Abstract
The genus Arthrospira has a long history of being used as a food source in different parts of the world. Its mass cultivation for production of food supplements and additives has contributed to a more detailed study of several species of this genus. In contrast, the type species of the genus (A. jenneri), has scarcely been studied. This work adopts a polyphasic approach to thoroughly investigate environmental samples of A. jenneri, whose persistent bloom was noticed in an urban reservoir in Poland, Central Europe. The obtained results were compared with strains designated as A. platensis, A. maxima, and A. fusiformis from several culture collections and other Arthrospira records from GenBank. The comparison has shown that A. jenneri differs from popular species that are massively utilized commercially with regard to its cell morphology, ultrastructure and ecology, as well as its 16S rRNA gene sequence. Based on our findings, we propose the establishment of a new genus, Limnospira, which currently encompasses three species including the massively produced L. (A.) fusiformis and L. (A.) maxima with the type species Limnospira fusiformis.
Introduction
Among the simple trichal cyanobacteria, three genera possess helically-coiled trichomes as a prominent diacritical feature: Spirulina Turpin ex Gomont 1892, Halospirulina Nübel, Garcia-Pichel et Muyzer 2000, and Arthrospira Stizenberger ex Gomont 1892. The latter is a widely-known taxon with long history of use as a food source, for example as dihé in Africa or tecuitlatl in Mexico1–3. Presently, the members of this genus are still used as food, but a substantial part of Arthrospira production is sold in a form of food supplements or food additives4,5; furthermore, under the commercial name of Spirulina, it is the most widely-produced microalgae in large-scale production6. Latest reports even mention the value of Arthrospira in bio-nanotechnology - the coiled shape of its trichomes and their gliding motility with rotation have been used as a biological matrix for creating biohybrid microbots for imaging-guided therapy7.
In most industrial applications, Arthrospira is used under the common name “Spirulina”, which may be easier to pronounce and remember, and thus appear more suitable from a marketing point of view. However, it is only remotely related to the true Spirulina, and despite great efforts, it was not possible to identify any facility producing any species of this genus anywhere in the world. Besides its marketing value, the name is used for historical reasons. In the nomenclatoric starting point for simple trichal cyanobacteria, Gomont8 mentions both the genera Arthrospira and Spirulina. However, Arthrospira was not accepted as a separate genus by Geitler9 and was hence merged with Spirulina. The issue of these two genera was well described by Komárek and Lund10, and by Sili and others6. Nowadays, a wide array of studies have confirmed that Arthrospira and Spirulina represent two independent genera, each classified in different orders, and apart from possessing helically-coiled trichomes, the two share a minimal morphological resemblance11.
One of the most prominent morphological features of the genus Arthrospira is the spiral coiling of multicellular trichomes with easily visible cross-walls6,12. The distance between spirals and width of individual coils, both often mentioned in literature as a typical attribute of particular taxa, cannot be considered a permanent morphological feature, since it may change in each trichome. Some authors even describe the ability of this genus to form completely straight and linear trichomes13,14. Twenty-three species, either forming mats or living as solitary trichomes, are currently taxonomically accepted within Arthrospira12,15. Three species are marine, three come from waters with elevated pH and/or electric conductivity, and the rest are freshwater types. The species have been recorded in all continents except Antarctica. In warmer regions, the planktic types often form heavy blooms and are traditionally harvested by local people for direct consumption2.
According to the current taxonomic classification, Arthrospira is one of the nineteen genera in the Microcoleaceae family within the order Oscillatoriales11. Even though several recent studies have dealt with the phylogenetic relationship of Arthrospira to other taxa11,16, none of these works include molecular data for the type species A. jenneri Stizenberger ex Gomont. Unlike more popular species cultivated commercially, A. jenneri originates from freshwater ecosystems with low salinity17. Strains/organisms determined as A. maxima Setchell & Gardner, A. fusiformis (Voronichin) Komárek & Lund, and A. platensis Gomont have often been isolated from highly alkaline and saline inland habitats in tropical and subtropical climate zones18,19; this knowledge, combined with the lack of information on the type species incorrectly suggests that the preference for high pH and high salinity and/or electrical conductivity is a general feature shared by the entire genus6. To better understand not only the ecological requirements of the whole genus Arthrospira but also its relationships with other taxa within the order Oscillatoriales, it is necessary to study the type species A. jenneri to which the genus name is connected in more detail.
In June 2015, a phytoplankton biodiversity survey of an urban reservoir in Poland (Central Europe) revealed the presence of small mats of A. jenneri floating in the water column. Further observations found a thick layer of the organism to be covering the whole bottom of the reservoir. Since then, the presence of A. jenneri has been constant: in winter, it creates a visible benthic mat under the ice cover, while in summer, when the air temperature increases to 28 °C, it detaches from the bottom of the reservoir and large lobes of its mat float to the surface.
The present study investigated the relationship of the type species of the genus Arthrospira, i.e. A. jenneri, to the massively-produced species A. fusiformis and A. maxima, using traditional and molecular methods. Based upon our findings, we amend the description of the genus Arthrospira and delimit the commercially produced species to a new genus, Limnospira.
Results
Arthrospira jenneri was first mentioned under the name Spirillum jenneri by Hassall in 1845 from Tunbridge, UK. The genus itself was later established by Stizenberger20, who compared his own collections from a pond in vicinity of Konstanz, Germany with Hassall’s17 description. Gomont8, in the literature designated as a starting point for the nomenclature of simple filamentous cyanobacteria, accepts the genus and indicates that this species can be found in various pools and garden lakes in different parts of Europe. Gardner21 designated A. jenneri as a type species of the whole genus Arthrospira, and thus connected the generic name with this organism. None of these authors suggest that A. jenneri should inhabit highly saline or alkaline waters. The morphology, ecology and to an extent, the geographical origin of the population of A. jenneri collected in Tomaszowska Reservoir, Poland, studied in this paper, correspond well with those given in the original descriptions in previous papers. It can hence be regarded as a reliable candidate for type species of Arthrospira in investigating its relationship to other species currently included in this genus.
