Abstract
Brewer’s spent grain (BSG) is a promising substrate for the production of biocontrol fertilizer (BF). The effects of temperature, water content and fermentation time on the conidiation and germination rate of the entomopathogenic fungi Beauveria bassiana (BbQLU1) were modeled in a 3 × 3 × 3 factorially designed experiment. The optimum conditions for BF production (60% water content at 25 °C for 12 days) resulted in a conidiation of 0.85 × 108 spores/g and a germination rate of 98.68%. BF at a concentration of 1 × 10−2 g/ml prompted plant growth and exhibited high toxicity against Galleria mellonella with an LT50 of 3.6 days. GC-MS analysis found 2-piperidone; benzoic acid, 3-methyl-, methyl ester; and other compounds to be potentially related to the toxicity and enhanced plant growth. These findings provide substantial evidence to support the production of BF.
Introduction
Brewer’s spent grain (BSG) is a brewing byproduct available throughout the year at low cost and in large amounts. For each hectoliter of brew, 15–20 kg of wet BSG is produced1. The annual global production of BSG is estimated at 39 million tons2. BSG has traditionally been used as animal feed due to its high fiber and protein content as well as its low cost2,3. This use has proved beneficial; however, the Food and Drug Administration (FDA) proposed to restrict the utilization of BSG as animal feed4. Its supply can often exceed its demand2, and excess BSG is deposited in landfill5. BSG has therefore been utilized as a feedstock for the production of other value-added bioproducts such as lactic acid, ethanol and biogas6–8.
In recent years, demand for the production of biopesticides is an increasing global concern, because some synthetic pesticides damage the environment, food safety and health9. Additionally, pests cause global crop losses and develop high resistance to the chemical pesticides10. The wide expansion of biopesticides is consequently an alternative and permissible in integrated pest management as part of the National Organic Program11,12. The market in biopesticides is growing annually at a rate of 10–44%, depending on the country, e.g., the growth rate is 20% in Europe and Oceania11. More than 150 commercial products are available for use as biocontrol products of phytopathogens13.
Biopesticides are mainly becoming more accepted based on the advantages of entomopathogenic fungi, which are easy to cultivate, yield greater biomass and exhibit strong pathogenicity14. The substrate of rice can provide the essential nutrients for fungal growth via solid-state fermentation (SSF)15,16. Lowering the costs of substrates used for SSF has mainly focused on maximizing the yield of infective spores and enhancing their storage stability. The most used substrates are agroindustrial wastes such as coconut fiber, banana wastes, tea and coffee wastes, yucca wastes, and oil palm wastes17–19. The conidiation and germination of suitable fungal strains should also be considered in deciding SSF conditions, primarily the combination of moisture, temperature, and time20. The development of novel methods for optimizing both conidium production and germination are therefore essential for the supply of biopesticides based on Beauveria bassiana spores.
The purpose of the present work was to evaluate the feasibility of using BSG as a substrate for the production of biocontrol fertilizer (BF) via SSF employing the fungal strain BbQLU1. The specific process parameters for the production of BF were optimized by the surface response methodology. Additionally, the effective compounds and their effects on plant growth, which were assisted by BF, were explored.
Results and Discussion
Verification of the predicted optimal experimental conditions
When BSG was inoculated with BbQLU1 spores, the conidiation and germination rate fluctuated under the different treatments, indicating that temperature (A), water content (B), and time (C) have an interactive effect on the optimal conditions (Table s1). Three-dimensional graphs were generated for pair wise combinations of the three factors, with the third factor in each case fixed at its optimal level.
With regards to conidiation, response surface methodology (Table 1, Eq. 1; Fig. 1) determined that the response and test variables were related by the following second-order polynomial equation:
| 1 |
Table 1.
Compositions of the biocontrol fertilizer determined by GC-MS.
| Peak | Retention time (min) | Molecular formula | Compound name | Molecular weight | Similarity index |
|---|---|---|---|---|---|
| 1 | 7.900 | C8H24O4Si4 | Cyclotetrasiloxane, octamethyl- | 296 | 96 |
| 2 | 10.733 | C10H30O5Si5 | Decamethylcyclopentasiloxane | 370 | 95 |
| 3 | 11.700 | C5H9NO | 2-Piperidinone | 99 | 97 |
| 4 | 11.833 | C10H8 | Azulene | 128 | 94 |
| 5 | 11.950 | C11H16 | Diethylmethylbenzene | 148 | 83 |
| 6 | 12.067 | C9H10O2 | Methyl 3-methylbenzoate | 150 | 94 |
| 7 | 13.292 | C11H16 | Benzene, pentamethyl- | 148 | 93 |
| 8 | 13.433 | C12H36O6Si6 | Cyclohexasiloxane, dodecamethyl- | 444 | 93 |
| 9 | 13.625 | C11H10 | Naphthalene, 2-methyl- | 142 | 96 |
| 10 | 13.875 | C11H10 | Naphthalene, 1-methyl- | 142 | 96 |
Figure 1.
