functional abnormalities could be responsible for the biological and behavioral outcomes of ASD.
In another example, the integrin, αIIbβ3 directly interacts with SERT at its C terminus to increase SERT surface expression and promote activity [131]. A human family-based study identified multiple gene variants for αIIbβ3 as susceptibility genes for ASD [100]. Integrin αIIbβ3 KO mice exhibit typical social interaction preference, but fail to display a normal preference for social novelty, while also demonstrating excessive repetitive grooming behaviors, consistent with autism-like stereotypy [102]. Thus, reductions in proteins such as NSF or αIIbβ3 (which both facilitate SERT surface expression and function) are associated with ASD associated behaviors. Such findings complement those from constitutive SERT KO mice, which display impaired social interaction behavior [88,94]. As a disorder with multifactorial causes, more studies should consider the role that secondary influencing factors of SERT may play in the complicated picture of serotonergic disruption in ASD to form a more complete picture of the factors necessary to result in a complete ASD phenotype.
4. Evidence for Bidirectional Serotonin Involvement in Autism Spectrum Disorder Pathophysiology from Treatment Attempts with Selective Serotonin Reuptake Inhibitors
Another means of understanding the persistent changes in 5-HT signaling in ASD is by examining studies that have investigated the effectiveness of treatment with selective serotonin reuptake inhibitors (SSRIs). SSRIs block 5-HT reuptake through pharmacologic inhibition of SERT, elevating extracellular 5-HT. Increased extracellular 5-HT through treatment with SSRIs improves symptoms in some ASD patients [132], but lacks therapeutic efficacy in others [133,134]. For example, only a minority (approximately 35%) of children and adolescents [132] with ASD exhibit reduced irritability, stereotypies, and inappropriate speech and hyperactivity when receiving SSRI treatment [132–135]. While the reason for this disparity in response remains unclear, one plausible reason for limited or lack of therapeutic benefit of SSRIs in some individuals with ASD, is that these individuals already have high extracellular 5-HT [136]. For example, these non-responders could harbor hypofunctional SERT gene variants, and therefore have higher baseline extracellular 5-HT, rendering additional SERT blockade from pharmaceuticals ineffective. Decreases in 5-HT uptake (platelet hyposerotonemia) can also lead to deficits in neuronal network formation, given the critical neurodevelopmental role of 5-HT, and may also contribute to the pathophysiology of ASD [65]. Conversely, and in agreement with the hypothesis that bidirectional disruptions in 5-HT enhance risk of autism, patients that positively respond to SSRI treatment may otherwise exhibit platelet hyperserotonemia. Therefore, in these individuals, blockade of hyperactive SERT function may be beneficial through facilitation of extended extracellular 5-HT signaling [63]. To support this, one study found that some children with ASD exhibit a 25% decrease in brain 5-HT synthesis compared to controls [137], which can occur under conditions of high intracellular 5-HT. Researchers have suggested that this may be associated with hyperfunctional variants in SERT that could cause increased 5-HT uptake [79,138,139], but does not account for the approximately 65% of individuals who do not respond to SSRIs, leading to continued discord within the clinical literature. While, for example, in a clinical study of paroxetine treatment for depression, individuals with higher baseline platelet 5-HT were better responders [140], surprisingly, few studies report relationships between therapeutic response to SSRIs in autism patients and modulation of platelet hypo- or hyperserotonemia. Such studies would be of great interest, as they would help determine the potential impact of both low and high expressing/functioning SERT gene variants in response to treatments with SSRIs on core autism symptoms. Moreover, this kind of investigation could reveal platelet 5-HT as a tool for predicting SSRI treatment response. Although therapeutic serotonergic manipulations with SSRIs at later life stages may temporarily alleviate ASD related symptoms, that these symptoms can only be improved ephemerally suggests ASD involves a fundamental developmental component that cannot be undone by manipulations later in life.
5. Possible Compensation by Secondary Transporters of Serotonin in Autism Spectrum Disorder
Outside of the typically described SERT in 5-HT transport and recycling, there is another class of 5-HT transporters whose possible role in ASD should be explored. When primary, or “uptake 1” monoamine transporters (high affinity, low capacity; e.g., SERT) are overwhelmed or impaired (as in the case of functional polymorphisms or SSRI exposure), ancillary transporters classified as “uptake 2” engage in clearance. These “uptake 2” transporters function with low affinity, but high capacity, to expedite clearance of monoamines such as 5-HT [141–144]. They include organic cation transporters (OCTs 1-3), and the plasma membrane monoamine transporter (PMAT). Any transporter, including SERT, is considered “uptake 2” when it transports a nonpreferred monoamine with lower affinity but high capacity (e.g., in mice, the dopamine transporter is an “uptake 2” transporter for 5-HT under conditions of impaired SERT function) [145,146]. In rodents, long-term impairment or inhibition of SERT function can lead to these ancillary uptake mechanisms for 5-HT, such as OCTs or PMATs, being upregulated to manage extracellular 5-HT [147,148]. The engagement of “uptake 2” transporters could provide one explanation for the often-reported decreasing therapeutic efficacy of SERT-targeting antidepressant treatments over time in humans [143,149], or explain how most individuals with ASD do not exhibit overt CSF or platelet 5-HT abnormalities compared to controls [150]. Though in the case of an environmental exposure disrupting 5-HT levels, the causative factor may be relatively fleeting (as compared to a constitutive genetic change in SERT function), the disruption could nevertheless establish permanent changes that lead to the long-term symptoms of ASD. This might be especially true when compensatory mechanisms to effectively regulate 5-HT levels and neurotransmission are recruited and become permanently up- or down-regulated.
5.1. Preclinical Evidence for “Uptake 2” Transporters in Serotonin Clearance
In the context of functional SERT genetic polymorphisms, it is important to consider the response that may occur by secondary 5-HT clearance mechanisms, which can be directly assessed in preclinical settings. Pharmacological blockade of “uptake 2” has been shown to produce antidepressant-like activity in mice with constitutively reduced SERT expression, modeling reduced SERT function conferred by the human 5-HTTLPR “S” allele. Similarly, in wild-type mice given a combined sub-effective dose of the SSRI fluvoxamine combined with the OCT and PMAT blocker decynium-22 (D-22) [151]. These studies suggest that “uptake 2” mechanisms, putatively OCT3, may play a significant role in 5-HT signaling and homeostasis, particularly when SERT function is reduced [147,152,153]. Further, uptake by OCT2 [154,155] and OCT3 is inhibited by corticosterone [148,156,157]. Therefore, hormonal interference of “uptake 2” clearance in later life could contribute to stress-exacerbated symptoms of autism, especially during perceived stressful social situations in cases where these secondary transporters may have been permanently upregulated to compensate for impaired SERT function. Alternatively, OCT3 has been shown to be inhibited by estradiol as well [158], and may help to explain the disparity in ASD incidence between males and females, and may even exacerbate symptoms in hyposerotonemia populations, given that males have high levels of brain estradiol during development [159,160]. Given the association between low expressing/functioning gene variants of SERT and ASD, it is also possible that compensation in expression and/or function of ancillary 5-HT transporters may persist beyond their usefulness or otherwise endure to promote a state of pathophysiology. Studies comparing density of these transporters in ASD patients and unaffected controls could prove very telling of the physiological compensatory pathways which occur in response to the abnormalities that are typically studied in relation to ASD. Information on the expression and function of these transporters in ASD may help further explain the heterogeneity in response to treatments.
5.2. Considering the Functional Polymorphisms of “Uptake 2” Transporters in Autism Spectrum Disorder
Aside from the polymorphisms discovered in SERT, functional polymorphisms have been detected in “uptake 2” transporters as well. For example, a recent study discovered 3 non-synonymous single nucleotide polymorphisms in the human PMAT gene (SLC29A4), two of which resulted in functionally impaired 5-HT transport. However, these polymorphisms were present in patients with ASD as well as their unaffected parents, indicating that impaired PMAT-mediated 5-HT uptake alone likely does not contribute to ASD development [54]. Future studies would have to confirm if PMAT polymorphisms could have an exacerbation effect on the 5-HT system and ASD incidence when combined with a SERT polymorphism and/or early life 5-HT disruptions. The effects of OCT polymorphisms have not yet been studied with respect to autism, but may prove to be an important consideration for 5-HT-related risk factors of ASD, as evidence suggests they may be important therapeutic targets for other 5-HT related disorders such as anxiety and depression [161,162].
5.3. Vesicular Monoamine Transporter Uptake of Serotonin in Autism Spectrum Disorder
Although not responsible for extracellular clearance of 5-HT, vesicular monoamine transporters (VMAT) play a role in 5-HT metabolism as an intracellular regulator of 5-HT recycling. Recent studies demonstrate that certain VMAT polymorphisms may be either protective against, or linked to ASD, suggesting that VMAT dysregulation could be another avenue by which 5-HT disruption could occur to play a role in ASD pathophysiology [163]. Greater consideration of factors that have a more tangential or up/downstream role in 5-HT signaling and metabolism may prove to be an important next step in understanding the causes and potential treatments for ASD.
5.4. Targeting Alternative Serotonin Transporters in the Treatment of Autism Spectrum Disorder
The relationship between both high and low expressing SERT variants and the development or treatment of ASD could be significant, as exposure to either low or high levels of extracellular 5-HT could cause abnormal signaling and wiring of critical neural networks, leading to ASD associated symptoms [65]. The general consensus is that the most prevalent gene polymorphisms impacting SERT expression alone are not a cause of autism. Nonetheless, it remains likely that varied SERT function interferes with treatment approaches in certain subsets of individuals with autism, and that genetic variance in SERT function interacts with environmental risk factors (e.g., dietary TRP deficiency), or additional genetic factors, to facilitate ASD pathology. Further, recruitment of “uptake 2” transporters during development and beyond may either help compensate for 5-HT signaling disruptions, or potentially make them worse, depending on the manner in which the serotonergic system has been perturbed.
Aside from using transporter genotypes to personalize treatment, direct examination of OCT blockade (with the available compound D-22) in animal models of autism-like behaviors with low SERT function to alleviate symptoms could reveal a broader, novel treatment strategy (at least until personalized medicine becomes more feasible).
Having abnormally high SERT function, as with Ala56, can be detrimental to 5-HT signaling due to hyper-efficient removal of extracellular 5-HT, decreasing 5-HT receptor activation and resultant downstream effects. It is in these situations where SSRIs hypothetically should have beneficial effects, as they would reduce 5-HT clearance and thereby prolong 5-HT signaling. On the other hand, when SERT function is decreased by pharmacologic treatment and/or genetic variation (e.g., short allele of 5-HTTLPR), “uptake 2” transporter function may increase to manage the elevated extracellular concentrations of 5-HT [149]. When SSRIs are employed as chronic treatments, SSRI effectiveness could be undermined by the subsequent recruitment of “uptake 2” transporters to manage extracellular 5-HT levels [143]. Alternatively, if “uptake 2” transporters could be pharmacologically targeted to enhance 5-HT clearance in cases of genetically reduced SERT function, activation of these secondary transporters might be a promising alternative strategy to alleviate associated ASD symptoms. Thus, “uptake 2” transporters represent a highly understudied mechanism of extracellular 5-HT signaling regulation that might hold great promise for treatments and a better understanding of ASD etiologies.
With our expanding knowledge of functional transporter polymorphisms, it could be possible in the future to personalize treatment plans for individuals with different polymorphism and/or biomarker profiles. It may be more efficacious to treat serotonergic disruptions with a compound(s) that targets multiple 5-HT transporters, avoiding undesirable up- or down-regulation of compensatory transporters or cell surface receptors that undermine therapeutic benefits. In addition, advancing our neurophysiological knowledge about the interactive impacts of SERT, PMAT, and OCT functional polymorphisms in ASD could also facilitate personalized patient treatment approaches for individuals under this variable umbrella diagnosis.
This evidence also demonstrates how variations in multiple divergent factors can lead to similar behavioral outcomes, and forces us to consider how combinations of these factors confer heterogeneity in core phenotypes. Outside of genetic changes, which may result in ASD phenotypes, various environmental variables have also been studied to demonstrate similar outcomes. The remainder of this review will focus on perinatal environmental factors hypothesized to facilitate the symptomatic disruptions associated with autism, including in utero exposure to pharmacological SERT inhibition through maternal use of SSRIs, or maternal TRP amino acid insufficiency or excess. The presence of combinations of such factors may help explain the variation observed in 5-HT physiology and the associated behavioral outcomes in ASD (reviewed in [49]).
