Abstract
Introduction:
Visceral leishmaniasis (VL) is a fatal parasitic disease caused by parasite belonging to Leishmania donovani complex and transmitted by infected female Phlebotomous argentipes sand flies. VL elimination strategy in the Indian subcontinent, that has a current goal of reducing the incidence of VL to below 1/10,000 of population by the year 2020, consists of rapid detection and treatment of VL to reduce the number of human reservoirs, and vector control using indoor residual spraying (IRS). However, as the incidence of VL declines towards the elimination goal, greater targeting of control methods will be required to ensure appropriate early action to prevent the resurgence of VL.
Area Covered:
We discuss the current progress and challenges in VL elimination program, and strategies to be employed to ensure sustained elimination of VL.
Expert opinion:
VL elimination initiative has saved many human lives; however, for VL elimination to become a reality in a sustained way, an intense effort is needed, as substantial numbers of endemic sub-districts (PHC blocks level) are yet to reach the elimination target. In addition to effective epidemiological surveillance, appropriate diagnostic and treatment services for VL at primary health centers will be needed to ensure long term sustainability and prevent re-emergence of VL.
Keywords: Visceral leishmaniasis, Elimination, Surveillance, Resurgence
1. Introduction
Visceral leishmaniasis (VL, also known as kala-azar) is the most severe form of Leishmaniasis with fatal outcomes if untreated. In the Indian subcontinent (ISC), VL is caused by parasites belonging to Leishmania donovani complex , which is transmitted from human to human by sand fly Phlebotomous argentipes, without a known animal reservoirs[1].The clinical syndrome is characterized by fever, splenomegaly, weight loss, hepatomegaly and anemia. Though the disease is endemic in over 60 countries, 90% of all reported cases occur in just six countries: Bangladesh, Brazil, Ethiopia, India, Sudan and Sudan [2, 3]. The disease affects mainly poor rural communities; of all the cases reported from India, majority are from the state of Bihar [4]. VL is known to have occurred in the Indian subcontinent for centuries, but after extensive insecticide spraying in the 1950s by the National Malaria Eradication Programme, there was a dramatic decline in its incidence [5, 6]. However, resurgence was noted from early 1970s onwards from a small area of North Bihar, and in the next 10–15 years it spread to the entire state of Bihar, a few districts of the newly created state Jharkhand and of the adjoining state West Bengal, plus the Eastern districts of Uttar Pradesh [7]. Neighbouring countries Nepal and Bangladesh were also affected. As a result, a joint VL elimination initiative supported by the World Health Organization (WHO) was launched in 2005 by the governments of India, Bangladesh and Nepal. The target for elimination was the reduction of annual VL incidence below 1/10,000 people at upazilla level in Bngladesh, sub-districts [(block public health centre (PHC)] level in India and district level in Nepal by the year 2015; a deadline later reset to 2020 [8]. Elimination of VL was considered technically feasible and operationally achievable goal for the following reasons: i) Anthroponotic (confined to human only) nature of VL by L.donovani; ii) availablity of point of care rapid diagnosis and effective treatment with oral Miltefosine; iii) Transmission is very focused to well defined number of districts; iv) P.argentipes is the only vector which is susceptible to insecticide and; v) strong political commitment of the governments (7, 9). Therefore, elimination program comprises of strategic components which were decided to implement in four phases: 1. Preparatory phase (2 years) that began after the plan has been prepared and approved by the three countries and includes the preparations useful in identifying constraint and operational difficulties; 2. Attack phase (5 years) which includes implementation and monitoring to bring the reduction of VL burden to less than 1/10,000; 3. Consolidation phase (3 years) to maintain control the burden; and 4. Maintenance phase (3 years) that will be followed by certification of elimination status. Major adopted strategies in VL control program are: i) effective case management through early diagnosis along with complete treatment of VL cases; ii) integrated vector management; iii) effective disease surveillance; iv) social mobilization and behavioral changes; and v) operational research [9].
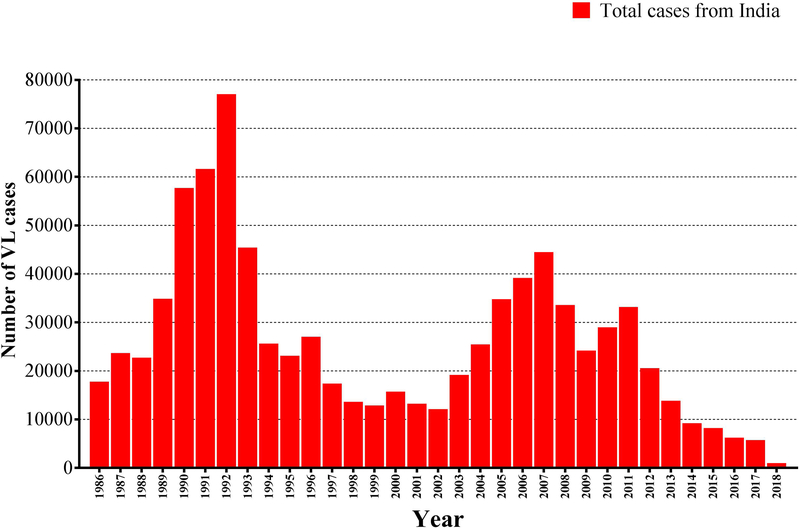
The number of new VL cases on the Indian subcontinent has decreased substantially since 2005 through the elimination efforts, though natural incidence cycles that rise and fall with a slow periodicity, a well known phenomenon in the history of Indian kala-azar, may also be playing a role (Figure-1). Nepal has reached and maintained the target for the last 3 years, Bangladesh has achieved it in over 90% of its endemic subdistricts and India in 70% of PHC blocks [5, 8]. The number of VL cases in India is declining steadily, from a peak of over 77000 reported cases in 1992 to below 6000 cases in 2017 followed by below 3000 cases in 2018 (till July 2018) (Figure-1). Similarly, in Bangladesh number of VL cases has also declined dramatically from 9,379 in 2006 to only 255 in 2016 and less than 200 cases in 2017. However, in Nepal, the number of VL cases has been continuously declining since 2003, with only 150 cases reported in 2016 [10].
