Abstract
The absence of companion may jeopardize mental health in social animals. Here, we tested the hypothesis that social isolation impairs social recognition memory by altering the excitability and the dialog between the olfactory bulb (OB) and the dorsal hippocampus (dHIP). Adult male Swiss mice were kept grouped (GH) or isolated (SI) for 7 days. Social memory (LTM) was evaluated using social recognition test. SI increased glutamate release in the OB, while decreased in the dHIP. Blocking AMPA and NMDA receptors into the OB or activating AMPA into the dHIP rescued LTM in SI mice, suggesting a cause-effect relationship between glutamate levels and LTM impairment. Additionally, during memory retrieval, phase-amplitude coupling between OB and dHIP decreased in SI mice. Our results indicate that SI impaired the glutamatergic signaling and the normal communication between OB and HIP, compromising the persistence of social memory.
Introduction
An ever-growing body of evidence has been showing that social isolation (SI) in general, and perceived social isolation (loneliness) in particular, have significant negative effects on human health1–4. There is also anatomical evidence suggesting that white matter density in areas related to social cognition is negatively correlated with Loneliness Scale responses in adults5. Additionally, loneliness might mediate the relationship between the amygdala volume and social distress scores6.
Rodents are also sensitive to SI and, as socially innate animals, prefer social housing, instead of isolation7. Particularly the post-winning SI induces long-lasting effects on cerebral function8–10, emotional behaviors11–13 and learning and memory14,15. On contrary, much less is known about the mechanisms underlying SI effects on adult animals. A brief period of SI, such as 24 h, is enough to impair social memory16 and to cause synaptic changes in dopaminergic neurons from the dorsal raphe nucleus17. Interestingly, activating those dopaminergic neurons increased social preference in mice17. Longer periods of SI, such as one week, impaired social memory persistence and olfaction, without compromising other hippocampus-dependent memories18,19.
Regarded as the first relay in the olfactory neural pathway, the olfactory bulb (OB) holds functional units, known as glomeruli, where the olfactory sensory neurons synapse with mitral cells. Mitral and tufted cells direct their glutamatergic projections to a wide number of brain regions20, such as the dentate gyrus of the hippocampus (HIP) via the lateral entorhinal cortex and the lateral perforant pathway21. In turn, part of the top-down modulation of the OB originates from the olfactory and limbic system and are essentially glutamatergic22. Mitral/tufted cells dendrites can also excite each other via glutamate spillover23. Therefore, any disturbance in the glutamatergic signaling would compromise the OB function. For instance, tufted and periglomerular cells from mice that underwent to olfactory deprivation presented higher amplitude of excitatory postsynaptic current (mEPSCs), mediated by the glutamate receptor AMPA24.
Social memory can be defined as the ability to recognize conspecifics25,26 and may be considered a hippocampus-dependent memory16,27–29. Furthermore, social memory processing relies on olfactory cues30–32 and protein synthesis in the OB27. However, it is yet to be determined whether OB and hippocampus interact to modulate social memory, although the relationship between those neural substrates had been already observed during a discriminative olfactory task33.
Here, we tested the hypothesis that social isolation alters the excitability and the dialog between the OB and HIP, limiting the duration of social memory. As an indicative for excitability, we evaluated the glutamate release in synaptosomes from OB and HIP. We also tested whether glutamate levels were driving the social memory consolidation by site-target injections of specific agonists and antagonist of AMPA and NMDA receptors. In addition, we evaluated whether the SI would also disturb the neural circuits connecting the OB and the HIP during social memory recollection. Our results show that SI changed the OB and HIP glutamatergic tonus, in opposite direction, causing a social memory deficit. We suggest that disturbing glutamatergic signaling compromised the normal communication between OB and HIP, impairing social memory persistence.
Methods
Subjects
Adult (8–12 weeks old) male Swiss mice were kept in groups of 4–5 animals (group-housed, GH) or alone (social isolated, SI) during 7 days, in a standard plastic cage (28 × 17 × 12 cm). Juveniles (21 days old) of the same strain and sex were kept in a standard plastic cage (28 × 17 × 12 cm). All animals were maintained in a climate-controlled environment (22 ± 2 °C, humidity at 55 ± 10%) under a 12 h dark- 12 h light cycle. All behavioral experiments were performed during the light phase. Both food and water were available ad libitum.
All experimental procedures were approved by The Animal Use Ethics Committee of Universidade Federal de Minas Gerais (CEUA), under the protocol number 55/2015. All experiments were performed in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Synaptosomes isolation
Animals were decapitated and had their hippocampus and olfactory bulb removed and homogenized in 1:10 (w/v) 0.32 M sucrose solution containing dithiothreitol (0.25 mM; MOLECULAR PROBES) and EDTA (1 mM; SIGMA-ALDRICH). Homogenates were submitted to low-speed centrifugation (1000 × g × 10 min) and synaptosomes were purified from the supernatant by discontinuous Percoll-density gradient centrifugation [SIGMA-ALDRICH34]. The isolated nerve terminals were re-suspended in Krebs–Ringer–HEPES solution (KRH; 124 mM NaCl, 4 mM KCl, 1.2 mM MgSO4, 10 mM glucose, 25 mM HEPES, pH 7.4) without adding CaCl2, to a concentration of approximately 10 mg/mL. For measurement of glutamate release, aliquots of 30 μl were prepared and kept on ice until use.
Measurement of continuous glutamate release
Glutamate release was measured by the increase of fluorescence due to the production of NADPH in the presence of type II glutamate dehydrogenase (SIGMA-ALDRICH) and NADP+35. This assay is based on the reaction involving GDH (glutamate dehydrogenase), NADP+ (nicotinamide adenine dinucleotide phosphate), NADPH and glutamate. When glutamate is released by synaptosomes it undergoes oxidation by the enzyme GDH and NADP+ is the acceptor of the electron oxidation. The NADPH being excited by light at a wavelength of 360 emits light at the wavelength of 450 nm, which is detected by a photomultiplier in the spectrofluorimeter. Thus, the glutamate released by the synaptosomes is quantified35. Since the reaction can occur both for the formation of α-Ketoglutarate or L-glutamate, an excess of NADP+ favors the direction of the reaction for the formation of α-ketoglutarate.
The procedure was performed as follows: the reaction medium containing a mixture of synaptosomes (approximately 30 μg of protein/well), CaCl2 (1 mM) and NADP+ (1 mM; SIGMA-ALDRICH) in KRH were transferred to Elisa microplates (300 μl/well) attached to a spectrofluorimeter (SYNERGY 2). After 3 min, glutamate dehydrogenase (35 units per well) was added and the reading was restarted until the fluorescence reached balance (approximately 10 min). Synaptosomes were depolarized with 33 mM KCl. The experimental data were expressed in nmol of glutamate released per mg of protein. The experiments were performed at 37 °C for 30–50 min with excitation wavelength of 360 nm and emission of 450 nm.
Cannula Implantation
Mice were anesthetized with a mixture of ketamine (80 mg/Kg) and xylazine (10 mg/kg) injected intraperitoneally and placed in a stereotaxic apparatus. Small holes (0.9 mm) were drilled directed toward the CA1 region of dorsal hippocampus [dHIP: AP, −1.9; LL, ±1.6; DV, −1.0 36] or main olfactory bulb [MOB: AP, +4.0; LL, ±1.0; DV, −3.0 27]. Bilateral guide cannulae (22 G, 7 mm) containing dummy cannulae were inserted and fixed in the skull with zinc cement followed by dental acrylic. Coordinates were chosen based on Paxinos37. At least 7 days after, animals were used to pharmacological experiments.
