Abstract
The global prevalence of type 2 diabetes is increasing rapidly; consequently there is great need for new and novel therapeutic options. Gynostemma pentaphyllum (GP) is a traditional medicinal plant, mainly present in Southeast Asian countries, that has been reported to exert antidiabetic effects, by stimulating insulin secretion. The specific compound responsible for this effect is however as yet unidentified. Screening for discovery and identification of bioactive compounds of an herbal GP extract, was performed in isolated pancreatic islets from spontaneously diabetic Goto-Kakizaki (GK) rats, a model of type 2 diabetes, and from non-diabetic control Wistar rats. From this herbal extract 27 dammarane-type saponins, including two novel compounds, were isolated and their structure was elucidated by mass spectrometry and NMR spectroscopy. One of the dammarane-type triterpenoid showed a glucose-dependent insulin secretion activity. This compound, gylongiposide I, displays unique abilities to stimulate insulin release at high glucose levels (16.7 mM), but limited effects at a low glucose concentration (3.3 mM). Further studies on this compound, also in vivo, are warranted with the aim of developing a novel anti-diabetic therapeutic with glucose-dependent insulinogenic effect.
Introduction
Gynostemma pentaphyllum (Thunb.) Makino (Cucurbitaceae), GP, a traditional medicinal plant found in China, Vietnam, Korea and Japan, is known for containing a large amount of biologically active saponins known as dammarane-type glycosides1,2. The beneficial effects reported for the dammarane-type glycosides are against numerous diseases, such as cardiovascular disease, hyperlipidemia, inflammations, diabetes and cancer2. Currently, more than 170 unique saponins have been isolated from GP extracts2–11.
One of the dammarane-type glycosides isolated from GP extract is phanoside, a substance with identified structure and potent insulin secretory effect12. However, this effect is not glucose-dependent, i.e. it is stimulating insulin release at both low and high glucose concentrations12. Such an action can potentially induce severe hypoglycemia, similar to that of the well-known antidiabetic drug sulfonylurea. Other studies on effects of identical GP extract in patients with drug-naïve type 2 diabetes have demonstrated reduced glycemia and HbA1c levels, and that these effects could be attributed mainly to improved insulin sensitivity13,14. This was in agreement with results from a study in spontaneously type 2 diabetic Goto-Kakizaki (GK) rats, in which the GP extract reduced hepatic glucose output, i.e. exerted an antidiabetic effect by improving hepatic insulin sensitivity15. In addition, GP extract improved glycemic control in type 2 diabetic patients who were also treated with the sulfonylurea compound gliclazide16. Taken together, these findings indicate that the active component(s) in GP can be delivered by oral administration of the extract. However, to determine the exact mechanism of action of the extract, or the active ingredient(s) responsible for the antidiabetic effect(s), we decided to isolate and characterize the active substance(s) with focus on insulin-stimulatory effects. For that purpose, we developed a system with isolated pancreatic islets from either diabetic GK rats15 and healthy, control Wistar (W) rats. GK rats are non-obese and were developed by repeated breeding of W rats, selected by higher-than-normal serum glucose levels in oral glucose tolerance tests. The GP extract was separated by HPLC into several fractions that were tested for their potential to stimulate insulin secretion from W or GK islets at a low (3.3 mM) or a high (16.7 mM) glucose concentration. One fraction was shown to have a beneficial activity on insulin secretion and therefore the saponins comprising the fraction were further separated and their individual activity tested. By this bioassay-guided investigation of the GP extract, 27 saponins including two new compounds were isolated and the saponin component responsible for the biological activity of the GP extract was identified. The structures of the 27 compounds were elucidated using NMR, LC-MS and GC-MS analysis. To facilitate further studies on structural characterization of dammarane-type saponins, a library consisting of MS data, NMR 1H and 13C chemical shifts and HSQC NMR spectra was established for the 27 compounds.
Results
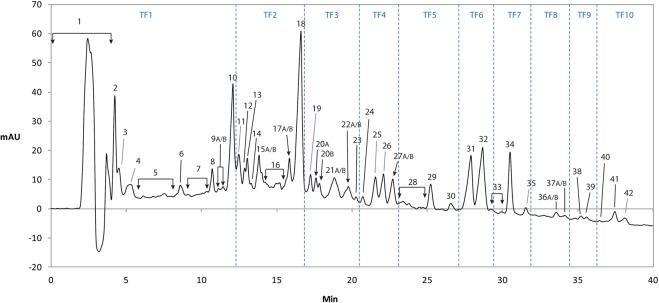
In order to isolate and characterize the maximum number of saponin structures in the extract, an optimized chromatographic method was developed using a C-18 reverse-phase column (Fig. 1). The extract was fractionated by repeated preparative chromatography and the fractions were further purified by a final isocratic elution. Twenty-seven saponins were characterized and two of them, fractions 12 and 34 had new structures not previously reported. (Table 1 and Fig. 2). A compilation of the structures, NMR 1H and 13C chemical shifts and HSQC NMR spectra is available in the Supplementary Information.
Figure 1.
Preparative HPLC chromatogram, showing the ten sequencial time fractions TF1-TF10 as well as the fractions 1–42.
Table 1.
Saponins isolated and characterized from the GP extract.
