Abstract
The fungal pathogen Rhizoctonia solani causes devastating diseases in hundreds of plant species. Among these, R. solani causes sheath blight, one of the three major diseases in rice. To date, few genes have been reported that confer resistance to R. solani. Here, rice-FOX Arabidopsis lines identified as having resistance to a bacterial pathogen, Pseudomonas syringae pv. tomato DC3000, and a fungal pathogen, Colletotrichum higginsianum were screened for disease resistance to R. solani. BROAD-SPECTRUM RESISTANCE2 (BSR2), a gene encoding an uncharacterized cytochrome P450 protein belonging to the CYP78A family, conferred resistance to R. solani in Arabidopsis. When overexpressed in rice, BSR2 also conferred resistance to two R. solani anastomosis groups. Both Arabidopsis and rice plants overexpressing BSR2 had slower growth and produced longer seeds than wild-type control plants. In contrast, BSR2-knockdown rice plants were more susceptible to R. solani and displayed faster growth and shorter seeds in comparison with the control. These results indicate that BSR2 is associated with disease resistance, growth rate and seed size in rice and suggest that its function is evolutionarily conserved in both monocot rice and dicot Arabidopsis.
Introduction
Rhizoctonia solani, belonging to the sub-division Basidiomycota, is a globally distributed necrotrophic soil-borne fungus. The host-range of R. solani spans numerous plant families including, but not limited to, wheat, rice, barley, canola, soybean, corn, potato and sugar beet1. R. solani colonizes different plant organs depending on the plant species; for example, leaves are infected in rice, whereas roots are infected in plants such as soybean, sugar beet and tomato. Because R. solani produces basidiospores under certain conditions, the fungus can be subdivided into anastomosis groups (AGs) based on hyphal anastomosis and physiological-biochemical characteristics2. To date, isolates of R. solani from plant species have been assigned to 13 AGs (AG1 to AG13), some of which include several subgroups3,4. Rice sheath blight, one of the major three diseases of rice, is caused by R. solani AG-1 IA and rice brown sheath blight is caused by R. solani AG-2-2 IIIB.
Despite the severe damage caused by R. solani, conventional breeding has had limited efficacy in introducing genetic resistance to R. solani in any crop species5. There is no rice cultivar that is fully resistant to R. solani; therefore, it is difficult to breed rice resistance to sheath blight using conventional breeding and selection methods. Genetic overexpression of several rice genes have been shown to confer resistance to R. solani, including OsACS26, Os2H167, OsWRKYs8–10 and OsASR211. The transcription factors OsWRKY80 and OsASR2 positively regulate OsWRKY4 and Os2H16, respectively8,11.
To date, several loss-of-function and gain-of-function mutant lines have been developed to isolate and investigate the functions of genes in rice12,13. Because approximately 30% of rice genes are predicted to be members of clustered and redundant gene families14, it can be difficult, if not impossible, to determine their functions using loss-of-function (silencing) approaches. In contrast, gain-of-function approaches enable direct screening of phenotypes of interest such as disease resistance.
Ichikawa et al.15 developed the Full-length cDNA OvereXpressing (FOX)-hunting system as an alternative to activation-tagging in Arabidopsis. In this system, transcriptomes of full-length cDNAs from another plant species are ectopically expressed in Arabidopsis. The short lifespan and small stature of Arabidopsis allow rapid screening for desirable transgenic phenotypes using this approach. A rice-FOX Arabidopsis population of 23,000 lines was previously generated by introducing 13,000 full-length rice cDNAs under the control of the CaMV 35S promoter into Arabidopsis ecotype Columbia16. Several screenings have been performed on these lines to date, and genes related to heat stress tolerance (OsHsfA2e17), salt tolerance (OsSMCP118; OsNAC06319; OsCEST20 and JAmyb21), secondary metabolism (OsLBD3722), disease resistance (BSR123) and photosynthesis (FNR1/FNR224) have been identified.
We previously screened 20,000 rice-FOX Arabidopsis lines for resistance to a bacterial pathogen, Pseudomonas syringae pv. tomato DC3000 (Pst DC3000), and then a fungal pathogen, Colletotrichum higginsianum, to find rice genes conferring broad-spectrum disease resistance23. We identified BROAD-SPECTRUM RESISTANCE1 (BSR1), a gene encoding a receptor-like cytoplasmic kinase. BSR1 conferred resistance to Pst DC3000 and C. higginsianum when overexpressed in Arabidopsis, and to Xanthomonas oryzae, Pyricularia oryzae (syn. Magnaporthe oryzae), Burkholderia glumae, and Cochliobolus miyabeanus when overexpressed in rice23,25. However, neither BSR1-overexpression (BSR1-OX) Arabidopsis nor rice displayed resistance to R. solani. To identify genes conferring resistance to R. solani from rice, we hypothesized that some of the other FOX lines showing resistance to both Pst DC3000 and fungal C. higginsianum might also display resistance to the fungus R. solani, even though Colletotrichum ascomycetes and the basidiomycete R. solani are not closely related according to their taxonomic classification. We decided to screen FOX lines showing resistance to both Pst DC3000 and C. higginsianum against R. solani.
In this study, before starting R. solani screening, we conducted Pst DC3000 screening using an additional 1,000 Rice-FOX Arabidopsis lines to increase the number of candidate resistant lines. By rescreening the FOX lines showing resistance to both Pst DC3000 and C. higginsianum, we succeeded in identifying a novel P450 gene, named BROAD-SPECTRUM RESISTANCE2 (BSR2). BSR2 conferred resistance to R. solani in both Arabidopsis and rice. BSR2 was ultimately found from the additional 1,000 lines that were screened. Surprisingly, both BSR2-OX Arabidopsis and rice displayed slower growth and longer seeds in comparison with the wild-type (WT). Our findings suggest that BSR2 is involved in pleiotropic functions in the monocot rice and strongly suggest that the molecular mechanism underlying these fundamental functions is conserved in the dicot Arabidopsis as well.
Results
Screening of additional FOX lines for resistance to Pst DC3000
We previously screened 20,000 rice-FOX Arabidopsis lines and obtained 72 lines that were resistant to infection by the Pst DC3000 bacterium23. More than 10 of these lines also showed resistance to infection by the fungus C. higginsianum. Here, we screened an additional 1,000 rice-FOX Arabidopsis lines and obtained one line, K02919, which showed clear resistance against PstDC3000. All the WT (Col-0) plants were susceptible to PstDC3000 and died, but the K02919 and resistant cpr5-2 control plants partly survived after inoculation (Fig. 1a). The K02919 line overexpressed a full-length rice cDNA AK072163 (Os08g0547300) that we termed BSR2. We generated six independent retransformed Arabidopsis lines (RT:BSR2-OX#1–#6) that overexpressed AK072163 (Supplementary Fig. S1). The K17903 line, another original FOX line overexpressing AK072163 cDNA, was also used (Supplementary Fig. S1). Because of the low fertility associated with BSR2 overexpression and the consequent shortage of transgenic seeds, different transgenic lines overexpressing AK072163 cDNA were used in later experiments.
Figure 1.
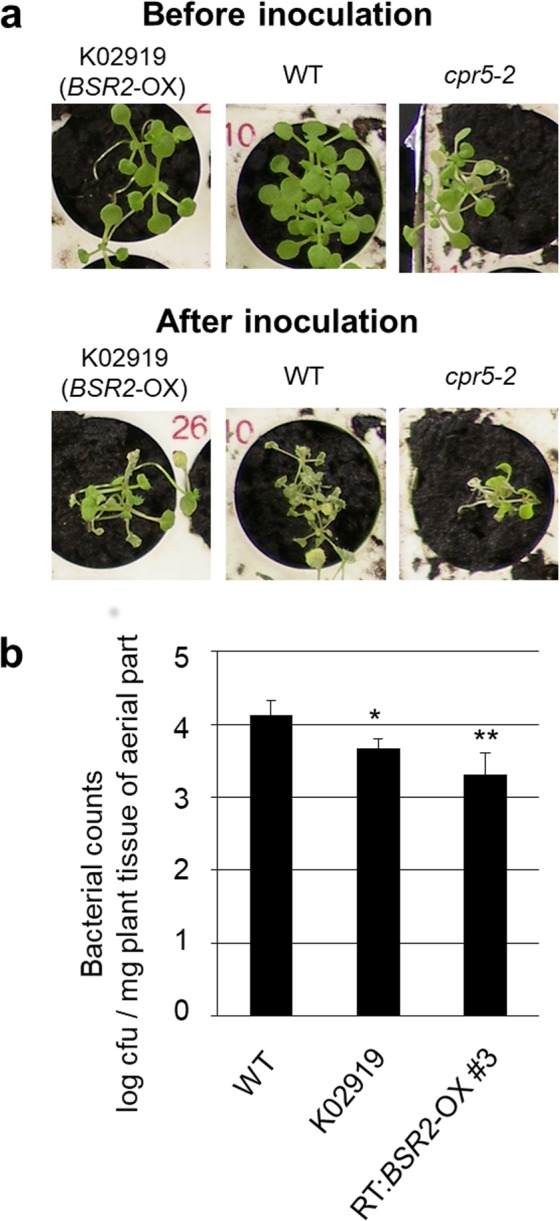
Resistance to Pst DC3000 in BSR2-OX Arabidopsis lines. (a) Phenotypic responses to Pst DC3000 inoculation. Three-week-old plants were inoculated with 2 × 108 cfu/mL of Pst DC3000. The wild-type (WT; Col-0) plants died, but the K02919 (a rice-FOX Arabidopsis T2 line; BSR2-OX) and cpr5-2 (control mutant resistant to Pst DC3000) plants remained green. (b) Growth of Pst DC3000 bacteria in plants. Arabidopsis plants at about the 20-leaf stage were inoculated with Pst DC3000 (106 cfu/mL) by dipping, and the numbers of bacteria in the aerial part of the plants were counted after 4 d. The bacteria in 8 replicates were counted and the differences between WT and two independent BSR2-OX lines (K02919 and RT:BSR2-OX#3) were significant according to a t-test (*P < 0.05; **P < 0.01). Error bars represent the standard deviation.
