Abstract
There has been increasing interest in the human anaerobic colonic bacterium Oxalobacter formigenes because of its ability to metabolize oxalate, and its potential contribution to protection from calcium oxalate kidney stones. Prior studies examining the prevalence of this organism have focused on subjects in developed countries and on adults. Now using O. formigenes-specific PCR, we have compared the prevalence of these organisms among subjects in two remote areas in which modern medical practices have hardly been present with a USA group of mothers and their infants for the first three years of life. Among the Amerindians of the Yanomami-Sanema and Yekwana ethnic groups in Venezuela and the Hadza in Tanzania, O. formigenes was detected in 60–80% of the adult subjects, higher than found in adults from USA in this and prior studies. In young children, the prevalence was much lower in USA than in either tribal village. These data extend our understanding of the epidemiology of O. formigenes carriage, and are consistent with the hypothesis that the rising incidence of kidney stones is associated with the progressive loss of O. formigenes colonization in populations that have been highly impacted by modern medical practices.
Introduction
Oxalobacter formigenes are gram-negative anaerobes that inhabit the intestinal tract of humans and other mammals1,2. O. formigenes is unique in that it utilizes oxalate as its sole carbon and energy source3,4, which it metabolizes into formate and CO25. In mammals, oxalate is an end-product of metabolism and must be eliminated either in the intestine or via urinary excretion6,7. The renal excretion can lead to nephrotoxicity, including calcium oxalate kidney stones8; thus, regulation of oxalate homeostasis has important pathophysiologic consequences.
One hypothesis is that intestinal colonization with O. formigenes helps protect against calcium oxalate nephrolithiasis9,10. O. formigenes utilizes dietary oxalate, reducing the amount absorbed into the circulation, and recent evidence suggests that it induces colonic oxalate transporters (SLC26), acting as a sink for systemic oxalate11–14. Studies comparing adults with recent nephrolithiasis and healthy adults showed a lower rate of O. formigenes colonization in the stone formers, consistent with a protective role for the organism10,15. Since ~75% of kidney stones are composed of calcium oxalate16, and since nephrolithiasis incidence is substantially increasing in both adults and children17–19, a focus on O. formigenes may be of interest for therapeutic applications.
Prior studies of the prevalence of O. formigenes colonization showed considerable differences, which might reflect true variation or technical issues. Studies in healthy adults suggest prevalence in the United States is between 31–38%10,20,21 but as high as 60–77% in Korea, Japan, and India22–24. Such variation could also reflect medical practice differences, as O. formigenes is known to be susceptible to many commonly prescribed antibiotics25. In one prospective study, antibiotic treatment resulted in lasting suppression of O. formigenes26. Patients with cystic fibrosis or inflammatory bowel disease, who receive multiple antibiotic courses also have lower O. formigenes prevalence than controls27,28. However, little is known about the transmission of O. formigenes and its timing, and the factors influencing colonization.
To further address these questions, we examined O. formigenes colonization in a small cohort of USA mothers and their infants longitudinally from birth through three years of life. To investigate the hypothesis that modern practices may be impacting O. formigenes colonization, in parallel, we examined adults and children in two remote populations: the Hadza, a tribe of hunter-gatherers in Tanzania27, and a group of remote Amerindians in Venezuela29.
