Abstract
The objective was to summarize existing data on the prevalence of active tobacco smoking among patients with hypertension or diabetes mellitus in Africa. We searched PubMed, EMBASE, and AJOL to include studies published from January 01, 2000 to August 23, 2017 reporting on the prevalence of active smoking in individuals aged ≥15 years with hypertension or diabetes mellitus residing inside Africa. We used a random-effects meta-analysis model to pool studies. The pooled prevalence of active smoking among patients with hypertension or diabetes was 12.9% (95%CI: 10.6–15.3; 50 studies; 16,980 patients) and 12.9% (95%CI: 9.6–16.6; 42 studies; 18,564 patients), respectively. For both conditions, the prevalence of active smoking was higher in males than in females (p < 0.001), and in Northern compared to sub-Saharan Africa (p < 0.001). There was no difference between urban and rural settings, and between community-based and hospital-based studies, except for patients with diabetes for whom the prevalence was higher in hospital-based studies (p = 0.032). The prevalence of active smoking is high among patients with hypertension or diabetes mellitus in Africa, with the heaviest burden in Northern Africa. Interventions for smoking prevention or cessation should be implemented in these high risk populations, targeting particularly the males.
Introduction
The burden of cardiovascular diseases (CVD) has dramatically risen in Africa over the past decade, and CVD and there is an epidemiological transition in which the burden of CVD is overtaking that of infectious diseases on the continent by 20301. In sub-Saharan Africa (SSA) for instance, CVD were responsible for nearly 1 million deaths in 2013, representing 38.3% of non-communicable disease-related deaths and 11.3% of all-cause mortality2. This surge in the burden of CVD is driven by the increasing prevalence in cardiovascular risk factors1.
Hypertension, diabetes mellitus, hypercholesterolemia, obesity, and smoking are the five major modifiable traditional cardiovascular risk factors3–5. At least one of these five risk factors is present in 80% to 95% of individuals who experienced a fatal or non-fatal cardiovascular event4,5. The most recent data from the Global Burden of Disease study showed that hypertension, diabetes mellitus and smoking remain among the five leading factors contributing to the global burden of disease6. Much more, the interaction between these three risk factors is devastating. Indeed, all forms of smoking amplifies markedly the risk of all-cause, CVD and non-CVD morbidity and mortality in patients with hypertension and diabetes7,8.
Smoking cessation and prevention is therefore a crucial component in the management of hypertension and diabetes mellitus7. In Africa where hypertension and diabetes mellitus are highly prevalent, the magnitude of active smoking in patients with these conditions is not well known. We present here a systematic review and meta-analysis which estimates the prevalence of active smoking among patients with hypertension or diabetes in Africa.
Methods
This review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was published in a peer-reviewed journal9, and is registered with PROSPERO (Registration number CRD42016052560). For this review, we used the same method as in previously published meta-analysis of prevalence studies10–13.
Literature search
We searched PubMed, Excerpta Medica Database (EMBASE), and African Journals Online (AJOL) to identify all relevant articles published from January 01, 2000 to August 23, 2017 on the prevalence of active smoking in individuals with hypertension or diabetes mellitus in Africa. No language restriction was applied. The full search strategy was published in the study protocol9. The reference list of all relevant articles were screened to identify other potential data sources.
Selection of studies for inclusion in the review
Cross-sectional and cohort studies reporting on the prevalence of active smoking in individuals aged more than 15 years with hypertension or diabetes mellitus residing in African continent or enough data to compute it were included. Hypertension had to be defined as the presence of systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or being on any antihypertensive treatment14. Diabetes mellitus was defined according to one of the following diagnostic criteria: being on any antidiabetic treatment, A1c haemoglobin ≥6.5%or fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or 2 hours plasma glucose ≥200 mg/dL (11.1 mmol/L) or random plasma glucose ≥200 mg/dL (11.1 mmol/L) in the presence of classic symptoms of hyperglycemia15. Active smoking was defined as current use of any tobacco product in either smoked or smokeless form16. We excluded studies conducted among populations of African origin residing outside Africa, studies on non-systemic hypertension (intracranial hypertension, pulmonary hypertension) or studies on gestational diabetes, letters, case series with small sample size (less than 50 participants), reviews, commentaries and editorials. For studies published in more than one paper, the most comprehensive one reporting the largest sample size was considered.
Titles and abstracts of articles retrieved from literature search were independently screened by two investigators (JJN and JJB), and the full-texts of those potentially eligible were obtained and further assessed for final inclusion. Disagreements were resolved through consensus.
Assessment of methodological quality and reporting of data
Methodological quality of included studies was evaluated using the tool developed by Hoy and colleagues17. A score of 1 (yes) or 0 (no) was assigned for each item, and scores summed across items to generate an overall quality score that ranged from 0 to 10. Studies were then classified as having a low (>8), moderate (6–8), or high (≤5) risk of bias. Three investigators (UFN, AN and JRNkeck) independently assessed study methodological quality of a third of included studies for each of them, and all the assessments were independently reviewed by a fourth investigator (JJN) with disagreements being resolved through consensus.
Data extraction and management
A preconceived and standardized Google online data extraction form was used to collect information on first author’s name, study country, African sub-region (Northern Africa vs sub-Saharan Africa), year of publication, study design (cross-sectional, cohort or case-control), setting (population-based vs hospital-based), area (rural vs urban), number of participants, mean or median age of the population, proportion of males, definition of smoking and the prevalence of active smoking. Three investigators (UF, AN and JRNkeck) extracted the data from individual studies, and all extracted data were crosschecked by a fourth investigator (JJN) with disagreements being resolved through consensus.
Data synthesis and analysis
We performed statistical analysis with R version 3.5.1 (The R Foundation for statistical computing, Vienna, Austria). Meta-analyses were conducted with the package ‘meta’. Unadjusted prevalence was recalculated based on the information of crude number of cases and sample size provided by each individual study. Each prevalence was reported with its 95% confidence interval (95%CI). The variance of each included study was stabilized with the Freeman-Tukey double arcsine transformation before meta-analysis. This was done to keep the effect of studies with extremely small or extremely large prevalence estimates on the overall estimate to a minimum18. Random-effects analysis was used to pool data. Funnel plot was drawn to investigate any asymmetry. The formal Egger’s test was used to definitively identify publication bias if p value < 0.1019. Heterogeneity was evaluated by the χ² test on Cochrane’s Q statistic20. The I² statistic, used to quantify heterogeneity, estimated the percentage of total variation across studies due to true between-study differences rather than chance. The I² values greater than 60–70% indicated the presence of substantial heterogeneity21. We also used H statistics to quantify heterogeneity. Subgroup analyses were performed for the following subgroups: sex (male versus female), regions (northern versus sub-Sahara Africa), sub-regions (northern, southern, central, eastern, and western), areas (urban versus rural), and settings (population versus hospital-based studies). To test for an effect of study and participants’ characteristics (year of publication, proportion of males, regions, areas, setting, and sample size), we used univariable and multivariable meta-regression analyses. We applied a manual forward selection procedure to identify sources of heterogeneity independently associated with the variation of overall prevalence of active tobacco smoking. We included in multivariable meta-regression analysis, all variables associated (p value < 0.20) with the variation of prevalence in univariable analysis. For categorical variables with 3 or more categories, the global p value was considered for the inclusion in multivariable models. A 2-sided p value < 0.05 was considered statistically significant.
Role of funding source
This study received no funding. All authors had full access to all study data and the corresponding author had final responsibility for the decision to submit the paper for publication.
Results
The review process and study characteristics
Initially, 2,871 records were identified. After elimination of duplicates, 2,683 records remained. Titles and abstracts were screened and 2,559 irrelevant records were excluded. Of the remaining 124 papers (full texts) scrutinized for eligibility, 37 were excluded with reasons. Finally, 87 full texts were retained in the meta-analysis with 45 including data for hypertension only22–66, 37 for diabetes mellitus only67–103 and five including both conditions104–108 (Fig. 1).
