Abstract
Progesterone receptor membrane component1 (PGRMC1) is a heme-binding protein involved in cancers and Alzheimer’s disease. PGRMC1 consists of a short N-terminal extracellular or luminal domain, a single membrane-spanning domain, and a long cytoplasmic domain. Previously, we generated two monoclonal antibodies (MAbs) 108-B6 and 4A68 that recognize cell surface-expressed PGRMC1 (csPGRMC1) on human pluripotent stem cells and some cancer cells. In this study, flow cytometric analysis found that an anti-PGRMC1 antibody recognizing the N-terminus of PGRMC1 could not bind to csPGRMC1 on cancer cells, and 108-B6 and 4A68 binding to csPGRMC1 was inhibited by trypsin treatment, suggesting that the epitopes of 108-B6 and 4A68 are trypsin-sensitive. To examine the epitope specificity of 108-B6 and 4A68, glutathione-S-transferase (GST)-fused PGRMC1 mutants were screened to identify the epitopes targeted by the antibodies. The result showed that 108-B6 and 4A68 recognized C-terminal residues 183–195 and 171–182, respectively, of PGRMC1, where trypsin-sensitive sites are located. A polyclonal anti-PGRMC1 antibody raised against the C-terminus of PGRMC1 could also recognized csPGRMC1 in a trypsin-sensitive manner, suggesting that the C-terminus of csPGRMC1 is exposed on the cell surface. This finding reveals that csPGRMC1 has a non-conventional plasma membrane topology, which is different from that of intracellular PGRMC1.
Introduction
Progesterone receptor membrane component 1 (PGRMC1) is a multifunctional protein with a C-terminal cytochrome b5 domain1. PGRMC1 is highly expressed in multiple types of cancer, and represents a proliferation marker for various cancer cells1–4. PGRMC1 also increases the neuronal toxicity of amyloid β-peptides through binding to amyloid β oligomer in Alzheimer’s disease5,6. PGRMC1 is also involved in diverse biological functions, such as regulation of cytochrome P450, progesterone signaling, vesicle trafficking, steroidogenesis, cell cycle regulation, anchorage-independent growth, invasive growth, angiogenesis, hypoxic biology, and autophagy promotion1,7. PGRMC1 consists of a short N-terminal luminal or extracellular domain, a single membrane-spanning domain, and a long cytoplasmic domain8–11. Many studies have shown that PGRMC1 is localized at various subcellular compartments, such as endoplasmic reticulum, Golgi apparatus, plasma membrane, inner acrosomal membrane, nucleus, nucleolus, and mitochondria9,12–15. PGRMC1 regulates cell proliferation and apoptosis in granulosa and luteal cells via interaction between its cytoplasmic cytochrome b5 binding domain (amino acids 70–130) and plasminogen activator inhibitor RNA-binding protein-1 (PAIR-BP1)10,16. PGRMC1 can also interact or associate with other binding partners including epidermal growth factor receptor (EGFR)17, glucagon-like peptide-1 receptor (GLP-1R)18, insulin receptor19, glucose channels19, membrane progesterone receptor (mPRα/PAQR7)20, and P450 proteins21,22. A recent study revealed that the heme-mediated dimerization of adjacent PGRMC1 monomers in the cytoplasmic side leads PGRMC1 to interact with cytochromes P450 and EGFR, causing enhanced proliferation, anti-apoptosis, and chemoresistance of cancer cells22. However, the conclusion is challenged with some observations and thoughts that tyrosine 113 phosphorylation of PGRMC1 is required for membrane trafficking to co-localize PGRMC1 and EGFR23, and the cytoplasmic domain of PGRMC1 is located on the luminal side of microsomes in A549 cells17.
Previously, we generated a panel of murine monoclonal antibodies (MAbs) against the surface molecules on undifferentiated human pluripotent stem cells (hPSCs) by using a modified decoy immunization strategy24. Subsequent studies showed that 108-B6 and 4A68, two of the MAbs, bind to cell surface expressed-PGRMC1 (csPGRMC1) on hPSCs and some cancer cells25. PGRMC1 knockdown approach further revealed that PGRMC1 suppresses the p53 and Wnt/β-catenin pathways to promote hPSC self-renewal25. Meanwhile, flow cytometric analysis found that an anti-PGRMC1 antibody recognizing the N-terminal domain (residues 1–46) of PGRMC1 was not able to bind to csPGRMC1 on cancer cells, although it was able to recognize intracellular PGRMC1 in saponin-treated cells. Flow cytometric analysis also showed that 108-B6 and 4A68 binding to csPGRMC1 was inhibited by trypsin treatment, suggesting that the epitopes of 108-B6 and 4A68 is outside the N-terminal domain and have trypsin-sensitive sites within them. This observation led us to investigate the epitope of two MAbs on PGRMC1. The results revealed that 108-B6 recognized C-terminal residues 183–195 of PGRMC1, and 4A68 recognized C-terminal residues 171–182 of PGRMC1, where putative trypsin-sensitive sites are located. Thus, this finding reveals that the C-terminal domain of PGRMC1 is exposed on the cell surface, instead of the N-terminal domain of PGRMC1. A polyclonal anti-PGRMC1 raised against the C-terminal domain of PGRMC1 also recognized csPGRMC1, supporting that the C-terminal domain of PGRMC1 is exposed on the cell surface. Thus, epitope analysis of PGRMC1 antibodies reveals that csPGRMC1 has a different membrane topology from that of intracellular PGRMC1.