To quantify the bacterial resistance, the bacterial numbers in the inoculated plants were counted. The bacterial counts in the K02919 and RT:BSR2-OX#3 plants were significantly lower than those in the WT plants (Fig. 1b) and the BSR2 transcript levels in RT:BSR2-OX#3 plants were higher than those in the K02919 line plants (Supplementary Fig. S1). Overall, the plant bacterial resistance levels correlated well with the BSR2 transcript levels (Fig. 1b).
Resistance to C. higginsianum
To determine whether BSR2 overexpression is also effective against fungal pathogens, we tested the BSR2-OX lines for resistance against the ascomycete C. higginsianum. The WT plants completely died after inoculation, but the K02919 and the resistant ecotype (Eil-0) control plants survived (Fig. 2a). For quantitative evaluation, relative fungal growth was compared between the plant lines using qRT-PCR. The relative fungal growths in K02919 and the two retransformed BSR2-OX lines (RT:BSR2-OX#2 and #3) were significantly lower than those in the WT plants (Fig. 2b).
Figure 2.
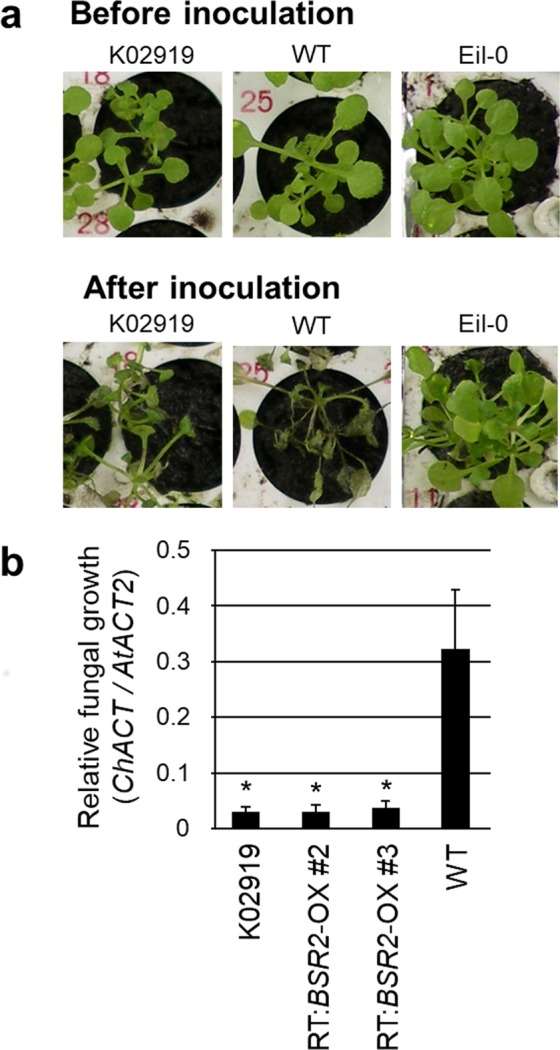
Resistance to C. higginsianum in BSR2-OX Arabidopsis lines. (a) Phenotypic responses to C. higginsianum inoculation. Three-week-old plants were inoculated with 105 conidia/mL of C. higginsianum. Unlike wild-type (WT; Col-0) plants, K02919 (a rice-FOX Arabidopsis T2 line; BSR2-OX) and Eil-0 (control ecotype resistant to C. higginsianum) plants were still green 13 d after inoculation. (b) Quantification of the relative fungal growth across plant lines. C. higginsianum Actin (ACT) DNA in the Arabidopsis plants were measured using quantitative real-time PCR 3 d after inoculation. The amounts of C. higginsianum genomic ACT DNA relative to Arabidopsis genomic ACT2 DNA in three independent BSR2-OX lines (K02919, RT:BSR2-OX#2 and RT:BSR2-OX#3) were significantly lower than those of WT plants (*P < 0.05 according to a t-test; n = 3–5; error bars represent the standard deviation).
Resistance to R. solani
R. solani, a fungal pathogen classified in the Basidiomycota, causes rice sheath blight, which is one of the three main diseases that devastate rice. We hypothesized that some FOX lines that show resistance to both bacterial Pst DC3000 and fungal C. higginsianum may also display resistance to the fungal pathogen R. solani. In addition to K02919, we previously identified 11 single-cDNA insert rice-FOX Arabidopsis lines that had resistance to both Pst DC3000 and C. higginsianum23 as shown in Supplementary Table S1. We further screened these FOX lines to identify the gene that conferred resistance to R. solani. We used the cpr5-2 mutant line with resistance to Pst DC3000 and the Eil-0 ecotype line with resistance to C. higginsianum as controls.
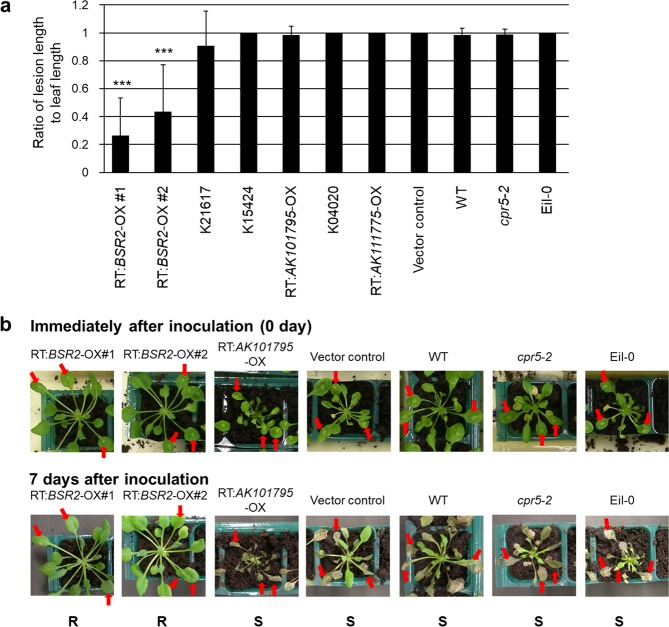
Because our main purpose was to identify rice cDNA that are effective against the R. solani isolate (MAFF243956; AG-1 IA) that causes rice sheath blight, we used this isolate to screen the FOX lines. This isolate can also infect Arabidopsis. Resistance to R. solani was evaluated by the ratio of disease lesion length to the drop-inoculated leaf length of six-week-old Arabidopsis plants. Most of the Arabidopsis lines tested — K21617 (AK103699-OX), K15424 (BSR1-OX), RT:AK101795-OX, K04020 (AK066139-OX) and RT:AK111775-OX — were susceptible to R. solani. Only two BSR2-OX lines (RT:BSR2-OX#1 and #2) displayed significant resistance to R. solani in comparison with WT, the vector control and other lines (Fig. 3). Therefore, overexpression of BSR2 conferred resistance to bacterial Pst DC3000, fungal C. higginsianum and R. solani in Arabidopsis.
Figure 3.
Screening rice-FOX Arabidopsis lines for resistance to R. solani. (a) Evaluation of R. solani resistance using the ratio of lesion length to leaf length. Six-week-old plants were used for drop inoculation with R. solani (MAFF243956; AG1-1A). Seven rice-FOX Arabidopsis lines showing resistance to both Pst DC3000 and C. higginsianum were used: RT:BSR2-OX#1 and #2, RT:AK101795-OX and RT:AK111775-OX are independent retransformed lines for AK072163, AK101795 and AK111775, respectively. K21617, K15424 and K04020 are independent transgenic rice lines with one rice cDNA insert for AK103699, BSR1 (AK070024) and AK066139, respectively. The lesion length and leaf length were measured 7 d after inoculation. Asterisks indicate that values are significantly different from the WT and vector control plants (***P < 0.001, according to a t-test; error bars represent the standard deviation; n = 3). (b) Representative Arabidopsis plants described in (a) immediately after inoculation and 7 d after inoculation. In comparison with the vector control and WT plants, the spread of disease symptoms stopped 7 d after inoculation in the BSR2-OX plants (RT:BSR2-OX#1 and #2). The arrows indicate the inoculation points. R, resistant; S, susceptible.
