Abstract
Aim
A comprehensive description of morbidity and mortality risk factors for post liver transplant has not been available to date. In this study, we established real‐time risk models of postoperative morbidities and mortality in liver transplant recipients using two Japanese nationwide databases.
Methods
Data from two Japanese nationwide databases were combined and used for this study. We developed real‐time prognostic models for morbidity and mortality from a derivation cohort (n = 1472) and validated the findings with an independent cohort (n = 395). Preoperative variables (C1), preoperative and intraoperative variables (C2), and all variables including postoperative morbidities within 30 days (C3) were analyzed to evaluate the independent risk factors for postoperative morbidity and mortality.
Results
We established real‐time risk models for morbidity and mortality. Areas under the curve (AUC) of C1 and C2 risk models for mortality were 0.74 (0.63‐0.82) and 0.79 (0.69‐0.86), respectively. Multivariate logistic analysis using C3 showed that hemoglobin <10 g/dL, operative time (hours), and five postoperative morbidities (prolonged ventilation >48 hours, coma >24 hours, renal dysfunction, postoperative systemic sepsis, and serum total bilirubin ≥10 mg/dL) represented independent risk factors for mortality (AUC = 0.87, 95% confidence interval [CI]: 0.78‐0.93).
Conclusions
Real‐time risk models of postoperative morbidities and mortality at various perioperative time points in liver transplant recipients were established. These novel approaches may improve postoperative outcomes of liver transplant recipients. Furthermore, these real‐time risk models may be applicable to other surgical procedures.
Keywords: benchmarking, feedback from database, prediction, risk calculator, surgical quality
1. INTRODUCTION
Liver transplant (LT), either from a deceased donor LT (DDLT) or a living donor LT (LDLT), is one of the most invasive gastroenterological surgeries. It has a substantially higher mortality rate than other procedures. Specifically, data from the Scientific Registry of Transplant Recipients (SRTR)1 and the European Liver Transplant Registry (ELTR)2, 3 showed 6‐month and 1‐year mortality rates of 10.6%‐12.0% and 12.7%‐18.0%, respectively. Additionally, data from the Japanese Liver Transplantation Society showed 1‐year mortality rates of 15.3% in 219 DDLT and 16.2% in 7255 LDLT between 1964 and 2013.4 The Adult‐to‐Adult Living Donor Liver Transplantation Study (A2ALL) showed that, in the USA, the 90‐day and 1‐year mortality rates of LDLT were 13% and 19%, respectively,5 with morbidity rates of 82.8% for LDLT and 78.2% for DDLT.6 The postoperative clinical course after LT should be determined by preoperative/postoperative recipient conditions and donor allograft conditions. Many studies have investigated the preoperative and intraoperative risk factors of recipient‐related or allograft‐related DDLT and LDLT recipients.2, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 However, to our knowledge, a large population study investigating both recipient and donor allograft conditions based on registry data has not been carried out to date. Furthermore, data on intraoperative and postoperative morbidity should dynamically influence the prognosis of LT recipients; however, as has been reviewed in the literature, morbidity outcomes have been overlooked in current and past studies.
For other gastroenterological surgeries, risk models of mortalities for eight procedures, including hepatectomy20 and Pancreatoduodenectomy,21 have been developed using preoperatively determined variables, based on nationwide clinical data registries, the National Clinical Database (NCD), along with implemented feedback reports by the participants.22 In contrast, the Japanese Liver Transplantation Society (JLTS) accumulated precise demographic data of all LT recipients and living donors in Japan from 2012. The data included graft weight and ABO compatibility,4 which is information not included in the NCD database. However, as opposed to the NCD database, the JLTS database did not record postoperative morbidities. Integration of two nationwide databases of LT recipients in a single registry may make up for these deficits.
In the present study, we used an integrated nationwide database to develop risk models of postoperative morbidity and mortality in LT recipients. We included preoperative variables as well as operative and procedural variables, such as estimated blood loss or operative duration. Furthermore, we developed real‐time risk models with postoperative morbidities, such as re‐intubation and sepsis, so that each time point of pre‐ and postoperative management could be precisely evaluated for mortality risk. Results were subsequently validated with an independent validation cohort.
2. MATERIALS AND METHODS
This study was approved by the project committee of the JLTS, the ethics committee of the Japan Society of Transplantation (JST), and the institutional review board of Osaka General Medical Center, Osaka, Japan.
2.1. Data collection and integration of two nationwide registry data: NCD and JLTS databases
All LT recipient surgeries, as well as living or cadaveric donor surgeries, that were registered in the NCD and/or JLTS databases between 2012 and 2015, were included as a derivation cohort. Surgeries registered in 2016 were included in this study as an independent dataset. NCD included 60 preoperative, 18 intraoperative, and 31 postoperative variables. The latter included morbidities within 30 days after surgery in both live, partial LDLT, and DDLT recipients. However, the NCD did not include the following variables: donor graft weight; ABO compatibility (identical, compatible, and incompatible); re‐transplant; and primary diagnosis. On the contrary, the JLTS registry did include these data, as well as donor graft weight from 2012. In the present study, we combined these two national registries and ensured protection of personal information by non‐linkable anonymization.
We recorded the clinical data of: patients who underwent LT between 2012 and 2015 and who were registered in the NCD (n = 1660) and JLTS (n = 1743); and patients who underwent LT in 2016 and who were registered in the NCD (n = 412) and JLTS (n = 438). Transplant and birth dates of recipients were used to identify the corresponding patients in the two registries. After exclusion of mismatched patients from both registries, a total of 1472 cases comprised the derivation cohort and 395 cases comprised the independent cohort of the integrated database of LT recipients (Figure 1).
Figure 1.
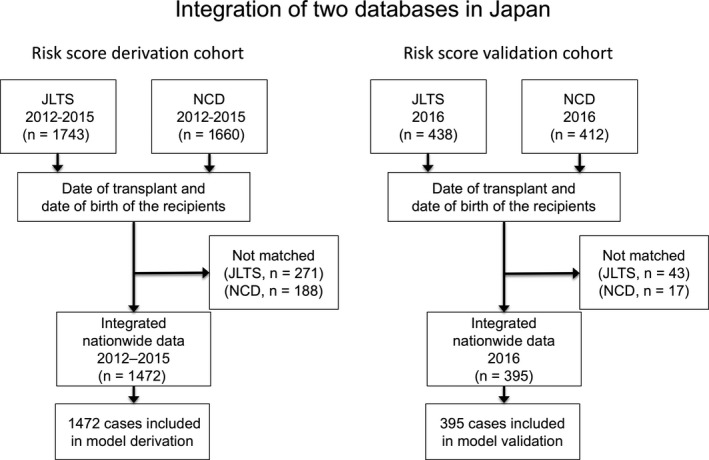
Integration of two databases in Japan. National Clinical Database (NCD) and Japanese Liver Transplantation Society (JLTS) database were integrated according to the year of transplant (2012‐15 and 2016) to a derivation cohort (n = 1472) and an independent cohort (n = 395)
The new integrated database included all data from the NCD. The JLTS database included data on: primary diagnosis of the recipients; ABO blood type compatibility; re‐transplant (history of past LT); deceased/living donor; and graft volume.4 In the present study, we used data from the integrated database, which included the following: 13 categorical and 13 continuous preoperative variables; six continuous intraoperative variables (Table 1 and Table S1); and 27 categorical variables on postoperative morbidity (Table 2) and mortality. Preoperative categorical variables included activities of daily living (ADL), which was defined as functional status either totally, partially dependent or independent. The former two categories (totally and partially dependent ADL) were considered as one category of “ADL with any assistance.”22 Continuous variables were divided into binary data. The best cutoff value was determined based on the least P‐value in the Pearson's chi‐squared test between the binary variable and death (Tables S1 and S3). Six variables (recipient age, donor age, model for end‐stage liver disease [MELD] score, the ratio of graft weight to standard liver volume [RGW/SLV], operative time, and intraoperative estimated blood loss) were used as continuous data with upper and lower limits (Table S2). Among the 13 preoperative continuous variables, nine were analyzed as binary data (Table 3, Nos 15‐23).
Table 1.
Pre‐ and intraoperative continuous variables according to survival in the derivation cohort
| Variable | Study population | Subgroup: death | Subgroup: no death | P‐value | ||
|---|---|---|---|---|---|---|
| Missing (%) | Median and quartiles | Median and quartiles | Median and quartiles | |||
| Preoperative continuous variables | ||||||
| Recipient age (y) | 0 (0.0%) | 49.1 (10.6‐59.6) | 50.9 (23.4‐61.1) | 48.5 (9.8‐59.3) | 0.116 | |
| Donor age (y) | 98 (6.7%) | 38.0 (30.0‐49.0) | 44.5 (34.3‐54.0) | 38.0 (30.0‐48.0) | 0.0001 | b |
| MELD score | 0 (0.0%) | 16.4 (12.0‐22.6) | 19.7 (14.4‐26.3) | 16.1 (11.8‐22.2) | 0.0001 | b |
| GW/SLV ratio | 2 (0.1%) | 0.49 (0.39‐0.71) | 0.46 (0.36‐0.63) | 0.50 (0.39‐0.73) | 0.007 | a |
| Intraoperative continuous variables | ||||||
| Operative time (h) | 2 (0.1%) | 12.4 (10.4‐14.8) | 13.6 (11.9‐16.4) | 12.2 (10.3‐14.6) | <0.0001 | b |
| Estimated blood loss (L) | 2 (0.1%) | 3.6 (1.2‐7.9) | 6.3 (2.7‐15.4) | 3.4 (1.2‐7.5) | <0.0001 | b |
P < 0.01
P < 0.001 (Student's t test)
GW/SLV, graft weight to standard liver volume; MELD, model for end‐stage liver disease.