| Compound 6A | ||||
| 3β,20ξ,21ξ,23ξ-tetrahydroxy-19-oxo-21,24ξ-cyclodammar-25-ene 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-α-L-arabinopyranoside | ||||
| White amorphous powder | C46H74O17 | [M + Na]+; calc. 921.4818 | [M + Na]+; measured 921.4789 | ref.7 |
| Compound 6B | ||||
| 3β,20ξ,21ξ,26-tetrahydroxy-19-oxo-21,23-epoxydammar-24-ene 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-α-L-arabinopyranoside | ||||
| White amorphous powder | C46H74O18 | [M + H]+; calc. 915.4948 | [M + H]+; measured 915.4930 | ref.35 |
| Compound 8 | ||||
| (20S)-3β,20,21-trihydroxy-19-oxodammar-23,25-diene 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-α-L-arabinopyranosyl-21-O-β-D-glucopyranoside | ||||
| White amorphous powder | C46H74O18 | [M + Na]+; calc. 1067.5397 | [M + Na]+; measured 1067.5351 | ref.36 |
| Compound 10 | ||||
| 3β,20ξ,21ξ-trihydroxy-19-oxodammar-24-ene 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-α-L-arabinopyranosyl-21-O-β-D-glucopyranoside | ||||
| White amorphous powder | C52H86O21 | [M + H]+; calc. 1069.5554 | [M + H]+; measured 1069.5663 | ref.26 |
| Compound 12 | ||||
| (3β,20S,23R)-3,20,23,26-tetrahydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]- β-D-glucopyranoside | ||||
| White amorphous powder | C47H76O18 | [M + Na]+; calc. 951.4924 | [M + Na]+; measured 951.4863 | |
| Compound 15A | ||||
| 3β,20,21-trihydroxydammar-24-en 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]- β-D-glucopyranosyl-21-O-β-D-glucopyranoside | ||||
| White amorphous powder | C53H90O21 | [M + Na]+; calc. 1085.5867 | [M + Na]+; measured 1085.5857 | ref.37 |
| Compound 17B | ||||
| 3β,20,21-trihydroxydammar-24-en 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]- 6-O-acetyl-β-D-glucopyranosyl-21-O-β-D-glucopyranoside | ||||
| White amorphous powder | C55H92O22 | [M + Na]+; calc. 1127.5972 | [M + Na]+; measured 1127.5899 | ref.37 |
| Compound 18 (major and minor) | ||||
| 3β,20ξ,21ξ-trihydroxy-19-oxo-21,23-epoxydammar-24-ene 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-α-L-arabinopyranoside | ||||
| White amorphous powder | C46H74O17 | [M−H2O]+; calc. 881.4893 | [M−H2O]+; measured 881.4901 | ref.7 |
| Compound 19 | ||||
| (3β,20S,23 S)-3,20,23trihydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-α-L-arabinopyranoside | ||||
| White amorphous powder | C46H72O17 | [M + Na]+; calc. 919.4662 | [M + Na]+; measured 919.4612 | refs21,22,35 |
| Compound 20A | ||||
| (3β,20S,23R)-3,20,23trihydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-α-L-arabinopyranoside | ||||
| White amorphous powder | C46H72O17 | [M + Na]+; calc. 919.4662 | [M + Na]+; measured 919.4625 | refs21,22 |
| Compound 20B | ||||
| (3β,20S)-3,20,21-trihydroxydammar-24-ene 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-α-L-arabinopyranoside | ||||
| White amorphous powder | C46H76O16 | [M + Na]+; calc. 907.5026 | [M + Na]+; measured 907.4995 | ref.17 |
| Compound 21 (major and minor) | ||||
| 23(R/S)-3β,20ξ,21(R/S)-trihydroxy-21,23-epoxydammar-24-ene 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-β-D- glucopyranoside | ||||
| White amorphous powder | C47H78O17 | [M - H2O]+; calc. 897.5206 | [M - H2O]+; measured 897.5219 | ref.7 |
| Compound 22 (major and minor) | ||||
| 23(R/S)-3β,20ξ,21(R/S)-trihydroxy-21,23-epoxydammar-24-ene 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-α-L-arabinopyranoside | ||||
| White amorphous powder | C46H76O16 | [M - H2O]+; calc. 867.5100 | [M - H2O]+; measured 867.5103 | ref.7 |
| Compound 23 | ||||
| (3β,20S,23S)-3,20,23-trihydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-glucopyranosyl-(1 → 3)]- β-D-glucopyranoside | ||||
| White amorphous powder | C48H78O18 | [M + H]+; calc. 943.5261 | [M + H]+; measured 943.5273 | ref.8 |
| Compound 25 | ||||
| (3β,20S,23R)-3,20,23-trihydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]- β-D-glucopyranoside | ||||
| White amorphous powder | C47H76O17 | [M + Na]+; calc. 913.5155 | [M + Na]+; measured 913.5150 | refs21,22 |
| Compound 26 | ||||
| (3β,20S,23S)-3,20,23-trihydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]- β-D-glucopyranoside | ||||
| White amorphous powder | C47H76O17 | [M + Na]+; calc. 913.5155 | [M + Na]+; measured 913.5144 | refs21,22 |
| Compound 27A | ||||
| (3β,20S,23R)-3,20,23-trihydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]- α-L-arabinopyranoside | ||||
| White amorphous powder | C46H74O16 | [M + Na]+; calc. 905.4869 | [M + Na]+; measured 905.4877 | refs21,22 |
| Compound 27B | ||||
| (3β,20S,23S)-3,20,23-trihydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]- α-L-arabinopyranoside | ||||
| White amorphous powder | C46H74O16 | [M + Na]+; calc. 905.4869 | [M + Na]+; measured 905.4877 | refs21,22 |
| Compound 29 | ||||
| 3β,20-dihydroxydammar-23,25-diene-21-carboxylic acid 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-β-D-glucopyranoside | ||||
| White amorphous powder | C47H76O17 | [M + Na]+; calc. 935.4975 | [M + Na]+; measured 935.4961 | ref.38 |
| Compound 31 | ||||
| (3β,20S,23R)-3,20,23-trihydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-6-O-acetyl-β-D-glucopyranoside | ||||
| White amorphous powder | C49H78O18 | [M + Na]+; calc. 977.5080 | [M + Na]+; measured 977.5033 | ref.8 |
| Compound 32 | ||||
| (3β,20S,23S)-3,20,23-trihydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-6-O-acetyl-β-D-glucopyranoside | ||||
| White amorphous powder | C49H78O18 | [M + Na]+; calc. 977.5080 | [M + Na]+; measured 977.5021 | ref.8 |
| Compound 34 | ||||
| 3β,20-dihydroxydammar-23,25-diene-21-carboxylic acid 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-β-D-6-O-acetylglucopyranoside | ||||
| White amorphous powder | C49H78O18 | [M + Na]+; calc. 977.5080 | [M + Na]+; measured 977.5047 | |
| Compound 39 | ||||
| (3β,20S,23S)-3,20,23-trihydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-6-O-acetyl-β-D-glucopyranoside | ||||
| White amorphous powder | C51H80O19 | [M + Na]+; calc. 1019.5186 | [M + Na]+; measured 1019.5140 | ref.8 |
| Compound 41 | ||||
| (23ξ)-21ξ-O-n-butyl-3β,20ξ,21ξ-trihydroxy-19-oxo-21,23-epoxydammar-24-ene 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-α-L-arabinopyranoside | ||||
| White amorphous powder | C50H82O17 | [M + Na]+; calc. 977.5444 | [M + Na]+; measured 977.5407 | ref.39 |
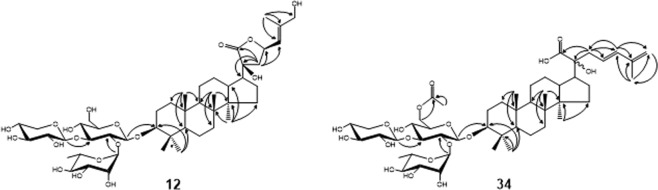
Figure 2.