Amended description of A. jenneri based on the studied population
Morphology
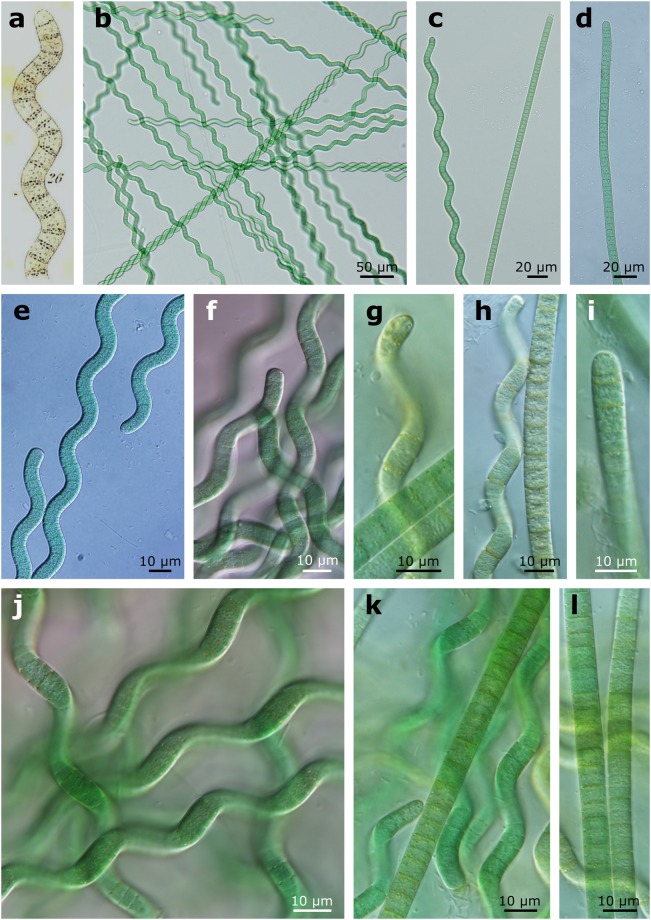
Trichomes are isopolar, cylindrical, spirally coiled or straight (Fig. 1, Fig. 2a–c). Trichomes of both types (spirally coiled and straight) are motile, unsheathed or with a thin, inconspicuous sheath, not or only slightly constricted at the cross walls and with rounded apical cells lacking calyptra or thickened cell wall (Fig. 1e–i; Fig. 2a,b). Cells of both trichome types are shorter than they are wide; 6–8 µm wide, 2.6–5.6 µm long. Both types of trichomes possess granulation at cross walls (Fig. 1g–i).
Figure 1.
The morphology of Arthrospira jenneri, (a) original drawing of A. jenneri by Gomont8, (b–l) morphology of straight and spiral trichomes of A. jenneri’s population from Tomaszowska Reservoir in LM.
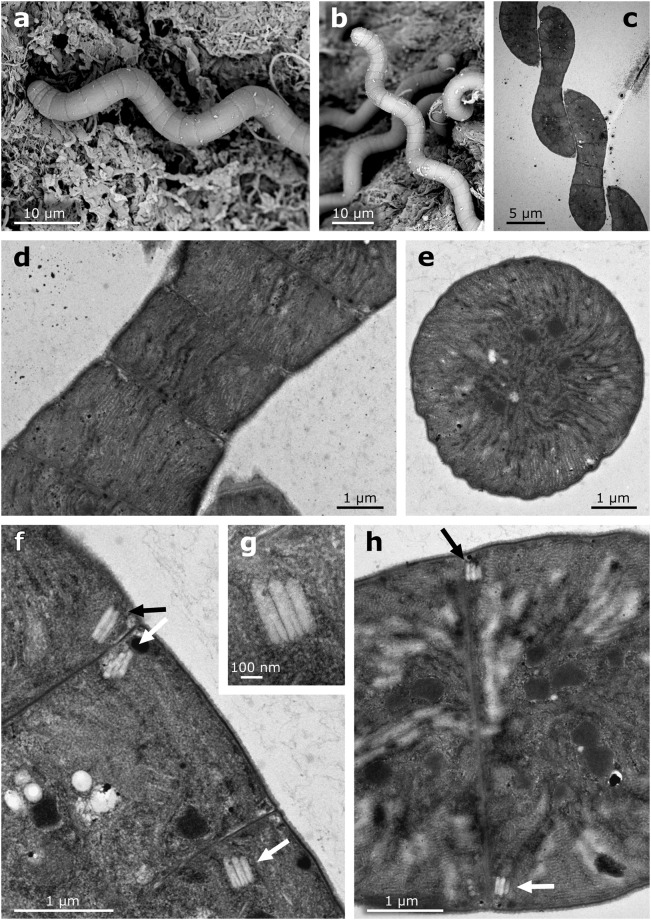
Figure 2.