Table 2.
Postoperative morbidity and mortality rates in the derivation cohort
| Postoperative morbidities | Incidence of morbidity (n) | Morbidity rate (%) | Mortality (n) | Mortality ratea (%) | P‐value | Selected as C3 variables |
|---|---|---|---|---|---|---|
| Postoperative occurrences (within 30 d) | 783 | 53.2 | 110 | 14.0 | <0.0001 | |
| Reoperation | 314 | 21.3 | 68 | 21.7 | <0.0001 | * |
| Superficial surgical site infection (SSI) | 70 | 4.8 | 21 | 30.0 | <0.0001 | |
| Deep incisional SSI | 43 | 2.9 | 17 | 39.5 | <0.0001 | |
| Organ space SSI | 37 | 2.5 | 6 | 16.2 | <0.0001 | |
| Wound disruption | 27 | 1.8 | 9 | 33.3 | <0.0001 | |
| Suture insufficiency | 51 | 3.5 | 7 | 13.7 | 0.012 | |
| Postoperative pneumonia | 122 | 8.3 | 46 | 37.7 | <0.0001 | * |
| Unplanned intubation | 139 | 9.4 | 56 | 40.3 | <0.0001 | * |
| On ventilator >48 h | 315 | 21.4 | 93 | 29.5 | <0.0001 | * |
| Renal dysfunction | 199 | 13.5 | 79 | 39.7 | <0.0001 | * |
| Urinary tract infection | 41 | 2.8 | 13 | 31.7 | <0.0001 | |
| Central nerve disorder | 17 | 1.2 | 7 | 41.2 | <0.0001 | |
| Coma >24 h | 109 | 7.4 | 48 | 44.0 | <0.0001 | * |
| Peripheral nerve disorder | 18 | 1.2 | 5 | 27.8 | 0.003 | |
| Cardiac arrest requiring resuscitation | 17 | 1.2 | 14 | 82.4 | <0.0001 | |
| Postoperative transfusion (>5 units) | 394 | 26.8 | 89 | 22.6 | <0.0001 | * |
| Postoperative systemic sepsis (including SIRS, sepsis, shock) | 194 | 13.2 | 79 | 40.7 | <0.0001 | * |
| Other morbidities (atelectasis) | 147 | 10.0 | 24 | 16.3 | 0.0003 | |
| Other morbidities (heart failure) | 7 | 0.5 | 4 | 57.1 | <0.0001 | |
| Other morbidities (i.p. hemorrhage) | 48 | 3.3 | 13 | 27.1 | <0.0001 | |
| Other morbidities (i.p. abscess) | 56 | 3.8 | 12 | 21.4 | 0.0004 | |
| Other morbidities (DIC) | 32 | 2.2 | 18 | 56.3 | <0.0001 | |
| Other morbidities (mechanical ileus) | 14 | 1.0 | 4 | 28.6 | 0.006 | |
| Other morbidities (serum bilirubin >10 mg/dL) | 119 | 8.1 | 58 | 48.7 | <0.0001 | * |
| Other morbidities (refractory ascites) | 186 | 12.6 | 37 | 19.9 | <0.0001 | |
| Other morbidities (dysuria) | 4 | 0.3 | 2 | 50.0 | 0.003 |
Mortality rate in the patients with morbidity.
DIC, disseminated intravascular coagulation; SIRS, systemic inflammatory response syndrome.
Table 3.
Preoperative binary variables and mortality rates in the derivation cohort
| Variables | Mortality (n, %) | Total (n) | P‐value |
|---|---|---|---|
| 1. Activities of daily living (ADL) | |||
| Partially or totally dependent | 64 (12.6%) | 507 | <0.0001 |
| Independent | 60 (6.2%) | 965 | |
| 2. Dyspnea (preoperative within 30 d) | |||
| Yes | 28 (15.1%) | 185 | 0.0004 |
| No | 96 (7.5%) | 1287 | |
| 3. Ventilator dependent (preoperative within 48 h) | |||
| Yes | 20 (19.0%) | 105 | <0.0001 |
| No | 104 (7.6%) | 1367 | |
| 4. Current pneumonia | |||
| Yes | 6 (22.2%) | 27 | 0.009 |
| No | 118 (8.2%) | 1445 | |
| 5. Ascites (preoperative within 30 d) | |||
| Yes | 85 (11.2%) | 757 | <0.0001 |
| No | 39 (5.5%) | 714 | |
| 6. Esophageal varices (preoperative within 6 mo) | |||
| Yes | 47 (11.8%) | 397 | 0.004 |
| No | 77 (7.2%) | 1075 | |
| 7. Acute renal failure (preoperative within 24 h) | |||
| Yes | 10 (19.6%) | 51 | 0.003 |
| No | 114 (8.0%) | 1421 | |
| 8. Dialysis (preoperative within 14 d) | |||
| Yes | 22 (15.7%) | 140 | 0.001 |
| No | 102 (7.7%) | 1332 | |
| 9. Long‐term steroid treatment | |||
| Yes | 18 (16.1%) | 112 | 0.002 |
| No | 106 (7.8%) | 1360 | |
| 10. Bleeding disorders prior to surgery | |||
| Yes | 71 (11.8%) | 601 | 0.0001 |
| No | 53 (6.1%) | 871 | |
| 11. Preop transfusion of ≥1 unit of whole/packed RBC 72 h before surgery | |||
| Yes | 30 (12.0%) | 249 | 0.024 |
| No | 94 (7.7%) | 1223 | |
| 12. Preoperative systemic sepsis | |||
| Yes | 6 (22.2%) | 27 | 0.009 |
| No | 118 (8.2%) | 1445 | |
| 13. Re‐transplant | |||
| Yes | 15 (23.4%) | 64 | <0.0001 |
| No | 109 (7.7%) | 1408 | |
| 14. ASA classification (ASA physical status) | |||
| ASA‐PS ≧4 | 42 (14.2%) | 296 | <0.0001 |
| ASA‐PS <4 | 82 (7.0%) | 1176 | |
| 15. Preoperative serum creatinine | |||
| >2.0 mg/dL | 11 (17.2%) | 64 | 0.010 |
| ≦2.0 mg/dL | 113 (8.1%) | 1403 | |
| 16. Preoperative hemoglobin | |||
| <10 mg/dL | 88 (11.6%) | 759 | <0.0001 |
| ≧10 mg/dL | 36 (5.1%) | 711 | |
| 17. Preoperative platelet count | |||
| <5 × 104/mm3 | 37 (10.9%) | 341 | 0.066 |
| ≧5 × 104/mm3 | 87 (7.7%) | 1130 | |
| 18. Preoperative serum albumin | |||
| <3.8 g/dL | 115 (9.3%) | 1233 | 0.006 |
| ≧3.8 g/dL | 9 (3.9%) | 233 | |
| 19. Preoperative total bilirubin | |||
| >3 mg/dL | 89 (10.0%) | 892 | 0.008 |
| ≦3 mg/dL | 35 (6.0%) | 579 | |
| 20. Preoperative BUN | |||
| >20 mg/dL | 42 (13.1%) | 321 | 0.0006 |
| ≦20 mg/dL | 81 (7.1%) | 1147 | |
| 21. International normalized ratio (INR) of PT values | |||
| >1.1 | 109 (9.5%) | 1151 | 0.004 |
| ≦1.1 | 13 (4.3%) | 301 | |
| 22. Preoperative aPTT | |||
| >40 s | 84 (11.1%) | 755 | <0.0001 |
| ≦40 s | 32 (4.8%) | 672 | |
| 23. Weight | |||
| ≧75 kg | 20 (12.3%) | 163 | 0.062 |
| <75 kg | 104 (8.0%) | 1307 | |
aPTT, activated partial thromboplastin time; ASA‐PS, American Society of Anesthesiologists physical status; PT, prothrombin time; RBC, red blood cells.
Variables were divided into three categories: (i) preoperative variables (C1); (ii) preoperative and intraoperative variables (C2); and (iii) all variables, including postoperative morbidity within 30 days (C3). All continuous variables were correlated with death. Among the binary variables, those correlated with death at a significant level (alpha) of 0.10 underwent multivariate logistic regression analysis.
2.2. Endpoints
Analysis endpoints were as follows: postoperative morbidities and mortality within 30 days for C1 and C2; mortality for C1, C2, and C3. Postoperative mortality included both in‐hospital deaths and deaths within 30 days post‐surgery.
