Abstract
Hand preference is a conspicuous variation in human behaviour, with a worldwide proportion of around 90% of people preferring to use the right hand for many tasks, and 10% the left hand. We used the large cohort of the UK biobank (~500,000 participants) to study possible relations between early life factors and adult hand preference. The probability of being left-handed was affected by the year and location of birth, likely due to cultural effects. In addition, hand preference was affected by birthweight, being part of a multiple birth, season of birth, breastfeeding, and sex, with each effect remaining significant after accounting for all others. Analysis of genome-wide genotype data showed that left-handedness was very weakly heritable, but shared no genetic basis with birthweight. Although on average left-handers and right-handers differed for a number of early life factors, all together these factors had only a minimal predictive value for individual hand preference.
Introduction
Roughly 90% of people have a preference for using the right hand for complex manual tasks1–3. A minority of roughly 10% prefer to use the left hand, and a smaller group of roughly 1% has no clear preference, the so-called ‘ambidextrous’ people. As a striking human behavioural polymorphism, handedness has attracted a lot of attention in both the scientific and popular literature. For example, personality traits and cognitive skills have been claimed to associate with handedness4,5. The prevalence of non-right-handedness has also been found to be increased in people with various cognitive or psychiatric disorders6,7.
Hand preference becomes established within the first two years of life, but prenatal observations using ultrasound scanning have indicated an earlier initiation of the trait8–10. Gene expression analysis has revealed left-right differences in the human central nervous system as early as four weeks post conception11, which indicates that laterality is an innate and pervasive property of the central nervous system. The strong skew towards right-hand preference at the population level suggests that right-hand-preference is the typical or default arrangement for humans, while left-hand preference may result from genetic, environmental or random perturbations that influence the central nervous system during early development (although alternatives to this view have been discussed12,13).
One biological effect on hand preference is known to be sex, with males more likely to be left-handed than females2,14. For example, in a U.S. dataset aged 10–86 years, the proportion of non-right-handers among 664,114 women was 9.9%, versus 12.6% among 513,393 men2. Previous studies have also shown that genetic variation contributes modestly to left-hand preference, with heritability estimates ranging from 0.03 for SNP-based heritability in the UK Biobank (N > 500,000)15, to 0.25 in twin studies16,17. A number of candidate genes or genetic pathways have been proposed to be involved in hand preference with varying degrees of statistical genetic support18–21, but no genetic mechanisms or biological processes have yet been implicated unambiguously. In addition, no clear markers of brain anatomical asymmetry have been found to associate with handedness22.
One of the problems with assessing hand preference is that, historically, people who are not right-handed have often been made to use their right hand for writing, handling cutlery, and various occupational tasks2,23. As a consequence, a proportion of otherwise left-handed or ambidextrous people has become right-handed, while possibly also a number of left-handed people have become ambidextrous through this enforcing24. The rate of enforced right-handedness varies between cultures25, but has typically shown a decline over recent decades: in many countries, proportions of left-handers have increased with time, probably because society has become more tolerant of variation2,26,27.
Among the early life factors that have been studied for associations with hand preference are the month of birth28–30, being part of a multiple birth31–35, birthweight12,16,36, breastfeeding37, and maternal smoking38,39. Effects of birthweight and multiple birth seem generally consistent throughout the literature; for example a recent study of two datasets of triplets, each numbering roughly 1000 participants, showed that lower birthweight was associated with non-right hand preference34. However, other effects remain equivocal. For example, previous studies have sometimes not taken the sex or age of participants into account, or have not accounted for the country of origin, so that the analyses may have been partly confounded. Some other studies have only considered university students or other convenient or biased sampling selections, which may have resulted in an incomplete picture. One recent, larger population-based study, based on over 12,000 teenagers and young adults, indicated that in a multivariable model being male, not being breastfed, having a lower birthweight, having a left-handed mother, and having an older mother increased the probability of developing left-hand preference, while maternal smoking had no effect40.
A very large, and well characterised, population-based cohort such as the UK Biobank, which includes hundreds of thousands of participants, allows multiple potential factors to be considered together, while providing unprecedented statistical power to begin to disentangle them. In this study, we analysed a number of early life factors that might influence adult hand preference in the UK Biobank dataset. In addition, as genome-wide association data are available for this dataset, we were able to assess the genetic correlations between hand preference and some of the early life measures, which can be heritable in their own right. Genetic correlation is a measure of the extent to which the same genetic variation, over the entire genome, affects two traits.
Results
Factors associated with left-hand preference
Data were obtained from the UK Biobank cohort, which is an adult population cohort41. In total, the dataset comprised 501,730 individuals (Table 1), but exclusions for high residual genetic relatedness (see Methods) left 421,776 individuals for whom demographic information (year of birth and sex) is illustrated in Figure S1, and further drop-out then varied according to the availability of each specific variable (see Tables 3 and 4; for example, information on birthweight was available for 62% of the females and 47% of the males). Exclusions for high relatedness included 95 persons who had an identical twin in the dataset (out of ~10,000 twins).
Table 1.
Distribution of responses to question about hand preference.
| Hand use | Males | Females | Total |
|---|---|---|---|
| Right-handed | 199,915 (87.4%) | 246,021 (90.1%) | 445,936 (89%) |
| Left-handed | 23,792 (10.4%) | 23,059 (8.4%) | 46,851 (9.3%) |
| Use both right and left hands equally | 4,847 (2.1%) | 3,813 (1.4%) | 8,660 (1.7%) |
| Prefer not to answer | 169 (0.007%) | 114 (0.004%) | 283 (0.005%) |
| TOTAL | 228,723 | 273,107 | 501,730 |
Table 3.
Univariable analysis of continuous early life variables and hand preference.
| TRAIT | N | p-value | Note | Effect on logit Right Hand preference |
|---|---|---|---|---|
| Year of birth | 421,667 | 1.0E-30 | Increase ~ 0.7percentage -points per decade | −0.007 yr−1 |
| Birthweight | 231,155 | 0.0009 | Left-handers are ~26 g lighter on average | 0.035 kg−1 |
| Cosine(month) | 421,667 | 0.004 | See Figure S4 | 0.022 |
Table 4.
