Abstract
Over the last 20 years, the sentinel node (SN) concept has been widely applied to the surgical staging of both breast cancer and melanoma. However, the validity of this concept has been controversial for esophageal cancer, because SN mapping for esophageal cancer is not considered to be technically easy because of the complicated multidirectional lymphatic networks of the esophagus and mediastinum. Nevertheless, studies including meta‐analyses indicated that SN mapping may be feasible in early esophageal cancer. Transthoracic esophagectomy with three‐field lymphadenectomy was developed as a potential curative procedure for thoracic esophageal cancer. However, this highly invasive procedure might increase morbidity and reduce patients’ quality of life (QOL) after esophagectomy. Although further validation based on multicenter trials using the standard protocol of SN mapping for esophageal cancer is required, SN navigation surgery would enable us to carry out personalized and limited lymph node dissection which might reduce morbidity and maintain patients’ QOL.
Keywords: esophageal cancer, micrometastasis, radioisotope, sentinel node
1. INTRODUCTION
Esophageal cancer is the eighth most common cancer worldwide, affecting more than 450 000 people per year and for which the incidence is increasing.1 Esophageal cancer also has one of the highest malignant potentials of any tumor. Its primary therapy is selected according to histological type, tumor stage and location, and patient's performance status and comorbidities.2 Although the efficacy of chemotherapy or chemoradiotherapy (CRT) for esophageal cancer has been reported, esophagectomy remains the mainstay of potential curative treatment for esophageal cancer.2
Lymph node metastasis is known as one of the useful indicators for predicting the long‐term outcome of esophageal cancer. Lymph node metastasis is not a rare event in esophageal cancer, with its incidence, even in submucosal tumors, reaching 45%.3 Other specific characteristics of esophageal cancer are multidirectional lymphatic flow from the primary tumor site and widespread and random patterns of lymph node metastasis from the neck to the abdominal areas. In fact, it was reported that anatomical skip metastases to the second or third compartment of regional lymph nodes were found in more than half of esophageal cancer cases.3 Middle thoracic esophageal cancer metastasized widely to the lymph nodes located from the neck (26%) to the abdomen (39%).3 Considering these specific characteristics, total or subtotal esophagectomy with three‐field lymphadenectomy has been recognized as a standard procedure for esophageal squamous cell carcinoma (SCC) in Japan.3, 4 However, esophagectomy with three‐field lymphadenectomy is one of the most invasive procedures in gastrointestinal (GI) surgeries even by minimally invasive esophagectomy such as thoracoscopic and laparoscopic approaches. Postoperative complications and mortality are known to be remarkably increased by three‐field lymphadenectomy.5 Sentinel node (SN) mapping may play a significant role in reducing the need for the uniform application of the highly invasive surgery by providing personal information to allow modifications of the surgical procedure for esophageal cancer patients.
The SN is defined as the first lymph node(s) receiving lymphatic drainage from the primary tumor site.6 The SN is considered to be the first possible site of lymph node micrometastasis from the primary tumor. If the SN is recognizable and pathologically negative for cancer metastasis, unnecessary radical lymphadenectomy could be omitted. SN navigation surgery is a type of less invasive surgery with modified or minimized lymphadenectomy based on the diagnosis of SN metastasis.
Sentinel node mapping and biopsy were first applied to breast cancer and melanoma and subsequently attempted for other solid tumors including GI cancers.6, 7, 8, 9, 10 SN mapping and biopsy results in reducing postoperative complications as a result of unnecessary extended lymphadenectomy in patients SN negative for cancer metastasis.6, 7
Sentinel node mapping for esophageal cancer is technically difficult in comparison with that for gastric cancer.10 However, SN mapping and biopsy might become useful tools for the accurate intraoperative diagnosis of lymph node metastasis and modification of the surgical procedures in minimally invasive surgery in patients with early‐stage esophageal cancer.11
2. SENTINEL NODE MAPPING AND BIOPSY PROCEDURES IN ESOPHAGEAL CANCER
A radio‐guided method is preferentially used to identify the SN in esophageal cancer.10, 11, 12 In our institution, technetium‐99m tin colloid solution as a radioisotope tracer is injected using an endoscopic puncture needle at four quadrants into the submucosal layer surrounding the primary tumor the day before surgery. Preoperative lymphoscintigraphy is usually carried out 3‐4 hours after injecting the tracer (Figure 1). In terms of the distribution of SN, they are spread widely from cervical to abdominal areas.
Figure 1.