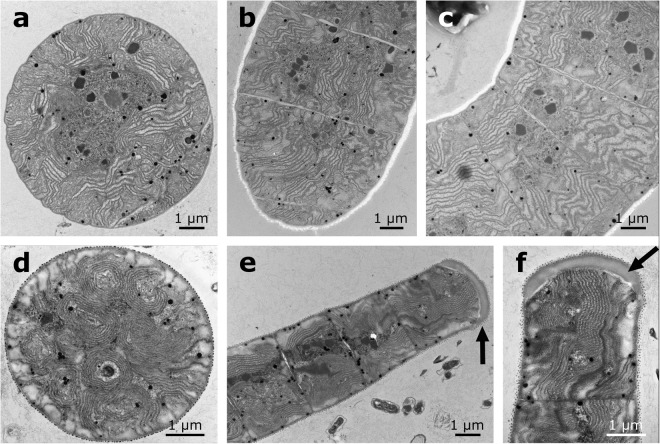
The morphology and ultrastructure of Arthrospira jenneri under electron microscope, (a,b) morphology of the trichomes in SEM, (c–h) ultrastructure of the trichomes in TEM, aerotopes indicated by arrows, (g) detail of an aerotope.
Ultrastructure
Cells possess radially-arranged thylakoids (Fig. 2e) and facultative gas vesicles forming aerotopes near the cross-walls (Fig. 2f–h).
Ecology
Benthic mats in standing waters in temperate zones with optimum growth between pH 6.8 and 7.5, and electrical conductivity between 250 and 445 µS.cm−1.
Phylogenetic analyses
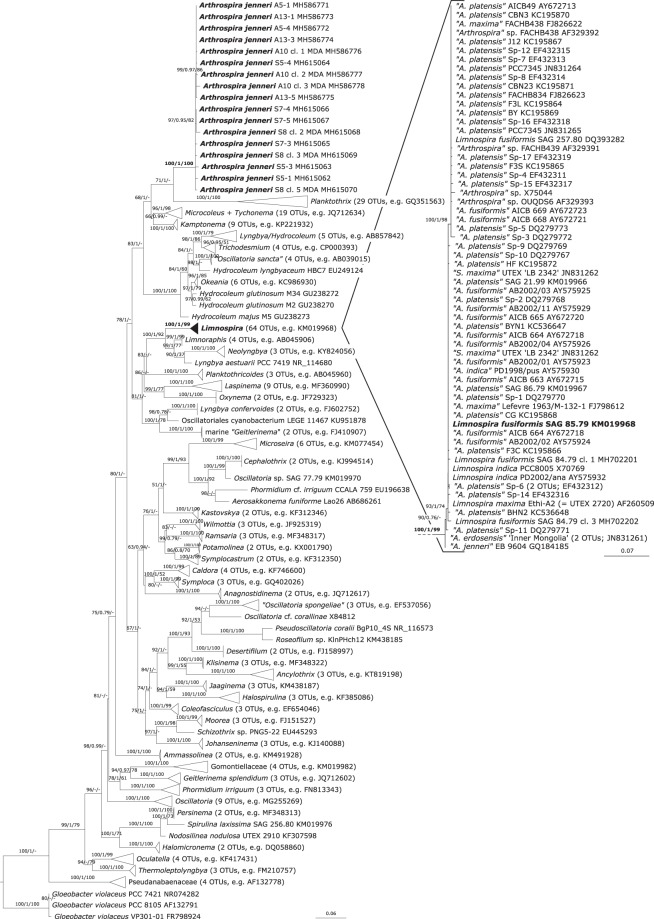
Phylogenetic analyses based on the 16S rRNA gene placed all sequences of A. jenneri from Tomaszowska Reservoir, Poland, within a well-supported clade well distant from all other sequenced genera (Fig. 3). With regard to the congruence of morphological and ecological data of the sequenced population with the original and starting point descriptions of the type species, this clade represents the genus Arthrospira. Maximum Likelihood and Bayesian inference placed the genus Planktothrix within the close vicinity of Arthrospira. All sequences of widely-consumed taxa in public sequence repositories determined as A. platensis, A. maxima, or A. fusiformis were grouped together with A. erdosensis, A. indica and ‘A. jenneri’, in a well-supported clade, distant from Arthrospira. The similarity between the sequences included in these two clades is below 91.21%.
Figure 3.
Phylogenetic tree based on 1800 bp fragment of the gene for 16S rRNA, including 291 sequences and showing the position of the genera Arthrospira and Limnospira. Branch support values (above 50 or 0.75) are shown as Maximum Likelihood, Bayesian posterior probability, and Maximum Parsimony bootstrap values, respectively. Note to Fig. 3: Quotation marks indicate the uncertainty of the real name of the sequenced organism.
Based on this information, we propose the establishment of a new genus, Limnospira, containing, inter alia, the mass-produced species of former “Arthrospira”. The sequence of A. jenneri (GQ184185) located in the Limnospira clade originates from organism inhabiting alkaline lakes, and thus cannot represent true A. jenneri. All performed analyses suggest that the new genus Limnospira is related to two recently-described genera, Limnoraphis (freshwater) and Neolyngbya (marine), together with the Lyngbya aestuarii PCC 7419 strain originating from a salt marsh in the USA (Fig. 3).
Limnospira gen. nov.
