Abstract
Pancreatic islet transplantation provides an effective treatment option for patients with type 1 diabetes (T1D) with intractable impaired awareness of hypoglycemia and severe hypoglycemic events. Currently, the primary goal of islet transplantation should be excellent glycemic control without severe hypoglycemia, rather than insulin independence. Islet transplant recipients were less likely to achieve insulin independence, whereas solid pancreas transplant recipients substantially had greater procedure‐related morbidity. Excellent therapeutic effects of islet transplantation as a result of accurate blood glucose level–reactive insulin secretion, which cannot be reproduced by current drug therapy, have been confirmed. Recent improvement of islet transplantation outcome has been achieved by refinement of the pancreatic islet isolation technique, improvement of islet engraftment method, and introduction of effective immunosuppressive therapy. A disadvantage of islet transplantation is that donors are essential, and donor shortage has become a hindrance to its development. With the development of alternative transplantation sites and new cell sources, including porcine islet cells and embryonic stem/induced pluripotent stem (ES/iPS)‐derived β cells, “On‐demand” and “Unlimited” cell therapy for T1D can be established.
Keywords: islet isolation, islet transplantation, regenerative medicine, severe hypoglycemic event, type 1 diabetes
1. INTRODUCTION
Type 1 diabetes (T1D) is a chronic metabolic disorder characterized by autoimmune‐mediated destruction of insulin‐secreting beta cells in the pancreatic islets.1 Estimated life expectancy for patients with T1D in a population‐based sample indicated lower life expectancy compared with the general population without T1D.2 Whole organ pancreas or pancreatic islet transplantation can potentially restore long‐term normoglycemia, and select patients with T1D can potentially become insulin independent. Pancreas transplantation is currently accepted as a commonly carried out therapy.3 To date, more than 35 000 pancreas transplants have been carried out worldwide4; however, the procedure is associated with significant mortality and morbidity in the early transplant period.5 A major benefit of islet transplantation is that it does not require major surgery. The treatment involves extracting sufficient numbers of pancreatic islets from the donor pancreas and infusing these through the portal vein into the patient's liver under local anesthesia. Excellent therapeutic effects of islet transplantation as a result of accurate blood glucose level–reactive insulin secretion, which cannot be reproduced by current drug therapy, have been confirmed. Currently, although all patients with T1D who underwent islet transplantation are not insulin independent, it is recognized as a safe and highly effective treatment for patients with impaired awareness of hypoglycemia (IAH) and severe hypoglycemic events (SHE). Meanwhile, some problems need to be solved, such as severe donor shortage for islet transplantation, necessity to search for alternative transplantation sites that can be more efficiently engrafted, and to develop alternative cell sources. In this review, we provide an outline on clinical islet transplantation (CIT). We also introduce the refinement that has been attempted to improve the clinical outcome, we then discuss future prospects based on the latest findings.
2. BRIEF OVERVIEW OF CLINICAL ISLET TRANSPLANTATION
For patients with T1D, lifetime exogenous insulin administration is required to control blood glucose level. Some patients are at risk of IAH and SHE,6 which have a negative impact on quality of life. In addition, blood glucose instability can cause various complications, such as retinopathy and neuropathy.7 Treatment to improve blood glucose instability of patients with T1D includes β‐cell replacement therapy, namely whole pancreas transplantation and pancreatic islet transplantation. Insulin independence can be achieved after pancreas transplantation from only one donor, and long‐term insulin independence can be expected. Numbers of transplantations have increased with induction of improved immunosuppressive regimens, progress of the organ preservation method, and improvement of operative techniques.8 Pancreas and combined kidney graft function has improved significantly over time. For instance, 1‐year primary pancreas graft function increased from 77.2% in 1987‐1993 to 85.5% in 2006‐2010 in patients who underwent simultaneous pancreas‐kidney (SPK) transplant.4 Although pancreas transplantation is considered a proven therapy, some physicians are reluctant to recommend this procedure to patients because of its complexity and risks, particularly for pancreas transplant alone.4 Islet transplantation is carried out to intraportally transfuse isolated pancreatic islets only, which constitute only 1%‐2% of the pancreas.9 This procedure was expected to be an ideal transplant therapy for patients with severe glycemic instability, and development aiming to improve this procedure has been undertaken worldwide. Ballinger and Lacy demonstrated a method for isolating pancreatic islets from rodents and confirmed in vivo function in 1972.10 By the establishment of the islet isolation method called the Ricordi method,11 the first case of clinical insulin independence was reported in 1990 by Scharp et al.12 The number of clinical transplantations gradually increased; however, insulin independence after islet allotransplantation was difficult to achieve in the earliest years. The International Islet Transplant Registry reported transplant outcomes of 237 patients with T1D who received adult islet allografts between 1990 and 1999. The 1‐year islet allograft survival (defined by basal C‐peptide ≧0.5 ng/mL) rate was 41%, and 11% of the recipients were insulin independent at 1 year post‐transplant.13 The result seemed to be insufficient for recognizing generalized treatment for T1D before 2000. In 2000, the “Edmonton Protocol” was seen as a key advance in islet transplantation when all seven patients treated achieved and maintained insulin independence.14 These patients subsequently received two or three different islet transplants, and the mean islet mass was 13 000 islet equivalents/kg in combination with a glucocorticoid‐free immunosuppressive regimen with anti‐interleukin‐2 receptor antagonist antibody therapy. This success increased the interest and activity among countries involved in the CIT program. Subsequently, an international multicenter trial was conducted to explore the feasibility and reproducibility using the Edmonton Protocol.15 This trial showed that the protocol could successfully restore long‐term endogenous insulin secretion and glycemic stability in patients with T1D; however, insulin independence was not sustainable. The protocol showed that the long‐term results were not enduring; hence, more advances were needed.