Structure of compounds 12 and 34, and the key HMBC correlations.
Biological activity
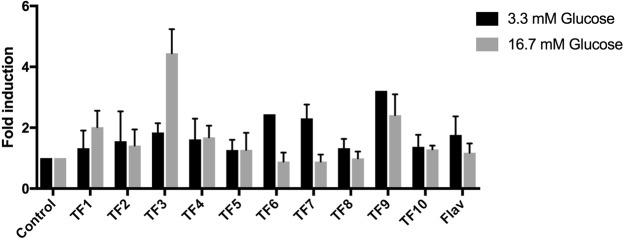
To locate the active saponins, ten sequential time fractions (Fig. 1) from the HPLC separation were collected and screened for biological activity. GP extract fractions or compounds isolated from the fractions were incubated with pancreatic islets of either W or GK rats to determine effects on insulin release at low (3.3 mM) and high (16.7 mM) glucose concentrations. No significant insulin stimulatory activity was found in TF1-TF2, TF4-TF8 and TF10, or the flavonoid fraction (Fig. 3), while such an activity was found in TF3 and TF9. TF9 enhanced insulin release to a similar extent regardless of in the presence of 3.3 or 16.7 mM glucose. TF3 dose-dependently increased insulin release at 16.7 mM glucose, while it did not enhance insulin release at 3.3 mM glucose (Fig. 4).
Figure 3.
Result of the screening of the time fraction for biological activity by incubating the extract of the ten time fraction with pancreatic islets of either W or GK rats to determine effects on insulin release at both low (3.3 mM) and high (16.7 mM) glucose concentrations.
Figure 4.
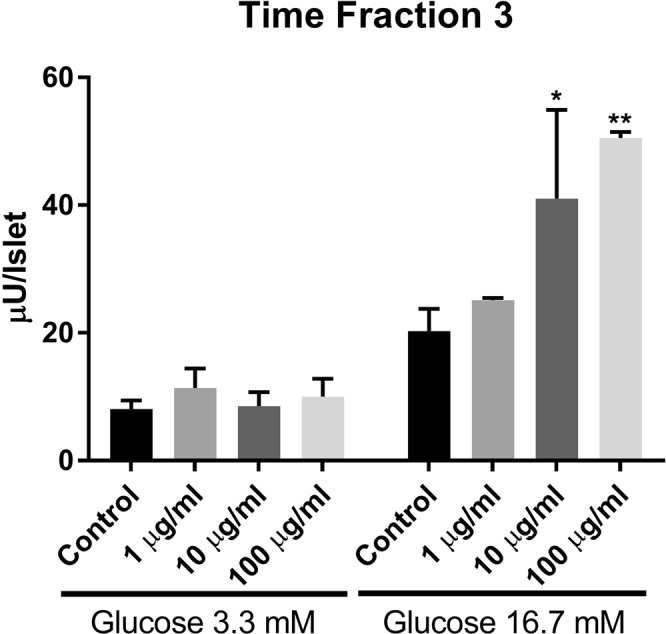
Effects of time fraction 3 (1, 10 and 100 µg/ml) on insulin release from isolated rat islets at either 3.3 or 16.7 mM glucose. *p < 0.05; **p < 0.01 or less.
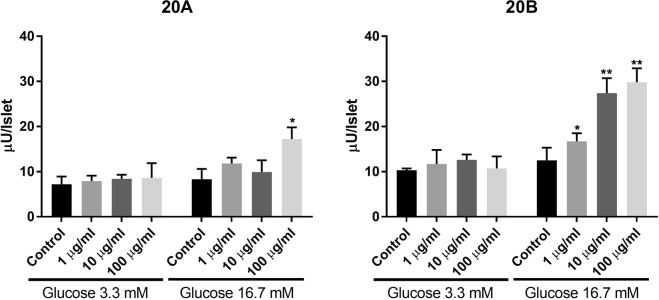
The saponins in fractions TF3 were further isolated and tested for their individual activity. Two compounds 20A and 20B in TF3 were found to stimulate insulin secretion. Compound 20B (Fig. 5) showed beneficial biological activity by only stimulating the insulin secretion at high glucose concentration (Fig. 6). This compound (20B) known as gylongiposide I has been isolated and its structure reported previously but has not been studied for its anti-diabetic activity17–20.
Figure 5.
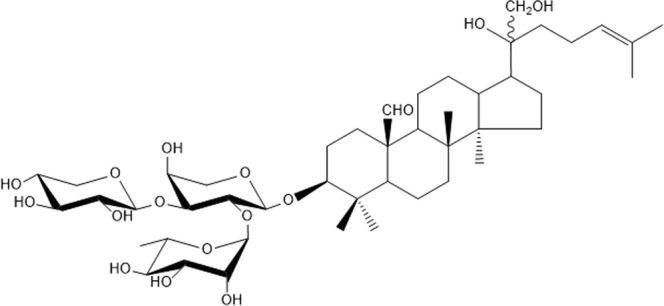
Structure of compound 20B (gylongiposide I).
Figure 6.
Effects of compounds 20A and 20B (1, 10 and 100 µg/ml) on insulin secretion at 3.3 and 16.7 mM glucose, from batch-incubated pancreatic islets isolated from spontaneously diabetic Goto-Kakizaki (GK) rats. *p < 0.05; **p < 0.01 or less.