Figure-1:
Trends of visceral leishmaniasis cases in India. (Source: adapted from National Vector-Borne Disease Control Programme, Directorate General of Health Services (DGHS), Ministry of Health and Family Welfare, New Delhi, Government of India.)
Importantly, with a decreasing number of active VL cases in the community, transmission may still take place, for instance from patients with Post-Kala-Azar Dermal Leishmaniasis (PKDL). Furthermore, HIV co-infected patients with their repeated clinical VL episodes have also been shown to be highly infectious [11, 12]. Mathematical modeling and observational evidences pointed out that asymptomatically infected person might also contribute to transmission, though their infectiousness is yet to be established [13]. Therefore, it is unlikely that achieving the low elimination target will result in cessation of disease transmission. In the present review, we discuss the various strategies that need to be adopted in control program in order to prevent resurgence of the VL epidemic.
2. Strategies for preventing resurgence
Successful strategies for preventing the re-emergence of VL in the context of the elimination program can be made by understanding the VL transmission dynamics followed by development of strategies to interrupt transmission.
2.1. Development of sensitive tools for early diagnosis and monitoring infections
The elimination initiative has so far not identified the tools to monitor its success, besides the elimination target that is expressed in clinical outcomes. There is no validated tool available to monitor leishmania infections[14]. Serological tests detecting L. donovani antibodies were designed to diagnose clinical VL, but they have also been used in many studies to identify sub-clinically infected individuals. Such individuals might be at increased risk of developing clinical VL and could play a role in the transmission of the disease. Still, we do not know if they are infectious, i.e., if they can infect sandflies, and this piece of evidence is crucially lacking to guide the VL elimination policy. From several studies, we know that a vast majority of healthy people with a positive leishmania serology will never progress to disease [15–17]. The serology for leishmaniasis includes immunofluorescence antibody test (IFAT), Enzyme linked immunosorbent assay (ELISA), Direct Agglutination test (DAT) and western blot assay. Various investigators have recommended ELISA based on different antigen preparations and DAT because of its rapidness. We established the value of DAT and the rK39-ELISA tests, as markers of L. donovani infection at population level. Healthy controls, drawn from the endemic regions, 6.2% and 5.9% tested positive on DAT and rK39 respectively, however agreement between tests was found weak (kappa=0.30) [18–20]. Most of these healthy controls were relatives of VL patients, often living in the same households, which may explain the very high percentage of sero-positivity in this group. In the absence of a gold standard test for measuring L.donovani infection, it is hard to know whether such seropositives subjects who remained healthy were genuinely infected with L. donovani or whether the serology results were just false positive results or prior infection that cleared [19].
Point of care diagnosis for VL has been made possible through the development of the rK39 rapid diagnostic test (RDT). This can be carried out in 20 minutes starting from a drop of blood, thereby reducing the delay in effective treatment with a single dose of intravenous AmBisome. In India, elimination initiatives has adopted rK39 rapid test and recommended in combination with a clinical case definition for diagnosis of VL[8, 14]. However, as countries move towards elimination target, proportion of VL patients among clinical suspects will decrease. In such situation the positive predictive value of the rK39 RDT will also decrease. Therefore, highly sensitive and specific diagnostic tests will be needed in future for accurate diagnosis and monitoring infections in the community. Molecular based diagnostic test have been shown highly sensitive and specific for diagnosis and to detect infection [14]. In India, Nepal, Bangladesh and Brazil, leishmania DNA was detected by polymerase chain reaction (PCR) in peripheral blood of sub-clinically infected patients[ reviewed in ref. 21] and Le Fichoux et al. cultured promastigotes of L. infantum from the buffy coat cells of 9 out of 76 asymptomatically infected blood donors in southern France[22]. In vitro T- lymphocyte tests (Modified QuantiFERON test ) based on leishmania antigen has been recently developed for leishmaniasis and might also be valuable for leishmaniasis[23].Validity of the diagnostic algorithm therefore needs to be monitored closely for the benefit of the patient who should not receive antileishmanial treatment inappropriately. For the VL elimination initiative in the Indian subcontinent, the infectiousness of such asymptomatically infected persons is a key question.