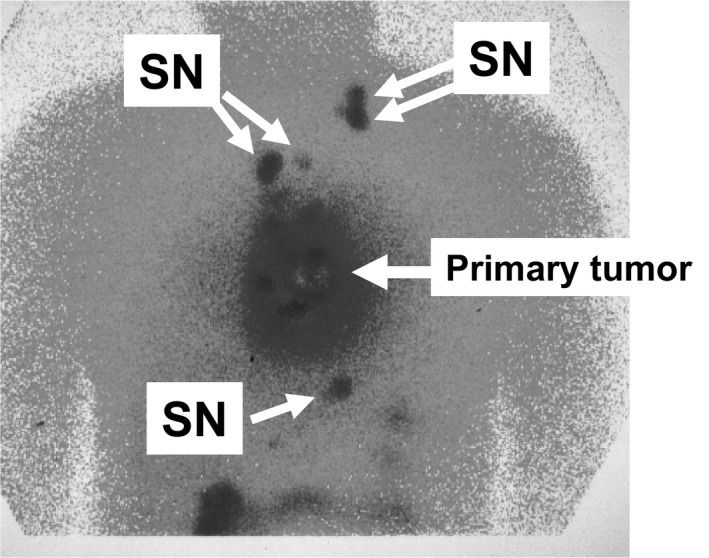
Preoperative lymphoscintigraphy in thoracic esophageal cancer. SN, sentinel node
We usually use a handheld gamma probe to accurately identify SN, which can be introduced from the trocar ports for thoracoscopic or laparoscopic intraoperative SN mapping (Figure 2). SN located in the neck can be easily identified by percutaneous gamma probing. After intraoperative SN mapping and biopsy, all SN in the resected specimens are confirmed using the gamma probe on a back table near the operating table and sent for intraoperative pathological examination. Finally, we confirm the absence of residual SN in the mediastinum or abdominal cavity using the gamma probe. In order to verify the accuracy of the SN mapping, intraoperative SN mapping and biopsy are generally followed by total or subtotal esophagectomy with regional lymphadenectomy based on the Japanese guidelines.12
Figure 2.
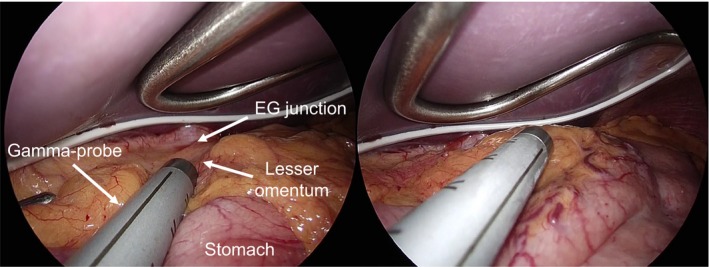
Intraoperative gamma probing during laparoscopic surgery. EG, esophagogastric
In contrast, a dual tracer method using radioactive tracer and blue dye (indocyanine green) is useful for SN detection for abdominal esophageal cancer or adenocarcinoma (AC) of the esophagogastric (EG) junction. The blue dye is injected into the submucosal layer around the primary tumor site endoscopically immediately after the start of surgery. Subsequently, the afferent lymphatics are visualized clearly even in laparoscopic observation, and blue‐stained nodes are identified as the SN approximately 15 minutes after the dye injection.
Dye‐only‐guided SN mapping is not recommended for thoracic esophageal cancer because lymph nodes in the mediastinum are frequently pigmented by anthracosis.13 Moreover, real‐time observation of the lymphatics using blue dye is sometimes difficult without operative mobilization of the esophagus, but the mobilization itself may disturb active lymphatic flow from the primary tumor site. However, the blue dye in addition to the radio‐guided method is useful in abdominal esophageal cancer or EG junction cancer, because it is relatively easy to identify blue‐stained lymphatic vessels and lymph nodes without the mobilization of the esophagus in the abdominal cavity compared with that in the mediastinum. Furthermore, pigmentation as a result of anthracosis is relatively rare in abdominal lymph nodes.
Endoscopic submucosal injection of tracer is useful for accurate SN mapping of esophageal cancer. The radioactive tracer, technetium‐99m tin colloid, has a relatively larger particle size (~200 nm in diameter) than blue dye.14 The radioactive tracer is known to migrate into the SN from the primary lesion within 2 hours after injection and accumulates in the SN without excessive diffusion. Radioactivity lasting at least 20 hours is sufficient for SN detection.15, 16 Preoperative lymphoscintigraphy is useful for detecting SN distant from the primary lesion before surgery (Figure 1). Furthermore, a handheld gamma probe is accurate and useful for intraoperative detection of SN in esophageal cancer.12 Gamma probing was shown to be feasible even in thoracoscopic or laparoscopic SN mapping.12
3. RESULTS OF SN MAPPING IN ESOPHAGEAL CANCER
To date, fewer studies have shown the feasibility and validity of the SN concept in esophageal cancer10, 12, 13, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 compared with studies on gastric cancer (Table 1). However, a number of single institution studies have indicated acceptable outcomes of SN mapping and biopsy for early‐stage esophageal cancer.31 In particular, a radio‐guided method appears to be superior regarding the SN detection rate and accuracy at predicting lymph node metastasis compared to the conventional dye‐guided method for esophageal cancer (Table 1).
Table 1.