Hering et al16 introduced refinements in induction immunosuppressive therapy using T cell–depleting antibody (thymoglobulin) and peritransplant management that increased the proportion of subjects maintaining insulin independence with a single‐donor islet infusion. The use of T cell–depleting antibody for induction immunosuppressive therapy has become mainstream since the publication of this successful result.
Based on a report of 677 cases of islet transplantation registered in the Collaborative Islet Transplant Registry (CITR: https://citregistry.org/)), the insulin independence rate was 27% after transplantation from 1999 to 2002, and it improved to 44% from 2007 to 2010. Release from SHE is maintained long‐term even in cases where insulin independence is not maintained. Multivariate analysis showed that factors influencing transplantation outcome were age of the recipient, transplanted islet yields, and viability of the pancreatic islet. Particularly, initial T cell–depleting therapy and tumor necrosis factor (TNF)‐α inhibition had mainly contributed to improving insulin independent rate.17
These findings are progressing forward in phase III trials to obtain Biological Licensure Application for an islet product from the FDA (CIT‐07: Islet Alone Licensure Study), supported through the CIT Consortium funded by the National Institutes of Health in the USA. In this trial, T cell–depleting therapy and TNF‐α inhibition induction combined with maintenance calcineurin inhibitor with mammalian target of rapamycin (mTOR) inhibitor or mycophenolate mofetil (MMF) were adopted as immunosuppressive therapy. This trial enrolled 48 patients and was completed in 2015. Although the insulin independence rate was approximately 50% 1 year after transplantation, islet transplantation was reported to provide glycemic control, restoration of hypoglycemia awareness, and protection from SHE in 87.5% of participants at 1‐year follow up.18 Improvement of health‐related quality of life has also been reported.19 The effectiveness of similar protocols, including thymoglobulin, tacrolimus, and MMF, has also been shown in multicenter trials in Australia. In this trial, 14 of 17 (82%) recipients achieved hemoglobin A1c (HbA1c) of <7% and cessation of severe hypoglycemia. Nine (53%) recipients achieved insulin independence for a median of 26 months (range, 7‐39 months).20 Recently, another phase III study has been completed. This was a multicenter, open‐label, randomized controlled trial to assess the efficacy and safety of islet transplantation compared with insulin therapy in patients with T1D with severe hypoglycemia or after kidney transplantation. This trial showed that islet transplantation effectively improves metabolic outcomes compared with insulin therapy.21 This is the first randomized controlled trial in the field of islet transplantation. Future research is necessary to establish the role of islet transplantation versus new technologies, such as sensor‐augmented pump therapy and automated insulin delivery.
Determining whether islet transplantation can suppress the onset of diabetic complications is important. A prospective, crossover, cohort study indicated that islet transplantation is associated with lower progression of microvascular complications, including nephropathy, retinopathy, and polyneuropathy, compared with intensive medical therapy.22 In addition, a health economic analysis showed that islet transplantation is cost‐effective in the short term and cost‐saving in the long term compared with standard insulin treatment.23 Because the clinical significance of the islet transplant is accumulating, islet transplantation is approved and reimbursable by insurance companies or covered by national health systems in several countries, including Canada, Australia, and several European countries.24 Table 1 summarizes the current and completed clinical trials and their results in islet transplantation.
Table 1.