Results
Monoclonal antibody against the N-terminus of PGRMC1 is not able to recognize csPGRMC1, whereas 108-B6 and 4A68 is able to recognize csPGRMC1
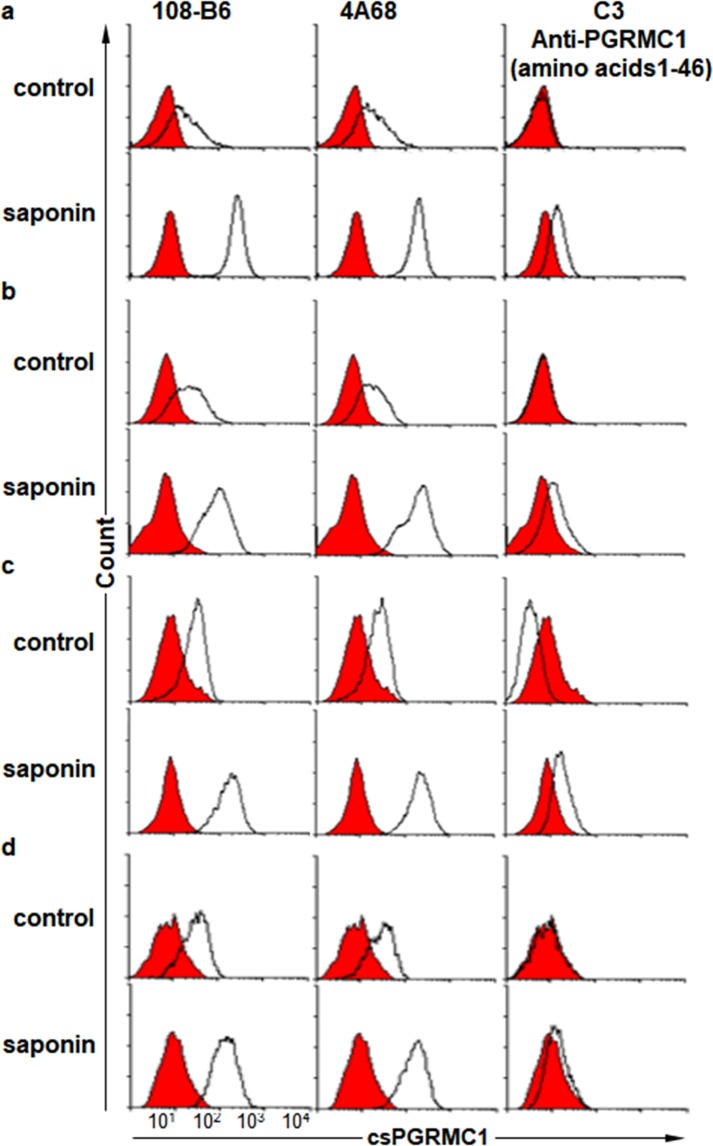
In the previous study, we generated two MAbs, 108-B6 and 4A68, against cell surface molecules on hPSCs, and found that both MAbs recognize csPGRMC1 on hPSCs and some cancer cells24,25. Many previous studies have shown that PGRMC1 has a short N-terminal luminal or extracellular domain (residues 1–20), a single membrane-spanning domain (residues 21–42), and a much longer cytoplasmic domain (residues 43–195)8–11,26. Therefore, we expected that 108-B6 and 4A68 recognized epitopes on the short N-terminal extracellular domain because they were able to recognize csPGRMC1. However, a commercially available antibody (C3) raised against the N-terminal domain (amino acids 1–46) of PGRMC1 was not able to recognize csPGRMC1 at all on the surface of H9, NT-2/D1, HEK293T and HepG2 cells while 108-B6 and 4A68 were able to recognize csPGRMC1 on the cell surface of all cells (Fig. 1a–d, upper panels). When PGRMC1 protein was immunoprecipitated with 108-B6 and 4A68, immnoprecipitated proteins were readily detected with C3, indicating that C3 is able to recognize 108-B6- and 4A68-reactive PGRMC1 protein in Western blot analysis (Supplementary Fig. 1). To further analyze whether the C3 antibody is functional in intracellular flow cytometric analysis, cells were analyzed in the presence of saponin detergent. The binding activity of 108-B6 and 4A68 was increased in all cells with saponin treatment, suggesting the increased accessibility of intracellular PGRMC1 (Fig. 1a–d, lower panels). The C3 antibody was also able to recognize intracellular PGRMC1 in the saponin-treated cells (Fig. 1a–d, lower panels). The results suggest that the C3 antibody is functional and is able to recognize the N-terminal domain of PGRMC1 when cells are permeabilized. Therefore, it is possible to speculate that the N-terminal domain of PGRMC1 may be located in the cytoplasmic side of H9, NT-2/D1, HEK293T and HepG2 cells.
Figure 1.
Monoclonal antibody C3 against the N-terminus of PGRMC1 is not able to recognize csPGRMC1, whereas 108-B6 and 4A68 is able to recognize csPGRMC1. (a–d) Flow cytometric analysis of H9 (a), NT-2/D1 (b), HEK293T (c), and HepG2 (d) with 108-B6, 4A68, and an anti-PGRMC1 antibody (C-3) in the absence (control) or presence of saponin (saponin). C3 is a monoclonal anti-PGRMC1 raised against the N-terminal residues 1–46. Red populations indicate fluorescence-conjugated secondary antibody staining as controls.
Trypsin treatment drastically abolishes binding reactivity of 108-B6 and 4A68 to csPGRMC1
Meanwhile, flow cytometric analysis showed that binding reactivity of 108-B6 and 4A68 to cancer cells was variable depending on detachment method. To figure out how the detachment method affected the binding reactivity of two MAbs to NT-2/D1 cells, cells were detached with trypsin or enzyme-free dissociation solution, and propidium iodide (PI)-negative live cells were subjected to flow cytometric analysis. Interestingly, trypsin treatment abolished the binding reactivity of two MAbs drastically, as compared with dissociation solution (Fig. 2a). The similar result was also observed with NCCIT cells, another human embryonal carcinoma cell line (Fig. 2b). The results suggest that the epitopes of 108-B6 and 4A68 is cell surface-exposed and trypsin-sensitive. Sequence analysis showed that there is no putative trypsin-sensitive site on the N-terminal domain of PGRMC1, further suggesting that the other domain of PGRMC1 is exposed on the extracellular side, instead of the N-terminal domain of PGRMC1.