According to the Rice Annotation Project (RAP) database, BSR2 encodes a cytochrome P450 protein (Supplementary Table S1). The BSR2 protein is classified as a CYP78A15 (CY78C5)-type cytochrome P45026–28, although its CYP78A15 sequence has an insertion of three additional amino acids. The sequence alignments and phylogenetic tree for BSR2 and its related P450 proteins are shown in Supplementary Fig. S2.
Rice resistance to R. solani (AG-1 IA) sheath blight
We examined whether overexpression of BSR2 also confers resistance to sheath blight in rice. The BSR2 cDNA, AK072163, was inserted downstream of the constitutive maize ubiquitin promoter, and this construct was used to generate transgenic rice lines overexpressing BSR2. At the organ regeneration step of tissue culture, shoots were produced from BSR2-OX calli but roots failed to form on both regeneration and hormone-free media. However, when the rootless regenerated shoots were transferred to soil and watered, roots formed and we were able to use the regenerated plants for subsequent analyses. Overexpression of the BSR2 cDNA was confirmed by quantitative real-time RT-PCR (qRT-PCR) analysis of the T0 plants (plants regenerated from transgenic calli), and their T1, T2 and T3 seeds were used for the experiments. The BSR2 expression levels in the BSR2-OX transgenic plants are presented in Supplementary Fig. S3. Because of the low fertility associated with BSR2 overexpression and the consequent shortage of transgenic seeds in rice as well as in Arabidopsis, different overexpressing lines were used in later experiments.
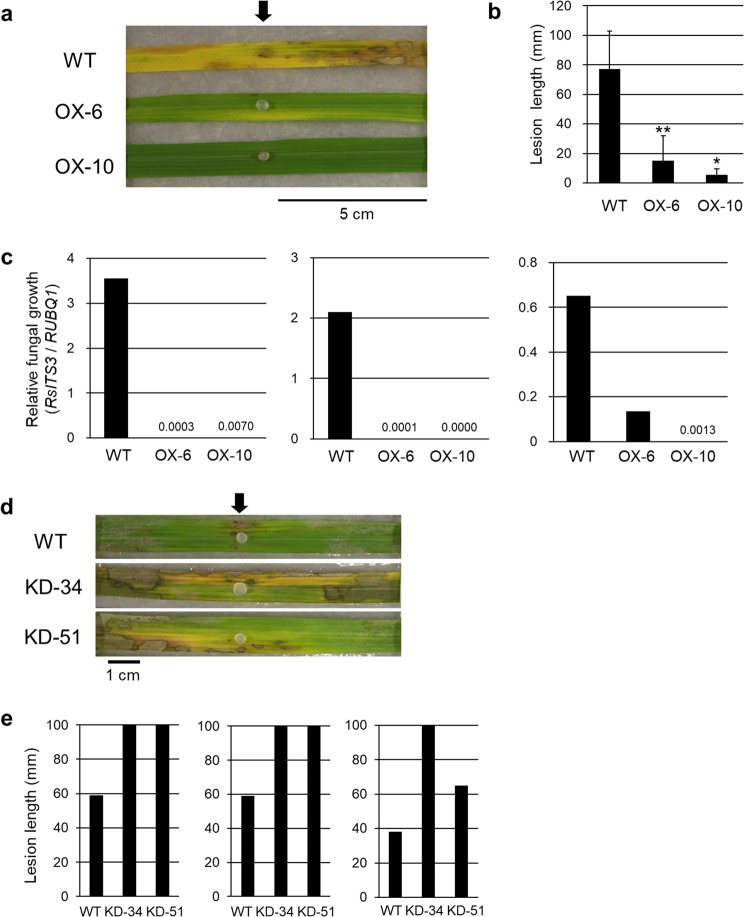
Leaves cut from the transgenic rice plants were used to evaluate their resistance to R. solani (MAFF243956; AG-1 IA). Whereas WT plants developed lesions that extended from the inoculation point, BSR2-OX lines showed only restricted lesion formation (Fig. 4a and Supplementary Fig. S4). The lesion lengths in the inoculated BSR2-OX leaves were significantly shorter than those in the WT leaves (Figs 4b and S4b). The relative fungal growths were compared between the plant lines using qRT-PCR. The relative fungal growths in two BSR2-OX lines were much lower than those in the WT plants in three independent experiments (Fig. 4c). A general sheath inoculation assay was also carried out to evaluate resistance to R. solani in whole plants. The lesion lengths of the two BSR2-OX lines were significantly shorter than those of the WT plants (Supplementary Fig. S4c). Together, these data confirm that the BSR2-OX rice lines have resistance to R. solani. Because the results obtained using the detached leaf inoculation assay and the sheath inoculation assay were similar, we used the only detached leaf inoculation assay for subsequent comparisons.
Figure 4.
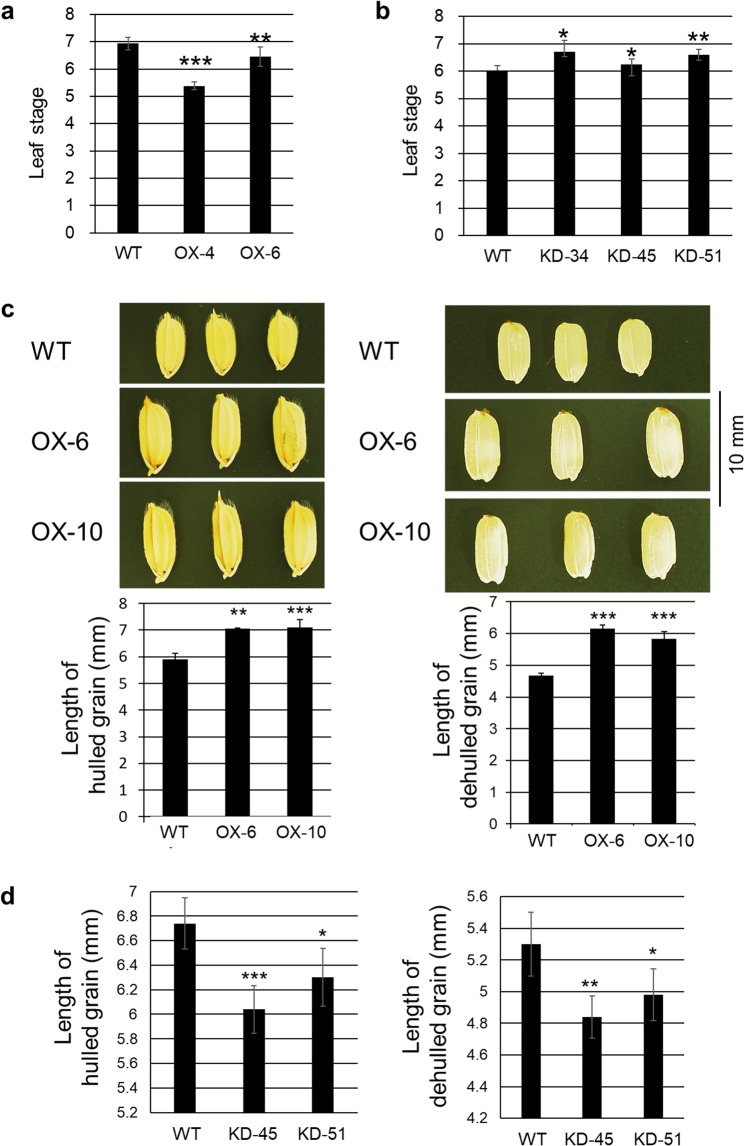
Resistance to R. solani (AG-1 IA) sheath blight in BSR2-OX and -KD rice lines. Comparisons of (a) inoculated leaves, (b) lesion lengths and (c) relative fungal growth of detached leaf blades from BSR2-OX lines 13 d after drop inoculation with R. solani (MAFF243956; AG1-1A). The second leaf blades from the top leaf at leaf stages 10–13 were used for inoculation. Asterisks indicate that values are significantly different from the WT (*P < 0.05; **P < 0.01, according to a t-test; error bars represent the standard deviation; n = 4). Measurements of relative fungal growth were performed three times with similar results. Comparison of (d) inoculated leaves and (e) lesion lengths of detached leaf blades of BSR2-KD lines 7 d after drop inoculation. The second leaf blades from the top leaf at leaf stages 10–13 were used for inoculation. The lesion lengths in the BSR2-KD plants were longer than those in the WT plants. Tests were performed three times with similar results. Arrows indicate the inoculation points.
Overexpression of genes sometimes induces artificial effects; therefore, loss-of-function experiments are generally more reliable for determining innate gene functions. To examine whether BSR2 is innately involved in rice defence against sheath blight, we generated BSR2-knockdown (KD) transgenic rice plants that suppress BSR2 using its 3′-untranslated region (~300 bp) as a trigger. BSR2 transcript levels in the generated BSR2-KD-34, -45 and -51 lines were successfully reduced (Supplementary Fig. S3). The lesion lengths in inoculated BSR2-KD rice leaf blades were longer than those in WT rice (Fig. 4d,e and Supplementary Fig. S5), indicating that BSR2 is innately involved in rice defence against sheath blight caused by R. solani.
