Abstract
Heterodera glycines is the most pervasive soybean pests worldwide. Biocontrol provides a strategy to sustainably control nematodes. In this study, 22 fungal isolates were obtained and identified from cysts of Heterodera spp. Among them, Aspergillus niger NBC001 showed high nematicidal activity against H. glycines. The 2-fold dilution of NBC001 culture filtrate caused 89% mortality of second-stage juveniles and inhibited more than 98% of egg hatching in vitro. In both pot and field experiments, the numbers of H. glycines cysts in soybean seedlings dressed with the the 5-fold concentrated culture filtrate of NBC001 were significantly reduced by 43% and 28%, respectively. In addition, application of NBC001 remarkably reduced the penetration of nematodes into the roots. Histochemical and fluorometric staining analyses indicate that application of NBC001 stimulated hydrogen peroxide activity in the roots and triggered callose deposition in the leaves and roots. Transcription of the PR1a and EREBP genes in the salicylic acid and ethylene signaling pathways was upregulated in soybean plants treated with NBC001. However, the application of concentrated culture filtrate of NBC001 had no significant impacts on the soil microbial community based on next generation DNA sequencing technology. In summary, NBC001 may be a good biocontrol agent against H. glycines via stimulation of the immunity/defense of the plant host.
Introduction
Soybean cyst nematodes (SCN, Heterodera glycines Ichinohe) are one of the most economically important pests causing considerable damage to soybean [Glycine max (L.) Merr.]1. SCN causes more than $120 million dollars in yield losses annually in China alone2,3. Chemical nematicides are the efficient ways to control H. glycines, but their application is being increasingly limited or banned due to their toxicity to humans and ecosystems4. Therefore, alternative safe methods are urgently required to control H. glycines. The use of biocontrol agents to suppress H. glycines could provide an alternative strategy to sustainably control H. glycines because of their eco-friendliness and general non-toxicity to humans.
Previously assessed biological control agents for managing H. glycines include nematophagous fungi, endoparasitic fungi, fungi that produce antibiotic substances, plant-growth-regulatory bacteria, and fungi or bacteria that induce systemic resistance (ISR) and plant defense responses in soybean5. Among these, Aspergillus species are very common in agricultural and non-agricultural soils, and are known to be lethal to a variety of plant-parasitic nematodes6. This fungus could significantly reduce the population of plant-parasitic nematodes in the soil and enhance the yield of plants7,8. Other biological control agents control nematodes by either producing antibiotic substances, parasitizing nematodes or inducing plant resistance to plant-parasitic nematodes. However, A. niger control nematodes by all the three ways. Oxalic acid, citric acid and undetermined compounds with molecule weight greater than 8000 have previously been reported to be nematicidal metabolites of A. niger7–9. The producation of NH3, HCN and siderophores may also contribute to the suppression of plant-parasitic nematodes10,11. In addition, A. niger has also been found to parasitize nematodes. Research has shown that the fungus grows and sporulates well on eggs, juveniles and adult females of root-knot nematodes11. Another mechanism of A. niger against plant-parasitic nematodes is that A. niger could induce the plant resistance. Li et al. (2011) reported that A. niger could reduce the nematode populations and promote the growth of tomato plants by increasing the activities of defense enzymes (phenylalanine ammonia, polyphenol oxidase, peroxidase, superoxide dismutase and catalase)12. This finding indicated that A. niger could markedly induce the resistance of tomatoes to plant-parasitic nematodes. However, the possibility that A. niger interacts with plant tissues and induces host plant resistance to pathogens has seldom been studied.
Most studies have focused on the effects of biocontrol microorganisms on the pathogens but neglected the responses of the host plant. Plants are able to recognize microbe and tailor their defense responses. These responses include the accumulation of phytoalexins, reinforcement of plant cell walls, production of reactive oxygen species (ROS) and callose, and synthesis of pathogenesis-related (PR) proteins, such as chitinases and glucanases13. Pathogen-induced systemic resistance, known as systemic acquired resistance (SAR), is commonly associated with salicylic acid (SA) and pathogenesis-related protein 1 (PR-1), while beneficial microorganisms trigger induced systemic resistance (ISR), which is often dependent on the jasmonate (JA) and/or ethylene (ET) signaling pathways with the concomitant induction of LOX and EREBP14. However, some ISR inducers also appear to activate the SA-dependent pathway, indicating that different signaling pathways may be operated when ISR is elicited15.
Soil microbes play a predominant role in agro-ecosystem. They can affect soil nutrient cycling, organic matter formation and decomposition and soil structure, thus influencing ecosystem stability16. The application of biocontrol agents could perturb the composition and function of soil microbial communities17. This might affect the soil quality for sustainable plant production and the promotion of plant health. Therefore, it is necessary to assess the effects of biocontrol agents on the soil microbial communities before they are used extensively.
In this study, we isolated strain A. niger NBC001 from Heterodera spp. cysts, and investigated the biocontrol efficacy of A. niger NBC001 against H. glycines on soybean. The ability of the ISR activity in soybean to suppress H. glycines infection was studied through the application of A. niger strain. In addition, we applied next generation sequencing (NGS) technology to compare the changes in soil microorganisms in response to the application of NBC001 and provide basic insights on risk assessment before the future commercialization of NBC001. These results will provide new strategies for the practical management of H. glycines and new insights into the mechanisms underlying the biocontrol processes of A. niger.
Results
Isolation and identification of fungi
Twenty-two fungal isolates were obtained from cysts of Heterodera spp. collected from the Xuchang District, Henan, China and Østfold, Norway. The fungal isolates represented ten genera, and Mortierella alpina was the most prominent species (Table 1). Thirteen representative isolates were selected for further study.
Table 1.
Fungal isolates obtained from Heterodera spp.