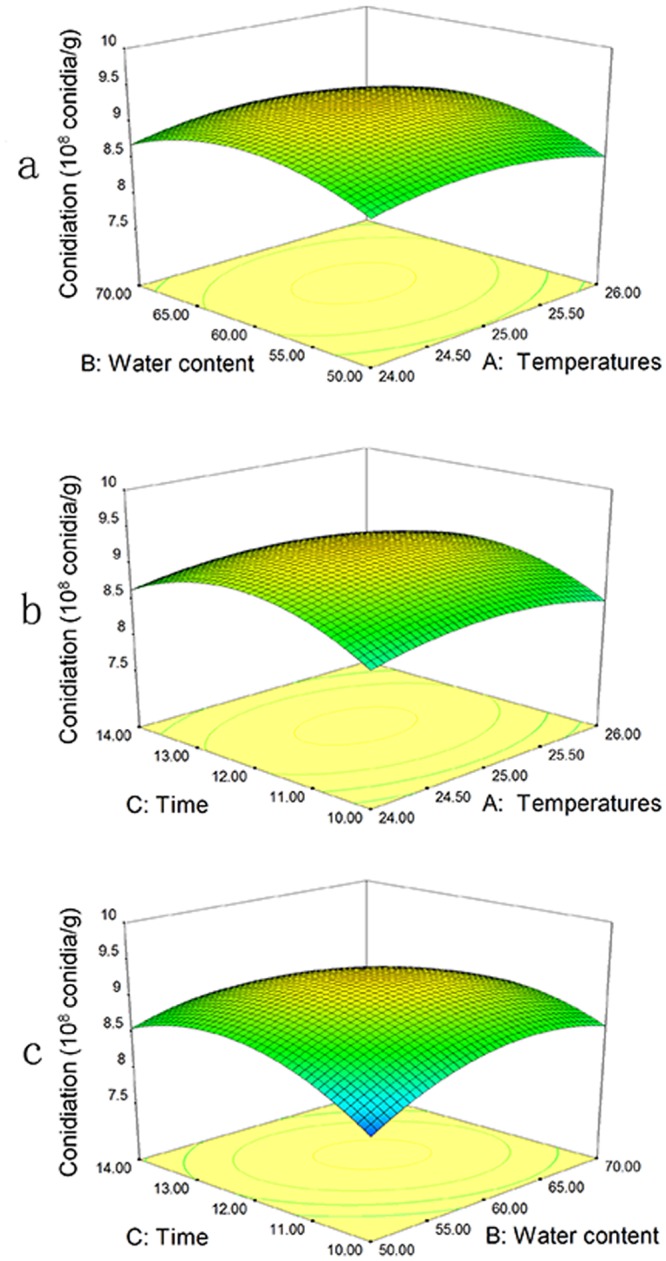
Response surface plots of conidiation of the strain BbQLU1. The interactions between (a) temperature and water content, (b) temperature and time, and (c) temperature and water content are shown.
From the ANOVA, the model F = 4.30, P(Prob > F) = 0.0338 (<0.05), indicating that the secondary model used in the experiment is statistically significant. An ANOVA revealed that the quadratic terms of water content (B2) and time (C2) significantly influenced conidiation (P < 0.05). The correlation measure for testing the goodness of fit of a regression equation is the adjusted determination coefficient, R2. Its value was only 0.8467, indicating that the correlation was not extremely high. In this case, the Lack of Fit F-value was 0.7220 (>0.05), which supports the model and the absence of Lack of Fit factors. The regression equation can therefore be used instead of the experimental points to analyze the experimental results, hinting that the effect of a specific experimental factor on response values is not a simple linear relationship. The fitted response for the above regression model, plotted in Fig. 1, demonstrates that the response surfaces for the two combinations were similar to each other. From the response surface in Fig. 1(c), fermentation time, compared to other variables, clearly had a significant effect on germination rate. The response surface diagram shows that within a certain range, the amount of sporulation is positively correlated with the temperature. When this range is exceeded, the amount of sporulation gradually decreases, indicating that the temperature increases to a certain value where sporulation peaks. The optimal temperature range, water content interval and fermentation time range were determined from Fig. 1 to be 24.47–25.47 °C, 53.38–63.38% and 11–13 days, respectively.
The second-order polynomial equation describing the germination rate (Table 1, Eq. 2; Fig. 2) was the following:
| 2 |
Figure 2.
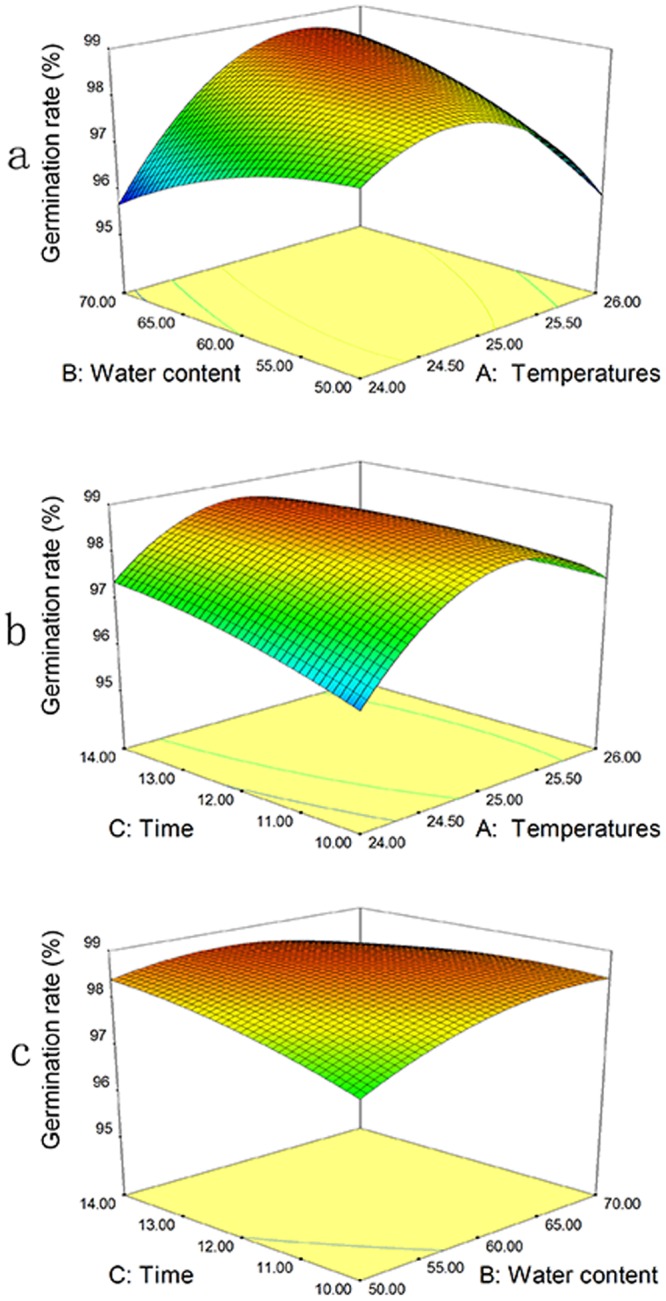
Response surface plots of the germination rate of the strain BbQLU1. The interactions between (a) temperature and water content, (b) temperature and time, and (c)temperature and water content are shown.