Figure 2.
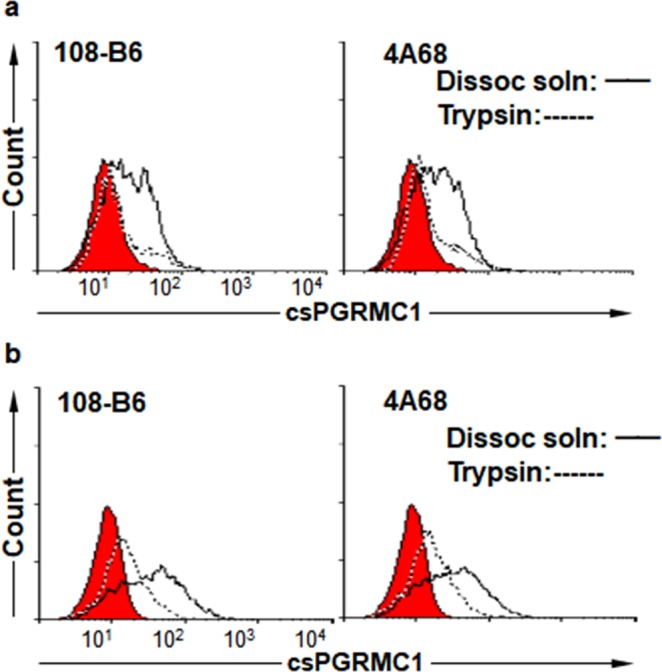
Trypsin treatment drastically abolishes binding reactivity of 108-B6 and 4A68 to csPGRMC1. (a) Flow cytometric analysis of NT-2/D1 cells with 108-B6 and 4A68 after detachment of cells with trypsin or enzyme-free dissociation solution. Red populations indicate fluorescence-conjugated secondary antibody staining as controls. (b) Flow cytometric analysis of NCCIT cells with 108-B6 and 4A68 after detachment of cells with trypsin or enzyme-free dissociation solution.
Comparison of amino acid sequences of 108-B6 and 4A68 variable regions
Although 108-B6 and 4A68 recognized the same csPGRMC1 protein, they showed slight different binding reactivity to U87-MG, NCI-H522 and A54925, suggesting that they recognize different binding epitopes on csPGRMC1 protein. The result also suggests that 108-B6 and 4A68 are different antibodies with different complementarity determining regions (CDRs). To compare amino acid sequence of 108-B6 and 4A68 variable regions, the heavy- and light-chain variable regions of two MAbs were cloned and sequenced by using universal degenerate primers27. The complementarity determining regions (CDRs) of 108-B6 and 4A68 were identified by using information of Abysis database (http://www.bioinf.org.uk). Although 108-B6 and 4A68 recognized the same PGRMC1 protein, sequence analysis showed that the CDRs of heavy and light chains of two MAbs were quite different to each other (Supplementary Fig. 2). Especially, the lengths and sequences of heavy chain CDR3s were completely different from each other, suggesting that they may recognize different epitopes on csPGRMC1.
Fine epitope mapping of 108-B6 and 4A68 antibodies
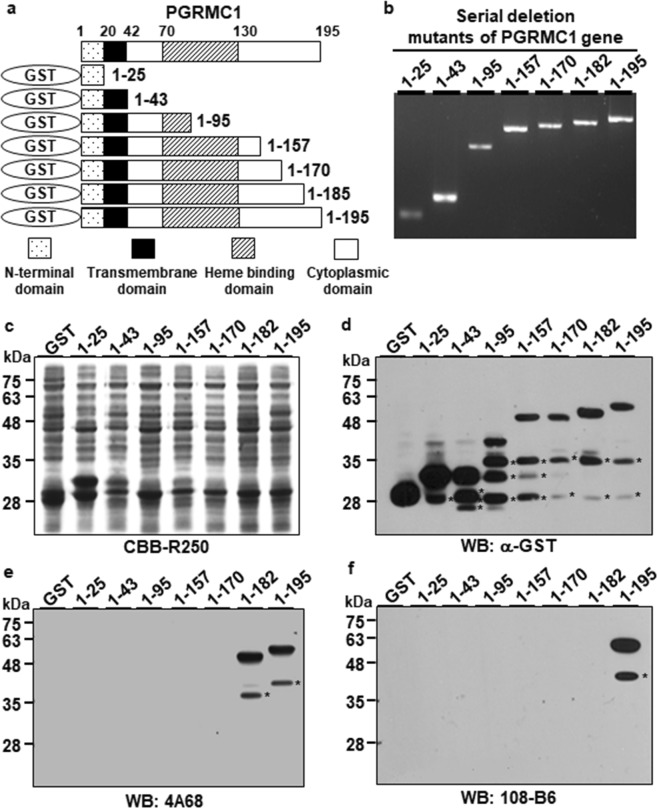
To examine the epitopes of 108-B6 and 4A68 on csPGRMC1 protein, a series of deletion mutants of PGRMC1 gene were generated as shown in Fig. 3a, and synthesized by PCR (Fig. 3b). Each cDNA was cloned into pGEX4T-2 cloning vector to tag glutathione-S-transferase (GST) gene and the fusion constructs were introduced into E. coli DH5α. The expression of a series of GST-fused PGRMC1 proteins were induced by isoprophyl-β-D-thiogalactopyranoside (IPTG) and judged by Coomassie Brilliant Blue (CBB) staining and Western blot analysis with anti-GST antibody, which showed the expression of expected sizes of GST-PGRMC1 fusion proteins, although the partially degraded forms of the serial deletion mutants of GST-PGRMC1 fusion proteins were also detected below the main GST-PGRMC1 fusion proteins (Fig. 3c,d). The same lysates were then subjected to Western blot analysis with 4A68 and 108-B6 (Fig. 3e,f). 4A68 recognized the wild-type PGRMC1 (residues 1–195) and one of deletion mutants (residues 1–182) but did not recognize the other deletion mutants (residues 1–25, 1–43, 1–95, 1–157 and 1–170), indicating that 4A68 recognizes the linear epitopes located between residues 171–182 of PGRMC1. 108-B6 recognized only the wild-type PGRMC1 (residues 1–195), but did not recognize any other deletion mutants (residues 1–25, 1–43, 1–95, 1–157, 1–170 and 1–182), indicating that 108-B6 recognizes the linear epitopes located between residues 183–195 of PGRMC1. The results suggest that the antigen binding sites of 4A68 and 108-B6 at least require residues 171–182 and 183–195, respectively, of PGRMC1 protein, although it could not exclude other residues from the body of the folded PGRMC1 protein. Generally, antibodies only access and recognize cell surface-exposed epitopes on live cells. Therefore, the results suggest that the epitope regions of 108-B6 and 4A68 are exposed on the cell surface.