Representative results of sentinel node mapping in esophageal cancer
| Author (Ref.) | Year | Tracer | Tumor depth | Number of patients | SN detection rate (%) | Sensitivity (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| Kitagawa10 | 2000 | RI (99mTc tin colloid) | cT1‐T3 | 27 SCC | 25/27 (93) | 14/16 (88) | 23/25 (92) |
| Kato17 | 2003 | RI (99mtechnetium rhenium sulfide) | pT1‐T4 | 25 SCC | 23/25 (92) | 13/15 (87) | 21/23 (91) |
| Yasuda18 | 2003 | RI (99mTc tin colloid) | pT1‐T3 | 23 SCC + AC | 23/23 (100) | 9/12 (75) | 20/23 (87) |
| Stein19 | 2004 | RI (99mTc colloid) + isosulfan blue | pT1‐T3 | 35 AC | 32/35 (91) | 2/4 (50) | 30/32 (94) |
| Udagawa20 | 2005 | RI (99mTc tin colloid and 99mTc phytate) | cT1 | 24 SCC | 24/24 (100) | 9/13 (69) | 20/24 (83) |
| Lamb21 | 2005 | RI (99mTc nanocolloid) | ND | 57 AC | 57/57 (100) | 35/37 (95) | 55/57 (96) |
| Arima22 | 2006 | RI (99mTc tin colloid) | pT1‐T3 | 19 SCC | 18/19 (95) | 14/18 (78) | 14/18 (78) |
| Takeuchi12 | 2009 | RI (99mTc tin colloid) | cT1‐T2 | 75 SCC + AC | 71/75 (95) | 29/33 (88) | 67/71 (94) |
| Grotenhuis13 | 2009 | Patent blue V | pT1‐T3 | 40 AC | 39/40 (98) | 27/33 (82) | 33/39 (85) |
| Bhat23 | 2010 | Methylene blue | pT1‐T3 | 32 SCC + AC | 26/32 (81) | 20/26 (77) | 14/20 (70) |
| Thompson24 | 2011 | RI (99mTc antimony colloid) | pT1a‐T3 | 16 SCC + AC | 14/16 (88) | 3/3 (100) | 16/16 (100) |
| Kim25 | 2011 | RI (99mTc neomannosyl HSA) | cT1‐T4 | 23 ESCC | 21/23 (91) | 8/8 (100) | 21/21 (100) |
| Uenosono26 | 2011 | RI (99mTc tin colloid) | cT1‐T3 | 134 SCC + AC | cT1: 56/60 (93) | cT1: 11/12 (92) | cT1: 55/56 (98) |
| cT2: 31/31 (100) | cT2: 12/18 (67) | cT2: 25/31 (81) | |||||
| cT3: 28/32 (88) | cT3: 13/24 (54) | cT3: 17/28 (61) | |||||
| CRT: 5/11 (46) | CRT: 0/3 (0) | CRT: 2/5 (40) | |||||
| Yuasa27 | 2012 | Indocyanine green fluorescence | cT1 | 20 | 19/20 (95%) | 3/4 (75%) | 18/19 (95) |
| Takeuchi28 | 2015 | RI (99mTc tin colloid) | pT1 | 70 SCC + AC | 65/70 (93) | 22/24 (92) | 63/65 (97) |
| Boone29 | 2016 | RI (99mTc nanocolloid) | cT1‐T3 | 8 SCC + AC | 8/8 (100) | 0/5 (0) | 3/8 (38) |
| Künzli30 | 2017 | RI (99mTc nanocolloid) | cT1 | 5 AC | 5/5 (100) | 0/1 (0) | 4/5 (80) |
AC, adenocarcinoma; CRT, chemoradiotherapy; HSA, human serum albumin; ND, not determined; RI, radioisotope; SCC, squamous cell carcinoma; SN, sentinel node; Tc, technetium.
Several types of radioisotope tracers have been used in SN studies for esophageal cancer (Table 1).17, 21, 25 Specifically, particle size of the radiocolloid is thought to be related to the time of tracer deposition in the lymph node; therefore, a larger particle such as tin colloid would be associated with a longer period of deposition. SN mapping with technetium‐99m colloidal rhenium sulfide was also reported to be useful to identify the lymphatic basin in 25 patients with esophageal cancer, and the results mostly matched those obtained with technetium‐99m tin colloid.17 However, further studies will be needed to ensure the optimal selection of radioisotope tracers.
Several contraindications against the recommendation of SN mapping in esophageal cancer have been reported.11 Patients with clinically apparent lymph node metastasis should be excluded from the indication for SN mapping because the purpose of SN mapping is to identify clinically undetectable lymph node involvement. Regarding tumor depth, clinically T1 esophageal cancers were reported to be suitable for SN mapping.10, 11, 26 In contrast, clinically T3/T4 tumors may cause higher false‐negative rates in SN mapping because original lymphatic drainage routes might be changed according to the tumor progression of esophageal cancer. In fact, SN mapping failure was more frequently reported in clinically T3/T4 tumors than in T1 tumors.26 Therefore, clinically T3 or T4 tumors should also be considered a contraindication for SN mapping in esophageal cancer.