Results
O. formigenes prevalence in the USA cohort
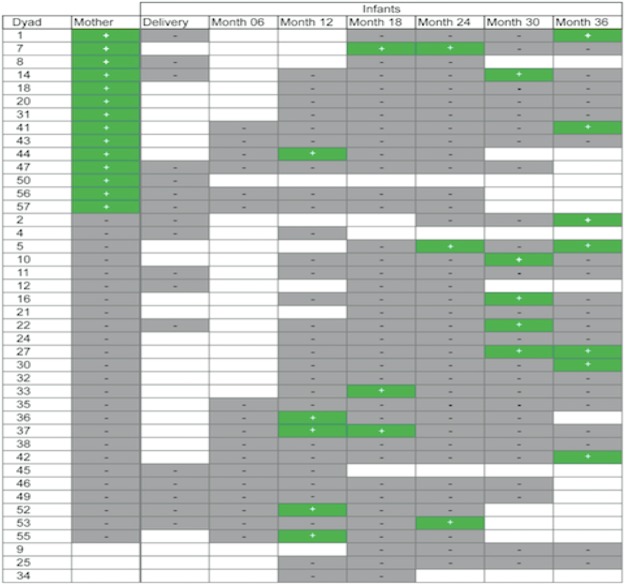
Among the 40 women who provided post-partum fecal samples, O. formigenes was detected in 15 (38%) (Table 1). Among the 42 infants, a total of 19 (45%) were positive at least once in the multiple determinations, and 4 (10%) were positive at least twice (Fig. 1). O. formigenes was only found in children greater than 1-year old and not in infants, and the prevalence appears to increase with age, although study numbers are small.
Table 1.
O. formigenes in USA maternal and infant fecal samples by PCR.
| Sample | N | % Positive |
|---|---|---|
| Mothers | 40 | 38 |
| Infants | ||
| Post-Delivery | 17 | 0 |
| Year 1 | 50 | 10 |
| Year 2 | 76 | 8 |
| Year 3 | 56 | 21 |
Figure 1.
Temporal distribution of O. formigenes in mother-infant dyads from USA. Figure represents 199 samples tested from 42 individual children. In total, 19 (45%) children were positive at ≥1 time point. Four (10%) children were positive at ≥2 time points.
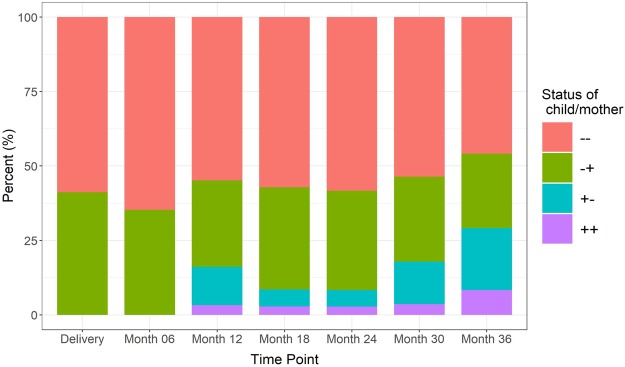
We then assessed whether there was a relationship between maternal and infant colonization status, using logistic regression with and without adjustments for maternal covariates (age, BMI, and race), calculating Cohen’s kappa statistic to examine the agreement between maternal and infant PCR results. We also used a generalized linear mixed model to analyze PCR results with or without adjustment for those covariates as well as maternal antibiotic use prior to pregnancy, during pregnancy, and during delivery. There was no statistically significant correlation between maternal and infant colonization, whether evaluating infant colonization at each time point or in infants positive at any point by either model (Fig. 2). Next, we asked whether O. formigenes status of the infants was correlated with their delivery method or gender. First, we used Fisher exact test and chi squared tests to assess the correlation between these variables and infants’ PCR results. Logistic regression and the generalized linear mixed model were also applied. We found no significant correlation between infant delivery method or gender and their O. formigenes colonization status at individual time points or positivity at any time, with or without adjustment for maternal covariates. We also asked whether O. formigenes colonization status was correlated with infant antibiotic use. We examined whether antibiotics given perinatally (intravenous antibiotics given to the newborn), systemic antibiotics given during the first, second, or third year of life, or systemic antibiotics given at any point correlated with infants’ O. formigenes status. Again there was no significant correlation between antibiotic use and colonization at subsequent time points by logistic regression or generalized mixed model with or without covariate adjustment.
Figure 2.
Concordance of maternal and child O. formigenes status over the first three years of life. O. formigenes detected by oxc PCR, as described in the Methods.