2.2. Improving treatment strategy to prevent drug resistance
Pentavalent antimonials (Sbv) have been the most common VL treatment for more than 70 years [24][25]. However, there is now considerable parasite resistance against these drugs, especially in India and neighboring country Nepal [26][27, 28]. Although these drugs are still employed to treat VL in Africa Middle East, central Asia and in most of Latin America; drugs such as amphotericin B, AmBisome (Liposomal formulation of Amphotericin B) Miltefosine and aminosidine (paromomycin) have been developed as alternative treatments against VL [26]. Furthermore, these drugs are far from ideal because of high cost, significant toxicity, development of drug resistance and prolonged duration of treatment [29]. Nowadays a single dose of AmBisome is sufficient to treat VL successfully, and it has now been recommended in elimination initiatives as a choice of treatment in India [30, 31]. Therefore, if implemented effectively in case management (including suspected case) at public health centre level, could eliminate this disease and parasite reservoirs from the most endemic parts of the countries. In endemic area, suspect case is defined as subject with fever history of more than 2 weeks and enlarged spleen and liver not responding to anti malaria. If found positive with rK39 rapid diagnostic test, such patients should be treated with AmBisome. However, there is a concern about emergence of drug resistance in future with monotherapy. Increasing drug resistance in endemic areas is a major problem in VL elimination and in maintenance phase after the goal of elimination is achieved. Further the pipeline for antileishmanial compounds is empty, thus there is a need for development and evaluation of new molecules. Importantly, there is no non-invasive tool to monitor the effectiveness of treatment in routine conditions. By providing knowledge and tools relevant for monitoring the effectiveness of the existing drugs, such tools will contribute to safeguard them and establish the bases for their longer-term and more rational use in future. Monitoring drug efficacy and early reporting are essential for course correction in the drug policy; this is even more important when the drug arsenal is limited as is the case in VL. This is very significant because now liposomal amphotericin B (AmBisome, Gilead Sciences, CO, US) is considered as first line drug for VL and this has significantly changed the way VL is managed [32]. This requires a standardized operating procedure to use it and a structure to implement it. For this, uninterrupted supply of quality drugs, promotion of the treatment adherence and the monitoring of treatment effectiveness and of drug resistance is pivotal. Knowledge on mechanisms of emergence of drug resistance, its dynamics and the impact of the introduction of new drugs is poor. Moreover, the region is confronted with an expanding HIV-epidemic, and we expect to see more HIV-VL co-infections which will generate major therapeutic challenges [33]. Thus, clinical and laboratory research is urgently needed to support the drug policy of the VL elimination program.
2.3. Strengthening VL surveillance program
Evaluating public health interventions in developing countries is one of the priorities of the WHO. However, assessing the impact of disease control strategies, and conducting epidemiological studies in areas where basic demographic, social and health indicators are not available, is challenging. VL surveillance system in India has greatly improved since VL elimination initiative was launched and underreporting of VL has diminished to a great extent [34]. However, as elimination is approaching, the proportion of vulnerable population will go up, and on the other hand political, social and clinical awareness will decline. Importantly, due to drastic decline in its transmission, diagnostic algorithm, currently used in primary healthcare setting, may become less accurate. Furthermore, human reservoirs such as PKDL, asymptomatic infected individuals and HIV-VL co-infections become important, and these may result in the resurgence of transmission. Therefore, a better surveillance system based on the absence of transmission rather than that of clinical VL needs to be developed. Without appropriate surveillance, which allows for timely response, the health gains of achieving elimination may be lost. It is thus important to develop an improved system for delivering primary health care in resource poor settings. The surveillance strategy should be in such that it is sustainable in the routine of the Indian PHC system. Importantly, beyond recording number of VL cases, population-based sero-surveillance can be a powerful tool to examine long-term trends in infection rates, and hence allows to examine the impact of the elimination initiative at population level.
2.4. Development of transmission dynamic modeling and spatial risk map
Mathematical models of disease transmission are useful tools to evaluate the feasibility of elimination [35]. Most models are though constrained by the paucity of data and the many pending knowledge gaps on critical biological parameters, such as duration of immunity, infectivity of distinct disease stages etc. There is a consensus amongst modelers about the need for xenodiagnostic (a diagnostic procedure in which the live insect vector is used for the detection of infection in a mammalian host) and longitudinal studies to enhance our understanding of asymptomatic infection and PKDL as reservoirs of infection. Robust mathematical models will enhance our understanding of the role of asymptomatic individuals in transmission and their progression to disease. It not only allows for impact evaluation and policy guidance in the ongoing VL elimination initiative but also helps for future development of both operational and scientific research. Better knowledge on the environmental determinants of leishmaniasis transmission and spatial risk maps will inform the control policy and may shape new intervention strategies.
2.5. Community engagement and participation:
The most important challenge with these VL control approaches is their long-term sustainability. Sooner or later, primary health care settings will need to be involved because passive case finding can not be effective in the long-term sustainability. However, current Indian VL control program is organized in a vertical way. In contrast to other vertical programs, physicians, nurses and auxillary staff of primary health centers (PHCs), accredited social health activists (ASHAs) are involved in the kala-azar Elimination Programme (KAEP). As they do not deal with one disease (i.e. VL only) but with patients presenting with a wide spectrum of diseases, it is a major handicap in KAEP. Among these clinical syndromes, they have to recognize the VL and differentiate it from a constellation of other illnesses including severe and treatable, severe and non-treatable and non-severe diseases[5]. To address this real challenge in clinical care of VL, there is an urgent need to re-examine and strengthen the approach through identification of the optimal mix between vertical and horizontal services. Better case management will promote confidence among the community and will make daily work in PHCs more satisfying. In reality, patients with febrile symptoms first present to private medical practitioners (frequently to unqualified practitioners in their villages) and thus patients experience a substantial delay between onset of symptoms and initiation of proper treatment [19, 36].Therefore, it is important to find the possible approaches to align private medical practitioners (qualified or unqualified) in the KAEP to ensure that appropriate and timely treatment is provided to these VL patients. Most importantly, for the elimination to be sustained in the long-term and to prevent any resurgence, control program needs to mobilize and integrate into the ongoing national health programs.