Class: Cyanophyceae
Order: Oscillatoriales
Family: Microcoleaceae
Morphology
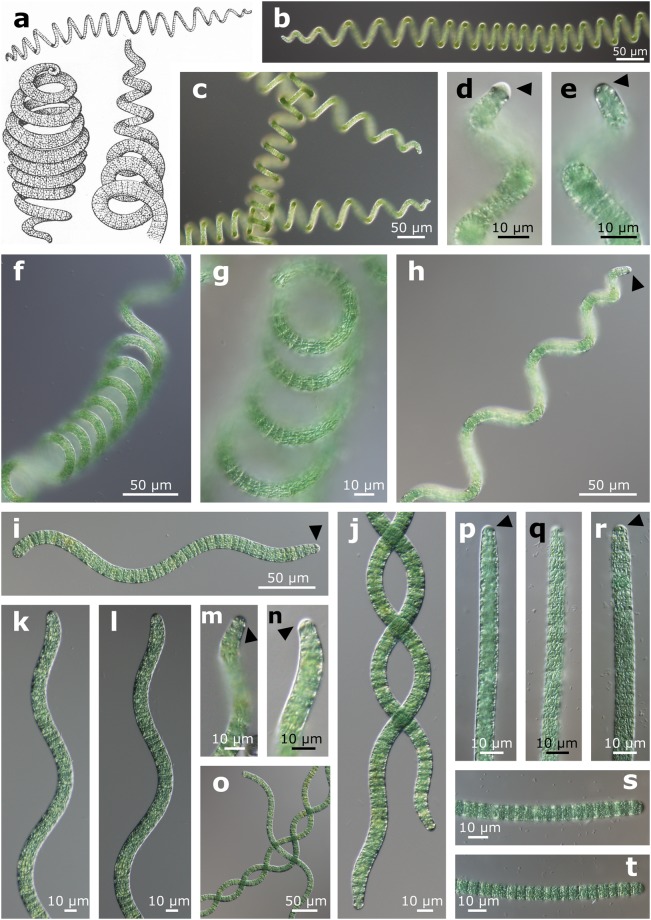
Trichomes isopolar, cylindrical, regularly spirally coiled with a tendency to loosening the coils, unbranched, more or less tapering towards the ends, not or slightly constricted at cross-walls, blue green or dark green. Cells always shorter than wide. Terminal cells rounded or subcapitate with thickened outer cell wall or calyptra (Figs 4d,e,h,i,m,n,p–r and 5e,f).
Figure 4.
The morphology of Limnospira, (a) original drawing of L. fusiformis (Spirulina fusiformis) by Voronichin22, (b–h) morphology of the L. fusiformis reference strain SAG 85.79 in LM (i–o) morphology of the L. fusiformis strain SAG 84.79 in LM, (p–t) morphology of the L. fusiformis strain SAG 257.80 in LM; black triangles point out calyptra/thickened cell wall.
Figure 5.
The ultrastructure of Limnospira in TEM, (a–c) the reference strain of L. fusiformis SAG 85.79, (d–f) the strain of L. fusiformis SAG 257.80, black arrows point out the calyptra/thickened cell wall at the end of the trichome.
Ultrastructure
Thylakoids have an irregular arrangement (Fig. 5). Aerotopes are facultatively present.
Ecology
Planktic in freshwater as well as in water with higher salinity, most of the species known so far prefer habitats with elevated pH and electrical conductivity.
Type species
Limnospira fusiformis (Voronichin) comb. nov.
Etymology
Límni- [Gr., λίμνη], lake, lagoon; -speíra [Gr., σπείρα] - spiral; the generic name refers to the morphology of trichomes and typical habitat, i.e. lakes.
Limnospira fusiformis (Voronichin) comb. nov
Basionym
Spirulina fusiformis Voronichin 1934, Trudy Soveta Izucheniu Prirodnych Resursov, Ser. Sibirsk. 8: 18222 Reference strain: SAG 85.79 = CCALA 026 = UTEX 2340 (Fig. 4b–h, Fig. 5a–c)
Limnospira maxima (Setchell et Gardner) comb. nov
Basionym
Arthrospira maxima Setchell et Gardner 1917, Univ. Calif. Publ. Bot. 6: 37723 Reference strain: UTEX 2720 (sub. “A. fusiformis” Ethi-A2).
Limnospira indica (Desikachary et Jeeji Bai) comb. nov
Basionym
Arthrospira indica Desikachary et Jeeji Bai 1992, ETTA Nat. Symp., Madras: 1524 Reference strain: PCC 8005.
Discussion
Our phylogenetic analyses allowed us to group former Arthrospira sequences from main public repositories (64 OTUs) into a single homogenous cluster, closely related to marine Neolyngbya, halophilic Lyngbya aestuarii PCC 7419 and freshwater cyanobacteria from the genus Limnoraphis; while the sequences obtained from both the spiral and straight trichomes of A. jenneri (17 OTUs) formed a coherent cluster related to the freshwater genus Planktothrix which were distant from the newly-established Limnospira (Fig. 3).
Most strains/species forming the genus Limnospira are well known for their preference of alkaline habitats (e.g. L. fusiformis). This is also one of the characteristics that makes the mass production of the varieties widely used in the food industry easier and less prone to contamination by other organisms5,6,25,26. Nevertheless, it seems that the ecological preferences of alkaline waters cannot be generalized for the whole genus. Based on our phylogenetic analyses, sequences representing A. indica also belong to the Limnospira clade (Fig. 3), and the authors of this taxon and Ballot and others27 report that this species comes from a freshwater environment. As it was impossible to track the origin of all of the other sequences grouped in Limnospira in the present study, there may be more representatives of this genus that do not require elevated pH for their growth.
The arrangement of thylakoids within the cells and the presence of calyptra/thickened cell wall in apical cells represent more stable and reliable features that can be used to distinguish Arthrospira from Limnospira. The ultrastructural investigations revealed that Arthrospira contains radially-arranged thylakoids (Fig. 2), while Limnospira possesses many irregular, whirl-like sections across the central part of the cell (Fig. 5). Whereas the number or density of thylakoids may be modified by environmental factors, the arrangement is very stable in genetic clusters within the 16S rRNA gene28,29. Furthermore, the presence of a thickened cell wall or calyptra seems to be present only in the genus Limnospira and was not observed in any of the Arthrospira trichomes, nor mentioned by the author of the original description of A. jenneri.