| Accession number | Fungal species | Sampling locations | Isolate from |
|---|---|---|---|
| NBC001 | Aspergillus niger | Østfold, Norway | H. avenae |
| NBC002 | Aspergillus flavus | Østfold, Norway | H. avenae |
| NBC003 | Aspergillus fumigatus | Østfold, Norway | H. avenae |
| NBC004 | Periconia macrospinosa | Østfold, Norway | H. avenae |
| NBC006 | Penicillium sp. | Østfold, Norway | H. avenae |
| NBC008 | Penicillium oxalicum | Østfold, Norway | H. avenae |
| NBC010 | Alternaria alternata | Østfold, Norway | H. avenae |
| NBC011 | Asperigillus fumigatus | Østfold, Norway | H. avenae |
| NBC012 | Penicillium oxalicum | Østfold, Norway | H. avenae |
| NBC013 | Doratomyces sp. | Østfold, Norway | H. avenae |
| NBC014 | Microdochium bolleyi | Østfold, Norway | H. avenae |
| NBC015 | Plectosphaerella cucumerina | Xu Chang district, Henan, China | H. filipjevi |
| NBC016 | Trichoderma brevicompactum | Xu Chang district, Henan, China | H. filipjevi |
| NBC017 | Mortierella alpina | Xu Chang district, Henan, China | H. filipjevi |
| NBC018 | Mortierella alpina | Xu Chang district, Henan, China | H. filipjevi |
| NBC019 | Mortierella alpina | Xu Chang district, Henan, China | H. filipjevi |
| NBC020 | Mortierella alpina | Xu Chang district, Henan, China | H. filipjevi |
| NBC021 | Mortierella alpina | Xu Chang district, Henan, China | H. filipjevi |
| NBC022 | Mortierella alpina | Xu Chang district, Henan, China | H. filipjevi |
| NBC023 | Mortierella alpina | Xu Chang district, Henan, China | H. filipjevi |
| NBC024 | Mortierella alpina | Xu Chang district, Henan, China | H. filipjevi |
| NBC025 | Fusarium oxyporum | Xu Chang district, Henan, China | H. filipjevi |
Effects of the isolated fungi on the J2s mortality of H. glycines in vitro
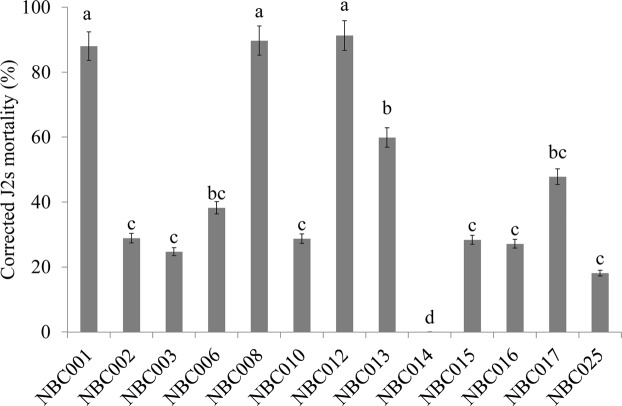
Thirteen fungal isolates were used to test for nematicidal activity in vitro. Although the nematicidal activity varied among the fungal species and isolates, almost of all the isolates could kill the J2s of H. glycines to different degrees (Fig. 1). Among them, the treatments of 2-fold dilutions of the culture filtrates of Aspergillus niger NBC001, Penicillium oxalicum NBC008 and Penicillium oxalicum NBC012 resulted in significantly higher corrected J2s mortalities of H. glycines than others, which were 88%, 90% and 92%, respectively (P < 0.05). NBC001 was selected for further study because some Aspergillus species have already been studied for their biocontrol potentials against plant parasitic nematodes18.
Figure 1.
Effects of isolated fungi on the J2s mortality of H. glycines. Corrected J2s mortalities of H. glycines after exposure to the culture filtrate of the isolated fungi. The error bars represent the standard deviation. The different letters on the bar represent significant differences at the 0.05 level based on Tukey’s multiple comparison test.
Effects of NBC001 on the J2s mortality and egg hatchability of H. glycines in vitro
As shown in Fig. 2, all of the culture filtrate of NBC001 at various concentrations had significant effects on the J2s mortality and egg hatchability of H. glycines. The 2 × , 10 × , and 20 × dilutions of the culture filtrate of NBC001 caused 89%, 45% and 31% corrected J2s mortality of H. glycines, respectively. The egg hatchability in all the treatments of the 2 × , 10 × , and 20 × dilutions of the culture filtrate of NBC001 was significantly lower than those in the control, and the correspondingly relative inhibitions of hatchability were 98%, 89%, 22%, respectively. These results indicate that NBC001 showed high nematicidal activity against H. glycines.
Figure 2.
Effect of Aspergillus niger NBC001 on the J2s mortality and hatchability of H. glycines. Corrected J2s mortality and inhibition of the hatchability of H. glycines after exposure to various concentrations of Aspergillus niger NBC001 culture filtrate. The error bars represent standard deviation. The different letters on each bar represent significant differences at the 0.05 level based on Tukey’s multiple comparison test.
Efficacy of NBC001 against H. glycines
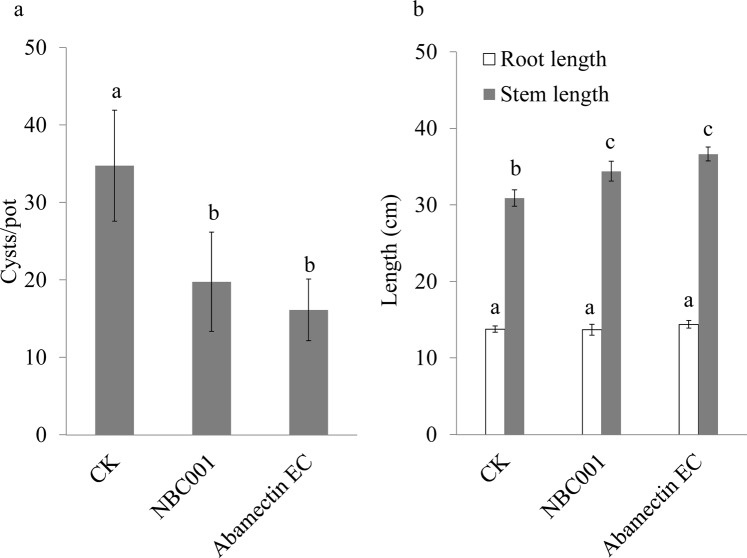
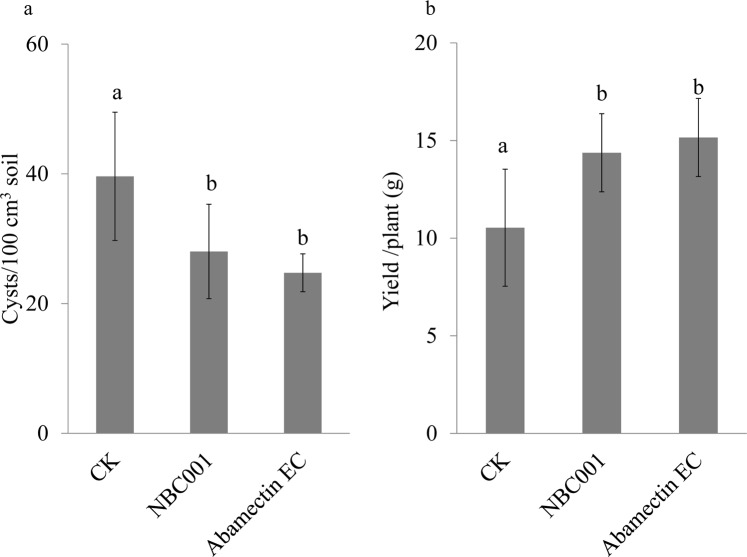
The efficacy of the 5-fold concentrated culture filtrate of NBC001 against H. glycines was tested in both the pot and the field (Figs 3 and 4). In the pot, the numbers of H. glycines cysts in the NBC001 treatment was significantly reduced by 43% at 30 d post-transplantation compared to that in the control (Fig. 3a, P < 0.05), but those cyst numbers in the NBC001 treatment showed no significant differences from those in the abamectin EC treatment (53% of reduction compared to the control). The stem lengths of the soybeans in both the NBC001 treatment and the abamectin EC treatment were significantly increased by 10% and 16%, respectively, compared to that in the control (Fig. 3b, P < 0.05). However, the root lengths of the soybean did not show significant differences among any of the treatments (Fig. 3b, P > 0.05). In the field, the NBC001 and abamectin EC treatments significantly reduced the numbers of H. glycines cysts by 28% and 38% at 90 d post-transplantation, and increased the yield by 37% and 44.8%, respectively (Fig. 4a,b, P < 0.05). These results indicate that soybean seedlings dressed with the culture filtrate of NBC001 could control H. glycines in both the pot and the field, and the control efficacy of NBC001 was not significantly different from that of the commercial pesticide abamectin EC.