2.6. Improving integrated vector management
Being a vector borne disease the distribution and occurrence of new VL cases is directly associated with the vector density and movement of the sand fly [37]. It is therefore important to design and implement innovative vector control programs, without which it will be hard to eliminate VL. Research into this vector has been neglected and there are major knowledge gaps [38]. An effective vector management is vital for the control of VL and prevention of its resurgence. Current dogma suggests that the habitat of P. argentipes, the only vector of L. donovani in India, is restricted to areas in and around human homes [39, 40]. Indoor residual insecticide spraying (IRS) is therefore assumed to be an effective vector control measure. Other interventions include long-lasting insecticide treated nets (LLIN) and environmental modification through plastering of walls [6]. However, except IRS, other measures are reported to be ineffective[41]. Alexander and Maroli [42], in their extensive review, observed that there are obvious gaps in current knowledge that limit our ability to implement effective vector control measures. In particular they mentioned the need for improved information on sand fly biting behavior, and on resting and breeding sites. Breeding sites of P. argentipes are still unknown. However, several reasons have been put forward to explain the failure of the current IRS strategy to control VL in the endemic districts, these include the assumption that P. argentipes is a poor flyer that breeds and rests mainly indoor and bites around midnight. Results from recent studies that sand flies can travel significantly further than 100m from their hatching site, have challenged the existing conventions [43]. Importantly, in Bihar P. argentipes has been found at a height of 18m in palm trees[44]. Refusal of IRS by the community in endemic areas, and emergence of dichloro-diphenyl-trichloroethane (DDT) resistance of sand flies in villages are other reasons for continued transmission in endemic areas. Poor IRS coverage (12%) was found in an endemic areas and use of alternative effective insecticides such as Alpha-cypermethrin ( synthetic pyrethroids) might be more costly even though such price differences are decreasing [40]. Therefore, to prevent resurgence of disease and improve the quality of IRS, an essential first step is to find out more about the reasons for the current low IRS coverage. In addition it is not known if the asymptomatically infected persons can be infectious to sand flies. There is thus an urgent need to challenge the conventions on vector behavior, vector abundance, biting behavior and timing of disease transmission for better IRS strategies and control measures.
2.7. Cause of disease outbreak
VL is a focal disease and over the last few years, the numbers of VL cases being reported from non endemic areas were increasing in India and Nepal, and represented a sizeable proportion of the disease burden. It is thus important to understand and verify whether local transmission could be responsible for the disease outbreaks. The presumed role of PKDL in disease outbreaks in west Bengal has been linked by the high rates of sand fly infections following exposure of the flies to the nodular PKDL lesions [45]. Therapy of PKDL is long (3–5 months) and arduous, further since the dermal lesions do not result in any disability, these patients do not seek treatment or do not complete the recommended regimen.
However, some researchers believe that HIV co-infected patients are responsible for disease outbreaks in naive population as many people in endemic regions are co-infected with multiple NTDs [46]. Similarly, these infections may interact and may worsen the prognosis of life threatening infections such as VL. Given the spatial clustering of VL cases, identification of factors associated with outbreaks are crucial to detect outbreaks early. Certification of sustained VL elimination and preventing resurgence of disease requires the capability to verify outbreak alerts.
2.8. Promote research on development of vaccines
Vaccination is the most straightforward option for sustaining reductions in transmission by eliciting long-lasting immune responses, but at the moment no Leishmania human vaccine is available [47]. Development of vaccines for VL is complicated as immunopathogenesis of VL remains to be completely unraveled. For effective vaccine development or immunochemotherapy, a perfect understanding of immunity of VL is mandatory. A major challenge in human vaccine design is to overcome variations in immune responses in a genetically heterogeneous population [48, 49]. While there are some facts known, most of the theories regarding the immuno-pathogenesis of VL rely on inferences drawn from human cutaneous leishmaniasis and clues obtained from murine models. No model mimics the human disease perfectly. A great deal of work has gone in to the study of the immunology of disease in various strains of mice. Although most of these efforts focus on Leishmania that cause cutaneous disease in humans, and extrapolation to the visceral disease can not be done [47]. Thus far only therapeutic use is being made of recombinant vaccines. Part of the problem may lie with vaccine delivery and eliciting sufficiently powerful or appropriately directed long term memory T-cell responses. Leish-111F/MPL-SE, a multi-subunit recombinant Leishmania vaccine, is the first defined vaccine candidate that has been progress to human phase-I and phase-II clinical trials in cured VL patients in India, CL and ML patients in Brazil and Peru and in healthy volunteers in USA [48, 50]. Further innovation in this field could also pave the way towards development of other and better vaccines.