2.3. Statistical analysis and real‐time risk model
Statistical analysis was carried out using two software programs (R, 64‐bit, version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria and JMP Pro, 64‐bit, version 13.2.0; SAS Institute Inc., Cary, NC, USA), and α was established a priori at 5%. An independent validation dataset was used to evaluate the predictive accuracy of the risk‐adjustment model by using receiver operator characteristic (ROC) curves, area under the curve (AUC), and calibration plots (Figure S1).23
Continuous and categorical variables with three or more levels were treated as binary variables with cutoff points being determined based on the smallest P‐value in the chi‐squared test between the binary variable and death. Four variables were used as Winsorized continuous variables (i.e. values below and above a predefined range replaced the threshold values). These variables included the following: donor age (20‐65 years); RGW/SLV (0.3‐0.5); operative time (8‐18 hours); and estimated intraoperative blood loss (0‐30 L; Table S2). Thresholds for Winsorization were determined based on the correlation between these continuous variables and death (data not shown). Missing values were replaced with the mode for binary variables and with the median for continuous variables. Fisher's exact U test was used for contingency analysis between categorical variables. Finally, Student's t test was used for comparison of continuous variables between two groups.
To create the real‐time risk models, with the exception of the risk model for mortality using C3 variables, all variables that significantly correlated with death at a significance level (alpha) of 0.10 were subjected to multivariate logistic regression analysis. Among four intraoperative variables with a P‐value <0.10 (Table 1 and Table S1), operative time and estimated blood loss were selected as candidate independent variables for logistic regression analysis. The remaining two variables (total volume of infusion during surgery and number of fresh frozen plasma units given intraoperatively) were not selected as candidate independent variables because they were both highly correlated (P < 0.0001) with the former two variables. With regard to the risk model for mortality using C3 variables, all C2 variables with a P‐value <0.10, postoperative morbidity variables with a P‐value <0.10, those with an incidence of >5% in all patients, and those with >20% conditional incidence of mortality were subjected to multivariate logistic analysis.
Logistic regression models were constructed using backward stepwise selection of predictors, with a criterion of P‐value <0.05. As a measure of model discrimination, C‐statistics (area under the ROC curve, AUC) were calculated for each risk model using an independent validation cohort. Calibration plots were drawn to visually examine the calibration of each model. Subjects were divided into 10 bins using threshold deciles of predicted risks. Each bin was represented by a dot, with the mean predicted risk of death on the horizontal axis and the observed proportion of death on the vertical axis. Error bars in the direction of the horizontal axis represented the range of predicted risk in each bin, whereas those in the direction of the vertical axis represented the 95% CI of the incidence of death in each bin. The latter was estimated assuming a binomial distribution.
2.4. Comparative analysis of equations used in previous studies versus those used in the current study
Numerous previous single‐center studies carried out risk factor analysis in LDLT with the use of preoperative variables. Yoshizumi et al24 reported that MELD score, donor age, and graft size were independent risk factors for graft loss after LDLT. Marubashi et al8 reported that MELD score and RGW/SLV were independent risk factors for small‐for‐size graft failure in LDLT. To evaluate the fitting of our real‐time risk model, we compared our results with those of the above‐mentioned studies, in particular the data on adult‐to‐adult LDLT. In order to compensate for the differences in calibrations between the model developed in this study and those of previous studies, we used recalibrated versions of previous univariate logistic models obtained using previous risk models as a single independent variable.
2.5. Validation analyses in the subgroups of deceased versus living donors and adult versus pediatric recipients
Postoperative morbidities and mortality could be influenced by types of donor, either deceased or living donors, and types of recipient, either adult versus pediatric recipients. To evaluate the accuracy of the real‐time risk models, c‐statistics (area under the ROC curve, AUC) were calculated for each risk model using the independent subgroups in 2016, adult/LDLT (n = 227), adult/DDLT (n = 46), and pediatric LDLT (n = 115).
3. RESULTS
3.1. Risk profiles and study population data
In a derivation cohort, a total of 1472 recipients (1057 [71.8%] adult and 415 [28.2%] pediatric patients) underwent DDLT (n = 153) and LDLT (n = 1319). Indication for LT was based on a primary diagnosis of cholestatic diseases (n = 483); hepatocellular diseases (n = 389); neoplastic diseases (n = 241); acute liver failure (n = 128); or re‐transplant for graft failure (n = 49; Table 4). Overall mortality rate was 8.4% (n = 124). Highest mortality rates were seen in recipients with a primary diagnosis of hepatocellular disease, neoplastic disease, acute liver failure, vascular disease, and re‐transplantation (23.4%), whereas lower mortality rates were seen in patients with cholestatic and metabolic disease (Table S3). Distributions of allograft lobes or segments in the derivation cohort can be observed in Table 5. In LDLT, most of the adult recipients received the right (n = 449, 48.4%) or the left lobe (n = 450, 48.5%), whereas the majority of the pediatric recipients received the left lateral section (n = 272, 69.4%).
Table 4.
Demographics, clinical and laboratory findings, and outcomes of derivation and validation cohorts
| Derivation cohort (n = 1472) | Validation cohort (n = 395) | |||
|---|---|---|---|---|
| Age | 49.1 (10.6‐59.6) | 45.8 (7.3‐57.3) | ||
| <18 y | 415 (28.2%) | 122 (30.9%) | ||
| Gender | ||||
| Male | 700 (47.6%) | 197 (49.9%) | ||
| Female | 772 (52.4%) | 198 (50.1%) | ||
| Weight (kg) | 54.0 (29.7‐65.4) | 53.8 (20.0‐65.9) | ||
| Primary diagnosis | ||||
| Cholestatic disease | 483 (32.8%) | 142 (35.9%) | ||
| Acute liver failure | 142 (9.6%) | 38 (9.6%) | ||
| Hepatocellular disease | 389 (26.4) | 99 (25.1%) | ||
| Metabolic disease | 78 (5.3%) | 24 (6.1%) | ||
| Neoplastic disease | 265 (18.0%) | 57 (14.4%) | ||
| Vascular disease | 27 (1.8%) | 8 (2.0%) | ||
| Re‐transplantation | 64 (4.3%) | 13 (3.3%) | ||
| Others | 24 (1.6%) | 14 (3.5%) | ||
| Mortality | 124 (8.4%) | 26 (6.6%) | ||
| Donor type | ||||
| Live | 1319 (89.6%) | 342 (86.6%) | ||
| Cadaveric | 153 (10.4%) | 53 (13.4%) | ||
| Activities of daily living (ADL) (prior to surgery) | ||||
| Independent | ||||
| Partially or totally dependent | 507 (34.4%) | 131 (33.2%) | ||
| ASA‐PS | ||||
| 1‐3 | 1176 (79.9%) | 316 (80.0%) | ||
| 4‐5 | 296 (20.1%) | 79 (20.0%) | ||
| MELD score | 16.4 (12.0‐22.6) | 16.8 (12.0‐21.8) | ||
| Bilirubin (mg/dL) | 4.3 (1.9‐12.7) | 5 (2.0‐12.2) | ||
| Creatinine (mg/dL) | 0.61 (0.34‐0.89) | 0.6 (0.28‐0.87) | ||
| PT‐INR | 1.38 (1.18‐1.69) | 1.36 (WNL‐1.70) | ||
| Hemoglobin (g/dL) | 9.9 (8.6‐11.6) | 9.9 (8.6‐12.2) | ||
Data are median (IQR) or n (%).
ASA‐PS, American Society of Anesthesiologists physical status; MELD, model for end‐stage liver disease; PT‐INR, prothrombin time‐international normalized ratio; WNL, within normal limit.
Table 5.
Characteristics of the liver grafts and recipients in the derivation cohort
| Liver segment | Living donor, Adult recipient (n) | Cadaveric donor, Adult recipient (n) | Living donor, pediatrics recipient (n) | Cadaveric donor, Pediatric recipients (n) | Total (n) |
|---|---|---|---|---|---|
| 1234 | 228 | 0 | 16 | 0 | 244 |
| 234 | 222 | 2 | 56 | 1 | 281 |
| 5678 | 449 | 5 | 6 | 0 | 460 |
| 23 | 0 | 0 | 272 | 11 | 283 |
| Mono‐segment (2 or 3) | 0 | 0 | 39 | 0 | 39 |
| 67 | 19 | 0 | 1 | 0 | 20 |
| 145678 | 0 | 9 | 1 | 0 | 10 |
| 45678 | 1 | 1 | 0 | 0 | 2 |
| 567 | 1 | 0 | 0 | 0 | 1 |
| 678 | 1 | 0 | 0 | 0 | 1 |
| 78 | 1 | 0 | 0 | 0 | 1 |
| Whole liver | 5 (domino) | 113 | 1 | 11 | 130 |
| Total | 927 | 130 | 392 | 23 | 1472 |
Preoperative characteristics of patients in the derivation cohort are shown in Tables 1 and 3. Although ABO blood type compatibility and deceased/living donor were not associated with mortality, the majority of the other pre‐ and intraoperative characteristics were linked to mortality (Table 3). Incidence of postoperative morbidities and mortality rates in the derivation cohort is reported in Table 2. Morbidities >5% (108 cases) of all 1472 patients, as well as those with a high mortality rate (>20%), included the following: reoperation; postoperative pneumonia; unintended re‐intubation; prolonged ventilation >48 hours; renal dysfunction (defined as the need for newly implemented dialysis or increase in serum creatinine >2 mg/dL post‐surgery); coma >24 hours; postoperative transfusion of >5 units; sepsis, including systemic inflammatory response syndrome (SIRS) and septic shock; and hyperbilirubinemia (>10 mg/dL). These variables were included in the C3 set of candidate independent variables.