6. Environmental Manipulation of Serotonin Availability
6.1. Exposure to Selective Serotonin Reuptake Inhibitors During Pre- and Postnatal Development
The SSRI class of psychoactive medications preferentially block SERT to reduce uptake of 5-HT, increasing extracellular 5-HT available to modulate neurotransmission [164]. Though use of SSRIs has been shown to be beneficial to some individuals with ASD, how exposure to these drugs affects the developing brain in utero remains controversial. This becomes significant to consider when in the United States, approximately 23% of pregnant women experience at least 1 depressive episode during pregnancy, and approximately 13% of these women are prescribed an SSRI during all or part of their pregnancy [164]. This SSRI prescription occurs to mitigate depressive episodes and behaviors that can lead to negative obstetrical outcomes if not otherwise treated [165]. Although still debated, the benefits of SSRI treatment for depression during pregnancy have generally been thought to outweigh the risks [166]. However, this view may be changing in light of mounting evidence for possible associations between gestational SSRI exposure and ASD (reviewed in [167,168]). Indeed, SSRIs cross the placental barrier, meaning that more than 200,000 babies per year in the US alone [164] are prenatally exposed to SSRIs and their 5-HT reuptake inhibiting actions. These babies may experience long-term impacts on 5-HT neurotransmission [169], though extensive, longitudinal studies are needed to evaluate this possibility. Currently, the literature remains divided on whether maternal SSRI use is linked to an increased incidence of ASD in exposed offspring [147,170–178] or not [179–186] (see Table 2 for summary).
Table 2. Summary of Studies Exploring the Association of Maternal Clinical Antidepressant Use with the Incidence of ASD Diagnosis in Prenatally Exposed Offspring.
Studies from the last ten years clearly show continued controversy with regard to whether or not gestational exposure to SSRIs increases ASD incidence or risk.
| Antidepressant Class | Offspring ASD Association? | Study Type | Statistical Ratios | Sample Size* |
Source |
|---|---|---|---|---|---|
| SSRIs | Yes | Population Based | OR 2.2, 95% CI 1.2-4.3 | 1,805 | Croen et al., 2011 [170] |
| SNRIs, SSRIs | Yes | Population Based | OR 3.34, 95% CI 1.50-7.47 | 47,749 | Rai et al., 2013 [171] |
| SSRIs, SNRIs, TCAs | No | Population Based | HR 1.5, 95% CI 1.2-1.9 | 655,615 | Sørensen et al., 2013 [179] |
| SSRIs | No | Population Based | RR 1.20, 95% CI 0.90-1.61 | 5,061,174 | Hviid et al., 2013 [180] |
| SSRIs | Yes | Population Based | OR 1.91, 95% CI 1.13-3.47 | 4264 | El Marroun et al., 2014 [172] |
| SSRIs | Yes | Retrospective | OR 2.0, 95% CI 1.6-2/6 | 628,408 | Gidaya et al., 2014 [173] |
| SSRIs | Yes | Population Based | OR 3.22, 95% CI: 1.7-8.84 | 966 | Harrington et al., 2014 [174] |
| Antidepressants NOS | No | Retrospective | OR 1.10, 95% CI: 0.70-1.70 | 5399 | Clements et al., 2015 [181] |
| SSRIs | Yes | Meta-analysis | OR 2.13, 95% CI 1.66-2.73 | 79,221 | Man et al., 2015 [175] |
| Antidepressants NOS | No | Replication Study | OR 0.90, 95% CI 0.50-1.54 | 4,650 | Castro et al., 2016 [182] |
| MAOIs, SSRIs, SNRIs, TCAs |
Yes | Population Based | HR 1.87, 95% CI 1.15-3.04 | 145,456 | Boukhris et al., 2016 [176] |
| SSRIs | No | Retrospective | HR 1.4, 9%% CI 1.02-1.92 | 64,754 | Malm et al., 2016 [[183] |
| SSRIs | Yes | Meta-analysis | OR 1.82, 95% CI 1.59-2.10 | 65,782 | Andalib et al., 2017 [177] |
| NDRIs, SSRIs, SNRIs, TCAs |
Yes | Population Based | F = 4.882, p = 0.027 | 2,748 | Ackerman et al., 2017 [147] |
| NDRIs, SSRIs, SNRIs, TCAs |
No | Population based | RR 1.23, 95% CI 0.96-1.57 | 179,077 | Viktorin et al., 2017 [184] |
| NDRIs, SSRIs, SNRIs, TCAs |
No | Retrospective | HR 0.83, 95% CI 0.62-1.13 | 1,580,629 | Sujan et al., 2017 [[185] |
| SNRIs, SSRIs | No | Retrospective | HR 1.61, 95% CI 0.997-2.59 | 35906 | Brown et al., 2017 [186] |
| SSRIs | Yes | Meta-analysis | HR 1.61, 95% CI 1.16-2-25 | 2,133,811 | Kaplan et al., 2017 [178] |
KEY: MAOI: Monoamine Oxidase Inhibitor; NOS: Not Otherwise Specified; SNRI: Serotonin and Norepinephrine Reuptake Inhibitor; NDRI: Norepinephrine and Dopamine Reuptake Inhibitor; SNRI: Serotonin-norepinephrine Reuptake Inhibitors; SSRI: Selective Serotonin Reuptake Inhibitor; TCA: Tricyclic Antidepressants. SSRIs may include: citalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline. F: F Ratio; HR: Hazards Ratio; OR: Odds Ratio; RR: Rates Ratio. See cited references for full study details.
Sample size only includes control populations and ASD relevant samples, not necessarily the total population studied in the citation (e.g., some papers included ADHD populations in addition to ASD).
Of the many available SSRIs, fluoxetine (Prozac) is most commonly prescribed to pregnant women battling depression. Exposure to fluoxetine in utero in both human studies and animals models has demonstrated, with some reproducibility, behavioral and psychological deficits including depression in adulthood, and has been implicated in an increased incidence of ASD [176,187–189]. This could stem from enduring morphological disturbances initiated by fluoxetine during development of neurons and excitatory synapses in brain regions important for social interactions and behavioral flexibility, such as the hippocampus [190,191]. Fluoxetine, and its metabolite norfluoxetine, which is also active at SERT to block 5-HT uptake, readily cross the placenta and assimilate into fetal tissues, with neonatal plasma levels reaching 65-72% of maternal levels [192]. The highest fetal accumulation of these compounds occurs in the brain and thymus [193,194].
Additionally, both fluoxetine and norfluoxetine are transferred to offspring though breast milk, making postnatal exposure another important aspect of neurodevelopment to study. The average total fluoxetine and norfluoxetine combined (as fluoxetine equivalents) infant exposure level through breast milk is 6.81% of weight-adjusted maternal dose, based on infant daily milk intake [195]. It has been considered safe for infants to reach weight-adjusted plasma levels of combined fluoxetine and norfluoxetine < 10% of the maternal dose [196], but controversy remains as to whether higher values would actually be detrimental to neonatal behavioral or neurochemical outcomes [197]. Further, most infants tolerate in utero exposure without any obvious postnatal symptoms; but infants who do present with symptoms related to in utero exposure, such as hyperactivity or colic, were often born prematurely or were additionally exposed postpartum through breast milk [195], confounding interpretations about the effects of in utero SSRI exposure.
However, despite evidence suggesting that exposure to fluoxetine may be detrimental to the fetus, fluoxetine remains classified by the US Food and Drug Administration as a category C drug during pregnancy, and continues to be prescribed. This means that even though evidence does suggest fluoxetine may have detrimental effects on fetal development in animal models, it has not been verified well enough in human cases to justify discontinuation, particularly in light of more abundant reproducible evidence demonstrating the negative obstetrical outcomes of untreated maternal depression [198]. The variability and lack of reproducibility between studies examining how SSRIs may influence ASD incidence (see Table 2) emphasizes how other mitigating factors, likely related to maternal depression and antidepressant use, and genetic factors (polymorphisms in transporters of 5-HT), specific to the mother and/or her offspring, combined may be necessary to disrupt brain development sufficiently to result in long-term ASD-relevant behaviors. By their nature, association studies do not manipulate such components, and cannot control for all of these possible factors in ASD. Further, most of these studies look at the association between a single exposure or factor and ASD, but future studies would be more informative by taking into consideration, for example, populations of individuals identified to have had the same medication exposures that may or may not be compounded by the presence of a genetic transporter or metabolic factor polymorphism. The estimates of magnitude of the associations represented in Table 2 are small, and may be vulnerable to interpretation bias due to unobserved or unaccounted confounding variables. However, that these associations persist in large meta-analysis studies suggests that there is at least some underlying link between antidepressant exposure and ASD risk. Future studies should assess SSRI exposure in the context of other factors modifying the 5-HT system, such as SERT gene variants or dietary TRP intake, the latter of which is discussed in the following section.
6.2. Reductions in Availability of the Serotonin Precursor Tryptophan in Autism Spectrum Disorder
Another critical player in the availability of 5-HT, and a potential component of ASD pathophysiology, is the essential amino acid precursor of 5-HT, tryptophan (TRP). TRP is ingested from protein-based foods. Dietary TRP deficiency is prevalent in populations affected by malnutrition (e.g., homelessness, anorexia, obesity) or in those with nutritional absorption disorders (e.g., Crohn’s disease) [199–201]. In addition to the accepted biomarker of hyperserotonemia [60,61,63], TRP insuffiency has emerged as a possible indicative marker for ASD as well. TRP levels in the blood of individuals with ASD have been found to be either elevated [202] or reduced [203,204] compared to unaffected individuals. TRP levels in general can be influenced by dietary supplementation of cofactors (such as B vitamins) that are important for 5-HT synthesis from TRP [205].
6.2.1. Evidence for the Role of Tryptophan in Autism Spectrum Disorder Relevant Pathophysiology
Dietary depletion of TRP has been shown to worsen ASD related behaviors in patients [105], as well as in adult mice [106], supporting a core contributing role of active 5-HT signaling in modulating behaviors characteristic of autism. An acute study of TRP depletion in adult patients with ASD evaluated behavioral changes following a 24-hour TRP depletion (achieving a 69% reduction in plasma free TRP and 86% total TRP) [104]. Here, 65% of patients showed an exacerbation of ASD related symptoms including pacing, whirling, self-hitting, rocking, and toe walking [104]. Knowing TRP insufficiency might exacerbate ASD symptoms should particularly be considered in circumstances where the individual exhibits rigid food selectivity, and may be nutritionally deprived of critical dietary components such as TRP [206]. In rodents, diets deficient in TRP (< 15% of daily recommended dose) reduce plasma TRP by 34–54% and brain 5-HT levels by 52–82% compared to controls [207,208]. Chronic dietary TRP depletion was found to reduce 5-HT neuron development in the raphe nuclei [209], and even acute TRP depletion reduced brain 5-HT turnover producing social interaction deficits in C57BL/6 and 129S mice [106]. TRP availability therefore, should be a factor considered in ASD development or pathology.
6.2.2. Potential to Alleviate Autism Spectrum Disorder Symptoms with Tryptophan Enhancement
Certainly, ASD cannot be cured with amino acid or vitamin supplementation. Nonetheless, evidence from clinical studies suggests therapeutic benefits of TRP supplementation can occur in some patients [205,210]. In humans, increases in available TRP result in increased 5-HT synthesis as well as production of its metabolite 5-hydroxyindoleacetic acid (5-HIAA) [211]. Therefore, TRP supplementation may have the potential to alleviate ASD symptoms caused by a lack of extracellular 5-HT (e.g. in cases of increased 5-HT reuptake) if the precursor is made more readily available.