3. Conclusion
Significant progress in reduction of VL incidence and mortality has been made through VL elimination initiatives. The regional VL elimination initiative is well on track to achieve the target, but even after it has been achieved, epidemiological surveillance of VL capable of monitoring VL elimination including outbreak investigations, and to conduct operational research on the quality of VL management in public and private health services have to be improved and continued in order to ensure sustained elimination and prevention of disease resurgence. There is indeed a need to learn from malaria control programs at this event, not only as a vision but as a realistic long term objective for the elimination of a vector borne disease.
4. Expert Commentary
Visceral leishmaniasis (VL) or kala-azar and its dermal sequel, post dermal kala-azar (PKDL) are parasitic disease occurring in the Indian subcontinent predominantly among poorest of the poor, causing an estimated 59, 000 deaths and 2.4 million disability-adjusted life years (DALYs) per year. Despite their high social, economic and public health burden, VL does not rank high on the international political agenda. Affected populations have low visibility and little political voice, which leads to its ignorance when public health priorities and health budgets are set. However, in the Indian subcontinent, the three countries affected by VL, India, Nepal and Bangladesh, aspired to eliminate VL from the subcontinent by 2020 through various control measures. Although no vaccine is available for VL, important advances have been made, and these, if implemented effectively, could eliminate this disease from this endemic region. These include the ability to carry out rapid diagnosis and complete treatment in one single day. Point of care diagnosis and treatment (PCDT) ensure 100% treatment compliance, something that was not possible with other available therapies. PCDT for VL has been made possible through the development of the rK39 rapid immunochromatographic test, making serodiagnosis possible in 20 minutes and the ability to treat patients effectively with a single dose of intravenous liposomal amphotericin B. PCDT, if done through active case detection in the villages, will also reduce the parasite reservoir in highly endemic areas since humans are the only provenreservoir for L. donovani in the Indian subcontinent. This will further reduce transmission in endemic areas. However, despite the success of the current program, and the potential for sustained reduction in transmission, VL has several epidemiological and biological characteristics that might result in the resurgence of the disease. Asymptomatically infected greatly outnumber clinical VL cases and therefore may play a major role in transmission, even if less infectious than clinical cases. For PKDL there are major knowledge gaps, little is known about the prevalence of different forms of PKDL in Bihar and even less about their relative importance in terms of infectiousness to sand flies. Recent theoretical and observational studies have indicated that transmission of VL can continue in low incidence areas [51], therefore the target of <1 case/10,000 individuals at block level may not be sufficient to prevent disease resurgence [52].Consequently, many questions that are integral to the outcome of an infection, its therapy or prophylaxis remain unanswered. Failure in transmission control operations, poor therapeutic control, lacunae in understanding of the disease and lack of political will lie at the root of the present situation. Therefore, quality of care in primary health care settings and developments of tools for disease burden estimates and pharmaco-vigilance should be given priority to prevent resurgence of disease in future. Strengthen research capacity in primary health centers should be prioritized in order to keep the community immobilization in the control program.
Five-year view
The World Health Organization supported visceral leishmaniasis elimination initiative in the Indian subcontinent does not aim at zero transmission of L.donovani, but at ‘reducing the VL incidence rates in the region below levels of public health problem’. These were empirically defined as an incidence rate below 1 per 10,000 population per year in each implementing unit (sub-district level in Bangladesh and India and district level in Nepal). The regional VL elimination initiative is well on track to achieve this target: Nepal has reached and maintained the target for the last 3 years; Bangladesh has achieved it in over 90% of its endemic sub-districts and India in 70%. The total reported VL case load in the region has diminished from over 50,000 in 2006 to less than 10,000 in 2017, with India reporting more than 90 percent of the latter cases. Importantly, in recent years, it has been found that epidemiology of VL in the Indian subcontinent appears to be changing. The Bihar state in India is characterized by a continuous transmission for more than 30 years, but the proportion of infected children has increased. Recently the disease is spreading to new areas and moving closer to urban areas which may challenge the elimination program in future. It is therefore very important to understand the nature and cause of distribution of this disease. Currently, the role and management of PKDL in the Indian subcontinent is considered a priority as PKDL has been identified as a potent threat to achieving the elimination target of VL and sustaining this. This is a very promising initiative as PKDL patients are the other sources of parasite reservoirs in the community. Setting the Post Elimination Agenda for Kala-azar in India (SPEAK India) consortium (https://speakindia.org.in/) has been established with researchers and technical experts working on VL elimination in India and funded by Bill & Melinda Gates Foundation (BMGF) in a partnership between Indian Council of Medical Research (ICMR), National Vector Borne disease Control Program (NVBDCP) of India and London School of Hygiene and Tropical Medicine (LSHTM, UK). This is a promising development that will provide the information’s on knowledge gaps and possible solutions in transmission to ensure VL transmission in India is successfully interrupted and elimination is sustained
Key issues:
-
❖
All suspect case of kala-azar should be screened and diagnosed in an endemic area in an individual who has fever of more than 2 weeks, splenomegaly and a positive serological rk39 test. Treatment is provided only if all the above criteria are present. Therefore, assessment of health care seeking behavior and management of VL suspects in public and private health sector is important for effective case management in endemic arears.