We previously reported a feasibility study of radio‐guided SN mapping and biopsy for 75 patients with clinically T1N0 or T2N0 esophageal cancer.12 Our study demonstrated acceptable SN detection in 71 (95%) of the 75 patients and successful diagnostic accuracy (94%) of nodal metastasis based on SN status. Mean number of identified SN was 4.7 per case in our study, but SN mapping was feasible even during thoracoscopic esophagectomy.12 In our study, the distribution of SN showed a wide spread from cervical to abdominal areas.12 In upper thoracic esophageal cancer, SN were frequently identified in the lymph nodes along the bilateral recurrent laryngeal nerve chain (stations #106recR and #106recL in the Japanese guidelines) and lymph nodes in the cervical area. In middle thoracic esophageal cancer, a wide distribution of SN was shown from cervical to abdominal areas. In particular, SN were frequently identified in the stations #106recR and #106recL, bifurcational and main bronchus lymph nodes (stations #107 and #109), and middle thoracic paraesophageal nodes (station #108) in middle thoracic esophageal cancer. However, more than 10% of the patients with middle thoracic esophageal cancer showed SN in the area along the cardia and lesser curvature of the stomach (stations #1, #2, #3, and #7). SN were mainly located in the lower and middle mediastinum and in the abdominal area in lower thoracic esophageal cancer, but some patients showed SN in the upper mediastinum. In more than 85% of patients with thoracic esophageal cancer, at least one SN was found to be located in the second or third compartment of regional lymph nodes.12 Generally, the lymph node stations that were frequently identified as SN showed a tendency to have a high incidence of metastasis, as detected pathologically.
In another study, 70 patients who underwent radio‐guided SN mapping and who were diagnosed with pathological T1 primary thoracic esophageal cancer were assessed in our institution.28 Results showed that 85% of the patients had no lymph node metastasis or had lymph node metastasis in SN only. Moreover, disease‐specific survival of the patients with metastatic non‐SN was significantly worse than that of the patients with no lymph node metastasis or lymph node metastasis in SN only. This study demonstrated that SN mapping is useful not only as an accurate diagnostic tool for detecting lymph node metastasis but also for prognostic stratification in patients with cN0 early esophageal cancer.
In comparison with SCC, distribution of SN is relatively localized in the lower mediastinum and in the abdominal area in the AC, but a previous study reported a high false‐negative rate reaching 15% in SN mapping in the AC.13 However, the lower accuracy may result from several concerns such as dye‐only‐guided SN mapping, use of transhiatal approach, and many patients with T3 tumors (65%) in the study. In contrast, Lamb et al21 reported acceptable outcomes of SN mapping in 57 patients with AC. Another group also demonstrated that SN mapping is feasible and accurate in patients with AC of the EG junction.32 We consider that SN mapping and biopsy are also applicable to AC of the distal esophagus or EG junction.
Nagajara et al31 reported a systematic review and meta‐analysis that evaluated the diagnostic value of SN mapping in esophageal cancer. Results of their meta‐analysis, which included 23 relevant studies with 623 patients, indicated that the SN detection rate and the accuracy of prediction of lymph node metastasis based on SN status were 93% and 88%, respectively, and were not markedly different between SCC and AC. Although they concluded that SN mapping is technically feasible in esophageal cancer, they also reported that the detection rate and accuracy were higher in radioisotope tracer in comparison with blue dyes.31
In contrast, in their meta‐analysis including 18 studies, Kakhki et al33 reported that the sensitivity to predict nodal metastasis was 84% in total and markedly higher for AC compared to SCC (91% vs 81%). Also, they indicated that the sensitivity tended to be lower according to tumor depth (T1: 88%, T2: 76%, T3: 50%) in the SCC patients. They concluded that cN0 patients with superficial primary tumors (T1 or T2) would be suitable for SN mapping for esophageal SCC.33
In general, distribution of nodal metastasis even after neoadjuvant therapy such as neoadjuvant chemotherapy or CRT could be useful information in the pathological staging of disease and in the planning of surgical procedures. Thompson et al24 found no remarkable differences in identifying SN between patients with and without neoadjuvant therapy. However, Uenosono et al26 reported that SN mapping is unacceptable for patients who have received neoadjuvant CRT because of the high false‐negative rate.
Recently, the utility of single‐photon emission computed tomography/computed tomography (SPECT/CT) for SN mapping has been reported in various solid tumors including esophageal cancer.34, 35 SPECT/CT for SN mapping could provide precise localization with 3‐D imaging compared to conventional lymphoscintigraphy. In contrast, Yuasa et al27 attempted intraoperative indocyanine green fluorescence imaging for SN biopsy in early‐stage esophageal cancer combined with preoperative CT lymphography. Although further studies will be required to verify the clinical utility of these new methods, in the future, technical innovations may improve the accuracy of SN mapping in esophageal cancer.
4. CLINICAL SIGNIFICANCE OF MICROMETASTASIS IN SN OF ESOPHAGEAL CANCER
Even now, positron emission tomography (PET)/CT scans still have limited sensitivity in detecting micrometastasis in esophageal cancer compared with conventional pathological examinations.36, 37, 38 Although SN mapping in esophageal cancer is clearly more invasive than other imaging, SN mapping and biopsy are believed to detect micrometastasis more accurately than other imaging procedures in patients with cN0 early‐stage esophageal cancer.28, 39
Several studies have demonstrated underestimation of the frequency of micrometastasis in regional lymph nodes by conventional pathological diagnosis using hematoxylin and eosin staining.40 In addition, immunohistochemical nodal micrometastasis is reported to be a significant prognostic indicator in patients with cN0 esophageal cancer.41, 42, 43 However, immunohistochemical examinations for all resected lymph nodes by esophagectomy are not practically feasible in clinical practice. SN mapping and biopsy can easily focus on the diagnosis of micrometastasis by assessing the small number of lymph nodes using immunohistochemistry. Immunohistochemistry and reverse transcription polymerase chain reaction (RT‐PCR) were reported to improve the sensitivity of SN mapping for predicting nodal metastasis in patients with esophageal cancer.26, 44 However, the clinical utility of RT‐PCR in SN of patients with early‐stage esophageal cancer remains controversial regarding its clinical impact, as well as its time‐ and labor‐consuming characteristics, and cost‐effectiveness.45 Further studies are required to evaluate the clinical significance of micrometastasis detected by RT‐PCR in SN in patients with early‐stage esophageal cancer.