Multivariable logistic model for right-hand preference, all participants.
| Estimate | S.E. | z | P | OR | OR 2.5% | OR 97.5% | |
|---|---|---|---|---|---|---|---|
| (Intercept) | 15.4 | 1.78 | 8.67 | 2.1E-18 | |||
| Categorical | |||||||
| Sex (Male) | −0.238 | 0.015 | −16.06 | 2.5E-58 | 0.79 | 0.77 | 0.81 |
| twin (Yes) | −0.141 | 0.044 | −3.238 | 0.0012 | 0.87 | 0.80 | 0.95 |
| breastfed (Yes) | 0.105 | 0.016 | 6.560 | 5.4E-11 | 1.11 | 1.08 | 1.15 |
| UKcountry-Ireland* | 0.238 | 0.095 | 2.496 | 0.013 | 1.27 | 1.06 | 1.54 |
| UKcountry-NI | 0.183 | 0.099 | 1.850 | 0.064 | 1.20 | 0.99 | 1.47 |
| UKcountry-Scotland | 0.250 | 0.029 | 8.699 | 1.7E-18 | 1.28 | 1.21 | 1.36 |
| UKcountry-Wales | 0.414 | 0.044 | 10.43 | 9.0E-26 | 1.51 | 1.40 | 1.64 |
| UKcountry-Elsewhere | 0.321 | 0.033 | 9.585 | 4.6E-22 | 1.38 | 1.29 | 1.47 |
| Continuous | |||||||
| year | −0.007 | 0.001 | −7.046 | 1.8E−12 | |||
| year^2 (scaled) | 6.798 | 3.497 | 1.988 | 0.048 | |||
| birthweight | 0.046 | 0.012 | 3.952 | 7.7E-05 | |||
| month.cos | 0.033 | 0.010 | 3.312 | 0.0009 | |||
| Model information | |||||||
| McFadden pseudo R2 | 0.005 | ||||||
| Log likelihood vs null | P = 1.1E−139 | ||||||
| Hosmer Lemeshow test | P = 0.10 | ||||||
| N | 219,994 | ||||||
*vs England.
The distribution of answers to the hand preference question for the complete cohort is shown in Table 1. The ambidextrous group was found to be inconsistent in their answers across timepoints (see Methods for details), so that we focussed here only on the binary trait of left-hand preference versus right-hand preference.
A number of early life variables were available in the UK biobank data. Here we analyzed all available variables relevant to the gestational period and the weeks following birth (Tables 2, 3 and 5), which were ‘breastfed as a baby’, ‘part of a multiple birth’, ‘maternal smoking around birth’, ‘birthweight’, ‘month of birth’, ‘year of birth’, and ‘country of birth’. We also included sex, as it is known to affect hand preference (see Introduction). For our primary analysis, month of birth was modelled using a cosine function to represent a continuous seasonal effect with extremes in the UK summer and winter (Methods). We did not analyze additional childhood variables which were assessed at later ages, by which time individual hand preference is already well established (such as ‘comparative body size at age 10’). We also left out a variable relating to adoption, since the age at adoption was not available.
Table 2.
Univariable analysis of categorical early life variables and hand preference. OR refers to the odds ratio, CI to the confidence interval.
| TRAIT | N | P | Frequency of left-hand preference | Notes | OR for right-hand preference (95% CI) |
|---|---|---|---|---|---|
| Sex (male) | 421,667 | 2.0E-113 | females = 8.6%, males = 10.6%, | 46% males | 0.79 (0.77–0.80) |
| Part of multiple birth | 414,560 | 5.9E-08 | single = 9.5%, multiple = 11.2% | 2.2% of participants are from multiple birth | 0.83 (0.78–0.89) |
| Maternal smoking | 363,866 | 0.102 | non-smoking = 9.4%; smoking = 9.6% | 29% of mothers smoked around pregnancy | 0.99 (0.99–1.00) |
| Breastfeeding | 322,576 | 1.55E-26 | breastfed = 9.1%, not breastfed = 10.3% | 72% of participants were breastfed | 1.15 (1.12–1.18) |
| Country of origin | 420,939 | 1.4E-150 | Lowest frequency left-handers born outside UK, highest in England | ||
| England | 322,287 | 10.1% | |||
| N. Ireland | 2,899 | 8.8% | |||
| Scotland | 34,424 | 8.1% | |||
| Wales | 18,370 | 7.3% | |||
| Rep. of Ireland | 4,801 | 7.3% | |||
| Elsewhere | 38,158 | 6.8% |
Table 5.
Variables included in the analysis. See Table 3a for sample sizes.
| Description | header | Type | Note |
|---|---|---|---|
| Country of birth | f.1647.0.0 | categorical | 4 UK countries, Republic of Ireland, Elsewhere |
| Breastfed as a baby | f.1677.0.0 | categorical | 1 = Yes,0 = no, −1 = do not know, −3 = prefer not to answer |
| Part of a multiple birth | f.1777.0.0 | categorical | 1 = Yes,0 = no, −1 = do not know, −3 = prefer not to answer |
| Maternal smoking around birth | f.1787.0.0 | categorical | 1 = Yes,0 = no, −1 = do not know, −3 = prefer not to answer |
| Birthweight | f.20022.0.0 | continuous | (kg) |
| Sex | f.31.0.0 | categorical | 0 = Female,1 = Male |
| Year of birth | f.34.0.0 | continuous (integer) | between 1934 and 1971 |
| Month of birth | f.52.0.0 | continuous* | 12 months |
*Transformed before analysis, see main text.
In univariable analyses, a higher probability of having left-hand preference was associated with being male, being part of a multiple birth, not being breastfed, having lower birthweight, being born in a more recent year, and being born in summer (all p-values < 0.05; Table 2 for categorical variables, Table 3 for continuous variables). The association of year of birth and left-hand preference is shown in Fig. S2, that of birthweight and left-hand preference in Fig. S3, and month of birth and left-hand preference in Fig. S4. The different countries within the UK also differed in rates of left-handers, with Wales having the lowest proportion, and people who were born outside the UK even lower (Table 2).
In separate univariable analyses of males and females (Tables S1 and S2), the cosine function of month of birth only had an effect in females (Fig. S4, p = 0.388 in males, p = 0.0012 in females), again such that females born in summer tended to have the highest rate of left hand preference.
Multivariable modelling and the relations between predictor variables
All predictor variables having shown nominally significant (p < 0.05) effects on hand preference in univariable testing (i.e. all but maternal smoking) were then included in the multivariable analysis, using general linear modelling (Methods), with hand preference as the dependent variable. In the multivariable model including both sexes, the probability of being left-handed was influenced by sex, year of birth, birthweight, country of birth, multiple birth, breastfeeding and the cosine function of month of birth (all p < 0.05) (Table 4). As the model fitting involved simultaneous entry, the significance of each of these variables indicates an independent effect after accounting for all others. All variables together significantly explained variation in hand preference (p = 1E-139), but the predictive power for individual hand preference was low (pseudo R2 MacFadden = 0.005).