O. formigenes prevalence in hunter-gatherer populations
We compared prevalence of O. formigenes colonization in the cohort of US children with that in tribal populations from Africa and South America. US children had the lowest prevalence (19%) (Fig. 3); compared with Amerindians (68%; p = 0.0013), and with Hadza, (82%; p < 0.0001, Two-sided Fisher exact test). There were no significant differences in prevalence between the Amerindian and Hadza children. While there was no evidence of colonization in the USA children until 12 months of age (0/17 were positive at 6 months (Fig. 1), O. formigenes was detected in some of the youngest subjects sampled in the Hadza and Amerindian populations: the earliest at 9 months and 3 months of age respectively (data not shown). We also examined O. formigenes prevalence in adulthood in 68 Amerindian and 116 Hadza subjects. Among the adults, 79% of Amerindians and 55% of Hadza subjects were PCR-positive. Comparing the two populations by age group (Fig. 4) demonstrated similar rates of colonization except among adolescents and young adults, in which prevalence in the Hadza was significantly lower. Regardless, the prevalence of O. formigenes among both healthy Amerindians and Hadza was substantially higher than that of healthy adults in the United States (ranging from 31% to 38%), as reported in three large and independent studies10,20,21, as well as the US mothers in this work.
Figure 3.
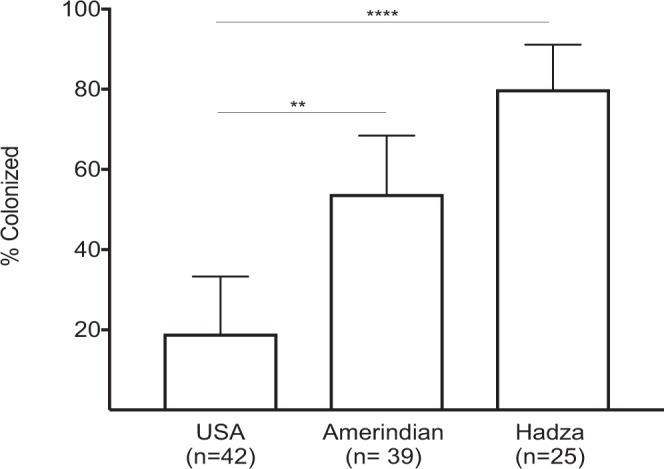
Percent of children less than 5 years old positive for O. formigenes in three populations. Only single samples were present from the Amerindian and Hadza children. Multiple samples were tested for the USA children, but only the sample from each child at the oldest age tested was used for the calculation. Percent of children colonized, with 95% CI shown (**p < 0.01; ****p < 0.001, by Fisher’s exact test).
Figure 4.
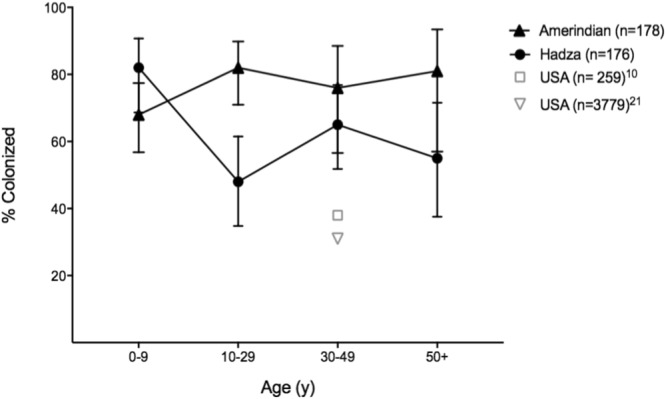
O. formigenes prevalence in traditional and USA populations, by age. Positivity for the Hadza and Amerindians determined by end point PCR using O. formigenes-specific primers, as described in Methods. Results for two prior US studies for comparison using different methodologies are shown at ~the mean age of the populations tested with the percent colonized indicated; the mean ages were 42 and 46, respectively10,21.