Figure 3.
Effect of Aspergillus niger NBC001 on H. glycines control in the pot. The numbers of H. glycines cysts in the soil (a) and stem and root lengths of soybean (b) at 30 d post-transplantation in the pot. The error bars represent the standard deviation. The different letters on each bar represent significant differences at the 0.05 level based on Tukey’s multiple comparison test.
Figure 4.
Effect of Aspergillus niger NBC001 on H. glycines control in the field. The numbers of H. glycines cysts in the soil at 90 d post-transplantation in the field. The error bars represent the standard deviation. The different letters on each bar represent significant differences at the 0.05 level based on Tukey’s multiple comparison test.
Effect of NBC001 on nematode development in soybean roots
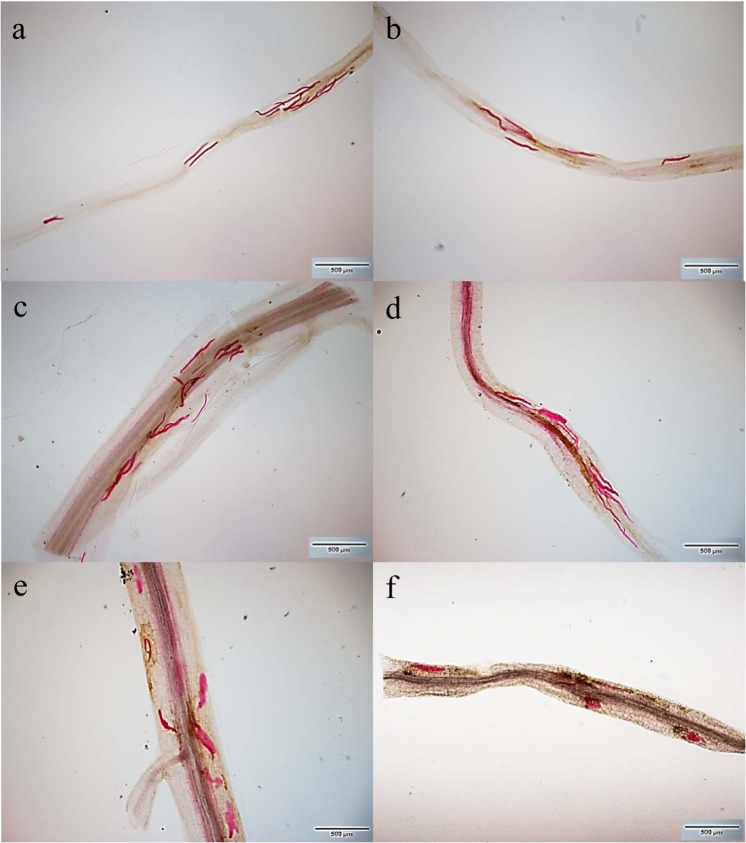
At 3, 7 and 14 d post-inoculation, significantly fewer juveniles were observed in the roots of the NBC001 treatment than in the roots of the control (Table 2, Fig. 5, P < 0.05). At 3 d post-inoculation, the juveniles in the soybean roots were all at the J2 stage (Fig. 5a,b). At 7 d post inoculation, significantly fewer J2 juveniles were observed in the NBC001-treated soybean roots than that in the control soybean roots (P < 0.05), but the numbers of J3 juveniles among the treatments were not significantly different (Table 2, Fig. 5c,d, P > 0.05). At 14 d post-inoculation, the numbers of J2 and J3 juveniles in the NBC001 treatment were significantly lower than those in the control roots (P < 0.05), but the numbers of J4 juveniles among the treatments were not significantly different (Table 2, Fig. 5e,f, P > 0.05). All the results indicate that the application of NBC001 significantly reduced the nematode penetration rates but did not affect nematode’s development inside the roots.
Table 2.
Numbers of H. glycines at different developmental stages in the soybean roots.
| Time* | Treatments | Numbers of different stages of juveniles | Numbers of total juveniles | ||
|---|---|---|---|---|---|
| J2 | J3 | J4 | |||
| 3 DPI | CK | 143 ± 41a | 0 ± 0 | 0 ± 0 | 143 ± 41b |
| NBC001 | 92 ± 33b | 0 ± 0 | 0 ± 0 | 92 ± 33c | |
| 7 DPI | CK | 132 ± 25a | 64 ± 31a | 0 ± 0 | 196 ± 53a |
| NBC001 | 93 ± 33b | 62 ± 28a | 0 ± 0 | 155 ± 60b | |
| 14 DPI | CK | 56 ± 5c | 60 ± 4a | 24 ± 1a | 140 ± 8b |
| NBC001 | 32 ± 5d | 32 ± 5b | 16 ± 4a | 80 ± 11c | |
The different letters on each bar represent significant differences at the 0.05 level based on Tukey’s multiple comparison test.
*DPI, days post-inoculation. Mean ± standard deviation of the mean from four soybean root replicates.
J2, second-stage juvenile; J3, third-stage juvenile; J4, fourth-stage juvenile.
Figure 5.
Penetration and development of H. glycines in soybean roots. Observations of juveniles of different developmental stages in the control soybean root group at 3 DPI (a), 7 DPI (c), and 14 DPI (e). Observations of juveniles of different developmental stages in the NBC001 treated soybean root group at 3 DPI (b), 7 DPI (d), and 14 DPI (f). Scale bars represent 500 μm.
Effects of NBC001 on callose deposition and ROS production in soybean
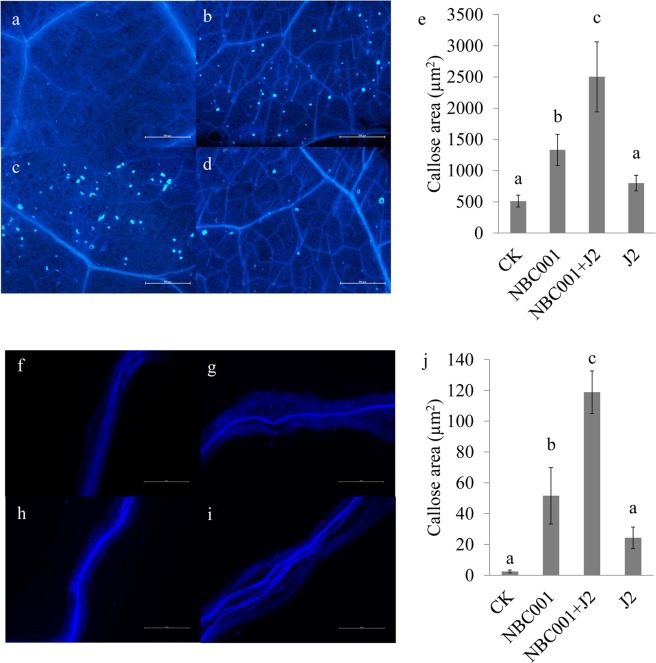
We hypothesized that NBC001 could activate the soybean defense responses. Therefore, callose deposition and ROS production in soybean were evaluated at 24 h post-inoculation. Treatment of the soybean seedlings with the culture filtrate of NBC001 led to an increase in the callose deposition in both soybean leaves and roots compared to that resulting from the control treatment (Fig. 6). Measurement of the total areas of callose deposition spots from 20 randomly selected leaf discs or roots from each treatment revealed that a larger area of callose deposition was significantly displayed in both the NBC001 treatment and the NBC001 and J2 treatments compared to those in the J2 treatment and control (Fig. 6e,j, P < 0.05).