3.2. Risk calculator models based on preoperative risk factors for morbidities and mortality: the C1 model
Risk models that used C1 categorical variables were created separately for morbidities and mortality with independent risk factors (Table 6). AUC of the risk calculator model for morbidities using the validation cohort ranged from 0.56 to 0.78, and that for mortality was 0.74 (95% CI: 0.63‐0.82). Independent risk factors for morbidity and mortality were slightly different: ADL with any assistance; preoperative recipient's weight ≥75 kg; activated partial thromboplastin time >40 seconds; re‐transplantation (preoperative recipient‐related variables); and RGW/SLV and donor age (donor‐related variables) were the independent risk factors for mortality.
Table 6.
Multivariate logistic regression analyses of pre‐, intra‐, and postoperative factors for morbidities and mortality after liver transplant
| Postoperative mortality | ||||||
|---|---|---|---|---|---|---|
| Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | Pre‐, intra‐, and postoperative variables (C3) | ||||
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Constant (β0) | 0.006 (0.003‐0.013) | <0.0001 | 0.002 (0.000‐0.006) | <0.0001 | 0.006 (0.002‐0.017) | <0.0001 |
| Preoperative variables | ||||||
| ADL (prior to surgery) | 2.17 (1.45‐3.25) | 0.0002 | 2.21 (1.45‐3.36) | 0.0002 | ||
| ASA‐PS ≥4 | 1.78 (1.15‐2.77) | 0.010 | ||||
| Preoperative dialysis <14 d | ||||||
| Acute renal failure (within 24 h) | ||||||
| Steroid use for chronic condition (history) | ||||||
| Dyspnea (within 30 d) | ||||||
| Current pneumonia | ||||||
| Ascites (within 30 d) | ||||||
| Esophageal varices | ||||||
| Bleeding disorders prior to surgery | ||||||
| Preoperative systemic sepsis | ||||||
| Preoperative transfusion (within 72 h) | ||||||
| Preoperative weight ≥75 kg | 1.75 (1.03‐3.00) | 0.040 | ||||
| Hemoglobin <10 g/dL | 1.95 (1.27‐2.99) | 0.002 | 1.86 (1.22‐2.84) | 0.004 | 1.74 (1.08‐2.82) | 0.021 |
| Platelet <50 000/μL | ||||||
| BUN >20 mg/dL | ||||||
| Creatinine >2.0 mg/dL | ||||||
| Total bilirubin >3 mg/dL | ||||||
| Albumin <3.8 g/dL | ||||||
| PT‐INR >1.1 | ||||||
| PTT >40 s | 1.78 (1.15‐2.76) | 0.010 | ||||
| Re‐transplant | 2.55 (1.32‐4.95) | 0.005 | ||||
| Donor variables | ||||||
| RGW/SLV (−0.1)a | 1.65 (1.25‐2.16) | 0.0003 | 1.53 (1.16‐2.02) | 0.003 | ||
| Donor age (5 y)a | 1.16 (1.08‐1.25) | 0.0001 | 1.16 (1.07‐1.25) | 0.0002 | ||
| Operative variables | ||||||
| Intraoperative estimated blood loss (L)a | 1.04 (1.02‐1.07) | 0.001 | ||||
| Operative time (h)a | 1.12 (1.04‐1.20) | 0.003 | 1.08 (1.00‐1.17) | 0.049 | ||
| Postoperative morbidities | ||||||
| Prolonged ventilation >48 h | 3.62 (2.09‐6.26) | <0.0001 | ||||
| Coma >24 h | 1.96 (1.09‐3.52) | 0.025 | ||||
| Serum total bilirubin >10 mg/dL | 2.24 (1.24‐4.05) | 0.008 | ||||
| Renal dysfunction | 2.69 (1.53‐4.72) | 0.001 | ||||
| Postoperative systemic sepsis | 3.35 (1.92‐5.83) | <0.0001 | ||||
| AUC | ||||||
| AUC using validation dataset | 0.74 (0.63‐0.82) | 0.79 (0.69‐0.86) | 0.87 (0.78‐0.93) | |||
| Unplanned reoperation within 30 d for intra‐abdominal bleeding | Unplanned reoperation within 30 d for reasons other than intra‐abdominal bleeding | |||||||
|---|---|---|---|---|---|---|---|---|
| Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | |||||
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Constant (β0) | 0.030 (0.017‐0.054) | <0.0001 | 0.008 (0.003‐0.022) | <0.0001 | 0.055 (0.032‐0.095) | <0.0001 | 0.021 (0.009‐0.052) | <0.0001 |
| Preoperative variables | ||||||||
| ADL (prior to surgery) | 1.45 (1.01‐2.07) | 0.045 | ||||||
| ASA‐PS ≥ 4 | 1.57 (1.07‐2.30) | 0.021 | 1.50 (1.02‐2.22) | 0.041 | ||||
| Preoperative dialysis <14 d | 1.95 (1.14‐3.38) | 0.016 | ||||||
| Acute renal failure (within 24 h) | ||||||||
| Steroid use for chronic condition (history) | 2.12 (1.31‐3.45) | 0.002 | 1.94 (1.19‐3.18) | 0.008 | ||||
| Dyspnea (within 30 d) | ||||||||
| Current pneumonia | ||||||||
| Ascites (within 30 d) | ||||||||
| Esophageal varices | ||||||||
| Bleeding disorders prior to surgery | ||||||||
| Preoperative systemic sepsis | 4.70 (1.40−15.8) | 0.012 | ||||||
| Preoperative transfusion (within 72 h) | 1.79 (1.20‐2.67) | 0.004 | 1.64 (1.08‐2.48) | 0.020 | ||||
| Preoperative weight ≥75 kg | ||||||||
| Hemoglobin <10 g/dL | ||||||||
| Platelet <50 000/μL | ||||||||
| BUN >20 mg/dL | 1.97 (1.34‐2.89) | 0.0005 | ||||||
| Creatinine >2.0 mg/dL | ||||||||
| Total bilirubin >3 mg/dL | ||||||||
| Albumin <3.8 g/dL | ||||||||
| PT–INR >1.1 | 2.38 (1.32‐4.30) | 0.004 | 1.89 (1.03‐3.46) | 0.039 | ||||
| PTT >40 s | ||||||||
| Re‐transplant | ||||||||
| Donor variables | ||||||||
| RGW/SLV (−0.1)a | 1.54 (1.20‐1.98) | 0.0007 | 1.51 (1.17‐1.95) | 0.002 | ||||
| Donor age (5 y)a | 1.08 (1.02‐1.15) | 0.015 | 1.08 (1.01‐1.15) | 0.019 | ||||
| Operative variables | ||||||||
| Intraoperative estimated blood loss (L)a | 1.06 (1.03‐1.08) | <0.0001 | ||||||
| Operative time (h)a | 1.10 (1.03‐1.18) | 0.007 | 1.07 (1.01‐1.13) | 0.014 | ||||
| Postoperative morbidities | ||||||||
| Prolonged ventilation >48 h | ||||||||
| Coma >24 h | ||||||||
| Serum total bilirubin >10 mg/dL) | ||||||||
| Renal dysfunction | ||||||||
| Postoperative systemic sepsis | ||||||||
| AUC | ||||||||
| AUC using validation dataset | 0.56 (0.46‐0.66) | 0.64 (0.54‐0.73) | 0.64 (0.55‐0.72) | 0.70 (0.65‐0.80) | ||||
| Pneumonia | Unplanned intubation | |||||||
|---|---|---|---|---|---|---|---|---|
| Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | |||||
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Constant (β0) | 0.006 (0.002‐0.017) | <0.0001 | 0.007 (0.003‐0.018) | <0.0001 | 0.032 (0.021‐0.049) | <0.0001 | 0.013 (0.005‐0.031) | <0.0001 |
| Preoperative variables | ||||||||
| ADL (prior to surgery) | ||||||||
| ASA‐PS ≥4 | 1.60 (1.02‐2.49) | 0.040 | 1.65 (1.06‐2.56) | 0.026 | 1.82 (1.20‐2.77) | 0.005 | 1.83 (1.20‐2.79) | 0.005 |
| Preoperative dialysis <14 d | ||||||||
| Acute renal failure (within 24 h) | ||||||||
| Steroid use for chronic condition (history) | 1.80 (1.03‐3.16) | 0.039 | ||||||
| Dyspnea (within 30 d) | 2.01 (1.25‐3.25) | 0.004 | 1.80 (1.11‐2.92) | 0.017 | 1.62 (1.01‐2.60) | 0.043 | 1.63 (1.02‐2.62) | 0.041 |
| Current pneumonia | 3.79 (1.54‐9.35) | 0.004 | 4.00 (1.63‐9.79) | 0.003 | 2.80 (1.08‐7.25) | 0.034 | 2.84 (1.09‐7.39) | 0.032 |