Rice resistance to R. solani (AG-2-2 IIIB) brown sheath blight
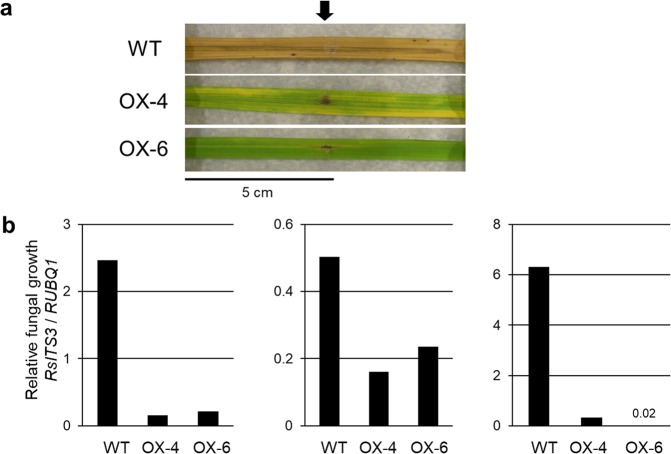
We investigated whether overexpression of BSR2 also conferred resistance to brown sheath blight, another disease that is caused by R. solani in rice. For this purpose, we used the R. solani Shimomura 9 isolate (AG-2-2 IIIB) instead of R. solani (MAFF243956; AG-1 IA). A detached leaf inoculation assay was performed to evaluate the rice resistance to the R. solani Shimomura 9 isolate. Whereas WT plants developed lesions that extended from the inoculation point, BSR2-OX lines had restricted lesion development (Fig. 5a). For quantitative evaluation, the relative fungal growths were compared using qRT-PCR. The relative fungal growths in the BSR2-OX lines were remarkably lower than those in the WT plants in three independent experiments (Fig. 5b). These results indicate that the BSR2-OX lines were resistant to brown sheath blight caused by R. solani (AG-2-2 IIIB). Therefore, in rice, BSR2 confers resistance to R. solani isolates belonging to two different AGs. To our knowledge, no other gene has been reported to confer resistance to R. solani isolates belonging to more than one AG.
Figure 5.
Resistance to R. solani (AG-2-2 IIIB) brown sheath blight in BSR2-OX rice lines. Comparison of (a) inoculated leaves and (b) relative fungal growths 11 d after drop inoculation with R. solani (Shimomura 9 isolate; AG-2-2 IIIB). The second leaf blades from the flag leaf at the heading stage were used for inoculation. The relative fungal growth levels in the BSR2-OX plants were lower than those in the WT plants. Tests were performed three times with similar results. The inoculation points are indicated by an arrow.
Changes in the growth rate, plant height and reproductive organ size caused by overexpression of BSR2 in Arabidopsis
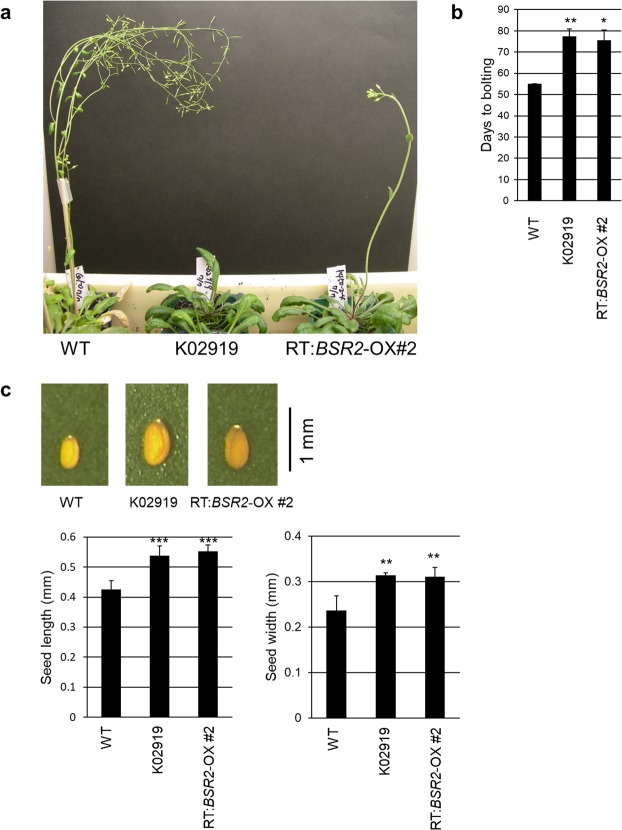
When BSR2 was overexpressed in Arabidopsis, morphological changes were observed in the leaves, flowers, siliques and seeds, and fertility was remarkably low. The BSR2-OX plants grew more slowly than the WT plants (Supplementary Fig. S6a), and they required a longer time to bolt in comparison with WT (Fig. 6a,b). However, the final heights of the BSR2-OX plants were taller than those of the WT plants (Supplementary Fig. S6). The flowers, siliques and seeds in the BSR2-OX plants were also remarkably larger than those observed in the WT plants (Supplementary Fig. S6 and Fig. 6c).
Figure 6.
Morphological traits of BSR2-OX Arabidopsis lines. (a) Representative BSR2-OX phenotypes 77 d after sowing and (b) the number of days to bolting. (c) The comparative sizes, lengths and widths of seeds. Asterisks indicate values that are significantly different from the WT (*P < 0.05, **P < 0.01, ***P < 0.001, according to a t-test; error bars represent the standard deviation; n = 3 (b) and 5 (c)).
Changes in the growth rate and reproductive organ size caused by overexpression or silencing of BSR2 in rice
When BSR2 was overexpressed in rice, the BSR2-OX plants grew more slowly than the WT plants just like in Arabidopsis. The leaf stages of the BSR2-OX-4 and -6 lines 40 d after sowing were 5.4 and 6.5, respectively. These values were significantly smaller than that of the WT plants (6.9; Fig. 7a). Further repeats of this experiment showed that the leaf stages of the BSR2-OX-6 and -2 plants were also smaller than that of the WT plants (Supplementary Fig. S7). These data indicate that the BSR2-OX rice grew more slowly than the WT rice plants. We examined whether the BSR2-KD rice had an opposite effect on the leaf growth rate. The leaf stages of three independent BSR2-KD lines developed faster than the leaves of the WT plants at the vegetative stage (Fig. 7b), indicating that BSR2-KD rice grew faster than WT.
Figure 7.
Morphological traits of BSR2-OX and -KD rice lines. Leaf stages of (a) BSR2-OX lines 40 d after sowing and (b) BSR2-KD lines 32 d after sowing in comparison with WT at the vegetative stage. (c) Comparison of hulled grain (left) and dehulled grain (right) of BSR2-OX and (d) the lengths of hulled grain and dehulled grain of BSR2-KD rice lines in comparison with WT. Asterisks indicate values that are significantly different from the WT (*P < 0.05, **P < 0.01, ***P < 0.001, according to a t-test; error bars represent the standard deviation; n = 5–12 (a), 4–13 (b), 4 (c) and 5 (d)).
The floral organs in the BSR2-OX plants were also larger than those of the WT plants at the flowering stage (19 weeks after sowing). The lemma, glume, anther and pistil lengths of the BSR2-OX plants were 13–19%, 25–30%, 29–53% and 22–39% longer than those of WT, respectively (Supplementary Fig. S8). The lengths of unhulled seed and dehulled rice grains from two independent BSR2-OX lines at the harvest stage were 20% and 25–32% longer than those of the WT (Fig. 7c). The fertility of the BSR2-OX rice plants was also notably low. Conversely, the lengths of unhulled seed and dehulled rice grains from two independent BSR2-KD lines were 7–10% and 6–9% shorter than those of the WT (Fig. 7d).
According to the Rice Expression Profile Database29 (http://ricexpro.dna.affrc.go.jp/), in WT ‘Nipponbare’ rice, BSR2 is expressed in reproductive organs such as inflorescences, pistils and lemmas as well as in vegetative organs such as leaves and roots (Supplementary Fig. S9). The spatio-temporal expression profile of BSR2 does not contradict the morphological phenotype of BSR2-OX or -KD rice plants. Therefore, it appears that BSR2 innately regulates the growth rate and seed size as well as disease resistance in rice.
Defence-related phytohormone levels in BSR2-OX Arabidopsis
To examine whether defence-related plant hormones are involved in disease resistance in BSR2-OX Arabidopsis, the salicylic acid (SA) and jasmonic acid (JA) levels were simultaneously measured from the same plant samples 2 d after Pst DC3000 inoculation (Supplementary Fig. S10). The SA levels with or without pathogen infection were similar and the JA levels without pathogen infection were similar between the vector control plants and the BSR2-OX plants. Although the JA content of the BSR2-OX#2 line after infection appeared to be higher than that of the vector control, the JA content of the BSR2-OX#3 line, which has strong resistance to Pst DC3000, was similar to that of the vector control. These results suggest that JA and SA are unlikely to be involved in the disease resistance conferred by BSR2-OX in Arabidopsis.
The ethylene (ET) emission levels were also measured, as described in the Supplementary Methods. The ET emission levels in the BSR2-OX and vector control plants were too low to detect even 3 d after Pst DC3000 infection. Together, these data show that the SA, JA and ET levels were not significantly different between the BSR2-OX plants and the vector control plants in our experiments. Therefore, the disease resistance mechanism associated with BSR2 may be unrelated to the disease resistance mechanisms mediated by these three phytohormones.