In support of this, TRP supplementation has been shown to mitigate some symptoms in patients with depression, presumably by increasing 5-HT available for transmission [212]. There could be beneficial effects of dietary TRP enhancement in ASD as well, as has been demonstrated by observations in a mouse model with face validity for autism-related behavior, the BTBRT+Itpr3tf/J, as increased TRP intake improved sociability [106]. However, caution should be heeded in taking large quantities of L-TRP supplements for extended durations. In the past, a by-product of TRP supplement production 1,1′-ethylidenebis L-tryptophan, was implicated in an alarming outbreak of eosinophilia-myalgia syndrome, and this triggered a flurry of research that resulted in reducing the upper limit for safe human consumption of L-TRP supplements to 8 grams/day for up to 8 weeks [213]. It must be emphasized that safety research requirements for amino acid supplements, particularly in the United States, are minimal as compared to the requirements for pharmaceuticals so further research is warranted, particularly before large scale use to treat children with autism spectrum disorders. Also use of L-TRP supplements should be carefully monitored and reported to physicians during pregnancy, as in mice a shift from 2-5% in diet been shown to restrict fetal growth [214].
6.2.3. Reduction of Tryptophan Availability In Utero
During fetal brain development 5-HT plays critical roles early (embryonic day 10.5-15.5 in mice; first and early second trimester in humans), even before the fetal hindbrain is capable of synthesizing 5-HT on its own (mouse embryonic day 16.5; later second trimester in humans) [215]. Interestingly, SERT and 5-HT receptors function in fetal brains even before neurons producing 5-HT are formed, highlighting their importance in neurodevelopment [42,43]. For example in mice lack of fetal SERT results in brain overgrowth and impaired social behavior [88,216,217]. Hence fetal SERT expression is critical to governing early brain growth and social interaction preference.
Recent studies demonstrate that maternal TRP, to a greater extent than maternal 5-HT, crosses the placenta [40,215]. L- amino acid transporters (LAT-1 (Light chain-type amino acid transporter 1) and LAT-2) are primarily responsible for TRP transport on placental brush border membranes, however additional transporters appear to be involved in its basal membrane transport [218,219]. There is some evidence that OCTs, which are richly expressed in placenta, may be among those involved in placental TRP transport as well as any 5-HT transport [220]. Given that OCT expression is promoted by insulin signaling [221,222] this indicates that conditions such as gestational diabetes may disrupt TRP and/or 5-HT transport to the fetus. Indeed associations between gestational diabetes and increased autism risk have been reported [223,224].
Within the placenta TRP is converted to 5-HT by placental TPH1 in early pregnancy (prior to GD 15.5) or transported to the fetal brain in later pregnancy (GD 16.5 to birth) when fetal THP2 is capable of converting TRP to 5-HT to supply fetal 5-HT requirements [40,215]. Either reducing or enhancing TRP availability prenatally or early postnatally in rodent models produces changes in 5-HT synthesis, metabolism, and transport that culminate into respectively worsened or improved adult behavioral phenotypes related to anxiety and depression [225–227].
Disruptions in fetal TRP availability could result not only from TRP deficiency in the maternal diet, but also from increased maternal TRP demand if 5-HT recycling is impaired (e.g., from maternal genetic or pharmacologic reductions in SERT function). During normal pregnancy, demand for TRP increases both for the mother and the fetus [228]. Moreover, non-pregnant women experience greater reductions in serum TRP than men under chronically depleted conditions [229], indicating that limited maternal TRP intake may have profound effects on both the mother and fetus. Further, fetal TRP concentrations are approximately twice as high in umbilical cord plasma compared to maternal circulation, suggesting TRP is preferentially transported to supply fetal needs in utero [230,231]. Fetal hindbrain 5-HT synthesis capacity increases throughout pregnancy [40], implicating placental TRP availability as an important limiting factor in fetal neurodevelopment. As a result, fetal neurodevelopmental processes influenced by 5-HT may go awry under conditions of reduced TRP availability, promoting long-term neurophysiological disruptions that culminate in behavioral deficits associated with autism. Such bidirectional findings are reminiscent of the dichotomous reports of hypo- or hyperserotonemia, providing further evidence that bidirectional serotonergic perturbations likely contribute to ASD pathophysiology.
6.2.4. Decreases in Tryptophan Availability Caused by Maternal Use of Selective Serotonin Reuptake Inhibitors
Acutely, SSRIs reduce peripheral TRP metabolism by inhibiting the TRP degrading liver enzyme indolylamine 2,3-dioxygenase, allowing for increased free TRP availability in the brain [232,233]. However, chronic SSRI effects on TRP levels are less well understood. The lack of knowledge on this topic is especially unfortunate, as exposure to SSRIs is not typically acute, and may have enduring effects on TRP metabolism and 5-HT signaling. How reduced or enhanced dietary TRP during pregnancy might interact with genetic and/or pharmacologic changes in SERT function to impact ASD incidence has also not been explored to our knowledge. Impaired maternal SERT function (genetic and/or pharmacologic) inhibits 5-HT recycling, thereby increasing maternal and fetal TRP demand for de novo 5-HT synthesis. Consequently, the influence of dietary TRP on 5-HT availability could be exacerbated or ameliorated by differences in SERT function, emphasizing the importance of considering multiple 5-HT influencing factors within both the maternal and fetal environments in the pathophysiology of ASD.
7. Considering Multiple Bidirectional Serotonin Influencing Factor Combinations in the Etiologies of Autism Spectrum Disorder
Genetically driven increases or decreases in SERT function in autistic individuals make treatment attempts challenging, as overwhelming evidence indicates that bidirectional serotonergic disruptions converge under a shared diagnosis and treatment strategy. Further, these genetic alterations in SERT function might either protect against, or exacerbate, additional genetic and environmental 5-HT-altering factors that enhance ASD vulnerability, potentially helping account for the wide variability in both diagnoses and treatment responses. Therefore, it becomes critical to more holistically investigate serotonergic system variability, as well as consider the influences of related components discussed herein, as well as others, such as the dopamine transporter [146], and 5-HT-modulating hormones (e.g., corticosterone, oxytocin) that under abnormal circumstances may also play an unexpected role in regulating 5-HT signaling [148,234]. Given the immense complexity of ASD, and the absence of a singular cause, it is indeed likely that numerous interfacing systems contribute to the development of this disorder. Future analyses should not utilize populations grouped together simply based on an ASD diagnosis, but instead based on functionally common genetic profiles and environmental exposures to better understand ontogeny of this complex disorder. Undoubtedly a great undertaking necessitating multiple site collaborations, such extensive studies will provide essential insight into how additive, synergistic, or protective these different conditions may be. In turn, parsing apart the multiple contributions of disrupted 5-HT regulation to neurodevelopmental processes and subsequent behavioral profiles will provide a greater understanding of the comprehensive conditions that fall under the umbrella ASD diagnosis.
Preclinical approaches to understanding how ASD emerges from early life serotonergic system disruptions should involve combined manipulation of two or more of the factors discussed in this review: SERT genotypes in fetus and mother, uptake 2 genotypes in fetus and mother, maternal SSRI use and dietary TRP content, and maternal and fetal TRP metabolism and/or 5-HT synthesis. For example, to our knowledge no studies have examined how functional disruptions of multiple 5-HT transporters (e.g., SERT and OCT3) might influence autism-related behaviors.
Our lab is beginning to decipher how a few of the numerous factors discussed in this review interact to impact autism-related behavior in rodents, in an effort to advance understanding of the complicated interplay between genotype, diet, and drug exposure. Such approaches are certainly challenging and laborious, but are essential for advancing our knowledge of this multifaceted disorder linked to so many varied risk factors. Indeed, current controversy in ASD literature might be at least partially resolved by considering that perhaps any one of the identified genetic variations or environmental exposures (such as SSRI use or dietary TRP deficiency) alone may not lead to increased incidence of ASD, but instead may become causative when combined in what has been termed the “two-hit” system of multiple genetic and/or environmental risk factors [147,152,153]. For example, the combination of a maternal TRP metabolic disorder with a SERT transporter polymorphism in the mother or offspring might be necessary to significantly influence 5-HT signaling within the developing brain to elicit autism-relevant behavioral disruptions.
Further candidate contributors, beyond the scope of this review, are melatonin and kynurenine metabolic pathways for which TRP is also the precursor [235]. These alternative pathways likely undergo compensatory shifts when TRP availability and demand are altered, and thus indirectly may affect the function of the 5-HT pathway by shunting TRP away from 5-HT synthesis. Indeed, future research must consider how several factors interactively influence 5-HT system function, rather than constraining observations to outcomes from manipulation of a single factor. Assessing these many facets in different combinations could reveal critical information about how multiple 5-HT-altering components interface to exacerbate or ameliorate autism-like behaviors. All of this work to date serves to demonstrate the immense complexity of ASD, and highlights how much is still to be explored, with the ultimate goals of better individualized treatments, and early recognition and intervention of high risk factor combinations.
Acknowledgments
Role of the Funding Source:
Funding sources had no roles in the writing of this report or in the decision to submit this report for publication.
Funding:
VRG is supported by a National Institute on Aging training grant (T32AG021890) to Nicolas Musi, and TLG is supported by a National Institute on Drug Abuse training grant (T32DA031115) to Charles P. France. The authors wish to acknowledge the following funding sources: Brain & Behavior Research Foundation and Vital Projects Fund, Inc., for a 2017 NARSAD Young Investigator Grant (26249) to TLG; National Institute of Mental Health grants (R01 MH093320 and R01 MH106978) to LCD; Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) grant (R21 HD081261) and Congressionally Directed Medical Research Program Autism Idea Award (AR110109) to GGG.
Footnotes
Conflict of Interest Statement:
None of the authors have any conflicts of interest to report.