| Ascites (within 30 d) | 2.04 (1.27‐3.26) | 0.003 | 2.12 (1.34‐3.36) | 0.001 | ||||
| Esophageal varices | 1.74 (1.19‐2.55) | 0.005 | 1.54 (1.04‐2.27) | 0.030 | ||||
| Bleeding disorders prior to surgery | 1.50 (1.02‐2.22) | 0.040 | ||||||
| Preoperative systemic sepsis | ||||||||
| Preoperative transfusion (within 72 h) | ||||||||
| Preoperative weight ≥75 kg | ||||||||
| Hemoglobin <10 g/dL | ||||||||
| Platelet <50 000/μL | 1.59 (1.06‐2.40) | 0.026 | ||||||
| BUN >20 mg/dL | 1.82 (1.20‐2.77) | 0.005 | 1.63 (1.09‐2.43) | 0.016 | 1.53 (1.03‐2.28) | 0.037 | ||
| Creatinine >2.0 mg/dL | ||||||||
| Total bilirubin >3 mg/dL | ||||||||
| Albumin <3.8 g/dL | ||||||||
| PT–INR >1.1 | 2.18 (1.06‐4.47) | 0.034 | 2.28 (1.12‐4.65) | 0.023 | ||||
| PTT >40 s | 1.70 (1.15‐2.53) | 0.008 | 1.52 (1.01‐2.27) | 0.043 | ||||
| Re‐transplant | ||||||||
| Donor variables | ||||||||
| RGW/SLV (−0.1)a | 1.33 (1.01‐1.77) | 0.046 | 1.41 (1.09‐1.82) | 0.010 | 1.34 (1.03‐1.74) | 0.029 | ||
| Donor age (5 y)a | 1.09 (1.01‐1.18) | 0.019 | 1.09 (1.01‐1.17) | 0.031 | ||||
| Operative variables | ||||||||
| Intraoperative estimated blood loss (L)a | 1.05 (1.03‐1.08) | <0.0001 | ||||||
| Operative time (h)a | 1.08 (1.01‐1.15) | 0.023 | ||||||
| Postoperative morbidities | ||||||||
| Prolonged ventilation >48 h | ||||||||
| Coma >24 h | ||||||||
| Serum total bilirubin >10 mg/dL) | ||||||||
| Renal dysfunction | ||||||||
| Postoperative systemic sepsis | ||||||||
| AUC | ||||||||
| AUC using validation dataset | 0.78 (0.68‐0.85) | 0.74 (0.62‐0.84) | 0.67 (0.58‐0.75) | 0.66 (0.57‐0.74) | ||||
| On ventilator greater than 48 h | Renal dysfunction including acute renal failure | |||||||
|---|---|---|---|---|---|---|---|---|
| Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | |||||
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Constant (β0) | 0.050 (0.032‐0.077) | <0.0001 | 0.015 (0.007‐0.032) | <0.0001 | 0.009 (0.004‐0.019) | <0.0001 | 0.001 (0.000‐0.003) | <0.0001 |
| Preoperative variables | ||||||||
| ADL (prior to surgery) | 1.44 (1.05‐1.98) | 0.023 | ||||||
| ASA‐PS ≥4 | 1.70 (1.23‐2.35) | 0.001 | 1.69 (1.22‐2.36) | 0.002 | ||||
| Preoperative dialysis < 14 d | ||||||||
| Acute renal failure (within 24 h) | 2.81 (1.47‐5.38) | 0.002 | 3.15 (1.62‐6.13) | 0.001 | ||||
| Steroid use for chronic condition (history) | 2.23 (1.44‐3.46) | 0.0004 | 2.06 (1.32‐3.22) | 0.002 | ||||
| Dyspnea (within 30 d) | 1.77 (1.22‐2.57) | 0.003 | ||||||
| Current pneumonia | 3.16 (1.33‐7.52) | 0.009 | 3.14 (1.32‐7.47) | 0.010 | 3.80 (1.49‐9.69) | 0.005 | ||
| Ascites (within 30 d) | 1.73 (1.16‐2.59) | 0.007 | 1.77 (1.18‐2.66) | 0.006 | ||||
| Esophageal varices | 1.50 (1.11‐2.02) | 0.007 | 1.44 (1.07‐1.95) | 0.017 | 1.64 (1.16‐2.33) | 0.005 | 1.44 (1.01‐2.07) | 0.045 |
| Bleeding disorders prior to surgery | 1.55 (1.17‐2.07) | 0.002 | 1.56 (1.17‐2.08) | 0.003 | 1.63 (1.15‐2.31) | 0.006 | 1.91 (1.34‐2.72) | 0.0003 |
| Preoperative systemic sepsis | 9.38 (1.94‐45.39) | 0.005 | 9.08 (1.83‐45.1) | 0.007 | 4.21 (1.21‐14.6) | 0.024 | ||
| Preoperative transfusion (within 72 h) | ||||||||
| Preoperative weight ≥75 kg | ||||||||
| Hemoglobin <10 g/dL | ||||||||
| Platelet <50 000/μL | 1.49 (1.05‐2.11) | 0.027 | ||||||
| BUN >20 mg/dL | 1.59 (1.16‐2.16) | 0.003 | 1.51 (1.11‐2.06) | 0.010 | 2.41 (1.70‐3.43) | <0.0001 | 2.12 (1.48‐3.05) | <0.0001 |
| Creatinine >2.0 mg/dL | ||||||||
| Total bilirubin >3 mg/dL | 1.63 (1.20‐2.21) | 0.002 | 1.55 (1.14‐2.12) | 0.006 | ||||
| Albumin <3.8 g/dL | ||||||||
| PT–INR >1.1 | 1.70 (1.12‐2.58) | 0.013 | 1.59 (1.04‐2.42) | 0.033 | 2.18 (1.21‐3.93) | 0.010 | 2.19 (1.21‐3.97) | 0.001 |
| PTT >40 s | ||||||||
| Re‐transplant | 2.23 (1.20‐4.12) | 0.011 | ||||||
| Donor variables | ||||||||
| RGW/SLV (−0.1)a | 1.27 (1.05‐1.55) | 0.016 | 1.27 (1.04‐1.55) | 0.022 | ||||
| Donor age (5 y)a | 1.12 (1.05‐1.20) | 0.0003 | 1.12 (1.05‐1.20) | 0.0003 | ||||
| Operative variables | ||||||||
| Intraoperative estimated blood loss (L)a | 1.03 (1.01‐1.05) | 0.010 | ||||||
| Operative time (h)a | 1.10 (1.05‐1.15) | 0.0001 | 1.16 (1.09‐1.24) | <0.0001 | ||||
| Postoperative morbidities | ||||||||
| Prolonged ventilation >48 h | ||||||||
| Coma >24 h | ||||||||
| Serum total bilirubin >10 mg/dL) | ||||||||
| Renal dysfunction | ||||||||
| Postoperative systemic sepsis | ||||||||
| AUC | ||||||||
| AUC using validation dataset | 0.68 (0.61‐0.74) | 0.70 (0.64‐0.76) | 0.69 (0.60‐0.76) | 0.73 (0.65‐0.80) | ||||
| Coma >24 h | Postoperative transfusion >5 units | |||||||
|---|---|---|---|---|---|---|---|---|
| Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | |||||
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Constant (β0) | 0.002 (0.001‐0.007) | <0.0001 | 0.002 (0.001‐0.007) | <0.0001 | 0.042 (0.023‐0.075) | <0.0001 | 0.033 (0.016‐0.069) | <0.0001 |
| Preoperative variables | ||||||||
| ADL (prior to surgery) | 2.28 (1.40‐3.70) | 0.001 | 2.28 (1.40‐3.70) | 0.001 | 1.52 (1.14‐2.02) | 0.004 | 1.53 (1.14‐2.04) | 0.004 |
| ASA‐PS ≥4 | 2.08 (1.30‐3.32) | 0.002 | 2.08 (1.30‐3.32) | 0.002 | ||||
| Preoperative dialysis < 14 d | ||||||||
| Acute renal failure (within 24 h) | ||||||||
| Steroid use for chronic condition (history) | 2.69 (1.53‐4.73) | 0.001 | 2.69 (1.53‐4.73) | 0.001 | 1.85 (1.19‐2.86) | 0.006 | 1.67 (1.06‐2.62) | 0.026 |
| Dyspnea (within 30 d) | 1.93 (1.16‐3.22) | 0.012 | 1.93 (1.16‐3.22) | 0.012 | ||||
| Current pneumonia | 3.41 (1.31‐8.85) | 0.012 | 3.41 (1.31‐8.85) | 0.012 | ||||
| Ascites (within 30 d) | 1.43 (1.08‐1.90) | 0.013 | ||||||
| Esophageal varices | 1.78 (1.15‐2.76) | 0.010 | 1.78 (1.15‐2.76) | 0.010 | 1.78 (1.35‐2.36) | <0.0001 | 1.69 (1.28‐2.23) | 0.0002 |
| Bleeding disorders prior to surgery | 1.40 (1.04‐1.85) | 0.015 | ||||||
| Preoperative systemic sepsis | ||||||||
| Preoperative transfusion (within 72 h) | 1.82 (1.30‐2.53) | 0.0004 | 1.80 (1.27‐2.54) | 0.001 | ||||
| Preoperative weight ≥75 kg | ||||||||
| Hemoglobin <10 g/dL | 1.29 (1.00‐1.67) | 0.050 | ||||||
| Platelet <50 000/μL | 1.60 (1.21‐2.11) | 0.001 | 1.39 (1.04‐1.85) | 0.025 | ||||
| BUN >20 mg/dL | 1.57 (1.18‐2.10) | 0.002 | 1.41 (1.05‐1.90) | 0.023 | ||||
| Creatinine >2.0 mg/dL | ||||||||
| Total bilirubin >3 mg/dL | ||||||||
| Albumin <3.8 g/dL | 1.78 (1.17‐2.69) | 0.007 | 1.62 (1.06‐2.46) | 0.025 | ||||
| PT‐INR >1.1 | 4.06 (1.61‐10.27) | 0.003 | 4.06 (1.61‐10.3) | 0.003 | ||||
| PTT >40 s | ||||||||