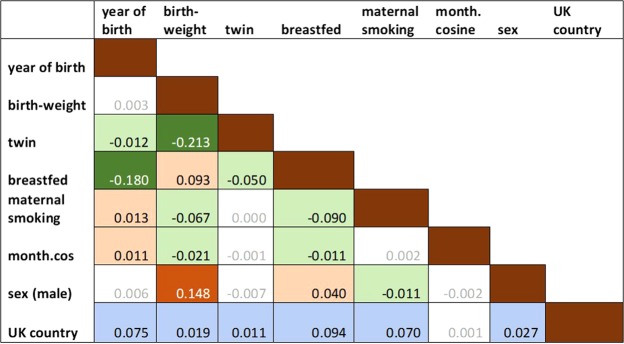
Although tests for variance inflation showed that there was no distorting collinearity in the model, with all inflation factors below 1.2, most predictor variables were correlated or associated with each other to some degree in pairwise univariable testing (see Fig. 1). For example, those from multiple births reported being born considerably lighter than singletons (2.46 kg vs 3.36 kg, p < 2.2e-16), while breastfed children were born heavier than non-breastfed children (3.39 kg vs 3.25 kg, p < 2.2e-16). In fact, birthweight was associated with all of the other variables (Fig. 1), apart from the year of birth (p = 0.75, Fig. S5). As regards birthweight and month of the year, the heaviest children were born in September-October (Fig. S6). Children from smoking mothers were born a little lighter than from non-smoking mothers (3.28 kg vs 3.37 kg, p < 2.2e-16). Males were heavier on average than females at birth (3.45 kg vs 3.25 kg, p < 2.2e-16), but still males showed a higher probability of left-hand preference than females in the multivariable model, i.e. opposite in direction to the association of birthweight and hand preference. Also sex was associated with a number of the other variables (Fig. 1). Year of birth was not correlated with birthweight, but was associated with among others.
Figure 1.
Associations between predictor variables. For associations between categorical variables, Cremer’s V is presented. Associations between continuous variables are shown as Pearson R. Associations between binary categorical and continuous variables are shown as Spearman rho. Associations between multi-category variable UK Country and continuous variables are shown as the ANOVA adjusted R. Colour and sign show the direction of the association between two binary variables, between two continuous variables or between binary and continuous variables (orange positive, green negative). Grey font indicates non-significant associations (p > 0.001).
Despite the associations between the predictors, all predictors had independent effects on hand preference in the multivariable model (Table 4). In the multivariable models that were fitted separately for males and females, again all included predictor variables were significant at p < 0.05 (Tables S3 and S4), although month of birth and year-squared were not included in the model for males, as these were not significant in univariable testing in males only (P > 0.05).
Heritability and genetic correlation
SNP-based heritability is a measure ranging from 0 to 1 which indicates the extent to which variation in a trait is influenced by the combined effects of variation at SNPs distributed over the genome42. Hand preference, birthweight, and being breastfed were previously reported to have low but significant SNP-based heritabilities in the UK biobank dataset (hand preference 1.8% (se = 0.00737), birthweight: 12% (se = 0.006); breastfed: 4.5% (se = 0.00674)20. Because we found that the latter two variables were associated with hand preference in the present study (see above), we re-calculated their SNP-based heritabilities and then measured their genetic correlations with hand preference, which had not been investigated before. Genetic correlation analysis measures the extent to which variability in a pair of traits is influenced by the same genetic variations over the genome.
Consistent with a previous analysis of the UKBiobank dataset20, we found low but significant SNP-based heritabilities of left-hand preference (4.35%), birthweight (15.47%), and being breastfed (5.94%) (see Supplementary Table S5). The analysis of genetic correlation between these measures was novel to the current study (Table S6), but there was no significant genetic correlation between hand preference and being breastfed, nor between hand preference and birthweight (Table S6; although note that we had limited power to detect a genetic correlation below 0.1 between hand preference and the binary trait of being breastfed (Fig. S7)).
Exploratory analyses
We explored additional functions for the month of birth using univariable analyses, in case seasonal effects might act at different stages during gestation, or have maxima and minima during other periods than summer or winter; Briefly, these analyses indicated that the summer-winter function chosen a priori for our primary analysis was the most relevant for females, while for males there was weaker evidence for a peak of left-handedness among those born in autumn, although a cosine function did not appear to describe the male data well (see Supplemental analyses).
We also explored whether there was evidence for an increased strength of association between hand preference and birthweight in those born at a weight of below two kilograms, and separately within singletons and those from multiple births, following a previous report of a large effect size on hand preference in very low birthweight triplets34; However these exploratory analyses indicated that the association of hand preference with birthweight in these subsets was not different to the rest of the dataset (see Supplemental analyses).
Discussion
In this study we assessed various early life factors in relation to the probability of developing left-hand preference. The large and well characterized dataset provided by the UK Biobank allowed the detection of very subtle associations, as well as the power to test for marginal effects of the individual factors after correction for all others. We confirmed a number of previously reported early life factors that influence hand preference, which we discuss in detail further below. In addition, we confirmed a very low SNP-heritability for left-hand preference, but found no genetic correlation with birthweight or being breastfed.
However, perhaps the most notable finding from our study is that, even when taken all together, the studied factors had only a tiny predictive effect for individual hand preference. The biological basis of hand preference therefore remains largely unexplained. It remains possible that some major, early life influences on hand preference do exist, but which were not assessed in the UK Biobank dataset, and do not correlate strongly with any of the early life variables that were available. However, other possibilities are also plausible, which are not necessarily mutually exclusive. Firstly, hand preference might be influenced by an accumulation of many, very small environmental influences, possibly having an effect during prenatal stages. What such environmental effects may be is currently unknown. Secondly, heterogeneous and rare genetic mutations may also be involved43,44, whose effects are not well captured by measures of SNP-based heritability, as the latter approach is focussed primarily on more common genetic variation42.
A random model of early embryonic development is also compatible with our observations. For example, if the brain’s left-right asymmetry arises from only a subtle left-right bias in the early embryo, such as a gene expression gradient that has a lateralized mean across embryos, but a variance that spans the point of symmetry, then a minority of embryos would experience a reversal of the foundational cues for left-right brain patterning. Subsequent steps in development might then reinforce upon the original cue, and result in the virtually bimodal trait of hand preference in adults. Assuming that hand dominance and/or division of labour between the hands is beneficial for fine motor control, then the fact that we have two hands essentially imposes a binary choice on a developmental program which may be more continuous in its original nature. The fact that human brain embryonic gene expression has been shown to be only very subtly lateralized is consistent with such a model45,46.
Notwithstanding the subtlety of the associations of predictor variables with hand preference that we found, some of these associations are consistent with the previous literature and relevant to remark on. Year and country of birth were among the strongest effects. The proportion of left-handers increased almost linearly with year of birth up to 1970, i.e. the birth year of the youngest participants, with ~ 0.7 percentage-points per decade (Fig. S2). We attribute this to a decline in enforcing right-handedness, as has been discussed before26,27,47, rather than reduced survival of non-right-handers48. However, as noted above, it is also possible that unknown environmental influences are involved in hand preference, especially prenatally, which might have changed over the decades.
As regards country of birth, while the average proportion of left-handers among people born outside the UK was 6.8%, it was 10.1% in England, and intermediate in the other UK countries. These differences between countries are likely to reflect mainly cultural effects. For example, forced hand switching during childhood may have been more prevalent outside of England, or may have continued for longer.