Figure 3.
Fine epitope mapping of 108-B6 and 4A68 antibodies. (a) Schematic diagram of recombinant PGRMC1 fragments (residues 1–25, 1–43, 1–95, 1–157, 1–170, 1–182 and 1–195) used in this study. (b) A series of depletion mutants of PGRMC1 gene were synthesized and separated by agarose gel electrophoresis. The deletion mutants of PGRMC1 genes were detected by ethidium bromide staining. (c) Individual fusion proteins were expressed in E. coli DH5α as fusion proteins with GST tag at the N-terminus, and stained with CBB R-250 after SDS-PAGE. (d,f) Western blot analysis of GST-PGRMC1 fusion proteins with anti-GST (d), 4A68 (e), and 108-B6 (f) antibodies. The asterisks indicate partial degradation products of GST-PGRMC1 fusion proteins.
Polyclonal antibody against the C-terminus of PGRMC1 is able to recognize csPGRMC1
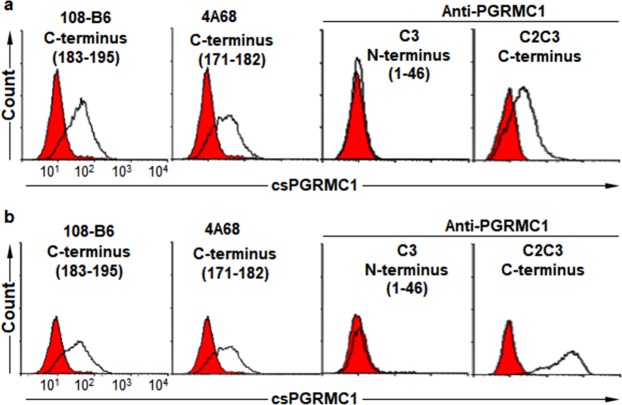
Residues 171–182 and 183–195, recognized by 4A68 and 108-B6, respectively, belong to the last C-terminal part of PGRMC1. Therefore, the present results strongly suggest that the C-terminal domain of PGRMC1 is exposed on the extracellular side, although previous studies have shown that an N-terminal domain of PGRMC1 is exposed on the cell surface8–11. Therefore, another commercially available polyclonal anti-PGRMC1 antibody (C2C3) raised against the C-terminal domain of PGRMC1 was also included in flow cytometric analysis. As expected, C2C3 was able to recognize csPGRMC1 on NT-2/D1 and H9 hPSCs while C3 was not able to recognize csPGRMC1 (Fig. 4). The same results were also obtained with A549 cells (Supplementary Fig. 3). Trypsin treatment decreased C2C3 binding to csPGRMC1 on A549 cells as well, suggesting that the epitope of C2C3 also contains trypsin-sensitive sites as the epitopes of 4A68 and 108-B6. Taken together, the results suggest again that the C-terminal domain of PGRMC1 is exposed on the cell surface, instead of the N-terminal domain of PGRMC1.
Figure 4.
Polyclonal antibody against the C-terminus of PGRMC1 is able to recognize csPGRMC1. Flow cytometric analysis of NT-2/D1 and H9 hPSCs with 108-B6, 4A68, and anti-PGRMC1 antibodies (C3 and C2C3) after detachment of NT-2/D1 (a) and H9 hPSCs (b) with dissociation solution. C2C3 is a rabbit polyclonal anti-PGRMC1 raised against the C-terminal region of PGRMC1. Red populations indicate fluorescence-conjugated secondary antibody staining as controls.