Figure 1.
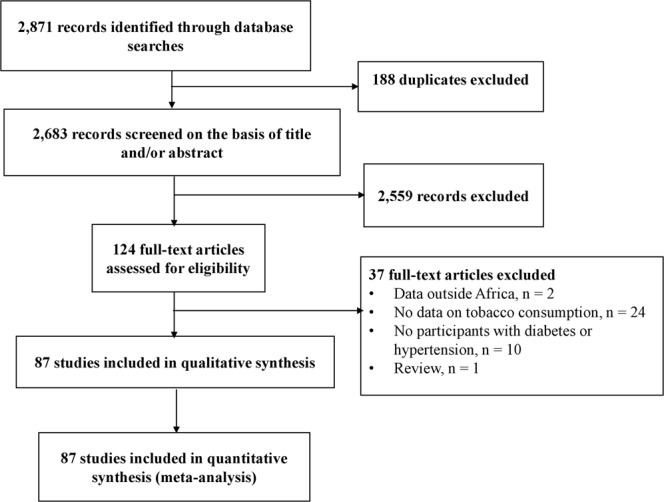
The review process.
Supplementary Table 1 (Appendix) summarizes characteristics of included studies. For hypertension, most studies were from western Africa, were multicentre, were conducted in urban areas, were population-based, and used prospective and consecutive sampling. For diabetes mellitus, most studies originated from Eastern and Northern Africa, were conducted in urban areas, in a single centre, were hospital-based, and used prospective and consecutive sampling. Forty-two (48.3%) studies had low, 38 (43.7%) studies had moderate and 7 (8.0%) studies had high risk of bias. Individual characteristics of each included study are shown in Supplementary Table 2 (Appendix).
Prevalence of active smoking in hypertension
In total, 16,980 participants were included from 18 countries. The prevalence varied widely from 0.5% to 58.4% both in Nigeria. Table 1 summarizes overall and subgroup statistics of the prevalence of active smoking in hypertension. The pooled overall prevalence of active smoking in hypertension was 12.9% (95%CI: 10.6–15.3; 50 studies) with substantial heterogeneity (Fig. 2). The prevalence was higher in males (27.6, 95%CI: 19.6–36.4) than in females (5.9, 95%CI: 4.1–8.0) (p < 0.0001) (Supplementary Figure 1, Appendix). The prevalence was higher in northern Africa (27.2%, 95%CI: 19.1–36.2) compared to sub-Saharan Africa (11.8%, 95%CI: 9.7–14.1) (p = 0.0002) (Fig. 2). This finding was confirmed for sub-regions analysis (Supplementary Figure 2, Appendix) and in meta-regression analysis (Supplementary Table 3, Appendix). There was no difference in smoking prevalence (p = 0.38 and 0.95, respectively) between rural (15.9%, 95%CI: 8.8–24.6) and urban dwellers (12.0%, 95%CI: 8.2–16.4) (Supplementary Figure 3, Appendix) and between hospital-based (12.8%, 95%CI: 9.0–17.0) and community-based studies (12.9%, 95%CI: 10.1–16.1) (Supplementary Figure 4, Appendix). There was no publication bias for overall (Supplementary Figure 5, Appendix) and subgroup analyses (Table 1).
Table 1.
Summary statistics of the prevalence of active tobacco smoking in people with hypertension in Africa.
| Prevalence % (95% confidence interval) | N Studies | N Participants | H (95% confidence interval) | I² (95% confidence interval) | p heterogeneity | p Egger | p difference subgroups | |
|---|---|---|---|---|---|---|---|---|
| Overall | 12.9 (10.6–15.3) | 50 | 16980 | 4.5 (4.1–4.9) | 95.1 (94.2–95.9) | <0.0001 | 0.686 | |
| By sex | ||||||||
| Male | 27.6 (19.6–36.4) | 8 | 1412 | 3.5 (2.7–4.5) | 91.6 (85.9–95.0) | <0.0001 | 0.741 | <0.0001 |
| Female | 5.9 (4.1–8.0) | 7 | 2384 | 1.9 (1.3–2.8) | 71.2 (37.5–86.8) | 0.002 | 0.948 | |
| By region | ||||||||
| Northern Africa | 27.2 (19.1–36.2) | 4 | 1580 | 3.4 (2.3–5.0) | 91.3 (80.9–96.1) | <0.0001 | 0.620 | 0.0002 |
| Sub-Saharan Africa | 11.8 (9.7–14.1) | 46 | 15400 | 4.2 (3.8–4.6) | 94.2 (93.0–95.2) | <0.0001 | 0.604 | |
| By sub-region | ||||||||
| Northern Africa | 27.2 (19.1–36.2) | 4 | 1580 | 3.4 (2.3–5.0) | 91.3 (80.9–96.1) | <0.0001 | 0.620 | 0.005 |
| Southern Africa | 14.2 (11.0–17.6) | 7 | 2666 | 2.3 (1.6–3.2) | 80.2 (59.7–90.3) | <0.0001 | 0.109 | |
| Central Africa | 14.0 (6.6–23.6) | 3 | 567 | 2.9 (6.6–23.6) | 87.7 (65.5–95.6) | 0.0003 | 0.538 | |
| Eastern Africa | 11.6 (8.6–15.1) | 16 | 5796 | 3.8 (3.2–4.5) | 93.0 (90.1–95.0) | <0.0001 | 0.195 | |
| Western Africa | 10.7 (6.4–15.8) | 18 | 5378 | 5.3 (4.7–6.1) | 96.5 (95.4–97.3) | <0.0001 | 0.489 | |
| By area | ||||||||
| Urban | 12.0 (8.2–16.4) | 18 | 5237 | 4.4 (3.8–5.1) | 94.9 (93.2–96.2) | <0.0001 | 0.447 | 0.388 |
| Rural | 15.9 (8.8–24.6) | 11 | 3588 | 6.3 (5.5–7.4) | 97.5 (96.6–98.2) | <0.0001 | 0.536 | |
| Setting | ||||||||
| Population-based | 13.0 (10.1–16.1) | 32 | 11639 | 4.7 (4.3–5.2) | 95.5 (94.5–96.3) | <0.0001 | 0.690 | 0.959 |
| Hospital-based | 12.8 (9.0–17.0) | 18 | 5341 | 4.3 (3.7–4.9) | 94.5 (92.6–95.9) | <0.0001 | 0.890 | |
Figure 2.