The current work proposes that only three former Arthrospira species be transferred to the newly-established genus Limnospira: L. fusiformis, L. maxima and L. indica. All three possess credible sets of morphological, molecular and ecological data on which their transfer can be based. Unfortunately, many other taxa currently classified as Arthrospira lack such necessary data. Despite the fact that many of the sequences forming the Limnospira clade are labelled as “A. platensis” (Fig. 3), this determination could not be verified by our results. According to the literature, A. platensis Gomont represents a benthic organism described from Uruguay with rare, relatively small and inconspicuous broadly rounded calyptras and probably no aerotopes10,30. Hence, based only on the presence of calyptra, this species should also be transferred to Limnospira. On the other hand, the benthic habitat and apparent absence of aerotopes would suggest a closer proximity to Arthrospira, as re-defined here. Regrettably, the available sequences designated as “A. platensis” do not seem to originate in the benthic habitats of Uruguay; on the contrary, all seem to represent planktic forms. Therefore, it is not certain whether the available molecular data really represents A. platensis and hence, whether the whole species should remain in Arthrospira or be transferred elsewhere.
Another example of unclear taxon requiring revision based on more detailed information is the species “A. erdosensis”, a type isolated from alkaline ponds in Erdos Plateau, Inner Mongolia, China. “A. erdosensis” has not been formally established and insufficient information is available to conclusively place it in a taxon. Hence, it was decided to present most of the names listed in the Limnospira clade, as currently named in GenBank/EML ENA/DDBJ, with quotation marks to indicate the uncertainty of their proper name (Fig. 3).
Despite the fact that such high morphological variability occurs in cyanobacteria, and certain morphological features can be used for the identification of taxa, the use of trichome spirality as a diacritical feature on its own may be misleading. As reported by several authors14 Limnospira is capable of reversible straightening and our results have shown the same for Arthrospira sensu stricto. This raises the question whether Hassall’s17 note in the very first description of A. jenneri “…mixed up with different species of Oscillatoriæ.” may apply to straight trichomes of the same species of Arthrospira. Non-heterocytous cyanobacteria exhibiting spiral trichomes were divided into three distinct genera outside of the order Synechococcales: the true genus Arthrospira, the closely-related Planktothrix (Fig. 3), which also contains species characterized by both straight (e.g. P. agardhii) and spiral (e.g. P. spiroides) trichomes, and the designated genus Limnospira. However, none of these genera seem to possess exclusively spiral trichomes, all of them either contain taxa with both coiled and straight trichomes, or the organisms themselves are able to change from one form to another. The only genus where the presence of straight and spiral trichomes has not yet been reported are Halospirulina and Spirulina, classified to the Synechococcales; however, partial straightening of trichomes has been observed in this case (Komárek pers. comm.). We therefore hypothesize, that some described species of the genus Oscillatoria Vaucher ex Gomont may represent the straight trichomes of Arthrospira or Limnospira, e.g. Oscillatoria curviceps Agardh ex Gomont.
The unsuitability of employing trichome spirality as a diacritical feature has also been demonstrated by strain SAG 31.96 maintained in the culture collection under the name Arthrospira massartii. Molecular and detailed morphological analyses place this strain within the genus Planktothrix Anagnostidis et Komárek and it probably represents species P. spiroides originally described from pond in China31. Since the strain SAG 31.96 was isolated from a pond with Microcystis spp. bloom (http://sagdb.uni-goettingen.de), the ecology does not seem to correspond with that described for A. massartii (i.e. clear springs). Hence, based on morphological and ecological data given in previous literature, A. massartii may truly belong to the genus Arthrospira12,32. However, to ultimately confirm whether the organism corresponds closely with the original description, with regard to its morphological and ecological characteristics, it needs to be sequenced.
The long history of the utilization of Limnospira under the commercial name “Spirulina” has resulted in many incorrect taxonomic designations. Despite the substantial number of scientific papers highlighting the clear separation of Arthrospira from Spirulina, Arthrospira/Limnospira platensis still occurs in many scientific studies under the invalid designation of “Spirulina platensis”33–35. Despite its value as a marketing tool, being easier to sell dietary products as “Spirulina” than “Arthrospira”, which calls to mind arthrosis, there is no scientific justification for using this invalid designation. Komárek29 argues that such common practice is a serious problem in modern science, and not using the formal nomenclatoric rules undermines the validity of research papers. Unfortunately, in some cases, culture collections also contribute to the confusion surrounding the species level nomenclature by offering strains with incorrect species designations: for example, UTEX LB 2720 is not L. (A.) fusiformis but L. (A.) maxima (according to Li and others36) or SAG 85.79 is not A. platensis according to its morphology or ecology.
Such nomenclature is also often incorrectly used in the case of commercial products. Biomass producers as well as retail products manufacturers cannot adopt nomenclatoric changes as easily as the scientific community due to several reasons, such as legislation and the need to avoid confusion by the consumer. On the other hand, it would be reasonable to expect them to follow long term trends in taxonomy which may take place over several decades. The polyphasic approach applied recently in cyanobacterial diversity research has resulted in many changes among the genera, families and orders, revealing more authentic phylogenetic relationships. For environmental scientists and specialists in monitoring laboratories who rely almost solely on traditional morphological traits, the molecular approach allows the creation of new taxonomic units which would be impossible to recognize without at least detailed ultrastructural studies. Fortunately, no such analysis is required for the newly-established genus Limnospira described herein, since some of its diacritic features are recognizable under the light microscope. Nevertheless, since modern criteria have been accepted within the classification system and have been applied to cyanobacterial taxonomy, it is essential that scientific papers and reports adopt the modern approach and use the names consistent with formal nomenclature.
