Abstract
Background and Purpose
Hydrogen sulfide (H2S) and polysulfides (H2Sn) are signalling molecules that mediate various physiological responses including cytoprotection. Their oxidized metabolite sulfite (SO3 2−) is found in blood and tissues. However, its physiological role remains unclear. In this study, we investigated the cytoprotective effect of sulfite on neurons exposed to oxidative stress caused by high concentrations of the neurotransmitter glutamate, known as oxytosis.
Experimental Approach
Concentrations of sulfite as well as those of cysteine and GSH in rats were measured by HPLC. Cytoprotective effects of sulfite on primary cultures of rat neurons against oxytosis was examined by WST‐8 cytoprotective and LDH cytotoxicity assays and compared with that of H2S, H2Sn and thiosulfate.
Key Results
Free sulfite, present at approximately 2 μM in the rat brain, converts cystine to cysteine more efficiently than H2S and H2Sn and facilitates transport of cysteine into cells. Physiological concentrations of sulfite protected neurons from oxytosis and were accompanied by increased intracellular concentrations of cysteine and GSH probably due to converting extracellular cystine to cysteine, more efficiently than H2S and H2Sn. In contrast, thiosulfate only slightly protected neurons from oxytosis.
Conclusions and Implications
Our present data have shown sulfite to be a novel cytoprotective molecule against oxytosis, through maintaining cysteine levels in the extracellular milieu, leading to increased intracellular cysteine and GSH. Although there may be adverse clinical effects in sensitive individuals, our results provide a new insight into the therapeutic application of sulfite to neuronal diseases caused by oxidative stress.
Linked Articles
This article is part of a themed section on Chemical Biology of Reactive Sulfur Species. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.4/issuetoc
Abbreviations
- 3MST
3‐mercaptopyruvate sulfurtransferase
- CAT
cysteine aminotransferase
- CBS
cystathionine β‐synthase
- CSE
cystathionine γ‐lyase
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- GSH
glutathione
- HPLC
high‐performance liquid chromatography
- Keap1
Kelch ECH‐associating protein 1
- LDH
lactate dehydrogenase
- mBBR
monobromobimane
- MEM
modified Eagle's medium
- Nrf2
nuclear factor erythroid 2‐related factor 2
- PTEN
tumour suppressor phosphatase and tensin homologue
- SQR
Sulfur quinone oxidoreductase
- WST‐8
4–3‐(2‐methoxy‐4‐nitrophenyl)‐2‐(4‐nitrophenyl)‐2H‐5‐tetrazokio‐1,3‐benzene disulfonate sodium salt
Introduction
Hydrogen sulfide (H2S), which is produced cystathionine ß‐synthase (CBS), cystathionine γ‐lyase (CSE), and 3‐mercaptopyruvate sulfurtransferase (MPST) together with cysteine aminotransferase (CAT), regulates neuronal transmission, vascular tone and inflammation; confers cytoprotection against oxidative stress; and acts as an oxygen sensors (Abe and Kimura, 1996; Hosoki et al., 1997; Zhao et al., 2001; Kimura and Kimura, 2004; Zanardo et al., 2006; Olson et al., 2006; Elrod et al., 2007; Shibuya et al., 2009; Singh et al., 2009; Peng et al., 2010).
Polysulfides (H2Sn), which have a higher number of (sulfane) sulfur than H2S, are produced by 3MST and by the chemical interaction between H2S and oxidants including NO (Kimura et al., 2013; Eberhardt et al., 2014; Cortese‐Krott et al., 2015; Kimura et al., 2015; Miyamoto et al., 2017; Kimura et al., 2017). They activate TRPA1 cation channels and protein kinase G1α to regulate the vascular tone (Kimura et al., 2013; Stubbert et al., 2014; Kimura et al., 2015; Kimura et al., 2017). Moreover, H2Sn regulate the activity of a tumour suppressor phosphatase and tensin homologue and GAPDH (Greiner et al., 2013; Jarosz et al., 2015).
There are two forms of glutamate toxicity: receptor‐initiated excitotoxicity (Choi, 1988) and non‐receptor‐mediated oxidative glutamate toxicity called oxytosis (Murphy et al., 1989; Tan et al., 2001), which is a well‐studied programmed cell death pathway that is independent of ionotropic glutamate receptors (Murphy et al., 1989; Tan et al., 2001; Maher and Davis, 1996; Kimura and Kimura, 2004). Oxytosis is induced by high concentrations of extracellular glutamate in newly plated primary cultures of neurons that have not yet expressed ionotropic glutamate receptors (Choi, 1987; Schubert and Piasecki, 2001; Kimura and Kimura, 2004). High concentrations of glutamate suppress the cystine/glutamate transporter xCT (SLC7A11) to decrease the incorporation of cystine, which is reduced to cysteine, a substrate to produce an intracellular major antioxidant GSH (Bannai and Kitamura, 1980), resulting in the vulnerability of cells to oxidative stress.
H2S protects neurons by increasing the intracellular levels of GSH through enhancing the activity of xCT as well as glutamate cysteine ligase, a rate‐limiting enzyme for GSH production (Bannai and Kitamura, 1980; Kimura and Kimura, 2004; Kimura et al., 2010). H2S also facilitates the cysteine transport possibly through the excitatory amino acid transporter EAAT3 (SLC1A1), which was initially identified as transporting aspartate and glutamate, but not through the alanine/serine/cysteine (ASC) transporter (Chen and Swanson, 2003; Kimura and Kimura, 2004; Kimura et al., 2010). On the other hand, H2Sn protects cells by activating a Kelch ECH‐associating protein 1 (Keap1)/nuclear factor erythroid 2‐related factor 2 (Nrf2) pathway through the sulfuration of Keap1, leading to the activation of antioxidant genes including those for GSH production (Koike et al., 2013).
Endogenous sulfite is present in the serum under physiological conditions, at concentrations typically between 0.2 and 4.87 μM (Togawa et al., 1992; Ji et al., 1995; Kajiyama et al., 2000; Meng et al., 2005). The extracellular milieu where cystine should be a dominant form of cysteine, is more oxidized than the intracellular milieu and the serum contains approximately 11–19 μM of cysteine and 40–77 μM of cystine (Brigham et al., 1960; Jones et al., 2011). Sulfite reacts with cystine to convert it into cysteine which can be more efficiently transported into neurons than cystine and used for GSH production (Clarke, 1932; Kimura et al., 2010). Sulfite can also be involved in maintaining cysteine levels in the extracellular milieu such as serum.