Structural elucidation
Compound 12 (Fig. 2) was obtained as a white, amorphous powder, the molecular formula was established as C47H76O18 and a molecular ion peak was observed by both positive and negative-mode ESI-MS (m/z 951.4863 [M + Na]+; calc. 951.4924). The 1H-13C multiplicity edited HSQC NMR (CD3OD, 600 MHz) spectrum of 12 indicated the presence of seven methyl signals at δH 0.86, 0.89, 0.93, 1.03, 1.03, 1.21, 1.75, twelve methylene signals, and twenty-one methine signals.
The NMR data of the aglycone moiety (Supporting Information S5e) showed characteristic resonances of carbons from one lactone carbonyl carbon δc 180.43, one double bond (δC 123.96, 142.68). COSY and TOCSY cross-peaks of H2-1/H2-2/H-3; H-5/H2-6/H2-7; H-9/H2-11/H2-12/H-13/H-17/H2-16/H2-15 and H2-22/H-23/H-24 demonstrated the presence of vicinal coupling system. In the HMBC spectrum, two and three bond correlations of H3-18/C-7, C-8, C-9, and C-14; H-19/C-1, C-5, C-9, and C-10; H3-27/C-24, C-25 C-26; H3-28, H3-29/C-3, C-4, and C-5; H3-30/C-8, C-13, C-14, and C-15; H2-22/C-17, C-20, C-21, C-23, and C-24, together with the chemical shift of all protons and carbons, reveal that compound 12 had a 3,20,23,26-tetrahydroxydammar-24-en-21-oic acid-21,23-lactone aglycone structure.
The NMR data of the glycoside moiety (Supporting Information S5e) showed three anomeric signals, at δH/C 4.44/105.08 (H1/C1 of Glc), δH/C 4.46/104.79 (H1/C1 of Xyl), and δH/C 5.42/101.70 (H1/C1 of Rha), two hydroxymethyl groups H6/C6 of Glc (3.67, 3.85/62.47) and H5/C5 of Xyl (3.28, 3.93/67.01), one methyl group C6 of Rha (1.21/17.88) and eleven oxygen-bearing sugar carbons and their attached protons in the region δC 69.94–87.95, δH 3.26–3.98. Three spin systems were identified through TOCSY experiment. The configuration was determined to be in the α and β form based on the magnitude of the H1-H2 coupling constant β-D-Xyl (H1, d, 3J = 7.6 Hz), β-D-Glc (H1, d, 3J = 7.6 Hz), and α-L-Rha (H-1, 3J = 1.5). HMBC correlations (Fig. 2) between H-1′′ Rha (δH 5.42) and C-2′ Glc (δC 77.85), H-1′′′ Xyl (δH 4.46) and C-3′ Glc (δC 87.95), H-1′ Glc (δH 4.44) and C-3 on the aglycone (δC 89.85), demonstrated that the β-D-glucopyranosyloxy unit was located at C-3 of the aglycone and that the α-L-rhamnopyranosyloxy and β-D-xylopyranosyloxy units were linked to C-2′ and C-3′ of the glucopyranosyloxy unit respectively.
The configuration at C-20 and C-23 were determined by comparison with the most recent studies of configuration at C-20 and C-23 carried out by electronic circular dichroism (ECD) and X-ray diffraction analysis21,22. Therefore, compound 12 was assigned to be (3β,20S,23R)-3,20,23,26-tetrahydroxydammar-24-en-21-oic acid-21,23-lactone 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]- β-D-glucopyranoside.
Compound 34 (Fig. 2) was obtained as a white, amorphous powder, the molecular formula was established as C49H78O18 and a molecular ion peak was observed by positive-mode ESI-MS (m/z 977.5047 [M + Na]+; calc. 977.5080). The 1H-13C multiplicity edited HSQC NMR (CD3OD, 600 MHz) spectrum of 34 indicated the presence of eight methyl signals at δH 0.85, 0.87, 0.89, 0.98, 1.03, 1.21, 1.79, 2.05, four olefin signals, eleven methylene signals, and nineteen methine signals.
The NMR data of the aglycone moiety (Supporting Information S21e) showed characteristic resonances of one double bond (δC 126.38, 136.79), one olefin carbon (δC 114.97) arising from the double bond between C-25 and C-26. COSY and TOCSY cross-peaks of H2-1/H2-2/H-3; H-5/H2-6/H2-7; H-9/H2-11/H2-12/H-13/H-17/H2-16/H2-15 and H2-22/H-23/H-24 demonstrated the presence of vicinal coupling system. In the HMBC spectrum, two and three bond correlation of H3-18/C-7, C-8, C-9 and C-14; H-19/C-1, C-5 and C-10; H2-26/C-27, C-24 and C-25; H3-27/C-24, C-25 and C-26; H3-28, H3-29/C-3, C-4 and C-5; H3-30/C-14 and C-15; H2-22/C-20, C-21, C-23 and C-24; H-24/C-22, C-25 and C-26, together with the chemical shift of all protons and carbons, reveal that 34 had a 3,20-dihydroxydammar-23,25-diene-21-carboxylic acid aglycone structure.
The NMR data of the glycoside moiety (Supporting Information S21e) showed three anomeric signals, at δH/C 4.44/105.01 (H1/C1 of Glc), δH/C 4.45/104.87 (H1/C1 of Xyl), and δH/C 5.41/101.79 (H1/C1 of Rha), two hydroxymethyl groups H6/C6 of Glc (4.23, 4.37/64.60) and H5/C5 of Xyl (3.27, 3.93/67.01), one methyl group C6 of Rha (1.21/17.97) and eleven oxygen-bearing sugar carbons and their attached protons in the region δC 70.16–87.73, δH 3.26–3.99. Three spin systems were identified through TOCSY experiment. An acetyl group at δH 2.05 and δC 172.33, 20.76, showed HMBC correlation between H-6 of Glc and the carbonyl carbon of this acetyl group. The configuration were determined to be in the α and β form based on the coupling constant β-D-Xyl (H1, d, 3J = 7.3 Hz), β-D-Glc (H1, d, 3J = 7.6 Hz), and α-L-Rha (H-1, 3J = 1.30). HMBC correlations (Fig. 2) between H-1′′ Rha (δH 5.41) and C-2′ Glc (δC 77.92), H-1′′′ Xyl (δH 4.45) and C-3′ Glc (δC 87.73), H-1′ Glc (δH 4.44) and C-3 on the aglycone (δC 90.44), demonstrated that the β-D-6-O-acetylglucopyranosyloxy unit was located at C-3 of the aglycone and that the α-L-rhamnopyranosyloxy and β-D-xylopyranosyloxy units were linked to C-2′ and C-3′ of the glucopyranosyloxy unit respectively.