From the ANOVA, the model F = 11.64, P (Prob > F) = 0.0019 (<0.05), indicating that the secondary model used in the experiment is statistically significant. An ANOVA revealed that the interaction of temperature (A) and water content (B) significantly influenced the sporulation quantity (P < 0.01). The value of R2 was 0.9374, indicating that the correlation was extremely high. In this case, the Lack of Fit F-value is 0.0702 (>0.05), which supports the model and the absence of Lack of Fit factors. The regression equation can therefore be used instead of the experimental points to analyze the experimental results.
From the response surface in Fig. 2(a–c), temperature, compared to other variables, clearly had a significant effect on the germination rate. The response surface curve shows that the minimum temperature at which conidial germination occurred was 10 °C. Fig. 2(a,b) shows that increases in temperature from 24 to 25 °C were accompanied by increases in conidial germination, followed by a gradual decrease in the percentage of germinated conidia as the temperature further increased from 25 to 26 °C. Higher percentages of germinated conidia were obtained by varying the concentration of water and the fermentation time (Fig. 2(c)), which demonstrates that the response surfaces for the two combinations were similar to each other. The effect of temperature on spore germination rate is more distinct than the effects of water content and fermentation time. The optimal temperature, water content, and fermentation time ranges were determined from Fig. 2 to be 24.5–25.5 °C, 58.29–63.29%, and 11.5–13.2 days, respectively.
The development of processes to produce B. bassiana has primarily focused on lowering cost by maximizing the yield of effective conidial preparations. Some agro-industrial wastes such as rice straw, wheat bran, and grains of millet have been used as solid substrates for B. bassiana conidium production16,21. However, the use of BSG for BF production through the fermentation of B. bassiana, an entomopathogenic fungus, has not been reported. BSG is usually full of rich starch and fibre that can facilitate B. bassianaconidium germination and mycelial growth7,22. BSG has rich inert lignocellulose, which can retain water and increase the porosity in the substrate while maintaining a supply of moisture to promote fungal growth23,24. We used discarded BSG as the main raw material in this study to improve the hydrophobicity in SSF. Hydrophobicity is strikingly important in solid state culture25. The radial extension rate was somewhat considered an estimation of fungal ability to colonize the substrate on which it grows26. The radial extension rates were markedly enhanced after the addition of liquid mediaor standard Sabouraud Dextrose broth27.
The conditions that produced B. bassiana conidia should be considered for maintaining high levels of germination and constant quality while maximizing production15. The optimal fermentation conditions are temperature of 25.10 °C, water content of 61.55%, and time of 12.17 days, which resulted in a spore germination rate of 98.68% and conidiation of 0.85 × 108 spores/g. Furthermore, several investigators have reported that the optimum temperatures for B. bassiana mycelial growth, conidial germination, sporulation, and virulence are in the range from 20–30 °C21–28. Two optimized parameters were a 40% moisture content and 25 °C as the culture temperature when grown in a solid-state culture using rice as the B. bassiana substrate29. As another example, the optimum temperature for conidial germination and vegetative growth in several strains of B. bassiana is approximately 25 °C30.
Biocontrol efficacy of BF
The system of G. mellonella infection was used to bioassay the virulence of the BF. The results indicate that the concentration of BF remarkably affected the G. mellonella larvae mortality (Fig. 3, F3,8 = 509.5300, p < 0.001). Analyses of virulence by BF against G. mellonella larvae by cuticle penetrationat 1 × 10−2 g/ml (containing the 0.8 × 106spores) resulted in an LT50 of 3.2–4.1days (Fig. 3a). There was no significant difference in insecticidal effect, compared with the same concentration of spores cultured on SDAY. Based on the LT50 means, the mortality was 1.6-, 3.5, and 4.7-fold slower than this ratewhen treatments of 1 × 10−3, 1 × 10−4, and 1 × 10−5g/ml, respectively, were applied. All these estimates implicated significant virulence against G. mellonella larvae at all tested concentrations of BF. When the dead G. mellonella larvae were cultivated in moisturized filter paper for 2 days, their bodies gradually shrank and became black-gray. In addition, they were soon covered with white and fluffy mycelia, hinting that they had become infected and killed by B. bassiana spores (Fig. 3b). Microscopic observation of the dead larva clearly distinguished blastospores on the basis of size and morphology (Fig. 3c).
Figure 3.
Influence of biocontrol fertilizer on Galleria mellonella larvae following treatment with different concentrations (a) Median lethal time (LT50) for biocontrol fertilizer against G. mellonella larva (F3,8 = 509.5300, p < 0.001), (b) G. mellonella larvae treated with biocontrol fertilizer for 8 days, and (c) the B. bassiana in the G. mellonella larval tissues under an electron microscope are shown.
Previous studies have reported that the virulence of entomopathogenic fungi is affected by biotic and abiotic factors31.These biotic factors include the isolated fungus, the target species and another host. The pathogenicity of identified B. bassiana isolates was evaluated at a concentration of 109 conidia/ml against the G. mellonella, yielding a lethal time (LT50) of 1.7days32. On the other hand, the biotic factors primarily depend on environment conditions such as humidity and temperature in controlling post-harvest insects33,34.