An effect of the cosine-transformed month of birth on hand preference was found in women, such that left-hand preference was associated with being born in the summer. The effect of season of birth on hand preference has been unclear in the literature. In a number of studies, a stronger seasonal effect was found in males than in females49,50. In other studies, more left-handers were found among children born in March-July28,29,51, but in other studies in winter49,50,52. In yet other studies, no effect of season was detected30,53–55. A meta-analysis published in 2008, based on data from more than 40,000 participants, found primarily that left-handed men had a slight tendency to have been born from March–July (in the northern hemisphere)51. In our exploratory analysis of additional functions to represent seasonal effects, we found that a cosine curve with a summertime peak of left-handedness was the best fit for the data on women, as we had used for our primary analysis. However, there was not a clear cosine function that captured the male data well (Supplementary Fig. 4), while males showed an autumn peak of left-handedness if anything. Given the conflicting results across studies, seasonal effects on hand preference remain tentative. Our analyses suggest that any seasonal effects on handedness are most pronounced in females, and likely to operate primarily on a summer-winter cycle, in the weeks either around the time of birth or else roughly six months before (i.e. three months of gestation). We cannot speculate on possible mechanisms, but note that possible seasonal variations in maternal testosterone or anxiety have been discussed in the literature51,56.
We confirmed that having a higher birthweight, not being part of a multiple birth, and being breastfed, all increase the probability of being right-handed, consistent with previous literature (see Introduction). Birthweight is a complex trait, which reflects not only healthy variation but also non-optimal development or pathology. Insofar as lower birthweight was associated with left-handedness, this suggests that a minority of left-handers may be linked etiologically to developmental insults, as has been discussed elsewhere13,57,58. As regards multiple birth, in a previous study, the effect of being part of a multiple birth was no longer detectable after accounting for the effects of birthweight and APGAR (Appearance, Pulse, Grimace, Activity, Respiration) score; the latter is an indicator of various adverse circumstances that could occur during pregnancy or birth33. We saw a significant effect of multiple birth on hand preference remaining after correction for birthweight, but the UK Biobank includes no APGAR scores or other indicators of prenatal or birth conditions, so we could not assess the relevance of such conditions. We saw no evidence that the relationship between handedness and birthweight became stronger in low birthweight individuals, in contrast to a previous report based on triplets34. Note that the UK Biobank variable ‘Part of a multiple birth’ makes no distinction between twins, triplets, quadruplets etc., although the large majority are expected to be twins.
Interestingly, the postnatal behaviour of breastfeeding was associated with right-hand preference and was also positively associated with birthweight: non-breastfed children were lighter at birth. The probability that mothers breastfeed their children may, among other things59, be associated with mother or baby health, which in turn may be partly reflected in birthweight. Even after accounting for birthweight, a significant association of breastfeeding with hand preference remained, as has been found before (Denny, 2011). Whether this is due to an underlying prenatal factor that affects both hand preference and breastfeeding, or a post-natal behavioural effect, cannot be inferred from the UK Biobank data.
With regard to maternal smoking around the time of birth, we found that this was not significantly associated with left-hand preference. An effect of maternal smoking on hand preference had been reported suggestively before38, but not found consistently in all studies39,40.
In addition to the factors investigated in this study, other early life factors have been reported in the literature, including birth order49,60–62, prenatal testosterone exposure63–65, maternal age40,49,53,66,67, maternal stress during pregnancy12,68, and birth events such as caesarean delivery or prolonged labour13,57,69. Though not all studies have found significant effects of these variables, a general interpretation of the literature is that less benign conditions are associated with higher proportions of left-handedness. Some of these factors may partly influence hand preference through effects via birthweight. For example, second and third births were reported to result more often in right-handed children than first births, and births subsequent to third49,60,67, while more left-handers were reportedly born to relatively young mothers or older mothers, than to mothers of intermediate age67,70. Birthweight first increases with maternal age and subsequently decreases71,72, and low-weight children and preterm births are more common among young (<20) and older (>30) mothers than mothers aged in-between71,73. Birth order necessarily correlates with maternal age, with births two and three occurring more often in the intermediate age range. However, birthweight has been shown to vary with birth order even after correction for maternal age72,74. Unfortunately, information on maternal age and birth order were not available for the UK Biobank dataset.
We observed a weak SNP-based heritability for hand preference (4.35%) which was consistent with previous reports, but there was no genetic overlap between hand preference and birthweight or being breastfed. For hand preference and birthweight, we had 80% statistical power to detect a genetic correlation as low as 0.18, so that the phenotypic correlation between hand preference and birthweight that we observed is likely due to an underlying environmental cause, rather than common genetic factors. However, it is well established that SNP-based heritability can only capture a proportion of total heritability, i.e. which is caused by common polymorphisms tagged on genotyping arrays42. In large twin studies, the heritability of hand preference was higher, around 20–25%16,17,24. The same was the case for birthweight, which had a twin heritability ~25% or higher75,76. Therefore genetic effects mediated by rare genetic variation, which was not well captured in this dataset, may also be relevant to the heritability of hand preference and birthweight, and in some cases might link these two traits.
The UK Biobank participants are older than the general population (birth years between 1934 and 1971), so that some effects in this cohort may be quantitatively or qualitatively different in younger cohorts77. The UK Biobank cohort, ranging over 30 years in age differences, and collected cross-population, is also more heterogeneous than some other previously investigated cohorts for hand preference. This may make our results broadly applicable to the general population, but on the other hand, it may mean that some effects were obscured by variation in factors that were not accounted for. A previous multivariable analysis for hand preference40, based on a sample of 12,686 Americans who were 14–21 years old in January 1979, found results similar to ours, with being male, not being breastfed, and lower birthweight increasing the probability of developing left-hand preference. In addition, they found that being black, having a left-handed mother and having an older mother significantly increased the probability of having left-hand preference. These factors were not available in the UK Biobank dataset. Maternal smoking had no significant effect in the American study, just like in the UK Biobank dataset. Despite the more homogeneous dataset with respect to age in that study40, and the additional significant factors in the model, the pseudo- R2 was still only 0.016, as compared to 0.005 in the present study, i.e. all factors combined still had a minimal predictive value for individual hand preference.
In the present study of the UK Biobank dataset, all variables, except sex and year of birth, were self-reported. This may introduce inaccuracies as recall may be imperfect. Also, it is possible that cultural differences affect recall, for example between geographical regions, different ages, or between the sexes. This may have reduced the estimates of effects of early life factors on hand preference, compared to if they had been recorded from direct observation. There is, however, no reason to expect left- and right-handers to differ in their self-report accuracy. UK Biobank is not fully representative of the sampling population; there is evidence of a “healthy volunteer” selection bias77. If some common negative health aspects are strongly associated with hand preference, this may have reduced the explanatory power of the model. We excluded outlier datapoints on the basis that they would influence the model fit, but this is also likely to have removed some errors in the dataset.
The present study treated hand preference as a categorical trait, which is supported by the bimodal distribution of overall hand preference when compiled across a number of tasks, and its robust test-retest repeatability78–80. However, some aspects of hand preference might be more accurately defined by degree and not category.