Compound 34 were assigned to be 3β,20-dihydroxydammar-23,25-diene-21-carboxylic acid 3-O-[α-L-rhamnopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 3)]-β-D-6-O-acetylglucopyranoside.
Discussion
Biological activity
We have identified and characterized the dammarane-type triterpene saponin responsible for the insulin secretion induced by a Chinese extract of the Gynostemma Pentaphyllum (GP) herbal plant. Although several of the pooled time fractions, TFs, obtained from the HPLC chromatogram, had varying degree of effect on insulin secretion from isolated islets of control W and diabetic GK rats, significant effects on the insulin-producing beta-cells were seen with one of the fractions, TF3. In addition, we found that TF3 had the most favourable antidiabetic effect by enhancing insulin release significantly only at high glucose but not at low glucose concentrations. This is in contrast to the well-known antidiabetic sulfonylurea drugs, that stimulate insulin secretion also at normoglycemia or even at lower glucose levels23 and thereby can cause serious hypoglycemia in patients. By further chromatographic separation of the saponins present in TF3 followed by bio-assay testing we were able to determine that this favourable activity, i.e. a glucose-dependent stimulation of insulin release was entirely related to the saponin compound 20B.
In this context, it is of great importance to note that this compound most likely retains its biological activity following oral administration, since we have shown prominent antidiabetic activity in diabetic GK rats, treated orally with GP extract (0.3 g/kg body weight) for 2 weeks24. In these animals, the improvement of glucose tolerance was demonstrated in parallel with augmented plasma insulin levels. A similar effect was previously reported by another compound isolated from GP extract, i.e. phanoside12,25. However, in contrast to the present saponin 20B, phanoside did not stimulate insulin secretion in a glucose-dependent manner. Recent mechanistic studies on the GP extract demonstrated that the stimulation of insulin secretion from rat islets was mediated by effects on several steps in the stimulus-secretion coupling to glucose in the pancreatic beta cells24. Among these steps are the K-ATP channels, the L-type Ca2+ channels, the protein kinase A activity, and pertussis-toxin sensitive exocytotic Ge-proteins.
Structure and activity
Gynostemma pentaphyllum extracts are known to possess numerous biological activities through their dammarane-type triterpene saponins. The possible structural-activity relationships (SAR) is related to the number and nature of the sugars, their acylation, the types of aglycones and the stereochemistry. A large number of studies have been conducted on anti-tumor, anti-inflammatory and anti-diabetic activities, where different structural components have been pointed out as important for the activities2,24,26. The presence of a hydroxyl group at position C-39, a double bond between C20-C22 or C20-C2127, and a five membered unsaturated ketone in the side chain28 in the dammarane-type structure has been mentioned as possible structural components responsible for anti-tumor activity. These results are from studies carried out on different cancer cell lines and are therefore difficult to compare. The differences in anti-inflammatory abilities have been explained by differences in sugar moieties29.
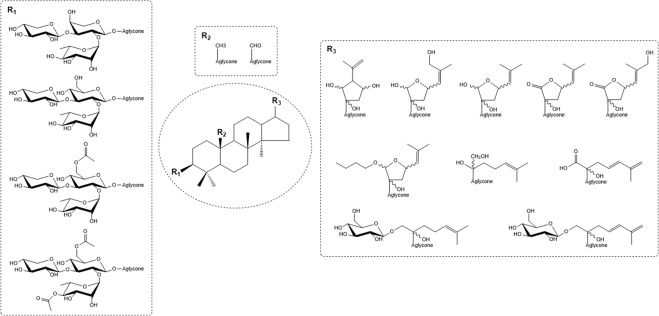
From the present work, some structural features are worth noticing: The glycoside moiety at the C-3 position on the aglycone of the isolated saponins consists of a branched trisaccharide either α-L-Rha-(1 → 2)-[β-D-Xyl-(1 → 3)]-α-L-Ara, or α-L-Rha-(1 → 2)-[β-D-Xyl-(1 → 3)]-β-D-Glc. More seldom α-L-Rha-(1 → 2)-[β-D-Xyl-(1 → 3)]-β-D-Glc with acetylation on C6 of glucose and in some cases also on C4 of rhamnose. In some cases, as in compounds 8, 10, 15 and 17 an additional glucopyranose unit is attached at C-21. The aglycone moiety shows structural variability at two positions. The substituent at C-19 can be either a methyl or an aldehyde group. The greatest variability between the structures is found in the side chain attached to C-17. Ten different side chains attached to C-17 were found, some composed of five membered rings, either as lactone or hemiacetal, other as open structures with different number of double bonds and/or additional glycoside moiety attached at C-21 (Fig. 7).
Figure 7.
Structural components of the dammarane-type saponins isolated and characterized in the GP extract. The chemical structures of the 27 isolated compounds are reported in the Supporting Information S1a–S23a.
Interestingly, the potential to stimulate insulin secretion at high glucose concentration is limited to one single compound, 20B, while several saponins can stimulate insulin secretion independent of glucose concentration. Comparison of the structures of the different saponins shows that compound 10 has a structure very similar to that of 20B with the same trisaccharide unit at C-3, an aldehyde group at C-19 and the same open chain attached at C-17 with a double bond between C-24 and C-25. The only difference between the two compounds is the presence of an additional glucose residue at C-21 in compound 10. Thus, the presence of an additional sugar appears to suppress the insulin secretion at high glucose concentration.
Because of the increasing amount of data on the biological activities of saponins, rapid screening methods including activity and structural data such as LC-MS and NMR are valuable30. The 1H-13C HSQC NMR spectra of the 27 identified saponins together with the MS data and 1H and 13C NMR chemical shifts reported in the present work should help in structural analysis when investigating new extract, and may allow for more rapid screening of already known compounds.