| Re‐transplant | ||||||||
| Donor variables | ||||||||
| RGW/SLV (−0.1)a | 1.67 (1.24‐2.25) | 0.001 | 1.67 (1.24‐2.25) | 0.001 | 1.60 (1.32‐1.91) | <0.0001 | 1.54 (1.28‐1.85) | <0.0001 |
| Donor age (5 y)a | 1.12 (1.03‐1.21) | 0.006 | 1.12 (1.03‐1.21) | 0.006 | 1.06 (1.01‐1.11) | 0.020 | ||
| Operative variables | ||||||||
| Intraoperative estimated blood loss (L)a | 1.05 (1.02‐1.07) | <0.0001 | ||||||
| Operative time (h)a | 1.04 (1.00‐1.10) | 0.041 | ||||||
| Postoperative morbidities | ||||||||
| Prolonged ventilation >48 h | ||||||||
| Coma >24 h | ||||||||
| Serum total bilirubin >10 mg/dL) | ||||||||
| Renal dysfunction | ||||||||
| Postoperative systemic sepsis | ||||||||
| AUC | ||||||||
| AUC using validation dataset | 0.65 (0.51‐0.77) | 0.65 (0.51‐0.77) | 0.64 (0.58‐0.70) | 0.68 (0.62‐0.74) | ||||
| Sepsis (including septic shock) | Serum bilirubin >10 mg/dL | |||||||
|---|---|---|---|---|---|---|---|---|
| Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | Preoperative variables (C1) | Preoperative and intraoperative variables (C2) | |||||
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Constant (β0) | 0.016 (0.008‐0.032) | <0.0001 | 0.001 (0.000‐0.004) | <0.0001 | 0.003 (0.001‐0.008) | <0.0001 | 0.001 (0.000‐0.004) | <0.0001 |
| Preoperative variables | ||||||||
| ADL (prior to surgery) | 1.37 (1.08‐2.58) | 0.022 | 1.84 (1.20‐2.84) | 0.006 | 1.67 (1.08‐2.58) | 0.022 | ||
| ASA‐PS ≥4 | ||||||||
| Preoperative dialysis <14 d | ||||||||
| Acute renal failure (within 24 h) | ||||||||
| Steroid use for chronic condition (history) | 1.85 (1.01‐3.38) | 0.047 | ||||||
| Dyspnea (within 30 d) | ||||||||
| Current pneumonia | 3.46 (1.36‐8.78) | 0.009 | 3.87 (1.46‐10.3) | 0.006 | 3.31 (1.23‐8.90) | 0.018 | 3.87 (1.46‐10.26) | 0.006 |
| Ascites (within 30 d) | 2.14 (1.31‐3.47) | 0.002 | 2.18 (1.34‐3.56) | 0.002 | 2.14 (1.31‐3.47) | 0.002 | ||
| Esophageal varices | 1.90 (1.25‐2.90) | 0.003 | ||||||
| Bleeding disorders prior to surgery | 2.11 (1.46‐2.05) | <0.0001 | 1.64 (1.07‐2.51) | 0.024 | 1.64 (1.07‐2.51) | 0.024 | ||
| Preoperative systemic sepsis | 6.78 (1.97‐23.3) | 0.002 | ||||||
| Preoperative transfusion (within 72 h) | ||||||||
| Preoperative weight ≥75 kg | ||||||||
| Hemoglobin <10 g/dL | ||||||||
| Platelet <50 000/μL | 1.59 (1.09‐2.31) | 0.015 | 1.72 (1.13‐2.60) | 0.011 | ||||
| BUN >20 mg/dL | 2.53 (1.75‐3.64) | <0.0001 | ||||||
| Creatinine >2.0 mg/dL | ||||||||
| Total bilirubin >3 mg/dL | 1.66 (1.04‐2.64) | 0.034 | 1.72 (1.08‐2.73) | 0.023 | 1.66 (1.04‐2.64) | 0.035 | ||
| Albumin <3.8 g/dL | ||||||||
| PT‐INR >1.1 | ||||||||
| PTT >40 s | 1.52 (1.03‐2.25) | 0.036 | ||||||
| Re‐transplant | 2.30 (1.21‐4.38) | 0.011 | ||||||
| Donor variables | ||||||||
| RGW/SLV (−0.1)a | 1.43 (1.11‐1.83) | 0.007 | 1.60 (1.21‐2.13) | 0.001 | 1.70 (1.26‐2.21) | 0.0004 | 1.60 (1.21‐2.13) | 0.001 |
| Donor age (5 y)a | 1.08 (1.01‐1.16) | 0.021 | 1.17 (1.08‐1.26) | <0.0001 | 1.18 (1.10‐1.28) | <0.0001 | 1.17 (1.08‐1.27) | 0.0001 |
| Operative variables | ||||||||
| Intraoperative estimated blood loss (L)a | 1.04 (1.02‐1.07) | 0.002 | 1.04 (1.02‐1.07) | 0.002 | ||||
| Operative time (h)a | 1.10 (1.02‐1.19) | 0.012 | 1.10 (1.02‐1.19) | 0.012 | ||||
| Postoperative morbidities | ||||||||
| Prolonged ventilation >48 h | ||||||||
| Coma >24 h | ||||||||
| Serum total bilirubin >10 mg/dL | ||||||||
| Renal dysfunction | ||||||||
| Postoperative systemic sepsis | ||||||||
| AUC | ||||||||
| AUC using validation dataset | 0.69 (0.58‐0.78) | 0.66 (0.56‐0.75) | 0.67 (0.53‐0.78) | 0.71 (0.59‐0.80) | ||||
ADL, activities of daily living; ASA‐PS, American Society of Anesthesiologists physical status; AUC, area under the curve; BUN, blood urea nitrogen; CI, confidence interval; OR, odds ratio; PT‐INR, prothrombin time‐international normalized ratio; PTT, prothrombin time; RGW/SLV, ratio of graft weight to standard liver volume.
Data are expressed by odds ratio (95% confidence interval) and P‐value.
Units of odds ratios for continuous variables are denoted in parentheses.
3.3. Comparison between risk models developed in previous studies and those in the current study
Previously reported formulas for risk score (−0.203 × MELD +0.136 × GW/SLV [%] + 1.509)8 and predictive score formulas (0.011 × GW/SLV − 0.0016 × donor age [years] − 0.008 × MELD − 0.15 × shunt [if present] + 1.757)25 were compared with our own risk models after recalibration against the current sample. For these comparisons, AUC were calculated for each model based on a population for which the previous scores were developed (i.e. adult LDLT recipients); in the current derivation cohort, there were 1319 patients. AUC of the recalibrated risk models from previous studies were 0.62 (95% CI: 0.57‐0.67) and 0.62 (95% CI: 0.57‐0.67). These values were lower than our observation in the current study (0.71, 95% CI: 0.64‐0.77).
3.4. Risk calculator models using preoperative and intraoperative risk factors for morbidities and mortality: the C2 model
Risk models based on the C2 variables were created separately for morbidities and mortality using independent risk factors (Table 6). AUC of the risk calculator model that used the validation cohort for morbidities (range, 0.64‐0.74) and mortality (0.79, 95% CI: 0.69‐0.86) were higher than those of the C1 model.
Variables independently associated with mortality were as follows: three preoperative recipient‐related variables (ADL with any assistance prior to the surgery, ASA‐PS ≥ 4, and hemoglobin <10 g/dL); two donor‐related variables (RGW/SLV and donor age); and two intraoperative variables (estimated blood loss and operation time). Most of the factors in the risk models that used C2 variables for morbidities were similar to those that used C1 variables for morbidities.
3.5. Risk calculator model using preoperative, intraoperative, and postoperative risk factors for mortality: the C3 model
Multivariate analysis using C3 variables showed that the following variables represented independent risk factors for mortality: one preoperative variable (Hb <10 g/dL); one intraoperative variable (operative time); and five postoperative morbidities (prolonged ventilation >48 hours, coma >24 hours, renal dysfunction, postoperative systemic sepsis, and serum total bilirubin ≥10 mg/dL) (Table 6). These had an AUC of 0.87 (95% CI: 0.78‐0.93) using the validation cohort.