Declarations of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee L-C, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehom A, Shenouda J, Hall-Lande J, Van, Naarden, Braun K, Dowling NF, Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2014, MMWR. Surveill. Summ 67 (2018) 1–23. 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Howlander N, Noone A, Krapcho M, Miller D, Bishop K, Kosary C, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K, (eds.), SEER Cancer Statistics Review, 1975–2014, (2017). https://seer.cancer.gov/csr/1975_2014/. [Google Scholar]
- [3].American Psychiatric Association, Diagnostic and statistical manual of mental disorders, 5th ed., American Psychiatric Association, Arlington, VA, 2013. [Google Scholar]
- [4].Christensen DL, Baio J, Braun KVN, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius-Spencer M, Lee L-C, Pettygrove S, Robinson C, Schulz E, Wells C, Wingate MS, Zahorodny W, Yeargin-Allsopp M, Centers for Disease Control and Prevention (CDC), Prevalence and characteristics of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 Sites, United States, 2012, MMWR. Surveill. Summ 65 (2016) 1–23. 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cholemkery H, Medda J, Lempp T, Freitag CM, Classifying autism spectrum disorders by ADI-R: subtypes or severity gradient?, J. Autism Dev. Disord 46 (2016) 2327–2339. 10.1007/s10803-016-2760-2. [DOI] [PubMed] [Google Scholar]
- [6].Mundy P, Sigman M, Ungerer J, Sherman T, Defining the social deficits of autism: the contribution of non-verbal communication measures, J. Child Psychol. Psychiatry. 27 (1986) 657–69. [DOI] [PubMed] [Google Scholar]
- [7].Volkmar FR, Nelson DS, Seizure disorders in autism, J. Am. Acad. Child Adolesc. Psychiatry. 29 (1990) 127–9. 10.1097/00004583-199001000-00020. [DOI] [PubMed] [Google Scholar]
- [8].Kaufinann WE, Kidd SA, Andrews HF, Budimirovic DB, Esler A, Haas-Givler B, Stackhouse T, Riley C, Peacock G, Sherman SL, Brown WT, Berry-Kravis E, Autism spectrum disorder in fragile X syndrome: cooccurring conditions and current treatment, Pediatrics. 139 (2017) S194–S206. 10.1542/peds.2016-1159F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gillott A, Fumiss F, Walter A, Anxiety in high-functioning children with autism, Autism. 5 (2001) 277–286. 10.1177/1362361301005003005. [DOI] [PubMed] [Google Scholar]
- [10].Reisinger DL, Roberts JE, Differential relationships of anxiety and autism symptoms on social skills in young boys with fragile X syndrome, Am. J. Intellect. Dev. Disabil 122 (2017) 359–373. 10.1352/1944-7558-122.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim JA, Szatmari P, Bryson SE, Streiner DL, Wilson FJ, The prevalence of anxiety and mood problems among children with autism and Asperger syndrome, Autism. 4 (2000) 117–132. 10.1177/1362361300004002002. [DOI] [Google Scholar]
- [12].Reiersen AM, Todd RD, Co-occurrence of ADHD and autism spectrum disorders: phenomenology and treatment, Expert Rev. Neurother 8 (2008) 657–69. 10.1586/14737175.8.4.657. [DOI] [PubMed] [Google Scholar]
- [13].Stores G, Wivggs L, Abnormal sleep patterns associated with autism, Autism. 2 (1998) 157–169. 10.1177/1362361398022004. [DOI] [Google Scholar]
- [14].Burack JA, Selective attention deficits in persons with autism: preliminary evidence of an inefficient attentional lens, J. Abnorm. Psychol 103 (1994) 535–43. [DOI] [PubMed] [Google Scholar]
- [15].Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, Kripke C, The health status of adults on the autism spectrum, Autism. 19 (2015) 814–823. 10.1177/1362361315577517. [DOI] [PubMed] [Google Scholar]
- [16].Thijssen AY, Mujagic Z, Jonkers DMAE, Ludidi S, Keszthelyi D, Hesselink MA, Clemens CHM, Conchillo JM, Kruimel JW, Masclee AAM, Alterations in serotonin metabolism in the irritable bowel syndrome, Aliment. Pharmacol. Ther 43 (2016) 272–282. 10.1111/apt.13459. [DOI] [PubMed] [Google Scholar]
- [17].Chan CK, Zhao Y, Liao SY, Zhang YL, Lee MYK, Xu A, Tse HF, Vanhoutte PM, A-FABP and oxidative stress underlie the impairment of endothelium-dependent relaxations to serotonin and the intima-medial thickening in the porcine coronary artery with regenerated endothelium, ACS Chem. Neurosci 4 (2013) 122–129. 10.1021/cn3000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Buczko W, [Serotonin--blood platelets, vessels], Acta Haematol. Pol 25 (1994) 61–5. [PubMed] [Google Scholar]
- [19].Shajib MS, Baranov A, Khan WI, Diverse effects of gut-derived serotonin in intestinal inflammation, ACS Chem. Neurosci 8 (2017) 920–931. 10.1021/acschemneuro.6b00414. [DOI] [PubMed] [Google Scholar]
- [20].Narita N, Kato M, Tazoe M, Miyazaki K, Narita M, Okado N, Increased monoamine concentration in the brain and blood of fetal thalidomide- and valproic acid-exposed rat: putative animal models for autism, Pediatr. Res 52 (2002) 576–9. 10.1203/00006450-200210000-00018. [DOI] [PubMed] [Google Scholar]
- [21].Jaiswal P, Guhathakurta S, Singh AS, Verma D, Pandey M, Varghese M, Sinha S, Ghosh S, Mohanakumar KP, Rajamma U, SLC6A4 markers modulate platelet 5-HT level and specific behaviors of autism: a study from an Indian population, Prog. Neuropsychopharmacol. Biol. Psychiatry. 56 (2015) 196–206. 10.1016/j.pnpbp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- [22].Kiser D, SteemerS B, Branchi I, Homberg JR, The reciprocal interaction between serotonin and social behaviour, Neurosci. Biobehav. Rev 36 (2012) 786–798. 10.1016/j.neubiorev.2011.12.009. [DOI] [PubMed] [Google Scholar]
- [23].Hollander E, Phillips A, Chaplin W, Zagursky K, Novotny S, Wasserman S, Iyengar R, A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism, Neuropsychopharmacology. 30 (2005) 582–9. 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]
- [24].Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD, Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors, Am. J. Hum. Genet 77 (2005) 265–79. 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sangkuhl K, Klein TE, Altman RB, Selective serotonin reuptake inhibitors pathway, Pharmacogenet. Genomics. 19 (2009) 907–909. 10.1097/FPC.0b013e32833132cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hirst WD, Price GW, Rattray M, Wilkin GP, Serotonin transporters in adult rat brain astrocytes revealed by [3H]5-HT uptake into glial plasmalemmal vesicles, Neurochem. Int 33 (1998) 11–22. 10.1016/S0197-0186(98)00003-5. [DOI] [PubMed] [Google Scholar]
- [27].Miner LH, Schroeter S, Blakely RD, Sesack SR, Ultrastructural localization of the serotonin transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to dopamine terminals, J. Comp. Neurol 427 (2000) 220–234. . [DOI] [PubMed] [Google Scholar]
- [28].Narboux-Nême N, Pavone LM, Avallone L, Zhuang X Gaspar P, Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs), Neuropharmacology. 55 (2008) 994–1005. 10.1016/j.neuropharm.2008.08.020. [DOI] [PubMed] [Google Scholar]
- [29].Mercado CP, Kilic F, Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels, Mol. Interv 10 (2010) 231–41. 10.1124/mi.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S, Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene, J. Clin. Invest 105 (2000) 1555–62. 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].VMortensen O, Kristensen AS, Rudnick G, Wiborg O Molecular cloning, expression and characterization of a bovine serotonin transporter, Mol. Brain Res 71 (1999) 120–126. 10.1016/S0169-328X(99)00178-3. [DOI] [PubMed] [Google Scholar]
- [32].Gaspar P, Cases O, Maroteaux L, The developmental role of serotonin: news from mouse molecular genetics, Nat. Rev. Neurosci 4 (2003) 1002–1012. 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- [33].Daubert EA, Condron BG, Serotonin: a regulator of neuronal morphology and circuitry, Trends Neurosci 33 (2010) 424–34. 10.1016/j.tins.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lesch K-P, Waider J, Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders, Neuron 76 (2012) 175–91. 10.1016/j.neuron.2012.09.013. [DOI] [PubMed] [Google Scholar]
- [35].Kepser L-J, Homberg JR The neurodevelopmental effects of serotonin: a behavioural perspective, Behav. Brain Res 277 (2015) 3–13. 10.1016/j.bbr.2014.05.022. [DOI] [PubMed] [Google Scholar]
- [36].Hendricks TJ, V Fyodorov D, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES, Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior, Neuron 37 (2003) 233–47. [DOI] [PubMed] [Google Scholar]
- [37].Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F, Hall SF, Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release, J. Neurosci 21 (2001) 6862–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sundström E, Kölare S, Souverbie F, Samuelsson EB, Pschera H, Lunell NO, Seiger A, Neurochemical differentiation of human bulbospinal monoaminergic neurons during the first trimester, Brain Res. Dev. Brain Res 75 (1993) 1–12. [DOI] [PubMed] [Google Scholar]
- [39].Teissier A, Soiza-Reilly M, Gaspar P, Refining the role of 5-HT in postnatal development of brain circuits, Front. Cell. Neurosci 11 (2017) 139 10.3389/fncel.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P. A transient placental source of serotonin for the fetal forebrain, Nature. 472 (2011) 347–50. 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen X, Ye R, Gargus JJ, Blakely RD, Dobrenis K, Sze JY, Disruption of transient serotonin accumulation by non-serotonin-producing neurons impairs cortical map development, Cell Rep 10 (2015) 346–358. 10.1016/j.celrep.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Choi DS, Ward SJ, Messaddeq N, Launay JM, Maroteaux L, 5-HT2B receptor-mediated serotonin morphogenetic functions in mouse cranial neural crest and myocardiac cells, Development. 124 (1997) 1745–55. [DOI] [PubMed] [Google Scholar]
- [43].Fiorica-Howells E, Maroteaux L, Gershon MD, Serotonin and the 5-HT(2B) receptor in the development of enteric neurons, J. Neurosci 20 (2000) 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Whitaker-Azmitia PM, Serotonin and brain development: role in human developmental diseases, Brain Res. Bull 56 (2001) 479–85. [DOI] [PubMed] [Google Scholar]
- [45].Côté F, Fligny C, Bayard E, Launay J-M, Gershon MD, Mallet J, Vodjdani G, Maternal serotonin is crucial for murine embryonic development, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 329–34. 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kligman D, R Marshak D, Purification and characterization of a neurite extension factor from bovine brain, Proc. Natl. Acad. Sci. U. S. A 82 (1985) 7136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hernández Rodríguez J, Serotonin as a neurotrophic factor in the fetal brain: binding, capture and release in centers of axonal growth, Gac. Med. Mex 130 (1994) 246–52. [PubMed] [Google Scholar]
- [48].Anitha A, Nakamura K, Yamada K, Suda S, Thanseem I, Tsujii M, Iwayama Y, Hattori E, Toyota T, Miyachi T, Iwata Y, Suzuki K, Matsuzakl H, Kawai M, Sekine Y, Tsuchiya K, Sugihara G-I, Ouchi Y, Sugiyama T, Koizumi K, Higashida H, Takei N, Yoshikawa T, Mori N, Genetic analyses of roundabout (ROBO) axon guidance receptors in autism, Am. J. Med. Genet. B. Neuropsychiatr. Genet 147B (2008) 1019–27. 10.1002/ajmg.b.30697. [DOI] [PubMed] [Google Scholar]
- [49].Daws LC, Gould GG, Ontogeny and regulation of the serotonin transporter: providing insights into human disorders, Pharmacol. Ther 131 (2011) 61–79. 10.1016/j.pharmthera.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Anderson GM, Gutknecht L, Cohen DJ, Brailly-Tabard S, Cohen JHM, Ferrari P, Roubertoux PL, Tordjman S, Serotonin transporter promoter variants in autism: functional effects and relationship to platelet hyperserotonemia, Mol. Psychiatry 7 (2002) 831–6. 10.1038/sj.mp.4001099. [DOI] [PubMed] [Google Scholar]
- [51].Cohen IL, Liu X, Schutz C, White BN, Jenkins EC, Brown WT, Holden JJA, Association of autism severity with a monoamine oxidase A functional polymorphism, Clin. Genet 64 (2003) 190–7. [DOI] [PubMed] [Google Scholar]