The effect of the BF on plant growth
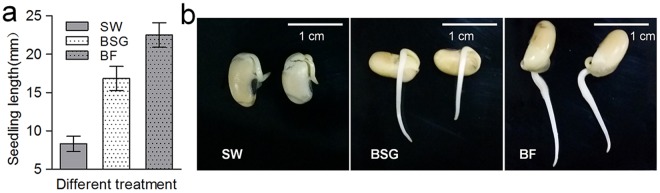
The effect of the BF on plant growth was determined by measuring root length, which was measured from the soybean surface to the tip of the sprout. Both BSG and BF had significant effects on almost all of the seedling growth parameters sampled 24 hours post-inoculation in both experimental replicates (Fig. 4a). The average seedling growth with sterilized water was 8.3 ± 0.6 mm; this value was 16.8 ± 0.9 mm when the sample was treated with BSG and, more significantly, 25.2 ± 0.9 mm when the sample was treated with BF. Treatment with BF significantly enhanced the percent seedling length (F2,6 = 75.592; P < 0.001), indicating that this experiment generated an extremely significant difference. Figure 4(b) shows that the root length of soybean was twice as long in the sample treated with BSG than that treated with sterile water and that the root length was 50% greater in the sample treated with BF than that treated with BSG. BSG is a novel rich and low-cost source of fiber and protein22, so the seedling can substantially absorb nutrients to increase the length of its root.
Figure 4.
Effects of 24 h of treatment of different seed inoculants (SW: sterile water, BSG: brewer’s spent grain, and BF: biocontrol fertilizer) on mean (±SE) seedling height. (a) A column diagram (F2,6 = 75.592; P < 0.001) and (b) a picture of grown plants are shown. Bars with different letters differ significantly from all seed treatment durations at the P = 0.05 level (Duncan’s Multiple Range Test after two-way ANOVA).
The root growth of seedlings is closely related to microgradients in the BF. These microgradients are involved in not only the rich BSG but also plant growth promoters35,36. In addition, they can directly affect colonization by mycelia and conidiation37. On the other hand, BF affected seedling growth more positively than BSG in our study. The tested endophyte B. bassiana plays a key role in growth promotion and productivity in BF38. Similar reports of plant growth promotion by endophytic fungal entomopathogens have been documented. Inoculation of wheat plants with B. bassiana has a significant impact, causing their root weight to become much greater than the root weight of control plants39.Entomopathogenic fungi can produce “siderophores” in soil to promote plant growth by increasing the pH value of the culture medium40,41. Furthermore, B. bassiana can colonize not only roots but also stems and leaves42,43. According to related literature, BF contains not only active B. bassiana microorganisms but also the metabolic pathways related to various organic substances. B. bassiana is present in roots to promote root growth, showing that greater speed and colonization are closely related. Several different endophytic fungi that exhibit tissue specificity possess have somewhat adapted to particular conditions present inside specific plant tissues. Many studies have reported that different fungi have varying ability to colonize different tissues of plants. For instance, Metarhizium anisopliaeICIPE 20 is seriously affected by the bean stem maggot, demonstrating that fungal endophytes can be promising tools for management44. Moreover, B. bassiana has been detected in both old and young stem/leaf tissuesin the fungus-treated maizeplants45. The tested benefits of BF on plant growth and nutrition to provide support for more sustainable biocontrol agents is thus currently underway.
Identification of the effective compounds in the BF
Identification of an unknown component in BF usingGC-MS depends on its retention time and mass spectra. Through GC-MS analysis, we were able to identify 10 low-molecular-weight polar metabolites, which are not easily determined by an LC-MS approach, using the unknown samples (Fig. 5a) BSG and (Fig. 5b) BF, respectively (Table 1).
Figure 5.
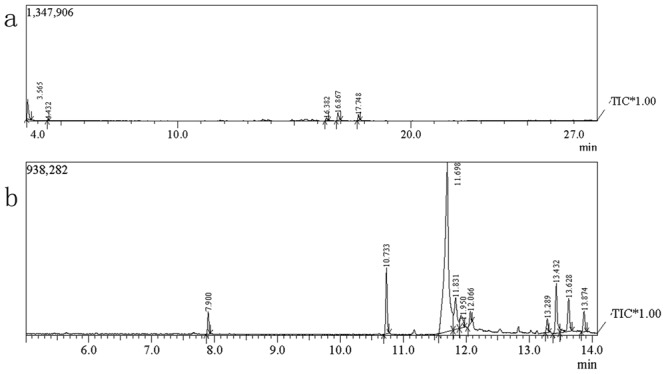
Total ion chromatograms for the gas chromatography-mass spectrometry analysis of (a) BSG and (b) BF.
The chemical constituents were identified by GC-MS, and the chemical components detected in peaksIII and VI could be insecticidally effective substances. The position of peak III, whose relative content was higher than that of peak VI, was 11.698 min, and this compound was identified as 2-piperidone by the GC-MS mass library. The position of peak VI, whose relative content was low, was 12.066 min, and this compound was identified as methyl 3-methylbenzoate by the GC-MS mass spectrometry library.
Several entomopathogenic fungus toxic bioactive compounds such as small secondary metabolites, cyclic peptides and macromolecular proteins can be purified46. For example, cyclicpeptides with immunosuppressive properties, cyclosporins A and C, were detected in B. bassiana mycelia, have an insecticidal effect on mosquito larvae and induce cuticular blackening47,48.An insect toxin protein, Bb70p, which was recently purified from B. bassiana 70, caused high mortality in G.mellonella and demonstrated a potential to enhance virulence49. Although 2-piperidone and methyl 3-methylbenzoate are shown above to be toxic components, their roles remain poorly understood. In the present study, the GC-MS mass spectra of BF have many small spectrogram peaks that are absent from the spectra of BSG, hinting that B. bassiana synthesized other unknown metabolites. Although very little is known about the secretion of bioactive metabolites by B. bassiana during growth metabolism or infection, we acknowledge that BF potentially increases plant growth and controls pests. The combination of pest management strategies currently requires the use of safe and environmentally sound biological control agents, so it is more efficient and cost effective than the fungicides in controlling pests.