Discussion
To our knowledge, this is the first study to examine early life O. formigenes colonization in more than 20 years and the only study that has examined a cohort of mothers and children longitudinally from birth through the first years of life. The other study of O. formigenes colonization in early life was a cross-sectional evaluation of children ages 0–12 in a remote area of the Ukraine where antibiotic use was considered to be limited30. In that study, Sidhu et al. found that 72% of children were PCR-positive, with no evidence of colonization before the age of one year, but reaching 90% by age 2; nearly all children appeared to be colonized by age 8, after which rates declined. Similar to the findings in Ukrainian infants, we found no evidence of colonization in children less than one year in our U.S. cohort. This may be explained by O. formigenes acquisition from the environment, as infants begin to become more mobile at this age, as proposed30 and demonstrated in rodent models31. The lack of association between maternal and infant colonization in this study also supports the hypothesis of horizontal transmission. Alternatively, O. formigenes may be acquired at birth from mothers but only at low abundance, blooming later in life with advancement of infants’ diet or with maturation of the microbiome and the acquisition of commensal species required for O. formigenes colonization success. The current study did not provide any direct support for this hypothesis, but more prolonged cohort studies with a larger number of subjects may be needed to better address this question. All of the children who were found to be O. formigenes-positive, also had negative samples at the earliest time points. Our past work20,21 showed that the relative abundance of O. formigenes in fecal specimens varies over >3 log10. Children with low abundances may be near the threshold of detection, with the variation in positivity that we observed consistent with our prior findings21.
Several studies have examined the intestinal microbiome of the Hadza29,32–34 and to a lesser extent, the Amerindians of Venezuela35. Since these groups have had only recent exposure to Western medicine, their results provide a window on the pre-industrial intestinal microbiome of humans. Similar to our prior study of a small uncontacted Amerindian population using high throughput sequencing, the prevalence of O. formigenes was substantially higher than that of USA subjects35. Now we provide evidence of higher rates in a larger population of both children and adults in remote Amerindian and African populations than in the USA, including those in this study as well as in the literature10,21,36. A possible explanation for this difference is that developments associated with the Westernization, such as changes in diet or antibiotic use may contribute to the loss of this commensal organism. A recent report of the association of kidney stones with prior antibiotic treatments37, across multiple antibiotic classes, is also consistent with that hypothesis.
Limitations of this study include the relatively small number of subjects to address associations with covariates. On average, the subjects in the USA were of higher socioeconomic status, more urban, and better-educated38 than the general population, so they were not fully representative of the USA population. Comparing fecal samples from the immediately post-partum women in the USA to populations of largely non-pregnant women is an intrinsic limitation as well. Individual dietary information was generally not available, so oxalate exposure differences could not be evaluated. Nevertheless, this study used the same O. formigenes-specific methodologies to examine specimens across three different populations in large cross-sectional surveys; this is the first report in the literature of such comparisons. In conclusion, O. formigenes was present at much lower prevalence in the USA subjects compared to remote indigenous groups, consistent with the hypothesis that O. formigenes is a part of the ancestral human gut microbiota, and that it may be disappearing in the context of socioeconomic advances and medical treatments, potentially contributing to the rise in nephrolithiasis.
Methods
Populations tested
All experiments were performed in accordance with relevant guidelines and regulations concerning human subjects (Table 2). United States. Fecal samples were collected from a cohort of mothers and infants in New York City as part of the Early Childhood Antibiotics and the Microbiome (ECAM) study38. The specimens used in this study were obtained under the ECAM protocol, approved by the NYU Langone Medical Center IRB, as reported in detail38. Informed consent was obtained from all subjects, or if subjects were under 18, from a parent and/or legal guardian. Forty of the 52 mothers enrolled in the study during their pregnancy self-collected fecal samples during the first month post-partum (Mean 9 +/−12 days (SD) post-partum). Fecal samples were collected from their infants within the first postpartum day and usually represented meconium, and then regularly through three years of life. Mothers were provided sterile supplies and instructions for sanitary collection of their own and their infant’s stools at home. Fecal samples were immediately chilled to −2 to −8 C, and delivered within 24–48 hours to the laboratory where they were frozen to −80 C. The maternal post-partum (n = 40) and infant delivery (n = 17) samples, as well as those obtained at ~6 months (n = 17), 12 m (n = 33), 18 m (n = 38), 24 m (n = 38), 30 m (n = 30) and 36 m (n = 26) were examined for O. formigenes colonization.