Figure 6.
Histochemical analysis of cell wall reinforcement in the leaves and roots of soybean at 24 h post-inoculation. (a,f) Control, (b,g) NBC001 treatment, (c,h) NBC001 + J2 treatment, and (d,i) J2 treatment. Scale bars represent 500 μm. (e,j) Callose deposits were quantified using ImagePro 5.0 software. The values are the mean callose deposits area ± standard deviation from 20 pieces of leaves (diameter, 1 cm) or the roots from five plants.
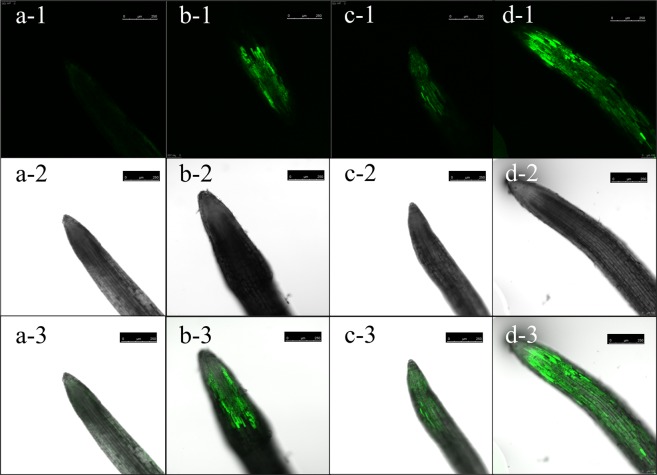
We labeled soybean roots with 5-(and-6)-chloromethyl-2′, 7′- dichlorodihydrofluorescein diacetate (CM-H2DCFDA), which fluoresces when activated by ROS in living cells. We used confocal laser scanning fluorescence microscopy to monitor the fluorescence in roots labeled with CM-H2DCFDA at 24 h post-innoculation. As shown in Fig. 7, the extent of CM-H2DCFDA fluorescence was increased in roots treated with both NBC001 and J2 and treated with only NBC001 compared to those in the roots treated with J2 or the control. These results indicate that treating soybean seedlings with the culture filtrate of NBC001 triggers ROS production in the soybean roots.
Figure 7.
Histochemical analysis of the production of reactive oxygen species in the leaves of soybean at 24 h post-inoculation. (a) Control, (b) NBC001 treatment, (c) J2 treatment, and (d) NBC001 + J2 treatment. Scale bars represent 250 μm.
Expression analysis of plant defense-related genes
To determine the signaling pathways elicited by NBC001, the expression of GmPR1a, GmLOX and GmEREBP as SA-responsive, JA-responsive and ET-responsive marker genes, respectively, was analyzed at 0, 24 and 48 h post-inoculation using real-time quantitative PCR. The increased expression of GmPR1a, GmLOX and GmEREBP significantly enhanced plant defense19. At 24 and 48 h post-inoculation, the expression of GmPR1a and GmEREBP in the roots treated with NBC001 or NBC001 with J2 appeared to be significantly higher than that in either the roots treated with J2 or the control (Fig. 8a,c, P < 0.05). However, GmLOX was constantly expressed at a low level and did not show any differences among any of the treatment (Fig. 8b, P > 0.05). The increased expression of GmPR1a and GmEREBP, typical SA- or ET-responsive marker gene, suggests that the ISR response induced by NBC001 was dependent on both SA and ET signal pathways. These data further confirm that NBC001 can activate the soybean defense responses.
Figure 8.
Expression of plant defense genes in soybean roots in different treatments after H. glycines infection at different time intervals. (a) GmPR1a, (b) GmLOX, and (c) GmEREBP. The data shown represent the average values from three independent experiments. The different letters on each bar represent significant differences at the 0.05 level based on Tukey’s multiple comparison test.
Effect of NBC001 on the soil microbial communities
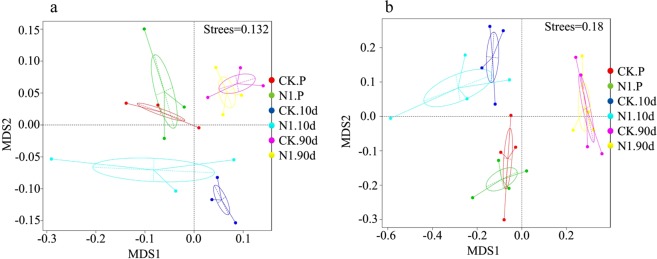
Bacterial and fungal microbial richness and diversity are shown in Fig. 9. Regarding bacteria and fungi, the observed species, Shannon and Simpson indices were not significantly different in the NBC001 treatment and the control before transplantation, or at 10 and 90 d post-transplantation. Nonmetric multidimensional scaling (NMDS) analysis showed that the bacterial and fungal communities changed along with different soybean growth stages, but the bacterial and fungal communities were similar between the NBC001 treatment and the control (Fig. 10). These results suggested that the application of NBC001 has no significant impacts on the soil microbial communities.
Figure 9.
Diversity of soybean rhizosphere microorganisms in the different treatments. The numbers of observed species and the Shannon and Simpson indices were calculated from bacterial (a–c) and fungal (d–f) OTUs of soybean rhizosphere microorganisms. CK, control treatment. N1, NBC001 treatment. P, before transplantation. 10 d and 90 d, 10 and 90 days post-ransplantation, respectively.
Figure 10.
NMDS ordination plots for the soybean rhizosphere bacterial (a) and fungal (b) communities in the different treatments. The proximity between the samples in the plot corresponds to high community similarity. CK, control treatment. N, NBC001 treatment. P, before transplantation. 10 d and 30 d, 10 and 30 days post-transplantation, respectively.
Discussion
H. glycines is one of the most economically important pathogens of soybean and is difficult to control. The management of H. glycines with biocontrol microorganisms is an eco-friendly method. In this study, we isolated a fungal strain, NBC001, from cysts of Heterodera spp. The strain NBC001 was identified as an A. niger species and found to induce the resistance of soybean against H. glycines.
A. niger is very common in all types of soils, and it has been found to effectively suppress plant parasitic nematodes and enhance the yield of plants11. In the greenhouse, the application of A. niger significantly reduced the soil populations of M. javanica and root galling and increased the plant height and fresh weight of mungbean shoots20. In the field, A. niger PD-42 significantly reduced tomato and pepper root galling due to Meloidogyne incognita. In this study, H. glycines in the soybean seedlings dressed with the culture filtrate of A. niger NBC001 was controlled in both the pot and field experiments.
