Abstract
BACKGROUND:
Do men have worse health than women? This question is addressed by examining sex differences in mortality and the health dimensions of the morbidity process that characterize health change with age. We also discuss health differences across historical time and between countries.
CONTENT:
Results from national-level surveys and data systems are used to identify male/female differences in mortality rates, prevalence of diseases, physical functioning, and indicators of physiological status. Male/female differences in health outcomes depend on epidemiological and social circumstances and behaviors, and many are not consistent across historical time and between countries. In all countries, male life expectancy is now lower than female life expectancy, but this was not true in the past. In most countries, women have more problems performing instrumental activities of daily living, and men do better in measured performance of functioning. Men tend to have more cardiovascular diseases; women, more inflammatory-related diseases. Sex differences in major cardiovascular risk factors vary between countries—men tend to have more hypertension; women, more raised lipids. Indicators of physiological dysregulation indicate greater inflammatory activity for women and generally higher cardiovascular risk for men, although women have higher or similar cardiovascular risk in some markers depending on the historical time and country.
SUMMARY:
In some aspects of health, men do worse; in others, women do worse. The lack of consistency across historical times and between countries in sex differences in health points to the complexity and the substantial challenges in extrapolating future trends in sex differences.
Common generalizations are that men live shorter but healthier lives and that women live longer lives but in worse health (1). Such generalizations are an oversimplification, and sex differences in health cannot be described so succinctly (2). Here we demonstrate the complexity of male/female health differences by examining differences in both mortality rates and multiple dimensions of morbidity at older ages. We also demonstrate the complexity of sex differences in health at older ages by showing that sex differences vary across dimensions of health, historical time, and between countries.
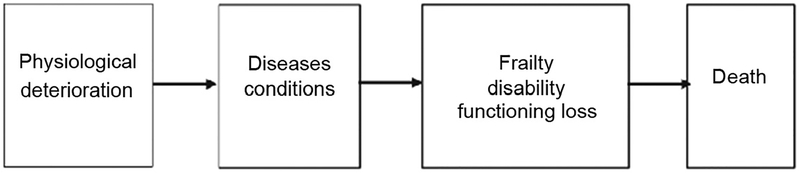
Our analysis of sex differences in health used the dimensions of the morbidity process (Fig. 1), which categorized multiple health indicators according to the process of health change with age at the population level (3). At the population level, health change with age is initiated by physiological dysregulation such as increased blood pressure and increasing concentrations of total cholesterol; these changes are followed by increases in the diagnoses of diseases and conditions, both physical and mental, which are then followed by increases in disability and loss of physical functioning and finally death. For individuals, the ordering of the process may differ; they may not experience some of the dimensions, and they may also experience reversals in the process. Dimensioning the nonmortality aspects of health change is important both to understanding the aspects of health in which men and women do better and worse, and to understanding how sex differentials in mortality rates arise. Although changes in the dimensions of the process are related, previous work has shown that population differentials and changes over time in these dimensions of morbidity are not necessarily similar (4). Our goal here was to investigate how these dimensions of health differ for men and women and whether the sex differences were similar across historical time and between countries. We did not expect the differences between men and women to be the same across dimensions of health, nor did we expect the differences to be the same over historical time and between countries in the world. Our aim was to show that, although generalizations can be made about sex differences in health and mortality rates, most differences cannot be described with a simple statement that describes differences at all historical times and in all countries. Understanding where in the process of health change men and women differ by (a) comprehensively examining historical trends in life expectancy and sex differences in prevalence of disease and physical functioning, and (b) incorporating recent evidence on biomarkers and other physiological status, adds substantially to knowledge of the sources and pathways to sex differences in health.
Fig. 1. Dimensions of the morbidity process.
Dimensions indicate time patterns of aspects of change in health with age at the population level. Reproduced with permission from Crimmins et al. (3).
There is agreement on the broad causes of sex differences in health and mortality. Some are biological, innate, or related to sex differences in genetics and hormones. The fact that women have 2 X chromosomes may provide advantageous redundancy because women have a second X to compensate for a mutation, whereas men do not. Asymmetric maternal inheritance of mitochondria may benefit women by providing deleterious mutations to men, causing a strong sexual dimorphism in aging and disadvantage in survival among men. Female hormones may provide protection against some conditions; in addition, women may have more responsive immune functioning, and women’s abilities to maintain homeostasis and reduce oxidative stress may differ from men’s (5–10). Other factors are behavioral, such as men being more likely to engage in risky and dangerous behavior and women more likely to engage in health-seeking behavior (11). Macro factors also affect how basic biological and behavioral factors influence health outcomes. In the past, infectious disease was important in determining life expectancy even beyond childhood. However, in today’s world, cardiovascular disease has become more important, and the importance of biological differences between men and women may have changed with the changing importance of diseases (12). The economic structure also influences health of men and women because of differences in occupations, economic well-being, and familial responsibilities and involvement, all of which can have long-term health consequences. Thus, there is reason to suspect that male and female health differences will likely vary across historical time and between countries and by type of health outcome investigated. We use the terms sex and sex differences instead of gender and gender differences throughout this review for consistency, although sex differences discussed here include differences resulting from both biological and social characteristics of men and women.