3.6. Validation analyses in the subgroups of deceased versus living donors and adult versus pediatric recipients
c‐Statistics (AUC) of postoperative mortality using whole validation cohort (n = 395), adult/LDLT (n = 227), adult/DDLT (n = 46), and pediatric LDLT (n = 115) were similar among the subgroups; 0.74 (0.63‐0.82), 0.72 (0.60‐0.82), 0.73 (0.73‐0.73), and 0.89 (0.89‐0.89) using C1 variables, 0.79 (0.69‐0.86), 0.74 (0.62‐0.83), 0.91 (0.91‐0.91), and 0.91 (0.91‐0.91) using C2 variables, and 0.87 (0.78‐0.93), 0.85 (0.74‐0.92), 1.00 (1.00‐1.00), and 1.00 (1.00‐1.00) using C3 variables, respectively.
4. DISCUSSION
In the present study, we used a combination of two Japanese nationwide databases to develop risk models of postoperative morbidity and mortality in LT recipients. To this end, we used three variable categories (C1, C2, and C3) for mortality and two variable categories (C1 and C2) for 10 postoperative morbidities. These models showed excellent discrimination and calibration, as confirmed by the independent validation cohort. To the best of our knowledge, this is the first study to develop “real‐time” risk calculator models of postoperative morbidities and mortality (Figure 2). Results from our studies enabled us to determine the real‐time risk for morbidity and mortality at each time point, ranging from the preoperative and immediate postoperative periods to the postoperative period.
Figure 2.
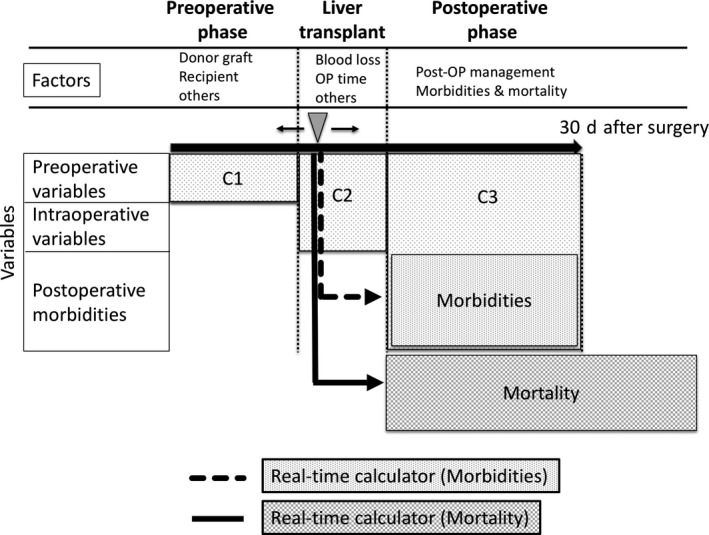
Schematic concept of “real‐time” risk calculator models of postoperative morbidities and mortality. “Real‐time” risk models provide the expected risk of morbidities and mortality at any time point from pre‐, intra‐, and postoperative periods within 30 d after the surgery. We used three variable categories (C1, C2, and C3) for mortality and two variable categories (C1 and C2) for 10 postoperative morbidities. C1, preoperative variables; C2, C1 + intraoperative variables; C3, C2 + postoperative morbidities
With the availability of real‐time risk models of postoperative morbidity and mortality at each time point post‐surgery, treatment team and caregivers might be encouraged to pay attention and possibly prevent or enhance recovery from specific morbidities and avoid mortality. Creation of an online feedback system or an automatic indication of high‐risk morbidities which includes laboratory tests and treatment strategies through electronic medical records would be the next viable step based on our findings. Currently, an online real‐time risk calculator is available for NCD users (https://registry3.ncd.or.jp/karte/page/feedback/index). The website calculates the probabilities for both morbidity and mortality in response to the clinical data input of C1, C2, or C3 variables. Additionally, benchmarks on morbidity incidence, rate of failure to rescue, and mortality based on risk‐adjusted comparison among hospitals could be established. Through these feedback and benchmarking systems, the outcomes of LT recipients could probably be improved as reported under the similar system of National Surgical Quality Improvement Program (NSQIP), American College of Surgeons.26, 27 Further, we should evaluate the impact of these risk calculators on clinical outcome in the future.
Several studies used either single‐center analysis8, 9 or registry data2, 7 to focus on the C1 risk model for mortality after LT. Importantly, although previous risk factor analyses included the MELD score as a preoperative predictor using C1 variables,8, 9, 11, 12, 13, 15, 25, 28, 29 in the present study, similar to a previous meta‐analysis,30 it was not an independent risk factor. Observation of a significantly improved AUC of the C1 risk calculator model for mortality versus the previously reported equations from single‐center analyses8, 9 indicates an effectiveness of these novel risk calculator models. In another words, compared with such risk models, our risk calculator was based on Japanese nationwide registry data and was more informative in terms of the data on the AUC.
Among the preoperative (C1) variables, re‐transplant (odds ratio, 2.55) and patients with ADL with any assistance (input to the NCD registry based on data collected prior to LT) (odds ratio, 2.17) had the highest risk for mortality using C1 variables. The other independent risk factors for mortality included donor age, allograft volume ratio to SLV, which were well‐known risk factors for allograft failure in LDLT.8, 9, 11, 25 One of the possible explanations for missing MELD score as an independent preoperative (C1) risk factor for mortality was that combination of other variables, including ADL and re‐transplant, was more important than MELD score.
Notably, real‐time risk model was more accurate in the C3 model (AUC 0.87, 95% CI 0.78‐0.93) than in the C2 (AUC 0.79, 95% CI 0.69‐0.86) and C1 models (AUC 0.74, 95% CI 0.63‐0.82). The majority of the most accurate risk factors for mortality, when using C3 variables, were postoperative morbidities. This indicates that postoperative events were more important than preoperative recipient or donor variables in predicting mortality after LT. When predicting mortality in C3 variables, prolonged ventilation >48 hours after transplant (OR = 3.62) and postoperative systemic sepsis (OR = 3.35) were the most important risk factors. This observation indicates that these morbidities were more important among all variables, and that they were directly associated with mortality compared with preoperative variables. The latter were indirectly associated with mortality through postoperative morbidities.
Similar to previous findings from a single‐center study,31 our results confirmed that hyperbilirubinemia following LT was a highly accurate marker for mortality, with an odds ratio of 2.24. Additional factors such as ADL with any assistance, preoperative weight ≥75 kg, RGW/SLV, and donor age were indirectly associated with mortality by variables such as hyperbilirubinemia, prolonged ventilation, coma, renal dysfunction, and postoperative systemic sepsis as shown in Table 6.
DDLT and LDLT ratios are quite different in Japan versus in other countries. In the present study, similar to a previous report,4 LDLT was more common (89.6%) in the derivation cohort. However, mortality risk was similar among donor types (P‐value = 0.973, data not shown). Types of recipient, either adult versus pediatric recipients, were also not independent risk factors for mortality and morbidities. Therefore, these variables were not included in the real‐time risk models. However, we further evaluated the accuracy of the risk models in these subgroups of DDLT versus LDLT and adult versus pediatric recipients using 2016 data, showing that our risk models, although they did not discriminate between these types of donors and recipients, could accurately determine the risks of each subgroup.
Although marginal allograft, such as severe steatosis and extended ischemia time, might influence the postoperative morbidity and mortality in deceased donors,18, 29 in the present study, we used exclusively donor age and graft volume as donor variables. In the majority of cases, allograft qualities such as cold ischemic time, steatosis, and fibrosis were sufficient and not marginal as a result of the nature of LDLT, which represented the majority of LT in this cohort. In Western countries where DDLT is the main procedure, our risk calculator would not be valid for LT recipients in its current form. However, the results of this study that postoperative morbidities and mortality were able to be accurately calculated using the simple data sets of C1, C2, and C3 variables, as well as the concept of these real‐time risk models, could still be applicable, and regional real‐time risk models could be developed in a similar way using, for example, big national registry data.
National registry data, which we used, were developed following the best field practices in each hospital. Importantly, hospital factors, such as high‐ or low‐volume center, were not included in this study.
A limitation of the present study was that our compiled database contained only in‐hospital morbidities and 30‐day mortality post‐surgery. As a consequence, the risk of mortality from morbidities beyond 30 days post‐surgery could not be evaluated using our database. Another limitation was that we did not include the exact time points of the occurrence of morbidities and their severities, as well as the specific variables for LT such as biliary/vascular complications. Unfortunately, as these variables were not available in the NCD and JLTS databases, we could not evaluate them in the current study. An additional limitation was that important variables in DDLT such as donor status, cause of death, cold ischemia time, or extent of steatosis of allograft were not included in this analysis. Using these specific variables with more DDLT cases will allow further refinement to the risk calculators for DDLT in the future. Another additional limitation was that in this study, we did not take into consideration the institutional disparities of surgical outcomes. This should be one of the next aspects to be evaluated for an accurate prediction of postoperative morbidities and mortality. Furthermore, our sample size was small compared with a previous registry‐based study.2 Nevertheless, an important advantage of the present study was the use of recent national data and the exclusion of results from the earlier periods when LT was evolving and developing.