In conclusion, our detailed investigations of the type species of the genus Arthrospira based on a polyphasic approach revealed clear differences between A. jenneri and organisms produced under the common name “Spirulina”, hence the need for the establishment of a new genus. We therefore propose the name Limnospira for the new genus that encompasses species with high industrial utilization and potential.
Materials and Methods
Study material and environmental background
Tomaszowska Reservoir (51°43′48′′N; 19°31′47′′E), where a massive occurrence of A. jenneri was noted, is located in the margins of the city of Łódź (Central Poland). It is a flow-through man-made reservoir located on the Olechówka River, with a surface area of 1 ha, average depth of 1.75 m and capacity of 17500 square m37. Hydrochemical data is presented in Table S1.
The A. jenneri samples were collected from the benthos of the reservoir once every two months for a year, beginning December 2016. Samples for analyses were taken from 25 cm2 of the bottom sediment using a plastic frame and small shovel.
Fresh samples of A. jenneri were examined by light microscopy (LM), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). In addition, strains of “A. platensis” SAG 257.80, SAG 85.79 and “A. maxima” SAG 84.79 were analysed in TEM. The LM observations were performed using an Olympus BX51 light microscope equipped with Nomarski DIC optics and an Olympus DP71 digital camera. The surface of trichomes was visualized using a Phenom Pro-X Scanning Electron Microscope. For ultrastructural observations in TEM fresh sample was fixed in 6% glutaraldehyde, left for several hours at room temperature and washed with 0.05 M phosphate buffer (pH 7.2). Then the sample was postfixed with 2% osmium tetroxide in the same buffer and temperature for two hours and repeatedly washed. Trichomes were dehydrated in isopropanol at gradually increasing concentration series and embedded in Spurr’s resign. Ultrathin sections were cut and contrasted with 2.5% uranyl acetate and analysed using a digital JEOL JEM–1010 Transmission Electron Microscope.
16S rRNA gene sequencing
Individual trichomes or trichome fragments were isolated from natural material using glass microcapillary as described in Zapomělová and others38, and Mareš and others39. Every trichome was washed in 6 drops of sterile TE buffer, subsequently placed to sterile 0.2 ml PCR tube and kept frozen (−20 °C) until further use. Three PCR tubes per morphotype were used for the molecular analyses to confirm that the same 16S rRNA is amplified from all of them. Direct PCR was applied to 2 of the PCR tubes using primers 359F40 and 23S30R41 in 50 μl reaction volume. The PCR mix contained 25 µl of Plain PP Master Mix (Top Bio, Prague, Czech Republic), 1.5 μl of each primer (concentration 5 pmol·μl−1), and 21 μl of PCR grade water. The cycling conditions were: 5 min denaturation at 95 °C, continued with 45 cycles of 94 °C for 1 min 30 s, 54 °C for 1 min 30 s, and 72 °C for 2 min, and final elongation at 72 °C for 10 min. The genomic DNA from the third trichome was amplified by the MDA method42 using Repli-g Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol, followed by PCR using primers 16S27F/f D141,43 and 23S30R41 in 20 μl reaction volume. The PCR mix contained 10 µl of Plain PP Master Mix (Top Bio, Prague, Czech Republic), 1.2 μl of each primer (concentration 5 pmol·μl−1), and 6.6 μl of PCR grade water. The cycling conditions were: 5 min initial denaturation at 95 °C, continued with 40 cycles of 94 °C for 1 min, 57 °C for 45 s, and 72 °C for 2 min, and a final elongation step at 72 °C for 10 min. After staining by Sybr Green (Lonza, Rockland, ME, USA) the PCR products were cut out of low melting agarose gel (1.5%, 60 V, 1 hour). The bands of interest were cloned into plasmids with pGEM®-T Easy vector system (Promega Corp., Madison, WI, USA). Three bacterial colonies containing the PCR product were purified from each plate and then sequenced commercially using following primers: T7 (5′-TAA TAC GAC TCA CTA TAG GG-3′), SP6 (5′-TAT TTA GGT GAC ACT ATA G-3′), and when needed also internal primer CYA781F(a)40. The obtained sequences were submitted to the NCBI GenBank database (www.ncbi.nlm.nih.gov): MH586771-78 and MH615062-70. The sequences were assembled in Geneious version 944 (http://www.geneious.com).
Phylogenetic Analyses
For phylogenetic analysis, the 16S rRNA sequences of A. jenneri obtained by us together with other Arthrospira records and representatives from simple trichal groups available in GenBank, and the outgroup taxa - Gloeobacter violaceus (NR_074282; AF132791; FR798924) were aligned using MAFFT v. 745 (http://mafft.cbrc.jp/alignment/server/). A total of 291 sequences were included in the analysis. Phylogenetic calculations were performed using maximum likelihood analysis (ML) in IQ-TREE web server46 (http://iqtree.cibiv.univie.ac.at/), Bayesian inference (BI) in MrBayes 3.2.247 and maximum parsimony analysis (MP) in MEGA 748. The ML tree was constructed applying the GTR + I + Γ model chosen according to Akaike Information Criterion provided by jModelTest 2 software49 and 1000 bootstrap replicate searches were performed to evaluate the relative support of branches. The MP involved 1000 replicate searches using the tree bisection-reconnection (TBR) branch-swapping algorithm. A total of 1000 replications were run to evaluate the relative branch support.
Electronic supplementary material
Acknowledgements
Very much appreciated financial support was obtained from GA CR 15-11912 S and GA CR 15-00113S and from the University of Łódź task grant for young researchers: no. B1711000001489.02 (2017). We gratefully acknowledge the Laboratory of EM, Biology Centre of CAS, institution supported by the MEYS CR (LM2015062 Czech-BioImaging) for the EM microphotographs. Phylogenetic analyses were computed at MetaCentrum Infrastructure (supported by programme LM2010005). We would also like to thank Professor Jiří Komárek for valuable consultations.