- [52].Coon H, Dunn D, Lainhart J, Miller J, Hamit C, Battaglia A, Tancredi R, Leppert MF, Weiss R, McMahon W, Possible association between autism and variants in the brain-expressed tryptophan hydroxylase gene (TPH2), Am. J. Med. Genet. B. Neuropsychiatr. Genet 135B (2005) 42–6. 10.1002/ajmg.b.30168. [DOI] [PubMed] [Google Scholar]
- [53].Boccuto L, Chen C-F, Pittman AR, Skinner CD, McCartney HJ, Jones K, R Bochner A, Stevenson RE, Schwartz CE, Decreased tryptophan metabolism in patients with autism spectrum disorders, Mol. Autism 4 (2013) 16 10.1186/2040-2392-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Adamsen D, Ramaekers V, Ho HT, Britschgi C, Rüfenacht V, Meili D, Bobrowski E, Philippe P, Nava C, Van Maldergem L, Bruggmann R, Walitza S, Wang J, Grünblatt E, Thöny B, Autism spectrum disorder associated with low serotonin in CSF and mutations in the SLC29A4 plasma membrane monoamine transporter (PMAT) gene, Mol. Autism 5 (2014) 43 10.1186/2040-2392-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Muller CL, Anacker AMJ, Veenstra-VanderWeele J, The serotonin system in autism spectrum disorder: From biomarker to animal models, Neuroscience. 321 (2016) 24–41. 10.1016/j.neuroscience.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lam KSL, Aman MG, Arnold LE, Neurochemical correlates of autistic disorder: a review of the literature, Res. Dev. Disabil 27 (2006) 254–89. 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- [57].Schain RJ, Freedman DX, Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children, J. Pediatr 58 (1961) 315–20. [DOI] [PubMed] [Google Scholar]
- [58].Anderson GM, Home WC, Chatteijee D, Cohen DJ, The hyperserotonemia of autism, Ann. N. Y. Acad. Sci 600 (1990) 331–40–2. [DOI] [PubMed] [Google Scholar]
- [59].Roll LH; Haarmann FY, Grotemeyer KH, Kehrer H, Serotonin and amino acid content in platelets of autistic children, Acta Psychiatr. Scand 87 (1993) 312–6. [DOI] [PubMed] [Google Scholar]
- [60].Leboyer M, Philippe A, Bouvard M, Guilloud-Bataille M, Bondoux D, Tabuteau F, Feingold J, Mouren-Simeoni MC, Launay JM, Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives, Biol. Psychiatry. 45 (1999) 158–63. [DOI] [PubMed] [Google Scholar]
- [61].Cross S, Kim S-J, Weiss LA, Delahanty RJ, Sutcliffe JS, Leventhal BL, Cook EH, Veenstra-Vanderweele J, Molecular genetics of the platelet serotonin system in first-degree relatives of patients with autism, Neuropsychopharmacology. 33 (2008) 353–60. 10.1038/sj.npp.1301406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Spivak B, Golubchik P, Mozes T, Vered Y, Nechmad A, Weizman A, Strous RD, Low platelet-poor plasma levels of serotonin in adult autistic patients, Neuropsychobiology. 50 (2004) 157–60. 10.1159/000079108. [DOI] [PubMed] [Google Scholar]
- [63].Gabriele S, Sacco R, Persico AM, Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis, Eur. Neuropsychopharmacol 24 (2014) 919–29. 10.1016/j.euroneuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- [64].Shufffey LC, Guter SJ, Delaney S, Jacob S, Anderson GM, Sutcliffe JS, Cook EH, Veenstra-VanderWeele J, Is there sexual dimorphism of hyperserotonemia in autism spectrum disorder?, Autism Res 10 (2017) 1417–1423. 10.1002/aur.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang C-J, Tan H-P, Du Y-J, The developmental disruptions of serotonin signaling may involved in autism during early brain development, Neuroscience. 267 (2014) 1–10. 10.1016/j.neuroscience.2014.02.021. [DOI] [PubMed] [Google Scholar]
- [66].Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT, Serotonin and dopamine transporter binding in children with autism determined by SPECT, Dev. Med. Child Neurol 50 (2008) 593–7. 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- [67].Chugani DC, Muzik O, Rothermel R, Behen M, Chakraborty P, Mangner T, Da Silva EA, Chugani HT, Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys, Ann. Neurol 42 (1997) 666–669. 10.1002/ana.410420420. [DOI] [PubMed] [Google Scholar]
- [68].Connors SL, Matteson KJ, Sega GA, Lozzio CB, Carroll RC, Zimmerman AW, Plasma serotonin in autism, Pediatr. Neurol 35 (2006) 182–186. 10.1016/j.pediatrneurol.2006.02.010. [DOI] [PubMed] [Google Scholar]
- [69].McNamara IM, Borella AW, Bialowas LA, Whitaker-Azmitia PM, Further studies in the developmental hyperserotonemia model (DHS) of autism: social, behavioral and peptide changes, Brain Res 1189 (2008) 203–14. 10.1016/j.brainres.2007.10.063. [DOI] [PubMed] [Google Scholar]
- [70].Yang SY, Yoo HJ, Cho IH, Park M, Kim SA, Association with tryptophan hydroxylase 2 gene polymorphisms and autism spectrum disorders in Korean families, Neurosci. Res 73 (2012) 333–336. 10.1016/J.NEURES.2012.05.012. [DOI] [PubMed] [Google Scholar]
- [71].Egawa J, Watanabe Y, Nunokawa A, Endo T, Kaneko N, Tamura R, Sugiyama T, Someya T, A detailed association analysis between the tryptophan hydroxylase 2 (TPH2) gene and autism spectrum disorders in a Japanese population, Psychiatry Res 196 (2012) 320–322. 10.1016/j.psychres.2011.09.001. [DOI] [PubMed] [Google Scholar]
- [72].Ramoz N, Cal G, Reichert JG, Corwin TE, Kryzak LA, Smith CJ, Silverman JM, Hollander E, Buxbaum JD, Family-based association study of TPH1 and TPH2 polymorphisms in autism, Am. J. Med. Genet. Part B Neuropsychiatr. Genet 141B (2006) 861–867. 10.1002/ajmg.b.30356. [DOI] [PubMed] [Google Scholar]
- [73].Tassone F, Qi L, Zhang W, Hansen RL, Pessah IN, Hertz-Picciotto I, MAOA, DBH, and SLC6A4 variants in CHARGE: a case-control study of autism spectrum disorders, Autism Res 4 (2011) 250–261. 10.1002/aur.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cohen I, Liu X, Lewis M, Chudley A, Forster-Gib son C, Gonzalez M, Jenkins E, Brown W, Holden J, Autism severity is associated with child and maternal MAOA genotypes, Clin. Genet 79 (2011) 355–362. 10.1111/j.1399-0004.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- [75].Saito M, Yamagata T, Matsumoto A, Shiba Y, Nagashima M, Taniguchi S, Jimbo E, Momoi MY, MAOA/B deletion syndrome in male siblings with severe developmental delay and sudden loss of muscle tonus, Brain Dev 36 (2014) 64–69. 10.1016/j.braindev.2013.01.004. [DOI] [PubMed] [Google Scholar]
- [76].Salem AM, Ismail S, Zarouk WA, Abdul Baky O, Sayed AA, Abd El-Hamid S, Salem S, Genetic variants of neurotransmitter-related genes and miRNAs in Egyptian autistic patients, ScientificWorldJournal. 2013 (2013) 670621 10.1155/2013/670621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Park Y, Won S, Nam M, Chung J-H, Kwack K, Interaction between MAOA and FOXP2 in association with autism and verbal communication in a Korean population, J. Child Neurol 29 (2014) NP207–NP211. 10.1177/0883073813511301. [DOI] [PubMed] [Google Scholar]
- [78].Kane MJ, Angoa-Peréz M, Briggs DI, Sykes CE, Francescutti DM, Rosenberg DR, Kuhn DM, Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism, PLoS One. 7 (2012) e48975 10.1371/journal.pone.0048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, lessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD, Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 5469–74. 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Yirmiya N, Pilowsky T, Nemanov L, Arbelle S, Feinsilver T, Fried I, Ebstein RP, Evidence for an association with the serotonin transporter promoter region polymorphism and autism, Am. J. Med. Genet 105 (2001) 381–6. [DOI] [PubMed] [Google Scholar]
- [81].Boylan CB, Blue ME, Hohmann CF, Modeling early cortical serotonergic deficits in autism, Behav. Brain Res 176 (2007) 94–108. 10.1016/j.bbr.2006.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].V Savelieva K, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthom TH, Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants, PLoS One. 3 (2008) e3301 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N, Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin, Transl. Psychiatry. 2 (2012) e122 10.1038/tp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cases O, Seif I; Grimsby J, Gaspar P, Chen K, Poumin S, Müller M, Aguet M, Babinet C, Shih JC, Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA, Science. 268 (1995) 1763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chen K, Holschneider DP, Wu W, Rebrin I, Shih JC, A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior, J. Biol. Chem 279 (2004) 39645–52. 10.1074/jbc.M405550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bortolato M, Godar SC, Alzghoul L, Zhang J, Darling RD, Simpson KL, Bini V, Chen K, Wellman CL, Lin RCS, Shih JC, Monoamine oxidase A and A/B knockout mice display autistic-like features, Int. J. Neuropsychopharmacol 16 (2013) 869–888. 10.1017/S1461145712000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Fox MA, Andrews AM, R Wendland J, Lesch K-P, Holmes A, Murphy DL, A pharmacological analysis of mice with a targeted disruption of the serotonin transporter, Psychopharmacology (Berl). 195 (2007) 147–66. 10.1007/s00213-007-0910-0. [DOI] [PubMed] [Google Scholar]
- [88].Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, R Magnuson T, Lauder JM, Social approach in genetically engineered mouse lines relevant to autism, Genes. Brain. Behav 8 (2009) 129–42. 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dufour-Rainfray D, Vourc’h P, Le Guisquet A-M, Garreau L, Temant D, Bodard S, Jaumain E, Gulhan Z, Belzung C, R Andres C, Chalon S, Guilloteau D, Behavior and serotonergic disorders in rats exposed prenatally to valproate: A model for autism, Neurosci. Lett 470 (2010) 55–59. 10.1016/j.neulet.2009.12.054. [DOI] [PubMed] [Google Scholar]
- [90].Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC, Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior, J. Neurochem 116 (2011) 291–303. 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM, Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression, J. Neurosci. Methods. 140 (2004) 169–181. 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- [92].Kalueff AV; Fox MA, Gallagher PS, Murphy DL, Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice, Genes, Brain Behav 6 (2007) 389–400. 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- [93].Ariefi Z; Kaur M, Gameeldien H, van derMerwe L, Bajic VB, 5-HTTLPR polymorphism: analysis in South African autistic individuals, Hum. Biol 82 (2010) 291–300. 10.3378/027.082.0303. [DOI] [PubMed] [Google Scholar]
- [94].Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch K-P, R Wendland J, How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems, Neuropharmacology. 55 (2008) 932–60. 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tordjman S, Gutknecht L, Carlier M, Spitz E, Antoine C, Slama F, Carsalade V, Cohen DJ, Ferrari P, Roubertoux PL, Anderson GM, Role of the serotonin transporter gene in the behavioral expression of autism, Mol. Psychiatry 6 (2001) 434–439. 10.1038/sj.mp.4000873. [DOI] [PubMed] [Google Scholar]
- [96].Kyzar EJ, Pham M, Roth A, Cachat J, Green J, Gaikwad S, Kalueff AV, Alterations in grooming activity and syntax in heterozygous SERT and BDNF knockout mice: The utility of behavior-recognition tools to characterize mutant mouse phenotypes, Brain Res. Bull 89 (2012) 168–176. 10.1016/J.BRAINRESBULL.2012.08.004. [DOI] [PubMed] [Google Scholar]
- [97].Siemann JK, Muller CL, Forsberg CG, Blakely RD, Veenstra-VanderWeele J, Wallace MT, An autism-associated serotonin transporter variant disrupts multisensory processing, Transl. Psychiatry. 7 (2017) e1067 10.1038/tp.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mosienko V, Beis D, Alenina N, Wöhr M, Reduced isolation-induced pup ultrasonic communication in mouse pups lacking brain serotonin, Mol. Autism. 6 (2015) 13 10.1186/s13229-015-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Chakraborti B, Verma D, Karmakar A, Jaiswal P, Sanyal A, Paul D, Sinha S, Singh AS, Guhathakurta S, Roychowdhury A, Panda CK, Ghosh S, Mohanakumar KP, Mukhophadhyay K, Rajamma U, Genetic variants of MAOB affect serotonin level and specific behavioral attributes to increase autism spectrum disorder (ASD) susceptibility in males, Prog. Neuro-Psychopharmacology Biol. Psychiatry 71 (2016) 123–136. 10.1016/j.pnpbp.2016.07.001. [DOI] [PubMed] [Google Scholar]