Table 2.
Demographics of the 442 subjects in three locales.
| Region | No. subjects | Age: range (y), mean ±SD | Sex: % female | BMI*: mean ±SD | Delivery: % vaginal | Race or Ethnicity: (%) | |
|---|---|---|---|---|---|---|---|
| Tanzania | 182 | 0–80, 28.3 ± 19.7 |
48.3 | 119.5 ± 3.4 | 100 | Hadza | 100 |
| Venezuela | 178 | 0–62, 18.3 ± 16.2 |
54.5 | 18.9 ± 5.1 | 100 | Sanema | 54 |
| Yekwana | 46 | ||||||
| New York-Mothers | 40 | 23–40, 35.8 ± 4.5 |
100 | 24.7 ± 4.3 | NA | African-Am | 5 |
| Asian | 18 | ||||||
| Hispanic | 10 | ||||||
| White | 65 | ||||||
| Other | 3 | ||||||
| New York-Infants | 42 | 0–3, NAŧ |
33.3 | NA | 55 | African-Am | 5 |
| Asian | 17 | ||||||
| Hispanic | 10 | ||||||
| White | 67 | ||||||
| Other | 2 | ||||||
*BMI included for subjects over age 5 years.
ŧSamples tested at multiple time points over first three years of life.
Tanzania
The Hadza live in north-central Tanzania around Lake Eyasi in the central Rift Valley. There are currently ~1,200 Hadza with fewer than 200 following a traditional foraging lifestyle that includes obtaining ~80–90% of their caloric needs from hunted and foraged resources. Fecal samples were collected from 182 traditional subjects of the Hadza tribe across wet and dry seasons29. The Hadza are among the last remaining hunter-gatherer populations in Africa, and reside in the eastern Rift Valley of Tanzania near Lake Eyasi39. Traditional Hadza live in camps averaging 20–30 people, with numbers varying according to season and availability of resources. Six camps were represented in this study (Kipamba, Makao, Mwamudu, Onowas, Sengeli, Ukamako)29. Until recently, Hadza had limited access to medical dispensaries and rural hospitals and thus western medications. However, recent encroachment into Hadzaland by pastoralists and other populations has increased access to western medical care – with medical posts within a day’s walk from the camps sampled. Permission for the study was obtained from the National Institute of Medical Research (MR/53i 100/83, NIMR/HQ/R.8a/Vol.IX/1542) and the Tanzania Commission for Science and Technology. As per our IRB, Informed Consent was obtained. Prior to sample collection, a brief explanation of the project and its objectives was given in a way that could be easily understood in Swahili via a translator. Each participant then signed a Consent Form either with a signature or thumb print in the presence of a witness to confirm her/his willingness to participate. In the case of minors, consent was provided by a parent or legal guardian29. The number of samples collected was not pre-specified for ensuring adequate statistical power. Subjects ranged from 6 months to 80 years of age; the age of six subjects was unknown and those samples were omitted from related analyses. Of the 182 subjects tested, 8 were pregnant women.
Fecal samples were self-collected by subjects, frozen in liquid nitrogen within 0–2 hours, and maintained frozen during all transport and storage until processed for analysis in the United States.