-
❖
Given the focal distribution and spatial clustering of VL cases, there is a clear potential for development of geospatial tools to detect outbreaks early.
-
❖
Vector control through IRS is one of the key components of the current VL control strategy; therefore, it is important to monitor not only the process of IRS implementation but also the outcome of IRS in order to sustain elimination.
-
❖
Mathematical modeling and xenodiagostic studies can confirm whether asymptomatically infected persons contribute to transmission or not, though at present several challenges complicate addressing this issue.
-
❖
As VL is approaching, the main transmission foci are shifting and the number of active VL cases is going down, however, certain specific subgroups of the disease, in particular post kala azar dermal leishmaniasis (PKDL) and VL-HIV co-infection, will become increasingly important because of their potential to trigger resurgence.
Acknowledgement:
This work was supported by the Extramural Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (TMRC grant number U19AI074321) and grants from the Bill & Melinda Gates Foundation (OPP 1117011).The funders had no role in design, decision to publish, or preparation of the report.
Footnotes
Conflict of interest: We declare that we have no conflict of interest.
References:
- [1].Singh OP, Singh B, Chakravarty J, Sundar S. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infect. Dis. Poverty 2016;March 8;5:19. doi: 10.1186/s40249-016-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012;7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Postigo JA. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int J Antimicrob Agents 2010;36 Suppl 1: S62–5. [DOI] [PubMed] [Google Scholar]
- [4].Boelaert M, Meheus F, Sanchez A, Singh SP, Vanlerberghe V, Picado A, Meessen B, Sundar S. The poorest of the poor: a poverty appraisal of households affected by visceral leishmaniasis in Bihar, India. Trop Med Int Health 2009;14: 639–44. [DOI] [PubMed] [Google Scholar]
- [5].Singh OP, Hasker E, Boelaert M, Sundar S. Elimination of visceral leishmaniasis on the Indian subcontinent. Lancet Infect Dis 2016;December;16(12):e304–e309. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ostyn B, Vanlerberghe V, Picado A, Dinesh DS, Sundar S, Chappuis F, Rijal S, Dujardin JC, Coosemans M, Boelaert M, Davies C. Vector control by insecticide-treated nets in the fight against visceral leishmaniasis in the Indian subcontinent, what is the evidence? Trop Med Int Health 2008;13: 1073–85. [DOI] [PubMed] [Google Scholar]
- [7].Control of the leishmaniases. World Health Organisation Techechnical Report Ser (2011) http://apps.who.int/iris/handle/10665/44412.
- [8].Accelerated Plan for Kala-azar Elimination-2017. http://nvbdcp.gov.in/Doc/Accelerated-Plan-Kala-azar1-Feb2017.pdf.
- [9].WHO-TDR. Research to support the elimination of visceral leishmaniasis http://www.who.int/tdr/publications/about-tdr/annual-reports/bl10-annual-report/en/2010.
- [10].WHO. Global Health Observatory Data Repository (Last updated: 2017–09-15). . http://apps.who.int/gho/data/node.main.NTDLEISHVNUM?lang=en.
- [11].Burza S, Mahajan R, Sinha PK, van Griensven J, Pandey K, Lima MA, Sanz MG, Sunyoto T, Kumar S, Mitra G, Kumar R, Verma N, Das P. Visceral leishmaniasis and HIV co-infection in Bihar, India: long-term effectiveness and treatment outcomes with liposomal amphotericin B (AmBisome). PLoS Negl Trop Dis 2014;8: e3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Molina R, Lohse JM, Pulido F, Laguna F, Lopez-Velez R, Alvar J. Infection of sand flies by humans coinfected with Leishmania infantum and human immunodeficiency virus. Am J Trop Med Hyg 1999;60: 51–3. [DOI] [PubMed] [Google Scholar]
- [13].Stauch A, Sarkar RR, Picado A, Ostyn B, Sundar S, Rijal S, Boelaert M, Dujardin JC, Duerr HP. Visceral leishmaniasis in the Indian subcontinent: modelling epidemiology and control. PLoS Negl Trop Dis 2011;5: e1405.** First mathemetical modelling in VL suupprting the role of asymptomatics in disease transmisssion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sundar S, Singh OP. Molecular Diagnosis of Visceral leishmaniasis. Mol Diagn Ther 2018;June 19. doi: 10.1007/s40291-018-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ostyn B, Gidwani K, Khanal B, Picado A, Chappuis F, Singh SP, Rijal S, Sundar S, Boelaert M. Incidence of symptomatic and asymptomatic Leishmania donovani infections in high-endemic foci in India and Nepal: a prospective study. PLoS Negl Trop Dis 2011;5: e1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Singh OP, Hasker E, Sacks D, Boelaert M, Sundar S. Asymptomatic Leishmania Infection: A New Challenge for Leishmania Control. Clin Infect Dis 2014. [DOI] [PMC free article] [PubMed]