Overview of selected clinical trials in islet transplantation
| Trial ID | Patient N | Investigators | Transplant type | Transplant site | Induction | Maintenance | Status | Primary endpoint | Percentage of participants that achieved the primary endpoint | Insulin independent rate (at any point throughout the trial) | Year of publicationRef |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N/A | N = 7 | Alberta | ITA | Liver | Daclizumab | Tacrolimus, sirolimus | Completed | N/A | N/A | 7/7 (100%) | 200014 |
| NCT00014911 | N = 36 | Multicenter (Alberta etc.) | ITA | Liver | Daclizumab | Tacrolimus, sirolimus | Completed | Insulin independence with adequate glycemic control 1 y after the final transplantation | 44% | 21/36 (58%) | 200615 |
| NCT00285194 | N = 6 | Minnesota | ITA | Liver | Anti‐CD3 | Tacrolimus, sirolimus | Completed | Safety, tolerability, immune activity, and pharmacokinetics of hOKT3γ1 (Ala‐Ala) antibody induction therapy | N/A | 4/6 (67%) | 200425 |
| N/A | N = 8 | Minnesota | ITA | Liver | ATG, daclizumab, etanercept | Tacrolimus, sirolimus or MMF | Completed | Proportion of recipients who achieve insulin independence in the first year after a single‐donor islet transplant | 100% | 8/8 (100%) | 200516 |
| NCT00434811 | N = 48 | Multicenter (Minnesota etc.) | ITA | Liver | ATG, daclizumab, etanercept | Tacrolimus, sirolimus or MMF | Completed | Achievement of HbA1c <7.0% at day 365 and freedom from severe hypoglycemic events from day 28 to day 365 after the first transplant | 87.5% | 25/48 (52.1%) | 201618 |
| ACTRN083020 | N = 17 | Australia, multicenter | ITA | Liver | ATG, daclizumab, etanercept | Tacrolimus, sirolimus, MMF | Completed | HbA1c of <7% and cessation of severe hypoglycemia | 82% | 9/17 (53%) | 201320 |
| NCT01148680 | N = 25 vs 24 (RCT) | France, multicenter | ITA and IAK | Liver | ATG, daclizumab, etanercept | Tacrolimus, MMF | Completed | Proportion of patients with a modified β‐score (in which an overall score of 0 was not allocated when stimulated C‐peptide was negative) of 6 or higher at 6 mo after first islet infusion | 64% vs 0% (Immediate transplantation group vs 6 mo after randomization in the insulin group) | 11/25 (44%) | 201821 |
| UMIN000003977 | N = 20 | Japan, multicenter | ITA and IAK | Liver | ATG, daclizumab, etanercept | Tacrolimus or cyclosporine, MMF | Recruiting | Achievement of HbA1c <7.4% at day 365 and freedom from severe hypoglycemic events from day 28 to day 365 after the first transplant | Not yet | Not yet | Not yet |
| NCT00468117 | N = 24 | Multicenter (Minnesota etc.) | IAK | Liver | ATG, daclizumab, etanercept | Tacrolimus, sirolimus or MMF | Completed | Proportion of patients with HbA1c </= 6.5% and an absence of severe hypoglycemic events or a reduction in HbA1c of at least 1 point and an absence of severe hypoglycemic events | Not yet | Not yet | Not yet |
| N/A | N = 8 | San Francisco | ITA | Liver | ATG | Efalizumab, sirolimus or MMF | Completed | N/A | N/A | 8/8 (100%) | 201026 |
| NCT01722682 | N = 9 | Italy | ITA | Bone marrow vs liver | Unknown | Unknown | Completed | Insulin secretion under stimulation | Not yet | Not yet | Not yet |
| NCT02213003 | N = 6 | Miami | ITA | Omentum | ATG, etanercept | Tacrolimus, MMF | Recruiting | HbA1c </= 6.5% and no severe hypoglycemia | Not yet | Not yet | Not yet |
| NCT02064309 | N = 4 | Uppsala | ITA | Subcutaneous, macroencapsulation devices | No immunosuppression | No immunosuppression | Completed | Safety of device, as evaluated by incidence of adverse events or serious adverse events judged probable or highly probable related to the device | N/A | 0/4 (0%) | 201827 |
| NCT02239354 | N = 65 | Multicenter (Alberta etc.) | hESC‐derived pancreatic beta cells | Subcutaneous, macroencapsulation devices | No immunosuppression | No immunosuppression | Active, not recruiting |
1. Incidence of all adverse events 2. Change in C‐peptide |
Not yet | Not yet | Not yet |
| NCT01739829 | N = 8 | Argentina | Porcine islets | Microencapsulation, intraperitoneal | No immunosuppression | No immunosuppression | Completed | Reduction in the unaware hypoglycemic event rate combined with no increase in HbA1c | 4/4 (100%; High‐dose group) | 0/8 (0%) | 201628 |
ATG, antithymocyte globulin; HbA1c, hemoglobin A1c; hESC, human embryonic stem cell; IAK, islet after kidney; ITA, islet transplant alone; MMF, mycophenolate mofetil; N/A, not applicable.
3. REFINEMENTS IN ISLET TRANSPLANTATION
Figure 1 shows the possible factors that can lead to loss of pancreatic islets before, during, and after intraportal transplantation. Many improvements have been attempted to improve the loss of islet mass as a result of these factors.
Figure 1.