In conclusion, we established real‐time risk models of postoperative morbidities and mortality for LT recipients at various perioperative time points using the combined data of the NCD and JLTS databases in Japan. Risk models and real‐time risk calculators are novel and viable tools aimed at improving the postoperative outcomes of LT recipients. These real‐time risk models could likewise be applicable and useful for several additional surgical procedures, which maintain certain risks for morbidity and mortality.
DISCLOSURE
Funding: This work was supported with a grant from the Japan Agency for Medical Research and Development (AMED).
Conflicts of interest: Authors declare no conflicts of interest for this article.
Ethical statement: This study was approved by the project committee of the JLTS, the ethics committee of the Japan Society of Transplantation (JST), and the institutional review board of Osaka General Medical Center, Osaka, Japan.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by a grant from the Japan Agency for Medical Research and Development (AMED). The authors thank Dr Norihiro Kokudo (ethics committee, the Japan Society for Transplantation); Dr Kenji Yuzawa (registration committee, the Japan Society for Transplantation); Dr Shinji Uemoto (immediate past president, Japanese Liver Transplant Society); Dr Yukihiro Inomata (registration committee, Japanese Liver Transplant Society); and Dr Hiroyuki Furukawa (project committee, Japanese Liver Transplant Society) for their outstanding contributions to this study.
Marubashi S, Ichihara N, Kakeji Y, et al. “Real‐time” risk models of postoperative morbidity and mortality for liver transplants. Ann Gastroenterol Surg. 2019;3:75–95. 10.1002/ags3.12217
REFERENCES
- 1. Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the united states, 1999‐2008. Am J Transplant. 2010;10:1003–19. [DOI] [PubMed] [Google Scholar]
- 2. Burroughs AK, Sabin CA, Rolles K, Delvart V, Karam V, Buckels J, et al. 3‐month and 12‐month mortality after first liver transplant in adults in europe: Predictive models for outcome. Lancet. 2006;367:225–32. [DOI] [PubMed] [Google Scholar]
- 3. Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, et al. Evolution of indications and results of liver transplantation in europe. A report from the european liver transplant registry (eltr). J Hepatol. 2012;57:675–88. [DOI] [PubMed] [Google Scholar]
- 4. Umeshita K, Inomata Y, Furukawa H, Kasahara M, Kawasaki S, Kobayashi E, et al. Liver transplantation in japan: Registry by the japanese liver transplantation society. Hepatol Res. 2016;46:1171–86. [DOI] [PubMed] [Google Scholar]
- 5. Olthoff KM, Merion RM, Ghobrial RM, Abecassis MM, Fair JH, Fisher RA, et al. Outcomes of 385 adult‐to‐adult living donor liver transplant recipients: A report from the a2all consortium. Ann Surg. 2005;242:314–23, discussion 323‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freise CE, Gillespie BW, Koffron AJ, Lok AS, Pruett TL, Emond JC, et al. Recipient morbidity after living and deceased donor liver transplantation: Findings from the a2all retrospective cohort study. Am J Transplant. 2008;8:2569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adam R, Cailliez V, Majno P, Karam V, McMaster P, Caine RY, et al. Normalised intrinsic mortality risk in liver transplantation: European liver transplant registry study. Lancet. 2000;356:621–27. [DOI] [PubMed] [Google Scholar]
- 8. Marubashi S, Nagano H, Eguchi H, Wada H, Asaoka T, Tomimaru Y, et al. Minimum graft size calculated from preoperative recipient status in living donor liver transplantation. Liver Transpl. 2016;22:599–606. [DOI] [PubMed] [Google Scholar]
- 9. Yoshizumi T, Taketomi A, Soejima Y, Uchiyama H, Ikegami T, Harada N, et al. Impact of donor age and recipient status on left‐lobe graft for living donor adult liver transplantation. Transpl Int. 2008;21:81–8. [DOI] [PubMed] [Google Scholar]
- 10. Alves RC, Fonseca EA, Mattos CA, Abdalla S, Goncalves JE, Waisberg J. Predictive factors of early graft loss in living donor liver transplantation. ArqGastroenterol. 2012;49:157–61. [DOI] [PubMed] [Google Scholar]
- 11. Ikegami T, Imai D, Wang H, Yoshizumi T, Yamashita Y, Ninomiya M, et al. D‐meld as a predictor of early graft mortality in adult‐to‐adult living‐donor liver transplantation. Transplantation. 2014;97:457–62. [DOI] [PubMed] [Google Scholar]
- 12. Schlegel A, Linecker M, Kron P, Gyori G, De Oliveira ML, Mullhaupt B, et al. Risk assessment in high‐ and low‐meld liver transplantation. Am J Transplant. 2017;17:1050–63. [DOI] [PubMed] [Google Scholar]
- 13. Wronka KM, Grat M, Stypulkowski J, Bik E, Krasnodebski M, Masior L, et al. Liver transplantation outcomes in recipients with high model for end‐stage liver disease (meld) scores: The relevance of meld scores. Ann Transplant. 2017;22:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lau L, Kankanige Y, Rubinstein B, Jones R, Christophi C, Muralidharan V, et al. Machine‐learning algorithms predict graft failure after liver transplantation. Transplantation. 2017;101:e125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stratigopoulou P, Paul A, Hoyer DP, Kykalos S, Saner FH, Sotiropoulos GC. High meld score and extended operating time predict prolonged initial icu stay after liver transplantation and influence the outcome. PLoS One. 2017;12:e0174173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waki K. Chapter 2. In Unos liver registry: Ten year survivals. Clinical Transplants 2006; p. 29–39. [cited 2018 Aug 1]. Available from https://terasaki.org/store/CH2-Waki_2006 [PubMed] [Google Scholar]
- 17. Petrowsky H, Rana A, Kaldas FM, Sharma A, Hong JC, Agopian VG, et al. Liver transplantation in highest acuity recipients: Identifying factors to avoid futility. Ann Surg. 2014;259:1186–94. [DOI] [PubMed] [Google Scholar]
- 18. Flores A, Asrani SK. The donor risk index: A decade of experience. Liver Transpl. 2017;23:1216–25. [DOI] [PubMed] [Google Scholar]
- 19. Asrani SK, Saracino G, O'Leary JG, Gonzales S, Kim PT, McKenna GJ, et al. Recipient characteristics and morbidity and mortality after liver transplantation. J Hepatol. 2018;69(1):43–50 [DOI] [PubMed] [Google Scholar]
- 20. Kenjo A, Miyata H, Gotoh M, Kitagawa Y, Shimada M, Baba H, et al. Risk stratification of 7,732 hepatectomy cases in 2011 from the national clinical database for japan. J Am Coll Surg. 2014;218:412–22. [DOI] [PubMed] [Google Scholar]
- 21. Matsubara N, Miyata H, Gotoh M, Tomita N, Baba H, Kimura W, et al. Mortality after common rectal surgery in japan: A study on low anterior resection from a newly established nationwide large‐scale clinical database. Dis Colon Rectum. 2014;57:1075–81. [DOI] [PubMed] [Google Scholar]
- 22. Gotoh M, Miyata H, Hashimoto H, Wakabayashi G, Konno H, Miyakawa S, et al. National clinical database feedback implementation for quality improvement of cancer treatment in japan: From good to great through transparency. Surg Today. 2016;46:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (roc) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 24. Yoshizumi T, Ikegami T, Bekki Y, Ninomiya M, Uchiyama H, Iguchi T, et al. Re‐evaluation of the predictive score for 6‐month graft survival in living donor liver transplantation in the modern era. Liver Transpl. 2014;20:323–32. [DOI] [PubMed] [Google Scholar]
- 25. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001;33:464–70. [DOI] [PubMed] [Google Scholar]
- 26. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the american college of surgeons national surgical quality improvement program: An evaluation of all participating hospitals. Ann Surg. 2009;250:363–76. [DOI] [PubMed] [Google Scholar]
- 27. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: The american college of surgeons national surgical quality improvement program approach. Adv Surg. 2010;44:251–67. [DOI] [PubMed] [Google Scholar]
- 28. Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–13. [DOI] [PubMed] [Google Scholar]
- 29. Bonney GK, Aldersley MA, Asthana S, Toogood GJ, Pollard SG, Lodge JP, et al. Donor risk index and meld interactions in predicting long‐term graft survival: A single‐centre experience. Transplantation. 2009;87:1858–63. [DOI] [PubMed] [Google Scholar]
- 30. Klein KB, Stafinski TD, Menon D. Predicting survival after liver transplantation based on pre‐transplant meld score: A systematic review of the literature. PLoS One. 2013;8:e80661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marubashi S, Dono K, Nagano H, Asaoka T, Hama N, Kobayashi S, et al. Postoperative hyperbilirubinemia and graft outcome in living donor liver transplantation. Liver Transpl. 2007;13:1538–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