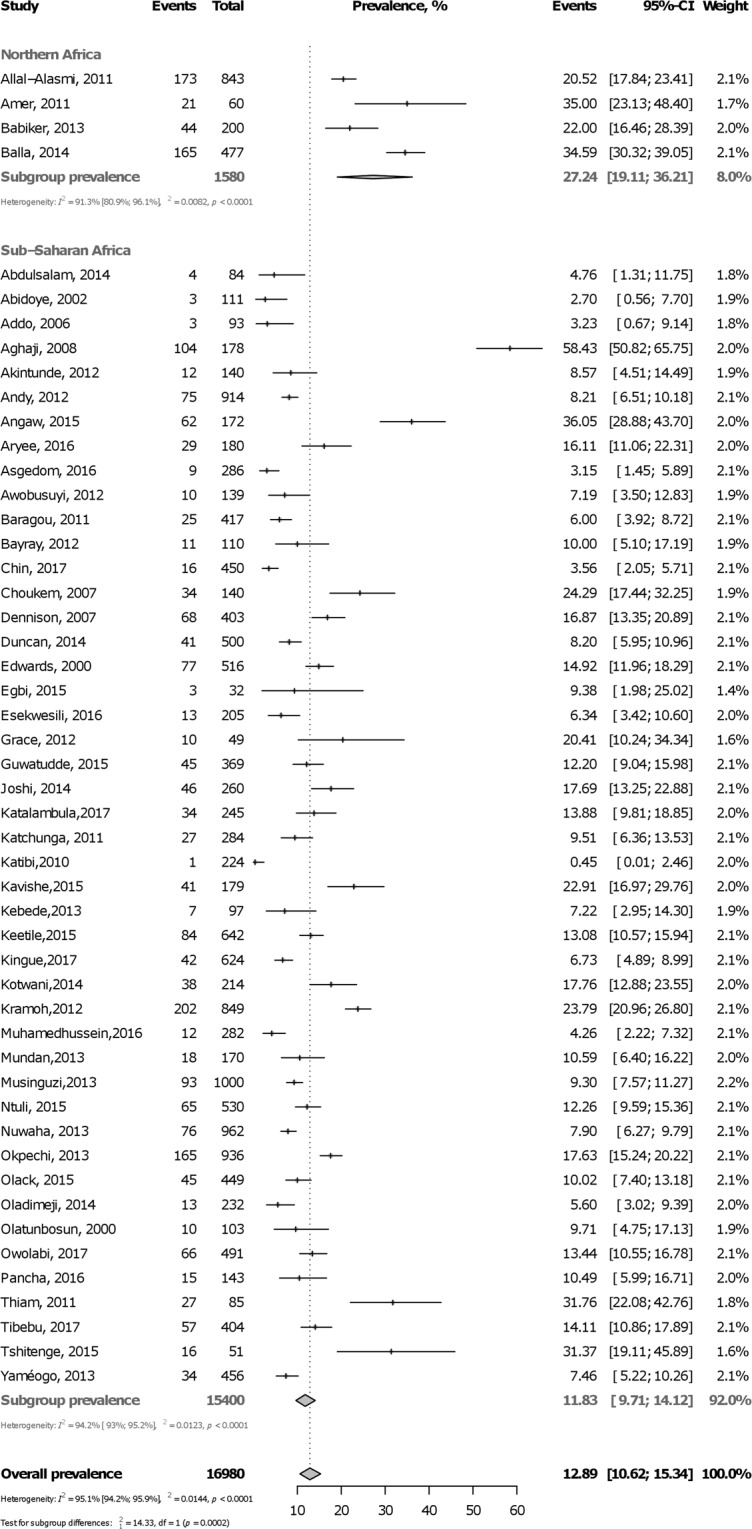
Forest plot of the meta-analysis prevalence of active smoking among people with hypertension in Africa.
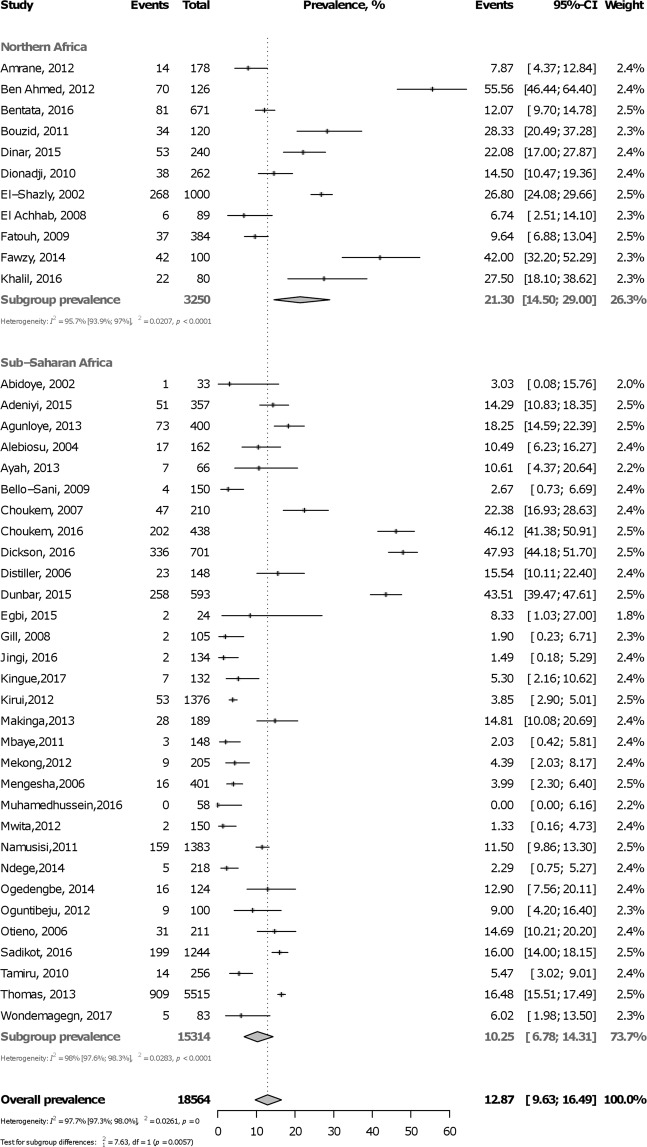
Prevalence of active smoking in diabetes mellitus
In total, 18,564 participants were included from 18 countries. The prevalence varied widely, from 0.0% in Tanzania to 55.7% in Tunisia. Table 2 summarizes overall and subgroup statistics of the prevalence of active smoking in diabetes mellitus. The pooled overall prevalence of active smoking in diabetes mellitus was 12.9% (95%CI: 9.6–16.6; 42 studies) with substantial heterogeneity (Fig. 3). The prevalence was higher in males (18.6%, 95%CI: 9.7–29.6) than in females (2.1%, 95%CI: 0.0–6.5) (p = 0.0006) (Supplementary Figure 6, Appendix). The prevalence was higher in northern Africa (21.3%, 95%CI: 14.5–29.0) compared to sub-Saharan Africa (10.3%, 95%CI: 6.8–14.3) (p = 0.0002) (Fig. 3). This finding was confirmed for sub-regions analysis (Supplementary Figure 7, Appendix) and in meta-regression analysis (Supplementary Table 4, Appendix). There was no difference (p = 0.19) in the prevalence of active smoking between urban (11.7%, 95%CI: 7.7–16.3) and rural dwellers (3.8%, 95%CI: 0.0–16.0) (Supplementary Figure 8, Appendix). The prevalence of active smoking was higher (p = 0.032) in hospital-based (14.3%, 95%CI: 10.5–18.6) compared to community-based studies (6.6%, 95%CI: 2.5–12.3) (Supplementary Figure 9, Appendix). There was no publication bias for overall (Supplementary Figure 10, Appendix) analysis; however, there was publication bias for some subgroup analyses including females, central Africa, rural areas, and population-based studies (Table 2).
Table 2.
Summary statistics of the prevalence of active tobacco smoking in people with diabetes mellitus in Africa.