In this study, we tested the effects of the culture filtrate of A. niger NBC001 on the J2s mortality and egg hatchability of H. glycines in vitro. The results showed that the culture filtrate of NBC001 caused 89% mortality of J2s and inhibited more than 98% of the eggs from hatching. These results indicate that NBC001 showed high nematicidal activity against H. glycines. In addition, the J2s mortality and inhibition of hatchability decreased as the dilution factor of NBC001 increased. This result indicated that NBC001 might produce nematicidal metabolites to suppress H. glycines. When the dilution factor of NBC001 was increased, the concentration of the nematicidal metabolites was reduced. Therefore, the nematicidal activity decreased. A. niger has been reported to produce toxic microbial exometabolites to kill plant parasitic nematodes21,22. The nematicidal metabolites in the A. niger NBC001 culture filtrate is being investigated.
In addition to direct antagonism, some biocontrol microorganisms can protect plants indirectly via the stimulation of inducible defense responses that render the hosts more resistant to further pathogen ingression23. Such an induction of enhanced defensive capacity, which is known as ISR, can be systemic and is effective against a broad range of diseases. These mechanisms include the reinforcement of plant cell walls, the production of antimicrobial phytoalexins and the synthesis of PR proteins24. However, the knowledge that biocontrol agents induced systemic resistance towards plant parasitic nematodes is rare25. Munif et al. (2001) reported that induced systemic resistance is considered a possible control mechanism of four endophytic bacteria, including Pantoea agglomerans MK-29, Cedeca davisae MK-30, Enterobacter spp. MK-42 and Pseudomonas putida MT-19, against root-knot nematodes26. The ability of the fungal inoculants to stimulate ISR against the nematodes was demonstrated27. Vu et al. (2006) found that Fusarium oxysporum control R. similis by inducing the systemic resistance of banana28.
Recently, Li et al. (2011) found that A. niger could reduce the nematode populations and promote the tomato plant growth by increasing the activities of defense enzymes12. These findings indicate that A. niger could induce the resistance of tomato to plant-parasitic nematodes. Therefore, in this study, we tested whether the A. niger NBC001 filtrate could activate soybean defense responses at the cellular and molecular levels. The rapid release of ROS, superoxide dismutase and hydrogen peroxide is ubiquitous in the early responses following the recognition of microorganisms29. ROS has direct antimicrobial activity and mediates other defense responses, including the oxidative crosslinking of plant cell walls, callose deposition and hypersensitive cell death30. Cell walls may be reinforced via the deposition of lignin, phenolic compounds, suberin and callose29. In this study, the application of A. niger NBC001 stimulated hydrogen peroxide activity and callose deposition, suggesting that A. niger NBC001 activated the cellular defenses of plant cells, thus contributing to the resistance to H. glycines. In addition, soybean seedlings dressed with the culture filtrate of A. niger NBC001 exhibited increased callose deposition in the leaves, demonstrating that NBC001 was able to activate plant induced systemic resistance against H. glycines.
Nematode infestation could activate plant defense responses, including the release of ROS, antimicrobial secondary metabolites, hydrolytic enzymes, and local fortifications of plant cell walls by lignification and callose deposits31. In this study, inoculation of H. glycines J2s stimulated the hydrogen peroxide activity and callose deposition in soybean. Moreover, the ROS and callose deposit area were increased in roots treated with both NBC001 and J2 compared to those in the NBC001 and J2 alone treatments. These results indicate that activation of plant defense responses by NBC001 and J2 was cooperative rather than antagonistic.
Biocontrol microorganisms commonly activate an ISR response in plants via the jasmonate (JA) and/or ethylene (ET) signaling pathways32. However, there are examples of SA-dependent defense responses19,32. In this study, we tested three genes related to the typical signaling pathways and found that the SA-regulated defense-related gene GmPR1a and the ET-regulated defense-related gene GmEREBP were activated by NBC001. However, the JA-regulated defense-related gene GmLOX did not show any significant differences in the roots treated with PDA and with NBC001 combined with H. glycines infection. This result indicates that the disease resistance induced by NBC001 may be controlled by the SA- and ET-dependent signaling pathways. The results are consistent with the point that the SA- and JA/ET- signaling pathways in plant protection provided by biocontrol microorganisms are cooperative rather than antagonistic33–35. The induction of ISR by the rhizobacteria N11.37 in Arabidopsis thaliana against Xanthomonas campestris is SA- and ET- dependent36.
Introduced biocontrol agents may have a short- or long-term effect on the soil microbial community37. In this study, we did not observe any definable risk for the soil microbe communities arising from the application of NBC001. Some researchers also found no effects on the soil microbial communities following inoculation with biocontrol agents, such as Pseudomonas fluorescens 2P24, P. fluorescens CPF10 and Bacillus subtilis Jdm238–40.
These results indicate that A. niger NBC001 has potential as a commercial biological control agent to control H. glycines. Aspergillus spp. are known to be opportunistic pathogens of human beings41. A. fumigatus is the most common mold pathogen of human beings and can cause both invasive disease in immunocompromised patients and allergic disease in patients with atopic immune systems42. However, to our knowledge, there are no reports on the effect of A. niger on human beings. However, it is necessary to assess the effects of NBC001 on human beings before it is used extensively.
In summary, NBC001 may be a good biocontrol agent against H. glycines via stimulation of plant host defense. In addition, the application of NBC001 has no effects on the soil microbial communities. To increase the field performance of biocontrol agents, a detailed knowledge of environmental adaptations is required.
Materials and Methods
Isolation of fungi
Soil samples were collected from fields in the Xuchang District, Henan, China and Østfold, Norway in 2015. Cysts were collected from the soil samples by suspending the soil in water and filtering the samples through nested 710-μm-pore and 250-μm -pore sieves43. The cysts were treated with 0.5% sodium hypochlorite (NaClO) for 5 min for surface disinfestation and washed three times with sterile distilled water to remove residual NaClO44. Subsequently, the cysts were broken open with a rubber plug to release the eggs and passed through a 200-μm -pore sieve and collected on a 30-μm -pore sieve45. Free eggs were smeared on Petri dishes containing potato dextrose agar (PDA; Oxoid Ltd, Basingtoke, Hampshire, England) and inoculated at 26 °C in complete darkness for 5 d45. Hyphae that emerged from the eggs were transferred to PDA plates for purification and identification.
Identification of the fungi
A single spore culture was established for each isolate, and the isolates were identified by blasting the sequences of the internal transcribed spacers (ITSs) of the rDNA regions as described by Tendulkar et al.46. Amplification of the fungal ITS fragment was performed using a PCR approach with the primers pairs ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′)47.
Nematode and fungus culture
Cysts of H. glycines were collected from Langfang, Hebei, China using the method described above. The J2s of H. glycines were obtained by incubating the cysts on a sieve (30-μm pore size) in 3 mM ZnCl2 at 26 °C48. The hatched J2s were collected daily.
The isolated fungi were cultured on a potato dextrose agar (PDA; Shanghai Bioway Technology Co., Ltd, Shanghai, China) plate and inoculated at 26 °C in complete darkness45,49. After 10 d, a 5-mm diameter mycelial plug of each tested fungus was inoculated into 200 mL of PDB in a 500 mL Erlenmeyer flask and incubated at 26 °C for 120 h at 150 r/min.