5. FUTURE PERSPECTIVES OF SN MAPPING IN ESOPHAGEAL CANCER
Radical esophagectomy with extended three‐field lymphadenectomy has been established as a potential curative surgical procedure for thoracic esophageal SCC in Japan.1, 2 Esophagectomy with three‐field lymphadenectomy may be reasonable on account of the wide distribution of SN and unpredictable metastatic nodes.12, 46 Regarding this point, the clinical application of SN mapping and biopsy in esophageal cancer may be unexpectedly limited. However, uniform application of esophagectomy with three‐field lymphadenectomy might increase the postoperative complications and remarkably reduce quality of life (QOL) after surgery. SN mapping and biopsy may provide significant information to carry out personalized selective lymph node dissection that could reduce postoperative complications without a negative impact on the prognosis.47 For example, if SN were identified in the mediastinum or abdominal area only and all SN were pathologically negative for cancer metastasis in patients with cT1N0 middle or lower thoracic esophageal cancer, cervical lymphadenectomy would be omitted.12 However, we also consider that SN mapping and biopsy will even be applicable to two‐field lymphadenectomy and provide useful information regarding the extent of lymphadenectomy.12 For instance, if SN is identified along the recurrent laryngeal nerves in upper mediastinum and is positive for cancer metastasis, extended lymphadenectomy for the upper mediastinum might be considered even in Ivor Lewis esophagectomy.
SN mapping and biopsy will also be applicable to AC of the distal esophagus or EG junction to adjust and modify the surgical procedures.12 For instance, if all SN were identified in the abdominal area only and were negative for metastasis by intraoperative pathological diagnosis in a patient with cT1N0 AC of distal esophagus, the patient could be treated with limited resection of the distal esophagus by a laparoscopic transhiatal approach without extensive mediastinal lymphadenectomy.28, 48 In contrast, if the SN(s) were positive for metastasis, the patient should be treated with thoracoscopic mediastinal lymphadenectomy.49 Although we need to evaluate the survival benefit of modified lymph node dissection based on the SN concept, the new surgical procedure might reduce the morbidity and mortality without a negative impact on the QOL for early esophageal cancer patients with pathologically negative SN.
Definitive CRT was reported to be a good option for patients with cT1N0M0 esophageal SCC who do not want surgical treatment.50 However, how to design the optimal irradiation field is still controversial, because larger fields might be better regarding control of subclinical lymph node metastasis, but may result in more adverse effects during long‐term follow up.51 We have carried out concurrent CRT for 16 patients with cT1N0M0 esophageal SCC with minimized irradiation fields that contain SN identified by lymphoscintigraphy to achieve local control of subclinical metastasis in SN.52 Of the 16 patients, there were nine cases of definitive CRT and seven of adjuvant CRT after endoscopic resections. The results showed that all patients with definitive CRT achieved complete remission. At the median follow up of 77 months, there was no lymph node recurrence. Prophylactic irradiation of the hot spots in lymphoscintigraphy may be effective for controlling subclinical micrometastasis in SN, although further evaluation concerning the validity of irradiation field settings based on lymphoscintigraphy is needed in patients with superficial esophageal SCC.
In addition, further investigations should clarify the feasibility and efficacy of novel esophagus‐preserving treatments, such as endoscopic submucosal dissection in patients with cT1b esophageal SCC or in EG junction cancer patients having pathologically negative SN confirmed by thoracoscopic or laparoscopic SN mapping and biopsy (Figure 3). However, the details of SN mapping techniques such as the choice of tracers and the timing of tracer injection need to be standardized and evaluated carefully for the clinical application of SN mapping and biopsy in early‐stage esophageal cancer. Until now, no multicenter prospective trials have been conducted to validate the SN concept in esophageal cancer because a standard protocol for SN mapping procedures has not yet been established. Accumulation of evidence based on multicenter prospective trials would be expected to verify the feasibility and reliability of SN mapping in early esophageal cancer.
Figure 3.