We allowed for possible non-linear effects of continuous predictor variables in our analyses, but did not include interaction terms between predictors in our multivariable models, in order to avoid collinearity, overfitting, and very extensive multiple testing. As regards sex, we performed some analyses separately within the two sexes to allow for potentially different effects, and we presented some descriptive comparisons between the sexes, but again did not test formally for interaction effects that involve sex. The male and female cohorts differed in a number of respects. For example, the proportion of males was not constant across the years of birth (Fig. S1), while women more often than men originated from the Republic of Ireland or elsewhere outside of the UK (Tables S1 and S2), and a much larger proportion of women than men reported their birthweight. Future hypothesis-driven work may investigate specific potential interactions of the various factors studied here.
Methods
Data were obtained from the UK Biobank cohort, as part of research application 16066, with Clyde Francks as the principal applicant. The data collection in the UK Biobank, including the consent procedure, has been described elsewhere41. Informed consent was obtained by the UK Biobank for all participants. All methods were performed in accordance with the relevant guidelines and regulations. For this study of early life factors we used measurements taken during the first visit, i.e. variables identified in the database by 0.0. In total, data were available for 501,730 individuals (Table 1). To avoid using non-independent data, we excluded randomly one individual from each pair of participants whose genetic relatedness was inferred to be 3rd degree or closer, on the basis of genotype data at single nucleotide polymorphisms (SNPs) spanning the genome, as previously calculated by Bycroft et al.81. This left 421,776 individuals.
For a subset of 9,856 participants, answers to the question about hand preference were available for both the initial (0.0) and the second follow-up visit (2.0). We compared these answers for consistency. While right-handed and left-handed persons were mostly consistent (only 0.7% and 2.6% changed their answer respectively), out of 156 people who had initially answered “use both right and left hands equally”, 64 (41%) gave a different answer during follow-up. Note that all visits were as adults, so this inconsistency does not imply forced hand switching, which is known as a feature of childhood in some countries/cultures (see Introduction). We therefore excluded all people who answered “use both right and left hands equally” at their first visit from further analyses, as well as the small number of people who had ticked ‘Prefer not to answer” (Table 1).
The early life variables which were available for this study are shown in Table 2, as well as sex, which was also used as a predictor variable for left-handedness. As also noted in the Results, we analyzed all available variables in the UK Biobank dataset relevant to the gestational period and the weeks following birth. All variables were self-reported, except sex and date of birth (see UK Biobank Showcase; http://www.ukbiobank.ac.uk/), although sex information could be updated by the participants.
The entries “do not know” and “prefer not to answer” for all variables were treated as missing values.
As months of the year are not independent categories (neighbouring months are more similar to each other with respect to e.g. temperature and day length), one approach is to model the effects of season as a waveform function of the month82. For our primary analysis we followed this approach, as described in the referenced paper, and tested:
where ti is an integer from 1 to 12 representing the month of birth. This cosine function has extremes in summer and winter, coinciding with peaks in UK daylight and temperature at time of birth. As seasonal effects may not necessarily be limited to the timing of birth, but could instead impact at other moments during pregnancy, we also performed some exploratory analyses of other possible curves. These extra models and results are described in Supplementary Information: Supplementary Analyses.
We excluded individuals with reported birthweight heavier than 6.0 kg to. Some may have been self-report errors, others accurate, but we cannot assess this probability, and the use of this cut-off was to reduce outlier effects in the model fitting.
Statistical analysis
All statistical analyses were performed with Rstudio, using R version 3.4.0.
Univariable analysis of categorical predictors of hand preference
Associations between hand preference and each of the categorical variables (country of birth, breastfed, multiple birth, maternal smoking, sex) were investigated with chi-square tests of independence.
Univariable analysis of continuous predictors of hand preference
For testing univariable associations between hand preference and continuous variables (birthweight, year of birth and cosine of month of birth), logistic regression was used. In addition, univariable effects on proportions of left-handed people were visualised to assess whether non-linear relations were playing a role (Figs S2 and S3). A model including either birthweight squared, or year of birth squared (as orthogonal vectors created by R function poly() from the ‘stats’ package), was compared to the corresponding model with the single variable to establish whether the squared predictor made a significant additional contribution.
The above analyses of hand preference were also carried out separately within the two sexes. Because previous studies have suggested that the effect of birthweight on hand preference may be more pronounced at the low end of the distribution33,34, we performed additional analyses on a subgroup with birthweight below 2 kg (Supplement).
Multivariable analysis of hand preference predictors
For multivariable analysis, glm (general linear model) was used in R v3.4.0, for the binomial family of models. Participants with missing values for any of the predictor variables were excluded. The threshold for significance in the multivariable model was set at 0.05, i.e. testing whether each variable made a contribution beyond the combined effects of all others, in simultaneous entry. Collinearity was checked with the VIF (Variance Inflation Factor) function in R. Model fit was estimated with the Hosmer-Lemeshow test, using 15 quantiles, while the log likelihood of the full model vs the null model (with no predictors for hand preference) was also estimated. In addition, the McFadden pseudo R2 was computed.
In the multivariable model, 219,994 participants without missing values were included: 83,506 males and 136,488 females. Multivariable analysis was also repeated for males and females separately.
Further statistical analysis
We investigated the pairwise relations between predictor variables as follows: For categorical pairs of variables the Chi square test was used to calculate Cramer’s V (i.e. a statistic scaled from 0 to 1 as an indication of the degree of non-independence). The R command assocstats was used for these calculations. For continuous pairs of variables the Pearson correlation coefficient R was calculated. When one of a pair of variables was dichotomous and the other continuous, Spearman’s rho was calculated. For Country of Birth in relation to continuous variables, ANOVA was used in which the adjusted R was calculated.
Genetic analysis
For this purpose, in addition to removing one from each pair of related subjects (see above), participants were also excluded when there was a mismatch of their reported and genetically inferred sex (N = 378), putative aneuploidy (N = 652), excessively high genomewide heterozygosity (>0.19) or genotype missingness (missing rate > 0.05) (N = 986), and we also restricted the analysis to participants with British ancestry as used by Bycroft et al.81. After this genetic quality control, there were a maximum of 335,998 participants per variable (see Table S5 for sample sizes per variable).
We then calculated the SNP-based heritabilities and genetic correlations between two traits using Restricted Maximum Likelihood estimation implemented as –reml in BOLT-LMM (v2.3)83. We used a genetic relationship matrix that included 547,108 genotyped SNPs (Minor Allele Frequency (MAF) >1% and genotyping rate across subjects >99%), and the pre-computed linkage disequilibrium (LD) scores based on 1000 Genomes European-descent data (https://data.broadinstitute.org/alkesgroup/LDSCORE/). The top ten principal components capturing genetic diversity in the genome-wide genotype data, calculated using fastPCA84 and provided by the UKBiobank81, were included as covariates to control for population structure, as well as sex, age, genotyping array, and assessment centre. For the binary traits hand preference and being breastfed, the population and sample prevalence were assumed to be the same, in order to transform the SNP-based heritabilities to the liability scale using the R code provided by Pulit et al.85.
Power analyses for assessing genetic correlations between hand preference and the two other traits (i.e. birthweight and being breastfed) were performed using the GCTA-GREML power calculator86, considering the relevant sample sizes, SNP-based heritabilities and trait prevalences (Table S5). Since BOLT-REML heritability estimates and standard errors are close to GCTA-REML estimates87 this calculator gives an indicative estimate. Results of the power analysis are shown in Fig. S7.