Sulfite protects hepatocytes against the toxicity of menadione, a precursor of vitamin K (Sun et al., 1990), and suppresses the formation of amyloid and aggregation of transthyretin, which transports thyroid hormones and retinal (Gales et al., 2007). Sulfite additives are used widely in food as antioxidant and preservatives. However, exposure to sulfite has been reported to cause a range of adverse reactions including dermatitis, flushing, asthma and anaphylactic reactions in sensitive individuals (Vally and Misso, 2012).
Thiosulfate, another oxidized product of H2S and H2Sn, was recently reported to protect neuroblastoma cells and primary neuronal cultures from oxygen glucose deprivation followed by reoxygenation and was proposed as a chemical entity to mediate H2S cytoprotection (Marutani et al., 2015). Sulfite was a more effective protector of neurons against oxytosis than thiosulfate.
Our present results showed that sulfite increased cysteine levels in the extracellular milieu and the intracellular cysteine and GSH to protect neurons from oxidative stress induced by oxytosis. It also provided a new insight into the therapeutic approach to neuronal diseases caused by oxidative stress.
Methods
Measurement of endogenous sulfite
Brains of postnatal day10 Sprague Dawley rats were homogenized in 9 volume of 50 mM Tris HCl buffer (pH 8.5). Homogenates were purged with nitrogen gas before sealing with a cap then centrifuged at 9,600× g for 10 min at 4°C. The supernatant was transferred to new tubes and incubated for 30 min at room temperature in the presence or absence of 100 mM NaBH4. Samples (100 μL) of reduced (total sulfite = free + bound forms) or non‐reduced (free form) materials were derivatized with monobromobimane (mBBr). The derivatized samples were then analysed by HPLC as described below for intracellular cysteine.
Cell culture and toxicity assay
All animal care and experimental procedures were approved by the National Institute of Neuroscience Animal Care and Use Committee of National Center of Neurology and Psychiatry (Approval number: 2017020). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Primary cortical neurons were prepared from embryonic day 17 Sprague–Dawley rats as described (Kimura and Kimura, 2004). In brief, cells were dissociated from the cortex and maintained in modified Eagle's medium (MEM) supplemented with 30 mM glucose, 2 mM glutamine, 1 mM pyruvate and 10% fetal calf serum in atmosphere with 10% CO2. The next day, the medium was changed to MEM supplemented with N1 (Sigma‐Aldrich, St. Louis, MO, USA). For toxicity studies, cells were seeded on poly‐D‐lysine‐coated 96‐well microtiter dishes at 5×4 cells per 100 μL in each well and exposed to 1 mM glutamate in the presence or absence of Na2S, Na2SO3, Na2SO4, Na2S2, Na2S3 and Na2S2O3 for 24 h. The WST‐8 viability assay was performed with the cell‐counting kit‐8 (Dojindo, Kumamoto, Japan) 24 h after the application of glutamate together with Na2S, Na2S2, Na2S3, Na2SO3, Na2S2O3 and Na2SO4. WST‐8 (10μL of 10 mM) was added to each well, the cells were incubated for 2 h at 37°C, and absorption values at 450 nm were measured.
The results obtained by WST‐8 assay were confirmed by LDH assays. Twenty four hours after the addition of glutamate together with Na2S, Na2S2, Na2S3, Na2SO3, Na2S2O3 and Na2SO4, the microtiter plate was centrifuged at 250× g for 10 min, and the cell supernatant was used for the LDH assay [Cytotoxicity Detection kit (LDH), Roche Diagnostics, Basel, Switzerland]. Background control is medium only, and high control is cells lysed with Triton X‐100 (final 2%) to determine the maximum releasable LDH enzyme activity. Low control is untreated cells used to determine the spontaneous LDH release. Per cent toxicity was calculated by the equation,
Measurement of intracellular cysteine and GSH levels
The intracellular amount of cysteine and GSH was measured by the method described previously (Kimura and Kimura, 2004). Briefly, 4 h after the treatment of glutamate in the presence or absence of Na2S, Na2S2, Na2S3, Na2SO3, Na2S2O3 or Na2SO4, cells were washed twice with ice‐cold PBS and harvested in phosphate buffer (0.1 M NaH2PO4, pH 5.8, 2 mM EDTA). After sonication, cell lysates were centrifuged at 16 000× g for 10 min, and supernatants were derivatized for HPLC. Samples of the supernatant (75 μL) were mixed with 0.5 M CHES [2‐(cyclohexylamino)‐ethanesulfonic acid], pH 8.4, then derivatized with 4 μL of 50 mM mBBr for 15 min in the dark. The reaction was terminated by adding 10 μL of 30% (v/v) acetic acid. Samples were analysed with a Waters Symmetry C18 (250 × 4.6 mm ID) column. The mBBr adduct was monitored by scanning fluorescence detector (Waters 474) with an excitation wavelength at 370 nm and an emission wavelength at 485 nm.
Measurement of cysteine levels in the culture medium
The amount of cysteine in culture medium was measured by the method described previously (Kimura et al., 2010). In brief times (2, 10, 20, 60, 120 and 240 min) after the addition of Na2S, Na2S2, Na2S3, Na2SO3, Na2S2O3 or Na2SO4 to the medium, 75 μL of culture medium was collected in a 1.5 mL Eppendorf tube containing 26 μL of 0.5 M CHES (pH 8.4) then derivatized with mBB. The derivatized samples were then analysed by HPLC as described above for intracellular cysteine.
Calcium imaging
The cells were washed once with basal salt solution (BSS) consisting of 130 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 5.5 mM glucose and 10 mM HEPES‐NaOH (pH 7.3) and were loaded with 1 μM calcium green‐1/acetoxymethyl ester (Thermo Fischer Scientific, Waltham, MA, USA) and 0.01% (v/v) cremophorEL (Nacalai Tesque, Kyoto, Japan) in BSS solution for 50 min at 37°C. The dishes were mounted on an upright fixed stage microscope (Leica DMLFS; Leica Microsystems, Wetzlar, Germany) and perfused at 2 mL·min−1. The images were acquired using a water‐immersion objective (20×; 0.5 NA; Leica Microsystems) and a CCD camera (C4742‐95‐12ER; Hamamatsu Photonics, Shizuoka, Japan). The frame duration ranged from 147 to 207 ms, and each image was acquired at 4 s intervals and 4 × 4 binning. Images were acquired and analysed using the Aquacosmos software ver. 2.0 (Hamamatsu Photonics). Changes in the intracellular calcium concentrations were monitored as a change in the fluorescence intensity (F) relative to the control image (F 0) that was acquired before stimulation.