Our approach is to discuss each dimension of the morbidity process in turn, beginning with life expectancy and ending with physiological differences. We analyzed data from many sources, all from nationally representative sources, which was necessary for making population-level generalizations. Although reliable mortality data have long been available from national and international agencies for most countries, data on dimensions of health that are nationally representative have been lacking. In the past 2 decades, many countries have undertaken national-level surveys of their middle-aged and older populations, which have included data on multiple dimensions of morbidity for large samples of both sexes (13). For our discussion of sex differences in health, we use individual-level survey data on the older population from China [China Health and Retirement Longitudinal Study (CHARLS)], Korea [Korean Longitudinal Study of Aging (KLoSA)], India and Russia [World Health Organization Study on Global AGEing and Adult Health (WHO SAGE)], several European countries [Survey of Health, Ageing and Retirement in Europe (SHARE)], the US [Health and Retirement Study (HRS)], England [English Longitudinal Study of Ageing (ELSA)], Indonesia [Indonesian Family Life Survey (IFLS)], Taiwan [Social Environment and Biomarkers of Aging Study (SEBAS)], and Mexico [Mexican Health and Aging Study (MHAS)]. Many of these data sets are available in the Gateway to Global Aging Data (14). We used individual-level data obtained from each study. Each of these studies is a large nationally representative sample of older persons ≥50 years of age. Data are similar across the countries because many of them have been harmonized for cross-country comparison. We compared men and women on the morbidity dimensions in these countries. From the WHO database on risk factors, we used national-level data on cardiovascular disease risk factors. We supplemented this with some data on risk factors that reflected basic mechanisms of aging from the US HRS. We also examined life expectancy for men and women from 198 countries from the World Bank database.
Male/Female Differences in Life Expectancy/Mortality
In most countries, mortality rates have been decreasing steadily for both men and women for more than a century. Male life expectancy is lower than female life expectancy in all countries (15, 16). Although the idea that men’s mortality rates exceed those of women has been routinely observed in recent decades, differences between male and female mortality rates changed considerably during the 20th century (12). The ratio of male mortality rates relative to female rates from middle age onward increased markedly during much of the century in most developed countries for which there are reliable data spanning a long period. Beltrán-Sánchez et al. examined about 1600 birth cohorts of men and women born from 1800 to 1935 in 13 countries and found that male and female mortality rates from 45 to 90 years of age were roughly at parity for cohorts born up to 1880; after this time, the male mortality rate relative to that of women rose among those above age 45 years so that the mortality rate became twice as high for men in old age in the latter part of the 20th century (12). This long-term divergence in male/female mortality rates resulted from men’s greater vulnerability to cardiovascular disease and differential uptake of smoking.
Whereas this finding highlighted the importance of behavioral changes in explaining time differences in male/female mortality ratios, another study suggested the observed change in infancy could not be because of behavior differences (17). This study analyzed the sex ratio of infant mortality in 15 countries during the period from 1751 to 2004 and reported marked changes in the sex ratio of mortality among infants, an age when the effect of behavioral differences should be minimal. As infant mortality rates declined over 2 centuries, the excess of male infant mortality rates increased from only 10% in 1751 to >30% by approximately 1970; since 1970, the male disadvantage in most countries fell back to lower levels. These changes have been attributed to both the changing importance of infectious disease on male and female infants and improvement in obstetrical and neonatal care.
In recent years, some countries have seen a decrease in the female advantage in life expectancy. In the US, the changing differential between men and women has also been related to differential change by socioeconomic status, with particularly poor performance among women of lower socioeconomic status (18). The difference in life expectancy at birth between white men and women declined from 7.4 years longer lives for women in 1980 to 4.7 years in 2013. For African American men and women, the life expectancy difference decreased from 8.7 to 6.1 years during the same period (19). Trovato and Lalu attributed similar convergence of male and female mortality rates from 38 countries over 20 years to a nation’s level of social and economic development (20). On the other hand, some studies have highlighted the importance of shift in health behaviors, such as the effect of smoking behaviors among women on the patterns of male/female mortality differentials between countries and over time (21). Analysis focusing on the US has also demonstrated the importance of an increasing similarity in smoking between men and women in causing the recent convergence of life expectancy by sex (22).