| Prevalence % (95% confidence interval) | N Studies | N Participants | H (95% confidence interval) | I² (95% confidence interval) | p heterogeneity | p Egger | p difference subgroups | |
|---|---|---|---|---|---|---|---|---|
| Overall | 12.9 (9.6–16.6) | 42 | 18564 | 6.7 (6.1–7.1) | 97.7 (97.3–98.0) | <0.0001 | 0.372 | |
| By sex | ||||||||
| Male | 18.6 (9.7–29.6) | 6 | 1130 | 4.0 (3.0–5.3) | 93.6 (88.8–96.4) | <0.0001 | 0.752 | 0.0006 |
| Female | 2.1 (0.0–6.5) | 6 | 1470 | 3.6 (2.7–4.9) | 92.3 (86.0–95.8) | <0.0001 | 0.049 | |
| By region | ||||||||
| Northern Africa | 21.3 (14.5–29.0) | 11 | 3250 | 4.8 (4.0–5.8) | 95.7 (93.9–97.0) | <0.0001 | 0.671 | 0.006 |
| Sub-Saharan Africa | 10.3 (6.8–14.3) | 31 | 15314 | 7.0 (6.5–7.6) | 98.0 (97.6–98.3) | <0.0001 | 0.194 | |
| By sub-region | ||||||||
| Northern Africa | 21.3 (14.5–29.0) | 11 | 3250 | 4.8 (4.0–5.8) | 95.7 (93.9–97.0) | <0.0001 | 0.671 | 0.021 |
| Southern Africa | 16.8 (8.427.3) | 6 | 7203 | 7.6 (6.3–9.2) | 98.3 (97.5–98.8) | <0.0001 | 0.958 | |
| Central Africa | 15.0 (0.9–40.6) | 4 | 987 | 9.0 (7.3–11.2) | 98.8 (98.1–99.2) | <0.0001 | 0.065 | |
| Western Africa | 7.7 (3.5–13.3) | 8 | 1141 | 2.8 (2.1–3.8) | 87.6 (77.8–93.1) | <0.0001 | 0.232 | |
| Eastern Africa | 7.3 (1.8–15.8) | 11 | 4607 | 8.5 (7.5–9.6) | 98.6 (98.2–98.9) | <0.0001 | 0.612 | |
| By area | ||||||||
| Urban | 11.7 (7.7–16.3) | 24 | 7010 | 5.4 (4.8–6.0) | 96.5 (95.7–97.2) | <0.0001 | 0.570 | 0.191 |
| Rural | 3.8 (0.0–16.0) | 3 | 520 | 4.1 (2.7–6.3) | 94.0 (85.8–97.4) | <0.0001 | 0.090 | |
| Setting | ||||||||
| Population-based | 6.6 (2.5–12.3) | 7 | 1989 | 3.3 (2.5–4.4) | 91.0 (84.0–94.9) | <0.0001 | 0.029 | 0.032 |
| Hospital-based | 14.3 (10.5–18.6) | 35 | 16575 | 7.0 (6.5–7.6) | 98.0 (97.6–98.3) | <0.0001 | 0.669 | |
Figure 3.
Forest plot of the meta-analysis prevalence of active smoking among people with diabetes mellitus in Africa.
Discussion
This systematic review and meta-analysis aimed to estimate the prevalence of active tobacco smoking among patients with hypertension or diabetes mellitus living in Africa. We compiled data from about 20,000 patients for each condition, and obtained a pooled prevalence of 13%. There was a wide variation between countries, from 0.5% to 58.4% both in Nigeria for patients with hypertension, and from 0.0% in Tanzania to 55.7% in Tunisia for patients with diabetes mellitus, with substantial heterogeneity between studies. Additionally, we found for both conditions that the prevalence of active smoking was higher in males than in females and in Northern Africa than in sub-Saharan Africa. However, there was no difference in prevalence estimates between urban and rural settings, and between community-based and hospital-based studies, except for patients with diabetes mellitus for whom the prevalence of active smoking was higher in hospital-based compared to community-based studies.
The prevalence of 13% found in this review concurs roughly with other reports from hypertensive or diabetes populations residing outside Africa109–111. Likewise, our estimates align with what has been reported by the World Health Organization (WHO) for the prevalence of smoking among the African general population, around 12%16. Similarly, the wide variation of prevalence estimates observed between studies or countries was previously reported16,112. For instance, in a meta-analysis compiling data from 13 African countries mostly from Eastern, Western and Southern Africa, it was found that the prevalence of active tobacco smoking in the general population varied immensely, from 1.8 to 25.8%112. Notwithstanding, the prevalence of active tobacco smoking in Africa seems lower than in European or American countries16,112,113, though all forms of tobacco consumption need to be taken into account.
The higher prevalence of smoking in men compared to women is unsurprising and corroborates previous reports16,112. Indeed, the global prevalence of smoking is about five times higher in men (37%) than in women (7%). In Africa specifically, it is 22% of males in comparison to only 2% of females who smoke16. Although reasons for this huge discrepancy between men and women’s attitude towards smoking remains mostly unexplored in Africa, it was hypothesized that there might be an influence of the culture or societal behaviour which discourages women from smoking114. On the other hand, the absence of difference between rural and urban settings corroborates previous observations regarding the prevalence of smoking in the Sub-Saharan African general population112.
Moreover, our prevalence estimates of active smoking were more than twice higher in Northern Africa than in sub-Saharan Africa both for patients with hypertension (27.2 vs. 11.8%; p = 0.0002) or diabetes mellitus (21.3 vs. 10.3%; p = 0.0002). This similar prevalence in hypertensive and diabetes populations suggests that tobacco burden might be lower in the general population in sub-Saharan Africa compared to Northern Africa. In fact, the global status report on tobacco clearly shows a lower prevalence of smoking in low-income countries including most sub-Saharan African countries, as compared to middle-income countries, in which Northern African countries are classified16. This might be explained by the fact that cigarette is more affordable for populations with higher socioeconomic status. Furthermore, Northern African countries might be culturally more prone to smoke.
Accordingly, special attention should be given to Northern Africa when monitoring the policies and interventions to reduce tobacco use on the continent. Considering the current previsions which announce an exponential increment in the prevalence of tobacco in Africa by 22% by 2030115, it is likely that the prevalence of smoking in patients with hypertension or diabetes mellitus will also increase sharply. Despite these projections and up till now, tobacco control has received very low priority in Africa115. Indeed, Africa is still very far behind full implementation of the WHO Framework Convention on Tobacco Control guidelines116, particularly when it comes to protection from exposure to tobacco smoke, packaging and labelling of tobacco products, and tobacco advertising, promotion and sponsorship117. Most importantly, raising taxes on tobacco products which is the best cost-effective strategy to reduce the burden of tobacco consumption is weakly and sparsely implemented in Africa16,117,118.
Hence, it is high time African countries start adopting and implementing or reinforcing tobacco control strategies to reduce the current and/or future tobacco burden in the continent16,119,120. This will contribute substantially in preventing people from starting to smoke. On the other hand and singularly, context-specific interventions for smoking cessation should be implemented, especially in hypertensive and diabetes populations, considering the devastating interaction between smoking, hypertension and/or diabetes, resulting in a sharp increase in all-cause and cardiovascular morbidity and mortality6,7. Indeed, smoking cessation is associated with many important improvements in health and quality of life and is pivotal in cardiovascular disease prevention121,122. Several smoking cessation interventions including pharmacological treatment, physical exercise, individual and telephone have been shown to be efficacious123,124. Furthermore, concerns have been raised that some smoking cessation therapies such as nicotine replacement therapy, bupropion or varenicline may raise the risk of major cardiovascular disease events associated within the quitting period. However, it has been shown that these therapies do not increase the risk of cardiovascular disease123,125. Patients should be continuously educated, and care givers trained and well-equipped to provide adequate support to their patients for smoking prevention and cessation, including pharmacological and behavioural therapies.
However, our findings should be interpreted in the context of some drawbacks. For instance and common to the majority of meta-analyses of this type, we found a substantial heterogeneity between studies; but we undertook sub-group and meta-regression analyses which contributed significantly in identifying the major sources of variability. Moreover, African sub-regions were disproportionally represented and a high number of studies were hospital-based or used consecutive sampling, which may have led to an overestimation of prevalence estimates in individual studies or may have hindered the translatability of our results to the entire African continent. Despite these limitations and to the very best of our knowledge, this is the first systematic review and meta-analysis which gives a clear and comprehensive estimation of the burden of active smoking in people with hypertension and/or diabetes mellitus residing in Africa. We used rigorous methodological procedures and robust statistical analyses to generate our estimates. Additionally, most studies that were included had a low risk of bias in their methodological quality.
Conclusion
This first systematic review and meta-analysis on the prevalence of active tobacco smoking among patients with hypertension or diabetes mellitus in Africa figured out a high burden of smoking in these populations. Accordingly, specific and effective interventions should be initiated or reinforced in these patients with either or both conditions, to prevent them from smoking or help them to be delivered from tobacco addiction. Special attention should be deserved to men and those living in Northern Africa.