Data and statistical analysis
All results are expressed as mean ± SEM. The data from multiple groups were compared by using Statcel2 software (OMS Japan) for one‐way ANOVA with post hoc testing by using Turkey‐Kramer test. Statistical analysis between two groups was performed by Student's t‐test. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Materials
Na2S, Na2SO3, Na2SO4 were from Wako Pure Chemical Industries Ltd (Osaka, Japan); Na2S2, Na2S3 from Dojindo, and Na2S2O3 from Sigma‐Aldrich, who also supplied glutamate, as L‐glutamic acid monosodium salt and CHES (2‐[cyclohexylamino]‐ethanesulfonic acid). Monobromobimane (mBBr) was supplied by Millipore Corp. (Bilerica, USA).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http: www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d).
Results
Endogenous concentrations of sulfite in rat brain
The endogenous concentrations of sulfite in the brain homogenates of neonatal (10 day old) rats were determined using HPLC. The concentration of free sulfite is approximately 2 μM and comprised approximately 6% of the total sulfite, including a bound form (Figure 1).
Figure 1.
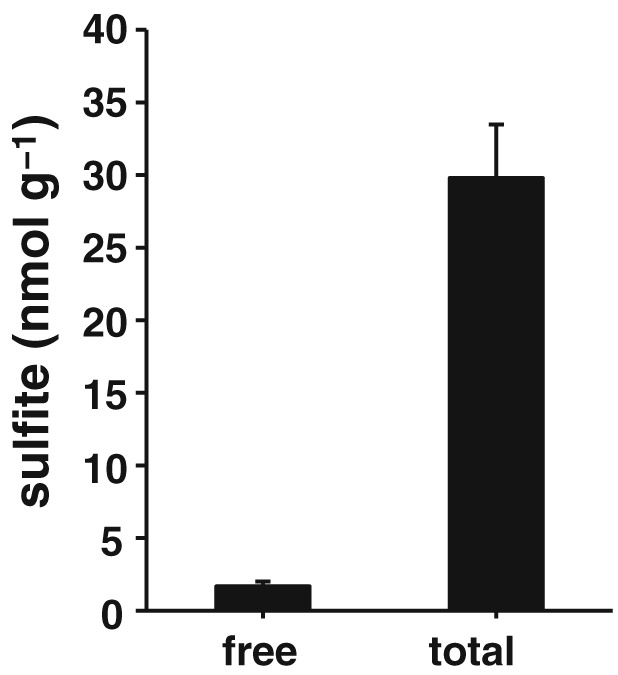
Concentrations of endogenous sulfite in the rat brain. Free and total sulfite in the brain were measured by HPLC (n = 8). All data shown are means ± SEM and are expressed as nmol g‐1 of tissue (wet weight).
Sulfite protects neurons from oxytosis
Because sulfite has been reported to protect hepatocytes from menadione‐induced cytotoxicity (Sun et al., 1990), we examined its protective effects against oxytosis in primary neuronal cultures compared with that of H2S and H2Sn using a cell survival assay based on WST‐8, a tetrazolium salt that is reduced by the dehydrogenase activity of cells to form intensely coloured formazan proportional to the number of living cells (Ishiyama et al., 1997; Umemura and Kimura, 2007). After 24 h of exposure of neurons to 1 mM glutamate, WST‐8 values decreased to approximately 55% of controls (Figure 2A). The addition of 1 μM of sulfite (as Na2SO3) completely protected the neurons against oxytosis (Figure 2A). In contrast, much higher concentrations (10 μM) of Na2S and Na2S3 were required to exert complete protection, while 3 μM of Na2S2 was required for complete protection (Figure 2B–D). These observations suggested that sulfite efficiently protects neurons from oxytosis.
Figure 2.
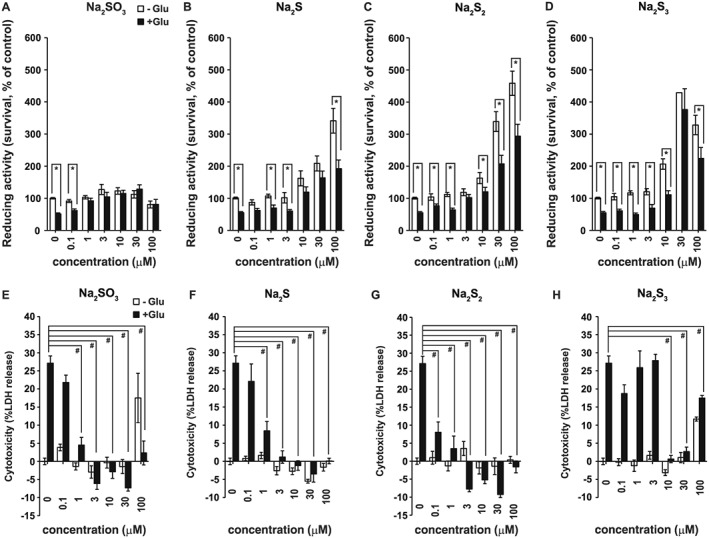
Comparison of the cytoprotective effect of sulfite, H2S, H2S2 and H2S3. WST‐8 values were shown for primary neuronal cultures 24 h after the addition of 1 mM glutamate (Glu) or without glutamate (−Glu) together with Na2SO3 (A), Na2S (B), Na2S2 (C) and Na2S3 (D) (n = 8). *P < 0.05, as indicated; Student's t‐test. LDH values were shown for primary neuronal cultures 24 h after the addition of 1 mM glutamate (Glu) or no glutamate (−Glu) together with Na2SO3 (E), Na2S (F), Na2S2 (G), and Na2S3 (H) (n = 8). Cytotoxicity [% = (Sample OD490 − low control/ (high control – low control) × 100 showed negative values at higher concentrations of Na2SO3, Na2S, Na2S2, and Na2S3. Low control corresponds to untreated cells used to determine spontaneous LDH release. The negative cytotoxicity % values are caused by the values of samples being lower than the low control value. Even the spontaneous release of LDH was suppressed by Na2SO3, Na2S, NaS2, and Na2S3 possibly due to their cytoprotective effects on cells. All data shown are means ± SEM. In A‐D, *P < 0.05, significantly different as indicated; Tukey−Kramer test. In E‐H, # P < 0.05, significantly different as indicated, for values with glutamate. OD490, optical density at 490 nm.