Ethics statement
This study utilized deidentified data from the baseline assessment of the UK Biobank, a prospective cohort study of 500,000 individuals (age 40–69 years) recruited across Great Britain during 2006–201041. The protocol and consent were approved by the UK Biobank’s Research Ethics Committee.
Supplementary information
Acknowledgements
CGFdK was supported by an Open Programme grant (824.14.005) to CF from the Netherlands Organization for Scientific Research (NWO). ACC was funded by a grant to CF from the NWO (054–15–101) as part of the FLAG-ERA consortium project ‘MULTI-LATERAL’, a Partner Project to the European Union’s Flagship Human Brain Project. Additional support was from the Max Planck Society (Germany).
Author Contributions
CGFdK: Conceptualization, Formal Analysis, Methodology, Visualization, Writing – Original Draft Preparation. ACC: Data Curation, Formal Analysis, Writing – Original Draft Preparation. CF: Conceptualization, Funding Acquisition, Project Administration, Supervision, Writing – Review & Editing.
Data Availability
Data were obtained from the UK Biobank cohort, as part of research application 16066, with Clyde Francks as the principal applicant. Table 2 shows the field identifiers used. Summary and description of these data can be found at, http://biobank.ctsu.ox.ac.uk/crystal/. For the use of the data, application must be made to, http://www.ukbiobank.ac.uk/register-apply/.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37423-8.
References
- 1.Peters M, Reimers S, Manning JT. Hand preference for writing and associations with selected demographic and behavioral variables in 255,100 subjects: The BBC internet study. Brain and Cognition. 2006;62:177–189. doi: 10.1016/j.bandc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert AN, Wysocki CJ. Hand preference and age in the United States. Neuropsychologia. 1992;30:601–608. doi: 10.1016/0028-3932(92)90065-T. [DOI] [PubMed] [Google Scholar]
- 3.Kushner HI. Why are there (almost) no left-handers in China? Endeavour. 2013;37:71–81. doi: 10.1016/j.endeavour.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Sartarelli M. Handedness, Earnings, Ability and Personality. Evidence from the Lab. PLoS One. 2016;11:e0164412. doi: 10.1371/journal.pone.0164412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson JM, Lansky LM. Left-handedness among architects: partial replication and some new data. Perceptual and motor skills. 1977;45:1216–1218. doi: 10.2466/pms.1977.45.3f.1216. [DOI] [PubMed] [Google Scholar]
- 6.Deep-Soboslay A, et al. Handedness, heritability, neurocognition and brain asymmetry in schizophrenia. Brain: a journal of neurology. 2010;133:3113–3122. doi: 10.1093/brain/awq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markou P, Ahtam B, Papadatou-Pastou M. Elevated Levels of Atypical Handedness in Autism: Meta-Analyses. Neuropsychol Rev. 2017;27:258–283. doi: 10.1007/s11065-017-9354-4. [DOI] [PubMed] [Google Scholar]
- 8.Hepper PG, Shahidullah S, White R. Handedness in the human fetus. Neuropsychologia. 1991;29:1107–1111. doi: 10.1016/0028-3932(91)90080-R. [DOI] [PubMed] [Google Scholar]
- 9.Hepper PG. The developmental origins of laterality: Fetal handedness. Developmental Psychobiology. 2013;55:588–595. doi: 10.1002/dev.21119. [DOI] [PubMed] [Google Scholar]
- 10.Parma V, Brasselet R, Zoia S, Bulgheroni M, Castiello U. The origin of human handedness and its role in pre-birth motor control. Sci Rep. 2017;7:16804. doi: 10.1038/s41598-017-16827-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Kovel CGF, et al. Left-Right Asymmetry of Maturation Rates in Human Embryonic Neural Development. Biol Psychiatry. 2017;82:204–212. doi: 10.1016/j.biopsych.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Searleman A, Porac C, Coren S. Relationship between birth order, birth stress, and lateral preferences: a critical review. Psychol Bull. 1989;105:397–408. doi: 10.1037/0033-2909.105.3.397. [DOI] [PubMed] [Google Scholar]
- 13.Nicholls ME, Johnston DW, Shields MA. Adverse birth factors predict cognitive ability, but not hand preference. Neuropsychology. 2012;26:578–587. doi: 10.1037/a0029151. [DOI] [PubMed] [Google Scholar]
- 14.Papadatou-Pastou M, Martin M, Munafo MR, Jones GV. Sex differences in left-handedness: a meta-analysis of 144 studies. Psychol Bull. 2008;134:677–699. doi: 10.1037/a0012814. [DOI] [PubMed] [Google Scholar]
- 15.Ge T, Chen CY, Neale BM, Sabuncu MR, Smoller JW. Phenome-wide heritability analysis of the UK Biobank. PLoS Genet. 2017;13:e1006711. doi: 10.1371/journal.pgen.1006711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medland SE, et al. Genetic influences on handedness: data from 25,732 Australian and Dutch twin families. Neuropsychologia. 2009;47:330–337. doi: 10.1016/j.neuropsychologia.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki K, Ando J. Genetic and environmental structure of individual differences in hand, foot, and ear preferences: A twin study. Laterality: Asymmetries of Body, Brain and Cognition. 2014;19:113–128. doi: 10.1080/1357650X.2013.790396. [DOI] [PubMed] [Google Scholar]
- 18.Francks C. Exploring human brain lateralization with molecular genetics and genomics. Annals of the New York Academy of Sciences. 2015;1359:1–13. doi: 10.1111/nyas.12770. [DOI] [PubMed] [Google Scholar]
- 19.Ocklenburg S, Beste C, Güntürkün O. Handedness: A neurogenetic shift of perspective. Neuroscience & Biobehavioral Reviews. 2013;37:2788–2793. doi: 10.1016/j.neubiorev.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Neale, B. Rapid GWAS of thousands of phenotypes for 337,000 samples in the UK Biobank, http://www.nealelab.is/blog/2017/7/19/rapid-gwas-of-thousands-of-phenotypes-for-337000-samples-in-the-uk-biobank (2017).