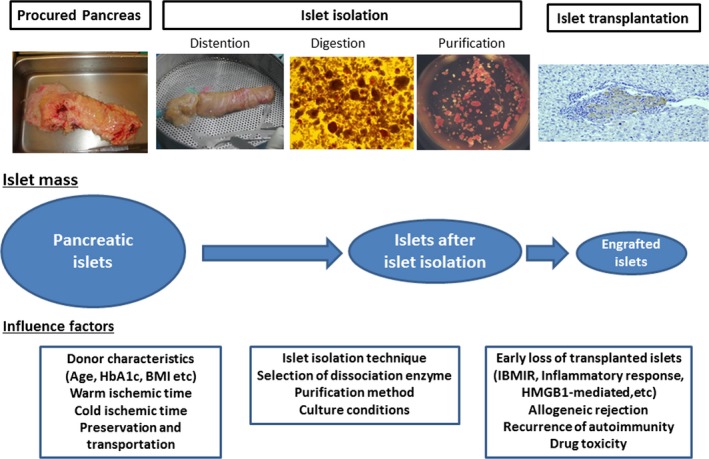
Process to islet transplantation and factors that contribute to islet loss. Procurement of pancreas from the deceased donor and preservation, islet isolation; pancreatic islets are then transplanted into the portal vein. Islets are lost during these processes due to the influence of many factors. Attempts to avoid loss as a result of these factors are important for improving islet transplantation outcome. BMI, body mass index; HbA1c, hemoglobin A1c; HMGB1, high‐mobility group box 1; IBMIR, instant blood‐mediated inflammatory reaction
3.1. Islet isolation
Transplantation of a sufficient mass of viable islets is essential to achieve better graft survival. Although great progress has been made in the standardization of islet isolation, improvements are still needed. In brief, islet isolation methods are as follows. The procured and cold‐preserved pancreas is distended with cold enzyme solution through the pancreatic duct in the cell processing unit. The distended pancreas is digested using the semiautomated method.11 The pancreatic digest is purified by continuous density gradient on a COBE 2991 cell processor (Terumo BCT, Gambro BCT, Inc., Lakewood, CO) under cold conditions. Liberated islets from grafts are cultured, and the islets are transplanted if the following releasing criteria are met: islet mass ≥5000 islet equivalents (IE)/kg (recipient body weight), islet purity ≥30%, membrane integrity‐viability ≥70%, packed‐tissue volume <10 mL, negative Gram stain, and content ≤5 endotoxin U/kg (recipient body weight). The islet isolation procedure seriously damages cellular and noncellular components of the pancreas.29, 30 The graft may be damaged by hypoxia, warm ischemia, activated proteolytic enzymes released from the acinar cells, mechanical stress, and oxidative stress. The pancreas digestion step, in particular, remains an empirical undertaking dependent on the activity of tissue‐digesting enzymes. Research efforts should focus on understanding the detailed molecular ultrastructure of the pancreatic islet‐exocrine matrix31 within the full range of donors (age, body mass index etc.), and on developing new, targeted clinical grade enzyme (recombinant) blends that can be used on all available donor pancreata.32
Purification outcome after density gradient separation is highly variable as a result of inconsistencies in tissue densities. Prior measurement of densities of islet and acinar tissue to customize the gradient range for actual purification likely maximizes islet recovery. A test gradient approach using multiple discontinuous gradients33 and an analytical/continuous test gradient method, for predicting pancreatic tissue densities before large‐scale purification,34 have been successfully introduced to minimize islet loss during purification.
3.2. Islet engraftment
The isolated islets are transplanted from a catheter placed in the portal vein percutaneously under local anesthesia by infusing the product suspended in the transplant media by gravity. General anesthesia and laparotomy are unnecessary, and transplantation is completed in a short time. The portal vein can be accessed by interventional radiology under ultrasonographic and fluoroscopic guidance. If this approach is not possible, such as in cases of large hepatic hemangioma, the portal vein system can be accessed surgically by limited laparotomy with i.v. infusion following catheterization of the mesenteric vein.35
Approximately 25%‐50% of the transplanted islets have been reported to maintain their functional reserve based on metabolic tests after transplantation.36 Instant blood‐mediated inflammatory reaction (IBMIR) is considered a main reason of substantial loss of islets during intraportal transplantation.37 IBMIR is characterized by platelet activation, coagulation, and complement systems triggered when islets are exposed to ABO‐compatible blood.37 Further studies showed that tissue factor plays an important role in mediating IBMIR.38 Several approaches demonstrated value in preventing IBMIR, including low molecular weight dextran sulfate,39 islet surface heparinization,40 and activated protein C.41
Strategies to inhibit inflammatory cytokines, including TNF‐α and interleukin 1 (IL‐1), have been considered therapeutic targets to improve islet engraftment. Hering et al16 applied TNF‐α receptor antibody and etanercept in the clinical setting, and etanercept has currently been included in many islet transplant protocols.17 IL‐1 receptor antagonist, anakinra, has also been applied in the clinical setting.42 Combination of etanercept and anakinra led to the improvement in islet engraftment by the marginal‐dose transplantation model.43
Yasunami et al44 have shown that early graft loss after islet transplantation is caused by natural killer T (NKT) cell–dependent interferon (IFN)‐γ production by Gr‐1+CD11b+ cells and is successfully prevented by treatment of NKT cells with repeated stimulation with their synthetic ligand, α‐galactosylceramide (α‐GalCer), to downregulate IFN‐γ production of NKT cells, or by depletion of Gr‐1+CD11b+ cells with anti‐Gr‐1 antibody. In addition, treatment with high‐mobility group box 1 (HMGB1)–specific antibody inhibited IFN‐γ production by NKT cells and Gr‐1+CD11b+ cells and prevented early islet graft loss. HMGB1‐mediated pathway is a potential therapeutic target to improve the efficiency of islet engraftment.45 The CXCL1‐CXCR1/2 axis was indicated as a therapeutic target for improving engraftment. The CXCR1/2 inhibitor significantly reduces post‐transplant recruitment of polymorphonuclear leukocytes and NKT cells. The CXCR1/2 allosteric inhibitor reparixin was already used for a phase II randomized, open‐label pilot study of CIT and indicated improved outcome.46 Identification of pathways that regulate post‐transplant detrimental inflammatory events seems to be a promising step toward improvement of islet transplantation outcome.