In the absence of glutamate, Na2SO3 barely altered the values obtained in the WST‐8 assay, whereas Na2S, Na2S2 and Na2S3 at concentrations greater than 10 μM, increased these values above the control levels (Figure 2). Because primary neuronal cultures do not proliferate, WST‐8 values above the control level may not reflect an increase in the number of cells, but the reducing activity of WST‐8 in neurons, as we previously reported on H2S (Umemura and Kimura, 2007).
The cytoprotective effect of sulfite was further examined by using the LDH assay. As a stable cytoplasmic enzyme present in all types of cells, LDH is released into the cell culture medium through damage to plasma membranes. Thus levels of released LDH are proportional to the number of damaged cells. Both WST‐8 and LDH assays have been performed to complementarily assess cell viability (Kimura et al., 2006). The results indicated that 1 μM of Na2SO3 significantly suppressed oxytosis, which was completely suppressed at a concentration of 3 μM (Figure 2E). Similar changes were observed with Na2S and Na2S2, whereas a higher concentration of Na2S3 (10 μM) was required to suppress oxytosis (Figure 2F–H). At 100 μM, both Na2SO3 and Na2S3 exerted toxic effects, similar to those observed in the WST‐8 assay (Figure 2A, D, E, H). These observations confirmed the cytoprotective effect of sulfite at low concentrations.
Because cytotoxicity (%) is calculated as (sample OD490 − low control)/(high control − low control), the negative values observed in Figure 2E–H are ascribed to sample values being lower than the low control values at concentrations greater than 3 μM. This observation suggests that sulfite, H2S and H2Sn can even suppress the spontaneous LDH release.
Increase in the levels of intracellular cysteine
Na2S increases the transport of cystine/cysteine and production of GSH, which peaks at 4 h after Na2S addition (Kimura and Kimura, 2004). Because Na2SO3 protected neurons from oxytosis more efficiently than did Na2S, Na2S2 and Na2S3, we examined the possibility that sulfite increases the intracellular levels of cysteine and GSH more effectively than do Na2S, Na2S2 and Na2S3.
The results revealed that incubation with extracellular sulfite markedly increased the intracellular concentrations of cysteine in neurons. At 10 μM, Na2SO3 and Na2S3, but not Na2S and Na2S2, significantly increased intracellular cysteine concentrations (Figure 3A–C). At 30 μM, both Na2SO3 and Na2S3 increased cysteine concentrations to more than 3‐fold, even in the presence of glutamate, whereas Na2S and Na2S2 elicited much weaker effects (Figure 3A–D).
Figure 3.
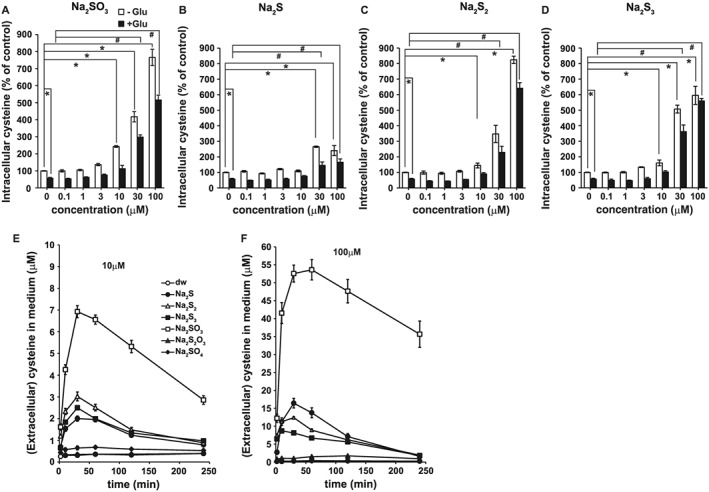
Increase in the intracellular concentrations of cysteine by sulfite compared with that by H2S, H2S2 and H2S3, and a time course of the conversion of cystine to cysteine in the culture medium induced by sulfur containing molecules. Intracellular concentrations of cysteine in primary neuronal cultures were measured by HPLC 4 h after the addition of Na2SO3 (A), Na2S (B), Na2S2 (C), and Na2S3 (D), with or without glutamate (Glu). All data shown are means ± SEM (n = 5). *P < 0.05, significantly different as indicated, for values without glutamate; Tukey−Kramer test. # P < 0.05, significantly different as indicated, for values with glutamate. Furthermore, 10 μM (E) and 100 μM (F) sulfur‐containing molecules indicated in (E) were applied to MEM and incubated in an atmosphere with 10% CO2, and the concentrations of cysteine in the medium were measured. All data shown are means ± SEM (n = 9).
Sulfite reacts with cystine to produce cysteine and S‐cysteinesulfonate (Clarke, 1932). Cysteine can be transported into cells by the cysteine transporters more efficiently than cystine transported by cystine/glutamate antipoter (Bussolati et al., 1992; Kimura et al., 2010). It is possible that the conversion of cystine to cysteine in the culture medium, corresponding to the extracellular milieu such as serum, is involved in the sulfite‐induced increase in the intracellular concentrations of cysteine.
The ability of sulfite to generate cysteine from cystine was examined by applying Na2SO3 to the culture medium in the absence of cells and was compared with the effects of Na2S, Na2S2, Na2S3, Na2S2O3 and Na2SO4. Our results revealed that Na2SO3 efficiently converted cystine to cysteine in the medium, whereas Na2S2 and Na2S3 resulted in approximately one‐third of the production induced by Na2SO3 (Figure 3E, F); 10 and 100 μM Na2SO3 generated approximately 7 and 54 μM cysteine, respectively, suggesting that approximately 50% of the applied concentrations of Na2SO3 were converted to generate cysteine in the medium. These observations suggested that the increase in the extracellular concentrations of cysteine may facilitate its transport into cells.