Electrode Implantation
Mice were anesthetized with a mixture of ketamine (80 mg/Kg) and xylazine (10 mg/kg) injected intraperitoneally and placed in a stereotaxic apparatus for implantation of three steel electrodes (0.005”, TeflonTM coated to a final thickness 0.007”, #7915, A-M SYSTEMS). One electrode was implanted in the dorsal HIP (AP −1.9; LL +1.6; DV −1.0), one in the ventral HIP (AP −2.8; LL +3.8; DV −1.0) and one in the mitral layer of the OB (AP +4.0; LL ±1.0; DV −3.0). One screw was used as reference and inserted in the contralateral occipital region, depth to dura mater; while a second one, used as ground screw, was inserted in the contralateral parietal region, also depth to dura mater. The electrodes and screws were positioned at specific depths and fixed to the skull with zinc cement. They were then welded to a pin connector, which allowed coupling to the electrophysiological recording system. At least 7 days after, animals were used to electrophysiology experiments.
Pharmacological Experiments
At the time of the infusions, mice were gently held, dummy cannulas were removed and a cannula (30 G, 8 mm) was coupled to the guide cannula. Drugs were injected using a 10 μL syringe (HAMILTON, USA) connected to a pump adjusted to a flow of 0.5 μL/minute (min). After the end of microinjection, the syringe remained connected to the cannula for approximately 1 min to avoid reflux of the drug. All animals were gently restrained during the injection procedures. The experimental group received (0.5 μl/side) of NMDA (SIGMA, 1 μg/μL), AP5 (SIGMA, 1 μg/μL)38, AMPA (SIGMA, 1 μg/1 μL) or NBQX (SIGMA, 2 μg/μL)39. The control group received saline (0.5 μl/side).
Social Recognition Test
Swiss juvenile male mice were used as intruders and were presented to the adult mouse inside a transparent acrylic cylinder (10 cm diameter) containing 60 holes equally distributed. Habituation phase consisted of introducing the adult mouse inside a clean standard cage containing an empty cylinder for 20 min. Training session (TR) lasted 5 min and consisted of replacing the empty cylinder by the one containing the juvenile mouse. Social investigation was quantified every time the resident animal introduced its nose and/or whiskers inside any of the cylinder’s holes. To assess social short-term memory (STM), 1:30 h after TR, mice were habituated exactly as describe above and the social investigation was scored. The social long term-memory (LTM) was measured 24 h after TR40. None of the juvenile mice were used more than three times.
Electrophysiological recordings
A set of thin and light copper wires was developed for making it possible to proceed with the freely moving recordings. Local field potentials (LFP), recorded in the dorsal HIP and OB, were pre-amplified (gain of 2000), filtered (0.1 Hz high-pass filter and 500 Hz, low-pass filter) and digitized with a sampling frequency of 1 kHz (NATIONAL INSTRUMENTS). Data were visualized using the software KANANDA LTDA (Brazil) and recorded in a dedicated computer. Synchronized with the recording, live image of the animal was captured through a video camera (TVnPC P6).
Electroencephalographic analysis (power spectrum and cross-frequency coupling)
All the analyses were performed using MATLAB R2013b, with codes available to the scientific community upon request. Sniffing segments were selected according to visual inspection of the video-EEG.
Spectral analysis was done using the short time Fourier transform (STFT), a time-frequency decomposition method, using windows with 1024 samples and 50% overlap. The energy of different LFP rhythms was extracted from each analysis segment (sniffing or no-sniffing), and mean values for each rhythm were taken for each recording and normalized by the respective baseline values (60 seconds before to juvenile exposition). Frequency bands were chosen as Delta 1–4 Hz; Theta 4–10 Hz; Alpha 10–16 Hz; Beta 17–30 Hz; slow Gamma 30–85 Hz and fast Gamma 90–140 Hz.
Functional connectivity between rhythms was assessed by estimating the degree of phase-amplitude coupling (PAC), a type of interaction which has received increasing interest because of its occurrence in several contexts, including the OB41. Furthermore, there are indications that this kind of coupling may play a key role in cognition and information processing42–45. PAC was measured between OB and dHIP using the Modulation Index (MI) proposed by Tort and colleagues46. The phase of the OB was obtained from the theta-filtered signal (4–10 Hz, the modulation band) and dHIP amplitude from the respective gamma-filtered signal (90–140 Hz), resulting in phase-amplitude distribution from which the MI was calculated. Thus, strong phase-amplitude modulation happens when gamma activity is concentrated in specific phases of the theta cycle, resulting in high MI values.
To account for the different exploration periods, MI was calculated in sliding windows of 1.8 seconds and 90% overlap. The mean values across windows of each sniffing segment were then obtained.
Histology
The procedures for post-mortem verification of cannula and electrodes placements were made at the end of the behavioral tests, when animals were euthanized. The encephalon was removed and fixed with 4% PFA overnight, followed by placement in a 30% sucrose solution in PBS. The brains were maintained at 4 °C for 3 days. For the histological examination, 40 μm coronal brain slices were obtained using a cryostat. Only data from animals with the correct implantation of the guide cannula or electrodes were included in statistical analysis.
Statistical analysis
Statistical analyses were performed using Prism 5 software (GRAPHPAD SOFTWARE). The data were analyzed by Two-way ANOVA and the post-hoc Bonferroni test for multiple comparisons. Unpaired t test was used to compare groups regarding glutamate release. Pearson correlation was performed between modulation index, theta or gamma power and recognition index. Significance level was set at p < 0.05.
Results
Region-specific effect of social isolation on glutamate release
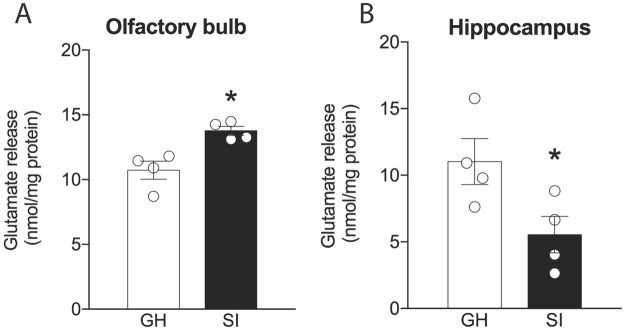
Glutamate signaling has been linked to the maintenance of hippocampus-dependent memories47–49. As socially isolated animals (SI) are unable to retain social memory for 24 h16,18, we tested whether SI would compromise neuronal excitability by altering the glutamate release. We prepared synaptosomes from olfactory bulb (OB) and hippocampus (HIP) of naïve group-housed (GH) and isolated (SI) mice. High potassium stimulated synaptosomes from the OB of SI mice released more glutamate compared to group-housed (GH) animals [t(6) = 3.9, p = 0.007; Fig. 1A]. On the contrary, glutamate release decreased in hippocampal synaptosomes from SI mice [t(7) = 2.5, p = 0.04; Fig. 1B]. Thus, our results suggest that SI increases the glutamatergic tonus in the OB, while decreases in the HIP.
Figure 1.