- 21.Scerri TS, et al. PCSK6 is associated with handedness in individuals with dyslexia. Hum Mol Genet. 2011;20:608–614. doi: 10.1093/hmg/ddq475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guadalupe T, et al. Differences in cerebral cortical anatomy of left- and right-handers. Front Psychol. 2014;5:261. doi: 10.3389/fpsyg.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galobardes B, Bernstein MS, Morabia A. The association between switching hand preference and the declining prevalence of left-handedness with age. Am J Public Health. 1999;89:1873–1875. doi: 10.2105/AJPH.89.12.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuoksimaa E, Koskenvuo M, Rose RJ, Kaprio J. Origins of handedness: A nationwide study of 30161 adults. Neuropsychologia. 2009;47:1294–1301. doi: 10.1016/j.neuropsychologia.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raymond M, Pontier D. Is there geographical variation in human handedness? Laterality. 2004;9:35–51. doi: 10.1080/13576500244000274. [DOI] [PubMed] [Google Scholar]
- 26.Salive ME, Guralnik JM, Glynn RJ. Left-handedness and mortality. American Journal of Public Health. 1993;83:265–267. doi: 10.2105/AJPH.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerhan JR, Folsom AR, Potter JD, Prineas RJ. Handedness and mortality risk in older women. American journal of epidemiology. 1994;140:368–374. doi: 10.1093/oxfordjournals.aje.a117259. [DOI] [PubMed] [Google Scholar]
- 28.Rogerson PA. On the relationship between handedness and season of birth for men. Perceptual and motor skills. 1994;79:499–506. doi: 10.2466/pms.1994.79.1.499. [DOI] [PubMed] [Google Scholar]
- 29.Martin M, Jones GV. Handedness and season of birth: a gender-invariant relation. Cortex. 1999;35:123–128. doi: 10.1016/S0010-9452(08)70790-1. [DOI] [PubMed] [Google Scholar]
- 30.Abel EL, Kruger ML. Relation of handedness with season of birth of professional baseball players revisited. Perceptual and motor skills. 2004;98:44–46. doi: 10.2466/pms.98.1.44-46. [DOI] [PubMed] [Google Scholar]
- 31.Davis A, Annett M. Handedness as a function of twinning, age and sex. Cortex. 1994;30:105–111. doi: 10.1016/S0010-9452(13)80326-7. [DOI] [PubMed] [Google Scholar]
- 32.Ellis SJ, Ellis PJ, Marshall E. Hand preference in a normal population. Cortex. 1988;24:157–163. doi: 10.1016/S0010-9452(88)80025-X. [DOI] [PubMed] [Google Scholar]
- 33.Heikkila K, et al. Higher Prevalence of Left-Handedness in Twins? Not After Controlling Birth Time Confounders. Twin Res Hum Genet. 2015;18:526–532. doi: 10.1017/thg.2015.53. [DOI] [PubMed] [Google Scholar]
- 34.Heikkila K, et al. Triplets, birthweight, and handedness. Proc Natl Acad Sci USA. 2018;115:6076–6081. doi: 10.1073/pnas.1719567115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sicotte NL, Woods RP, Mazziotta JC. Handedness in twins: a meta-analysis. Laterality. 1999;4:265–286. doi: 10.1080/713754339. [DOI] [PubMed] [Google Scholar]
- 36.Hay DA, Howie PM. Handedness and differences in birthweight of twins. Perceptual and motor skills. 1980;51:666. doi: 10.2466/pms.1980.51.2.666. [DOI] [PubMed] [Google Scholar]
- 37.Denny K. Breastfeeding predicts handedness. Laterality. 2012;17:361–368. doi: 10.1080/1357650x.2011.579131. [DOI] [PubMed] [Google Scholar]
- 38.Dragovic M, Milenkovic S, Kocijancic D, Zlatko S. Etiological aspect of left-handedness in adolescents. Srpski arhiv za celokupno lekarstvo. 2013;141:354–358. doi: 10.2298/SARH1306354D. [DOI] [PubMed] [Google Scholar]
- 39.Olsen J. Is left-handedness a sensitive marker of prenatal exposures or indicators of fetal growth? Scandinavian journal of social medicine. 1995;23:233–235. doi: 10.1177/140349489502300403. [DOI] [PubMed] [Google Scholar]
- 40.Johnston, D. W., Nicholls, M. E. R., Shah, M. A. & Shields, M. A. Handedness, Health and Cognitive Development: Evidence from Children in the NLSY. (Forschungsinstitut zur Zukunft der Arbeit, Bonn, Germany, 2010).
- 41.Sudlow C, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS medicine. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vinkhuyzen AA, Wray NR, Yang J, Goddard ME, Visscher PM. Estimation and partition of heritability in human populations using whole-genome analysis methods. Annu Rev Genet. 2013;47:75–95. doi: 10.1146/annurev-genet-111212-133258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kavaklioglu T, Ajmal M, Hameed A, Francks C. Whole exome sequencing for handedness in a large and highly consanguineous family. Neuropsychologia. 2016;93:342–349. doi: 10.1016/j.neuropsychologia.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Armour JA, Davison A, McManus IC. Genome-wide association study of handedness excludes simple genetic models. Heredity (Edinb) 2014;112:221–225. doi: 10.1038/hdy.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ocklenburg, S. et al. Epigenetic regulation of lateralized fetal spinal gene expression underlies hemispheric asymmetries. Elife6, 10.7554/eLife.22784 (2017). [DOI] [PMC free article] [PubMed]
- 46.de Kovel, C. G. F., Lisgo, S. N., Fisher, S. E. & Francks, C. Subtle left-right asymmetry of gene expression profiles in embryonic and foetal human brains. bioRxiv, 10.1101/263111 (2018). [DOI] [PMC free article] [PubMed]
- 47.Harris, L. J. Do left-handers die sooner than right-handers? Commentary on Coren and Halpern’s (1991) “Left-handedness: a marker for decreased survival fitness”. Psychol Bull114, 203–234; discussion 235–247 (1993). [DOI] [PubMed]
- 48.Coren S, Halpern DF. Left-handedness: a marker for decreased survival fitness. Psychol Bull. 1991;109:90–106. doi: 10.1037/0033-2909.109.1.90. [DOI] [PubMed] [Google Scholar]
- 49.Badian NA. Birth order, maternal age, season of birth, and handedness. Cortex. 1983;19:451–463. doi: 10.1016/S0010-9452(83)80027-6. [DOI] [PubMed] [Google Scholar]
- 50.Stoyanov Z, Nikolova P, Pashalieva I. Season of birth, Geschwind and Galaburda hypothesis, and handedness. Laterality. 2011;16:607–619. doi: 10.1080/1357650X.2010.506689. [DOI] [PubMed] [Google Scholar]
- 51.Jones GV, Martin M. Seasonal anisotropy in handedness. Cortex. 2008;44:8–12. doi: 10.1016/j.cortex.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Tran US, Stieger S, Voracek M. Latent variable analysis indicates that seasonal anisotropy accounts for the higher prevalence of left-handedness in men. Cortex. 2014;57:188–197. doi: 10.1016/j.cortex.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Karev GB. Season of birth and parental age in right, mixed and left handers. Cortex. 2008;44:79–81. doi: 10.1016/j.cortex.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Tonetti L, Adan A, Caci H, Fabbri M, Natale V. Season of birth and handedness in young adults. Laterality. 2012;17:597–601. doi: 10.1080/1357650x.2011.599118. [DOI] [PubMed] [Google Scholar]
- 55.Cosenza RM, Mingoti SA. Season of birth and handedness revisited. Perceptual and motor skills. 1995;81:475–480. doi: 10.2466/pms.1995.81.2.475. [DOI] [PubMed] [Google Scholar]