Venezuela
Fecal samples were collected from 178 Amerindians in seven villages in the Yanomami-Sanema and Yekwana territory located in the rain forest of the High Caura river basin, from Bolivar state in Venezuela, under Venezuelan Institute of Scientific Research (IVIC) institutional review board (IRB) approval (project Dir0229/10 approval granted to M. Contreras)40. Informed consent was obtained according to the approved IRB protocol, first from the community leaders, then from the individual volunteers who were explained the study, and who signed or stamped their thumb print on the consent form. Parents signed to consent their infants and children. Villagers lack a market economy, and live in relative isolation in villages from 15 to 100 people, with access to the closest town by river requiring travel for one week. They maintain the traditional lifestyle of hunter-gatherer–gardeners common in the remote villages in this mountainous region. There are no health posts in Sanema villages, and one health post in one Yekwana village, while the others receive no medical care, except by infrequent visits from physicians. Amerindian subjects ranged from 3 months to 62 years of age; pregnant women were not sampled. Fecal samples were collected in the field and immediately frozen in liquid nitrogen, and remained frozen until the time of DNA extraction.
PCR methods
DNA extraction from fecal swabs was performed using the PowerSoil DNA Extraction Kit (MoBio, Carlsbad CA), per the manufacturer’s protocol. All extracted DNA was stored at −80 C. Primers were designed based on the sequence alignment of the first 500 bp of the oxc gene of O. formigenes. The specificity of these primers [5′-ATGTAGAGTTGACTGATGGC-3′ (forward) and 5′-TTGATG CTGTTGATACG-3′ (reverse)] was confirmed by examining their homology with all other bacterial sequences deposited at the National Center for Biotechnology Information Basic Local Alignment Search Tool website (http://www.ncbi.nlm.nih.gov/BLAST). PCR was performed using Taq DNA Polymerase and 10x CoralLoad PCR buffer (Qiagen, Valencia CA). Each 50 ul reaction contained 1ul of extracted DNA, 0.2 uM of each primer and 6.3 uM DNTPs. PCR cycling conditions included an initial denaturation at 95 C for 3 min and 39 cycles of denaturation at 95 C for 30 s, annealing at 54 C for 30 s, and extension at 72 C for 1 min. PCR products were analyzed by electrophoresis using 2% agarose gels.
Statistical Analysis
For the USA studies in which more extensive metadata were available, logistic regression was applied to explore the relationship between the O. formigenes status of each infant and of their mother. Analyses were performed with and without adjustment for the mother’s covariates (including age, BMI, race, antibiotic use prior to pregnancy, antibiotic use status during pregnancy, and antibiotics use status during delivery). Due to small sample size, race was defined as a binary variable with white = 1 and nonwhite = 0. Infants’ O. formigenes status was their status at each time point or being positive at any time. As a sensitivity analysis, the agreement of infant and mother O. formigenes status pair also was evaluated by Cohen’s kappa test statistic41. Fisher exact test, chi-squared test and logistic regression with or without adjustment for mother’s covariates were applied for testing whether the O. formigenes status of infants at each time or being positive at any time was related to their birth method, antibiotic use status, and gender, respectively. The generalized linear mixed model was used to determine whether the infants’ O. formigenes status during 0–36 months was related to their baseline variates and time dependent covariates such as antibiotic usage, with or without adjustment for their mother’s covariates. Time points were converted to 0–36 months, and missing data were omitted.
Acknowledgements
Supported in part by RO1DK090989, U01 AI22245 (to MJB) T35-DK007421-32 (to AP), R01DK110014 (to HL), the Oxalosis and Hyperoxaluria Foundation-American Society of Nephrology career development Award (to LN), and by the Emch and C&D Funds (to MGDB). This study was also supported by Rare Kidney Stone Consortium grant U54KD083908, a part of the Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, the National Center for Advancing Translational Sciences (NCATS). This consortium is funded through a collaboration between the NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases. We thank Nora Henderson, Jennifer Chung and Yi Cai for their technical assistance.