Effect of social isolation on the release of glutamate in the (A) olfactory bulb and (B) hippocampus. (n = 4/group). Results expressed as mean ± standard error of the mean. *Indicates difference between groups.
Blocking AMPA and NMDA receptors into the OB allows social memory persistence in SI mice
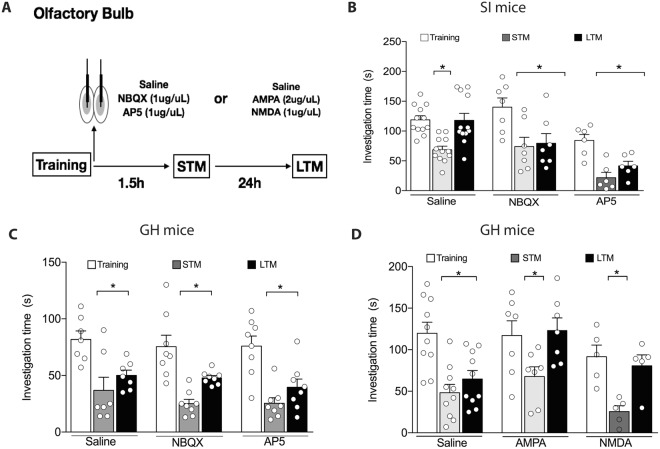
Next, we tested whether the higher levels of glutamate release into the OB were driving the memory deficits in SI mice, by blocking AMPA (NBQX) and NMDA (AP5) receptors directly into the OB (Fig. 2A). As we predicted, the intra-OB injection (immediately after the TR) of NBQX and AP5 rescued LTM (tested 24 h after training) in SI mice, without affecting STM (tested 1 h 30 min after training) [Interaction: F(4,46) = 3.6, p = 0.01; Drug: F(2,23) = 7.3, p = 0.003; Trial: F(2,46) = 37.5, p < 0.0001; Fig. 2B].
Figure 2.
Effect of NBQX (AMPA receptor antagonist) and AP5 (NMDA receptor antagonist) administration in the olfactory bulb (OB), immediately after the training session on the social recognition task. (A) Schematic representation of the experimental design. (B) Blockade of AMPA (NBQX) and NMDA (AP5) receptors into the OB recovered the social long-term memory (LTM) in social isolated (SI) mice without affecting social short-term memory (STM) (n = 6–12/group). (C) NBQX and AP5 into the OB did not affect STM and LTM in group-housed (GH) mice (n = 7–8/group). (D) AMPA and NMDA agonists impaired LTM in GH animals (n = 5–10/group). Results are expressed as mean ± standard error of the mean. *Indicates difference between the training and test sessions, within the same group.
To test whether glutamatergic signaling in the OB is necessary to social memory consolidation, we administered the same antagonists, NBQX and AP5, in group-housed animals (GH). Our results showed that AMPA and NMDA activation in the OB is not necessary to social memory consolidation [Interaction: F(4,40) = 0.2, p = 0.9; Drug: F(2,20) = 0.9, p = 0.3; Trial: F(2,40) = 39.4, p < 0.0001; Fig. 2C].
Next, we designed a pharmacological experiment to reinforce the cause-effect relationship between high glutamate levels into the OB and social memory persistence. AMPA and NMDA agonists were administered directly into the OB of GH animals, immediately after training. Our results showed that AMPA and NMDA [Interaction: F(4,38) = 2.6, p = 0.05; Drug: F(2,19) = 3.3, p = 0.05; Trial: F(2,38) = 23.6, p < 0.0001; Fig. 2D] impaired LTM, but not STM, suggesting that increasing glutamatergic tonus into the OB, either by AMPA and NMDA agonists compromised social memory persistence.
Activating AMPA receptors in the dorsal hippocampus (dHIP) allows social memory persistence in SI mice
The activation of glutamatergic receptors into the hippocampus seems essential for the consolidation of LTM50,51. However, fear conditioning was the behavioral paradigm used to demonstrate this dependency. Thus, before testing whether decreased glutamate release from hippocampal synaptosomes would have a cause-effect relationship with LTM impairment, we tested the hypothesis that hippocampal glutamatergic signaling is essential for the formation of long-lasting social memory. To address this question, AMPA and NMDA receptors antagonists were administered into the CA1 region of the dHIP, immediately after TR in the social recognition test (Fig. 3A).
Figure 3.
Effect of AMPA and NMDA receptors agonists administration in the dorsal hippocampus (dHIP), immediately after the training session on the social recognition task. (A) Schematic representation of the experimental design. (B) AMPA agonist into the dHIP recovered the social long-term memory (LTM) deficit of social isolated mice (SI). NMDA agonist did not recover LTM (n = 7–8/group) of SI mice. (C) Administration of NBQX impairs the consolidation of LTM in group-housed animals (GH), while, the administration of AP5 did not affect LTM (n = 6–16/group). Results are expressed as mean ± standard error of the mean. *Indicates difference between the training and test sessions, within the same group.
NBQX impaired the consolidation of LTM in GH animals, though AP5 had no effect [Interaction: F(4,56) = 7.6, p < 0.0001; Drug: F(2,28) = 10.5, p = 0.0004; Trial: F(2,56) = 39.1, p < 0.0001], Fig. 3C.
After, we verified whether the activation of AMPA and NMDA receptors in the hippocampus of isolated animals would restore LTM in these animals. Intra-hippocampal AMPA activation recovered LTM in SI mice [Interaction: F(4,40) = 0.49, p = 0.74; Drug: F(2,20) = 0.22, p = 0.8; Trial: F(2,40) = 25.93, p < 0.0001], while NMDA had no effect (Fig. 3B).
Altogether, our results suggest that the hippocampal activation of AMPA receptors, rather than NMDA receptors, in the early stages of consolidation is necessary for the formation of LTM.
Social exploration increases gamma, but not theta power into the OB and dHIP
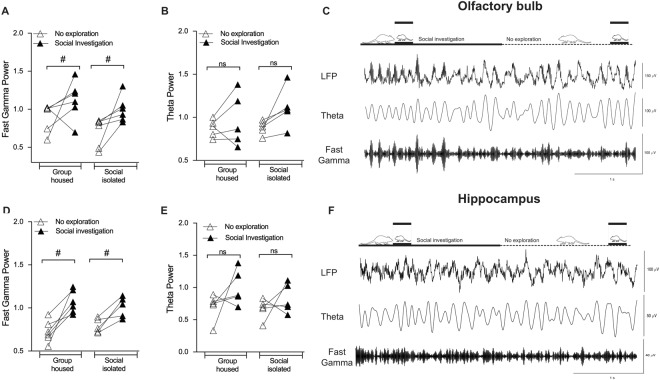
Glutamatergic signaling may drive gamma oscillations in the OB41 reviewed by33 and theta oscillations in the HIP52. Furthermore, in humans, it was showed a robust relationship between hippocampal glutamate levels and frontal theta activity53. As we showed evidences that SI changed glutamatergic transmission in the OB and HIP, compromising social memory persistence. For that purpose, we recorded the local field potential (LFP) in both areas, during the social recognition test. First, we verified whether social exploration would change brain activity in both groups. We observed that social investigation increased the fast gamma power into the OB of both GH and SI animals [Interaction: F(1,18) = 0.06, p = 0.8; Environment condition: F(1,18) = 6.0, p = 0.02; Behavior: F(1,18) = 16.6, p = 0.0007; Fig. 4A,C]. No effect on theta power was observed [Interaction: F(1,16) = 0.5, p = 0.4; Environment condition: F(1,16) = 0.6, p = 0.4; Behavior: F(1,16) = 3.1, p = 0.09; Fig. 4B,C].