Discussion
The mechanism of broad-spectrum disease resistance conferred by BSR2
In this study, we describe BSR2, a gene that encodes a P450 protein that is involved in innate disease resistance to the necrotrophic fungal pathogen R. solani, the causal pathogen of sheath blight in rice. In addition, BSR2-OX rice showed resistance to another isolate of R. solani that belongs to a different AG and causes brown sheath blight, a different rice disease. In Arabidopsis, BSR2 expression also conferred remarkable resistance to the hemibiotrophic bacterial pathogen Pst DC3000, a hemibiotrophic fungal pathogen C. higginsianum, and to R. solani. To our knowledge, there are few genes that confer such broad-spectrum disease resistance.
To date, several R. solani resistance genes have been identified in rice6–8,10,11. Some reports suggest the importance of ET/JA signalling pathway in R. solani resistance5,6,8. Transgenic rice lines with inducible ET production were previously generated by expressing rice ACS2 (encoding 1-aminocyclopropane-1-carboxylic acid synthase, a key ET biosynthesis enzyme) under the control of a strong pathogen-inducible promoter6. These OsACS2-overexpression lines had significantly increased levels of endogenous ET and exhibited increased resistance to a field isolate of R. solani, as well as to different races of P. oryzae. These results suggest that pathogen-inducible ET production in transgenic rice can enhance resistance to necrotrophic R. solani as well as to hemibiotrophic P. oryzae. ET signalling is also important for isoflavonoid-mediated resistance to R. solani in the roots of Medicago truncatula5. Overexpression of OsWRKY30 increases endogenous JA accumulation and resistance to R. solani and P. oryzae9. OsWRKY4 protein, which is induced upon R. solani infection, specifically binds to the promoter regions of pathogenesis-related (PR) genes. Overexpression of OsWRKY4 increases the resistance to R. solani10. Moreover, OsWRKY80, which is induced by R. solani, JA and ET, but not by SA, binds to the promoter regions of OsWRKY4. Similar to OsWRKY4, overexpression of OsWRKY80 increases resistance to R. solani, while suppression of OsWRKY80 by RNAi reduces resistance to R. solani. These studies suggest that the OsWRKY80-OsWRKY4 module functions as a positive regulatory circuit for rice defence against R. solani that acts via the JA/ET-dependent signalling pathway8.
Other reports describe the contribution of SA-dependent immunity to resistance against R. solani30,31. Kouzai et al.31 recently found that foliar pretreatment with SA can induce R. solani sheath blight resistance in rice and Brachypodium distachyon. They also showed the involvement of SA in R. solani resistance using SA-deficient NahG transgenic rice. Because SA-dependent plant immunity generally targets biotrophs and hemi-biotrophs, their findings suggest a preceding biotrophic stage and a subsequent necrotrophic stage in the R. solani infection process31. Moreover, overexpression of Arabidopsis NPR1, which is responsible for SA signalling, confers resistance to R. solani in rice30. NPR1 may also target the biotrophic stage of R. solani.
The disease resistance mechanism of BSR2 is still unknown. Because BSR2 encodes a P450 enzyme, it should be involved in the biosynthesis of certain chemical compounds. Although several reports implicate known defence hormones, the measurement of phytohormones in the present study suggested that BSR2 overexpression does not affect the production of SA, JA or ET (Supplementary Fig. S10). Some P450 proteins are responsible for the biosynthesis of phytoalexins; for example, PAD3 (CYP71B15) for camalexin32, CYP76M7 for phytocassane33 and CYP82C2 for 4-hydroxyindole-3-carbonyl nitrile (4-OH-ICN)34. However, there are no known chemicals, including plant hormones, that can explain the concomitant phenotypes of seed size and growth rate in addition to the broad-spectrum disease resistance in BSR2-OX plants. Therefore, we think that BSR2 may be involved in the biosynthesis of novel chemical compounds that function pleiotropically in disease resistance, seed size and the plant growth rate.
Positive effect of BSR2 on the reproductive organ size
Seeds of BSR2-OX Arabidopsis and -OX rice are longer than WT seeds and those of BSR2-KD rice are shorter, which suggests that BSR2 is involved in the development of reproductive organs (Figs 6 and 7, Supplementary Figs S6 and S8). There are many reports on the morphological effects of the CYP78A family proteins. KLUH(KLU)/CYP78A5 was the first member of the CYP78A family to be identified in Arabidopsis. Whereas CYP78A5 loss-of-function mutants form smaller organs than WT, CYP78A5 overexpression leads to larger organs with more cells. Therefore, it seems that CYP78A5 controls the sizes of plant organs such as leaves, sepals, petals, ovules and seeds, by promoting cell proliferation35–37.
Arabidopsis CYP78A6, A8 and A9 are homologous with BSR2 (Supplementary Fig. S2). Overexpression of CYP78A9 induces longer sepals, petals and pistils, shorter stamens, larger fruit and seed, and reduced fertility38,39. The cyp78a8 and cyp78a9 double mutant has reduced seed set due to development arrest of the outer ovule integument that leads to female sterility39. Similarly, overexpression of EOD/CYP78A6 increases seed size by promoting both cell proliferation and cell expansion, whereas knockout mutants form small seeds. CYP78A6 functions redundantly with CYP78A9 to affect seed growth40. Together, these studies show that CYP78A5, A6, A8 and A9 also have positive effects on plant reproductive organ size.
In the monocot rice, GIANT EMBRYO (GE) is essential for controlling the size balance between embryo and endosperm, and has been reported as encoding CYP78A13 (Supplementary Fig. S2)41,42. In contrast to the loss-of-function mutant that had large embryos and small endosperms, GE overexpression led to the production of small embryos and enlarged endosperms41,42. Moreover, GE overexpression promoted cell proliferation and cell expansion that resulted in longer leaves and bigger spikelet hulls42. When CYP78A10, an Arabidopsis ortholog for GE, was overexpressed in Arabidopsis, the seeds were much larger than those of the WT plants42. Furthermore, CYP78A13 rescues the phenotype of short sepals and siliques in a CYP78A5 mutant, Arabidopsis klu-4, suggesting a conserved role in reproductive organ development in both monocots and dicots43. In the dicot tomato, SIKLUH regulates fruit size, which is a domestication trait44. In soybean and wheat, GmCYP78A10, GmCYP78A72 (homologous to CYP78A5) and TaCYP78A5 regulate seed size45–47. Together, these data suggest that the positive effects of CYP78As on the sizes of the reproductive organs are likely to be conserved in angiosperms. Therefore, the positive effect of BSR2 on reproductive organ size seems to be a typical effect of CYP78A proteins acting to increase cell proliferation and/or expansion. However, in contrast to GE/CYP78A13, BSR2 overexpression has little effect on the size balance between the embryo and the endosperm (Fig. 7c, right).
Negative effect of BSR2 on the growth rate
BSR2-OX Arabidopsis and BSR2-OX rice showed slow growth, while BSR2-KD rice displayed fast growth (Fig. 7, Supplementary Figs S6 and S7). A PLA1/CYP78A11 loss-of-function mutant in rice has rapid leaf initiation and a shortened plastochron, the time interval during which new leaves are produced48. In contrast, transgenic maize constitutively overexpressing maize PLA1 grow more slowly49. A KLU/CYP78A5 loss-of-function mutant in Arabidopsis also showed an accelerated leaf initiation rate, suggesting that KLU/CYP78A5 affects the plastochron as well as the organ size50. The shortened plastochron duration was mostly due to the increased cell division rate. Wang et al.50 also suggested the possibility that the plastochron duration and organ size are coordinately regulated by an unknown common regulator. Due to its sequence similarity at the protein level, the BSR2 protein may produce chemicals similar to the PLA1 or KLU proteins. Such chemicals may be responsible for the repression of leaf initiation observed and allow for the maintenance of an appropriate plastochron duration. BSR2 overexpression could increase the production of such chemicals. This might suppress the rate of leaf initiation by decreasing the rate of cell division, leading to a longer plastochron and slower growth.
Possible function of BSR2
Although some members of the CYP78As, such as CYP78A5, are known to affect seed size and plastochron, no member of the CYP78As has been reported as being involved in disease resistance. BSR2-KD rice displayed susceptibility to R. solani and had shorter seeds and faster growth than WT plants, probably due to a shorter plastochron. These data suggest that BSR2 is involved in pleiotropic functions such as innate immunity, reproductive organ size and growth rate in rice.
Our discovery and characterization of BSR2 adds new insight into the biological roles of CYP78As, because, to our knowledge, no other CYP78A has such pleiotropic function. CYP78As are found in most angiosperm species and moss, suggesting that they have an ancient conserved role28. This is the first report on disease resistance found by overexpression of a CYP78A (BSR2), and it is quite possible that other members of the CYP78A family play key roles in disease resistance as well.