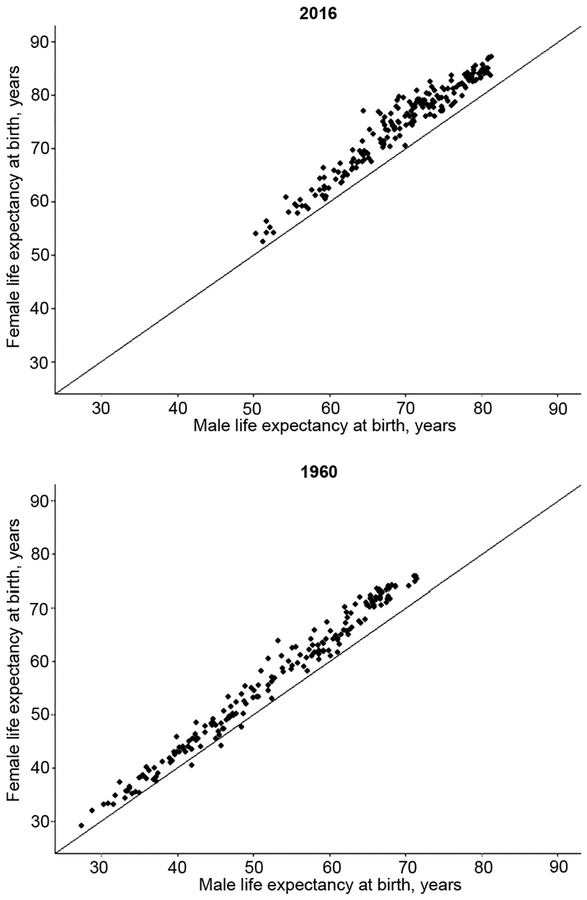
The relationship between male and female mortality rates clearly depends on the epidemiological circumstances and behavioral differences. When mortality is more heavily weighted by infectious conditions, male/female mortality rates are generally more similar, and there have been historical times and geographic places where male life expectancy exceeded that of women (14, 23). Fig. 2 shows data for male and female life expectancy for 198 countries in 2016 and 1960 to illustrate the differences found in male and female life expectancy during this nearly 60-year period. Female life expectancy exceeds that of men in every country in 2016 as indicated by the fact that every point is above the line of equality (the top graph in Fig. 2); the mean difference over 198 countries is 4.85 years. The deficit in male life expectancy differs widely between countries. The sex gap in life expectancy is particularly large in Eastern Europe: 10.5 years in Kazakhstan, 8.44 in the Kyrgyz Republic, 8.12 in the Russian Federation, and 8.0 in Estonia. On the other hand, the difference is small in several Asian countries: 0.22 in the Maldives, 0.26 in Nepal, and 0.37 in Pakistan. In 1960, life expectancy was lower in all countries, and the average difference between men and women across countries was 3.76 years. Female life expectancy was lower than that of men in 3 countries (i.e., India, Iran, and Iraq) in 1960. The comparison of life expectancy in 1960 and 2016 for many countries indicates that with the overall increase in life expectancy, the sex difference has gotten larger, on average, and the survival disadvantage of men has increased. It is true, however, that reductions in the male and female difference in life expectancy have been seen in several individual countries recently, including the US.
Fig. 2. Male and female life expectancy in 1960 and 2016 (198 countries).
Each dot represents male and female life expectancy in an individual country; the line indicates equal life expectancy. Source of data: The World Bank Life Expectancy at Birth, Male and Female (available from https://data.worldbank.org/indicator/SP.DYN.LE00.IN).
The differences between countries and the changes over time clearly point to the fact that the difference between male/female mortality rates is highly contingent on the circumstances in which people live and mortality-related epidemiological conditions such as disease dominance, public health infrastructure, and healthcare resources. In the period when the parity in male/female mortality rates at mature ages shifted to men having mortality rates twice as high, chronic conditions—particularly cardiovascular conditions and cancers—supplanted infectious diseases as major causes of death. However, the change in the relative level of mortality rates for men and women does not merely reflect epidemiological changes in the distribution of cause of death over time, but also differential changes in behaviors and exposures to risk for men and women (24).
Male/Female Differences in Disability and Functioning Loss
Examinations of sex differences in disability and physical functioning ability generally show that men have better physical functioning and report less disability. The finding that women have more functional limitations than men is almost as universal as the finding that men have higher mortality rates. Because so many studies of health and aging rely on physical functioning and disability measures to indicate health, this is the source of the statements about men having better health and higher mortality rates.
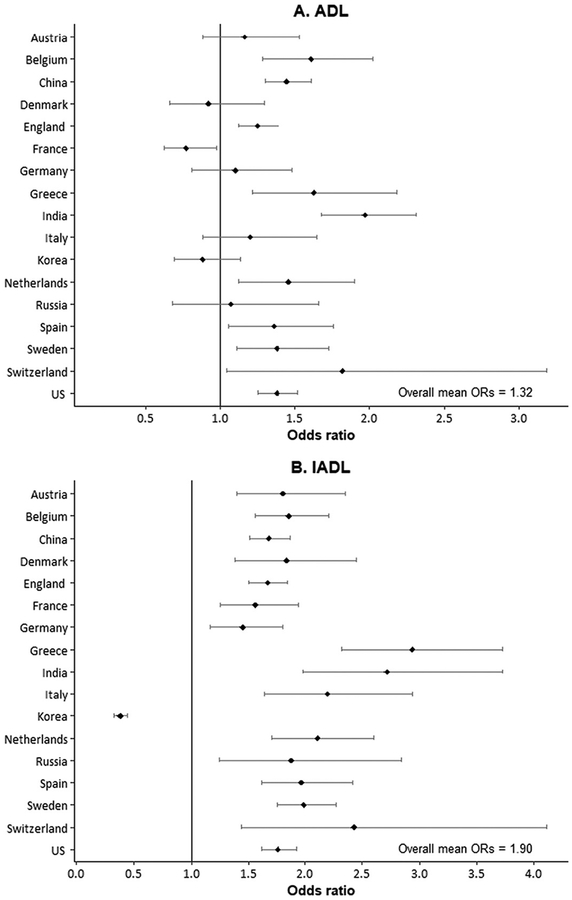
Disability and problems with physical functioning among mature populations in national population surveys are typically measured using self-reports of ability to perform tasks needed for self-care and independent living, which have been called activities of daily living (ADLs) and instrumental activities of daily living (IADLs). Crimmins et al. examined measures of ability to perform ADL and IADL functioning in 13 countries (Austria, Belgium, Denmark, England, France, Germany, Greece, Italy, the Netherlands, Spain, Sweden, Switzerland, and the US) (25). Sex differences in difficulty performing ADL and IADL activities for these countries, along with additional data for China, Korea, India, and Russia, are shown in Fig. 3, A and B. The mean odds ratio for the effect of being a woman controlled for age was 1.9 for IADL problems, indicating that the likelihood of having difficulties in carrying out daily activities and functioning problems was about 2-fold higher for women around the world. For all but 1 of the 34 country comparisons by sex, women have worse IADL functioning; only in Korea was IADL difficulty more prevalent among men. On the other hand, ADL functioning difficulties, which reflect ability to bathe, dress, eat, toilet, get in and out of a bed, and walk across a room, are not as differential by sex. The mean odds ratio is 1.3, and women are likely to have significantly more difficulty in only 10 of the 17 countries; men report more ADL difficulty in France.