Experimental Procedures
Material and reagents
Gynostemma Pentaphyllum (GP) herbal extract was purchased from Hanzhong TRG Biotech Co.,Ltd. Solid phase extraction column Hypersep C-18 was purchased from Thermo scientific. Deionized water was purified by Milli-Q system from Millipore. Ethanol absolute 100% Ph.Eur, acetonitrile (HPLC grade) and methanol (HPLC grade) were purchased from VWR chemicals. Methanol D4 99.80% D (NMR) were purchased from Euriso-top. Collagenase, HEPES, bovine albumin, D-glucose, L-glutamine, RPMI 1640 culture medium, and fetal calf serum were obtained from Sigma-Aldrich, Sweden.
Extraction and isolation
The dried and powdered GP extract was dissolved in 30% ethanol solution, and applied to a Hypersep C-18 cartridge. The column was washed with 30% ethanol solution (flavonoid fraction). The saponin fraction was collected by elution with 100% ethanol. The saponin fractions were dried and further separated through preparative HPLC (Gilson 305/306 system) on a 20 × 150 mm C-18 column (Kromasil 100-5C18 HiCHROM, UK) with a binary solvent system of an aqueous (Milli-Q) 0.1% formic acid solution (A) and acetonitrile (B), with the following gradient elution: initially 30% (B) – 70% (A), changed to 50% (B) after 7 min, 50% (B) to 15 min, 60% (B) to 35 min, 80% (B) to 40 min and returned to 30% (B) after 41 min. The detection (Gilson 118 UV/VIS detector) was done at 210 nm, and a flow rate of 10 ml/min was used.
Ten sequential time fractions from the HPLC separation were collected (Fig. 1), lyophilized and stored at 4 °C until activity testing.
Furthermore, fractions 1–42 (Fig. 1) were collected and lyophilized, through repeated injection to receive sufficient material for NMR analysis to determine if the fractions contain flavonoids, saponins or other components. Some of the 42 screened compounds were not saponins and are therefore not reported. Enough material were collected through repeated injections for exhaustive structural analysis of 27 saponins fractions. The fractions were then lyophilized and purified by isocratic elution according table S24 available in the Supporting Information. Fractions 20A and 20B were co-eluting and separated by an isocratic elution (50:50) of a binary solvent system of an aqueous 0.1% formic acid solution (A) and 80:20% acetonitrile:methanol (B). The purified fractions were stored at 4 °C until NMR and LC-MS analysis.
NMR
The NMR data were recorded at 298 K with a Bruker AVANCE™ III 600 MHz spectrometer using a 5 mm 1H/13C/15N/31P cryoprobe or a 5 mm broadband observe detection SmartProbe, both equipped with z-gradient. The 13C and 1H chemical shifts were measured using CD3OD as an internal standard (δ = 3.31 and 49.15 ppm for proton and carbon respectively). The data were acquired and processed using Bruker software. The 1H and 13C resonances were assigned using homonuclear 1H–1H COSY, TOCSY and NOESY and heteronuclear 1H–13C HSQC, HSQC–TOCSY and HMBC experiments from the Bruker pulse sequence library.
LC-MS
The LCMS analysis were carried out on isolated fractions from the crude extract with an Agilent 1100 liquid chromatography system equipped with a variable UV wavelength detector and a Kromasil® C-18 reverse-phase (4.6 mm × 150, 5 µm) column using suitable isocratic condition for each isolated fraction of aqueous:organic solvent with the inclusion of 0.1% formic acid into both aqueous (Milli-Q) and organic phase in which the later phase consists of acetonitrile and methanol 8:2 (v/v). The flow rate was set at 1 ml/min and the UV detection wavelength was set at 210 nm to verify and screen UV peak of the compounds with their respective total ion chromatogram (TIC) peak.
The accurate mass analysis on the saponins were determined using Maxis Impact Bruker® MS-QTOF coupled to the LC system and using Compass® Bruker data processing software.
Animals and pancreatic islet isolation
Male diabetic GK rats and control Wistar (W) rats were used. The GK rats were bred within the department31, while the W rats were purchased from Charles River Ltd. The rats were maintained in a 12 h light and dark cycle and allowed food and water ad libitum. The GK rats (n = 25) had body weights ranging from 160 to 277 g, and their non-fasted plasma glucose levels were 6.4–10.9 mM, while W rats (n = 8) were 199–267 g with plasma glucose levels 4.4–5.9 mM. The animals were sacrificed by CO2 to allow removal of pancreata for islet isolation by collagenase digestion as described32. Isolated islets were handpicked under a stereo microscope, maintained for 24 h at 37 °C and 5% CO2 in culture dishes with RPMI 1640 medium, containing 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, and 11 mM glucose prior to further experiments with incubations to determine insulin secretion25,31,33. All methods and experimental protocols were carried out in concert with relevant guidelines and regulations. In addition, the studies were performed after approval by the laboratory animal ethics committee of the Karolinska Institutet, Stockholm.
Islet batch incubations
Isolated islets were pre-incubated for 45 min in Krebs-Ringer bicarbonate (KRB) buffer25,33, pH 7.4, and supplemented with 10 mM HEPES, 0.2% bovine albumin and 3.3 mM glucose. Thereafter, batches of three islets were incubated in KRB buffer with 10 mM HEPES, 0.2% albumin and either 3.3 or 16.7 mM glucose. In addition, GP fractions were filtered through a 0.20 μm syringe filter prior to the incubations to remove any debris from the sample. The GP fractions were diluted to final concentrations of 100 µg/ml, 10 µg/ml, 1 µg/ml, and a control of 0 µg/ml in buffer with either glucose concentration. Each GP fraction was tested in triplicates, and each batch of islets was incubated for 60 min in 300 µl buffer in a shaking water bath at 37 °C, and then 200 µl of the incubation media was removed and stored at −18 °C until later determination of insulin. Experiments were repeated two times, and hence there were 3–6 observations from each fraction. Insulin was determined by radioimmunoassay as described34.
Statistical analysis
All results from insulin secretion experiments were analysed and presented as mean and standard error of the mean (SEM): One-way Anova was performed to evaluate statistical significance of results. When this analysis indicated statistical significance, the results were further analysed by Fisher’s least significant difference. Statistical analysis was conducted on each of the individual batch incubations and therefore n = 3–6 for each of the fractions tested at each concentration. The n value varied depending on the number of replicas of the experiment conducted with triplicates. A p value < 0.05 was considered statistically significant. To allow comparisons between the groups, the mean results for each concentration of the pooled fraction, or pure saponin, were divided by their respective control at low and high glucose levels. This allowed a direct comparison between the groups since the absolute values of insulin secretion from the islets varied between rats. The results were therefore presented as fold induction of insulin release compared against 3.3 mM or 16.7 mM glucose control values.