Increase in the levels of intracellular GSH
Because Na2SO3 increases the intracellular concentrations of cysteine, a component of GSH, it is possible that Na2SO3 also increases the production of GSH. At 1, 3 and 10 μM, Na2SO3 increased GSH concentrations significantly to higher levels than incubation with 10 μM Na2S2 or Na2S3, whereas 10μM Na2S did not significantly change intracellular GSH. These results showed that low concentrations of Na2SO3 efficiently increased the neuronal concentrations of GSH (Figure 4). Under oxytosis conditions (in the presence of 1mM glutamate), GSH concentrations were decreased to approximately 70% of controls. This depressed levels of GSH was significantly raised by 3μM Na2S3, 10 μM Na2SO3 or Na2S2, and 30 μM Na2S (Figure 4). These observations suggested that sulfite increased the intracellular concentrations of GSH efficiently even under conditions of oxytosis.
Figure 4.
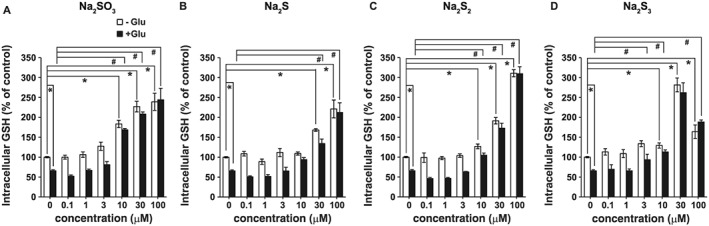
Increase in the intracellular concentrations of GSH after incubation with sulfite, compared with those after H2S, H2S2, or H2S3. Intracellular concentrations of GSH in primary neuronal cultures were measured by HPLC 4 h after the application of Na2SO3 (A), Na2S (B), Na2S2 (C), and Na2S3 (D) with or without glutamate. All data shown are means ± SEM (n = 5). *P < 0.05, significantly different as indicated, for values without glutamate; Tukey−Kramer test. # P < 0.05, significantly different as indicated, for values with glutamate.
Sulfuration is not involved in the cytoprotective effect of sulfite
The polysulfides, H2Sn, can sulfurate cysteine residues of target proteins, followed by the formation of cysteine disulfide bonds, leading to conformational changes that modify the activity of the protein (Kimura, 2015). For example, H2Sn activate TRPA1 channels by sulfurating two cysteine residues at the amino terminus of the channels (Kimura et al., 2013; Hatakeyama et al., 2015). Sulfuration of the Keap1/Nrf2 complex leads to the induction of GSH production (Koike et al., 2013). Sulfuration is a chemical reaction, that takes places rapidly and is maintained, while the induced molecules remain in the medium. We examined whether sulfite can sulfurate cysteine residues by assessing the activation of TRPA1 channels in astrocytes as a sensitive assay (Kimura et al., 2013; Hatakeyama et al., 2015). In our present experiments, even at 500 μM, Na2SO3 did not activate TRPA1 channels (Figure 5). In contrast, 10 μM Na2S3, which sulfurates cysteine residues, effectively activated the channels (Kimura et al., 2013; Hatakeyama et al., 2015). These observations indicated that sulfite does not sulfurate cysteine residues and suggested that sulfuration of cysteine residues of proteins such as Keap1 may not be involved in the cytoprotective effect of sulfite.
Figure 5.
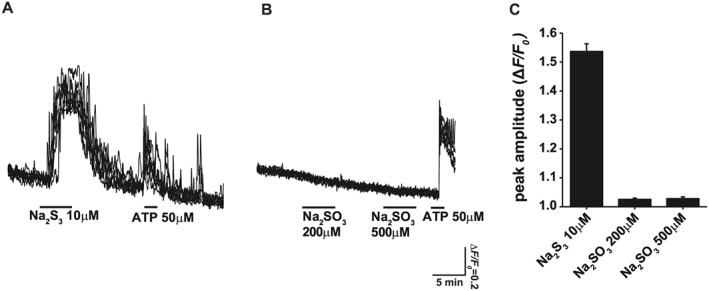
The inability of sulfite to sulfurate the cysteine residues. Ca2+ influx induced in astrocytes was used as an index of sulfuration on cysteine residues of TRPA1 channels (Kimura et al., 2013; Hatakeyama et al., 2015). Na2S3 induced Ca2+ influx (A), but even 500 μM of Na2SO3 did not (B). Responses from seven individual cells were superimposed. (C) The comparison between Na2S3 (six to seven cells picked up for Ca2+ imaging from each plate, and a total number of cells was 62) and Na2SO3 (number of cells: 84) with respect to the induction of Ca2+ influx in astrocytes. ATP was applied as a positive control. All data are expressed as mean ± standard error of the mean.
Thiosulfate exerts a weak cytoprotective effect against oxytosis
The cytoprotective effect of H2S against oxygen glucose deprivation followed by reoxygenation was reported to be mediated by thiosulfate sequentially produced by the oxidation of H2S in culture medium (Marutani et al., 2015). We examined whether or not thiosulfate protects neurons from oxytosis. The sequential generation of sulfite and thiosulfate from Na2S in a culture medium was examined in the absence of cells. The concentrations of Na2S were markedly decreased immediately after its addition to the medium, as previously reported (Kimura et al., 2006). The concentrations of sulfite gradually increased, peaked 30 min after Na2S addition and declined thereafter (Figure 6A). In contrast, the concentrations of thiosulfate started increasing 1 h after the addition of Na2S and peaked after 2 h (Figure 6A).
Figure 6.