- [17].Das S, Matlashewski G, Bhunia GS, Kesari S, Das P. Asymptomatic Leishmania infections in northern India: a threat for the elimination programme? Trans R Soc Trop Med Hyg 2014;108: 679–684. [DOI] [PubMed] [Google Scholar]
- [18].Hasker E, Kansal S, Malaviya P, Gidwani K, Picado A, Singh RP, Chourasia A, Singh AK, Shankar R, Menten J, Wilson ME, Boelaert M, Sundar S. Latent infection with Leishmania donovani in highly endemic villages in Bihar, India. PLoS Negl Trop Dis 2013;7: e2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hasker E, Malaviya P, Gidwani K, Picado A, Ostyn B, Kansal S, Singh RP, Singh OP, Chourasia A, Kumar Singh A, Shankar R, Wilson ME, Khanal B, Rijal S, Boelaert M, Sundar S. Strong association between serological status and probability of progression to clinical visceral leishmaniasis in prospective cohort studies in India and Nepal. PLoS Negl Trop Dis 2014;8: e2657. ** Established the association between serology tests and progression to diseases in healthy subjects living in disease endemic areas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Singh OP, Gidwani K, Kumar R, Nylen S, Jones SL, Boelaert M, Sacks D, Sundar S. Reassessment of immune correlates in human visceral leishmaniasis as defined by cytokine release in whole blood. Clin Vaccine Immunol 2012;19: 961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sundar S and Singh OP. Molecular diagnosis of visceral leishmaniasis. Mol. Diagn. Ther 2018; 22: 443–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].le Fichoux Y, Quaranta JF, Aufeuvre JP, Lelievre A, Marty P, Suffia I, Rousseau D, Kubar J. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J Clin Microbiol 1999;37: 1953–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Singh OP, Sundar S. Whole blood assay and visceral leishmaniasis: Challenges and promises. Immunobiology 2014;219: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Haldar A, Sen P, Roy S. Use of antimony in the treatment of leishmaniasis: Current status and future directions. Molecular Biology International 2011;2011. [DOI] [PMC free article] [PubMed]
- [25].Boelaert M, Criel B, Leeuwenburg J, Van Damme W, Le Ray D, Van der Stuyft P. Visceral leishmaniasis control: a public health perspective. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94: 465–471. [DOI] [PubMed] [Google Scholar]
- [26].Croft S, Sundar S, Fairlamb A. Drug resistance in leishmaniasis. Clinical Microbiology Reviews 2006;19: 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, Kumar PC, Murray HW. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis 2000;31: 1104–7. ** Clinical evidence for pentavalent antimony resistance in VL [DOI] [PubMed] [Google Scholar]
- [28].Rijal S, Bhandari S, Koirala S, Singh R, Khanal B, Loutan L, Dujardin JC, Boelaert M, Chappuis F. Clinical risk factors for therapeutic failure in kala-azar patients treated with pentavalent antimonials in Nepal. Transactions of the Royal Society of Tropical Medicine and Hygiene 2010;104: 225–229. [DOI] [PubMed] [Google Scholar]
- [29].Duncan R, Gannavaram S, Dey R, Debrabant A, Lakhal-Naouar I, Nakhasi H. Identification and Characterization of Genes Involved in Leishmania Pathogenesis: The Potential for Drug Target Selection. Molecular Biology International 2011;2011. [DOI] [PMC free article] [PubMed]
- [30].Sundar S, Chakravarty J. Leishmaniasis: an update of current pharmacotherapy. Expert Opinion on Pharmacotherapy 2013;14: 53–63. [DOI] [PubMed] [Google Scholar]
- [31].Sundar S, Sinha P, Rai M, Verma D, Nawin K, Alam S, Chakravarty J, Vaillant M, Verma N, Pandey K, Kumari P, Lal C, Arora R, Sharma B, Ellis S, Strub-Wourgaft N, Balasegaram M, Olliaro P, Das P, Modabber F. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. The Lancet 2011;377: 477–486.* Clinical trial for combination therapy in VL
- [32].Matlashewski G, Arana B, Kroeger A, Battacharya S, Sundar S, Das P, Sinha PK, Rijal S, Mondal D, Zilberstein D, Alvar J. Visceral leishmaniasis: elimination with existing interventions. Lancet Infect Dis 2011;11: 322–5. [DOI] [PubMed] [Google Scholar]
- [33].Burza S, Mahajan R, Sanz MG, Sunyoto T, Kumar R, Mitra G, Lima MA. HIV and visceral leishmaniasis coinfection in Bihar, India: an underrecognized and underdiagnosed threat against elimination. Clin Infect Dis 2014;August 15;59(4):552–5. doi: 10.1093/cid/ciu333. Epub 2014 May 10. [DOI] [PubMed] [Google Scholar]