The biochemical function of BSR2 is unknown. Zea mays CYP78A1 has been reported as having lauric acid 12-monooxygenase activity51. Similarly, CYP78A5, A7 and A10 have short-chain fatty acid hydroxylase activities52. CYP78A5, A6, A9 and A11 act non-cell-autonomously; therefore, they are also thought to be involved in generating a novel mobile growth signal that is distinct from the classical phytohormones38,40,48. Analyses of gain-of-function and loss-of-function mutants of CYP78A9 (one of the closest homologues of BSR2) have revealed perturbations in the flavonol biosynthesis pathway39. The same study found no clear overlap between the CYP78A9-regulated genes and known phytohormone-responsive genes39. Moreover, an earlier study found that no known phytohormone treatment could mimic the entire phenotype of CYP78A9-OX Arabidopsis38. No known phytohormone could also mimic the pleiotropic phenotype of BSR2-OX plants. Because CYP78As are involved in fatty acid and flavonol biosynthesis, it is conceivable that BSR2 overexpression led to the activation of a novel biosynthetic pathway. Such a pathway could have several bioactive products that affect the phenotypes associated with BSR2 expression; including reproductive organ size, growth rate and disease resistance. In future, the biochemical function of BSR2 should be characterized. RNA sequencing analysis coupled with a metabolomic assay would shed light on which genes or gene products are activated by BSR2 overexpression during the vegetative and reproductive stages and before and after infection by R. solani.
Applications for disease resistant crops
BSR2 may be useful for generating crop plants that have broad-spectrum disease resistance. Because BSR2 was effectively expressed in both the monocot rice and the dicot Arabidopsis, it may be useful for generating many monocot and dicot crops. However, because BSR2 overexpression led to notably reduced fertility in both Arabidopsis and rice, it would be useful to use promoters that prevent BSR2 expression in the reproductive organs. Alternatively, the adoption of pathogen-inducible promoters like PR1b53 may be useful for preventing the growth retardation and low fertility that result from constitutive expression of BSR2. However, constitutive BSR2 expression may still be applicable to crops that are generally propagated without reproductive organ development; for example, potato, sweet potato and sugarcane. In Arabidopsis, BSR2 overexpression also resulted in enlarged siliques (fruit) and flowers. Therefore, BSR2 expression may be beneficial in ornamental flower and fruit tree species, in addition to the broad-spectrum disease resistance that it confers.
Methods
Plant materials and culture conditions
Arabidopsis thaliana ecotype Columbia (Col-0) was used as the WT control. A mutant line, cpr5-2 (a gift from Dr. B.N. Kunkel, Washington University, USA), with very high resistance to Pst DC3000 was used as positive control. The Arabidopsis ecotype Eil-0, which is highly resistant to C. higginsianum, was also used as positive control. The candidate full-length rice cDNAs identified by Pst DC3000 screening were re-transformed in Arabidopsis as previously described23. Transgenic Arabidopsis plants were grown on half-strength MS medium (Wako Pure Chemicals, Osaka, Japan) with 1% sugar, B5 vitamins (0.04% myo-inositol, 0.0004% nicotinic acid, 0.0004% pyridoxine hydrochloride, 0.004% vitamin B1 hydrochloride), 0.05% MES, 10 µg/mL hygromycin B (Wako Pure Chemicals) and 0.8% agar adjusted to pH 5.7. Col-0, cpr5-2 and Eil-0 seeds were sown in the same medium but without the hygromycin. For the R. solani resistance assay, 2-week-old plants were transferred to pots containing sterile moistened black peat moss (Super Mix; Sakata, Yokohama, Japan) and grown under short-day conditions (9 h light and 15 h dark) at 22 °C. To observe morphological traits or harvest seeds, 3- to 4-week-old plants were transferred to pots and grown under long-day conditions (16 h light and 8 h dark) at 22 °C.
Rice (Oryza sativa L.) cultivar ‘Nipponbare’ was used as the WT control. Dehusked seeds were surface sterilized and sown on half-strength MS medium containing 3% (w/v) sucrose and 0.4% (w/v) Gelrite (Wako Pure Chemicals) in sterile Petri dishes. The dishes were incubated in a growth chamber at 28 °C in the dark for 3 d, followed by 4–7 d at 25 °C under long-day conditions (16 h light [60–70 µmol m −2 s −1] and 8 h dark). For transgenic selection, hygromycin B (30–50 µg/mL) was added to the medium. WT seedlings and hygromycin-resistant transgenic seedlings were transplanted to pots containing soil (Bonsol No. 2, Sumitomo Kagaku Kougyo, Osaka, Japan) and cultivated in a greenhouse at 27–30 °C.
Pathogen strains and cultures
The bacterial pathogen Pst DC3000 and the fungal pathogen C. higginsianum (MAFF305635) were used for disease resistance screening in Arabidopsis. The fungal pathogen R. solani (MAFF243956) was used for both disease resistance screening in Arabidopsis and evaluation of sheath blight disease resistance in rice. In addition, the R. solani Shimomura 9 isolate was kindly provided by Dr. Toshiyuki Morikawa (Toyama Prefectural Agricultural, Forestry and Fisheries Research Center) and used to evaluate resistance to brown sheath blight in rice.
The protocols for Pst DC3000 culture and inoculation are as previously described23. The fungi C. higginsianum and R. solani were cultured on PDA agar plates (0.39% potato extract, 2.1% glucose and 1.41% agar, adjusted to pH 5.6; Nissui, Tokyo, Japan) at 28 °C under dark conditions for 2 weeks and 3 d, respectively, before inoculation.
Pst DC3000 and C. higginsianum screening
Arabidopsis Col-0 was used as the WT (negative control), and cpr5-2 (a Pst DC3000 resistant mutant line) and Eil-0 (a C. higginsianum resistant ecotype) were used as positive controls. The control ecotype, cpr5-2 and an additional 1,000 rice-FOX Arabidopsis lines16,23, which were different from those screened in the previous report23, were sown at 5 seeds per well in 60-well plates containing pre-sterilized moist black peat moss. Screening for Pst DC3000 and C. higginsianum, and the bacterial growth assay for Pst DC3000 were performed as previously described23.
Quantification of relative fungal growth by quantitative real-time PCR
Quantification of C. higginsianum growth was performed as follows. Four- to five-week-old Arabidopsis plants were inoculated by spraying the rosette leaves with C. higginsianum (3.2 × 106 conidia/mL). C. higginsianum infection was quantified by measuring C. higginsianum genomic DNA relative to Arabidopsis genomic DNA using quantitative real-time PCR (qRT-PCR) analysis. Total DNA was extracted from the aerial parts of the plants using extraction buffer containing 0.5% (w/v) sodium dodecyl sulfate, 250 mM EDTA, 25 mM NaCl, and 200 mM Tris-HCl (pH 8.0), as previously described54. Disease severity was evaluated by comparing the amounts of C. higginsianum genomic ACT DNA relative to Arabidopsis genomic ACT2 (At3g18780) DNA 3 d after inoculation.
Quantification of R. solani in rice was performed as follows. Total DNA was extracted from a previously inoculated leaf, excluding 5-mm portions on either side of the inoculation point. The disease severity was evaluated by the amounts of R. solani ITS3 of rDNA relative to rice Rubq1 (Os06g0681400) using qRT-PCR. The same primers were used for two different R. solani strains. The primer sequences are listed in Supplementary Table S2.
Assay for resistance to R. solani in Arabidopsis
Arabidopsis plants were grown under aseptic short-day conditions for 6 weeks. Three-day-old R. solani (MAFF243956) on PDA medium (50 mL) was crushed using a mortar and pestle and mixed with an equal volume of sterile water (50 mL). A drop (5 μL) of the mycelial agar liquid suspension was applied to each of the middle rosette leaves of the Arabidopsis plants. The plants were then incubated under humid short-day conditions at 22 °C and the disease lesion length was measured one week after inoculation.
Plasmid construction and rice transformation
For the BSR2 overexpression lines, the full-length AK072163 cDNA (kindly provided by the Rice Genome Resource Center, NARO, Japan) was inserted into the SfiI site of the pRiceFox vector55 between the maize (Zea mays) ubiquitin promoter and the nopaline synthase terminator. For the BSR2 knockdown lines, a PCR product that was designed to trigger RNAi was first subcloned into the pENTR/D-TOPO entry vector (Invitrogen). The PCR product was then amplified using the following primers: 5′-GTGGGACTAAGACGAGGAGA-3′ and 5′-GAGCTATTCTACACTCATCA-3′. The fragment was used to make an inverted repeat construct in the pANDA destination vector through a LR clonase reaction by the Gateway system56,57. To generate overexpression and knockdown rice plants, the constructed vectors were transformed into rice using Agrobacterium-mediated method58. The T0 transformant plants and their progeny were used for morphological observations and to test R. solani resistance.
Detached leaf inoculation assay for R. solani in rice
The detached leaf inoculation assay using R. solani was performed as follows. Leaf blades were detached from rice plants at the tillering or heading stages. Three-day-old R. solani (MAFF243956 or Shimomura 9 isolate) growing on PDA medium (50 mL) was crushed using a mortar and pestle and mixed with sterile water (25 or 50 mL). Detached leaves were placed on a wet paper towel in Petri dishes, and 30 μL of the mycelial agar liquid suspension was drop-inoculated onto the middle parts of the leaves. Inoculated leaves were incubated in humid long-day conditions at 25 °C and after 7–13 d disease development was evaluated either by measuring the lesion length or by quantifying the relative fungal growth using qRT-PCR.