Supplementary information
Acknowledgements
The authors thank Dr. Aurel Tankeu for their role during the conception of the study and development of the protocol. There was no funding source for this study.
Author Contributions
J.J.N., J.J.B. and J.R.N.ansseu conceived the study and, together with FTE and A.D.K. developed the protocol. J.J.N. and J.J.B. conducted the literature search and selected the studies. U.F.N., A.N., J.R.N.keck, and J.J.N. extracted the relevant information. J.J.N. and J.J.B. synthesised the data. J.J.N., J.R.N.ansseu, F.T.E. and J.J.B. wrote the first draft of the paper. J.J.N., J.J.B., U.F.N., A.D.K., J.R.N.ansseu, J.R.N.keck and F.T.E. critically revised successive drafts of the paper and approved its final version. JJN is the guarantor of the review.
Availability of Data and Material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anderson Ngouo, Jan René Nkeck and Ulrich Flore Nyaga contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37858-z.
References
- 1.WHO. Global status report on noncommunicable diseases, http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1 (2014).
- 2.Mensah GA, et al. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013. Cardiovascular journal of Africa. 2015;26:S6–10. doi: 10.5830/cvja-2015-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canto JG, Iskandrian AE. Major risk factors for cardiovascular disease: debunking the “only 50%” myth. Jama. 2003;290:947–949. doi: 10.1001/jama.290.7.947. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. Jama. 2003;290:891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 5.Khot UN, et al. Prevalence of conventional risk factors in patients with coronary heart disease. Jama. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 6.Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England)390, 1345–1422, 10.1016/s0140-6736(17)32366-8 (2017). [DOI] [PMC free article] [PubMed]
- 7.Fagard RH. Smoking amplifies cardiovascular risk in patients with hypertension and diabetes. Diabetes care. 2009;32(Suppl 2):S429–431. doi: 10.2337/dc09-S354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsiki N, Papadopoulou SK, Fachantidou AI, Mikhailidis DP. Smoking and vascular risk: are all forms of smoking harmful to all types of vascular disease? Public health. 2013;127:435–441. doi: 10.1016/j.puhe.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Wafeu GS, et al. Prevalence and associated factors of active smoking among individuals living with hypertension and/or diabetes in Africa: a systematic review and meta-analysis protocol. BMJ open. 2017;7:e015444. doi: 10.1136/bmjopen-2016-015444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bigna JJ, Nkeck JR, Ngouo A, Nyaga UF, Noubiap JJ. Hepatitis B virus and HIV coinfection among adults residing in Cameroon: A systematic review and meta-analysis of prevalence studies. Infection, Disease & Health. 2018;23:170–178. doi: 10.1016/j.idh.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Kenmoe S, et al. Prevalence of human respiratory syncytial virus infection in people with acute respiratory tract infections in Africa: A systematic review and meta-analysis. Influenza and other respiratory viruses. 2018;12:793–803. doi: 10.1111/irv.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noubiap JJ, et al. Prevalence of dyslipidaemia among adults in Africa: a systematic review and meta-analysis. The Lancet. Global health. 2018;6:e998–e1007. doi: 10.1016/s2214-109x(18)30275-4. [DOI] [PubMed] [Google Scholar]
- 13.Noubiap, J. J., Nansseu, J. R., Nkeck, J. R., Nyaga, U. F. & Bigna, J. J. Prevalence of white coat and masked hypertension in Africa: A systematic review and meta-analysis. Journal of clinical hypertension (Greenwich, Conn.), 10.1111/jch.13321 (2018). [DOI] [PMC free article] [PubMed]
- 14.James PA, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 15.Diagnosis and classification of diabetes mellitus. Diabetes care37 (Suppl 1), S81–90, 10.2337/dc14-S081 (2014). [DOI] [PubMed]
- 16.WHO. Global Status Report on Noncommunicable Diseases 2014: Attaining the Nine Global Noncommunicable Diseases Targets; A Shared Responsibility, https://reliefweb.int/report/world/global-status-report-noncommunicable-diseases-2014-attaining-nine-global (2015).
- 17.Hoy D, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. Journal of clinical epidemiology. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. Journal of epidemiology and community health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochran WG. The Combination of Estimates from Different Experiments. Biometrics. 1954;10:101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Abdulsalam S, Olugbenga-Bello A, Olarewaju O, Abdus-Salam I. Sociodemographic correlates of modifiable risk factors for hypertension in a rural local government area of oyo state South west Nigeria. International journal of hypertension. 2014;2014:842028. doi: 10.1155/2014/842028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan PR, Howe LD, Manukusa Z, Purdy S. Determinants of obesity and perception of weight in hypertensive patients in rural South Africa. Journal of Clinical Nutrition. 2014;27:56–62. [Google Scholar]
- 24.Edwards R, et al. Hypertension prevalence and care in an urban and rural area of Tanzania. Journal of hypertension. 2000;18:145–152. doi: 10.1097/00004872-200018020-00003. [DOI] [PubMed] [Google Scholar]
- 25.Addo J, Amoah AG, Koram KA. The changing patterns of hypertension in Ghana: a study of four rural communities in the Ga District. Ethnicity & disease. 2006;16:894–899. [PubMed] [Google Scholar]
- 26.Aghaji, M. N. Hypertension and Risk Factors Among Traders in Enugu, Nigeria. International Journal of Medicine and Health Development13 (2008).
- 27.Akintunde AA, Oyedeji AT, Familoni OB, Ayodele OE, Opadijo OG. QT Interval prolongation and dispersion: Epidemiology and clinical correlates in subjects with newly diagnosed systemic hypertension in Nigeria. Journal of cardiovascular disease research. 2012;3:290–295. doi: 10.4103/0975-3583.102705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allal-Elasmi M, et al. Prehypertension among adults in Great Tunis region (Tunisia): A population-based study. Pathologie-biologie. 2012;60:174–179. doi: 10.1016/j.patbio.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Amer MS, et al. Association of high-sensitivity C-reactive protein with carotid artery intima-media thickness in hypertensive older adults. Journal of the American Society of Hypertension: JASH. 2011;5:395–400. doi: 10.1016/j.jash.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Chin JH, et al. Determinants of Raised Blood Pressure in Urban Uganda: A Community-Based Case-Control Study. Ethnicity & disease. 2017;27:15–20. doi: 10.18865/ed.27.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andy JJ, et al. Prevalence and correlates of hypertension among the Ibibio/Annangs, Efiks and Obolos: a cross sectional community survey in rural South-South Nigeria. Ethnicity & disease. 2012;22:335–339. [PubMed] [Google Scholar]
- 32.Mundan V, Muiva M, Kimani S. Physiological, Behavioral, and Dietary Characteristics Associated with Hypertension among Kenyan Defence Forces. ISRN preventive medicine. 2013;2013:740143. doi: 10.5402/2013/740143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musinguzi G, Nuwaha F. Prevalence, awareness and control of hypertension in Uganda. PloS one. 2013;8:e62236. doi: 10.1371/journal.pone.0062236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tibebu A, Mengistu D, Negesa L. Adherence to recommended lifestyle modifications and factors associated for hypertensive patients attending chronic follow-up units of selected public hospitals in Addis Ababa, Ethiopia. Patient preference and adherence. 2017;11:323–330. doi: 10.2147/PPA.S126382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tshitenge, S. & Mabuza, L. H. A survey of risk factors associated with hypertension in the adult population of Kang, Kgalagadi North, Botswana. Family Practice57, 10.1080/20786190.2014.976963 (2015).