3.3. Immunosuppression
As mentioned earlier, the Edmonton Protocol has shown the usefulness of steroid‐free immunosuppressive therapy,14, 15 and the usefulness of T cell–depleting antibody has been demonstrated in recent clinical outcomes.17 Alemtuzumab (anti‐CD52 antibody) is also used similarly to T cell–depleting antibody, apart from thymoglobulin, which adopted CIT‐07.18 The Edmonton group reported that the immunosuppressive method using alemtuzumab and etanercept induction combined with maintenance tacrolimus and MMF was well tolerated and showed higher 5‐year insulin independence rates than the conventional Edmonton Protocol.24 T cell–depleting antibody might suppress autoimmunity, and development of future research is expected.
Calcineurin inhibitor (tacrolimus) is mainly used in maintenance immunosuppressive therapy. In addition, sirolimus, an inhibitor of mTOR or MMF, is used in combination with calcineurin inhibitor. Side‐effects of calcineurin inhibitor, such as nephrotoxicity and impaired glucose tolerance, are of concern, but, currently, it shows the most effective maintenance immunosuppressive effect. Several attempts have been made to establish more effective immunosuppressive therapy by calcineurin inhibitor/steroid‐sparing protocols. The University of California, San Francisco group proposed two immunosuppressive regimens based on the costimulation blocker belatacept or antileukocyte functional antigen‐1 antibody efalizumab.47 This group described the efficacy of two immunosuppression regimens that consisted of antithymocyte globulin induction and maintenance with sirolimus or mycophenolate and belatacept or efalizumab. Both calcineurin inhibitor/steroid‐sparing protocols could achieve long‐term insulin independence after one or two islet transplantations. Unfortunately, efalizumab was withdrawn from clinical use because of a rare incidence of progressive multifocal leukoencephalopathy when used in a different clinical setting. However, these findings showed that transplantation results can be improved by novel maintenance immunosuppressive therapy, and further development in the future is possible.
4. FUTURE PROSPECTS
4.1. Alternative transplant site
The liver might not be the “ideal site” for islet transplantation because early graft loss can occur by IBMIR, it is inaccessible for graft biopsy, and graft removal is impossible. Research should focus on alternative sites that ensure that islet transplantation remains a safer, well tolerated, and minimally invasive treatment, but that ensures improved islet graft survival.
Several clinical trials have been conducted or are ongoing to verify the safety and efficacy of an alternative transplant site. In a pilot study of autologous islet transplantation to test feasibility and safety of the bone marrow as a site for islet transplantation in humans, islets were successfully engrafted as shown by measurable C‐peptide levels and histopathological evidence of insulin‐producing cells in the biopsy of four patients who developed diabetes after total pancreatectomy.48 This group started a clinical trial to evaluate safety and efficacy of the bone marrow as the site for pancreatic islet transplantation for T1D in humans (NCT01722682). In contrast, islets transplanted in the bone marrow seem to be less protected from the adaptive immune response in the presence of anti‐CD3 treatment in a mouse model.49 At the moment, the evidence is poor, and the expectation as an alternative transplant site is not very high, but future research is expected.
The omentum, which covers the abdominal organs, is highly vascularized, easily accessible, and drains into the portal system, is a part considered as an effective transplant site. Recent approach using an in situ‐generated adherent, resorbable plasma‐thrombin biological scaffold showed the potency of islets implanted onto the omentum by evaluation in diabetic rat and non‐human primate (NHP) models.50 Based on feasibility/efficacy data from this study, they proceeded to a pilot phase I/II clinical trial for allogenic islet transplantation (NCT02213003). In this trial, the authors reported a case of insulin independence under induction immunosuppression consisting of antithymocyte globulin and etanercept and maintenance immunosuppression consisting of tacrolimus and mycophenolate sodium.51 This trial is still under investigation.