RNA extraction and quantitative real-time RT-PCR analysis
Total RNA was extracted and purified from rice leaves as described previously25, or by using Sepasol-RNA Super G reagent (Nacalai Tesque, Kyoto, Japan) according to the manufacturers protocol. Total RNA was extracted from Arabidopsis leaves using an RNeasy mini kit (Qiagen, Valencia, CA, USA). First-strand cDNAs were synthesized from equal amounts of total RNA in 10 or 20 μL using a PrimeScript II first-strand cDNA synthesis kit (Takara, Tokyo, Japan) or a ReverTra Ace qPCR RT Master Mix with gDNA Remover Kit (Toyobo, Osaka, Japan), according to the manufacturers’ protocols. Thermal Cycler Dice TP800 system (Takara) and a Kapa SYBR FAST qPCR kit (Kapa Biosystems, Cape Town, South Africa) were used for the qRT-PCR, as described by the manufacturer. The list of primers used for qRT-PCR are included in Supplementary Table S2. The transcript levels of each gene were normalized to an endogenous rice reference gene, Rubq159. Actin2 was used as the reference gene in Arabidopsis and for quantifying the relative fungal growth (Supplementary Table S2). The relative expression level of each gene was calculated using the 2−ΔΔCt expression ratio, which corrects for gene-specific PCR amplification efficiencies60.
Sequence alignment and phylogenetic analysis
The sequence alignment and phylogenetic analysis were performed as described in Supplementary Methods.
R. solani sheath inoculation assay in rice
The R. solani sheath inoculation assay was performed in rice as described in Supplementary Methods.
Phytohormone measurements
Phytohormones were measured as described in Supplementary Methods.
Supplementary information
Acknowledgements
This work was supported by a Special Coordination Fund for Promoting Science and Technology (Japan Science and Technology Agency), by a grant for Genomics-based Technology for Agricultural Improvement (Grant number GMO-1006a; Ministry of Agriculture, Forestry, and Fisheries of Japan) and by a grant for Research Program on Development of Innovative Technology (NARO Bio-oriented Technology Research Advancement Institution). We thank Dr. Yuji Kamiya and Yumiko Takebayashi (RIKEN, Japan) for the phytohormone analysis. We thank Dr. Brian J. Staskawicz (UC Berkeley, USA) for providing the Pst DC3000, Dr. Toshiyuki Morikawa (Toyama Prefectural Agricultural, Forestry and Fisheries Research Center, Japan) for providing Shimomura 9 isolate of R. solani and Dr. Barbara N. Kunkel (Washington University, USA) for donating the cpr5-2 Arabidopsis mutant. We thank late Dr. Ko Shimamoto (Nara Institute of Science and Technology, Japan) for providing pANDA vector. We thank Dr. Takahito Nomura (Utsunomiya University, Japan) for kind suggestions for phytohoromone analysis. We also thank Emi Mizutani (NIAS, Japan) for plasmid construction for RNAi, Miki Ohtake, Lois Ishizaki, Tomiko Senba and Chiyoko Umeda (NIAS, Japan) for their support in Arabidopsis and rice transformation and for overall technical assistance. We also thank Shelley Robison, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author Contributions
M.M.o. conceived and together with K.O., M.M.a. and H.H. designed the experiments. S.M., J.G.D., Y.K., Y.J. and S.S. performed the experiments. K.O. and M.M.a. contributed materials. S.M., Y.J., S.S. and M.M.o. analyzed the data. S.M., J.G.D. and M.M.o. wrote the paper.
Data Availability
All data generated or analysed during this study are included in this article and its Supplementary Information files.
Competing Interests
K.O., M.M.a., H.H. and M.M.o. were funded by Japan Science and Technology Agency. M.M.o. was funded by the Ministry of Agriculture, Forestry, and Fisheries of Japan and has been funded by NARO Bio-oriented Technology Research Advancement Institution. S.M., J.G.D., Y.K., Y.J. and S.S. declare no potential conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37365-1.
References
- 1.Hane JK, Anderson JP, Williams AH, Sperschneider J, Singh KB. Genome sequencing and comparative genomics of the broad host-range pathogen Rhizoctonia solani AG8. PLoS Genet. 2014;10:e1004281. doi: 10.1371/journal.pgen.1004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budge GE, Shaw MW, Colyer A, Pietravalle S, Boonham N. Molecular tools to investigate Rhizoctonia solani distribution in soil. Plant Pathol. 2009;58:1071–1080. doi: 10.1111/j.1365-3059.2009.02139.x. [DOI] [Google Scholar]
- 3.Carling DE, Baird RE, Gitaitis RD, Brainard KA, Kuninaga S. Characterization of AG-13, a Newly Reported Anastomosis Group of Rhizoctonia solani. Phytopathology. 2002;92:893–899. doi: 10.1094/PHYTO.2002.92.8.893. [DOI] [PubMed] [Google Scholar]
- 4.Yang, G. & Li, C. General Description of Rhizoctonia Species Complex. Plant Pathology 41–52 (2012).
- 5.Liu Y, et al. Ethylene Signaling Is Important for Isoflavonoid-Mediated Resistance to Rhizoctonia solani in Roots of Medicago truncatula. Mol Plant Microbe Interact. 2017;30:691–700. doi: 10.1094/MPMI-03-17-0057-R. [DOI] [PubMed] [Google Scholar]
- 6.Helliwell EE, Wang Q, Yang Y. Transgenic rice with inducible ethylene production exhibits broad-spectrum disease resistance to the fungal pathogens Magnaporthe oryzae and Rhizoctonia solani. Plant Biotechnol J. 2013;11:33–42. doi: 10.1111/pbi.12004. [DOI] [PubMed] [Google Scholar]
- 7.Li N, et al. Overexpression of Os2H16 enhances resistance to phytopathogens and tolerance to drought stress in rice. Plant Cell, Tissue and Organ Culture (PCTOC) 2013;115:429–441. doi: 10.1007/s11240-013-0374-3. [DOI] [Google Scholar]
- 8.Peng X, et al. OsWRKY80-OsWRKY4 Module as a Positive Regulatory Circuit in Rice Resistance Against Rhizoctonia solani. Rice (N Y) 2016;9:63. doi: 10.1186/s12284-016-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng X, et al. Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Planta. 2012;236:1485–1498. doi: 10.1007/s00425-012-1698-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, et al. Rice WRKY4 acts as a transcriptional activator mediating defense responses toward Rhizoctonia solani, the causing agent of rice sheath blight. Plant Mol Biol. 2015;89:157–171. doi: 10.1007/s11103-015-0360-8. [DOI] [PubMed] [Google Scholar]
- 11.Li N, et al. OsASR2 regulates the expression of a defence-related gene, Os2H16, by targeting the GT-1 cis-element. Plant Biotechnol J. 2018;16:771–783. doi: 10.1111/pbi.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirochika H, et al. Rice mutant resources for gene discovery. Plant Mol Biol. 2004;54:325–334. doi: 10.1023/B:PLAN.0000036368.74758.66. [DOI] [PubMed] [Google Scholar]
- 13.Abe K, Ichikawa H. Gene Overexpression Resources in Cereals for Functional Genomics and Discovery of Useful Genes. Front Plant Sci. 2016;7:1359. doi: 10.3389/fpls.2016.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IRGSP The map-based sequence of the rice genome. Nature436, 793–800 (2005). [DOI] [PubMed]
- 15.Ichikawa T, et al. The FOX hunting system: an alternative gain-of-function gene hunting technique. Plant J. 2006;48:974–985. doi: 10.1111/j.1365-313X.2006.02924.x. [DOI] [PubMed] [Google Scholar]
- 16.Kondou Y, et al. Systematic approaches to using the FOX hunting system to identify useful rice genes. Plant J. 2009;57:883–894. doi: 10.1111/j.1365-313X.2008.03733.x. [DOI] [PubMed] [Google Scholar]
- 17.Yokotani N, et al. Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta. 2008;227:957–967. doi: 10.1007/s00425-007-0670-4. [DOI] [PubMed] [Google Scholar]
- 18.Yokotani N, et al. Overexpression of a rice gene encoding a small C2 domain protein OsSMCP1 increases tolerance to abiotic and biotic stresses in transgenic Arabidopsis. Plant Mol Biol. 2009;71:391–402. doi: 10.1007/s11103-009-9530-x. [DOI] [PubMed] [Google Scholar]
- 19.Yokotani N, et al. Tolerance to various environmental stresses conferred by the salt-responsive rice gene ONAC063 in transgenic Arabidopsis. Planta. 2009;229:1065–1075. doi: 10.1007/s00425-009-0895-5. [DOI] [PubMed] [Google Scholar]
- 20.Yokotani N, et al. A novel chloroplast protein, CEST induces tolerance to multiple environmental stresses and reduces photooxidative damage in transgenic Arabidopsis. J Exp Bot. 2011;62:557–569. doi: 10.1093/jxb/erq290. [DOI] [PubMed] [Google Scholar]
- 21.Yokotani N, et al. Role of the rice transcription factor JAmyb in abiotic stress response. J Plant Res. 2013;126:131–139. doi: 10.1007/s10265-012-0501-y. [DOI] [PubMed] [Google Scholar]