Author Contributions
A.P., L.N. and M.B. planned the study. M.J., O.N.-A., M.C., O.L., J.L., M.G.D.B. collected and analyzed the specimens. A.P. performed the experiments. A.P., C.W. and H.L. performed the analyses. All authors wrote the manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allison MJ, Dawson KA, Mayberry WR, Foss JG. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Archives of Microbiology. 1985;141:1–7. doi: 10.1007/BF00446731. [DOI] [PubMed] [Google Scholar]
- 2.Allison MJ, Cook HM, Milne DB, Gallaher S, Clayman RV. Oxalate degradation by gastrointestinal bacteria from humans. Journal of Nutrition. 1986;116:455–460. doi: 10.1093/jn/116.3.455. [DOI] [PubMed] [Google Scholar]
- 3.Cornick NA, Allison MJ. Assimilation of oxalate, acetate, and CO2 by Oxalobacter formigenes. Can J Microbiol. 1996;42:1081–1086. doi: 10.1139/m96-138. [DOI] [PubMed] [Google Scholar]
- 4.Cornick NA, Allison MJ. Anabolic Incorporation of Oxalate by Oxalobacter formigenes. Appl Environ Microbiol. 1996;62:3011–3013. doi: 10.1128/aem.62.8.3011-3013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allison MJ, Dawson KA, Mayberry WR, Foss JG. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch.Microbiol. 1985;141:1–7. doi: 10.1007/BF00446731. [DOI] [PubMed] [Google Scholar]
- 6.Holmes RP. Oxalate synthesis in humans: assumptions, problems, and unresolved issues. Mol.Urol. 2000;4:329–332. [PubMed] [Google Scholar]
- 7.Holmes RP, Assimos DG. The impact of dietary oxalate on kidney stone formation. Urol Res. 2004;32:311–316. doi: 10.1007/s00240-004-0437-3. [DOI] [PubMed] [Google Scholar]
- 8.Hoppe B, et al. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transplant. 2011;26:3609–3615. doi: 10.1093/ndt/gfr107. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe B, Dittlich K, Fehrenbach H, Plum G, Beck BB. Reduction of plasma oxalate levels by oral application of Oxalobacter formigenes in 2 patients with infantile oxalosis. Am J Kidney Dis. 2011;58:453–455. doi: 10.1053/j.ajkd.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman DW, et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol. 2008;19:1197–1203. doi: 10.1681/ASN.2007101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. American journal of physiology. Gastrointestinal and liver physiology. 2011;300:G461–469. doi: 10.1152/ajpgi.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatch M, Freel RW. A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis. 2013;41:379–384. doi: 10.1007/s00240-013-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatch M, et al. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 2006;69:691–698. doi: 10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 14.Arvans D, et al. Oxalobacter formigenes-Derived Bioactive Factors Stimulate Oxalate Transport by Intestinal Epithelial Cells. Journal of the American Society of Nephrology: JASN. 2017;28:876–887. doi: 10.1681/ASN.2016020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siener R, et al. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 2013;83:1144–1149. doi: 10.1038/ki.2013.104. [DOI] [PubMed] [Google Scholar]
- 16.Hesse A, Siener R. Current aspects of epidemiology and nutrition in urinary stone disease. World J Urol. 1997;15:165–171. doi: 10.1007/BF02201853. [DOI] [PubMed] [Google Scholar]
- 17.Scales CD, Jr., Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. European urology. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dwyer ME, et al. Temporal trends in incidence of kidney stones among children: a 25-year population based study. The Journal of urology. 2012;188:247–252. doi: 10.1016/j.juro.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kittanamongkolchai W, et al. The Changing Incidence and Presentation of Urinary Stones Over 3 Decades. Mayo Clin Proc. 2018;93:291–299. doi: 10.1016/j.mayocp.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnett C, Nazzal L, Goldfarb DS, Blaser MJ. The Presence of Oxalobacter Formigenes in the Microbiome of Healthy Young Adults. The Journal of urology. 2015 doi: 10.1016/j.juro.2015.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, et al. Oxalobacter formigenes-associated host features and microbial community structures examined using the American Gut Project. Microbiome. 2017;5:108–125. doi: 10.1186/s40168-017-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwak C, et al. Molecular identification of Oxalobacter formigenes with the polymerase chain reaction in fresh or frozen fecal samples. BJU Int. 2001;88:627–632. doi: 10.1046/j.1464-4096.2001.02395.x. [DOI] [PubMed] [Google Scholar]