The subcutaneous space has been considered to have several advantages as a promising site for pancreatic islet transplantation, offering better acceptability, possible graft removal, and better safety. However, as a result of poor vascularization and low oxygenation, engrafting the islet in the subcutaneous space is difficult. Various approaches, such as islet encapsulation in a semipermeable membrane (bioartificial pancreas)52 or prevascularization before islet transplant, have been carried out. A particularly interesting approach is a method proposed by Luan and Iwata to achieve long‐term allogeneic islet graft survival in prevascularized subcutaneous sites of diabetic rats without immunosuppression. Agarose rods with basic fibroblast growth factor and heparin were implanted in subcutaneous sites before transplantation. The islets were transplanted into the prevascularized sites after rod removal. Allogeneic islets transplanted into this site showed long‐term graft survival and function in a diabetic rat model without immunosuppression.53 This prevascularized method led to granulomatous tissue formation containing regulatory T cells that suppressed immune reactions for the grafts.54 A similar approach was reported from the Edmonton group. They reported “device‐less” islet transplantation into a prevascularized, subcutaneous site created by temporary placement of a vascular access catheter.55
Other clinical reports of islet transplantation to intramuscular56 or intraperitoneal28 sites and other similar procedures have been made, and future development of an ideal transplantation site is desired.
4.2. Alternative cell sources
Donor shortage is a major problem for patients who need islet transplantation. Realization of xenogeneic (porcine) islet transplantation and stem cell–derived β cells has been expected to overcome donor shortage and to achieve on‐demand cell supply. Recent research results have been clinically developed and become a realistic option.
Pig insulin has shown clinical efficacy because it was the main insulin treatment for diabetes before the advent of genetic recombinant technology in the 1980s. In addition, there are several advantages, such as unlimited and on‐demand supply, and it is ethically acceptable.57 For the success of xenogeneic islet transplantation, control of xenogeneic immune reaction, including hyperacute rejection and the possibility of zoonotic infection of porcine endogenous retrovirus (PERV), has been regarded as obstacles to clinical development. To prevent xenogeneic immune reaction, a method of giving a plurality of immunosuppressive agents to NHP was attempted, and success in the NHP model has been reported.58 More recently, the utilization of new technologies has been studied, including genetic engineering,59 cellular therapy,60 and encapsulating devices.61 Clinical trials have already been conducted in Oceania and South America for intraperitoneal porcine islet transplantation using microencapsulation.28 The transmission of PERV has been a major safety concern; however, no transmission has been observed to date in human or NHP recipients.57 Recent advances in genome‐editing technology may become a major solution in the near future.62
Research on ES/iPS cell–derived pancreatic β‐cell (insulin‐producing cells) transplantation has been rapidly progressing in recent years. The possibility of curing diabetes at the small animal level has been shown.63, 64 Establishment of a functional and large amount of insulin‐producing cell preparation technology and development of an effective and safe transplantation method has been considered to be necessary for clinical application. Phase I/II trial has already been undertaken (NCT02239354) wherein human embryonic stem cell (ESC) is implanted at the differentiation stage of endocrine progenitors. The authors adopted a method of subcutaneous implantation using a macroencapsulation device to mitigate the risk of tumor formation and protect cells from allogeneic immunity and autoimmunity.65, 66 Furthermore, next‐generation devices that enable angiogenesis of encapsulated ESC‐derived β cells have been developed, and clinical trials using such devices have also been approved (NCT03162926). For the development of stem cell–derived β‐cell transplantation, clarification of the significance of cell therapy is necessary based on the clinical results of islet transplantation and the development of a safe and effective alternative transplant site.
5. CONCLUSIONS
During the last 10 years, islet allotransplantation has developed into an established treatment modality for patients with T1D complicated by IAH and SHE. Further improvement of islet transplantation outcome is expected in the near future as a result of development of pancreatic islet isolation technique, improvement of islet engraftment method, and refinement of immunosuppressive therapy. In addition, with the development of alternative transplantation sites and new cell sources, including porcine islet cells and ES/iPS‐derived β cells, “On‐demand” and “Unlimited” cell therapy for diabetes will be established.
The minimally invasive nature of the procedure leads to the possibility of expanding the target disease in the future. For example, in the future, we can discuss strategies for implementing islet transplantation for children, as well as for conditions with severe β‐cell failure other than T1D (ie, type 2 diabetes, cystic fibrosis, after pancreatectomy). To achieve target expansion, alternative transplant sites and alternative cell sources of pancreatic β cells are clearly a key to implementing this goal.
DISCLOSURE
Funding: This work was supported in part by JSPS KAKENHI Grant Number 18K08593 from the Ministry of Health, Labour and Welfare of Japan.
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Anazawa T, Okajima H, Masui T, Uemoto S. Current state and future evolution of pancreatic islet transplantation. Ann Gastroenterol Surg. 2019;3:34–42. 10.1002/ags3.12214
REFERENCES
- 1. Maahs DM, West NA, Lawrence JM, Mayer‐Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008‐2010. JAMA. 2015;313(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiang JL, Kirkman MS, Laffel LM, Peters AL, Type 1 Diabetes Sourcebook Authors . Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty‐four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2011;8(1):6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gruessner RW, Sutherland DE, Troppmann C, et al. The surgical risk of pancreas transplantation in the cyclosporine era: an overview. J Am Coll Surg. 1997;185(2):128–44. [DOI] [PubMed] [Google Scholar]
- 6. Hopkins D, Lawrence I, Mansell P, et al. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes: the U.K. DAFNE experience. Diabetes Care. 2012;35(8):1638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diabetes Control and Complications Trial Research Group , Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. [DOI] [PubMed] [Google Scholar]
- 8. Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233(4):463–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Kort H, de Koning EJ, Rabelink TJ, Bruijn JA, Bajema IM. Islet transplantation in type 1 diabetes. BMJ. 2011;342:d217. [DOI] [PubMed] [Google Scholar]
- 10. Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72(2):175–86. [PubMed] [Google Scholar]
- 11. Ricordi C, Lacy P, Scharp D. Automated islet isolation from human pancreas. Diabetes. 1989;38(Suppl 1):140–2. [DOI] [PubMed] [Google Scholar]
- 12. Scharp DW, Lacy PE, Santiago JV, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes. 1990;39(4):515–8. [DOI] [PubMed] [Google Scholar]
- 13. Brendel MD HB, Schultz AO, Bretzel RG. International Islet Transplant Registry. Newsletter # 9. University Hospital Giessen. 2018. http://www.med.uni-giessen.de/itr/newsletter/no_9/news_9.pdf. Accessed June 3, 2018.