Conclusions
This study is first to use BSG to produce BF through the fermentation of B. bassiana. BSG is usually full of rich starch and fibre that can facilitate B. bassiana conidium germination and mycelial growth. This experiment optimizes the culture conditions by the response surface analysis method, which not only optimizes the main factors controlling SSF and shortens the testing period but also effectively evaluates the interactions between factors. The optimum conditions for BF production are following the 60% water content at 25 °C for 12 days. In addition to the main factors of temperature, water content and fermentation time, the oxygen concentration, osmotic pressure and other factors will also alter the effects of BF50–53, and their ranges should thus be considered.
BSG enhances the nutritional value of BF, which facilitates conidial germination and speeds. Applications of BF on the G. mellonella larvae can be an effective and efficient biological control strategy for the management of soil-born insects. BF affected seedling growth more positively than BSG in our study. The tested endophyte B. bassiana may plays a key role in growth promotion and productivity in BF. Through GC-MS analysis, we were able to identify 10 low-molecular-weight polar metabolites, in which 2-piperidone and methyl 3-methylbenzoate could be insecticidally effective substances.
These results elucidate the production and function of BF, and add the new knowledge to what are known about the virulence and potential promotion of bioagent. Such theory and technique would be helpful for enriching the type of fertilizer and the biocontrol of soil-borne plant pathogens, which can propell the future industrialization of microbial fertilizers forward.
Methods
Test strains
The fungal strain BbQLU1 (China General Microbiology Culture CollectionCenter–CGMCC; Accession No. 11618) was cultured at 25 °C on Sabouraud dextrose agarplus yeast extract (SDAY, 4% glucose, 1% peptone, 1.5% agar and 1% yeast extract) medium for normal growth.
BSG
The BSG samples were collected from the Tsingtao Brewery located in Jinan, China, and stored at −20 °C until they were used. The fresh BSG was presented the following composition (w/w): 70.16% water, 16.92% nitrogen-free extract, 8.48% crude protein, 1.53% crude fat and 1.34% crude fiber. As soon as obtained, the material was dried at 50 ± 5 °C until approximately 15% moisture content. The BSG was not milled before pretreatment.
Inoculation of BSG and experimental design
Cultures of BbQLU1 were prepared by shaking 50 ml of SDAY at a concentration of 104 conidia per milliliter in 250 ml flasks at 25 °C for 3days, followed by inoculation. The combination of the cultures and the BSG was incubated under the different conditions listed in Table s1. The experimental design was a 3 × 3 × 3 factorial that includedthree fermentation temperatures, three water contents and three fermentation times. In total, 17 runs were performed as apart of the experimental design. The parameters of fermentation temperature, water content and fermentation time were chosen as the main variables and designated A, B, and C, respectively. Low, middle and high values of fermentation temperature, water content and fermentation time were designated −1, 0, and 1, respectively. The water content of BSG was adjusted by adding sterilized distilled water. The fermentations of BSG were incubated on plates (9 cm diameter) and incubated for 10 days. All experiments were performed in triplicate, and the average values of conidiation and germination rates were used to select the optimal conditions.
Assessment of conidiation capacity and germination rate
Conidia on each plate were washed off in 100 ml of 0.02% Tween 80 by 10 min of vibrations. Conidial concentrations were determined in the suspension by using microscopic counts with a hemocytometer and converted to the number of conidia pergramas an index of conidial yield. The conidia cultured on BSG were suspended at a concentration of 106 conidia/ml using 0.5% Tween-80 sterile solution. Next, 50 μL aliquots of conidial suspension was spread onto plates (9 cm) of GM (2% sucrose, 0.5% peptone and 1.5% agar), and the plates were incubated for 24 h at 25 °C and in a 12:12 h cycle. From 4 h onwards, the percent germination on each plate was assessed every 2 h using three microscopic counts.
Assessment of the virulence of conidia and the BF
G. mellonella infected by topical application was used to bioassay the virulence of BF via cuticle penetration. Briefly, batches of 30–40 G. mellonella larvae (~300 mg per larva) were immersed for ~10 s in 30 ml of 1 × 10−5, 1 × 10−4, 1 × 10−3,and 1 × 10−2 g/ml suspension with BF (treatment) or BSG with sterile distilled water (control). Each batch of the immersed larvae were incubated in Petri dishes (15 cm diameter) for 35 days at 25 °C and examined at 12 h intervals for mortality records. The median lethal time (LT50, days) of each strain against G. mellonella larvae was estimated by probit analysis of time-mortality trends from the bioassays. All the values and estimates from three repeated assays of the tested strains were analyzed via an analysis of variance (ANOVA).
The effect of BF on soybean growth
Surface-sterilized soybean seeds were immersed in 60% alcohol solution for 10 min and treated with sterile distilled water, BSG (0.01 g/ml), or BF (0.01 g/ml) for 8 h (n = 30 for each treatment). The seeds were maintained in the incubators at 25 °C for the designated duration. All experiments were performed in triplicate. Each batch of the immersed seeds was incubated in Petri dishes (15 cm diameter) at 25 °C, and their root lengths were examined.