- 56.Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Archives of neurology. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- 57.Bailey LM, McKeever WF. A large-scale study of handedness and pregnancy/birth risk events: implications for genetic theories of handedness. Laterality. 2004;9:175–188. doi: 10.1080/13576500342000013. [DOI] [PubMed] [Google Scholar]
- 58.Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and mixed-handedness. Pediatric research. 2007;62:586–590. doi: 10.1203/PDR.0b013e3181558678. [DOI] [PubMed] [Google Scholar]
- 59.Colombo, L. et al. Breastfeeding Determinants in Healthy Term Newborns. Nutrients10, 10.3390/nu10010048 (2018). [DOI] [PMC free article] [PubMed]
- 60.Bakan P. Left handedness and birth order revisited. Neuropsychologia. 1977;15:837–839. doi: 10.1016/0028-3932(77)90018-5. [DOI] [PubMed] [Google Scholar]
- 61.Bakan P. Handedness and birth order. Nature. 1971;229:195. doi: 10.1038/229195a0. [DOI] [PubMed] [Google Scholar]
- 62.Hicks RA, Evans EA, Pellegrini RJ. Correlation between handedness and birth order: compilation of five studies. Perceptual and motor skills. 1978;46:53–54. doi: 10.2466/pms.1978.46.1.53. [DOI] [PubMed] [Google Scholar]
- 63.Elkadi S, Nicholls ME, Clode D. Handedness in opposite and same-sex dizygotic twins: testing the testosterone hypothesis. Neuroreport. 1999;10:333–336. doi: 10.1097/00001756-199902050-00023. [DOI] [PubMed] [Google Scholar]
- 64.Vuoksimaa E, Eriksson CJ, Pulkkinen L, Rose RJ, Kaprio J. Decreased prevalence of left-handedness among females with male co-twins: evidence suggesting prenatal testosterone transfer in humans? Psychoneuroendocrinology. 2010;35:1462–1472. doi: 10.1016/j.psyneuen.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lust JM, et al. Differential effects of prenatal testosterone on lateralization of handedness and language. Neuropsychology. 2011;25:581–589. doi: 10.1037/a0023293. [DOI] [PubMed] [Google Scholar]
- 66.Coren S, Porac C. Birth factors and laterality: effects of birth order, parental age, and birth stress on four indices of lateral preference. Behav Genet. 1980;10:123–138. doi: 10.1007/BF01066263. [DOI] [PubMed] [Google Scholar]
- 67.McKeever WF, Suter PJ, Rich DA. Maternal age and parity correlates of handedness: gender, but no parental handedness modulation of effects. Cortex. 1995;31:543–553. doi: 10.1016/S0010-9452(13)80065-2. [DOI] [PubMed] [Google Scholar]
- 68.Reissland N, Aydin E, Francis B, Exley K. Laterality of foetal self-touch in relation to maternal stress. Laterality. 2015;20:82–94. doi: 10.1080/1357650x.2014.920339. [DOI] [PubMed] [Google Scholar]
- 69.Van der Elst W, et al. On the association between lateral preferences and pregnancy/birth stress events in a nonclinical sample of school-aged children. Journal of clinical and experimental neuropsychology. 2011;33:1–8. doi: 10.1080/13803391003757825. [DOI] [PubMed] [Google Scholar]
- 70.Domellof E, Johansson AM, Ronnqvist L. Handedness in preterm born children: a systematic review and a meta-analysis. Neuropsychologia. 2011;49:2299–2310. doi: 10.1016/j.neuropsychologia.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 71.Restrepo-Méndez MC, et al. The Association of Maternal Age with Birthweight and Gestational Age: A Cross-Cohort Comparison. Paediatric and Perinatal Epidemiology. 2015;29:31–40. doi: 10.1111/ppe.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swamy GK, Edwards S, Gelfand A, James SA, Miranda ML. Maternal age, birth order, and race: differential effects on birthweight. Journal of Epidemiology and Community Health. 2012;66:136–142. doi: 10.1136/jech.2009.088567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fall CH, et al. Association between maternal age at childbirth and child and adult outcomes in the offspring: a prospective study in five low-income and middle-income countries (COHORTS collaboration) The Lancet. Global health. 2015;3:e366–377. doi: 10.1016/s2214-109x(15)00038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dahly DL, Adair LS. Does lower birth order amplify the association between high socio-economic status and central adiposity in young adult Filipino males? International journal of obesity (2005) 2010;34:751–759. doi: 10.1038/ijo.2009.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magnus P, Gjessing HK, Skrondal A, Skjaerven R. Paternal contribution to birth weight. J Epidemiol Community Health. 2001;55:873–877. doi: 10.1136/jech.55.12.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG: an international journal of obstetrics and gynaecology. 2000;107:375–381. doi: 10.1111/j.1471-0528.2000.tb13234.x. [DOI] [PubMed] [Google Scholar]
- 77.Fry A, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan U. Normal distribution of hand preference and its bimodality. Int J Neurosci. 1993;68:61–65. doi: 10.3109/00207459308994260. [DOI] [PubMed] [Google Scholar]
- 79.McManus, I. C. The inheritance of left-handedness. Ciba Found Symp162, 251–267; discussion 267–281 (1991). [DOI] [PubMed]
- 80.Ransil BJ, Schachter SC. Test-retest reliability of the Edinburgh Handedness Inventory and Global Handedness preference measurements, and their correlation. Perceptual and motor skills. 1994;79:1355–1372. doi: 10.2466/pms.1994.79.3.1355. [DOI] [PubMed] [Google Scholar]
- 81.Bycroft, C. et al. Genome-wide genetic data on ~500,000 UK Biobank participants. bioRxiv, 10.1101/166298 (2017).
- 82.Lyall LM, et al. Seasonality of depressive symptoms in women but not in men: A cross-sectional study in the UK Biobank cohort. J Affect Disord. 2018;229:296–305. doi: 10.1016/j.jad.2017.12.106. [DOI] [PubMed] [Google Scholar]
- 83.Loh PR, et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet. 2015;47:284–290. doi: 10.1038/ng.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galinsky KJ, et al. Fast Principal-Component Analysis Reveals Convergent Evolution of ADH1B in Europe and East Asia. American journal of human genetics. 2016;98:456–472. doi: 10.1016/j.ajhg.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pulit, S. L. et al. Shared Genetic Contributions to Atrial Fibrillation and Ischemic Stroke Risk. bioRxiv, 10.1101/239269 (2018).
- 86.Visscher PM, et al. Statistical power to detect genetic (co)variance of complex traits using SNP data in unrelated samples. PLoS Genet. 2014;10:e1004269. doi: 10.1371/journal.pgen.1004269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loh PR, et al. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat Genet. 2015;47:1385–1392. doi: 10.1038/ng.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were obtained from the UK Biobank cohort, as part of research application 16066, with Clyde Francks as the principal applicant. Table 2 shows the field identifiers used. Summary and description of these data can be found at, http://biobank.ctsu.ox.ac.uk/crystal/. For the use of the data, application must be made to, http://www.ukbiobank.ac.uk/register-apply/.