- 22.Albinsky D, et al. Metabolomic screening applied to rice FOX Arabidopsis lines leads to the identification of a gene-changing nitrogen metabolism. Mol Plant. 2010;3:125–142. doi: 10.1093/mp/ssp069. [DOI] [PubMed] [Google Scholar]
- 23.Dubouzet JG, et al. Screening for resistance against Pseudomonas syringae in rice-FOX Arabidopsis lines identified a putative receptor-like cytoplasmic kinase gene that confers resistance to major bacterial and fungal pathogens in Arabidopsis and rice. Plant Biotechnol J. 2011;9:466–485. doi: 10.1111/j.1467-7652.2010.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higuchi-Takeuchi M, et al. Functional analysis of two isoforms of leaf-type ferredoxin-NADP(+)-oxidoreductase in rice using the heterologous expression system of Arabidopsis. Plant Physiol. 2011;157:96–108. doi: 10.1104/pp.111.181248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda S, Hayashi N, Sasaya T, Mori M. Overexpression of BSR1 confers broad-spectrum resistance against two bacterial diseases and two major fungal diseases in rice. Breed Sci. 2016;66:396–406. doi: 10.1270/jsbbs.15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson DR, Schuler MA, Paquette SM, Werck-Reichhart D, Bak S. Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol. 2004;135:756–772. doi: 10.1104/pp.104.039826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson DR. The cytochrome p450 homepage. Human genomics. 2009;4:59–65. doi: 10.1186/1479-7364-4-1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson D, Werck-Reichhart D. A P450-centric view of plant evolution. Plant J. 2011;66:194–211. doi: 10.1111/j.1365-313X.2011.04529.x. [DOI] [PubMed] [Google Scholar]
- 29.Sato Y, et al. RiceXPro version 3.0: expanding the informatics resource for rice transcriptome. Nucleic Acids Res. 2013;41:D1206–1213. doi: 10.1093/nar/gks1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molla KA, et al. Tissue-specific expression of Arabidopsis NPR1 gene in rice for sheath blight resistance without compromising phenotypic cost. Plant Sci. 2016;250:105–114. doi: 10.1016/j.plantsci.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Kouzai Y, et al. Salicylic acid-dependent immunity contributes to resistance against Rhizoctonia solani, a necrotrophic fungal agent of sheath blight, in rice and Brachypodium distachyon. New Phytol. 2018;217:771–783. doi: 10.1111/nph.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou N, Tootle TL, Glazebrook J. Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell. 1999;11:2419–2428. doi: 10.1105/tpc.11.12.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swaminathan S, Morrone D, Wang Q, Fulton DB, Peters RJ. CYP76M7 is an ent-cassadiene C11alpha-hydroxylase defining a second multifunctional diterpenoid biosynthetic gene cluster in rice. Plant Cell. 2009;21:3315–3325. doi: 10.1105/tpc.108.063677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajniak J, Barco B, Clay NK, Sattely ES. A new cyanogenic metabolite in Arabidopsis required for inducible pathogen defence. Nature. 2015;525:376–379. doi: 10.1038/nature14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zondlo SC, Irish VF. CYP78A5 encodes a cytochrome P450 that marks the shoot apical meristem boundary in Arabidopsis. Plant J. 1999;19:259–268. doi: 10.1046/j.1365-313X.1999.00523.x. [DOI] [PubMed] [Google Scholar]
- 36.Anastasiou E, et al. Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Dev Cell. 2007;13:843–856. doi: 10.1016/j.devcel.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Adamski NM, Anastasiou E, Eriksson S, O’Neill CM, Lenhard M. Local maternal control of seed size by KLUH/CYP78A5-dependent growth signaling. Proc Natl Acad Sci USA. 2009;106:20115–20120. doi: 10.1073/pnas.0907024106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito T, Meyerowitz EM. Overexpression of a gene encoding a cytochrome P450, CYP78A9, induces large and seedless fruit in arabidopsis. Plant Cell. 2000;12:1541–1550. doi: 10.1105/tpc.12.9.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sotelo-Silveira M, et al. Cytochrome P450 CYP78A9 is involved in Arabidopsis reproductive development. Plant Physiol. 2013;162:779–799. doi: 10.1104/pp.113.218214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang W, Wang Z, Cui R, Li J, Li Y. Maternal control of seed size by EOD3/CYP78A6 in Arabidopsis thaliana. Plant J. 2012;70:929–939. doi: 10.1111/j.1365-313X.2012.04907.x. [DOI] [PubMed] [Google Scholar]
- 41.Nagasawa N, et al. GIANT EMBRYO encodes CYP78A13, required for proper size balance between embryo and endosperm in rice. Plant J. 2013;75:592–605. doi: 10.1111/tpj.12223. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, et al. Control of rice embryo development, shoot apical meristem maintenance, and grain yield by a novel cytochrome p450. Mol Plant. 2013;6:1945–1960. doi: 10.1093/mp/sst107. [DOI] [PubMed] [Google Scholar]
- 43.Xu F, et al. Variations in CYP78A13 coding region influence grain size and yield in rice. Plant Cell Environ. 2015;38:800–811. doi: 10.1111/pce.12452. [DOI] [PubMed] [Google Scholar]
- 44.Chakrabarti M, et al. A cytochrome P450 regulates a domestication trait in cultivated tomato. Proc Natl Acad Sci USA. 2013;110:17125–17130. doi: 10.1073/pnas.1307313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, et al. Evolution and association analysis of GmCYP78A10 gene with seed size/weight and pod number in soybean. Mol Biol Rep. 2015;42:489–496. doi: 10.1007/s11033-014-3792-3. [DOI] [PubMed] [Google Scholar]
- 46.Zhao B, et al. Arabidopsis KLU homologue GmCYP78A72 regulates seed size in soybean. Plant Mol Biol. 2016;90:33–47. doi: 10.1007/s11103-015-0392-0. [DOI] [PubMed] [Google Scholar]
- 47.Ma M, et al. TaCYP78A5 regulates seed size in wheat (Triticum aestivum) J Exp Bot. 2016;67:1397–1410. doi: 10.1093/jxb/erv542. [DOI] [PubMed] [Google Scholar]
- 48.Miyoshi K, et al. PLASTOCHRON1, a timekeeper of leaf initiation in rice, encodes cytochrome P450. Proc Natl Acad Sci USA. 2004;101:875–880. doi: 10.1073/pnas.2636936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun X, et al. Altered expression of maize PLASTOCHRON1 enhances biomass and seed yield by extending cell division duration. Nat Commun. 2017;8:14752. doi: 10.1038/ncomms14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang JW, Schwab R, Czech B, Mica E, Weigel D. Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell. 2008;20:1231–1243. doi: 10.1105/tpc.108.058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imaishi H, Matsuo S, Swai E, Ohkawa H. CYP78A1 preferentially expressed in developing inflorescences of Zea mays encoded a cytochrome P450-dependent lauric acid 12-monooxygenase. Biosci Biotechnol Biochem. 2000;64:1696–1701. doi: 10.1271/bbb.64.1696. [DOI] [PubMed] [Google Scholar]
- 52.Kai K, et al. Metabolomics for the characterization of cytochromes P450-dependent fatty acid hydroxylation reactions in Arabidopsis. Plant Biotechnol-Nar. 2009;26:175–182. doi: 10.5511/plantbiotechnology.26.175. [DOI] [Google Scholar]
- 53.Goto S, et al. Development of disease-resistant rice by pathogen-responsive expression of WRKY45. Plant Biotechnol J. 2016;14:1127–1138. doi: 10.1111/pbi.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokotani N, et al. OsNAC111, a blast disease-responsive transcription factor in rice, positively regulates the expression of defense-related genes. Mol Plant Microbe Interact. 2014;27:1027–1034. doi: 10.1094/MPMI-03-14-0065-R. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura H, et al. A genome-wide gain-of function analysis of rice genes using the FOX-hunting system. Plant Mol Biol. 2007;65:357–371. doi: 10.1007/s11103-007-9243-y. [DOI] [PubMed] [Google Scholar]
- 56.Miki D, Shimamoto K. Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol. 2004;45:490–495. doi: 10.1093/pcp/pch048. [DOI] [PubMed] [Google Scholar]
- 57.Miki D, Itoh R, Shimamoto K. RNA silencing of single and multiple members in a gene family of rice. Plant Physiol. 2005;138:1903–1913. doi: 10.1104/pp.105.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toki S, et al. Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J. 2006;47:969–976. doi: 10.1111/j.1365-313X.2006.02836.x. [DOI] [PubMed] [Google Scholar]
- 59.Jiang CJ, et al. Abscisic acid interacts antagonistically with salicylic acid signaling pathway in rice-Magnaporthe grisea interaction. Mol Plant Microbe Interact. 2010;23:791–798. doi: 10.1094/MPMI-23-6-0791. [DOI] [PubMed] [Google Scholar]
- 60.Yuan JS, Reed A, Chen F, Stewart CN., Jr. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this article and its Supplementary Information files.