- 36.Yameogo NV, et al. Factors associated with poor blood pressure control in hypertensive black Africans: cross-sectional study of 456 hypertensive patients from Burkina Faso. Annales de cardiologie et d’angeiologie. 2013;62:38–42. doi: 10.1016/j.ancard.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Angaw K, Dadi AF, Alene KA. Prevalence of hypertension among federal ministry civil servants in Addis Ababa, Ethiopia: a call for a workplace-screening program. BMC cardiovascular disorders. 2015;15:76. doi: 10.1186/s12872-015-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ntuli ST, Maimela E, Alberts M, Choma S, Dikotope S. Prevalence and associated risk factors of hypertension amongst adults in a rural community of Limpopo Province, South Africa. African journal of primary health care & family medicine. 2015;7:847. doi: 10.4102/phcfm.v7i1.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nuwaha F, Musinguzi G. Pre-hypertension in Uganda: a cross-sectional study. BMC cardiovascular disorders. 2013;13:101. doi: 10.1186/1471-2261-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aryee, C. et al. An Analysis of Anthropometric Indicators and Modifiable Lifestyle Parameters Associated with Hypertensive Nephropathy. International Journal of2016, 10.1155/2016/6598921 (2016). [DOI] [PMC free article] [PubMed]
- 41.Dennison CR, et al. Cardiovascular risk and comorbid conditions among Black South Africans with hypertension in public and private primary care settings: the HiHi study. Ethnicity & disease. 2007;17:477–483. [PubMed] [Google Scholar]
- 42.Asgedom SW, Gudina EK, Desse TA. Assessment of Blood Pressure Control among Hypertensive Patients in Southwest Ethiopia. PloS one. 2016;11:e0166432. doi: 10.1371/journal.pone.0166432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Awobusuyi, J. O., Adebola, A. P. & Ajose, F. Prevalence and socio-demographic profile of hypertensive patients in a Nigerian general out-patients’ department. Internet Journal of Third World Medicine10, 10.5580/2c6e (2011).
- 44.Ezekwesili CN, Ononamadu CJ, Onyeukwu OF, Mefoh NC. Epidemiological survey of hypertension in Anambra state, Nigeria. Nigerian journal of clinical practice. 2016;19:659–667. doi: 10.4103/1119-3077.188710. [DOI] [PubMed] [Google Scholar]
- 45.Babiker FA, Elkhalifa LA, Moukhyer ME. Awareness of hypertension and factors associated with uncontrolled hypertension in Sudanese adults. Cardiovascular journal of Africa. 2013;24:208–212. doi: 10.5830/cvja-2013-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshi MD, et al. Prevalence of hypertension and associated cardiovascular risk factors in an urban slum in Nairobi, Kenya: a population-based survey. BMC public health. 2014;14:1177. doi: 10.1186/1471-2458-14-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okpechi IG, et al. Blood pressure gradients and cardiovascular risk factors in urban and rural populations in Abia State South Eastern Nigeria using the WHO STEPwise approach. PloS one. 2013;8:e73403. doi: 10.1371/journal.pone.0073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olack B, et al. Risk factors of hypertension among adults aged 35-64 years living in an urban slum Nairobi, Kenya. BMC public health. 2015;15:1251. doi: 10.1186/s12889-015-2610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oladimeji AM, Fawole O, Nguku P, Nsubuga P. Prevalence and factors associated with hypertension and obesity among civil servants in Kaduna, Kaduna State, June 2012. The Pan African medical journal. 2014;18(Suppl 1):13. doi: 10.11694/pamj.supp.2014.18.1.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olatunbosun ST, Kaufman JS, Cooper RS, Bella AF. Hypertension in a black population: prevalence and biosocial determinants of high blood pressure in a group of urban Nigerians. Journal of human hypertension. 2000;14:249–257. doi: 10.1038/sj.jhh.1000975. [DOI] [PubMed] [Google Scholar]
- 51.Owolabi EO, Goon DT, Adeniyi OV, Seekoe E. Social epidemiology of hypertension in Buffalo City Metropolitan Municipality (BCMM): cross-sectional study of determinants of prevalence, awareness, treatment and control among South African adults. BMJ open. 2017;7:e014349. doi: 10.1136/bmjopen-2016-014349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pancha Mbouemboue O, Derew D, Tsougmo JO, Tangyi Tamanji M. A Community-Based Assessment of Hypertension and Some Other Cardiovascular Disease Risk Factors in Ngaoundere, Cameroon. International journal of hypertension. 2016;2016:4754636. doi: 10.1155/2016/4754636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katalambula LK, et al. Dietary pattern and other lifestyle factors as potential contributors to hypertension prevalence in Arusha City, Tanzania: a population-based descriptive study. BMC public health. 2017;17:659. doi: 10.1186/s12889-017-4679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katchunga PB, et al. Hypertension in the adult Congolese population of Southern Kivu: Results of the Vitaraa Study. Presse medicale (Paris, France: 1983) 2011;40:e315–323. doi: 10.1016/j.lpm.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 55.Thiam M, Ndiaye A, Wade B, Perret JL. Descriptive study of complicated hypertension. Experience of the Principal Hospital of Dakar. Dakar medical. 2001;46:86–88. [PubMed] [Google Scholar]
- 56.Katibi IA, Olarinoye JK, Kuranga SA. Knowledge and practice of hypertensive patients as seen in a tertiary hospital in the middle belt of Nigeria. Nigerian journal of clinical practice. 2010;13:159–162. [PubMed] [Google Scholar]
- 57.Kavishe B, et al. High prevalence of hypertension and of risk factors for non-communicable diseases (NCDs): a population based cross-sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC medicine. 2015;13:126. doi: 10.1186/s12916-015-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gudina, E. K., Michael, Y. & Assegid, S. Prevalence of hypertension and its risk factors in southwest Ethiopia: a hospital-based cross-sectional survey. Integrated blood pressure control6, 111–117, 10.2147/ibpc.s47298 (2013). [DOI] [PMC free article] [PubMed]
- 59.Keetile M, Navaneetham K, Letamo G. Patterns and determinants of hypertension in Botswana. Journal of Public Health (Germany) 2015;23:311–318. doi: 10.1007/s10389-015-0682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grace J, Semple S. The prevalence of cardiovascular disease risk factors in normotensive, pre-hypertensive and hypertensive South African colliery executives. International journal of occupational medicine and environmental health. 2012;25:375–382. doi: 10.2478/s13382-012-0045-3. [DOI] [PubMed] [Google Scholar]
- 61.Guwatudde D, et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC public health. 2015;15:1211. doi: 10.1186/s12889-015-2546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balla SA, Abdalla AA, Elmukashfi TA, Ahmed HA. Hypertension among rural population in four states: Sudan 2012. Global journal of health science. 2014;6:206–212. doi: 10.5539/gjhs.v6n3p206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baragou S, et al. Prevalence of cardiovascular risk factors in an urban area of Togo: a WHO STEPS-wise approach in Lome, Togo. Cardiovascular journal of Africa. 2012;23:309–312. doi: 10.5830/cvja-2011-071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bayray A, Berhe H. Nutrition status and major risk factors of hypertension among adults in tigray, North Ethiopia; A case control study. International Journal of Pharmaceutical Sciences and Research. 2012;3:4206–4212. [Google Scholar]