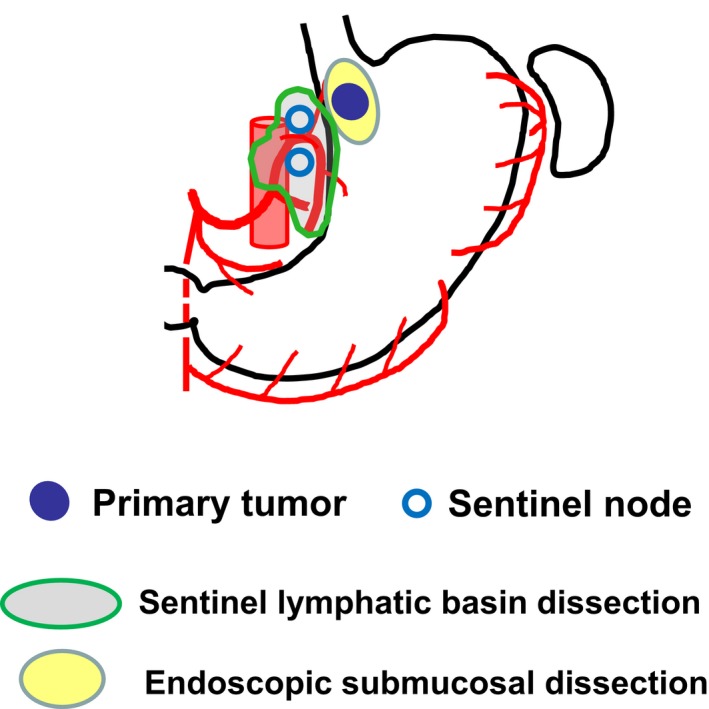
Endoscopic submucosal dissection combined with sentinel node mapping and biopsy in cT1bN0M0 esophagogastric junction cancer
Moreover, further improvement in surgical approaches should be achieved for less invasive and more accurate SN mapping. Recently, Fujiwara et al53 developed a lymphadenectomy method in the left upper mediastinum using a left cervical approach with a single‐port mediastinoscopic technique. The new surgical techniques enable us to access #106recL easily without mobilization of the esophagus by a right thoracoscopic approach (Figure 4). The combination of the mediastinoscopic SN mapping and biopsy techniques might revolutionize SN navigation surgery for early‐stage esophageal cancer.
Figure 4.
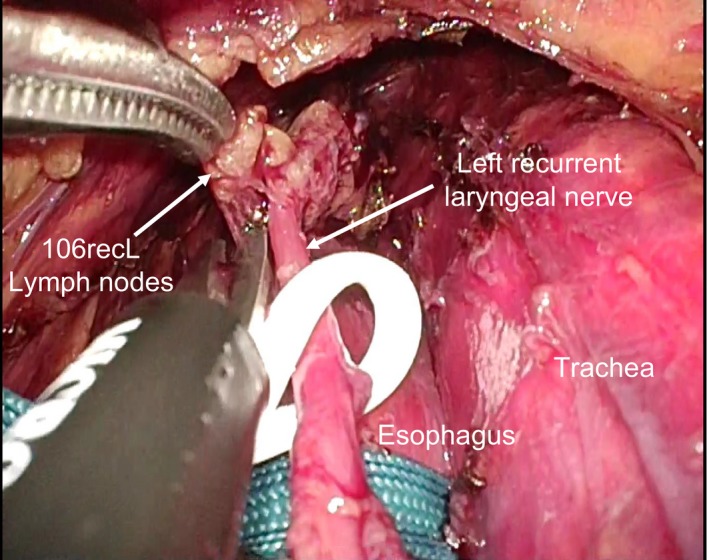
Mediastinoscopic sampling of lymph node along the left recurrent laryngeal nerve (#106recL) from the left cervical incision
6. CONCLUSION
The incidence of lymph node metastasis is relatively high even in cN0 early‐stage esophageal cancer. Therefore, SN mapping must be accurate for carrying out SN navigation surgery in esophageal cancer. Previous studies suggested that the SN concept appears to be valid and radio‐guided SN mapping may be feasible in cT1N0 esophageal cancer. Further accumulation of evidence based on multicenter trials using a standard protocol of SN mapping is needed, but SN navigation surgery will become an ideal less invasive personalized treatment for early‐stage esophageal cancer.
DISCLOSURE
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Author Contributions: HT wrote the manuscript and made tables and figures. YK supervised the whole editing.
Takeuchi H, Kitagawa Y. Sentinel node navigation surgery in esophageal cancer. Ann Gastroenterol Surg. 2019;3:7–13. 10.1002/ags3.12206
REFERENCES
- 1. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. [DOI] [PubMed] [Google Scholar]
- 2. Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D; ESMO Guidelines Committee . Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27(Suppl 5):v50–7. [DOI] [PubMed] [Google Scholar]
- 3. Ando N, Ozawa S, Kitagawa Y, Shinozawa Y, Kitajima M. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg. 2000;232:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220:360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three‐field lymphadenectomy with two‐field lymphadenectomy. Ann Surg. 1995;222:654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9. [DOI] [PubMed] [Google Scholar]
- 7. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer–a multicenter validation study. N Engl J Med. 1998;339:941–6. [DOI] [PubMed] [Google Scholar]
- 9. Bilchik AJ, Saha S, Wiese D, et al. Molecular staging of early colon cancer on the basis of sentinel node analysis: a multicenter phase II trial. J Clin Oncol. 2001;19:1128–36. [DOI] [PubMed] [Google Scholar]
- 10. Kitagawa Y, Fujii H, Mukai M, et al. The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin North Am. 2000;80:1799–809. [DOI] [PubMed] [Google Scholar]
- 11. Takeuchi H, Kawakubo H, Takeda F, Omori T, Kitagawa Y. Sentinel node navigation surgery in early‐stage esophageal cancer. Ann Thorac Cardiovasc Surg. 2012;18:306–13. [DOI] [PubMed] [Google Scholar]
- 12. Takeuchi H, Fujii H, Ando N, et al. Validation study of radio‐guided sentinel lymph node navigation in esophageal cancer. Ann Surg. 2009;249:757–63. [DOI] [PubMed] [Google Scholar]
- 13. Grotenhuis BA, Wijnhoven BP, van Marion R, et al. The sentinel node concept in adenocarcinoma of the distal esophagus and gastroesophageal junction. J Thorac Cardiovasc Surg. 2009;138:608–12. [DOI] [PubMed] [Google Scholar]
- 14. Jimenez IR, Roca M, Vega E, et al. Particle sizes of colloids to be used in sentinel lymph node radiolocalization. Nucl Med Commun. 2008;29:166–72. [DOI] [PubMed] [Google Scholar]
- 15. Kitagawa Y, Saikawa Y, Takeuchi H, et al. Sentinel node navigation in early stage gastric cancer – updated data and current status. Scand J Surg. 2006;95:256–9. [DOI] [PubMed] [Google Scholar]