GC-MS analysis
BF (1 g) was immersed in 100 ml of sterile water for 12 h, and the mixture was then centrifuged at 5000 r/min for 15 min. After that,100 ml of 75% ethanol was added, and the sample was allowed to precipitate for 24 h at room temperature. The sample was vortexed and then centrifuged at 5000 r/min for 15 min. The upper layer of the sample was added to twice its volume of ethyl acetate to soak overnight, and the upper, organic phase was taken in layers. The lower, aqueous phase was washed with twice its volume of ethyl acetate. This extraction procedure was repeated three times.The combined fractions of the upper, organic-phase ethyl acetate were concentrated under reduced pressure. The crude extract of the toxin was eluted by thin-layer chromatography to obtain pure toxin. After the toxin had been diluted with twice its volume of ethyl acetate, the concentrated solution was filtered through a 0.45-μm filter to obtain sample for spotting. The injection volume was 1.0 μL, and injections were made in splitless mode. The following oven temperature program was used with helium as the carrier gas: the temperature was maintained at 50 °C for 3 min, increased to 250 °C at a ramp rate of 10 °C/min and then held for 5 min, giving a total run time of 28 min. Electron impact ionization mode was used for ionization. The ionizing energy was 70 eV. Qualitative analysis was conducted in full scan mode (m/z range: 20–650).GC-MS data analysis was conducted by integrating eachresolved chromatogram peak. A 2-piperidone standard (purity 98%) was verified on a gas chromatograph to elute at 11.609 min. The results showed that the peak time for the standard and peak III was approximately the same. A methyl 3-methylbenzoate standard (purity 99%) was verified on a gas chromatograph to elute at 12.042 min. Identification of toxic components was performed by searching for mass spectra using the NIST08 and the Golm (http://gmd.mpimp-golm.mpg.de/) libraries and when available, by comparison with analytical standards.
Statistics
Differences between the results from treatments were calculated and statistically analyzed using ANOVA and Box-Behnken tests (P < 0.05). Surface response methodologywas applied to evaluate the effects of the three factors on BbQLU1conidiation and germination rate. The parameters oftemperature, water content and time were chosen as the mainvariables and designated A, B and C, respectively. Low, middleand high values oftemperature, water content and time were designated −1, 0 and 1, respectively. Response surface models were constructedusing Design-Expertsoftware(trial version 8.0.5.0; Stat-Ease, Inc.,Minneapolis, MN, USA).
Supplementary information
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31600063), the Shandong Provincial Natural Science Foundation, China (ZR201702070054) and the Project of Shandong Province Higher Educational Science and Technology Program (J16LE05).
Author Contributions
The design of research was planned by J.J.W. and L.Q. The experiments were performed by J.J.L. and Z.L. Results were discussed by all co-authors. The manuscript was written by.J.J.W. and revised by all the authors. L.Q. coordinated the whole work.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Qiu, Jiao-Jiao Li and Zhen Li contributed equally.
Contributor Information
Lei Qiu, Email: qiulei.2005@163.com.
Juan-Juan Wang, Email: wjj880414@163.com.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36949-1.
References
- 1.Vieira E, et al. Valuation of brewer’s spent grain using a fully recyclable integrated process for extraction of proteins and arabinoxylans. Ind Crop Prod. 2014;52:136–143. doi: 10.1016/j.indcrop.2013.10.012. [DOI] [Google Scholar]
- 2.Lynch KM, Steffen EJ, Arendt EK. Brewers’ spent grain: a review with an emphasis on food and health. J Inst Brew. 2016;122:553–568. doi: 10.1002/jib.363. [DOI] [Google Scholar]
- 3.Ben-Hamed U, Seddighi H, Thomas K. Economic returns of using brewery’s spent grain in animal feed. World Acad Sci Eng Technol. 2011;5:595–598. [Google Scholar]
- 4.Byrne, J. FDA reviewing part of animal feed rule after fury over spent grains proposal.Availableat: http://www.feednavigator.com/Regulation/FDAreviewing-part-of-animal-feed-rule-after-fury-over-spent-grains-proposal accessed (2014).
- 5.Liguori R, Soccol CR, Vandenberghe LPD, Woiciechowski AL, Faraco V. Second generation ethanol production from brewers’ spent grain. Energies. 2015;8:2575–2586. doi: 10.3390/en8042575. [DOI] [Google Scholar]
- 6.Xiros C, Topakas E, Katapodis P, Christakopoulos P. Hydrolysis and fermentation of brewer’s spent grain by Neurospora crassa. Bioresour Technol. 2008;9:5427–5435. doi: 10.1016/j.biortech.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Mussatto SI. Brewer’s spent grain: a valuable feedstock for industrial applications. J Sci Food Agric. 2014;94:1264–1275. doi: 10.1002/jsfa.6486. [DOI] [PubMed] [Google Scholar]
- 8.Malakhova DV, Egorova MA, Prokudina LI, Netrusov AI, Tsavkelova EA. The biotransformation of brewer’s spent grain into biogas by anaerobic microbial communities. World J Microbiol Biotechnol. 2015;31:2015–2023. doi: 10.1007/s11274-015-1951-x. [DOI] [PubMed] [Google Scholar]
- 9.Das R, Das SJ, Das AC. Effect of synthetic pyrethroid insecticides on N-2- fixation and its mineralization in tea soil. Eur J Soil Biol. 2016;74:9–15. doi: 10.1016/j.ejsobi.2016.02.005. [DOI] [Google Scholar]