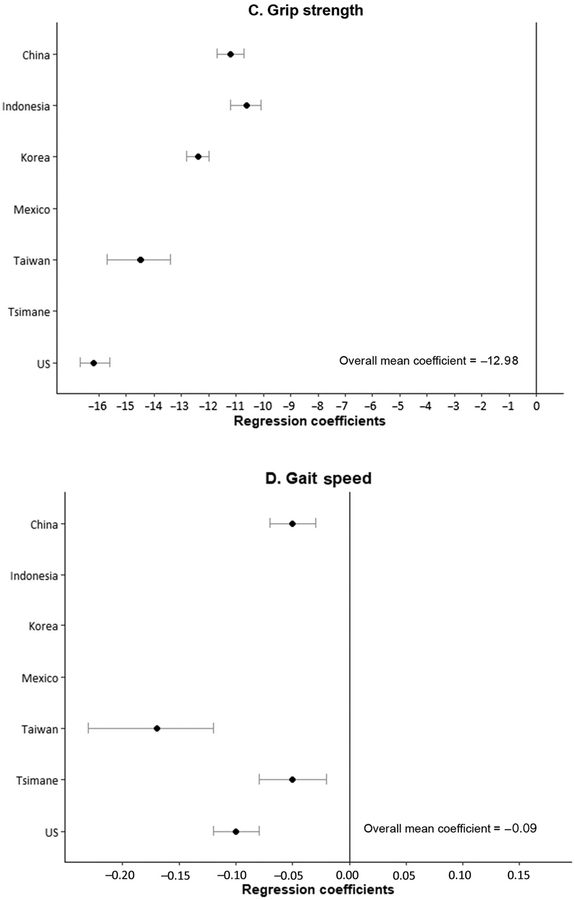
Fig. 3. Odds ratios and regression coefficients of effect of being a woman on functioning difficulties and performance.
(A and B), odds ratios (ORs) indicating effect of being a woman on self-reports of functioning difficulties: ADL and IADL (population age, ≥50 years). (C and D), regression coefficients indicating effect of being a woman on measured functioning performance (population age, ≥50 years): grip strength and gait speed.
ADL is the ability to bathe, dress, eat, toilet, get in and out of a bed, and to walk across a room; IADL is the ability to make telephone calls, take medications, manage money, prepare a hot meal, shop for groceries, and use a map to figure out how to get around in a strange place.
IADLs also include doing work around the house or garden in SHARE and community activities and concentration for 10 minutes instead of medication and managing money in SAGE. Grip strength, average of 2 or 3 trials in kilograms; gait speed, timed walk in seconds over 2 trials. Vertical line represents equality of men and women. Source of data (A and B): Odds ratios from logistic regressions of age on ADL and IADL; China, CHARLS (2011); Korea, KLoSA (2010); India and Russia, WHO SAGE (2007–2010); SHARE (2004), HRS (2004), and ELSA (2004) from Crimmins et al. (25); (C and D): Coefficients from Wheaton and Crimmins (26).
Researchers have questioned whether the differential functioning of men and women reflects reporting differences. Crimmins et al. found that when controls for the presences of diseases were included in the analysis, the sex differences disappeared, suggesting that the worse functioning of women was explained by having more conditions that affect functioning rather than differential reporting (25). To address potential bias from self-reports, Wheaton and Crimmins examined sex differences in performance measures of functioning, including gait speed, grip strength, and indicators of balance (tandem stand) and mobility (chair stand) for a limited number of countries (26). Participants were asked to perform these physical tests, and the results were recorded by the interviewer to provide an objective measure of functioning. Data derived from this analysis showed sex differences in gait speed and grip strength (Fig. 3, C and D). Women have lower grip strength (mean, 12.62 kg) and slower gait speed (0.07 s slower). Results for chair stands and tandem stand (not shown) indicated men were more mobile and had better balance. Although the differences between countries in the performance of men and women are variable, we can conclude that men are stronger and faster. It is likely that these abilities influence ability to perform the disability and functioning measures shown in Fig. 3, A and B.
These results lead to the conclusion that there is a great similarity in the magnitude and direction of sex differences in functioning and disability between countries despite the considerable differences in context. Women perform worse on IADL tasks and in many countries on ADL tasks. Clearly, men have better physical performance, although the size of the differences varies between countries.