- 16. Kitagawa Y, Fujii H, Mukai M, Kubota T, Otani Y, Kitajima M. Radio‐guided sentinel node detection for gastric cancer. Br J Surg. 2002;89:604–8. [DOI] [PubMed] [Google Scholar]
- 17. Kato H, Miyazaki T, Nakajima M, et al. Sentinel lymph nodes with technetium‐99 m colloidal rhenium sulfide in patients with esophageal carcinoma. Cancer. 2003;98:932–9. [DOI] [PubMed] [Google Scholar]
- 18. Yasuda S, Shimada H, Chino O, et al. Sentinel lymph node detection with Tc‐99 m tin colloids in patients with esophagogastric cancer. Jpn J Clin Oncol. 2003;33:68–72. [DOI] [PubMed] [Google Scholar]
- 19. Stein HJ, Burian M, Feith M, Piert M, Naehrig J, Siewert JR. First experience with sentinel lymphadenectomy in early adenocarcinoma of the distal esophagus and esophagogastric junction. Gastroenterology. 2004;126:769. [Google Scholar]
- 20. Udagawa H. Sentinel node concept in esophageal surgery: an elegant strategy. Ann Thorac Cardiovasc Surg. 2005;11:1–3. [PubMed] [Google Scholar]
- 21. Lamb PJ, Griffin SM, Burt AD, Lloyd J, Karat D, Hayes N. Sentinel node biopsy to evaluate the metastatic dissemination of oesophageal adenocarcinoma. Br J Surg. 2005;92:60–7. [DOI] [PubMed] [Google Scholar]
- 22. Arima H, Natsugoe S, Uenosono Y, et al. Area of nodal metastasis and radioisotope uptake in sentinel nodes of upper gastrointestinal cancer. J Surg Res. 2006;135:250–4. [DOI] [PubMed] [Google Scholar]
- 23. Bhat MA, Naikoo ZA, Dass TA, Lone RA, Dar AM. Role of intraoperative sentinel lymph node mapping in the management of carcinoma of the esophagus. Saudi J Gastroenterol. 2010;16:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thompson SK, Bartholomeusz D, Devitt PG, Lamb PJ, Ruszkiewicz AR, Jamieson GG. Feasibility study of sentinel lymph node biopsy in esophageal cancer with conservative lymphadenectomy. Surg Endosc. 2011;25:817–25. [DOI] [PubMed] [Google Scholar]
- 25. Kim HK, Kim S, Park JJ, Jeong JM, Mok YJ, Choi YH. Sentinel node identification using technetium‐99 m neomannosyl human serum albumin in esophageal cancer. Ann Thorac Surg. 2011;91:1517–22. [DOI] [PubMed] [Google Scholar]
- 26. Uenosono Y, Arigami T, Yanagita S, et al. Sentinel node navigation surgery is acceptable for clinical T1 and N0 esophageal cancer. Ann Surg Oncol. 2011;18:2003–9. [DOI] [PubMed] [Google Scholar]
- 27. Yuasa Y, Seike J, Yoshida T, et al. Sentinel lymph node biopsy using intraoperative indocyanine green fluorescence imaging navigated with preoperative CT lymphography for superficial esophageal cancer. Ann Surg Oncol. 2012;19:486–93. [DOI] [PubMed] [Google Scholar]
- 28. Takeuchi H, Kawakubo H, Nakamura R, et al. Clinical significance of sentinel node positivity in patients with superficial esophageal cancer. World J Surg. 2015;39:2941–7. [DOI] [PubMed] [Google Scholar]
- 29. Boone J, Hobbelink MG, Schipper ME, et al. Sentinel node biopsy during thoracolaparoscopic esophagectomy or advanced esophageal cancer. World J Surg Oncol. 2016;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Künzli HT, van Berge Henegouwen MI, Gisbertz SS, et al. Pilot‐study on the feasibility of sentinel node navigation surgery in combination with thoracolaparoscopic lymphadenectomy without esophagectomy in early esophageal adenocarcinoma patients. Dis Esophagus. 2017;30:1–8. [DOI] [PubMed] [Google Scholar]
- 31. Nagajara V, Eslick G, Cox M. Sentinel lymph node in oesophageal cancer—a systematic review and meta‐analysis. J Gastrointest Oncol. 2014;5:127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burian M, Stein HJ, Sendler A, et al. Sentinel node detection in Barrett's and cardia cancer. Ann Surg Oncol. 2004;3:255S–8S. [DOI] [PubMed] [Google Scholar]
- 33. Kakhki VR, Bagheri R, Tehranian S, et al. Accuracy of sentinel node biopsy in esophageal carcinoma: a systematic review and meta‐analysis of the pertinent literature. Surg Today. 2014;44:607–19. [DOI] [PubMed] [Google Scholar]