- 10.Nagaraju J, Gopinath G, Sharma V, Shukla JN. Lepidopteran sex determination: a cascade of surprises. Sex Dev. 2014;8:104–112. doi: 10.1159/000357483. [DOI] [PubMed] [Google Scholar]
- 11.Quiroz RD, et al. Challenges and opportunities of the bio-pesticides production by solid-state fermentation: filamentous fungi as a model. Crit Rev Biotechnol. 2015;35:326–333. doi: 10.3109/07388551.2013.857292. [DOI] [PubMed] [Google Scholar]
- 12.Pretty J, Bharucha ZP. Integrated pest management for sustainable intensification of agriculture in Asia and Africa. Insects. 2015;6:152–182. doi: 10.3390/insects6010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faria MR, Wraight SP. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control. 2007;43:237–256. doi: 10.1016/j.biocontrol.2007.08.001. [DOI] [Google Scholar]
- 14.Zhang JX, et al. Bacillus amyloliquefaciens, GB1 can effectively control apple valsa canker. Biol Control. 2015;88:1–7. doi: 10.1016/j.biocontrol.2015.04.022. [DOI] [Google Scholar]
- 15.Lopez-Perez M, Rodriguez-Gomez D, Loera O. Production of conidia of beauveria bassiana in solid-state culture: current status and future perspectives. Crit Rev Biotechnol. 2015;35:334–341. doi: 10.3109/07388551.2013.857293. [DOI] [PubMed] [Google Scholar]
- 16.Kang SW, Lee SH, Yoon CS, Kim SW. Conidia production by Beauveria bassiana (for the biocontrol of a diamondback moth) during solid-state fermentation in a packed-bed bioreactor. Biotechnol Lett. 2005;27:135–139. doi: 10.1007/s10529-004-7871-8. [DOI] [PubMed] [Google Scholar]
- 17.Xie L, Chen HM, Yang JB. Conidia production by Beauveria bassiana on rice in solid-state fermentation using tray bioreactor. Adv Mater Res. 2013;610:3478–3482. doi: 10.1002/adma.201300886. [DOI] [Google Scholar]
- 18.Mascarin GM, Jaronski ST. The production and uses of Beauveria bassiana, as a microbial insecticide. World J Microbiol Biotechnol. 2016;32:177–203. doi: 10.1007/s11274-016-2131-3. [DOI] [PubMed] [Google Scholar]
- 19.Thomas L, Larroche C, Pandey A. Current developments in solid state fermentation. Biochem Eng J. 2013;81:146–161. doi: 10.1016/j.bej.2013.10.013. [DOI] [Google Scholar]
- 20.Muñiz-Paredes F, Miranda-Hernández F, Loera O. Production of conidia by entomopathogenic fungi: from inoculants to final quality tests. World J Microbiol Biotechnol. 2017;33:57–66. doi: 10.1007/s11274-017-2229-2. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS, Skinner M, Parker BL. Influence of whey permeate and millet as substrates on thermotolerance of beauveria bassiana and metarhizium anisopliae conidia during storage. Biocontrol Sci Technol. 2010;20:859–863. doi: 10.1080/09583157.2010.486072. [DOI] [Google Scholar]
- 22.Ktenioudaki A, Chaurin V, Reis SF, Gallagher E. Brewer’s spent grain as a functional ingredient for breadsticks. Int J Food Sci Tech. 2012;47:1765–1771. doi: 10.1111/j.1365-2621.2012.03032.x. [DOI] [Google Scholar]
- 23.Arzumanov T, Jenkins N, Roussos S. Effect of aeration and substrate moisture content on sporulation of Metarhizium anisopliae var. acridum. Process Biochem. 2005;40:1037–1042. doi: 10.1016/j.procbio.2004.03.013. [DOI] [Google Scholar]
- 24.Dorta B, Arcas J. Sporulation of Metarhizium anisopliae in solid state fermentation with forced aeration. Enzyme Microb Technol. 1998;23:501–505. doi: 10.1016/S0141-0229(98)00079-9. [DOI] [Google Scholar]
- 25.Holder DJ, Kirkland BH, Lewis MW, Keyhani NO. Surface characteristics of the entomopathogenic Fungus Beauveria (Cordyceps) bassiana. Microbology. 2007;53:3448–3457. doi: 10.1099/mic.0.2007/008524-0. [DOI] [PubMed] [Google Scholar]
- 26.Cortes-Espinosa DV, et al. Selection and identification of fungi isolated from sugarcane bagasse and their application for phenanthrene removal from soil. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41:475–86. doi: 10.1080/10934520500428351. [DOI] [PubMed] [Google Scholar]
- 27.Alves SB, Rossi LS, Lopes RB, Tamai MA, Pereira RM. Beauveria bassiana yeast phase on agar medium and its pathogenicity against Diatraea saccharalis (Lepidoptera Crambidae) and Tetranychus urticae (Acari: Tetranychidae) J Invertebr Pathol. 2002;81:70–77. doi: 10.1016/S0022-2011(02)00147-7. [DOI] [PubMed] [Google Scholar]
- 28.Tefera T, Pringle K. Germination, radial growth, and sporulation of beauveria bassiana and metarhizium anisopliae isolates and their virulence to chilo partellus (lepidoptera: pyralidae) at different temperatures. Biocontrol Sci Technol. 2003;13:699–704. doi: 10.1080/0958315031000151756. [DOI] [Google Scholar]
- 29.Pham TA, Kim JJ, Kim K. Optimization of solid-state fermentation for improved conidia production of Beauveria bassiana as a mycoinsecticide. Microbiology. 2010;38:137–143. doi: 10.4489/MYCO.2010.38.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumbos CI, Athanassiou CG. Use of entomopathogenic fungi for the control of stored-product insects:can fungi protect durable commodities. J Pest Sci. 2017;90:839–854. doi: 10.1007/s10340-017-0849-9. [DOI] [Google Scholar]
- 31.Ibrahim AA, Mohamed HF, El-Naggar SEM, Swelim MA, Elkhawaga OE. Isolation and Selection of Entomopathogenic Fungi as Biocontrol Agent against the Greater Wax Moth, Galleria mellonella L. (Lepidoptera: Pyralidae) Egypt J Biol Pest Co. 2016;26:249–253. [Google Scholar]
- 32.Ekesi S, Maniania NK, Ampong-Nyarko K. Effect of temperature on germination, radial growth and virulence of Metarhizium anisopliae and Beauveria bassiana on Megalurpthrups sjostedti. Biocontrol Sci Technol. 1999;9:177–185. doi: 10.1080/09583159929767. [DOI] [Google Scholar]