Male/Female Differences in the Prevalence of Diseases and Conditions
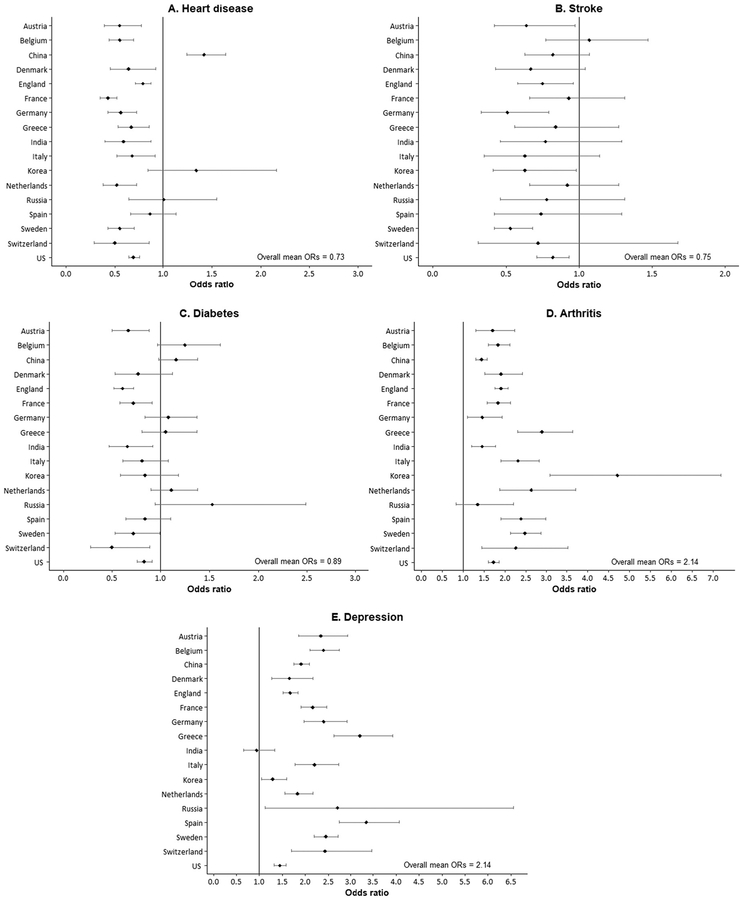
Turning to diseases and conditions, the differences between men and women are not as consistent. We examined differences by sex in major chronic conditions among people ≥50 years of age in 17 countries. The observed conditions include heart disease, stroke, diabetes, arthritis, and depression. Men are more likely to have heart disease, stroke, and diabetes, whereas women are more likely to have arthritis and depression, and there is considerable variation between countries in the differences between the sexes. Men are significantly more likely to report having heart disease in most countries; however, in Spain, Russia, and Korea, the differences between the sexes are not significant, and in China, women are more likely than men to have heart disease (Fig. 4). The mean difference between all countries is relatively large—women are about 27% less likely than men to have heart disease in the countries examined. Men everywhere but Belgium are more likely to report that they have had a stroke, but the sex differences are significant in only 6 of the 17 countries examined. Sex differences for the prevalence of diabetes are not significant in 11 countries, but in the 6 countries where they are significant, men are more likely to have diabetes.
Fig. 4. Odds ratios indicating effect of being a woman on presence of disease or condition (population age, ≥50 years).
Odds ratios from logistic regressions of sex and age on the presence of condition; vertical line indicates equality for men and women. Source of data: China, CHARLS (2011); Korea, KLoSA (2010); India and Russia, WHO SAGE (2007–2010); SHARE (2004), HRS (2004), and ELSA (2004) from Crimmins et al. (25).
Women are significantly more likely to have arthritis in all the countries shown except for Russia. Among these countries, the mean odds ratio is 2.14. Women are also more likely to have depressive symptoms in every country shown except India, and the mean odds ratio is again 2.14. Analysis of sex differences in depressive symptoms in a study of Japan, Denmark, and the US has shown women having higher levels of depression than men overall, although the age trajectories vary between countries (27). The comparison in Fig. 4 indicates that sex differences in prevalence of depression in the US are not as great as those found in most of the other 16 countries shown.
Although we considered only a few diseases and conditions, we found that men were generally more likely to have the lethal conditions, such as heart disease, stroke, and diabetes. Women were more likely to have debilitating, but seldom fatal, conditions, including arthritis and depression. This difference in the links between these diseases and the other dimensions of health is 1 reason that mortality differences and health differences do not necessarily coincide. Many chronic conditions are not strongly linked to mortality (e.g., arthritis and Alzheimer disease) but are strongly linked to disability and loss of functioning. Researchers have also argued that it is not just that men and women differ in the conditions they have, but they also differ in the outcomes associated with those conditions and that men may be more vulnerable to adverse effects on mortality of some of these lethal conditions and women may have stronger associations with disability (28, 29).
Male/Female Differences in Physiological Status
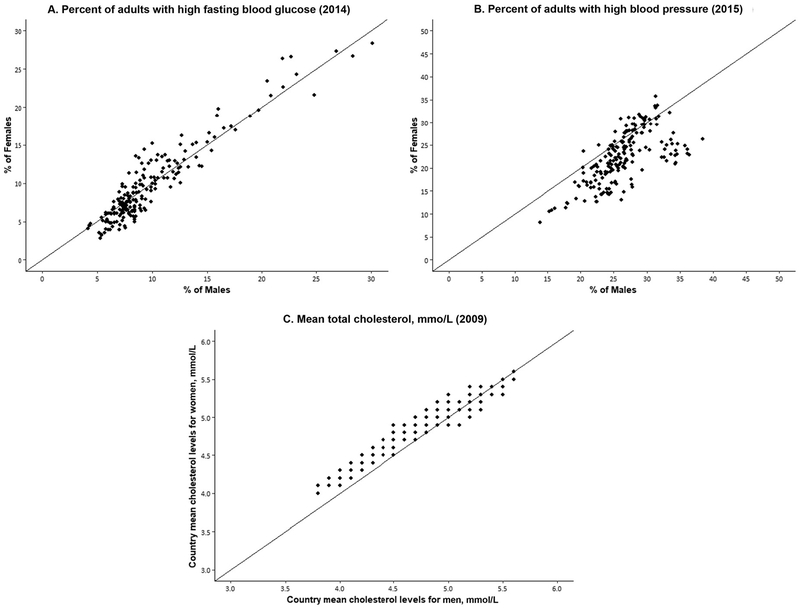
Indicators of physiological status include known risk factors for developing some of the above chronic conditions, so examining differentials by sex may help to clarify the mechanisms behind differentials in downstream dimensions of health and mortality. Here we present data for men and women from WHO on the measured prevalence of risk levels for glucose, hypertension, and cholesterol at the current time for approximately 190 countries. Comparing men and women within countries, national levels of high glucose are just about as likely to be higher for men as for women (Fig. 5). The level of hypertension for men exceeds that for women in most countries, but there are several countries where the prevalence of hypertension is higher for women. For high concentrations of total cholesterol, the patterns are reversed, with women having a higher prevalence of risk-level total cholesterol in most countries, yet in some countries men are higher.