- [100].Schuch JB, Muller D, Endres RG, Bosa CA, Longo D, Schuler-Faccini L, Ranzan J, Becker MM, dos Santos Riesgo R, Roman T, The role of β3 integrin gene variants in autism spectrum disorders — diagnosis and symptomatology, Gene 553 (2014) 24–30. 10.1016/j.gene.2014.09.058. [DOI] [PubMed] [Google Scholar]
- [101].Saito J, FGrota T, Kikunaga N, Otsubo K, Ieiri I, Interindividual differences in placental expression of the SLC22A2 (OCT2) gene: relationship to epigenetic variations in the 5’-upstream regulatory region, J. Pharm. Sci 100 (2011) 3875–83. 10.1002/jps.22595. [DOI] [PubMed] [Google Scholar]
- [102].Carter MD, Shah CR, Muller CL, Crawley JN, Carneiro AMD, Veenstra-VanderWeele J, Absence of preference for social novelty and increased grooming in integrin β3 knockout mice: initial studies and future directions, Autism Res 4 (2011) 57–67. 10.1002/aur.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Iwata K, Matsuzaki H, Tachibana T, Ohno K, Yoshimura S, Takamura H, Yamada K, Matsuzaki S, Nakamura K, Tsuchiya KJ, Matsumoto K, Tsujii M, Sugiyama T, Katayama T, Mori N, N-ethylmaleimide-sensitive factor interacts with the serotonin transporter and modulates its trafficking: implications for pathophysiology in autism, Mol. Autism 5 (2014) 33 10.1186/2040-2392-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].McDougle CJ, Naylor ST, Cohen DJ, Aghajanian GK, R Heninger G, Price LH, Effects of tryptophan depletion in drug-free adults with autistic disorder, Arch. Gen. Psychiatry. 53 (1996) 993–1000. [DOI] [PubMed] [Google Scholar]
- [105].Trotter PD, McGlone F, McKie S, McFarquhar M, Elliott R, Walker SC, Deakin JFW, Effects of acute tryptophan depletion on central processing of CT-targeted and discriminatory touch in humans, Eur. J. Neurosci 44 (2016) 2072–83. 10.1111/ejn.13298. [DOI] [PubMed] [Google Scholar]
- [106].Zhang WQ, Smolik CM, Barba-Escobedo PA, Gamez M, Sanchez JJ, Javors MA, Daws LC, Gould GG, Acute dietary tryptophan manipulation differentially alters social behavior, brain serotonin and plasma corticosterone in three inbred mouse strains, Neuropharmacology. 90 (2015) 1–8. 10.1016/j.neuropharm.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Uhl GR, Johnson PS, Neurotransmitter transporters: three important gene families for neuronal function, J. Exp. Biol 196 (1994) 229–36. [DOI] [PubMed] [Google Scholar]
- [108].Graff-Guerrero A, De la Fuente-Sandoval C, Camarena B, Gómez-Martin D, Apiquián R, Fresán A, Aguilar A, Méndez-Núñez JC, Escalona-Huerta C, Drucker-Colín R, Nicolini H, Frontal and limbic metabolic differences in subjects selected according to genetic variation of the SLC6A4 gene polymorphism, Neuroimage. 25 (2005) 1197–204. 10.1016/j.neuroimage.2004.12.020. [DOI] [PubMed] [Google Scholar]
- [109].Ho P-S, Shih M-C, Ma K-H, Huang W-S, Ho KK-J, Yen C-H, Lu R-B, Huang S-Y, Availability of the serotonin transporter in patients with alcohol dependence, World J. Biol. Psychiatry 12 (2011) 134–142. 10.3109/15622975.2010.503813. [DOI] [PubMed] [Google Scholar]
- [110].Pritchard AL, Pritchard CW, Bentham P, Lendon CL, Role of serotonin transporter polymorphisms in the behavioural and psychological symptoms in probable Alzheimer Disease patients, Dement. Geriatr. Cogn. Disord 24 (2007) 201–206. 10.1159/000107081. [DOI] [PubMed] [Google Scholar]
- [111].Cho IH, Yoo HJ, Park M, Lee YS, Kim SA, Family-based association study of 5-HTTLPR and the 5-HT2A receptor gene polymorphisms with autism spectrum disorder in Korean trios, Brain Res 1139 (2007) 34–41. 10.1016/j.brainres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- [112].Coutinho AM, Sousa I, Martins M, Correia C, Morgadinho T, Bento C, Marques C, Ataíde A, Miguel TS, Moore JH, Oliveira G, Vicente AM, Evidence for epistasis between SLC6A4 and ITGB3 in autism etiology and in the determination of platelet serotonin levels, Hum. Genet 121 (2007) 243–56. 10.1007/s00439-006-0301-3. [DOI] [PubMed] [Google Scholar]
- [113].Guhathakurta S, Sinha S, Ghosh S, Chatterjee A, Ahmed S, Gangopadhyay PK, Usha R, Population-based association study and contrasting linkage disequilibrium pattern reveal genetic association of SLC6A4 with autism in the Indian population from West Bengal, Brain Res 1240 (2008) 12–21. 10.1016/j.brainres.2008.08.063. [DOI] [PubMed] [Google Scholar]
- [114].Schauder KB, Muller CL, Veenstra-VanderWeele J, Cascio CJ, Genetic variation in serotonin transporter modulates tactile hyperresponsiveness in ASD, Res. Autism Spectr. Disord 10 (2015) 93–100. 10.1016/j.rasd.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP, Allelic variation of human serotonin transporter gene expression, J. Neurochem 66 (1996) 2621–4. [DOI] [PubMed] [Google Scholar]
- [116].Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH, Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels, Am. J. Psychiatry 155 (1998) 207–13. 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- [117].Canli T, Lesch K-P, Long story short: the serotonin transporter in emotion regulation and social cognition, Nat. Neurosci 10 (2007) 1103–9. 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- [118].van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, Baldwin RM, Innis RB, Gelemter J, Central serotonin transporter availability measured with [123I]beta-CIT SPECT in relation to serotonin transporter genotype, Am. J. Psychiatry. 161 (2004) 525–31. 10.1176/appi.ajp.161.3.525. [DOI] [PubMed] [Google Scholar]
- [119].Homberg JR, Lesch K-P, Looking on the bright side of serotonin transporter gene variation, Biol. Psychiatry 69 (2011) 513–519. 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- [120].Rose’meyer R, A review of the serotonin transporter and prenatal cortisol in the development of autism spectrum disorders, Mol. Autism 4 (2013) 37 10.1186/2040-2392-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gebril O, Khalil R, Meguid N, A study of blood serotonin and serotonin transporter promoter variant (5-HTTLPR) polymorphism in Egyptian autistic children, Adv. Biomed. Res 4 (2015) 94 10.4103/2277-9175.156658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kistner-Griffin E, Brune CW, Davis LK, Sutcliffe JS, Cox NJ, Cook EH, Parent-of-origin effects of the serotonin transporter gene associated with autism, Am. J. Med. Genet. Part B Neuropsychiatr. Genet 156 (2011) 139–144. 10.1002/ajmg.b.31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Gelemter J, Kranzler H, Cubells JF, Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects, Hum. Genet 101 (1997) 243–6. [DOI] [PubMed] [Google Scholar]
- [124].Noskova T, Pivac N, Nedic G, Kazantseva A, Gaysina D, Faskhutdinova G, Gareeva A, Khalilova Z, Khusnutdinova E, Kovacic DK, Kovacic Z, Jokic M, Seler DM, Ethnic differences in the serotonin transporter polymorphism (5-HTTLPR) in several European populations, Prog. Neuro-Psychopharmacology Biol. Psychiatry 32 (2008) 1735–1739. 10.1016/j.pnpbp.2008.07.012. [DOI] [PubMed] [Google Scholar]
- [125].Huang CH, Santangelo SL, Autism and serotonin transporter gene polymorphisms: A systematic review and meta-analysis, Am. J. Med. Genet. Part B Neuropsychiatr. Genet 147B (2008) 903–913. 10.1002/ajmg.b.30720. [DOI] [PubMed] [Google Scholar]
- [126].Zürcher NR, Bhanot A, McDougle CJ, Hooker JM, A systematic review of molecular imaging (PET and SPECT) in autism spectrum disorder: Current state and future research opportunities, Neurosci. Biobehav. Rev 52 (2015) 56–73. 10.1016/J.NEUBIOREV.2015.02.002. [DOI] [PubMed] [Google Scholar]
- [127].Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD, Enhanced activity of human serotonin transporter variants associated with autism, Philos. Trans. R. Soc. Lond. B. Biol. Sci 364 (2009) 163–73. 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Glatt CE, DeYoung JA, Delgado S, Service SK, Giacomini KM, Edwards RH, Risch N, Freimer NB, Screening a large reference sample to identify very low frequency sequence variants: comparisons between two genes, Nat. Genet 27 (2001) 435–438. 10.1038/86948. [DOI] [PubMed] [Google Scholar]
- [129].Veenstra-Vanderweele J, lessen TN, Thompson BJ, Carter M, Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD, Modeling rare gene variation to gain insight into the oldest biomarker in autism: construction of the serotonin transporter Gly56Ala knock-in mouse, J. Neurodev. Disord 1 (2009) 158–71. 10.1007/s11689-009-9020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kerr TM, Muller CL, Miah M, Jetter CS, Pfeiffer R, Shah C, Baganz N, Anderson GM, Crawley JN, Sutcliffe JS, Blakely RD, Veenstra-Vanderweele J, Genetic background modulates phenotypes of serotonin transporter Ala56 knock-in mice, Mol. Autism. 4 (2013) 35 10.1186/2040-2392-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Carneiro AMD, Cook EH, Murphy DL, Blakely RD, Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans, J. Clin. Invest 118 (2008) 1544–52. 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Hollander E, Soorya L, Chaplin W, Anagnostou E, Taylor BP, Ferretti CJ, Wasserman S, Swanson E, Settipani C, A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders, Am. J. Psychiatry 169 (2012) 292–9. 10.1176/appi.ajp.2011.10050764. [DOI] [PubMed] [Google Scholar]
- [133].Williams K, Brignell A, Randall M, Silove N, Hazell P, Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD), Cochrane Database Syst. Rev (2013) CD004677 10.1002/14651858.CD004677.pub3. [DOI] [PubMed] [Google Scholar]
- [134].Taylor JL, Dove D, Veenstra-VanderWeele J, Sathe NA, McPheeters ML, Jerome RN, Warren Z, Interventions for Adolescents and Young Adults With Autism Spectrum Disorders, Agency for Healthcare Research and Quality (US), 2012. [PubMed] [Google Scholar]
- [135].H Fatemi S, Realmuto GM, Khan L, Thuras P, Fluoxetine in treatment of adolescent patients with autism: a longitudinal open trial, J. Autism Dev. Disord 28 (1998) 303–7. [DOI] [PubMed] [Google Scholar]
- [136].Sugie Y, Sugie H, Fukuda T, Ito M, Sasada Y, Nakabayashi M, Fukashiro K, Ohzeki T, Clinical efficacy of fluvoxamine and functional polymorphism in a serotonin transporter gene on childhood autism, J. Autism Dev. Disord 35 (2005) 377–85. [DOI] [PubMed] [Google Scholar]
- [137].Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, Chugani HT, Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children, Ann. Neurol 45 (1999) 287–95. [DOI] [PubMed] [Google Scholar]
- [138].Adamsen D, Meili D, Blau N, Thöny B, Ramaekers V, Autism associated with low 5-hydroxyindolacetic acid in CSF and the heterozygous SLC6A4 gene Gly56Ala plus 5-HTTLPR L/L promoter variants, Mol. Genet. Metab 102 (2011) 368–73. 10.1016/j.ymgme.2010.11.162. [DOI] [PubMed] [Google Scholar]
- [139].Lesch KP, Wolozin BL, Murphy DL, Reiderer P, Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter, J. Neurochem 60 (1993) 2319–22. [DOI] [PubMed] [Google Scholar]
- [140].Goodnick PJ, Henry J, Kumar A, Neurochemistry and paroxetine response in major depression, Biol. Psychiatry. 37 (1995) 417–419. 10.1016/0006-3223(94)00312-Q. [DOI] [PubMed] [Google Scholar]
- [141].Burgen AS, Iversen LL, The inhibition of noradrenaline uptake by sympathomimetic amines in the rat isolated heart, Br. J. Pharmacol. Chemother 25 (1965) 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Busch AE, Quester S, Ulzheimer JC, Gorboulev V, Akhoundova A, Waldegger S, Lang F Koepsell H, Monoamine neurotransmitter transport mediated by the polyspecific cation transporter rOCT1, FEBS Lett 395 (1996) 153–6. [DOI] [PubMed] [Google Scholar]