Discussion
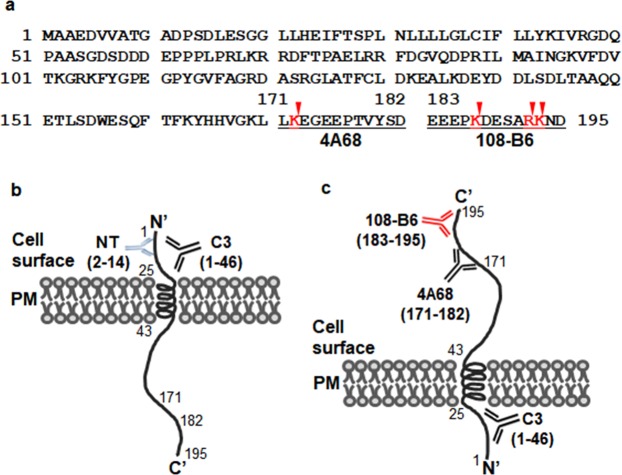
The present study found that two MAbs, 108-B6 and 4A68, recognized csPGRMC1 (Fig. 1), and their epitopes were located between residues 183–195, 171–182, respectively, of PGRMC1 (Fig. 3d–f). Generally, antibodies can access and recognize cell surface exposed-epitopes, and the residues 171–195 are located in the last C-terminal domain of PGRMC1. Therefore, the present results suggest that the C-terminal domain of csPGRMC1 is exposed on the cell surface. csPGRMC1 was also recognized by C2C3, a commercially available polyclonal anti-PGRMC1 antibody raised against the C-terminal domain of PGRMC1 (Fig. 4 and Supplementary Fig. 3). Furthermore, C3, another monoclonal anti-PGRMC1 antibody generated against the N-terminal domain (residues 1–46) of PGRMC1, was not able to detect csPGRMC1, although it was able to recognize the N-terminal domain of PGRMC1 after cell permeabilization with saponin (Fig. 1a–d). The results suggest that the C-terminal domain of PGRMC1 is exposed on the cell surface, but the N-terminal domain of PGRMC1 is not exposed on the cell surface. The binding reactivity of 108-B6 and 4A68 to NT-2/D1, NCCIT, and A549 cells was decreased with trypsin treatment. Sequence analysis revealed that trypsin-sensitive sites are present in the epitopes of 108-B6 and 4A68 (just behind residues 172, 187, 192 and 193) (Fig. 5a). Therefore, it is highly likely that decreased reactivity of 108-B6, 4A68 and C2C3 to csPGRMC1 on trypsin-treated cells is due to the cleavages of their epitopes by trypsin. Thus, the results suggest that the C-terminal domain (residues 171–195) of csPGRMC1 is at least exposed on the extracellular side. However, it has been known that PGRMC1 consists of a short N-terminal extracellular domain, a single membrane-spanning domain, and a long cytoplasmic domain (Fig. 5b)8–11. Therefore, characterizing the epitopes of 108-B6, 4A68 and C3 antibodies revealed the presence of the non-conventional reverse topology of csPGRMC1 on the surface of hPSCs and some cancer cells. A proposed model for the non-conventional plasma membrane topology of csPGRMC1 is therefore presented (Fig. 5c).
Figure 5.
Proposed model for the plasma membrane topology of csPGRMC1. (a) Amino acid sequences of PGRMC1. The epitopes determined in the present study are underlined. Putative trypsin sensitive sites on the epitopes are marked by arrow heads. (b) The membrane topology of csPGRMC1 based on the previous studies8–11,26. NT and C3 represent antibodies raised against the N-terminal domain of PGRMC1. (c) Newly proposed plasma membrane topology of csPGRMC1 based on the present study. The antigen binding sites of 4A68 and 108-B6 at least require residues 171–182 and 183–195, respectively, of PGRMC1 protein, although it could not exclude other residues from the body of the folded PGRMC1 protein.
PGRMC1 is mainly localized in the endoplasmic reticulum, mitochondria, nucleus membrane, and nucleolus in multiple cancer cells1,11. Some studies have also shown that PGRMC1 is expressed on the surface of cancer and neuronal cells5,17,28–30. PGMRC1 consists of a short N-terminal extracellular domain, a single membrane-spanning domain, and a much longer cytoplasmic domain8–11,26,31 (Fig. 5b). We found that 108-B6- and 4A68-reactive csPGRMC1 was expressed on hPSCs and a few cancer cell lines25. In the very beginning, we expected that 108-B6 and 4A68 recognized the short N-terminal extracellular domain of PGRMC1 in hPSCs and cancer cells, because the cytoplasmic side of membrane proteins remains on the cytoplasmic side while the luminal side of membrane proteins is exposed on the cell surface even during the process of cell surface translocation32,33. Contrary to our expectation, epitope mapping of 108-B6 and 4A68 reveals that the C-terminal domain of PGMRC1 is exposed on the cell surface, suggesting an unexpected membrane topology of PGRMC1 on the cell surface. A previous study demonstrated that PGRMC1 is not affected by proteinase K digestion of a microsomal fraction without detergent in A549 cells17, suggesting a luminal orientation for the cytoplasmic domain of PGRMC1 in A549 cells. The result also suggests that the C-terminal domain of PGRMC1 would be exposed on the cell surface of A549 cells during the process of cell surface translocation. In this study, we showed that the C-terminal domain of PGRMC1 is exposed on the cell surface of A549 cells (Supplementary Fig. 3), which is consistent with the previous prediction17. The other studies have also observed extracellular PGRMC1 on the surface of neurons5,34. The studies also suggest that the C-terminus of PGRMC1 is located extracellularly in neurons, which is consistent with the present finding.
The present study suggests the existence of the opposite membrane topology of PGRMC1 on the cell surface. Based on the present study, however, it could not exclude the possibility that both the N- and C-terminus of PGRMC1 could be simultaneously present on the extracellular surface, where the extracellular N-terminus could interact with a natural extracellular ligand and be inaccessible for C3 binding, while the C-terminus could interact with 108-B6, 4A68 and C2C3. The present findings could also be harmonized by the multiple topologies of PGRMC1. Multiple or dynamic topologies are found in some membrane proteins, such as ductin, cystic fibrosis transmembrane conductance regulator, aquaporin-1 and P-glycoprotein35–41. Actually, Cahill and Medlock also suggested the possibility of alternative post-translational topologies of PGRMC111. However, the possibility of multiple topologies of csPGRMC1 seems to be low because C3 recognizing the N-terminal domain of PGRMC1 was not able to recognize csPGRMC1 on hPSCs and some cancer cells (Figs 1 and 4). As described in many literatures1,11,42,43, the major function of PGRMC1 is based on the interaction between the cytoplasmic C-terminal domain of PGRMC1 and intracellular factors. Therefore, the biological significance of the opposite membrane topology of PGRMC1 will be the next interesting research subject.