Fig. 5. Percentage of men and women with high-risk levels of fasting glucose and high blood pressure and mean total cholesterol in individual countries.
Note: Each dot represents the percentage or mean level for men and women in an individual country. The number of countries is 191 for blood glucose and blood pressure (age, ≥18 years) and 189 countries for cholesterol (age, ≥25 years). All numbers are age-standardized. Source of data: WHO Global Health Observatory Data Repository (available from http://apps.who.int/gho/data/node.main.A867?lang-en). To convert cholesterol concentrations in mmol/L to mg/dL, multiply by 38.67.
Focusing on the US, there is strong evidence that sex differences in overall risk for disease, based on a summary indicator composed of markers including those examined above, are not constant over time within 1 country. A recent study examined a summary indicator of cardio-metabolic risk including adverse levels of systolic blood pressure, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, and glycohemoglobin from 1990 to 2010 for men and women at ages ≥40 years using data from the National Health and Nutrition Examination Survey (NHANES) (30), and showed that men and women differed in levels of overall risk in 1990 and 2000, such that men had higher risk until older ages. However, by 2010, there were no sex differences in mean age-specific cardiovascular risk based on these indicators at ages >50 years (30). This represents a remarkable change from what we think of as the traditional difference between cardiovascular risk profiles in men and women, in which risk for men rises at earlier ages and at the oldest ages risk is similar for both sexes. The change for both sexes in overall cardiovascular risk in the US was caused by the reduction in the prevalence of high-risk lipid concentrations and some reduction in blood pressure driven by greater use of effective prescription medication (30).
For the US, recent data from a national sample allow us to examine current sex differences in additional indicators of physiological functioning that are related to some of the proposed mechanisms underlying sex differences in health change with age. We have 3 markers of inflammatory responses, 1 of the mechanisms thought to underlie both aging and male/female differences in health: high sensitivity C-reactive protein (CRP), which is a general marker of inflammation; cytomegalovirus (CMV) seroprevalence, which can indicate the ability of the immune system to control CMV virus in older persons; and the lymphocyte count, which could reflect acute inflammatory response (Table 1). Two of the 3 indicators are higher for women than men, perhaps indicating a stronger inflammatory challenge among women; however, there is no gender difference in CRP, the most general indicator of inflammatory burden.
Table 1.
Regression coefficients indicating effect of being a woman on concentration of biomarkers: US HRS, 2016, age ≥56 years.
| N | Mean (SD) | Female coefficients | P value | ||
|---|---|---|---|---|---|
| Markers of inflammation | CRP (high sensitivity), mg/L | 8872 | 4.73 (11.69) | 0.25 | 0.3248 |
| Lymphocyte count (×10e9) | 8651 | 1.96 (2.15) | 0.15 | 0.0016 | |
| CMV IgG (mg/dL) | 8872 | 303.99 (392.29) | 103.44 | <0.0001 | |
| Marker of heart function | NT–proBNP, pg/mL | 8725 | 332.59(1210.66) | −27.30 | 0.2837 |
Regression coefficients are from equations with controls for age, race/ethnicity, and obesity.
Source of data: Health and Retirement Study data. Venous blood collection and assay protocol in the 2016 Health and Retirement Study. http://hrsonline.isr.umich.edu/modules/meta/vbs/2016/desc/HRS2016VBSDD.pdf.
Table 1 also shows the effect of being a woman on an indicator related to cardiovascular functioning. B-type natriuretic peptide is an indicator of heart failure (NT-proBNP), which does not differ significantly between men and women >56 years of age in the US. This is somewhat surprising given the previous evidence on higher cardiovascular problems among men.