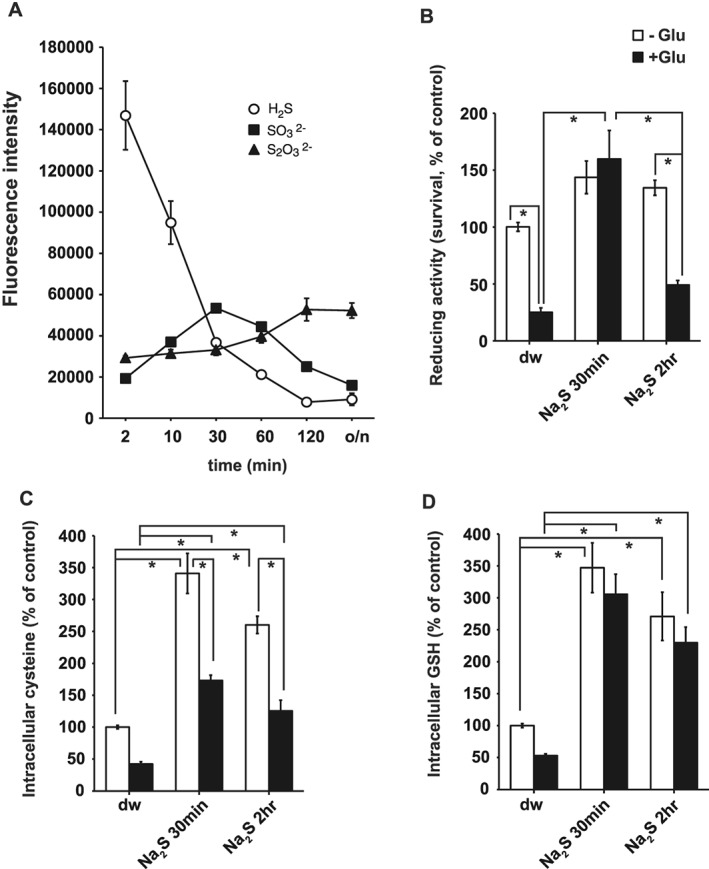
H2S is oxidized to sulfite and thiosulfate in culture medium. (A) The levels of H2S, sulfite (SO3 2‐), and thiosulfate (S2O3 2‐) after the application of 100 μM Na2S to the culture medium are shown (n = 6). o/n, overnight incubation. (B) The cytoprotective effect of culture medium 30 min and 2 h after the addition of 100 μM Na2S on primary cultures of cortical neurons was measured by the WST‐8 assay (n = 6). (C, D) Increase in intracellular cysteine (n = 7) (C) and GSH (n = 6) (D) was measured by HPLC 4 h after the application of medium 30 min and 2 h. *P< 0.05, significantly different as indicated; Tukey−Kramer test. All data shown are means ± SEM.
The cytoprotective effect of culture medium at 30 min (containing approximately 42 μM sulfite, 20 μM H2S, and 12 μM thiosulfate) and at 2 h (18 μM sulfite, 2 μM H2S, and 18 μM thiosulfate) after the addition of Na2S, was examined by adding them to neuronal cultures. The medium 30 min after adding Na2S markedly protected neurons (approximately 150%) from oxytosis, whereas only a weak effect was observed after the 2 h medium (approximately 50%; Figure 6B).
Changes in the intracellular concentrations of cysteine and GSH were also examined, on adding 30 min or 2 h medium to neuronal cultures. Both cysteine and GSH showed a tendency to be more increased by 30 min medium than by 2 h medium (Figure 6C, D). These observations suggest that sulfite rather than thiosulfate exerts cytoprotective effect against oxytosis.
Because the medium contains a mixture of H2S, sulfite and thiosulfate at 30 min and 2 h after the addition of Na2S, the effect of thiosulfate alone was examined. At 30 μM, thiosulfate showed a weak cytoprotective effect against oxytosis in primary neuronal cultures, as shown by the WST‐8 and LDH assays (Figure 7A, C). The intracellular concentrations of cysteine and GSH were not significantly increased in the presence of thiosulfate compared with the controls (Figure 7D, E). We also determined the effect of sulfate, which neither showed cytoprotective effects nor increased GSH production (Figure 7). These observations confirmed that sulfite, rather than thiosulfate, exerts the cytoprotective effects on neurons against oxytosis.
Figure 7.
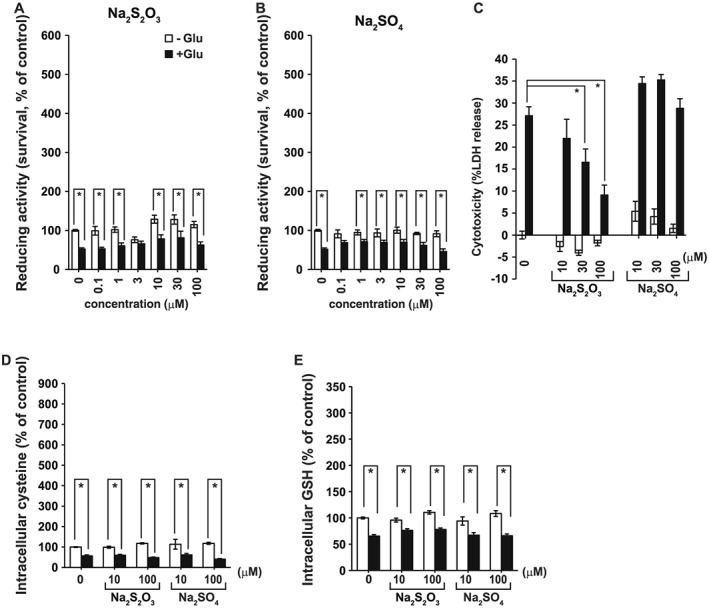
A weak protective effect of thiosulfate against oxytosis. The cytoprotective effect of Na2S2O3 (n = 5) (A) and Na2SO4 (n = 5) (B) measured by the WST‐8 cell survival assay. *P < 0.05, significantly different as indicated; Student's t‐test. (C) Suppression of glutamate cytotoxicity by Na2S2O3 and Na2SO4 was measured by the LDH assay (n = 8). Increase in the concentrations of cysteine (n = 6) (D) and GSH (n = 6) (E) induced by Na2S2O3 and Na2SO4 was measured by HPLC. All data shown are means ± SEM. *P < 0.05, significantly different as indicated; Tukey−Kramer test.
Discussion
Sulfite increases the intracellular concentrations of cysteine and GSH
Sulfite generates cysteine three times more effectively than do H2S, H2S2, and H2S3 (Figures 3E and F). The serum concentrations of sulfite (0.2–4.87 μM) previously reported (Togawa et al., 1992; Ji et al. 1995; Kajiyama et al., 2000) may therefore facilitate the conversion of cystine to cysteine. While the modified Eagle's medium (MEM) contains cystine but not cysteine, serum contains both cysteine and cystine at 11~19 and 40~77 μM, respectively (Brigham et al., 1960). The intracellular cysteine can be provided not only by cystine transported through the cystine/glutamate antiporter but also by the cysteine transporter (Bannai and Kitamura, 1980; Kimura and Kimura, 2004; Kimura, et al., 2006 and 2010). These observations suggested that sulfite may play a physiological role in maintaining cysteine in the extracellular milieu.