Supplementary information
Acknowledgements
This study was supported by grants (to CGÖ) from the Swedish Diabetes Foundation and Stiftelsen Olle Engkvist Byggmästare (SOEB), an EU Erasmus grant to support Darren Rattigan, and by an SLU infrastructure grant (LL, CS). The authors thanks Shervin Rashedi and Lars-Börje Sjöberg for providing the GP extract and valuable discussions.
Author Contributions
C.G.Ö., C.S. and L.L. conceived and designed the study. D.R. performed all the pancreatic islet experiments, and prepared Figure 3, 4 and 6. C.G.Ö. interpreted the data from the pancreatic islet experiments. L.L. performed the NMR experiments. E.E. and L.L. performed the LC-MS experiments. C.D. and L.L. performed the fractionation experiments of the saponins. L.L. and C.S. interpreted all NMR and LC-MS data. L.L. prepared Figure 1, 2, 5 and 7, and all figures and tables in Supplementary Information. C.G.Ö., C.S. and L.L. wrote the manuscript. All authors reviewed the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lena C. E. Lundqvist and Claes-Göran Östenson contributed equally.
Contributor Information
Lena C. E. Lundqvist, Email: Lena.Lundqvist@slu.se
Claes-Göran Östenson, Email: Claes-Goran.Ostenson@ki.se.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37517-3.
References
- 1.Prabhakar PK, Doble M. Mechanism of action of natural products used in the treatment of diabetes mellitus. Chinese Journal of Integrative Medicine. 2011;17:563–574. doi: 10.1007/s11655-011-0810-3. [DOI] [PubMed] [Google Scholar]
- 2.Razmovski-Naumovski V, et al. Chemistry and Pharmacology of Gynostemma pentaphyllum. Phytochemistry Reviews. 2005;4:197–219. doi: 10.1007/s11101-005-3754-4. [DOI] [Google Scholar]
- 3.Lee C, et al. Isolation and Characterization of Dammarane-Type Saponins from Gynostemma pentaphyllum and Their Inhibitory Effects on IL-6-Induced STAT3 Activation. Journal of Natural Products. 2015;78:971–976. doi: 10.1021/np500803e. [DOI] [PubMed] [Google Scholar]
- 4.Pham TK, et al. Dammarane-type saponins from Gynostemma pentaphyllum. Phytochemistry. 2010;71:994–1001. doi: 10.1016/j.phytochem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Ha TKQ, Shi YP, Oh WK, Yang JL. Hypoglycemic triterpenes from Gynostemma pentaphyllum. Phytochemistry. 2018;155:171–181. doi: 10.1016/j.phytochem.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Shi GH, Wang XD, Zhang H, Zhang XS, Zhao YQ. New dammarane-type triterpene saponins from Gynostemma pentaphyllum and their anti-hepatic fibrosis activities in vitro. Journal of Functional Foods. 2018;45:10–14. doi: 10.1016/j.jff.2018.03.016. [DOI] [Google Scholar]
- 7.Yin F, Zhang YN, Yang ZY, Hu LH. Nine new dammarane saponins from Gynostemma pentaphyllum. Chemistry & Biodiversity. 2006;3:771–782. doi: 10.1002/cbdv.200690079. [DOI] [PubMed] [Google Scholar]
- 8.Yin F, Hu LH. Six new triterpene saponins with a 21,23-lactone skeleton from Gynostemma pentaphyllum. Helvetica Chimica Acta. 2005;88:1126–1134. doi: 10.1002/hlca.200590083. [DOI] [Google Scholar]
- 9.Li N, et al. A New Dammarane-type Triterpene with PTP1B Inhibitory Activity from Gynostemma pentaphyllum. Bull. Korean Chem. Soc. 2014;35:3122–3124. doi: 10.5012/bkcs.2014.35.10.3122. [DOI] [Google Scholar]
- 10.Yang F, et al. Two new saponins from tetraploid jiaogulan (Gynostemma pentaphyllum), and their anti-inflammatory and alpha-glucosidase inhibitory activities. Food Chemistry. 2013;141:3606–3613. doi: 10.1016/j.foodchem.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Ma L, et al. New Dammarane-type Glycosides from the Roots of Gynostemma pentaphyllum. Planta Med. 2012;78:597–605. doi: 10.1055/s-0031-1298256. [DOI] [PubMed] [Google Scholar]
- 12.Norberg A, et al. A novel insulin-releasing substance, phanoside, from the plant Gynostemma pentaphyllum. Journal of Biological Chemistry. 2004;279:41361–41367. doi: 10.1074/jbc.M403435200. [DOI] [PubMed] [Google Scholar]
- 13.Huyen, V. T. T., Phan, D. V., Thang, P., Hoa, N. K. & Östenson, C. G. Gynostemma pentaphyllum tea improves insulin sensitivity in type 2 diabetic patients. Journal of Nutrition and Metabolism2013 (2013). [DOI] [PMC free article] [PubMed]
- 14.Huyen VTT, Phan DV, Thang P, Hoa NK, Ostenson CG. Antidiabetic Effect of Gynostemma pentaphyllum Tea in Randomly Assigned Type 2 Diabetic Patients. Hormone and Metabolic Research. 2010;42:353–357. doi: 10.1055/s-0030-1248298. [DOI] [PubMed] [Google Scholar]
- 15.Yassin K, Huyen V, Hoa NK, Ostenson CG. Herbal Extract of Gynostemma pentaphyllum Decreases Hepatic Glucose Output in Type 2 Diabetic Goto-Kakizaki Rats. Diabetes. 2010;59:A203–A203. [PMC free article] [PubMed] [Google Scholar]
- 16.Huyen, V. T. T. et al. Antidiabetic Effects of Add-On Gynostemma pentaphyllum Extract Therapy with Sulfonylureas in Type 2 Diabetic Patients. Evidence-Based Complementary and Alternative Medicine, 10.1155/2012/452313 (2012). [DOI] [PMC free article] [PubMed]