- 34. Vermeeren L, van der Ploeg IM, Olmos RA, et al. SPECT/CT for preoperative sentinel node localization. J Surg Oncol. 2010;101:184–90. [DOI] [PubMed] [Google Scholar]
- 35. Tsai JA, Celebioglu F, Lindblad M, et al. Hybrid SPECT/CT imaging of sentinel nodes in esophageal cancer: first results. Acta Radiol. 2013;54:369–73. [DOI] [PubMed] [Google Scholar]
- 36. van Vliet EP, Heijenbrok‐Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta‐analysis. Br J Cancer. 2008;98:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kato H, Kimura H, Nakajima M, et al. The additional value of integrated PET/CT over PET in initial lymph node staging of esophageal cancer. Oncol Rep. 2008;20:857–62. [PubMed] [Google Scholar]
- 38. Shimizu S, Hosokawa M, Itoh K, Fujita M, Takahashi H, Shirato H. Can hybrid DFG‐PET/CT detect subclinical lymph node metastasis of esophageal cancer appropriately and contribute to radiation treatment planning? A comparison of image‐based and pathological findings. Int J Clin Oncol. 2009;14:421–5. [DOI] [PubMed] [Google Scholar]
- 39. Takeuchi H, Kitagawa Y. Sentinel lymph node biopsy in esophageal cancer: has its time come? Ann Surg. 2010;252:413–5. [DOI] [PubMed] [Google Scholar]
- 40. Takeuchi H, Kitajima M, Kitagawa Y. Sentinel lymph node as a target of molecular diagnosis of lymphatic micrometastasis and local immunoresponse to malignant cells. Cancer Sci. 2008;99:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Komukai S, Nishimaki T, Suzuki T, Kanda T, Kuwabara S, Hatakeyama K. Significance of immunohistochemical nodal micrometastasis as a prognostic indicator in potentially curable oesophageal carcinoma. Br J Surg. 2002;89:213–9. [DOI] [PubMed] [Google Scholar]
- 42. Li SH, Wang Z, Liu XY, Liu FY, Sun ZY, Xue H. Lymph node micrometastasis: a predictor of early tumor relapse after complete resection of histologically node‐negative esophageal cancer. Surg Today. 2007;37:1047–52. [DOI] [PubMed] [Google Scholar]
- 43. Koenig AM, Prenzel KL, Bogoevsli D, et al. Strong impact of micrometastatic tumor cell load in patients with esophageal carcinoma. Ann Surg Oncol. 2009;16:454–62. [DOI] [PubMed] [Google Scholar]
- 44. Hagihara T, Uenosono Y, Arigami T, et al. Assessment of sentinel node concept in esophageal cancer based on lymph node micrometastasis. Ann Surg Oncol. 2013;20:3031–7. [DOI] [PubMed] [Google Scholar]
- 45. Kitagawa Y, Mukai M, Nakahara T, et al. Clinical significance of molecular metastasis in sentinel lymph nodes of esophageal cancer. Esophagus. 2007;4:19–22. [Google Scholar]
- 46. Takeuchi H, Kitagawa Y. Sentinel node navigation surgery for esophageal cancer. Gen Thorac Cardiovasc Surg. 2008;56:393–6. [DOI] [PubMed] [Google Scholar]
- 47. Kitajima M, Kitagawa Y. Surgical treatment of esophageal cancer – the advent of the era of individualization. N Engl J Med. 2002;347:1705–9. [DOI] [PubMed] [Google Scholar]
- 48. Pring C, Dexter S. A laparoscopic vagus preserving Merendino procedure for early esophageal adenocarcinoma. Surg Endosc. 2010;24:1195–9. [DOI] [PubMed] [Google Scholar]
- 49. Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus. Five‐year survival of a randomized clinical trial. Ann Surg. 2007;246:992–1001. [DOI] [PubMed] [Google Scholar]
- 50. Kato H, Sato A, Fukuda H, et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol. 2009;39:638–43. [DOI] [PubMed] [Google Scholar]
- 51. Ishikura S, Nihei K, Ohtsu A, et al. Long‐term toxicity after definitive chemoradiotherpay for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:2697–702. [DOI] [PubMed] [Google Scholar]
- 52. Kaburagi T, Takeuchi H, Fujii H, et al. Initial experience of individualized chemoradiotherapy for superficial esophageal cancers based on the sentinel lymph node concept. Esophagus. 2012;9:147–52. [Google Scholar]
- 53. Fujiwara H, Shiozaki A, Konishi H, et al. Perioperative outcomes of single‐port mediastinoscope‐assisted transhiatal esophagectomy for thoracic esophageal cancer. Dis Esophagus. 2017;30:1–8. [DOI] [PubMed] [Google Scholar]