Figure 4.
Effect of social exploration on fast gamma and theta oscillations in the (C) olfactory bulb (OB) and (F) dorsal hippocampus (dHIP). Social exploration increases fast gamma power in the (A) OB (n = 6/group) and (D) dHIP (n = 5/group), in both groups. #Indicates difference between no exploration and social exploration within the same group. There was no difference in theta power after social exploration in (B) OB (n = 6/group) and (E) dHIP (n = 5/group) of both groups.
In the HIP, social investigation increased fast gamma power in both groups [Interaction: F(1,18) = 1.4, p = 0.2; Environment condition: F(1,18) = 0.005, p = 0.9; Behavior: F(1,18) = 31.0, p < 0.0001; Fig. 4D,F]. Similar to OB, no effect of social exploration in hippocampal theta power was detected in GH and SI mice [Interaction: F(1,16) = 0.5, p = 0.4; Environment condition: F(1,16) = 0.9, p = 0.3; Behavior: F(1,16) = 5.4, p = 0.03; Fig. 4E,F].
Considering these results, we suggest that social exploration triggers fast gamma oscillations in the OB, regardless of whether the animal was submitted or not to social isolation.
Social isolation increased gamma power oscillations in the olfactory bulb, during long-term social memory retrieval
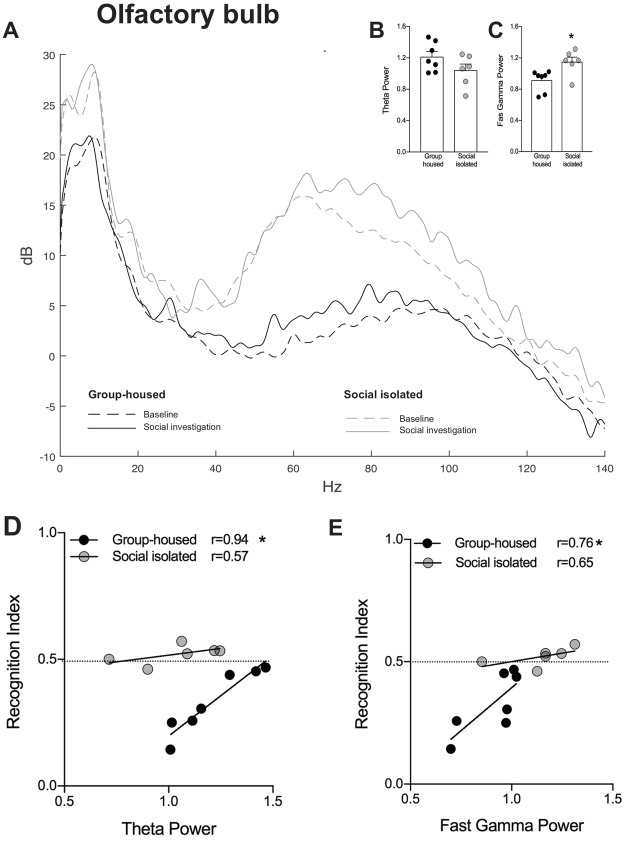
Next, we evaluated the OB oscillations during memory retrieval. No difference between groups was observed in STM (data not shown). Figure 5A shows the power spectral density (PSD) of LFP recordings in the OB of one animal from each group. We did not observe difference between group-housed and isolated mice for theta oscillations (t(11) = 1.6, p = 0.13; Fig. 5B). However, social isolation increased fast gamma oscillations during LTM retrieval (t(11) = 2.8, p = 0.01; Fig. 5C).
Figure 5.
Effect of social isolation on the olfactory bulb gamma and theta oscillations, during social long-term memory retrieval (LTM). (A) Power Spectral Density (PSD) from one representative animal from group-housed (dark lines) and social isolated (grey lines) groups. (B) Theta and (C) fast gamma power during LTM. *Indicates difference between groups. Correlation between LTM performance (recognition index) and (D) theta or (E) fast gamma power oscillation. *Indicates statistically significant correlation.
In our study, changes in theta and gamma power may not be related to speed of locomotion54,55, since no difference on speed of locomotion between GH and SI mice was detected (Fig. S1).
Next, we asked whether OB theta and gamma oscillations were driving the social memory retrieval in both conditions: group-housed and social isolation. Theta and gamma power linearly correlated with memory performance (recognition index) in group-housed animals (Theta: r = 0.94, p = 0.001; Fast gamma: r = 0.76, p = 0.04), but not in SI mice (Theta: r = 0.57, p = 0.23; Fast gamma: r = 0.65, p = 0.15), Fig. 5D,E.
Social isolation did not alter gamma and theta power oscillations in the dorsal hippocampus, during long-term social memory retrieval
To further explore the effect of SI on hippocampal oscillations, we quantified the fast gamma and theta power during the memory retrieval. No differences between groups were observed during STM (data not shown). Figure 6A shows the power spectral density (PSD) of LFP recordings in the dorsal hippocampus (dHIP) of one animal from each group. We did not observe statistical difference between groups regarding theta (t(10) = 0.67, p = 0.51; Fig. 6B) and fast gamma (t(11) = 1.0, p = 0.31; Fig. 6C) power oscillations.
Figure 6.
Effect of social isolation on the dorsal hippocampus fast gamma and theta oscillations, during social long-term memory retrieval (LTM). (A) Power Spectral Density (PSD) from one representative animal from group-housed (dark lines) and social isolated (grey lines) groups. (B) Theta and (C) fast gamma power during LTM. Correlation between LTM performance (recognition index) and (D) theta or (E) gamma power oscillation. *Indicates statistically significant correlation.
Next, we evaluated whether there is correlation between LTM and hippocampal oscillations, and whether social isolation plays a role in this correlation. There was a significant correlation between social memory and theta oscillations in group-housed mice (r = 0.83, p = 0.03; Fig. 6D), but not in SI mice (r = 0.76, p = 0.07; Fig. 6D). However, both groups (Group-housed: r = 0.80, p = 0.02; SI: r = 0.88, p = 0.01; Fig. 6E) showed significant correlation between LTM performance and dHIP fast gamma oscillations. The correlation analysis of group-housed animals indicates that social memory is evoked more efficiently the lower the value for dHIP gamma and theta power.
Social isolation decreased theta phase/gamma amplitude coupling between the OB and the dorsal HIP during long-term social memory retrieval
LFP analysis indicated that gamma and theta power oscillations, in both OB and dHIP, correlates with animal’s performance during LTM retrieval, in GH animals. However, on average, we only observed a difference between GH and SI animals in OB gamma power. Therefore, these results, though interesting, did not entirely explain why SI mice do not present LTM.