The culture filtrate of NBC001 was prepared by centrifuging at 2,000 rpm for 20 min50. Ten ml of the supernatant was filtered through a sterile 0.22 μm polyethersulfone filter (Whatman, Clifton, NJ, USA) for the in vitro experiments51. The other 10 L of supernatant was concentrated to 2 L using a vacuum freeze drier (Hitachi ES-2030) for the pot and field experiments.
Antagonistic effect experiments
The antagonistic effects of the culture filtrate of NBC001 on the J2s and hatchability of H. glycines were performed as described by Jin et al.51. Each treatment had three replicates, and each experiment was repeated twice.
Pot experiments
The experiment was conducted in a controlled environmental chamber with a 16 h photoperiod and a 26/20 °C day/night regimen with the soybean (Glycine max L. Merr., cv. Zhonghuang NO.13, susceptible to H. glycines). Soybean seeds were dressed with the 5-fold concentrated culture filtrate of NBC001, 5-fold concentrated PDB medium or 1.8% abamectin EC for 2 min. One soybean seed was sown in a 10-cm-diameter plastic pot filled with 1,600 cm3 of soil containing 280 cysts collected from fields in Langfang, Hebei, China. The treatment was conducted in a randomized complete block design (RCBD). Each treatment had ten pots and the experiment was repeated twice.
At 30 d post-transplantation, plant and rhizosphere soil samples were collected from each treatment. Cysts of H. glycines in the soil were extracted as described above. The recovered cysts were counted using a stereoscopic microscope (SZ61, Olympus Corp., Japan). The stem and root lengths were measured using a meter ruler.
Field experiments
Field experiments were conducted in a field naturally infested with H. glycines in Langfang, Hebei, China during the 2017 cropping season. Zhonghuang NO.13 seeds were transplanted into the field after being dressed with the culture filtrate of NBC001, PDB medium or 1.8% abamectin EC for 2 min. The treatments were arranged as a randomized complete block design (RCBD) with four blocks for each treatment.
At 90 d post-transplantation, the rhizosphere soil samples were collected from each treatment. Cysts of H. glycines in the soil were extracted using the method decreased above. The yield was recorded at 120 days post-transplantation.
Juvenile development analysis
The experiment was conducted as described above. Soybean seeds were dressed with the culture filtrate of NBC001 or PDB medium. One soybean seed was sown in a pot containing clay and sand at a 7:3 ratio (v/v). When the second leaf had emerged, 1,000 J2s of H. glycines were inoculated around the roots. The treatment was laid out in a randomized complete block design (RCBD), and each treatment had ten pots.
To observe the development of H. glycines in soybean roots, the roots were stained as described by Bybd et al. (1983) at 3, 7, and 14 d post inoculation52. After staining, juvenile at different developmental stages were examined for morphology and counted using a Leica dissecting microscope (Leica M165C, Wetzlar, Germany). Five replicates were performed for each treatment, and each assay was performed three times.
ISR assays
A pot experiment was conducted as described above. Twenty roots and mature leaves were sampled for callose and reactive oxygen species (ROS) detection at 24 h post-inoculation. This experiment was conducted with three independent biological replicates.
Callose deposition was detected using the aniline blue staining method53. The stained leaves and roots were visualized using an Olympus IX83 microscope with a UV filter (Olympus Corporation, Tokyo, Japan; excitation filter 350/50 nm, replicated DM 400 dichroic beam splitter and emission filter 460/50 nm). ImagePro 5.0 software (Media Cybernetics, Bethesda, MD) was used to convert the colored micrographs to binary, and pixel densitometry was performed across the 20 images from each treatment.
ROS production was examined with the oxidant-sensitive probe dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen, California, USA)54. The samples were imaged with a Carl Zeiss confocal laser scanning microscope (LSM 880; Carl Zeiss, Oberkochen, Germany).
Real-time quantitative PCR
To test whether NBC001 could activate soybean plant defenses, we characterized the expression of the defense genes GmPR1a, GmLOX and GmEREBP during H. glycines infection in the presence or absence of NBC001 using real-time quantitative PCR. The soybeans were treated as described above. Soybean roots were collected at 0, 24, and 48 h post-inoculation. Total RNA was extracted from the soybean roots using TRIzol reagent (Invitrogen, Carlsbad, CA). The total RNA was treated with recombinant DNase I (Takara, Shiga, Japan) and the recombinant RNase inhibitor (Takara, Shiga, Japan) according to the manufacturer’s instructions. The DNase-treated RNA (1 μg) was used for cDNA synthesis, which was performed using the M-MLV Reverse Transcriptase (Takara, Shiga, Japan) according to the manufacturer’s instructions. The cDNA template was amplified by real-time quantitative PCR using the SYBR® Premix Dimmer Eraser kit (Takara, Shiga, Japan) according to the manufacturer’s instructions, and the PCRs were performed on an ABI 7500 Real-Time PCR System (Applied Biosystems). The primers are listed in Table 3. Actin was used as an internal control. The results were analyzed using SDS 2.0 software (Applied BioSystems), and the gene expression changes were calculated using the 2−△△CT method.
Table 3.
Sequences of the gene-specific primers used for real-time quantitative PCR.
| Gene | Forward/reverse primers | Reference |
|---|---|---|
| GmActin | F: 5′-GAGCTATGAATTGCCTGATGG-3′ R: 5′-CGTTTCATGAATTCCAGTAGC-3′ |
55 |
| GmPR1a | F: 5′-GGGTGATGTTGCCTACGCTCAA-3′ R: 5′-CAGCAACCGTATCATCCCAAGC-3′ |
19 |
| GmLOX | F: 5′-TGGAGGTTTTAAGAGGAGATGG-3′ R: 5′-CCTGCGAGGGTAAGGATAGTTG-3′ |
19 |
| GmEREBP | F: 5′-GATTACTCCCACATCGCTACCC-3′ R: 5′-AGATTCTTCCTCTGCCTCTTCA-3′ |
19 |
Soil microbial community analysis
Field experiments were conducted as described above. Rhizosphere soil samples were collected from each plot immediately before treatment and at 10 and 90 days post-transplantation.
Total DNA was extracted from 0.5 g soil samples using the PowerSoil DNA isolation kit (Mo Bio Laboratories, Inc., Carlsbad, CA) according to the manufacturer’s instructions. The DNA extracted from each soil sample served as a template for the 16 S rRNA gene and ITS region amplification. The bacterium-biased primers 515 F/806 R and fungal-specific primers 1737F/2043 R were used. All the PCRs were performed using a Phusion® High-Fidelity PCR Master Mix (New England Biolabs). Sequencing libraries were conducted on the Ion S5TM XL platform (Life Technologies, USA, Novogene Bioinformatics Technology Co., Ltd, Beijing, China).
Data analyses
Alpha diversity was applied to analyze the complexity of the species diversity in each sample using three indices, including observed species, Shannon and Simpson. The community structure was analyzed using nonmetric multidimensional scaling (nMDS). They were calculated using QIIME (Version1.7.0) and displayed with R software (Version 2.15.3).
All other data were analyzed using SPSS 15.0 (SPSS Inc., IBM, USA). One-way analysis of variance (ANOVA) and Tukey’s multiple comparison test were conducted to test for significant differences among the treatments. All statistical tests were performed at a significance level of 0.05.