Methods
Cell Culture
H9 hPSCs were cultured on the irradiated mouse embryonic fibroblast (MEF) feeder cells in DMEM/F12 medium (WelGene, Daegu, Korea), supplemented with 20% serum replacement (Invitrogen, Seoul, Korea), 0.1 mM 2-mercaptoethanol, 1% non-essential amino acids, 32 mM sodium bicarbonate, and 4 ng/ml basic fibroblast growth factor (bFGF) (PeproTech, Rocky Hill, NJ)24,44. Human embryonal carcinoma cell lines NT-2/D1 and NCCIT were cultured according to the instructions provided by American Type Culture Collection (ATCC, Manassas, VA). The non-small cell lung carcinoma cell line A549 was obtained from ATCC and maintained according to the protocol provided by the supplier. HepG2 was purchased from Korean Cell Line Bank (Seoul, Korea). Hybridomas 108-B6 and 4A68 were cultured at 5% CO2, 37 °C in DMEM (WelGene) supplemented with 10% fetal bovine serum (WelGene).
Antibody Purification
MAbs were purified from the culture supernatant of hybridomas by Protein G-Agarose column chromatography as described previously24,45.
Flow cytometry
H9, NT-2/D1, HEK293T, HepG2, NCCIT and A549 cells were harvested as single cell suspensions using trypsin/EDTA (Welgene, Daegu, Korea) solution or enzyme-free dissociation solution (Millipore, Billerica, MA). Detached cells were immediately resuspended in PBA (1% bovine serum albumin, 0.02% NaN3 in phosphate buffered saline (PBS), pH7.4) and incubated for 20 min at 4 °C with 108-B6, 4A68, rabbit polyclonal-anti-PGRMC1 antibody (C2C3, GeneTex, Irvine CA), and mouse monoclonal anti-PGRMC1 (C3, Santa Cruz Biotechnologies, Santa Cruz, CA). The cells were then further incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG or FITC-conjugated anti-rabbit IgG (BD Biosciences, Seoul, Korea). After washing, propidium iodide (PI)-negative live cells were analyzed for antibody binding using FACS Calibur and Cell Quest software (BD Biosciences). For intracellular flow cytometric analysis, cells were fixed in 2% paraformaldehyde (PFA) in PBS (pH 7.4), permeabilized in 0.5% saponin (Sigma-Aldrich, Seoul, Korea) in PBA for 15 min at 4 °C, and then washed twice with PBA. The cells were incubated with 108-B6, 4A68 or C-3 antibodies for 15 min at 4 °C, subsequently incubated with FITC-conjugated mouse IgG (BD Biosciences) for 15 min at 4 °C, and analyzed for the antibody binding using FACS Calibur (BD Biosciences) and Cell Quest software (BD Biosciences).
Western blot analysis
Total extracts of various cells were obtained after lysis for 30 min at 4 °C in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% Doxycholic acid, 0.1% SDS, 50 mM Tris-HCl (pH 7.4)). Protein samples were fractionated by sodium dodecyl-sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% polyacrylamide gel under denaturing conditions and transferred to a nitrocellulose membrane. The membrane was blocked with 5% skim milk in PBST (PBS containing 0.1% Tween 20) at room temperature (RT) for 2 hrs. After washing with PBST, the membrane was incubated with various primary antibodies at RT for 1 hr, followed by horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Millipore). The stained bands were visualized by using ECL (Animal Genetics, Gyeonggi-do, Korea) detection reagent.
cDNA cloning and sequencing
Total RNAs were extracted from 108-B6 and 4A68 hybridoma cells with RNA iso plus reagent (TaKaRa, Otsu, Japan) according to the supplier’s protocol. cDNAs were generated from total RNAs by Prime Script RT Master Mix (TaKaRa), and used for polymerase chain reaction (PCR) amplification to obtain the coding regions of variable heavy and light chains of two MAbs by standard reverse transcriptase (RT)-PCR using specific primers as described previously27. The amplified gene segments were subcloned into pBluescript cloning vector and used to transform DH5α bacterial cells. Selected plasmids were sequenced using the M13 primers (Cosmo Genetech, Seoul, Korea).
Preparation and induction of GST-fusion protein
Serially truncated PGRMC1 proteins were expressed as fusion proteins with Glutathione-S-transferase (GST) proteins. The coding sequences of serially truncated and whole PGRMC1 genes were synthesized by PCR from the pCMV-SPORT6-PGRMC1 plasmid using 5′-primer and various 3′-primers and subcloned into the EcoRI/SalI sites of pGEX4T-2 (GE Healthcare, Seoul, Korea) to yield the expression plasmids. All primer sequences are listed in Table 1. Each expression plasmid was confirmed by DNA sequencing, and introduced into E. coli DH5α cells to express the GST-PGRMC1 fusion proteins. The expression of the fusion proteins was induced by 0.1 mM IPTG at 32 °C for 3 hrs. The induced bacterial cells were washed with pre-chilled PBS (pH 7.4), incubated with acetone on ice for 5 min, and lysed in 1% SDS supplemented with 100 μg/ml phenylmethanesulfonyl fluoride for 2 min at room temperature (RT). Proteins were clarified by centrifugation, and their concentration was measured by bicinchoninic assay (Thermo Scientific, Seoul, Korea). The cell lysates were subjected to 12% SDS-PAGE, stained with CBB R-250, and analyzed by western blot analysis as described above.
Table 1.