Because it is difficult to summarize the meaning of differences in many individual indicators of physiological functioning, several researchers have attempted to integrate multiple biomarkers from blood chemistries and examinations to construct summary indices, which have been called “allostatic load,” “biological age,” and “physiological dysregulation.” These summary indicators share a goal of attempting to quantify the aging process based on a set of biological parameters; however, they differ in their algorithms and the included biomarkers. Studies using these measures typically include age-related bio-markers that cross several domains including, but not limited to, cardiovascular, metabolic, and organ functioning. Yang and Kozloski examined differences among American men and women in indices of inflammation, metabolic syndrome, and a composite allostatic load measure including inflammation, metabolic syndrome, kidney, and lung function. Women had a worse inflammatory index and men had a worse metabolic index, with women having a worse overall index (31). The direction of the sex difference does not match that found by Levine and Crimmins, who included 8 markers of physiological status (glycohemoglobin, total cholesterol, systolic blood pressure, forced expiratory volume, serum creatinine, serum alkaline phosphatase, serum albumin, and CRP) in an index of biological age for men and women ≥20 years of age in the US in 1988 to 1994 and 2007 to 2010. Women had significantly lower biological ages than men in each age-group at each time, meaning women had a younger or better biological profile at a given age at both times (32). Over time, there was a reduction in biological age for both men and women and some narrowing of the sex gap. These somewhat conflicting results occur both because of the inclusion of different measures and the use of different methods, and because the differences between men and women are complex and inconsistent across markers.
Using summary indicators of physiological dysregulation like those described above, researchers have also examined change of sex difference over time or change with aging among individuals. They have also examined how mortality risk is associated with these summary measures by sex; findings are somewhat inconsistent. Arbeev et al. used data on blood pressure, heart rate, cholesterol, glucose, hematocrit, body mass index, and mortality in the Framingham original cohort and found women becoming dysregulated more quickly but men having a stronger link between dysregulation and mortality risk (33). Cohen et al. examined the process of change for men and women in 5 physiological systems (oxygen transport, electrolytes, hematopoiesis, lipids, and liver/kidney function) in the US and Italy and found higher dysregulation levels in men overall and in the oxygen transport and hematopoietic systems, but no sex differences in rates of change in dysregulation or in the likelihood of a mortality outcome (34). The 4 studies reporting on summary indices of biological differences between men and women were done on data from roughly the same period and based on primarily US data. Nonetheless, the findings indicate that the data on physiology are complex, and that measures chosen and the dates of measurement will influence the outcomes observed. This is an area of substantial current research and is likely to be a research focus in the coming decade as the focus shifts to the underlying mechanism of sex differences in health.
Our findings on the complexity of sex differences across dimensions of health do not support the statement that women have worse health but longer life than men. Our findings support that the differences in health and mortality between men and women are complex and depend on the social, behavioral, and epidemiological context in which they are investigated (1, 28). The 20th century was a period of growing sex disparities in life expectancy, which appear to have peaked recently and reversed for some countries, including the US. Growing male disadvantage in life expectancy came from changes in disease importance and behaviors. As cardiovascular disease retreats in importance as a cause of death, as risk for cardiovascular disease is controlled and treated, and as men and women behave more similarly, sex differences in disease prevalence and mortality rates may recede. Although women’s more responsive inflammatory functioning may have been more functional in a highly infectious world with high fertility, it may be less advantageous in the current epidemiological and low fertility environment. Men’s cardiovascular weaknesses may be lessened with the control and management of cardiovascular risk and changes in behavior. In a world dominated by cardiovascular disease and cancer, the role of differential behavior may increase as an explanation for differences in disease prevalence.
The treatment for risk factors can change male/female differentials in physiological indicators, as has happened for cardiovascular risk in the US. This has implications for future sex differences in downstream outcomes including disease and mortality. Because treatment controls risk, the sex differences in some diseases may disappear. The differential level of functioning problems that occur among men and women may also represent a treatable condition for which sex differences could change with interventions to maintain functioning with aging. Sex differences in risk factors point to the biological interventions that might reduce male/female differences and improve health. Around the world, men are more in need of blood pressure treatment and women are more in need of lipid management.
Deterring the underlying process of aging for both men and women will require both better data and better understanding of how to characterize the innate process of aging. Although our investigation of physiological dysregulation was cursory, it clarified that one sex was not better or worse in all areas of physiological dysregulation. Summarizing male and female physiology to clarify best how to improve the aging experience for all remains a challenge.
We conclude that men live shorter lives than women at present. Women have more functioning problems now at least partly because they are not as strong, mobile, or steady as men. Currently, men have more lethal conditions, whereas women have more disabling chronic conditions. Men and women have somewhat different health problems; one sex cannot be characterized as having better health. Our strongest conclusion is that male/female differences in health are highly dependent on historical time and geographic location.
Acknowledgments
E.M. Crimmins provided financial support.
Research Funding: E.M. Crimmins, National Institute on Aging (grant P30-AG017265, T32-AG000037).
Nonstandard abbreviations:
- CHARLS
China Health and Retirement Longitudinal Study
- KLoSA
Korean Longitudinal Study of Aging
- WHO SAGE
World Health Organization Study on Global AGEing and Adult Health
- SHARE
Survey of Health
- HRS
Health and Retirement Study
- ELSA
English Longitudinal Study of Ageing
- IFLS
Indonesian Family Life Survey
- SEBAS
Social Environment and Biomarkers of Aging Study
- MHAS
Mexican Health and Aging Study
- ADL
activities of daily living
- IADL
instrumental activities of daily living
- NHANES
National Health and Nutrition Examination Survey
- CRP
C-reactive protein
- CMV
cytomegalovirus
- NT-proBNP
B-type natriuretic peptide
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
Patents: None declared.