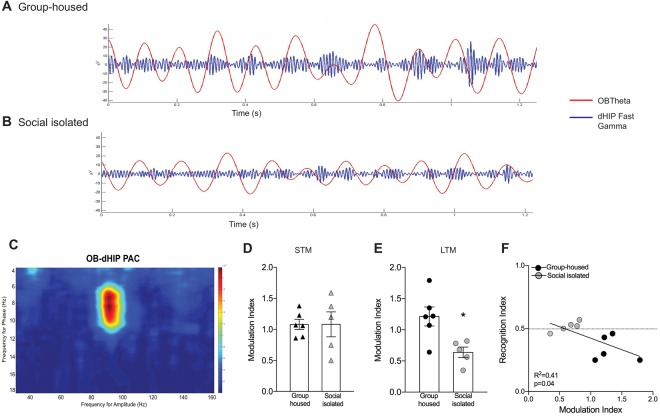
Thus, we decided to evaluate OB-dHIP circuits by analyzing the strength of coupling between LPF rhythms: theta phase and fast gamma amplitude (Fig. 7A,B). We did not find coupling between OB and ventral hippocampus and between both hippocampus (data not shown). The functional connectivity, measured by the interactions among frequency bands, revealed that OB modulates dHIP during STM (Fig. 7D) and LTM (Fig. 7E) in GH and SI animals. However, SI animals showed a lower coupling during LTM [t(9) = 3.0, p = 0.01], while were similar to GH during STM retrieval [t(9) = 0.0005, p = 0.3].
Figure 7.
Theta phase/gamma amplitude coupling between the olfactory bulb (OB) and dorsal hippocampus (dHIP) during the retrieval of short (STM) and long-term memory (LTM). Representative theta phase/gamma amplitude coupling between the OB and dHIP during LTM in (A) group-housed and (B) isolated animal. (C) Representative phase-amplitude comodulogram recorded during social exploration. Modulation index during (D) STM and (E) LTM. *Indicate difference between groups. (F) Positive correlation between LTM performance (recognition index) and coupling (modulation index).
To further characterize the effect of OB-dHIP coupling and LTM, we also analyzed the correlation between coupling (modulation index) and performance in LTM (recognition index). Correlation analysis shows that recognition index correlated with modulation index [r = −0.64; p = 0.04; Fig. 7F]. Also, the linear regression indicated that efficient coupling (high values of modulation index) was present in animals showing LTM (see black circles, GH animals, Fig. 7F). Our results indicated a significant coefficient of determination (R2), p = 0.04, with explanatory value for 53% of all cases (R2 = 0.41).
Discussion
The present study shows that social isolation (SI) potentiates the glutamatergic signaling in the OB, while reduces in the HIP. The glutamatergic unbalance lead to a deficit in LTM, which is accompanied by a decreased phase-amplitude coupling between dHIP and OB.
We found increased glutamate release from OB synaptosome after social isolation. In addition, blocking AMPA and NMDA receptors in the OB, after training, rescued social memory in SI mice, while did not change in GH animals. In line with these results, AMPA and NMDA activation mimicked the SI impairment in control animals. Regardless of whether the source of glutamate has not been accessed, increased glutamatergic signaling in the OB was deleterious to LTM. However, we wonder how would SI be changing the glutamatergic tonus in the OB?
Depriving neonatal rats of odors, through naris occlusion, during 3 days, increased the probability of glutamate release in the glomerulus, and also increased synaptic currents mediated by AMPA and NMDA receptors24. Thus, one possibility is that under SI, the animal is subjected to a restricted repertoire of odors, diminishing the sensorial input to the OB and leading to an upregulation of the glutamatergic system. Accordingly, we showed previously that if the SI is applied in combination with odors from conspecific, the LTM deficit is rescued18.
Nevertheless, the effects of social isolation go beyond the olfactory constraint. Mice rely on social contact to maintain mental and physical health56–58. One of the main mediators of sociability is the neuropeptide oxytocin (OXT). OXT administration usually increases pro-social behaviors in several species59–61. Furthermore, engaging in social interactions may trigger the production of OXT62,63.
Recently, it was found that OXT, acting on the anterior olfactory nucleus (AON), adjusts the feedback inhibition of mitral cells, improving the signal-to-noise ratio of odor responses64. Additionally, same authors found that deletion of OXT receptors in the AON increased olfactory exploration and impaired specifically the social recognition memory64. Accordingly, the activation of the par medialis portion of AON worsened social recognition65. Collectively, these studies reinforce the idea that centrifugal feedback excitatory projections to the OB are important to adjust olfactory sensitivity66 and, further, suggest that OXT may play a role in this process. We, therefore, speculate that SI may affect oxytocin production and/or signaling, ultimately leading to a glutamatergic unbalance in the OB. As a consequence, the signal-to-noise ratio for juvenile’s odors detection in mitral/tufted cells is compromised, as well as the social recognition memory.
Feedback inhibition of mitral cells firing by granule cells has been proposed as the possible generator of OB gamma oscillations22. Accordingly, odor stimulation, as well as olfactory learning, modify gamma oscillation in the OB67. During the TR, the adult mouse samples the juvenile, mainly using its olfactory system, likely reason why gamma power increased in OB, in response to the social investigation. At this point, social isolation had no effect, since no difference between groups was observed, suggesting that SI is affecting the storage of social memory rather than olfaction per se.
It has been reported that social recognition memory is a hippocampus-dependent memory16,28,68. Here we demonstrated that AMPA, but not NMDA receptors activation in the CA1 region of the dHIP is necessary to social recognition memory, reinforcing the idea of the hippocampus recruitment in this type of memory. Furthermore, SI decreased glutamate release from hippocampal synaptosomes. As we recovered the memory deficit of SI mice by the intra-hippocampal administration of AMPA, but not NMDA, we strongly suggest a cause-effect relationship between glutamatergic signaling and LTM consolidation in the dHIP. In line with these observations, extensive research has demonstrated that AMPA administration into the hippocampus improves memory69–72. Notably, odor discrimination learning is facilitated following injection of AMPA agonist73.
We argued before that OXT64 could be a candidate to participate on the effect of SI on the glutamatergic signaling in the OB. Thus, we wonder whether a similar mechanism could be proposed to explain the effects of SI in the hippocampus. Intranasal OXT increases both gamma power and activation, measured by functional magnetic resonance, of the dHIP74. It was recently demonstrated that hippocampal OXT receptors are necessary for discrimination of social stimuli75. Same authors identified that hippocampal OXT receptors are preferentially expressed in inhibitory interneurons, which reconcile with results showing that OXT receptors activation disinhibits CA1 neurons76. Therefore, in our study, we suggest that SI could be interfering in the oxytocin ability to modulate hippocampus excitability, compromising LTM.
Social memory can be studied using distinct paradigms. In the habituation-dishabituation protocol, memory is formed by short and repeated exposition to a conspecific, taking to the formation of a short-term memory trace77,78. It was shown that theta oscillations, in the main OB of rats, gradually decrease along the encounters in the habituation-dishabituation protocol, indicating a strong correlation between olfactory exploration and theta rhythms79. In our study, we did not observe a relation between OB or dHIP theta oscillations and social exploration, during the acquisition phase. However, during LTM retrieval, we observed a significant correlation in control animals, whereas animals with better performance were the ones with lower theta power in the OB and dHIP.
Theta oscillations is frequently associated with communication between distant areas80 and linked with cognitive performance79. Furthermore, simple home cage exploration may induce theta oscillations81. Alternatively, it has been argued that theta oscillations in areas such as dHIP and OB may be in fact a respiration-entrained rhythm [RR82–86]. We did not measure nasal respiration, though the quantification of social investigation resembles sniffing. Therefore, the theta power quantified here could be overestimated and be in fact a RR. If this was true, we should have seen higher theta power when animals were exploring the juvenile, such as in the training session. However, we did not find an increase in theta power in both OB and dHIP when animals were exploring the juvenile.