- 65.Kotwani P, et al. Evaluating linkage to care for hypertension after community-based screening in rural Uganda. Tropical medicine & international health: TM & IH. 2014;19:459–468. doi: 10.1111/tmi.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kramoh, K. E. et al. Management of hypertension in the elderly patient at Abidjan cardiology institute (Ivory Coast). International Journal of2012, 10.1155/2012/651634 (2012). [DOI] [PMC free article] [PubMed]
- 67.Adeniyi OV, Longo-Mbenza B, Ter Goon D. Female sex, poverty and globalization as determinants of obesity among rural South African type 2 diabetics: a cross-sectional study. BMC public health. 2015;15:298. doi: 10.1186/s12889-015-1622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agunloye AM, Adebakin AM, Adeleye JO, Ogunseyinde AO. Ultrasound prevalence of gallstone disease in diabetic patients at Ibadan, Nigeria. Nigerian journal of clinical practice. 2013;16:71–75. doi: 10.4103/1119-3077.106770. [DOI] [PubMed] [Google Scholar]
- 69.Alebiosu CO, Odusan O, Familoni OB, Jaiyesimi AE. Cardiovascular risk factors in type 2 diabetic Nigerians with clinical diabetic nephropathy. Cardiovascular journal of South Africa: official journal for Southern Africa Cardiac Society [and] South African Society of Cardiac Practitioners. 2004;15:124–128. [PubMed] [Google Scholar]
- 70.Amrane M, et al. Influence of retinopathy on plasma concentrations of total homocysteine and other biochemical parameters in algerian patients with type 2 diabetes mellitus. Pteridines. 2012;23:96–103. doi: 10.1515/pteridines.2012.23.1.96. [DOI] [Google Scholar]
- 71.Ayah R, et al. A population-based survey of prevalence of diabetes and correlates in an urban slum community in Nairobi, Kenya. BMC public health. 2013;13:371. doi: 10.1186/1471-2458-13-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bello-Sani F, Anumah F. Electrocardiographic abnormalities in persons with type 2 diabetes in Kaduna, Northern Nigeria. International Journal of and Metabolism. 2009;18:99–103. [Google Scholar]
- 73.Ben Ahmed H, et al. La chirurgie du pontage aorto coronaire chez les diabétiques: Résultats immédiats et à moyen terme. Tunisie Medicale. 2012;90:798–802. [PubMed] [Google Scholar]
- 74.Bentata Y, et al. Does smoking increase the risk of progression of nephropathy and/or cardiovascular disease in type 2 diabetic patients with albuminuria and those without albuminuria? American journal of cardiovascular disease. 2016;6:66–69. [PMC free article] [PubMed] [Google Scholar]
- 75.Bouzid K, et al. Study of cardiovascular risk factors in Tunisian patients with recent type 2diabetes. Annales de cardiologie et d’angeiologie. 2012;61:81–87. doi: 10.1016/j.ancard.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Choukem SP, et al. Hyperuricaemia in patients with type 2 diabetes in a tertiary healthcare centre in sub-Saharan Africa: prevalence and determinants. Tropical doctor. 2016;46:216–221. doi: 10.1177/0049475515626030. [DOI] [PubMed] [Google Scholar]
- 77.Dickson K. Prevalence of diabetes and its associated risk factors in south-western Uganda. Journal of Medicine. 2016;24:15–17. [Google Scholar]
- 78.Dinar Y, Elkhoudri N, Belahsen R. Effect of education on nutrition and diabetes status in type 2 diabetics in El Jadida province of Morocco. Mediterranean Journal of Nutrition and Metabolism. 2015;8:187–197. doi: 10.3233/MNM-150040. [DOI] [Google Scholar]
- 79.Dionadji M, Lamlad L, Achbi S, Belhous S, Boudiba A. Associative factors related to ischemia during rest electrocardiograms in type 2 diabetics. Le Mali medical. 2010;25:4–6. [PubMed] [Google Scholar]
- 80.Distiller LA, Joffe BI, Melville V, Welman T, Distiller GB. Carotid artery intima-media complex thickening in patients with relatively long-surviving type 1 diabetes mellitus. Journal of diabetes and its complications. 2006;20:280–284. doi: 10.1016/j.jdiacomp.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 81.Dunbar GL, Hellenberg DA, Levitt N. Diabetes mellitus and non-traumatic lower extremity amputations in four public sector hospitals in Cape Town, South Africa, during 2009 and 2010. Medical Journal. 2015;105:1053–1056. doi: 10.7196/SAMJ.2015.v105i12.9276. [DOI] [PubMed] [Google Scholar]
- 82.El AY, et al. Diabetes and erectile dysfunction in Morocco: epidemiological study among outpatients. Eastern Mediterranean health journal=La revue de sante de la Mediterranee orientale=al-Majallah al-sihhiyah li-sharq al-mutawassit. 2008;14:1090–1100. [PubMed] [Google Scholar]
- 83.el-Shazly M, Zaki A, Nicolucci A. Care-related risk factors for chronic diabetic complications in developing countries: a case from Egypt. Public health. 2002;116:289–296. doi: 10.1038/sj.ph.1900855. [DOI] [PubMed] [Google Scholar]
- 84.Fatouh NF, Nour El-Din MM. Quality of diabetes care in family health facilities in one health district in alexandria. The Journal of the Egyptian Public Health Association. 2009;84:457–478. [PubMed] [Google Scholar]
- 85.Fawzy OA, Arafa AI, El Wakeel MA, Abdul Kareem SH. Plantar pressure as a risk assessment tool for diabetic foot ulceration in egyptian patients with diabetes. Clinical medicine insights. Endocrinology and diabetes. 2014;7:31–39. doi: 10.4137/cmed.s17088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gill G, Gebrekidan A, English P, Wile D, Tesfaye S. Diabetic complications and glycaemic control in remote North Africa. QJM: monthly journal of the Association of Physicians. 2008;101:793–798. doi: 10.1093/qjmed/hcn096. [DOI] [PubMed] [Google Scholar]
- 87.Jingi AM, et al. Association of insulin treatment versus oral hypoglycaemic agents with diabetic retinopathy and its severity in type 2 diabetes patients in Cameroon, sub-SaharanAfrica. Annals of translational medicine. 2016;4:395. doi: 10.21037/atm.2016.08.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khalil NH. & Ramadan, R. A. Study of risk factors for pulmonary tuberculosis among diabetes mellitus patients. Egyptian Journal of Chest Diseases and Tuberculosis. 2016;65:817–823. doi: 10.1016/j.ejcdt.2016.05.009. [DOI] [Google Scholar]
- 89.Kirui NK, et al. Important co-morbidity in patients with diabetes mellitus in three clinics in Western Kenya. Public health action. 2012;2:148–151. doi: 10.5588/pha.12.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Makinga PN, Beke A. A cross-sectional survey on the lifestyle and healthseeking behaviour of Basotho patients with diabetes. Family Practice. 2013;55:190–195. [Google Scholar]
- 91.Mbaye MN, et al. Epidemiological aspects of diabetes in Senegal: Results of a survey on cardiovascular risk factors in Saint-Louis. Medecine des Maladies Metaboliques. 2011;5:659–664. [Google Scholar]
- 92.Mekong JO, Kengne AP, Dehayem MY, Sobngwi E, Mbanya JC. Cardiovascular preventative therapies and outcomes of care among urban sub-saharan Africans with type 2diabetes: A cross-sectional study in Cameroon. Journal of Clinical Outcomes Management. 2012;19:446–452. [Google Scholar]
- 93.Mengesha AY. Lipid profile among diabetes patients in Gaborone, Botswana. South African medical journal=Suid-Afrikaanse tydskrif vir geneeskunde. 2006;96:147–148. [PubMed] [Google Scholar]
- 94.Mwita JC, Mugusi F, Lwakatare J, Chiwanga F. Hypertension control and other cardiovascular risk factors among diabetic patients at Muhimbili NationalHospital, Tanzania. East African journal of public health. 2012;9:70–73. [PubMed] [Google Scholar]
- 95.Namusisi O, et al. Risk factors for non-communicable diseases in rural Uganda: a pilot surveillance project among diabetes patients at a referral hospital clinic. The Pan African medical journal. 2011;10:47. [PMC free article] [PubMed] [Google Scholar]
- 96.Ndege BW, Diero LO, Owiti MO, Anjichi G, Siika AM. Prevalence, Treatment and Control of Hypertension Among Type 2 Diabetic Patients At Moi Teaching and Referral Hospital, Eldoret, Kenya. East African medical journal. 2014;91:253–260. [PubMed] [Google Scholar]