Author Contributions
P.N.K. - collected the material, performed environmental measurements, contributed to L.M. analyses, performed SEM observations, contributed to processing of natural material in molecular lab, contributed to manuscript preparations; R.M. - contributed to L.M. analyses, processed natural material in molecular lab, contributed to manuscript preparations, prepared figures and plates in manuscript; T.H. - performed TEM observations, performed phylogenetic analyses, contributed to manuscript preparations.
Data Availability
The sequence data generated during the current study are available in the NCBI GenBank database (www.ncbi.nlm.nih.gov). Other data analysed during this study are included in this article and its Supplementary Information file.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36831-0.
References
- 1.Iltis, A. Algues des eaux natroné du Kanem (Tchad). Cah. O.R.S.T.O.M., Hydrobiol. 7(1), 25–54 (In French, with English summary) (1973).
- 2.Ciferri O. Spirulina the edible microorganism. Microbiol. Rev. 1983;47(4):551–578. doi: 10.1128/mr.47.4.551-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tefera G, Hailu D, Tsegaye Z. Importance of Arthrospira Spirulina in Sustainable Development. Int. J. Curr. Trend. Pharmacobiol. Med. Sci. 2016;1(2):60–68. [Google Scholar]
- 4.Wikfors GH, Ohno M. Impact of algal research in aquaculture. J. Phycol. 2002;37(6):968–974. doi: 10.1046/j.1529-8817.2001.01136.x. [DOI] [Google Scholar]
- 5.Koru, E. Earth food Spirulina (Arthrospira): production and quality standards. Food Additive. - InTech 191–202, 10.5772/31848 (2012).
- 6.Sili, C., Torzillo, G. & Vonshak, A. Arthrospira (Spirulina) (ed. B. A. Whitton, B. A.) (ed) 677–705 (Springer, 2012).
- 7.Yan X, et al. Multifunctional biohybrid magnetite microrobots for imaging-guided therapy. Science Robotics. 2017;2(12):eaaq1155. doi: 10.1126/scirobotics.aaq1155. [DOI] [PubMed] [Google Scholar]
- 8.Gomont M. Monographie des Oscillariées (Nostocacées Homocystées), Deuxième partie. - Lyngbyées. Annls. Sci. Nat. Bot. 1892;7(16):91–264. [Google Scholar]
- 9.Geitler, L. Cyanophyceae (ed. Pascher, A.) 1–450 (Gustav Fischer, 1925).
- 10.Komárek J, Lund JWG. What is “Spirulina platensis” in fact? Algol. Stud. 1990;58:1–13. [Google Scholar]
- 11.Komárek J, Kaštovský J, Mareš J, Johansen JR. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia. 2014;86:295–335. [Google Scholar]
- 12.Komárek, J. & Anagnostidis, K. Cyanoprokaryota II, Oscillatoriales, (eds Büdel, B., Gärtner, G., Krienitz, L. & Schagerl M.) 1–757 (Elsevier GmbH, 2005).
- 13.Van Eykelenburg C, Fuchs A. Rapid reversible macromorphological changes in Spirulina platensis. Naturwissenschaften. 1980;67:200–201. doi: 10.1007/BF01086309. [DOI] [Google Scholar]
- 14.Wang ZP, Zhao Y. Morphological reversion of Spirulina (Arthrospira) platensis (Cyanophyta): from linear to helical. J. Phycol. 2005;41:622–628. doi: 10.1111/j.1529-8817.2005.00087.x. [DOI] [Google Scholar]
- 15.Guiry, M. D. & Guiry, G. M. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org (2018).
- 16.Shih PM, et al. Improving the coverage of the cyanobacterial phylum using diversity driven genome sequencing. Proc. Natl. Acad. Sci. USA. 2013;15:1053–1058. doi: 10.1073/pnas.1217107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassall, A. H. A History Of The British Freshwater Algae, Including Descriptions Of The Desmideae And Diatomaceae, With Upwards Of One Hundred Plates Illustrating The Various Species, 1–462 (Spottishwoode A., New-Street-Square, 1845).
- 18.Melack JM. Photosynthesis and growth of Spirulina platensis (Cyanophyta) in an equatorial lake (Lake Simbi, Kenya) Limnol. Oceanogr. 1979;24:753–760. doi: 10.4319/lo.1979.24.4.0753. [DOI] [Google Scholar]
- 19.Okoth OE, et al. Spatial and seasonal variations in phytoplankton community structure in alkaline-saline Lake Nakuru, Kenya. Lake & Reservoirs Res. Manag. 2009;14:57–69. doi: 10.1111/j.1440-1770.2009.00392.x. [DOI] [Google Scholar]
- 20.Stizenberger E. Spirulina und Arthrospira (nov. gen.) Hedwigia. 1854;1(7):32–34. [Google Scholar]
- 21.Gardner, N. L. The Myxophyceae Of Porto Rico And The Virgin Islands, 1–311 (New York Academy of Sciences, 1932).
- 22.Voronichin NN. K biologii mineralizovanych vodojemov Kulundinskoj stepi. Trudy Soveta Izucheniu Prirodnych Resursov, Ser. Sibirsk. 1934;8:177–183. [Google Scholar]
- 23.Gardner NL. New Pacific coast marine algae I. Univ. Calif. Publ. Bot. 1917;6:377–416. [Google Scholar]
- 24.Desikachary, T. V. & Jeeji Bai, N. Studies in Spirulina. (eds Seshadri, C. V. & Jeeji Bai, N.) 12–21 (Spirulina. ETTA National Symposium MCRC, Madras, 1992).