- 14. Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid‐free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8. [DOI] [PubMed] [Google Scholar]
- 15. Shapiro A, Ricordi C, Hering B, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–30. [DOI] [PubMed] [Google Scholar]
- 16. Hering B, Kandaswamy R, Ansite J, et al. Single‐donor, marginal‐dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–5. [DOI] [PubMed] [Google Scholar]
- 17. Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999‐2010. Diabetes Care. 2012;35(7):1436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foster ED, Bridges ND, Feurer ID, et al. Improved health‐related quality of life in a phase 3 Islet Transplantation Trial in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2018;41:1001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Connell PJ, Holmes‐Walker DJ, Goodman D, et al. Multicenter Australian trial of islet transplantation: improving accessibility and outcomes. Am J Transplant. 2013;13(7):1850–8. [DOI] [PubMed] [Google Scholar]
- 21. Lablanche S, Vantyghem MC, Kessler L, et al. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;S2213–8587(18):30078. [DOI] [PubMed] [Google Scholar]
- 22. Thompson DM, Meloche M, Ao Z, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91(3):373–8. [DOI] [PubMed] [Google Scholar]
- 23. Beckwith J, Nyman JA, Flanagan B, Schrover R, Schuurman HJ. A health economic analysis of clinical islet transplantation. Clin Transplant. 2012;26(1):23–33. [DOI] [PubMed] [Google Scholar]
- 24. Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13(5):268–77. [DOI] [PubMed] [Google Scholar]
- 25. Hering B, Kandaswamy R, Harmon J, et al. Transplantation of cultured islets from two‐layer preserved pancreases in type 1 diabetes with anti‐CD3 antibody. Am J Transplant. 2004;4(3):390–401. [DOI] [PubMed] [Google Scholar]
- 26. Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti‐LFA‐1 antibody efalizumab. Am J Transplant. 2010;10(8):1870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carlsson PO, Espes D, Sedigh A, et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am J Transplant. 2018;18(7):1735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsumoto S, Abalovich A, Wechsler C, Wynyard S, Elliott RB. Clinical benefit of islet xenotransplantation for the treatment of type 1 diabetes. EBioMedicine. 2016;12:255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner‐Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45(9):1161–7. [DOI] [PubMed] [Google Scholar]
- 30. Rosenberg L, Wang R, Paraskevas S, Maysinger D. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery. 1999;126(2):393–8. [PubMed] [Google Scholar]
- 31. Cross SE, Vaughan RH, Willcox AJ, et al. Key matrix proteins within the pancreatic islet basement membrane are differentially digested during human islet isolation. Am J Transplant. 2017;17(2):451–61. [DOI] [PubMed] [Google Scholar]
- 32. Loganathan G, Subhashree V, Breite AG, et al. Beneficial effect of recombinant rC1rC2 collagenases on human islet function: efficacy of low‐dose enzymes on pancreas digestion and yield. Am J Transplant. 2018;18(2):478–85. [DOI] [PubMed] [Google Scholar]
- 33. Anderson J, Deeds M, Armstrong A, Gastineau D, Kudva Y. Utilization of a test gradient enhances islet recovery from deceased donor pancreases. Cytotherapy. 2007;9(7):630–6. [DOI] [PubMed] [Google Scholar]
- 34. Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation. 2011;91(5):508–14. [DOI] [PubMed] [Google Scholar]
- 35. Owen RJ, Ryan EA, O'Kelly K, et al. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: radiologic aspects. Radiology. 2003;229(1):165–70. [DOI] [PubMed] [Google Scholar]
- 36. Ryan EA, Lakey JR, Paty BW, et al. Successful islet transplantation: continued insulin reserve provides long‐term glycemic control. Diabetes. 2002;51(7):2148–57. [DOI] [PubMed] [Google Scholar]
- 37. Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105(2):125–33. [DOI] [PubMed] [Google Scholar]
- 38. Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039–45. [DOI] [PubMed] [Google Scholar]
- 39. Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low molecular weight dextran sulfate prevents the instant blood‐mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77(5):741–7. [DOI] [PubMed] [Google Scholar]
- 40. Cabric S, Sanchez J, Lundgren T, et al. Islet surface heparinization prevents the instant blood‐mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56(8):2008–15. [DOI] [PubMed] [Google Scholar]
- 41. Contreras JL, Eckstein C, Smyth CA, et al. Activated protein C preserves functional islet mass after intraportal transplantation: a novel link between endothelial cell activation, thrombosis, inflammation, and islet cell death. Diabetes. 2004;53(11):2804–14. [DOI] [PubMed] [Google Scholar]