- [34].Hirve S, Kroeger A, Matlashewski G, Mondal D, Banjara MR, Das P, Be-Nazir A, Arana B, Olliaro P. Towards elimination of visceral leishmaniasis in the Indian subcontinent-Translating research to practice to public health. PLoS Negl Trop Dis 2017; October 12;11(10):e0005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Le Rutte EA, Coffeng LE, Bontje DM, Hasker EC, Postigo JA, Argaw D, Boelaert MC, De Vlas SJ. Feasibility of eliminating visceral leishmaniasis from the Indian subcontinent: explorations with a set of deterministic age-structured transmission models. Parasit Vectors 2016;January 19;9:24. doi: 10.1186/s13071-016-1292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Medley GF, Hollingsworth TD, Olliaro PL, Adams ER. Health-seeking behaviour, diagnostics and transmission dynamics in the control of visceral leishmaniasis in the Indian subcontinent. Nature 2015;December 3;528(7580):S102–8. doi: 10.1038/nature16042. [DOI] [PubMed] [Google Scholar]
- [37].Tiwary P, Singh S, Kushwaha AK, Rowton E, Sacks D, Singh OP, Sundar S, Lawyer P. Establishing, Expanding, and Certifying a Closed Colony of Phlebotomus argentipes (Diptera: Psychodidae) for Xenodiagnostic Studies at the Kala Azar Medical Research Center, Muzaffarpur, Bihar, India. J Med Entomol 2017;54(5): 1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Alexander B, Jaramillo C, Usma MC, Quesada BL, Cadena H, Roa W, Travi BL. An attempt to control Phlebotomine sand flies (Diptera: Psychodidae) by residual spraying with deltamethrin in a Colombian village. Mem Inst Oswaldo Cruz 1995;90: 421–4. [DOI] [PubMed] [Google Scholar]
- [39].Dhiman RC, Pahwa S, Dhillon GP, Dash AP. Climate change and threat of vector-borne diseases in India: are we prepared? Parasitol Res 2010;106: 763–73. [DOI] [PubMed] [Google Scholar]
- [40].Poché DM, Garlapati RB, Mukherjee S, Torres-Poché Z, Hasker E, Rahman T, Bharti A, Tripathi VP, Prakash S, Chaubey R, Poché RM. Bionomics of Phlebotomus argentipes in villages in Bihar, India with insights into efficacy of IRS-based control measures. PLoS Negl Trop Dis 2018;e0006168. doi: 10.1371/journal.pntd.0006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Coleman M, Foster GM, Deb R, Singh RP, Ismail HM, Shivam P, Ghosh AK, Dunkley S, Kumar V, Coleman M, Hemingway J, Paine MJ, Das P. DDT-based indoor residual spraying suboptimal for visceral leishmaniasis elimination in India. Proc Natl Acad Sci U S A 2015;July 14;112(28):8573–8578.** Experimental report for the ineffectiveness of DDT-based IRS in India
- [42].Alexander B, Maroli M. Control of phlebotomine sandflies. Med Vet Entomol 2003;17: 1–18. [DOI] [PubMed] [Google Scholar]
- [43].Chowdhury R, Kumar V, Mondal D, Das ML, Das P, Dash AP, Koroeger A. Implication of vector charecteristics of Phlebotomous argentipes in the kala-azar elimination program in the Indian sub-continent. Pathogen Global Health 2016;110 (3): 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Poche D, Garlapati R, Ingenloff K, Remmers J, Poche R. Bionomics of phlebotomine sand flies from three villages in Bihar, India. J Vector Ecol 2011;36 Suppl 1: S106–17. [DOI] [PubMed] [Google Scholar]
- [45].Addy M, Nandy A. Ten years of kala-azar in west Bengal, Part I. Did post-kala-azar dermal leishmaniasis initiate the outbreak in 24-Parganas? Bull World Health Organ 1992;70: 341–6. [PMC free article] [PubMed] [Google Scholar]
- [46].van Griensven J Visceral leishmaniasis and HIV coinfection in Bihar, India: a wake-up call? Clin Infect Dis 2014. August 15;59(4):556–8. doi: 10.1093/cid/ciu334. [DOI] [PubMed] [Google Scholar]
- [47].Raman VS, Duthie MS, Fox CB, Matlashewski G, Reed SG. Adjuvants for Leishmania vaccines: from models to clinical application. Front Immunol 2012;3: 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kumar R, Engwerda C. Vaccines to prevent leishmaniasis. Clin Transl Immunology 2014;14; 3(3): e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Singh OP, Sundar S. Immunotherapy and targeted therapies in treatment of visceral leishmaniasis: current status and future prospects. Front Immunol 2014;5: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chakravarty J, Kumar S, Trivedi S, Rai VK, Singh A, Ashman JA, Laughlin EM, Coler RN, Kahn SJ, Beckmann AM, Cowgill KD, Reed SG, Sundar S, Piazza FM. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine for use in the prevention of visceral leishmaniasis. Vaccine 2011;29: 3531–7. [DOI] [PubMed] [Google Scholar]
- [51].Ostyn B, Uranw S, Bhattarai NR, Das ML, Rai K, Tersago K, Pokhrel Y, Durnez L, Marasini B, Van der Auwera G, Dujardin JC, Coosemans M, Argaw D, Boelaert M, Rijal S. Transmission of Leishmania donovani in the Hills of Eastern Nepal, an Outbreak Investigation in Okhaldhunga and Bhojpur Districts. PLoS Negl Trop Dis 2015;9(8):e0003966. doi: 10.1371/journal.pntd.0003966.* Outbreak report in visceral leishmaniasis
- [52].Le Rutte EA, Chapman LAC, Coffeng LE, Jervis S, Hasker EC, Dwivedi S, Karthick M, Das A, Mahapatra T, Chaudhuri I, Boelaert M, Medley GF, Srikantiah S, Hollingsworth TD, de Vlas SJ. Elimination of visceral leishmaniasis in the Indian subcontinent: a comparison of predictions from three transmission models. Epidemics 2017;18:67–80. doi: 10.1016/j.epidem.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]