- 25.Vonshak A, Abeliovich A, Boussiba S, Arad S, Richmond A. Production of Spirulina biomass: effects of environmental factors and population density. Biomass. 1981;2:175–185. doi: 10.1016/0144-4565(82)90028-2. [DOI] [Google Scholar]
- 26.Carvalho JCM, Francisco FR, Almeida KA, Sato S, Converti A. Cultivation of Arthrospira (Spirulina) platensis (Cyanophyceae) by fed-batch addition of ammonium chloride at exponentially increasing feeding rates. J. Phycol. 2004;40:589–597. doi: 10.1111/j.1529-8817.2004.03167.x. [DOI] [Google Scholar]
- 27.Ballot A, Dadheech PK, Krienitz L. Phylogenetic relationship of Arthrospira, Phormidium and Spirulina from Kenyan and Indian waterbodies. Algol. Stud. 2004;113:37–56. doi: 10.1127/1864-1318/2004/0113-0037. [DOI] [Google Scholar]
- 28.Komárek J. The modern classification of Cyanoprokaryotes (Cyanobacteria) Oceanol. Hydrobiol. Stud. 2005;34(3):5–17. [Google Scholar]
- 29.Komárek J. Cyanobacterial taxonomy: current problems and prospects for the integration of traditional and molecular approaches. Algae. 2006;21(4):349–375. doi: 10.4490/ALGAE.2006.21.4.349. [DOI] [Google Scholar]
- 30.Hindák F. Morphology of trichomes in Spirulina fusiformis Voronichin from Lake Bogoria, Kenya. Arch. Hydrobiol. Suppl. 1985;71:201–218. [Google Scholar]
- 31.Liu Y, Wang Z, Lin S, Yu G, Li R. Polyphasic characterization of Planktothrix spiroides sp. nov. (Oscillatoriales, Cyanobacteria), a freshwater bloom-forming alga superficially resembling Arthrospira. Phycologia. 2013;52(4):326–332. doi: 10.2216/13-142.1. [DOI] [Google Scholar]
- 32.Kufferath H. Contribution à l'étude de la flore algologique du Luxembourg meridionale. Chlorophycees (exclus. Desmidiacées), Flagellates et Cyanophycées. Ann. Biol. Lac. 1914;2:231–271. [Google Scholar]
- 33.Piñero Estrada JE, Bermejo Bescós P, Villar del Fresno AM. Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco. 2001;56(5-7):497–500. doi: 10.1016/S0014-827X(01)01084-9. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, et al. Optimizing light distribution and controlling biomass concentration by continuously pre-harvesting Spirulina platensis for improving the microalgae production. Bioresour. Technol. 2017;252:14–19. doi: 10.1016/j.biortech.2017.12.046. [DOI] [PubMed] [Google Scholar]
- 35.LewisOscar F, Nithya C, Alharbi SA, Alharbi NS, Thajuddin N. In vitro and in silico attenuation of quorum sensing mediated pathogenicity in Pseudomonas aeruginosa using Spirulina platensis. Microb. Pathog. 2018;116:246–256. doi: 10.1016/j.micpath.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 36.Li R, Jebessa H, Carmichael WW. Isolates identifiable as Arthrospira maxima and Arthrospira fusiformis (Oscillatoriales, Cyanobacteria) appear identical on the basis of a morphological study in culture and 16S rRNA gene sequences. Phycologia. 2001;4(4):367–371. doi: 10.2216/i0031-8884-40-4-367.1. [DOI] [Google Scholar]
- 37.Knysak P, Żelazna-Wieczorek J. Massive occurrence of the alien invasive Pleodorina indica (Volvocales, Chlorophyta) in a reservoir located in urban areas of Central Poland. Oceanol. Hydrobiol. Stud. 2017;46(1):116–122. doi: 10.1515/ohs-2017-0012. [DOI] [Google Scholar]
- 38.Zapomělová E, Řeháková K, Znachor P, Komárková J. Morphological diversity of coiled planktonic types of the genus Anabaena (cyanobacteria) in natural populations - taxonomic consequences. Cryptogam. Algol. 2007;28:353–371. [Google Scholar]
- 39.Mareš J, et al. Phylogenetic analysis of cultivation-resistant terrestrial cyanobacteria with massive sheaths (Stigonema spp. and Petalonema alatum, Nostocales, Cyanobacteria) using single-cell and filament sequencing of environmental samples. J. Phycol. 2015;51:288–297. doi: 10.1111/jpy.12273. [DOI] [PubMed] [Google Scholar]
- 40.Nübel U, Garcia-Pichel F, Muyzer G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997;63:3327–3332. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taton A, Grubisic S, Brambilla E, De Wit R, Wilmotte A. Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo dry valleys, Antarctica): A morphological and molecular approach. Appl. Environ. Microbiol. 2003;69:5157–5169. doi: 10.1128/AEM.69.9.5157-5169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasken RS. Genomic DNA amplification by the multiple displacement amplification (MDA) method. Biochem. Soc. Trans. 2009;37(2):450–453. doi: 10.1042/BST0370450. [DOI] [PubMed] [Google Scholar]
- 43.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kearse M, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katoh, K., Rozewicki, J. & Yamada, K. D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 1–7, 10.1093/bib/bbx108 (2017). [DOI] [PMC free article] [PubMed]
- 46.Trifinopoulos J, Nguyen LT, Von Haeseler A, Minh BQ. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44(W1):W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ronquist F, et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Dataset. Mol. Biol. Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9(8):772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence data generated during the current study are available in the NCBI GenBank database (www.ncbi.nlm.nih.gov). Other data analysed during this study are included in this article and its Supplementary Information file.