- [143].Daws LC, Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy, Pharmacol. Ther 121 (2009) 89–99. 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Shaskan EG, Snyder SH, Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake, J. Pharmacol. Exp. Ther 175 (1970) 404–18. [PubMed] [Google Scholar]
- [145].Zhou F-M, Liang Y, Salas R, Zhang L, DeBiasi M, Dani JA, Corelease of dopamine and serotonin from striatal dopamine terminals, Neuron 46 (2005) 65–74. 10.1016/j.neuron.2005.02.010. [DOI] [PubMed] [Google Scholar]
- [146].Mössner R, Simantov R, Marx A, Lesch KP, Seif I, Aberrant accumulation of serotonin in dopaminergic neurons, Neurosci. Lett 401 (2006) 49–54. 10.1016/j.neulet.2006.02.081. [DOI] [PubMed] [Google Scholar]
- [147].Ackerman S, Schoenbrun S, Hudac C, Bernier R, Adamsen D, Meili D, Blau N, Thöny B, Ramaekers V, Ho HT, Britschgi C, Rüfenacht V, Meili D, Bobrowski E, Philippe P, Nava C, Van Maldergem L, Bruggmann R, Walitza S, Wang J, Grünblatt E, Thöny B, Alwan S, Friedman JM, Chambers C, Andalib S, R Emamhadi M, Yousefzadeh-Chabok S, Shakouri SK, Høilund-Carlsen PF, Vafaee MS, Michel TM, Anderson GM, Stevenson JM, Cohen DJ, Home WC, Chatteijee D, Cohen DJ, Gutknecht L, Cohen DJ, Brailly-Tabard S, Cohen JHM, Ferrari P, Roubertoux PL, Tordjman S, Anitha A, Nakamura K, Yamada K, Suda S, Thanseem I, Tsujii M, Iwayama Y, Hattori E, Toyota T, Miyachi T, Iwata Y, Suzuki K, Matsuzaki H, Kawai M, Sekine Y, Tsuchiya K, Sugihara G-I, Ouchi Y, Sugjyama T, Koizumi K, Jfrgashida H, Takei N, Yoshikawa T, Mori N, Ariefi Z; Kaur M, Gameeldien H, van der Merwe L, Bajic VB, Badawy AA, Interactive effects of prenatal antidepressant exposure and likely gene disrupting mutation on the severity of autism spectrum disorder, J Autism Dev Disord 47 (2017) 3489–3496. 10.1007/s10803-017-3246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Baganz N, Horton R, Martin K, Holmes A, Daws LC, Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun, J. Neurosci 30 (2010) 15185–95. 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC, Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 18976–81. 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].V Shemer A, Azmitia EC, Whitaker-Azmitia PM, Dose-related effects of prenatal 5-methoxytryptamine (5-MT) on development of serotonin terminal density and behavior, Brain Res. Dev. Brain Res 59 (1991) 59–63. [DOI] [PubMed] [Google Scholar]
- [151].Horton RE, Apple DM, Owens WA, Baganz NL, Cano S, Mitchell NC, Vitela M, Gould GG, Koek W, Daws LC, Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: uncovering novel targets to treat depression, J. Neurosci 33 (2013) 10534–43. 10.1523/JNEUROSCI.5687-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Girirajan S, Rosenfeld JA, Cooper GM, Antonacci F, Siswara P, Itsara A, Vives L, Walsh T, McCarthy SE, Baker C, Mefford HC, Kidd JM, Browning SR, Browning BL, Dickel DE, Levy DL, Ballif BC, Platky K, Farber DM, Gowans GC, Wetherbee JJ, Asamoah A, Weaver DD, Mark PR, Dickerson J, Garg BP, Ellingwood SA, Smith R, Banks VC, Smith W, McDonald MT, Hoo JJ, French BN, Hudson C, Johnson JP, Ozmore JR, Moeschler JB, Surti U, Escobar LF, El-Khechen D, Gorski JL, Kussmann J, Salbert B, Lacassie Y, Biser A, McDonald-McGinn DM, Zackai EH, Deardorff MA Shaikh TH, Haan E, Friend KL, Fichera M, Romano C, Gécz J, DeLisi LE, Sebat J, King M-C, Shaffer LG, Eichler EE, A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay, Nat. Genet 42 (2010) 203–9. 10.1038/ng.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Leblond CS, Heinrich J, Delorme R, Proepper C, Betancur C, Huguet G, Konyukh M, Chaste P, Ey E, Rastam M, Anckarsäter H, Nygren G, Gillberg IC, Melke J, Toro R, Regnault B, Fauchereau F, Mercati O, Lemière N, Skuse D, Poot M, Holt R, Monaco AP, Järvelä I, Kantojärvi K, Vanhala R, Curran S, Collier DA, Bolton P, Chiocchetti A, Klauck SM, Poustka F, Freitag CM Waites R, Kopp M, Duketis E, Bacchelli E, Minopoli F, Ruta L, Battaglia A, Mazzone L, Maestrini E, Sequeira AF, Oliveira B, Vicente A, Oliveira G, Pinto D, Scherer SW, Zelenika D, Delepine M, Lathrop M, Bonneau D, Guinchat V, Devillard F, Assouline B, Mouren M-C, Leboyer M, Gillberg C, Boeckers TM, Bourgeron T, Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders, PLoS Genet 8 (2012) e1002521 10.1371/journal.pgen.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Koepsell H, Lips K, Volk C, Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications, Pharm. Res 24 (2007) 1227–1251. 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- [155].Miura Y, Yoshikawa T, Naganuma F, Nakamura T, Iida T, Kárpáti A, Matsuzawa T, Mogi A, Harada R, Yanai K, Characterization of murine polyspecific monoamine transporters, FEBS Open Bio 7 (2017) 237–248. 10.1002/2211-5463.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Hayer-Zillgen M, Brüss M, Bönisch H, Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3, Br. J. Pharmacol 136 (2002) 829–836. 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Couroussé T, Bacq A, Belzung C, Guiard B, Balasse L, Louis F, Le Guisquet A-M, Gardier AM, Schinkel AH, Giros B, Gautron S, Brain organic cation transporter 2 controls response and vulnerability to stress and GSK3β signaling, Mol. Psychiatry 20 (2015) 889–900. 10.1038/mp.2014.86. [DOI] [PubMed] [Google Scholar]
- [158].Gasser PJ, Lowry CA, Orchinik M, Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission, J. Neurosci 26 (2006) 8758–66. 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C, Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens, Nat. Neurosci 9 (2006) 220–226. 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- [160].Schwarz JM, McCarthy MM, Cellular mechanisms of estradiol-mediated masculinization of the brain, J. Steroid Biochem. Mol. Biol 109 (2008) 300–6. 10.1016/j.jsbmb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Daws LC, Koek W, Mitchell NC, Revisiting serotonin reuptake inhibitors and the therapeutic potential of “uptake-2” in psychiatric disorders, ACS Chem. Neurosci 4 (2013) 16–21. 10.1021/cn3001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Feng N, Mo B, Johnson PL, Orchinik M, Lowry CA, Renner KJ, Local inhibition of organic cation transporters increases extracellular serotonin in the medial hypothalamus, Brain Res 1063 (2005) 69–76. 10.1016/j.brainres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- [163].Noroozi R, Ghafouri-Fard S, Omrani MD, Habibi M, Sayad A, Taheri M, Association study of the vesicular monoamine transporter 1 (VMAT1) gene with autism in an Iranian population, Gene 625 (2017) 10–14. 10.1016/j.gene.2017.05.003. [DOI] [PubMed] [Google Scholar]
- [164].Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C, The management of depression during pregnancy: A report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists, Obstet. Gynecol 114 (2009) 703–713. 10.1097/AOG.0b013e3181ba0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Cooper WO, Willy ME, Pont SJ, Ray WA, Increasing use of antidepressants in pregnancy, Am. J. Obstet. Gynecol 196 (2007) 544.e1–544.e5. 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- [166].Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C, Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data, Arch. Gen. Psychiatry 63 (2006) 898 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- [167].Olivier JDA, Åkerud H, Kaihola H, Pawluski JL, Skalkidou A, Högberg U, Sundström-Poromaa I, The effects of maternal depression and maternal selective serotonin reuptake inhibitor exposure on offspring, Front. Cell. Neurosci 7 (2013) 73 10.3389/fncel.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Alwan S, Friedman JM, Chambers C, Safety of selective serotonin reuptake inhibitors in pregnancy: A review of current evidence, CNS Drugs. 30 (2016) 499–515. 10.1007/s40263-016-0338-3. [DOI] [PubMed] [Google Scholar]
- [169].Boyer EW, Shannon M, The serotonin syndrome, N. Engl. J. Med 352 (2005) 1112–1120. 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- [170].Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V, Antidepressant use during pregnancy and childhood autism spectrum disorders, Arch. Gen. Psychiatry 68 (2011) 1104 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- [171].Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C, Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study, BMJ. 346 (2013) f2059–f2059. 10.1136/bmj.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].El Marroun H, White TJH, van der Knaap NJF, R Homberg J, Fernández G, Schoemaker NK, V Jaddoe VW, Holman A, Verhulst FC, Hudziak JJ, Strieker BHC, Tiemeier H, Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children, Br. J. Psychiatry. 205 (2014) 95–102. 10.1192/bjp.bp.113.127746. [DOI] [PubMed] [Google Scholar]
- [173].Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, Newschaffer CJ, In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder, J. Autism Dev. Disord 44 (2014) 2558–2567. 10.1007/s10803-014-2128-4. [DOI] [PubMed] [Google Scholar]
- [174].Harrington RA, Lee L-C, Crum RM, Zimmerman AW, Hertz-Picciotto I, Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay, Pediatrics. 133 (2014) e1241–8. 10.1542/peds.2013-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Man KKC, Tong HHY, Wong LYL, Chan EW, Simonoff E, Wong ICK, Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: A systematic review and meta-analysis of observational studies, Neurosci. Biobehav. Rev 49 (2015) 82–89. 10.1016/j.neubiorev.2014.11.020. [DOI] [PubMed] [Google Scholar]
- [176].Boukhris T, Sheehy O, Mottron L, Bérard A, Antidepressant use during pregnancy and the risk of autism spectrum disorder in children, JAMA Pediatr 170 (2016) 117 10.1001/jamapediatrics.2015.3356. [DOI] [PubMed] [Google Scholar]
- [177].Andalib S, Emamhadi MR, Yousefcadeh-Chabok S, Shakouri SK, Høilund-Carl sen PF, Vafaee MS, Michel TM, Maternal SSRI exposure increases the risk of autistic offspring: A meta-analysis and systematic review, Eur. Psychiatry 45 (2017) 161–166. 10.1016/j.eurpsy.2017.06.001. [DOI] [PubMed] [Google Scholar]
- [178].Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K, Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: a meta-analysis of cohort studies, Br. J. Clin. Pharmacol 83 (2017) 2798–2806. 10.1111/bcp.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Sorensen MJ, Christensen J, Pamer ET, Grønborg TK, Vestergaard M, Schendel D, Pedersen LH, Antidepressant exposure in pregnancy and risk of autism spectrum disorders, Clin. Epidemiol 5 (2013) 449 10.2147/CLEP.S53009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Hviid A, Melbye M, Pasternak B, Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism, N. Engl. J. Med 369 (2013) 2406–2415. 10.1056/NEJMoa1301449. [DOI] [PubMed] [Google Scholar]
- [181].Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, Erb JL, Churchill SE, Kaimal AJ, Doyle AE, Robinson EB, Smoller JW, Kohane IS, Perlis RH, Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system, Mol. Psychiatry 20 (2015) 727–34. 10.1038/mp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Castro VM, Kong SW, Clements CC, Brady R, Kaimal AJ, Doyle AE, Robinson EB, Churchill SE, Kohane IS, Perlis RH, Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study, Transl. Psychiatry 6 (2016) e708–e708. 10.1038/tp.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, Weissman M, Wickramaratne P, Artama M, Gingrich JA, Sourander A, Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: A national register-based study, J. Am. Acad. Child Adolesc. Psychiatry 55 (2016) 359–66. 10.1016/j.jaac.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Viktorin A, Uher R, Reichenberg A, Levine SZ, Sandin S, Autism risk following antidepressant medication during pregnancy, Psychol. Med 47 (2017) 2787–2796. 10.1017/S0033291717001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Sujan AC, Rickert ME, Öberg AS, Quinn PD, Hernández-Díaz S, Almqvist C, Lichtenstein P, Larsson H, DOnofrio BM, Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring, JAMA. 317 (2017) 1553–1562. 10.1001/jama.2017.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Brown HK, Ray JG, Wilton AS, Lunsky Y, Gomes T, Vigod SN, Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children, JAMA. 317 (2017) 1544 10.1001/jama.2017.3415. [DOI] [PubMed] [Google Scholar]