- 33.Athanassiou CG, Steenberg T. Insecticidal effect of beauveria bassiana (balsamo) vuillemin (ascomycota: hypocreaes) in combination with three diatomaceous earth formulations against sitophilus granarius (l.) (coleoptera: curculionidae) Biol Control. 2007;40:411–416. doi: 10.1016/j.biocontrol.2006.12.001. [DOI] [Google Scholar]
- 34.Vassilakos TN, Athanassiou CG, Kavallieratos NG, Vayias BJ. Influence of temperature on the insecticidal effect of beauveria bassiana in combination with diatomaceous earth against rhyzopertha dominica and sitophilus oryzae on stored wheat. Biol Control. 2006;38:270–281. doi: 10.1016/j.biocontrol.2006.03.009. [DOI] [Google Scholar]
- 35.Liao XG, O’Brien TR, Fang WG, St Leger RJ. The plant beneficial effects of Metarhizium species correlate with their association with roots. Appl Microbiol Biotechnol. 2014;98:7089–7096. doi: 10.1007/s00253-014-5788-2. [DOI] [PubMed] [Google Scholar]
- 36.Sánchez–Rodríguez AR, et al. An endophytic Beauveria bassiana strain increases spike production in bread and durum wheat plants and effectively controls cotton leafworn (Spodoptera littoralis) larvae. Biol Control. 2018;116:90–102. doi: 10.1016/j.biocontrol.2017.01.012. [DOI] [Google Scholar]
- 37.Ortega-Sa´nchez E, Loera O, Viniegra-Gonza´lez G. The effect of the ratio between substrate concentration and specific area of the support on the biomass yield of fungal surface cultures. Rev Mex Ing Quim. 2012;11:485–494. [Google Scholar]
- 38.Parsa S, Ortiz V, Vega FE. Establishing fungal entomopathogens as endophytes: Towards endophytic biological control. J Vis Exp. 2013;74:e50360. doi: 10.3791/50360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurulingappa P, Sword GA, Murdoch G, McGee PA. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol Control. 2010;55:34–41. doi: 10.1016/j.biocontrol.2010.06.011. [DOI] [Google Scholar]
- 40.Raya DS, Quesada ME, Barrón V, Del Campillo MC, Sánchez Rodríguez AR. Redefining the dose of the entomopathogenic fungus Metarhizium brunneum (Ascomycota, Hypocreales) to increase Fe bioavailability and promote plant growth in calcareous and sandy soils. Plant Soil. 2017;418:387–404. doi: 10.1007/s11104-017-3303-0. [DOI] [Google Scholar]
- 41.Jirakkakul J, et al. Tenellin acts as an iron chelator to prevent iron-generated reactive oxygen species toxicity in the entomopathogenic fungus beauveria bassiana. Fems Microbiol Lett. 2015;362:1–8. doi: 10.1093/femsle/fnu032. [DOI] [PubMed] [Google Scholar]
- 42.Jaber LR. Grapevine leaf tissue colonization by the fungal entomopathogen Beauveria bassiana s.l. and its effect against downy mildew. BioControl. 2015;60:103–112. doi: 10.1007/s10526-014-9618-3. [DOI] [Google Scholar]
- 43.Akello J, et al. Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.) J Invertebr Pathol. 2007;96:34–42. doi: 10.1016/j.jip.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Mutune B, et al. Fungal endophytes as promising tools for the management of bean stem maggot ophiomyia phaseoli, on beans phaseolus vulgaris. J Pest Sci. 2016;89:1–9. doi: 10.1007/s10340-015-0725-4. [DOI] [Google Scholar]
- 45.Renuka S, Ramanujam B, Poornesha B. Endophytic Ability of Different Isolates of Entomopathogenic Fungi Beauveria bassiana (Balsamo) Vuillemin in Stem and Leaf Tissues of Maize (Zea mays L.) Indian J Microb. 2016;56:126–133. doi: 10.1007/s12088-016-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strasser H, Vey A, Butt TM. Are there any risks in using entomopathogenic fungi for pest control, with particular reference to the bioactive metabolites of metarhizium, tolypocladium and beauveria species. Biocontrol Sci Technol. 2000;10:717–735. doi: 10.1080/09583150020011690. [DOI] [Google Scholar]
- 47.Weiser J, Maťha V. The insecticidal activity of cyclosporines on mosquito larvae. J Invertebr Pathol. 1988;51:92–93. doi: 10.1016/0022-2011(88)90092-4. [DOI] [PubMed] [Google Scholar]
- 48.Weiser J, Matha V, Zizka Z, Jeqorov A. Pathology of cyclosporin A in mosquito larvae. Cytobios. 1989;59:238–239. [PubMed] [Google Scholar]
- 49.Khan S, et al. Identification and characterization of an insect toxin protein, Bb70p, from the entomopathogenic fungus, Beauveria bassiana, using Galleria mellonella as a model system. J Invertebr Pathol. 2016;133:87–94. doi: 10.1016/j.jip.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Tlecuitl-Beristain S, Viniegra-González G, Díaz-Godínez G, Loera O. Medium selection and effect of higher oxygen concentration pulses on metarhizium anisopliae var. lepidiotum conidial production and quality. Mycopathologia. 2010;169:387–394. doi: 10.1007/s11046-009-9268-7. [DOI] [PubMed] [Google Scholar]
- 51.Garza-López PM, Konigsberg M, Saucedo-Castaneda G, Loera O. Differential profiles of Beauveria bassiana (Bals.-Criv.) Vuill. as a response to CO2: production of conidia and amylases. Agrociencia-mexico. 2011;45:761–770. [Google Scholar]
- 52.Rodriguez-Gomez D, Loera O, Saucedo-Castan˜eda G, Viniegra-Gonzalez G. Substrate influence on physiology and virulence of Beauveria bassiana acting on larvae and adults of Tenebrio molitor. World J Microbiol Biotechnol. 2009;25:513–518. doi: 10.1007/s11274-008-9917-x. [DOI] [Google Scholar]
- 53.Kim JS, Kassa A, Skinner M, Hata T, Parker BL. Production of thermotolerant entomopathogenic fungal conidia on millet grains. J Ind Microbiol Biotechnol. 2011;38:697–704. doi: 10.1007/s10295-010-0850-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.