Finally, we tested the hypothesis that SI impaired LTM by compromising the communication between OB and dHIP. To test this hypothesis, we chose to analyze the phase-amplitude coupling, or cross-frequency coupling (CFC) between OB and dHIP and represent this result as modulation index [MI46]. Phase-amplitude coupling may be useful to assess the communication between brain areas, whereas slow oscillation phase (theta) modulates the amplitude of faster oscillation (gamma)43. Several studies have shown that theta-gamma coupling may facilitate transfer of information throughout entorhinal cortex-hippocampus network87. And recently, we found OB-dHIP CFC during a spatial olfactory task88.
We observed a theta phase-gamma amplitude coupling between OB and dHIP during social memory retrieval in GH animals. The involvement of an olfaction-hippocampal network has been shown in odor processing and discrimination33,89,90. Here, we indicate that during social memory retrieval, gamma oscillations in the dHIP are driven by the OB. Accordingly, a potential function for higher amplitudes of gamma oscillations at a particular theta phase is to facilitate memory recollection of earlier experiences91–94.
As predicted, MI during LTM, but not STM, was attenuated by SI. We also found that MI can predict the LTM performance, with explanatory value of 53%. There are several studies showing that impairment in hippocampal gamma amplitude modulation by theta phase relates to memory deficits95,96. However, this is the first time a study shows OB-dHIP coupling in social recognition memory.
Social isolation impaired the glutamatergic transmission in the OB and dHIP at the same time that decreased the theta-phase gamma-amplitude coupling between same areas, during LTM retrieval. In the present study we did not address whether the pharmacological manipulations of AMPA and NMDA receptors, performed immediately after training, would affect OB-dHIP coupling at the time that LTM is tested. However, there are some evidences showing that drugs that alter the glutamatergic tonus, in areas such as dorsal hippocampus97 and the olfactory bulb98, modified brain oscillations and related-behaviors.
Altogether, our results show that SI induced opposite glutamatergic tonus in OB and dHIP, which is one possible cause for the social memory deficit. Furthermore, we may suggest that disturbing glutamatergic signaling compromised the normal communication between OB and HIP, impairing social memory persistence.
Supplementary information
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES)-Finance code 001. This study was financially supported by CNPq, FAPEMIG and CAPES (Procad). M.F.D.M. and G.S.P. were supported by a fellowship from CNPq. A.F.S.A. was supported by a fellowship from CNPq (PVE) and CAPES (PNPD). The authors thank Dr. Pete Glower for language-editing service.
Author Contributions
A.F.S.A. performed and designed the experiments and wrote the manuscript. V.R.C. analyzed the electrophysiology data and wrote the manuscript. L.J. assisted in the execution of pharmacological experiments. C.M.C. assisted in the execution of biochemical experiments. H.P.P. assisted in the execution of electrophysiological experiments. T.P.D.O. performed the experiments of glutamate release. L.B.V. helped to design and provided the infrastructure for the measurement of glutamate release and wrote the manuscript. M.F.D.M. assisted in the design and execution of electrophysiological experiment and also provided the infrastructure for data acquisition and analysis. G.S.P. conceived and designed the study, supervised all data analysis and wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36871-6.
References
- 1.Chou KL, Cacioppo JT, Kumari M, Song YQ. Influence of social environment on loneliness in older adults: Moderation by polymorphism in the CRHR1. Am J Geriatr Psychiatry. 2014;22:510–518. doi: 10.1016/j.jagp.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51:S54–66. doi: 10.1177/0022146510383501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinrich LM, Gullone E. The clinical significance of loneliness: a literature review. Clin Psychol Rev. 2006;26:695–718. doi: 10.1016/j.cpr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa S, et al. White matter structures associated with loneliness in young adults. Sci Rep. 2015;5:17001. doi: 10.1038/srep17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian X, Hou X, Wang K, Wei D, Qiu J. Neuroanatomical correlates of individual differences in social anxiety in a non-clinical population. Soc Neurosci. 2016;11:424–437. doi: 10.1080/17470919.2015.1091037. [DOI] [PubMed] [Google Scholar]
- 7.Van Loo PL, Mol JA, Koolhaas JM, Van Zutphen BF, Baumans V. Modulation of aggression in male mice: influence of group size and cage size. Physiol Behav. 2001;72:675–683. doi: 10.1016/S0031-9384(01)00425-5. [DOI] [PubMed] [Google Scholar]
- 8.Hall FS, et al. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav. 1998;59:859–872. doi: 10.1016/S0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 9.Heidbreder CA, et al. Increased responsiveness of dopamine to atypical, but not typical antipsychotics in the medial prefrontal cortex of rats reared in isolation. Psychopharmacology (Berl) 2001;156:338–351. doi: 10.1007/s002130100760. [DOI] [PubMed] [Google Scholar]
- 10.Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/S0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- 11.Bibancos T, Jardim DL, Aneas I, Chiavegatto S. Social isolation and expression of serotonergic neurotransmission-related genes in several brain areas of male mice. Genes Brain Behav. 2007;6:529–539. doi: 10.1111/j.1601-183X.2006.00280.x. [DOI] [PubMed] [Google Scholar]
- 12.Pibiri F, et al. The combination of huperzine A and imidazenil is an effective strategy to prevent diisopropyl fluorophosphate toxicity in mice. Proc Natl Acad Sci USA. 2008;105:14169–14174. doi: 10.1073/pnas.0807172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDermott B, Berry H, Cobham V. Social connectedness: a potential aetiological factor in the development of child post-traumatic stress disorder. Aust N Z J Psychiatry. 2012;46:109–117. doi: 10.1177/0004867411433950. [DOI] [PubMed] [Google Scholar]
- 14.Lu L, et al. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183:600–609. doi: 10.1016/S0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- 15.Ibi D, et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 16.Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063. [DOI] [PubMed] [Google Scholar]
- 17.Matthews GA, et al. Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell. 2016;164:617–631. doi: 10.1016/j.cell.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gusmao ID, et al. Odor-enriched environment rescues long-term social memory, but does not improve olfaction in social isolated adult mice. Behav Brain Res. 2012;228:440–446. doi: 10.1016/j.bbr.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 19.Monteiro BM, Moreira FA, Massensini AR, Moraes MF, Pereira GS. Enriched environment increases neurogenesis and improves social memory persistence in socially isolated adult mice. Hippocampus. 2014;24:239–248. doi: 10.1002/hipo.22218. [DOI] [PubMed] [Google Scholar]
- 20.Wagner S, Gresser AL, Torello AT, Dulac C. A multireceptor genetic approach uncovers an ordered integration of VNO sensory inputs in the accessory olfactory bulb. Neuron. 2006;50:697–709. doi: 10.1016/j.neuron.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Vanderwolf CH. Behavior-related cortical activity and swim-to-platform performance in the aged rat. Behav Brain Res. 1992;52:153–158. doi: 10.1016/S0166-4328(05)80225-6. [DOI] [PubMed] [Google Scholar]