- 23.Kodama T, et al. Detection of Oxalobacter formigenes in human feces and study of related genes in a new oxalate-degrading bacterium. Hinyokika Kiyo. 2003;49:371–376. [PubMed] [Google Scholar]
- 24.Kumar R, et al. Role of Oxalobacter formigenes in calcium oxalate stone disease: a study from North India. European urology. 2002;41:318–322. doi: 10.1016/S0302-2838(02)00040-4. [DOI] [PubMed] [Google Scholar]
- 25.Lange JN, et al. Sensitivity of human strains of Oxalobacter formigenes to commonly prescribed antibiotics. Urology. 2012;79:1286–1289. doi: 10.1016/j.urology.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharlamb V, et al. Oral antibiotic treatment of Helicobacter pylori leads to persistently reduced intestinal colonization rates with Oxalobacter formigenes. J Endourol. 2011;25:1781–1785. doi: 10.1089/end.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidhu H, et al. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet. 1998;352:1026–1029. doi: 10.1016/S0140-6736(98)03038-4. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R, Ghoshal UC, Singh G, Mittal RD. Infrequency of colonization with Oxalobacter formigenes in inflammatory bowel disease: possible role in renal stone formation. J Gastroenterol Hepatol. 2004;19:1403–1409. doi: 10.1111/j.1440-1746.2004.03510.x. [DOI] [PubMed] [Google Scholar]
- 29.Smits SA, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science. 2017;357:802–806. doi: 10.1126/science.aan4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidhu H, et al. Evaluating children in the ukrain for colonization with the intestinal bacterium Oxalobacter formigenes, Using a polymerase chain reaction based detection system. Molecular Diagnosis. 1997;2:89–97. doi: 10.1016/S1084-8592(97)80015-X. [DOI] [PubMed] [Google Scholar]
- 31.Cornelius JG, Peck AB. Colonization of the neonatal rat intestinal tract from environmental exposure to the anaerobic bacterium Oxalobacter formigenes. Journal of medical microbiology. 2004;53:249–254. doi: 10.1099/jmm.0.05418-0. [DOI] [PubMed] [Google Scholar]
- 32.Schnorr SL, et al. Gut microbiome of the Hadza hunter-gatherers. Nature communications. 2014;5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turroni S, et al. Fecal metabolome of the Hadza hunter-gatherers: a host-microbiome integrative view. Sci Rep. 2016;6:32826. doi: 10.1038/srep32826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clemente, J. C. et al. The microbiome of uncontacted Amerindians. Science advances1, 10.1126/sciadv.1500183 (2015). [DOI] [PMC free article] [PubMed]
- 36.Barnett C, Nazzal L, Goldfarb DS, Blaser MJ. The presence of Oxalobacter formigenes in the microbiome of healthy young adults. The Journal of urology. 2015;195:499–506. doi: 10.1016/j.juro.2015.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tasian, G. E., Jemielita, T., Goldfarb, D. S., Gerber, J. & Wu, Q. Oral antibiotic exposure and kidney stone disease. J Amer Soc Nephrol (2018). [DOI] [PMC free article] [PubMed]
- 38.Bokulich NA, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Science translational medicine. 2016;8:343ra382. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marlowe, F. The Hadza: Hunter-Gatherers of Tanzania. First edition edn, (University of California Press, 2010).
- 40.Ruggles K, et al. Changes in the gut microbiota of urban subjects during an immersion in the traditional diet and lifestyle of a rainforest village. mSphere. 2018;3:3:e00193–18. doi: 10.1128/mSphere.00193-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.