Acknowledgements
This study was supported by the Special Fund for Agro-Scientific Research in the Public Interest (grant number 201503114).
Author Contributions
N.J. and D.L.P. designed the experiments and wrote the manuscript. N.J., Y.H.W., Y.P.C. and F.Y.G. performed the experiments. N.J., D.L.P., S.M.L., H.P., W.K.H., L.A.K.and H.J. analyzed the results. All authors have read and approved the final manuscript.
Data Availability
The datasets analyzed during the present study are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peng DL, et al. First report of soybean cyst nematode (Heterodera glycines) on soybean from Gansu and Ningxia China. Plant Dis. 2016;100:229. doi: 10.1094/PDIS-04-15-0451-PDN. [DOI] [Google Scholar]
- 2.Ou SQ, Peng DL, Liu XM, Li Y, Moens M. Identification of Heterodera glycines using PCR with sequence characterised amplified region (SCAR) primers. Nematology. 2008;10:397–403. doi: 10.1163/156854108783900212. [DOI] [Google Scholar]
- 3.Peng H, et al. Novel pectate lyase genes of Heterodera glycines play key roles in the early stage of parasitism. PLoS One. 2016;11:e0149959. doi: 10.1371/journal.pone.0149959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mwaheb MA, et al. Synergetic suppression of soybean cyst nematodes by chitosan and Hirsutella minnesotensis via the assembly of the soybean rhizosphere microbial communities. Biol. Control. 2017;115:85–94. doi: 10.1016/j.biocontrol.2017.09.011. [DOI] [Google Scholar]
- 5.Xiang N, Lawrence KS, Kloepper JW, Donald PA, McInroy JA. Biological control of Heterodera glycines by spore-forming plant growth-promoting rhizobacteria (PGPR) on soybean. PLoS One. 2017;12:e0181201. doi: 10.1371/journal.pone.0181201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arshad AM. A characterization of some isolates of Aspergillus niger and evaluation of their effects against Meloidogyne incognita. Annals of Applied Biology. 2006;27:33–34. [Google Scholar]
- 7.Zuckerman B, Matheny M, Acosta N. Control of plant-parasitic nematodes by a nematicidal strain of Aspergillus niger. J. Chem. Ecol. 1994;20:33–43. doi: 10.1007/BF02065989. [DOI] [PubMed] [Google Scholar]
- 8.Jang JY, et al. Biological control of Meloidogyne incognita by Aspergillus niger F22 producing oxalic acid. PLoS One. 2016;11:e0156230. doi: 10.1371/journal.pone.0156230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mankau R. Nematicidal activity of Aspergillus niger culture filtrates. Phytopathology. 1969;59:1170. [Google Scholar]
- 10.Nair MG, Burke BA. A new fatty acid methyl ester and other biologically active compounds from Aspergillus niger. Phytochemistry. 1988;27:3169–3173. doi: 10.1016/0031-9422(88)80021-9. [DOI] [Google Scholar]
- 11.Khan MR, Anwer MA. DNA and some laboratory tests of nematode suppressing efficient soil isolates of Aspergillus niger. Indian Phytopathology. 2008;61:212–225. [Google Scholar]
- 12.Li S, et al. Effects of adding secondary metabolites of Aspergillus niger on resistance to tomato root-knot nematode. China Veg. 2011;4:44–49. [Google Scholar]
- 13.Kano A, et al. D-Psicose induces upregulation of defense-related genes and resistance in rice against bacterial blight. J. Plant Physiol. 2011;168:1852–1857. doi: 10.1016/j.jplph.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Rahman A, Uddin W, Wenner NG. Induced systemic resistance responses in perennial ryegrass against Magnaporthe oryzae elicited by semi-purified surfactin lipopeptides and live cells of Bacillus amyloliquefaciens. Mol. Plant Pathol. 2015;16:546–558. doi: 10.1111/mpp.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-Gutiérrez L, et al. The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate and salicylic acid dependent defence responses. Micob. Biotechnol. 2013;6:264–274. doi: 10.1111/1751-7915.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cycoń M, Wójcik M, Borymski S, Piotrowska-Seget Z. Short-term effects of the herbicide napropamide on the activity and structure of the soil microbial community assessed by the multi-approach analysis. Appl. soil ecol. 2013;66:8–18. doi: 10.1016/j.apsoil.2013.01.014. [DOI] [Google Scholar]
- 17.Haas BJ, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui IA, Ehteshamul-Haque S. Suppression of the root rot–root knot disease complex by Pseudomonas aeruginosa in tomato: the influence of inoculum density, nematode populations, moisture and other plant-associated bacteria. Plant Soil. 2001;237:81–89. doi: 10.1023/A:1013313103032. [DOI] [Google Scholar]
- 19.Lu XX, et al. Isolation and characterization of Bacillus altitudinis JSCX-1 as a new potential biocontrol agent against Phytophthora sojae in soybean [Glycine max (L.) Merr.] Plant Soil. 2017;416:53–66. doi: 10.1007/s11104-017-3195-z. [DOI] [Google Scholar]
- 20.Eapen SJ, Beena B, Ramana K. Tropical soil microflora of spice-based cropping systems as potential antagonists of root-knot nematodes. J. Invertebr. Pathol. 2005;88:218–225. doi: 10.1016/j.jip.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui I, Ali N, Zaki M, Shaukat S. Evaluation of Aspergillus species for the biocontrol of Meloidogyne javanica in mungbean. Nematol. Medit. 2001;29:115–121. [Google Scholar]
- 22.Metcalf H. Cultural studies of a nematode associated with plant decay. Trans. Am. Microbiol. Soc. 1903;24:89–102. doi: 10.2307/3220858. [DOI] [Google Scholar]
- 23.Ongena M, et al. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007;9:1084–1090. doi: 10.1111/j.1462-2920.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- 24.Jeandet P, et al. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. BioFactors. 2010;36:331–341. doi: 10.1002/biof.108. [DOI] [PubMed] [Google Scholar]
- 25.Elsen A, Gervacio D, Swennen R, De Waele D. AMF-induced biocontrol against plant parasitic nematodes in Musa sp.: a systemic effect. Mycorrhiza. 2008;18:251–256. doi: 10.1007/s00572-008-0173-6. [DOI] [PubMed] [Google Scholar]
- 26.Munif A, Hallmann J, Sikora RA. Induced systemic resistance of selected endophytic bacteria against Meloidogyne incognita on tomato. Med. Facul. Landbouww. Rijksuniv Gent. 2001;66:663–669. [PubMed] [Google Scholar]
- 27.Siddiqui I, Shaukat S. Rhizobacteria‐mediated induction of systemic resistance (ISR) in tomato against Meloidogyne javanica. J. Phytopathol. 2002;150:469–473. doi: 10.1046/j.1439-0434.2002.00784.x. [DOI] [Google Scholar]