- 17.Wang TJ, Bian BL. Studies on the chemical constituents of Gynostemma longipes C. Y. Wu. Acta Pharmaceutica Sinica. 1997;32:524–529. [PubMed] [Google Scholar]
- 18.Yin F, et al. Novel dammarane-type glycosides from Gynostemma pentaphyllum. Chem. Pharm. Bull. 2004;52:1440–1444. doi: 10.1248/cpb.52.1440. [DOI] [PubMed] [Google Scholar]
- 19.Liu JQ, et al. Six New Triterpenoid Glycosides from Gynostemma pentaphyllum. Helvetica Chimica Acta. 2009;92:2737–2745. doi: 10.1002/hlca.200900100. [DOI] [Google Scholar]
- 20.Shi L, Cao JQ, Li W, Zhao H, Zhao YQ. A new dammarane-type triterpene saponin from Gynostemma pentaphyllum. Chin. Chem. Lett. 2010;21:699–701. doi: 10.1016/j.cclet.2010.01.006. [DOI] [Google Scholar]
- 21.Gan M, et al. Dammarane Glycosides from the Root of Machilus yaoshansis. Journal of Natural Products. 2012;75:1373–1382. doi: 10.1021/np300310a. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y-j, et al. Dammarane-Type Triterpenoids from Gentianella azurea. Journal of Natural Products. 2014;77:1201–1209. doi: 10.1021/np500077z. [DOI] [PubMed] [Google Scholar]
- 23.Bolen S, et al. Systematic review: Comparative effectiveness and safety of oral medications for type 2 diabetes Mellitus. Ann. Intern. Med. 2007;147:386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 24.Lokman EF, Gu HF, Mohamud WNW, Ostenson CG. Evaluation of Antidiabetic Effects of the Traditional Medicinal Plant Gynostemma pentaphyllum and the Possible Mechanisms of Insulin Release. Evidence-Based Complementary and Alternative Medicine. 2015 doi: 10.1155/2015/120572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoa NK, et al. The possible mechanisms by which phanoside stimulates insulin secretion from rat islets. J. Endocrinol. 2007;192:389–394. doi: 10.1677/joe.1.06948. [DOI] [PubMed] [Google Scholar]
- 26.Takemoto T, et al. Studies on the constitients of cucurbitaceae plants. 12. On the saponin constituents of Gynostemma pentaphyllum Makino. Yakugaku Zasshi-Journal of the Pharmaceutical Society of Japan. 1984;104:1155–1162. doi: 10.1248/yakushi1947.104.11_1155. [DOI] [PubMed] [Google Scholar]
- 27.Piao XL, Xing SF, Lou CX, Chen DJ. Novel dammarane saponins from Gynostemma pentaphyllum and their cytotoxic activities against HepG2 cells. Bioorg. Med. Chem. Lett. 2014;24:4831–4833. doi: 10.1016/j.bmcl.2014.08.059. [DOI] [PubMed] [Google Scholar]
- 28.Li N, et al. Triterpenes possessing an unprecedented skeleton isolated from hydrolyzate of total saponins from Gynostemma pentaphyllum. European Journal of Medicinal Chemistry. 2012;50:173–178. doi: 10.1016/j.ejmech.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 29.Yang F, Shi H, Zhang X, Yu L. Two Novel Anti-Inflammatory 21-Nordammarane Saponins from Tetraploid Jiaogulan (Gynostemma pentaphyllum) Journal of Agricultural and Food Chemistry. 2013;61:12646–12652. doi: 10.1021/jf404726z. [DOI] [PubMed] [Google Scholar]
- 30.Wang SF, et al. Rapid discovery and identification of anti-inflammatory constituents from traditional Chinese medicine formula by activity index, LC-MS, and NMR. Scientific Reports. 2016;6:13. doi: 10.1038/srep31000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostenson CG, et al. Abnormal insulin secretion and glucose metabolism in pancreatic islets from the spontaneously diabetic GK rat. Diabetologia. 1993;36:3–8. doi: 10.1007/bf00399086. [DOI] [PubMed] [Google Scholar]
- 32.Lacy, P. E. Method for the isolation of intact islets of Langerhans from the rat pancreas. Current Contents/Life Sciences, 21–21 (1981). [DOI] [PubMed]
- 33.Lokman, F. E. et al. Antidiabetic Effect of Oral Borapetol B Compound, Isolated from the Plant Tinospora crispa, by Stimulating Insulin Release. Evidence-Based Complementary and Alternative Medicine, 7, 10.1155/2013/727602 (2013). [DOI] [PMC free article] [PubMed]
- 34.Herbert V, Lau KS, Gottlieb CW, Bleicher S. J. Coated charcoal immunoassay of insulin. J. Clin. Endocrinol. Metab. 1965;25:1375−+. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 35.Shi L, Cao JQ, Li W, Zhao YQ. Three New Triterpene Saponins from Gynostemma pentaphyllum. Helvetica Chimica Acta. 2010;93:1785–1794. doi: 10.1002/hlca.200900455. [DOI] [Google Scholar]
- 36.Shi L, Tan DH, Liu YE, Hou MX, Zhao YQ. Two New Dammarane-Type Triterpenoid Saponins from Gynostemma pentaphyllum. Helvetica Chimica Acta. 2014;97:1333–1339. doi: 10.1002/hlca.201300446. [DOI] [Google Scholar]
- 37.Yin F, Hu LH, Lou FC, Pan RX. Dammarane-type glycosides from Gynostemma pentaphyllum. Journal of Natural Products. 2004;67:942–952. doi: 10.1021/np0499012. [DOI] [PubMed] [Google Scholar]
- 38.Shi L, Lu F, Zhao H, Zhao YQ. Two new triterpene saponins from Gynostemma pentaphyllum. Journal of Asian Natural Products Research. 2012;14:856–861. doi: 10.1080/10286020.2012.700925. [DOI] [PubMed] [Google Scholar]
- 39.Shi L, Cao JQ, Shi SM, Zhao YQ. Triterpenoid saponins from Gynostemma pentaphyllum. Journal of Asian Natural Products Research. 2011;13:168–177. doi: 10.1080/10286020.2010.547029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.