- 42. Matsumoto S, Takita M, Chaussabel D, et al. Improving efficacy of clinical islet transplantation with iodixanol‐based islet purification, thymoglobulin induction, and blockage of IL‐1β and TNF‐α. Cell Transplant. 2011;20(10):1641–7. [DOI] [PubMed] [Google Scholar]
- 43. McCall M, Pawlick R, Kin T, Shapiro AM. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am J Transplant. 2012;12(2):322–9. [DOI] [PubMed] [Google Scholar]
- 44. Yasunami Y, Kojo S, Kitamura H, et al. Valpha14 NK T cell‐triggered IFN‐gamma production by Gr‐1 + CD11b+ cells mediates early graft loss of syngeneic transplanted islets. J Exp Med. 2005;202(7):913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsuoka N, Itoh T, Watarai H, et al. High‐mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120(3):735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Citro A, Cantarelli E, Maffi P, et al. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012;122(10):3647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Posselt AM, Szot GL, Frassetto LA, et al. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor‐free immunosuppressive protocols based on T‐cell adhesion or costimulation blockade. Transplantation. 2010;90(12):1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes. 2013;62(10):3523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cantarelli E, Citro A, Pellegrini S, et al. Transplant site influences the immune response after islet transplantation: bone marrow versus liver. Transplantation. 2017;101(5):1046–55. [DOI] [PubMed] [Google Scholar]
- 50. Berman DM, Molano RD, Fotino C, et al. Bioengineering the endocrine pancreas: intraomental islet transplantation within a biologic resorbable scaffold. Diabetes. 2016;65(5):1350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baidal DA, Ricordi C, Berman DM, et al. Bioengineering of an Intraabdominal Endocrine Pancreas. N Engl J Med. 2017;376(19):1887–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Teramura Y, Iwata H. Bioartificial pancreas microencapsulation and conformal coating of islet of Langerhans. Adv Drug Deliv Rev. 2010;62(7–8):827–40. [DOI] [PubMed] [Google Scholar]
- 53. Luan NM, Iwata H. Long‐term allogeneic islet graft survival in prevascularized subcutaneous sites without immunosuppressive treatment. Am J Transplant. 2014;14(7):1533–42. [DOI] [PubMed] [Google Scholar]
- 54. Kuwabara R, Hamaguchi M, Fukuda T, et al. Long‐term functioning of allogeneic islets in subcutaneous tissue pretreated with a novel cyclic peptide without immunosuppressive medication. Transplantation. 2018;102(3):417–25. [DOI] [PubMed] [Google Scholar]
- 55. Pepper AR, Gala‐Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM. A prevascularized subcutaneous device‐less site for islet and cellular transplantation. Nat Biotechnol. 2015;33(5):518–23. [DOI] [PubMed] [Google Scholar]
- 56. Rafael E, Tibell A, Rydén M, et al. Intramuscular autotransplantation of pancreatic islets in a 7‐year‐old child: a 2‐year follow‐up. Am J Transplant. 2008;8(2):458–62. [DOI] [PubMed] [Google Scholar]
- 57. Schuetz C, Anazawa T, Cross SE, et al. β cell replacement therapy: the next 10 years. Transplantation. 2018;102(2):215–29. [DOI] [PubMed] [Google Scholar]
- 58. Hering B, Wijkstrom M, Graham M, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild‐type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–3. [DOI] [PubMed] [Google Scholar]
- 59. Bottino R, Wijkstrom M, van der Windt DJ, et al. Pig‐to‐monkey islet xenotransplantation using multi‐transgenic pigs. Am J Transplant. 2014;14(10):2275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shin JS, Kim JM, Kim JS, et al. Long‐term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015;15(11):2837–50. [DOI] [PubMed] [Google Scholar]
- 61. Dufrane D, Goebbels RM, Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozotocin‐induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010;90(10):1054–62. [DOI] [PubMed] [Google Scholar]
- 62. Yang L, Güell M, Niu D, et al. Genome‐wide inactivation of porcine endogenous retroviruses (PERVs). Science. 2015;350(6264):1101–4. [DOI] [PubMed] [Google Scholar]
- 63. Pagliuca FW, Millman JR, Gürtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin‐producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33. [DOI] [PubMed] [Google Scholar]
- 65. Agulnick AD, Ambruzs DM, Moorman MA, et al. Insulin‐producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Transl Med. 2015;4(10):1214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bruin JE, Rezania A, Xu J, et al. Maturation and function of human embryonic stem cell‐derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013;56(9):1987–98. [DOI] [PubMed] [Google Scholar]