Primer sequences for serial deletion mutants of PGRMC1.
| PGRMC1 cDNA | Sequence (5′– 3′) |
|---|---|
| PGRMC1 sense | 5′- CTCGAATTCTCATGGCTGCCGAG-3′ |
| (1–25) antisense | 5′-CGGCTCGAGTTAAATCTCATGCAGCAG-3′ |
| (1–43) antisense | 5′-GCGCTCGAGTTAGTAGAGCAGGAAGAT-3′ |
| (1–95) antisense | 5′-TCTCTCGAGTTAAAAGACCCCATACGG-3′ |
| (1–157) antisense | 5′-AGTCTCGAGTTACTCCCAGTCACT-3′ |
| (1–170) antisense | 5′- CCCCTCGAGTTACAGTTTGCCCAC-3′ |
| (1–182) antisense | 5′- TGGCTCGAGTTAATCTGAGTACAC-3′ |
| (1–195) antisense | 5′-CCCCTCGAGTTAATCATTTTTCCGGGC-3′ |
Supplementary information
Acknowledgements
We thank Dr. Hee Chul Lee for their comments and proofreading the manuscript. This study was supported by the National Research Foundation of Korea (2016M3A9C6918220).
Author Contributions
Ji Yea Kim: Collection of data, data interpretation; So Young Kim: Collection of data, data interpretation; Hong Seo Choi: Collection of data, data interpretation; Sungkwan An: Data interpretation; Chun Jeih Ryu: Conception and design, financial support, manuscript writing.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37441-6.
References
- 1.Cahill MA, Jazayeri JA, Catalano SM, Toyokuni S, Kovacevic Z, Richardson DR. The emerging role of progesterone receptor membrane component 1 (PGRMC1) in cancer biology. Biochim Biophys Acta. 2016;1866:339–349. doi: 10.1016/j.bbcan.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Peluso JJ, Liu X, Gawkowska A, Lodde V, Wu CA. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol Cell Endocrinol. 2010;320:153–161. doi: 10.1016/j.mce.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohe HJ, Ahmed IS, Twist KE, Craven RJ. PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol Ther. 2009;121:14–19. doi: 10.1016/j.pharmthera.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izzo NJ, et al. Alzheimer’s therapeutics targeting amyloid beta 1–42 oligomers II: Sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PLoS One. 2014;9:e111899. doi: 10.1371/journal.pone.0111899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin Y, et al. Progesterone attenuates Abeta(25–35)-induced neuronal toxicity via JNK inactivation and progesterone receptor membrane component 1-dependent inhibition of mitochondrial apoptotic pathway. J Steroid Biochem Mol Biol. 2015;154:302–311. doi: 10.1016/j.jsbmb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Mir SU, et al. Progesterone receptor membrane component 1/Sigma-2 receptor associates with MAP1LC3B and promotes autophagy. Autophagy. 2013;9:1566–1578. doi: 10.4161/auto.25889. [DOI] [PubMed] [Google Scholar]
- 8.Min L, et al. Characterization of the adrenal-specific antigen IZA (inner zone antigen) and its role in the steroidogenesis. Mol Cell Endocrinol. 2004;215:143–148. doi: 10.1016/j.mce.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Losel R, Dorn-Beineke A, Falkenstein E, Wehling M, Feuring M. Porcine spermatozoa contain more than one membrane progesterone receptor. Int J Biochem Cell Biol. 2004;36:1532–1541. doi: 10.1016/j.biocel.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Peluso JJ, Romak J, Liu X. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone’s antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology. 2008;149:534–543. doi: 10.1210/en.2007-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahill MA, Medlock AE. Thoughts on interactions between PGRMC1 and diverse attested and potential hydrophobic ligands. J Steroid Biochem Mol Biol. 2017;171:11–33. doi: 10.1016/j.jsbmb.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Falkenstein E, et al. Localization of a putative progesterone membrane binding protein in porcine hepatocytes. Cell Mol Biol (Noisy-le-grand) 1998;44:571–578. [PubMed] [Google Scholar]
- 13.Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci U S A. 2000;97:12816–12821. doi: 10.1073/pnas.97.23.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beausoleil SA, et al. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A. 2004;101:12130–12135. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piel RB, 3rd, et al. A Novel Role for Progesterone Receptor Membrane Component 1 (PGRMC1): A Partner and Regulator of Ferrochelatase. Biochemistry. 2016;55:5204–5217. doi: 10.1021/acs.biochem.6b00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peluso JJ, Pappalardo A, Losel R, Wehling M. Expression and function of PAIRBP1 within gonadotropin-primed immature rat ovaries: PAIRBP1 regulation of granulosa and luteal cell viability. Biol Reprod. 2005;73:261–270. doi: 10.1095/biolreprod.105.041061. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed IS, Rohe HJ, Twist KE, Craven RJ. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J Biol Chem. 2010;285:24775–24782. doi: 10.1074/jbc.M110.134585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng C, et al. Sigma-2 ligands induce tumour cell death by multiple signalling pathways. Br J Cancer. 2012;106:693–701. doi: 10.1038/bjc.2011.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampton KK, Anderson K, Frazier H, Thibault O, Craven RJ. Insulin Receptor Plasma Membrane Levels Increased by the Progesterone Receptor Membrane Component 1. Mol Pharmacol. 2018;94:665–673. doi: 10.1124/mol.117.110510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aizen J, et al. Roles of progesterone receptor membrane component 1 and membrane progestin receptor alpha in regulation of zebrafish oocyte maturation. Gen Comp Endocrinol. 2018;263:51–61. doi: 10.1016/j.ygcen.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szczesna-Skorupa E, Kemper B. Progesterone receptor membrane component 1 inhibits the activity of drug-metabolizing cytochromes P450 and binds to cytochrome P450 reductase. Mol Pharmacol. 2011;79:340–350. doi: 10.1124/mol.110.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabe Y, et al. Haem-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nat Commun. 2016;7:11030. doi: 10.1038/ncomms11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cahill MA, Jazayeri JA, Kovacevic Z, Richardson DR. PGRMC1 regulation by phosphorylation: potential new insights in controlling biological activity. Oncotarget. 2016;7:50822–50827. doi: 10.18632/oncotarget.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi HS, et al. Development of a decoy immunization strategy to identify cell-surface molecules expressed on undifferentiated human embryonic stem cells. Cell Tissue Res. 2008;333:197–206. doi: 10.1007/s00441-008-0632-6. [DOI] [PubMed] [Google Scholar]