An experiment to show suppression of sulfite neuroprotection by pharmacological or siRNA inhibition of the cysteine transporters such as xCT and EAAT3 (Murphy et al., 1989; Tan et al., 2001; Kimura and Kimura, 2004; Kimura et al., 2010) would support the cytoprotective effect of sulfite against oxytosis However, both these transporters are suppressed by glutamate, and their inhibitors are glutamate analogues (Chan and Swanson, 2003). Suppression of transporter activity by inhibitors or siRNA must induce oxytosis‐like cell death. For these reasons, it is difficult to show the suppression of the reinstating effect of sulfite on oxytosis by using inhibitors or siRNAs against these transporters.
Sulfite increased the levels of GSH more effectively than did H2S, H2S2, and H2S3 (Figure 4). Polysulfides, such as H2S2 and H2S3, can facilitate the production of GSH through the activation of Nrf2. In contrast, because sulfite cannot sulfurate cysteine residues, proteins such as Nrf2 whose activity is regulated by nucleophiles, including compounds that induce sulfuration, may not be involved in the effect of sulfite (Figure 5) (Kimura et al., 2013; Hatakeyama et al., 2015). The increase in GSH levels by sulfite may partly be induced by increased cysteine in the extracellular milieu. It is possible that sulfite enhances the activity of GSH‐producing enzymes in addition to increasing cysteine concentrations.
Cytoprotective effect of sulfite on neurons
Sulfite protected neurons without reducing WST‐8 as observed in cells treated with H2S, H2S2 and H2S3 (Figure 2) (Umemura and Kimura, 2007). The mechanism has not been elucidated yet. Because H2S, H2S2 and H2S3 were not in the culture medium 24 h after their addition, a possibility of the reduction of WST‐8 formazan by the remaining H2S, H2S2 and H2S3 could be excluded (Umemura and Kimura, 2007). The intracellular reducing substances, such as cysteine and GSH, were increased by sulfite 4 h after its addition, while the levels of both cysteine and GSH returned to the control level after 24 h (Kimura and Kimura, 2004). Therefore, cysteine and GSH may not be involved in the reduction of WST‐8. Because H2S2 and H2S3 readily react with cysteine and GSH to produce cysteine–persulfide, GSH–persulfide and persulfurated cysteine residues of proteins that have a strong reducing activity and more stable than H2S2 and H2S3, they may be involved in the reduction of WST‐8 (Kimura et al., 2017). Because sulfite does not sulfurate cysteine, GSH or cysteine residues, we assumed that it did not increase WST‐8 values through persulfide species (Figure 5).
At 1 μM, sulfite effectively protected neurons from oxytosis (Figure 2). The endogenous concentration of free sulfite in rat brain is approximately 2 μM, which is in the range of the effective concentrations (Figure 1). Sulfite may be involved in protecting neurons from oxidative stress. Although bound sulfite is more abundant than the free form, it may not contribute to the cytoprotective effect (Figure 1) (Meng et al., 2005).
It is also possible that sulfite may be taken up into cells and react with cysteine disulfide bonds in proteins to modify their activity. The emzyme 3MST produces H2Sn, cysteine‐persulfide, glutathione‐persulfide and persulfurated cysteine residues of proteins that can subsequently produce cysteine disulfide bonds (Kimura et al., 2015; Kimura et al., 2017; Koike et al., 2017; Nagahara et al., 2018). The expression of 3MST increases the levels of bound sulfane sulfur, which consists of H2Sn, cysteine‐persulfide, GSH‐persulfide and per‐sulfurated cysteine residues of proteins, while the brains of 3MST knockout mice show markedly decreased levels of bound sulfane sulfur (Shibuya et al., 2009; Kimura et al., 2017). 3MST supplies persulfide to thiolation of tRNA, and cysteinyl t‐RNA synthetase also produces these persulfurated molecules (Lipsett et al., 1967; Wong et al., 1974; Frasdorf et al., 2014; Akaike et al., 2017). For example, H2Sn activate TRPA1 channels by sulfurating two cysteine residues at the amino terminus of the channels (Kimura et al., 2013). H2Sn also regulate antioxidant genes by sulfurating two cysteine residues of Keap1 to release Nrf2 from Keap1/Nrf2 complex (Koike et al., 2013). The activity of these proteins can be modified by sulfite transported into cells.
Sulfite protects neurons more efficiently than does thiosulfate
Thiosulfate, which was reported to protect neurons from oxygen glucose deprivation and reoxygenation, showed relatively weak protection against oxytosis (Figure 7A, C). GSH levels were slightly reinstated by thiosulfate, leaving cysteine levels unchanged (Figure 7D, E). Marutani et al. (2015) reported that H2S is oxidized to thiosulfate, which was proposed as the chemical entity mediating the protective effect on neurons. However, the present study revealed that H2S was converted to sulfite before thiosulfate (Figure 6A), and sulfite exerted protective effects against oxytosis in neurons much more effectively than did thiosulfate (Figures 2A, E, 3A and 7). Sulfite markedly increased intracellular GSH, while thiosulfate did not (Figures 4A and 7E). Thiosulfate was reported to significantly increase the levels of GSH in SH‐SY5Y cells (Marutani et al., 2015), but not in primary neuronal cultures in the present study (Figure 7E). The discrepancy may be caused by the difference between a cell line and primary neuronal cultures.
Therapeutic potential and possible adverse effects of sulfite
S‐cysteinesulfonate produced from sulfite and cystine activates NMDA receptors as does glutamate (Kumar et al., 2018). Substantial neuronal excitotoxic injury is caused with 2 to 5 μM glutamate (Mark et al., 2001), and the pathophysiological extracellular concentrations of glutamate in human traumatic brain injury patients are approximately 20 μM (Bullock et al., 1998). Sulfite applied to culture medium generated approximately a half of its concentration as cysteine and the counterpart S‐cysteinesulfonate (Figure 3E). A low concentration of sulfite (1 μM) is enough to protect neurons from oxytosis (Figure 2A, B), and only approximately 0.5 μM S‐cysteinesulfonate can be produced at this concentration of sulfite. These observations suggest that any excitotoxic effects due to the presence of S‐cysteinesulfonate could be minimal. However, the administration of sulfite would need special attention in individuals sensitive to sulfite and those with molybdenum cofactor deficiency whose sulfite oxidase is deficient (Vally and Misso, 2012; Kumar et al., 2018). The adverse effect of S‐cysteinesulfonate on neurons may be alleviated by the application of antagonists to NMDA receptors.