References
- 1.Austad SN. Why women live longer than men: sex differences in longevity. Gend Med 2006;3:79–92. [DOI] [PubMed] [Google Scholar]
- 2.Macintyre S, Hunt K, Sweeting H. Gender differences in health: are things really as simple as they seem? Soc Sci Med 1996;42:617–24. [DOI] [PubMed] [Google Scholar]
- 3.Crimmins EM, Kim JK, Vasunilashorn S. Biodemography: new approaches to understanding trends and differences in population health and mortality. Demography 2010; 47:S41–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiers N, Jagger C, Clarke M. Physical function and perceived health: cohort differences and interrelationships in older people. J Gerontol B Psychol Sci Soc Sci 1996; 51:S233. [DOI] [PubMed] [Google Scholar]
- 5.Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab 2016;23:1022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff JN, Gemmell NJ. Mitochondria, maternal inheritance, and asymmetric fitness: why males die younger. Bioessays 2013;35:93–9. [DOI] [PubMed] [Google Scholar]
- 7.Frank SA, Hurst LD. Mitochondria and male disease. Nature 1996;383:224. [DOI] [PubMed] [Google Scholar]
- 8.Pérez-López FR, Larrad-Mur L, Kallen A, Chedraui P, Taylor HS. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod Sci 2010;17:511–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pomatto LC, Tower J, Davies KJ. Sexual dimorphism and aging differentially regulate adaptive homeostasis. J Gerontol A Biol Sci Med Sci 2017;73:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pomatto LC, Carney C, Shen B, Wong S, Halaszynski K, Salomon MP, et al. The mitochondrial Lon protease is required for age-specific and sex-specific adaptation to oxidative stress. Curr Biol 2017;27:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers RG, Everett BG, Saint Onge JM, Krueger PM. Social, behavioral, and biological factors, and sex differences in mortality. Demography 2010;47:555–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beltrán-Sánchez H, Finch CE, Crimmins EM. Twentieth century surge of excess adult male mortality. Proc Natl Acad Sci U S A 2015;112:8993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Research Council. Biosocial surveys. Washington (DC): National Academies Press; 2008. https://www.nap.edu/catalog/11939/biosocial-surveys (Accessed June 2018). [Google Scholar]
- 14.Gateway to Global Aging Data. Produced by the USC Program on Global Aging, Health & Policy, with funding from the National Institute on Aging (R01 AG030153), 2018. https://g2aging.org/ (Accessed June 2018).
- 15.Barford A, Dorling D, Smith GD, Shaw M. Life expectancy: women now on top everywhere: during 2006, even in the poorest countries, women can expect to outlive men. BMJ 2006;332:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leon DA. Trends in European life expectancy: a salutary view. Int J Epidemiol 2011;40:271–7. [DOI] [PubMed] [Google Scholar]
- 17.Drevenstedt GL, Crimmins EM, Vasunilashorn S, Finch CE. The rise and fall of excess male infant mortality. Proc Natl Acad Sci U S A 2008;105:5016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasson I Trends in life expectancy and lifespan variation by educational attainment: United States, 1990–2010. Demography 2016;53:269–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crimmins EM. Trends in mortality, disease, and physiological status in the older population In: National Academies of Sciences, Engineering, and Medicine Committee on Population, Hayward MD, Majmundar MK, editors. Future directions for the demography of aging proceedings of a workshop. Washington (DC): National Academies Press; 2018. [PubMed] [Google Scholar]
- 20.Trovato F, Lalu NM. Narrowing sex differentials in life expectancy in the industrialized world: early 1970s to early 1990s. Soc Biol 1996;43:20–37. [DOI] [PubMed] [Google Scholar]
- 21.Crimmins EM, Preston SH, Cohen B, editors. National Research Council, Committee on Population. Explaining divergent levels of longevity in high-income countries. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 22.Preston SH, Wang H. Sex mortality differences in the United States: the role of cohort smoking patterns. Demography 2006;43:631–46. [DOI] [PubMed] [Google Scholar]
- 23.Abubakar II, Tillmann T, Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet 2015;385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Kozloski M. Change of sex gaps in total and cause-specific mortality over the life span in the United States. Ann Epidemiol 2012;22:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crimmins EM, Kim JK, Solé-Auró A. Gender differences in health: Results from SHARE, ELSA and HRS. Eur J Public Health 2010;21:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheaton FV, Crimmins EM. Female disability disadvantage: a global perspective on sex differences in physical function and disability. Ageing Soc 2016; 36:1136–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oksuzyan A, Crimmins E, Saito Y, O’Rand A, Vaupel JW, Christensen K. Cross-national comparison of sex differences in health and mortality in Denmark, Japan and the US. Eur J Epidemiol 2010;25:471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Case A, Paxson C. Sex differences in morbidity and mortality. Demography 2005;42:189–214. [DOI] [PubMed] [Google Scholar]
- 29.Kingston A, Davies K, Collerton J, Robinson L, Duncan R, Bond J, et al. The contribution of diseases to the male-female disability-survival paradox in the very old: results from the Newcastle 85 study. PloS One 2014;9: e88016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JK, Ailshire JA, Crimmins EM. Twenty-year trends in cardiovascular risk among men and women in the United States. Aging Clin Exp Res 2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Kozloski M. Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci 2011;66:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine ME, Crimmins EM. Is 60 the new 50? Examining changes in biological age over the past two decades. Demography 2018;55:387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arbeev KG, Cohen AA, Arbeeva LS, Milot E, Stallard E, Kulminski AM, et al. Optimal versus realized trajectories of physiological dysregulation in aging and their relation to sex-specific mortality risk. Front Public Health 2016;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen AA, Legault V, Li Q, Fried LP, Ferrucci L. Men sustain higher dysregulation levels than women without becoming frail. J Gerontol A Biol Sci Med Sci 2017; 73:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]