- 25.Kim JY, et al. Progesterone Receptor Membrane Component 1 suppresses the p53 and Wnt/beta-catenin pathways to promote human pluripotent stem cell self-renewal. Sci Rep. 2018;8:3048. doi: 10.1038/s41598-018-21322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandes MS, Brosens JJ. Gellersen B. Honey, we need to talk about the membrane progestin receptors. Steroids. 2008;73:942–952. doi: 10.1016/j.steroids.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, et al. Universal PCR amplification of mouse immunoglobulin gene variable regions: the design of degenerate primers and an assessment of the effect of DNA polymerase 3′ to 5′ exonuclease activity. J Immunol Methods. 2000;233:167–177. doi: 10.1016/S0022-1759(99)00184-2. [DOI] [PubMed] [Google Scholar]
- 28.Zeng C, et al. Subcellular localization of sigma-2 receptors in breast cancer cells using two-photon and confocal microscopy. Cancer Res. 2007;67:6708–6716. doi: 10.1158/0008-5472.CAN-06-3803. [DOI] [PubMed] [Google Scholar]
- 29.Mir SU, Ahmed IS, Arnold S, Craven RJ. Elevated progesterone receptor membrane component 1/sigma-2 receptor levels in lung tumors and plasma from lung cancer patients. Int J Cancer. 2012;131:E1–9. doi: 10.1002/ijc.26432. [DOI] [PubMed] [Google Scholar]
- 30.Shin BK, et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003;278:7607–7616. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez J, Gharahdaghi F, Mische SM. Routine identification of proteins from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels or polyvinyl difluoride membranes using matrix assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS) Electrophoresis. 1998;19:1036–1045. doi: 10.1002/elps.1150190619. [DOI] [PubMed] [Google Scholar]
- 32.Singer SJ. The structure and insertion of integral proteins in membranes. Annu Rev Cell Biol. 1990;6:247–296. doi: 10.1146/annurev.cb.06.110190.001335. [DOI] [PubMed] [Google Scholar]
- 33.Shao S, Hegde RS. Membrane protein insertion at the endoplasmic reticulum. Annual review of cell and developmental biology. 2011;27:25–56. doi: 10.1146/annurev-cellbio-092910-154125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izzo NJ, et al. Alzheimer’s therapeutics targeting amyloid beta 1–42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits. PLoS One. 2014;9:e111898. doi: 10.1371/journal.pone.0111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Heijne G. Recent advances in the understanding of membrane protein assembly and structure. Quarterly reviews of biophysics. 1999;32:285–307. doi: 10.1017/S0033583500003541. [DOI] [PubMed] [Google Scholar]
- 36.Chen M, Zhang JT. Topogenesis of cystic fibrosis transmembrane conductance regulator (CFTR): regulation by the amino terminal transmembrane sequences. Biochemistry. 1999;38:5471–5477. doi: 10.1021/bi982153t. [DOI] [PubMed] [Google Scholar]
- 37.Dunlop J, Jones PC, Finbow ME. Membrane insertion and assembly of ductin: a polytopic channel with dual orientations. The EMBO journal. 1995;14:3609–3616. doi: 10.1002/j.1460-2075.1995.tb00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Turnbull IR, Bragin A, Carveth K, Verkman AS, Skach WR. Reorientation of aquaporin-1 topology during maturation in the endoplasmic reticulum. Molecular biology of the cell. 2000;11:2973–2985. doi: 10.1091/mbc.11.9.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han ES, Zhang JT. Mechanism involved in generating the carboxyl-terminal half topology of P-glycoprotein. Biochemistry. 1998;37:11996–12004. doi: 10.1021/bi980702p. [DOI] [PubMed] [Google Scholar]
- 40.Sadlish H, Skach WR. Biogenesis of CFTR and other polytopic membrane proteins: new roles for the ribosome-translocon complex. The Journal of membrane biology. 2004;202:115–126. doi: 10.1007/s00232-004-0715-6. [DOI] [PubMed] [Google Scholar]
- 41.von Heijne G. Membrane-protein topology. Nature reviews Molecular cell biology. 2006;7:909–918. doi: 10.1038/nrm2063. [DOI] [PubMed] [Google Scholar]
- 42.Cahill, M. A. The evolutionary appearance of signaling motifs in PGRMC1. Biosci Trends (2017). [DOI] [PubMed]
- 43.Ryu CS, Klein K, Zanger UM. Membrane Associated Progesterone Receptors: Promiscuous Proteins with Pleiotropic Functions - Focus on Interactions with Cytochromes P450. Front Pharmacol. 2017;8:159. doi: 10.3389/fphar.2017.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi, H. S., Lee, H. M., Jang, Y. J., Kim, C. H. & Ryu, C. J. Heterogeneous Nuclear Ribonucleoprotein A2/B1 Regulates the Selfrenewal and Pluripotency of Human Embryonic Stem Cells via the Control of the G1/S Transition. Stem Cells (2013). [DOI] [PubMed]
- 45.Kim WT, Seo Choi H, Min Lee H, Jang YJ, Ryu CJ. B-cell receptor-associated protein 31 regulates human embryonic stem cell adhesion, stemness, and survival via control of epithelial cell adhesion molecule. Stem Cells. 2014;32:2626–2641. doi: 10.1002/stem.1765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.