- 28.Vu T, Hauschild R, Sikora RA. Fusarium oxysporum endophytes induced systemic resistance against Radopholus similis on banana. Nematology. 2006;8:847–852. doi: 10.1163/156854106779799259. [DOI] [Google Scholar]
- 29.Ge YH, Bi Y, Guest DI. Defence responses in leaves of resistant and susceptible melon (Cucumis melo L.) cultivars infected with Colletotrichum lagenarium. Physiol. Mol. Plant Pathol. 2013;81:13–21. doi: 10.1016/j.pmpp.2012.09.002. [DOI] [Google Scholar]
- 30.Ray S, Mondal S, Chowdhury S, Kundu S. Differential responses of resistant and susceptible tomato varieties to inoculation with Alternaria solani. Physiol. Mol. Plant Pathol. 2015;90:78–88. doi: 10.1016/j.pmpp.2015.04.002. [DOI] [Google Scholar]
- 31.Goverse A, Smant G. The activation and suppression of plant innate immunity by parasitic nematodes. Annu Rev Phytopathol. 2014;52:243–265. doi: 10.1146/annurev-phyto-102313-050118. [DOI] [PubMed] [Google Scholar]
- 32.Ongena M, et al. Stimulation of the lipoxygenase pathway is associated with systemic resistance induced in bean by a nonpathogenic Pseudomonas strain. Mol. Plant Microbe Interact. 2004;17:1009–1018. doi: 10.1094/MPMI.2004.17.9.1009. [DOI] [PubMed] [Google Scholar]
- 33.Van Wees SCM, De Swart EAM, Van Pelt JA, VanLoon LC, Pieterse CMJ. Enhancement of induced disease resistance by simultaneous activation of salicylate-and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2000;97:8711–8716. doi: 10.1073/pnas.130425197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivas-Martínez S, Fernández-González F, Loidi J, Lousã M, Penas A. Syntaxonomical checklist of vascular plant communities of Spain and Portugal to association level. Itinera Geobot. 2001;14:5–341. [Google Scholar]
- 35.Leon-Reyes A, et al. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta. 2010;232:1423–1432. doi: 10.1007/s00425-010-1265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domenech J, Ramos S, Probanza A, Lucas G, Gutierrez M. Elicitation of systemic resistance and growth promotion of Arabidopsis thaliana by PGPRs from Nicotiana glauca: a study of the putative induction pathway. Plant Soil. 2007;290:43–50. doi: 10.1007/s11104-006-9089-0. [DOI] [Google Scholar]
- 37.Somasekhar N, Grewal PS, De Nardo EAB, Stinner BR. Non‐target effects of entomopathogenic nematodes on the soil nematode community. J. Appl. Ecol. 2002;39:735–744. doi: 10.1046/j.1365-2664.2002.00749.x. [DOI] [Google Scholar]
- 38.Du W, et al. Effects of Beauveria bassiana and acephate on enzyme activities and microbial diversity in paddy soil. Plant, soil and environment. 2013;59:562–567. doi: 10.17221/447/2013-PSE. [DOI] [Google Scholar]
- 39.Bongers T. The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia. 1990;83:14–19. doi: 10.1007/BF00324627. [DOI] [PubMed] [Google Scholar]
- 40.Yin DH, Wang N, Xia F, Li Q, Wang W. Impact of biocontrol agents Pseudomonas fluorescens 2P24 and CPF10 on the bacterial community in the cucumber rhizosphere. Eur. J. Soil Blol. 2013;59:36–42. doi: 10.1016/j.ejsobi.2013.09.001. [DOI] [Google Scholar]
- 41.Morehouse, L. G. & Wyllie, T. D. Mycotoxic Fungi and Chemistry of Mycotoxins in Mycotoxic fungi, mycotoxins, mycotoxicoses: an encyclopedic handbook (ed. Marcel, D.) (Marcel Dekker 1977).
- 42.Denning DW, Anderson MJ, Turner G, Latgé JP, Bennett JW. Sequencing the Aspergillus fumigatus genome. Lancet Infect. Dis. 2002;2:251–253. doi: 10.1016/S1473-3099(02)00243-8. [DOI] [PubMed] [Google Scholar]
- 43.Workneh F, Tylka GL, Yang XB, Faghihi J, Ferris JM. Regional assessment of soybean brown stem rot, Phytophthora sojae, and Heterodera glycines using area-frame sampling: prevalence and effects of tillage. Phytopathology. 1999;89:204–211. doi: 10.1094/PHYTO.1999.89.3.204. [DOI] [PubMed] [Google Scholar]
- 44.Douillet P. Disinfection of rotifer cysts leading to bacteria-free populations. J. Exp. Mar. Biol. Ecol. 1998;224:183–192. doi: 10.1016/S0022-0981(97)00200-1. [DOI] [Google Scholar]
- 45.Sun MH, Gao L, Shi YX, Li BJ, Liu XZ. Fungi and actinomycetes associated with Meloidogyne spp. eggs and females in China and their biocontrol potential. J. Invertebr. Pathol. 2006;93:22–28. doi: 10.1016/j.jip.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Tendulkar SR, Gupta A, Chattoo BB. A simple protocol for isolation of fungal DNA. Biotechnol. Lett. 2003;25:1941–1944. doi: 10.1023/B:BILE.0000003990.27624.04. [DOI] [PubMed] [Google Scholar]
- 47.White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics in PCR Protocols: a guide to methods and applications (eds Innis, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J.) 315–322 (Academic Press 1990).
- 48.Goverse A, et al. Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol. Plant Microbe Interact. 2000;13:1121–1129. doi: 10.1094/MPMI.2000.13.10.1121. [DOI] [PubMed] [Google Scholar]
- 49.Pu SY, Qin LL, Che JP, Zhang BR, Xu M. Preparation and application of a novel bioflocculant by two strains of Rhizopus sp. using potato starch wastewater as nutrilite. Bioresource Technol. 2014;162:184–191. doi: 10.1016/j.biortech.2014.03.124. [DOI] [PubMed] [Google Scholar]
- 50.Pu S, et al. Isolation, identification, and characterization of an Aspergillus niger bioflocculant-producing strain using potato starch wastewater as nutrilite and its application. PLoS One. 2018;13:e0190236. doi: 10.1371/journal.pone.0190236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin N, et al. Field evaluation of Streptomyces rubrogriseus HDZ-9-47 for biocontrol of Meloidogyne incognita on tomato. J. Integr. Agr. 2017;16:1347–1357. doi: 10.1016/S2095-3119(16)61553-8. [DOI] [Google Scholar]
- 52.Bybd D, Jr., Kirkpatrick T, Barker K. An improved technique for clearing and staining plant tissues for detection of nematodes. J. Nematol. 1983;15:142. [PMC free article] [PubMed] [Google Scholar]
- 53.Peng XL, et al. Mycotoxin ochratoxin A-induced cell death and changes in oxidative metabolism of Arabidopsis thaliana. Plant Cell Rep. 2010;29:153–161. doi: 10.1007/s00299-009-0808-x. [DOI] [PubMed] [Google Scholar]
- 54.Siddique S, et al. Parasitic worms stimulate host NADPH oxidases to produce reactive oxygen species that limit plant cell death and promote infection. Sci. Signal. 2014;7:33. doi: 10.1126/scisignal.2004777. [DOI] [PubMed] [Google Scholar]
- 55.Kõljalg U, et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013;22:5271–5277. doi: 10.1111/mec.12481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the present study are available from the corresponding author upon reasonable request.