- 22.Rojas-Libano D, Kay LM. Olfactory system gamma oscillations: the physiological dissection of a cognitive neural system. Cogn Neurodyn. 2008;2:179–194. doi: 10.1007/s11571-008-9053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb. Neuron. 1999;23:377–384. doi: 10.1016/S0896-6273(00)80787-4. [DOI] [PubMed] [Google Scholar]
- 24.Tyler WJ, Petzold GC, Pal SK, Murthy VN. Experience-dependent modification of primary sensory synapses in the mammalian olfactory bulb. J Neurosci. 2007;27:9427–9438. doi: 10.1523/JNEUROSCI.0664-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr WJ, Yee L, Gable D, Marasco E. Olfactory recognition of conspecifics by domestic Norway rats. J Comp Physiol Psychol. 1976;90:821–828. doi: 10.1037/h0077266. [DOI] [PubMed] [Google Scholar]
- 27.Pena RR, Pereira-Caixeta AR, Moraes MF, Pereira GS. Anisomycin administered in the olfactory bulb and dorsal hippocampus impaired social recognition memory consolidation in different time-points. Brain Res Bull. 2014;109:151–157. doi: 10.1016/j.brainresbull.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353:1536–1541. doi: 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luscher Dias T, Fernandes Golino H, Moura de Oliveira VE, Dutra Moraes MF, Schenatto Pereira G. c-Fos expression predicts long-term social memory retrieval in mice. Behav Brain Res. 2016;313:260–271. doi: 10.1016/j.bbr.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Ropartz P. Evidence for an increase of locomotor activity of a group of female mice in response to the odor of a group of unknown males. C R Acad Sci Hebd Seances Acad Sci D. 1968;267:2341–2343. [PubMed] [Google Scholar]
- 31.Matochik JA. Role of the main olfactory system in recognition between individual spiny mice. Physiol Behav. 1988;42:217–222. doi: 10.1016/0031-9384(88)90073-X. [DOI] [PubMed] [Google Scholar]
- 32.Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol. 1991;1:555–560. doi: 10.1016/0924-977X(91)90010-R. [DOI] [PubMed] [Google Scholar]
- 33.Martin C, Beshel J, Kay LM. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. J Neurophysiol. 2007;98:2196–2205. doi: 10.1152/jn.00524.2007. [DOI] [PubMed] [Google Scholar]
- 34.Dunkley PR, et al. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: homogeneity and morphology of subcellular fractions. Brain research. 1988;441:59–71. doi: 10.1016/0006-8993(88)91383-2. [DOI] [PubMed] [Google Scholar]
- 35.Nicholls DG, Sihra TS, Sanchez-Prieto J. Calcium-dependent and -independent release of glutamate from synaptosomes monitored by continuous fluorometry. J Neurochem. 1987;49:50–57. doi: 10.1111/j.1471-4159.1987.tb03393.x. [DOI] [PubMed] [Google Scholar]
- 36.Lazaroni TL, et al. Angiotensin-(1–7)/Mas axis integrity is required for the expression of object recognition memory. Neurobiol Learn Mem. 2012;97:113–123. doi: 10.1016/j.nlm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos, G., Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates. 2nd. edn, (Academic Press., 2001).
- 38.Siegmund A, Wotjak CT. Hyperarousal does not depend on trauma-related contextual memory in an animal model of Posttraumatic Stress Disorder. Physiol Behav. 2007;90:103–107. doi: 10.1016/j.physbeh.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 39.Xu A, Duan S, Tian Y. Effects of intracerebroventricular NMDA and non-NMDA receptor agonists or antagonists on general anesthesia of propofol in mice. Front Med China. 2007;1:207–210. doi: 10.1007/s11684-007-0039-x. [DOI] [PubMed] [Google Scholar]
- 40.Pereira-Caixeta AR, Guarnieri LO, Pena RR, Dias TL, Pereira GS. Neurogenesis Inhibition Prevents Enriched Environment to Prolong and Strengthen Social Recognition Memory, But Not to Increase BDNF Expression. Mol Neurobiol. 2017;54:3309–3316. doi: 10.1007/s12035-016-9922-2. [DOI] [PubMed] [Google Scholar]
- 41.Rojas-Libano D, Frederick DE, Egana JI, Kay LM. The olfactory bulb theta rhythm follows all frequencies of diaphragmatic respiration in the freely behaving rat. Front Behav Neurosci. 2014;8:214. doi: 10.3389/fnbeh.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Axmacher N, et al. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci USA. 2010;107:3228–3233. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Wingerden M, van der Meij R, Kalenscher T, Maris E, Pennartz CM. Phase-amplitude coupling in rat orbitofrontal cortex discriminates between correct and incorrect decisions during associative learning. J Neurosci. 2014;34:493–505. doi: 10.1523/JNEUROSCI.2098-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheffer-Teixeira, R. & Tort, A. B. On cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. Elife5, 10.7554/eLife.20515 (2016). [DOI] [PMC free article] [PubMed]
- 46.Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104:1195–1210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitsushima D, Ishihara K, Sano A, Kessels HW, Takahashi T. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc Natl Acad Sci USA. 2011;108:12503–12508. doi: 10.1073/pnas.1104558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi T. Mechanisms underlying contextual fear learning. Commun Integr Biol. 2011;4:726–727. doi: 10.4161/cib.17505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Migues PV, et al. Blocking Synaptic Removal of GluA2-Containing AMPA Receptors Prevents the Natural Forgetting of Long-Term Memories. J Neurosci. 2016;36:3481–3494. doi: 10.1523/JNEUROSCI.3333-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izquierdo I, et al. Sequential role of hippocampus and amygdala, entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. Eur J Neurosci. 1997;9:786–793. doi: 10.1111/j.1460-9568.1997.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 51.Izquierdo I, Schroder N, Netto CA, Medina JH. Novelty causes time-dependent retrograde amnesia for one-trial avoidance in rats through NMDA receptor- and CaMKII-dependent mechanisms in the hippocampus. Eur J Neurosci. 1999;11:3323–3328. doi: 10.1046/j.1460-9568.1999.00742.x. [DOI] [PubMed] [Google Scholar]
- 52.Robinson, J. M. et al Activation of Septal Glutamatergic Neurons Drive Hippocampal Theta Rhythms. J Neurosci, 10.1523/JNEUROSCI.2141-15 (2016). [DOI] [PMC free article] [PubMed]
- 53.Gallinat, J. K. et al glutamate concentration predicts cerebral theta oscillations during cognitive processing. Psychopharmacology (Berl) (2006). [DOI] [PubMed]
- 54.Wyble, B. P., Hyman, J. M., Rossi, C. A. & Hasselmo, M. E. Analysis of theta power in hippocampal EEG during bar pressing and running behavior in rats during distinct behavioral contexts. Hippocampus, 10.1002/hipo.20012 (2004). [DOI] [PubMed]
- 55.Sinnamon, H. M. Decline in hippocampal theta activity during cessation of locomotor approach sequences: amplitude leads frequency and relates to instrumental behavior. Neuroscience, 10.1016/j.neuroscience.2006.02.058 (2006). [DOI] [PubMed]
- 56.Siuda D, et al. Social isolation-induced epigenetic changes in midbrain of adult mice. J Physiol Pharmacol. 2014;65:247–255. [PubMed] [Google Scholar]
- 57.Matsumoto K, Puia G, Dong E, Pinna G. GABA(A) receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress. 2007;10:3–12. doi: 10.1080/10253890701200997. [DOI] [PubMed] [Google Scholar]