- [187].Oberlander TF, Gingrich JA, Ansorge MS, Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence, Clin. Pharmacol. Ther 86 (2009) 672–7. 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Glover ME, Clinton SM, Of rodents and humans: A comparative review of the neurobehavioral effects of early life SSRI exposure in preclinical and clinical research, Int. J. Dev. Neurosci 51 (2016) 50–72. 10.1016/j.ijdevneu.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Kobayashi T, Matsuyama T, Takeuchi M, Ito S, Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: A systematic review and meta-analysis, Reprod. Toxicol 65 (2016) 170–178. 10.1016/j.reprotox.2016.07.016. [DOI] [PubMed] [Google Scholar]
- [190].Zheng J, Xu D-F, Li K, Wang H-T, Shen P-C, Lin M, Cao X-H, Wang R Neonatal exposure to fluoxetine and fluvoxamine alteres spine density in mouse hippocampal CA1 pyramidal neurons, Int. J. Clin. Exp. Pathol 4 (2011) 162–8. [PMC free article] [PubMed] [Google Scholar]
- [191].Rubin RD, Watson PD, Duff MC, Cohen NJ, The role of the hippocampus in flexible cognition and social behavior, Front. Hum. Neurosci 8 (2014) 742 10.3389/fnhum.2014.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Heikkinen T, Ekblad U, Palo P, Laine K, Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation, Clin. Pharmacol. Ther 73 (2003) 330–7. [DOI] [PubMed] [Google Scholar]
- [193].Pohland RC, Byrd TK, Hamilton M, Koons JR, Placental transfer and fetal distribution of fluoxetine in the rat, Toxicol. Appl. Pharmacol 98 (1989) 198–205. [DOI] [PubMed] [Google Scholar]
- [194].Rampono J, Proud S, Hackett LP, Kristensen JH, Ilett KF, A pilot study of newer antidepressant concentrations in cord and maternal serum and possible effects in the neonate, Int. J. Neuropsychopharmacol 7 (2004) 329–34. 10.1017/S1461145704004286. [DOI] [PubMed] [Google Scholar]
- [195].Kristensen JH, Ilett KF, Hackett LP, Yapp P, Paech M, Begg EJ, Distribution and excretion of fluoxetine and norfluoxetine in human milk, Br. J. Clin. Pharmacol 48 (1999) 521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Begg EJ, Atkinson HC, Duflull SB, Prospective evaluation of a model for the prediction of milk:plasma drug concentrations from physicochemical characteristics, Br. J. Clin. Pharmacol 33 (1992) 501–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Gentile S, Rossi A, Bellantuono C, SSRIs during breastfeeding: spotlight on milk-to-plasma ratio, Arch. Womens. Ment. Health. 10 (2007) 39–51. 10.1007/s00737-007-0173-0. [DOI] [PubMed] [Google Scholar]
- [198].Prozac, Package Insert, Eli Lilly & Co; Indianapolis, IN: (2009). [Google Scholar]
- [199].Kertesz SG, Pellagra in 2 homeless men, Mayo Clin. Proc 76 (2001) 315–8. 10.4065/76.3.315. [DOI] [PubMed] [Google Scholar]
- [200].Jagielska G, Tomaszewicz-Libudzic CE, Brzozowska A, Pellagra: a rare complication of anorexia nervosa, Eur. Child Adolesc. Psychiatry 16 (2007) 417–420. 10.1007/s00787-007-0613-4. [DOI] [PubMed] [Google Scholar]
- [201].Rosmaninho A, Sanches M, Fernandes IC, Pinto-Almeida T, Vilaça S, Oliveira A, Selores M, Letter: Pellagra as the initial presentation of Crohn disease, Dermatol. Online J 18 (2012) 12. [PubMed] [Google Scholar]
- [202].Hoshino Y, Yamamoto T, Kaneko M, Kumashiro H, Plasma free tryptophan concentration in autistic children, Brain Dev 8 (1986) 424–7. [DOI] [PubMed] [Google Scholar]
- [203].D’Eufemia P, Finocchiaro R, Celli M, Viozzi L, Monteleone D, Giardini O, Low serum tryptophan to large neutral amino acids ratio in idiopathic infantile autism, Biomed. Pharmacother 49 (1995) 288–92. 10.1016/0753-3322(96)82645-X. [DOI] [PubMed] [Google Scholar]
- [204].Naushad SM, Jain JMN, Prasad CK, Naik U, Akella RRD, Autistic children exhibit distinct plasma amino acid profile, Indian J. Biochem. Biophys 50 (2013) 474–8. [PubMed] [Google Scholar]
- [205].Kałużna-Czaplińska J, Jóźwik-Pruska J, Chirumbolo S, Bjørklund G, Tryptophan status in autism spectrum disorder and the influence of supplementation on its level, Metab. Brain Dis 32 (2017) 1585–1593. 10.1007/s11011-017-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Marí-Bauset S, Llopis-González A, Zazpe-García I, Marí-Sanchis A, Morales-Suárez-Varela M, Nutritional status of children with autism spectrum disorders (ASDs): A case–control study, J. Autism Dev. Disord 45 (2015) 203–212. 10.1007/s10803-014-2205-8. [DOI] [PubMed] [Google Scholar]
- [207].De Marie ML, Enesco HE, Influence of diet on plasma tryptophan and brain serotonin levels in mice, Experientia. 41 (1985) 48–50. [DOI] [PubMed] [Google Scholar]
- [208].Segall PE, Ooka H, Rose K, Timiras PS, Neural and endocrine development after chronic tryptophan deficiency in rats: I. Brain monoamine and pituitary responses, Mech. Ageing Dev 7 (1978) 1–17. [DOI] [PubMed] [Google Scholar]
- [209].Flores-Cruz GM, Escobar A, Reduction of serotonergic neurons in the dorsal raphe due to chronic prenatal administration of a tryptophan-free diet, Int. J. Dev. Neurosci 30 (2012) 63–7. 10.1016/j.ijdevneu.2012.01.002. [DOI] [PubMed] [Google Scholar]
- [210].Guo M, Zhu J, Yang T, Lai X, Liu X, Liu J, Chen J, Li T, Vitamin A improves the symptoms of autism spectrum disorders and decreases 5-hydroxytryptamine (5-HT): A pilot study, Brain Res. Bull 137 (2017) 35–40. 10.1016/j.brainresbull.2017.11.001. [DOI] [PubMed] [Google Scholar]
- [211].Wurtman RJ, Hefti F, Melamed E, Precursor control of neurotransmitter synthesis, Pharmacol. Rev 32 (1980) 315–35. [PubMed] [Google Scholar]
- [212].Shabbir F, Patel A, Mattison C, Bose S, Krishnamohan R, Sweeney E, Sandhu S, Net W, Rais A, Sandhu R, Ngu N, Sharma S, Effect of diet on serotonergic neurotransmission in depression, Neurochem. Int 62 (2013) 324–329. 10.1016/j.neuint.2012.12.014. [DOI] [PubMed] [Google Scholar]
- [213].Oketch-Rabah HA, Roe AL, Gurley BJ, Griffiths JC, Giancaspro GI, The importance of quality specifications in safety assessments of amino acids: the cases of L-tryptophan and L-citrulline, J. Nutr 146 (2016) 2643S–2651S. 10.3945/jn.115.227280. [DOI] [PubMed] [Google Scholar]
- [214].Tsuji A, Nakata C, Sano M, Fukuwatari T, Shibata K, L-tryptophan metabolism in pregnant mice fed a high L-tryptophan diet and the effect on maternal, placental, and fetal growth, Int. J. Tryptophan Res 6 (2013) IJTR.S12715 10.4137/UTR.S12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Bonnin A, Levitt P, Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain, Neuroscience. 197 (2011) 1–7. 10.1016/j.neuroscience.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Page DT, Kuti OJ, Prestia C, Sur M, Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior, Proc. Natl. Acad. Sci 106 (2009) 1989–1994. 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Murphy DL, Lesch K-P, Targeting the murine serotonin transporter: insights into human neurobiology, Nat. Rev. Neurosci 9 (2008) 85–96. 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- [218].Kudo Y, Boyd CA, Characterisation of L-tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles, J. Physiol 531 (2001) 405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Gaccioli F, Aye ILMH, Roos S, Lager S, Ramirez VI, Kanai Y, Powell TL, Jansson T, Expression and functional characterisation of System L amino acid transporters in the human term placenta, Reprod. Biol. Endocrinol 13 (2015) 57 10.1186/s12958-015-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Song I-S, Lee DY, Shin M-H, Kim H, Ahn YG, Park I, Kim KH, Kind T, Shin J-G, Fiehn O, Liu K-H, Pharmacogenetics meets metabolomics: discovery of tryptophan as a new endogenous OCT2 substrate related to metformin disposition, PLoS One. 7 (2012) e36637 10.1371/journal.pone.0036637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Grover B, Buckley D, Buckley AR, Cacini W, Reduced expression of organic cation transporters rOCT1 and rOCT2 in experimental diabetes, J. Pharmacol. Exp. Ther 308 (2004) 949–56. 10.1124/jpet.103.058388. [DOI] [PubMed] [Google Scholar]
- [222].Jang E-H, Kim H-K, Park C-S, Kang J-H, Increased expression of hepatic organic cation transporter 1 and hepatic distribution of metformin in high-fat diet-induced obese mice, Drug Metab. Pharmacokinet 25 (2010) 392–7. [DOI] [PubMed] [Google Scholar]
- [223].Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, Buchanan TA, Coleman KJ, Getahun D, Association of maternal diabetes with autism in offspring, JAMA. 313 (2015) 1425 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- [224].Wan H, Zhang C, Li H, Luan S, Liu C, Association of maternal diabetes with autism spectrum disorders in offspring, Medicine (Baltimore). 97 (2018) e9438 10.1097/MD.0000000000009438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Huether G, Thömke F, Adler L, Administration of tryptophan-enriched diets to pregnant rats retards the development of the serotonergic system in their offspring, Brain Res. Dev. Brain Res 68 (1992) 175–81. [DOI] [PubMed] [Google Scholar]
- [226].Zhang L, Guadarrama L, Corona-Morales AA, Vega-Gonzalez A, Rocha L, Escobar A, Rats subjected to extended L-tryptophan restriction during early postnatal stage exhibit anxious-depressive features and structural changes, J. Neuropathol. Exp. Neurol 65 (2006) 562–70. [DOI] [PubMed] [Google Scholar]
- [227].Zoratto F, Berry A, Anzidei F, Fiore M, Alieva E, Laviola G, Macrì S, Effects of maternal L-tryptophan depletion and corticosterone administration on neurobehavioral adjustments in mouse dams and their adolescent and adult daughters, Prog. Neuropsychopharmacol. Biol. Psychiatry. 35 (2011) 1479–92. 10.1016/j.pnpbp.2011.02.016. [DOI] [PubMed] [Google Scholar]
- [228].Carretti N, Bertazzo A, Comai S, Costa CVL, Allegri G, Petraglia F, Serum tryptophan and 5-hydroxytryptophan at birth and during post-partum days, Adv. Exp. Med. Biol 527 (2003) 757–60. [DOI] [PubMed] [Google Scholar]
- [229].Delgado PL, Chamey DS, Price LH, Landis H, Heninger GR, Neuroendocrine and behavioral effects of dietary tryptophan restriction in healthy subjects, Life Sci 45 (1989) 2323–32. [DOI] [PubMed] [Google Scholar]
- [230].Tricklebank MD, Pickard FJ, de Souza SW, Free and bound tryptophan in human plasma during the perinatal period, Acta Paediatr. Scand 68 (1979) 199–204. [DOI] [PubMed] [Google Scholar]
- [231].Hernández J, Manjarréz GG, Chagoya G, Newborn humans and rats malnourished in utero: free plasma L-tryptophan, neutral amino acids and brain serotonin synthesis, Brain Res 488 (1989) 1–13. [DOI] [PubMed] [Google Scholar]
- [232].Bano S, Gitay M, Ara I, Badawy A, Acute effects of serotonergic antidepressants on tryptophan metabolism and corticosterone levels in rats, Pak. J. Pharm. Sci 23 (2010) 266–72. [PubMed] [Google Scholar]
- [233].Badawy AA-B, Tryptophan: the key to boosting brain serotonin synthesis in depressive illness, J. Psychopharmacol 27 (2013) 878–93. 10.1177/0269881113499209. [DOI] [PubMed] [Google Scholar]
- [234].Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K, Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice, J. Neurosci 29 (2009) 2259–71. 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Pagan C, Delorme R, Callebert J, Goubran-Botros H, Amsellem F, Drouot X, Boudebesse C, Le Dudal K, Ngo-Nguyen N, Laouamri H, Gillberg C, Leboyer M, Bourgeron T, Launay J-M, The serotonin-N-acetylserotonin–melatonin pathway as a biomarker for autism spectrum disorders, Transl. Psychiatry. 4 (2014) e479–e479. 10.1038/tp.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]