- 97.Ogedengbe, S. O. & Ezeani, I. U. Profile of metabolic abnormalities seen in patients with type 2 diabetes mellitus and their first degree relatives with metabolic syndrome seen in Benin City, Edo state Nigeria. Journal of and Metabolic Disorders13, 10.1186/2251-6581-13-61 (2014). [DOI] [PMC free article] [PubMed]
- 98.Oguntibeju OO, Odunaiya N, Oladipo B, Truter EJ. Health behaviour and quality of life of patients with type 2 diabetes attending selected hospitals in south western Nigeria. The West Indian medical journal. 2012;61:619–626. [PubMed] [Google Scholar]
- 99.Otieno CF, Vaghela V, Mwendwa FW, Kayima JK, Ogola EN. Cardiovascular risk factors in patients with type 2 diabetes mellitus in Kenya: levels of control attained at the Outpatient Diabetic Clinic of Kenyatta National Hospital, Nairobi. East African medical journal. 2005;82:S184–190. doi: 10.4314/eamj.v82i12.9380. [DOI] [PubMed] [Google Scholar]
- 100.Tamiru S, Alemseged F. Risk Factors for Cardiovascular Diseases among Diabetic Patients In Southwest Ethiopia. Ethiopian journal of health sciences. 2010;20:121–128. doi: 10.4314/ejhs.v20i2.69438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wondemagegn AT, et al. Undiagnosed Diabetes Mellitus and Related Factors in East Gojjam (NW Ethiopia) in 2016: A Community-Based Study. Journal of public health research. 2017;6:834. doi: 10.4081/jphr.2017.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hermans MP, et al. The normal-weight type 2 diabetes phenotype revisited. Diabetes & metabolic syndrome. 2016;10:S82–88. doi: 10.1016/j.dsx.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 103.Thomas RL, et al. Ethnic differences in the prevalence of diabetic retinopathy in persons with diabetes when first presenting at a diabetes clinic in South Africa. Diabetes care. 2013;36:336–341. doi: 10.2337/dc12-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kingue S, Rakotoarimanana S, Rabearivony N, Bompera FL. Prevalence of selected cardiometabolic risk factors among adults in urban & semi-urban hospitals in four sub-Saharan African countries. Cardiovascular Journal of. 2017;28:147–153. doi: 10.5830/CVJA-2016-072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muhamedhussein, M. S., Nagri, Z. I. & Manji, K. P. Prevalence, Risk Factors, Awareness, and Treatment and Control of Hypertension in Mafia Island, Tanzania. International Journal of2016, 10.1155/2016/1281384 (2016). [DOI] [PMC free article] [PubMed]
- 106.Abidoye RO, Izunwa RD, Akinkuade FO, Abidoye GO. Inter-relationships between lifestyle and diabetes mellitus, overweight/obesity and hypertension in Nigeria. Nutrition and health. 2002;16:203–213. doi: 10.1177/026010600201600306. [DOI] [PubMed] [Google Scholar]
- 107.Egbi OG, Ofili AN, Oviasu E. Hypertension and Diabetes Self-care Activities: A Hospital Based Pilot Survey in Benin City, Nigeria. The Nigerian postgraduate medical journal. 2015;22:117–122. [PubMed] [Google Scholar]
- 108.Choukem SP, Kengne AP, Dehayem YM, Simo NL, Mbanya JC. Hypertension in people with diabetes in sub-Saharan Africa: revealing the hidden face of the iceberg. Diabetes research and clinical practice. 2007;77:293–299. doi: 10.1016/j.diabres.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 109.White, W. B. et al. High-Intensity Cigarette Smoking Is Associated With Incident Diabetes Mellitus In Black Adults: The Jackson Heart Study. Journal of the American Heart Association7, 10.1161/jaha.117.007413 (2018). [DOI] [PMC free article] [PubMed]
- 110.Liu X, Byrd JB. Cigarette Smoking and Subtypes of Uncontrolled Blood Pressure Among Diagnosed Hypertensive Patients: Paradoxical Associations and Implications. American journal of hypertension. 2017;30:602–609. doi: 10.1093/ajh/hpx014. [DOI] [PubMed] [Google Scholar]
- 111.Stanton CA, et al. Trends in tobacco use among US adults with chronic health conditions: National Survey on Drug Use and Health 2005-2013. Preventive medicine. 2016;92:160–168. doi: 10.1016/j.ypmed.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brathwaite R, Addo J, Smeeth L, Lock K. A Systematic Review of Tobacco Smoking Prevalence and Description of Tobacco Control Strategies in Sub-Saharan African Countries; 2007 to 2014. PloS one. 2015;10:e0132401. doi: 10.1371/journal.pone.0132401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harper S, McKinnon B. Global socioeconomic inequalities in tobacco use: internationally comparable estimates from the World Health Surveys. Cancer causes & control: CCC. 2012;23(Suppl 1):11–25. doi: 10.1007/s10552-012-9901-5. [DOI] [PubMed] [Google Scholar]
- 114.Storr CL, et al. Smoking estimates from around the world: data from the first 17 participating countries in the World Mental Health Survey Consortium. Tobacco control. 2010;19:65–74. doi: 10.1136/tc.2009.032474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blecher, E. & Ross, H. Tobacco use in Africa: tobacco control through prevention, https://www.cancer.org/content/dam/cancer-org/cancer-control/en/reports/tobacco-use-in-africa-tobacco-control-through=prevention.pdf (2012).
- 116.WHO Framework Convention on Tobacco Control. Guidelines for implementation Article 5.3 | Article 8 | Articles 9 and 10 | Article 11 | Article 12 | Article 13 | Article 14, http://apps.who.int/iris/bitstream/handle/10665/75218/9789241501316_eng.pdf?sequence=1 (2011).
- 117.Tumwine J. Implementation of the framework convention on tobacco control in Africa: current status of legislation. International journal of environmental research and public health. 2011;8:4312–4331. doi: 10.3390/ijerph8114312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoffman SJ, Tan C. Overview of systematic reviews on the health-related effects of government tobacco control policies. BMC public health. 2015;15:744. doi: 10.1186/s12889-015-2041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nansseu JR, Bigna JJ. Electronic Cigarettes for Curbing the Tobacco-Induced Burden of Noncommunicable Diseases: Evidence Revisited with Emphasis on Challenges in Sub-Saharan Africa. Pulm Med. 2016;2016:4894352. doi: 10.1155/2016/4894352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jankowski P, et al. Practice setting and secondary prevention of coronary artery disease. Archives of medical science: AMS. 2018;14:979–987. doi: 10.5114/aoms.2017.65236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Athyros VG, Katsiki N, Doumas M, Karagiannis A, Mikhailidis DP. Effect of tobacco smoking and smoking cessation on plasma lipoproteins and associated major cardiovascular risk factors: a narrative review. Current medical research and opinion. 2013;29:1263–1274. doi: 10.1185/03007995.2013.827566. [DOI] [PubMed] [Google Scholar]
- 122.Mons, U. et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ (Clinical research ed.)350, h1551, 10.1136/bmj.h1551 (2015). [DOI] [PMC free article] [PubMed]
- 123.Suissa, K. et al. Efficacy and Safety of Smoking Cessation Interventions in Patients With Cardiovascular Disease: A Network Meta-Analysis of Randomized Controlled Trials. Circulation. Cardiovascular quality and outcomes10, 10.1161/circoutcomes.115.002458 (2017). [DOI] [PubMed]
- 124.Podgorski T, et al. Aerobic and concentration training and allele 7 in the dopamine receptor D4 (D4DR) gene increase chances of smoking cessation in young Polish women. Archives of medical science: AMS. 2018;14:199–206. doi: 10.5114/aoms.2018.72243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129:28–41. doi: 10.1161/circulationaha.113.003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.