- 58.Madden KS, Szpunar MJ, Brown EB. Early impact of social isolation and breast tumor progression in mice. Brain Behav Immun. 2013;30:S135–141. doi: 10.1016/j.bbi.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sobota R, Mihara T, Forrest A, Featherstone RE, Siegel SJ. Oxytocin reduces amygdala activity, increases social interactions, and reduces anxiety-like behavior irrespective of NMDAR antagonism. Behav Neurosci. 2015;129:389–398. doi: 10.1037/bne0000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson, K. J., Twiss, S. D., Hazon, N., Moss, S. & Pomeroy, P. P. Positive social behaviours are induced and retained after oxytocin manipulations mimicking endogenous concentrations in a wild mammal. Proc Biol Sci284, 10.1098/rspb.2017.0554 (2017). [DOI] [PMC free article] [PubMed]
- 61.Simpson EA, et al. Inhaled oxytocin increases positive social behaviors in newborn macaques. Proc Natl Acad Sci USA. 2014;111:6922–6927. doi: 10.1073/pnas.1402471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pournajafi-Nazarloo H, et al. Exposure to chronic isolation modulates receptors mRNAs for oxytocin and vasopressin in the hypothalamus and heart. Peptides. 2013;43:20–26. doi: 10.1016/j.peptides.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Crockford C, Deschner T, Ziegler TE, Wittig RM. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Front Behav Neurosci. 2014;8:68. doi: 10.3389/fnbeh.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oettl LL, et al. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron. 2016;90:609–621. doi: 10.1016/j.neuron.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aqrabawi AJ, et al. Top-down modulation of olfactory-guided behaviours by the anterior olfactory nucleus pars medialis and ventral hippocampus. Nat Commun. 2016;7:13721. doi: 10.1038/ncomms13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Markopoulos F, Rokni D, Gire DH, Murthy VN. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76:1175–1188. doi: 10.1016/j.neuron.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin C, Ravel N. Beta and gamma oscillatory activities associated with olfactory memory tasks: different rhythms for different functional networks? Front Behav Neurosci. 2014;8:218. doi: 10.3389/fnbeh.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davis CM, et al. A profile of the behavioral changes produced by facilitation of AMPA-type glutamate receptors. Psychopharmacology (Berl) 1997;133:161–167. doi: 10.1007/s002130050386. [DOI] [PubMed] [Google Scholar]
- 70.Granger R, et al. Facilitation of glutamate receptors reverses an age-associated memory impairment in rats. Synapse. 1996;22:332–337. doi: 10.1002/(SICI)1098-2396. [DOI] [PubMed] [Google Scholar]
- 71.Hampson RE, Rogers G, Lynch G, Deadwyler SA. Facilitative effects of the ampakine CX516 on short-term memory in rats: correlations with hippocampal neuronal activity. J Neurosci. 1998;18:2748–2763. doi: 10.1523/JNEUROSCI.18-07-02748.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 73.Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci USA. 1994;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galbusera A, et al. Intranasal Oxytocin and Vasopressin Modulate Divergent Brainwide Functional Substrates. Neuropsychopharmacology. 2017;42:1420–1434. doi: 10.1038/npp.2016.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Commun. 2017;8:2001. doi: 10.1038/s41467-017-02173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Owen SF, et al. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 78.Winslow JT, Camacho F. Cholinergic modulation of a decrement in social investigation following repeated contacts between mice. Psychopharmacology (Berl) 1995;121:164–172. doi: 10.1007/BF02245626. [DOI] [PubMed] [Google Scholar]
- 79.Tendler, A. & Wagner, S. Different types of theta rhythmicity are induced by social and fearful stimuli in a network associated with social memory. Elife4, 10.7554/eLife.03614 (2015). [DOI] [PMC free article] [PubMed]
- 80.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 81.Zhong W, et al. Selective entrainment of gamma subbands by different slow network oscillations. Proc Natl Acad Sci USA. 2017;114:4519–4524. doi: 10.1073/pnas.1617249114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kocsis B, Pittman-Polletta BR, Roy A. Respiration-coupled rhythms in prefrontal cortex: beyond if, to when, how, and why. Brain Struct Funct. 2018;223:11–16. doi: 10.1007/s00429-017-1587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lockmann AL, Laplagne DA, Leao RN, Tort AB. A Respiration-Coupled Rhythm in the Rat Hippocampus Independent of Theta and Slow Oscillations. J Neurosci. 2016;36:5338–5352. doi: 10.1523/JNEUROSCI.3452-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lockmann ALV, Tort ABL. Nasal respiration entrains delta-frequency oscillations in the prefrontal cortex and hippocampus of rodents. Brain Struct Funct. 2018;223:1–3. doi: 10.1007/s00429-017-1573-1. [DOI] [PubMed] [Google Scholar]
- 85.Lockmann, A. L. V., Laplagne, D. A. & Tort, A. B. L. Olfactory bulb drives respiration-coupled beta oscillations in the rat hippocampus. Eur J Neurosci, 10.1111/ejn.13665 (2017). [DOI] [PubMed]
- 86.Tort ABL, et al. Parallel detection of theta and respiration-coupled oscillations throughout the mouse brain. Sci Rep. 2018;8:6432. doi: 10.1038/s41598-018-24629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colgin LL. Theta-gamma coupling in the entorhinal-hippocampal system. Curr Opin Neurobiol. 2015;31:45–50. doi: 10.1016/j.conb.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pena RR, et al. Home-cage odors spatial cues elicit theta phase/gamma amplitude coupling between olfactory bulb and dorsal hippocampus. Neuroscience. 2017;363:97–106. doi: 10.1016/j.neuroscience.2017.08.058. [DOI] [PubMed] [Google Scholar]
- 89.Kay LM. Theta oscillations and sensorimotor performance. Proc Natl Acad Sci USA. 2005;102:3863–3868. doi: 10.1073/pnas.0407920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gourevitch B, Kay LM, Martin C. Directional coupling from the olfactory bulb to the hippocampus during a go/no-go odor discrimination task. J Neurophysiol. 2010;103:2633–2641. doi: 10.1152/jn.01075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mormann F, et al. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15:890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- 92.Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci USA. 2009;106:20942–20947. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Kahana MJ. Human neocortical oscillations exhibit theta phase differences between encoding and retrieval. Neuroimage. 2006;31:1352–1358. doi: 10.1016/j.neuroimage.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 96.Ittner AA, Gladbach A, Bertz J, Suh LS, Ittner LM. p38 MAP kinase-mediated NMDA receptor-dependent suppression of hippocampal hypersynchronicity in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun. 2014;2:149. doi: 10.1186/s40478-014-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee, J., Hudson, M. R., O’Brien, T. J., Nithianantharajah, J. & Jones, N. C. Local NMDA receptor hypofunction evokes generalized effects on gamma and high-frequency oscillations and behavior. Neuroscience, 10.1016/j.neuroscience.2017.06.039 (2017). [DOI] [PubMed]
- 98.Hunt, M. J. et al. The olfactory bulb is a source of high-frequency oscillations (130–180 Hz) associated with a subanesthetic dose of ketamine in rodents. Neuropsychopharmacology, 10.1038/s41386-018-0173-y (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.