Cytoprotective effects of sulfite have been reported for cells other than neurons. Sulfite protected hepatocytes from oxidative stress caused by menadione (Sun et al., 1990). Sulfite competes with GSH for conjugation with menadione and thus preserves intracellular GSH to suppress the reactive oxygen species formed by menadione.
In summary, our present results show clearly that sulfite protected neurons in culture from oxytosis and that this effect was accompanied by an increase in the intracellular levels of cysteine and GSH. The present study has provided a new insight into the protection of cells from oxidative stress and indicated a therapeutic potential of sulfite for treating diseases caused by oxidative stress.
Author contributions
Y.K. and H.K. conceived and designed the study. Y.K. conducted the experiments and analysed the data. Y.K., N.S. and H.K. discussed the study. Y.K. and H.K. authored the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was supported by KAKENHI (26460352 and 17K08613), a Grant‐in‐Aid for Scientific Research to Y.K.; KAKENHI (16K15123), a Grant‐in‐Aid for Scientific Research to N.S.; and KAKENHI (26460115 and 17K08331), a Grant‐in‐Aid for Scientific Research, the Strategic Research program for Brain Sciences from Japan Agency for Medical Research and development, AMED, the Uehara Memorial Foundation to H.K.
Kimura, Y. , Shibuya, N. , and Kimura, H. (2019) Sulfite protects neurons from oxidative stress. British Journal of Pharmacology, 176: 571–582. 10.1111/bph.14373.
References
- Abe K, Kimura H (1996). The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T, Ida T, Wei F‐Y, Nishida M, Kumagai Y, Alam MM et al (2017). Cysteinyl‐tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat Commun 8: 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S, Kitamura E (1980). Transport interaction of L‐cysteine and L‐glutamate in human diploid fibroblasts in culture. J Biol Chem 255: 2372–2376. [PubMed] [Google Scholar]
- Brigham P, Stein WH, Moore S (1960). The concentrations of cysteine and cystine in human blood plasma. J Clin Invest 39: 1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD et al (1998). Factors affecting excitatory amino acid release following severe human head injury. J Neurosurg 89: 507–518. [DOI] [PubMed] [Google Scholar]
- Bussolati O, Laris PC, Rotoli BM, Dall'Asta V, Gazzola GC (1992). Transport system ASC for neutral amino acids. J Biol Chem 267: 8330–8335. [PubMed] [Google Scholar]
- Chan Y, Swanson RA (2003). The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem 84: 1332–1339. [DOI] [PubMed] [Google Scholar]
- Choi DW (1987). Ionic dependence of glutamate neurotoxicity in cortical cell culture. J Neurosci 7: 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW (1988). Glutamate neurotoxicity and diseases of the nervous system. Neuron 1: 623–634. [DOI] [PubMed] [Google Scholar]
- Clarke HT (1932). The action of sulfite upon cystine. J Biol Chem 97: 235–248. [Google Scholar]
- Cortese‐Krott MM, Kuhnle GGC, Dyson A, Fernandez BO, Grman M, DuMond JF et al (2015). Key bioactive reaction products of the NO/H2S interaction are S/N‐hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci U S A 112: E4651–E4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt M, Dux M, Namer B, Jiljkovic J, Cordasic N, Will C et al (2014). H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO‐TRPA1‐CGRP signaling pathway. Nat Commun 5: 4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L et al (2007). Hydrogen sulfide attenuates myocardial ischemia‐reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A 104: 15560–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasdorf B, Radon C, Leimkuhler S (2014). Characterization and interaction studies of two isoforms of the dual localized 3‐mercaptopyruvate sulfurtransferase TUM1 from humans. J Biol Chem 289: 34543–34556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gales L, Saraiva MJ, Damas AM (2007). Structural basis for the protective role of sulfite against transthyretin amyloid formation. Biochim Biophys Acta 1774: 59–64. [DOI] [PubMed] [Google Scholar]
- Greiner R, Palinkas Z, Basell K, Becher D, Antelmann H, Nagy P et al (2013). Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal 19: 1749–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1,091–D1,106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama Y, Takahashi K, Tominaga M, Kimura H, Ohta T (2015). Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons. Mol Pain 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H (1997). The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531. [DOI] [PubMed] [Google Scholar]
- Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K (1997). A highly water‐soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 44: 1299–1305. [DOI] [PubMed] [Google Scholar]
- Jarosz AP, Wei W, Gauld JW, Auld J, Ozcan F, Aslan M et al (2015). Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) is inactivated by S‐sulfuration in vitro. Free Radic Biol Med 89: 512–521. [DOI] [PubMed] [Google Scholar]
- Ji AJ, Savon SR, Jacobsen DW (1995). Determination of total serum sulfite by HPLC with fluorescence detection. Clin Chem 41: 897–903. [PubMed] [Google Scholar]
- Jones DP, Park Y, Gletsu‐Miller N, Liang Y, Yu T, Accardi CJ et al (2011). Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition 27: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama H, Nojima Y, Mitsuhashi H, Ueki K, Tamura S, Sekihara T et al (2000). Elevated levels of serum sulfite in patients with chronic renal failure. J Am Soc Nephrol 11: 923–927. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H (2015). Signaling molecules: hydrogen sulfide and polysulfide. Antioxid Redox Signal 22: 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Dargusch R, Schubert D, Kimura H (2006). Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 8: 661–670. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Goto Y‐I, Kimura H (2010). Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 12: 1–13. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kimura H (2004). Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18: 1165–1167. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Koike S, Shibuya N, Lefer D, Ogasawara Y, Kimura H (2017). 3‐Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine‐ and glutathione‐persulfide (Cys‐SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Sci Rep 7: 10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka J‐I, Kimura H (2013). Polysulfides are possible H2S‐derived signaling molecules in rat brain. FASEB J 27: 2451–2457. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Toyofuku Y, Koike S, Shibuya N, Nagahara N, Lefer D et al (2015). Identification of H2S3 and H2S produced by 3‐mercaptopyruvate sulfurtransferase in the brain. Sci Rep 5: 14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Kawamura K, Kimura Y, Shibuya N, Kimura H, Ogasawara Y (2017). Analysis of endogenous H2S and H2Sn in mouse brain by high‐performance liquid chromatography with fluorescence and tandem mass spectrometric detection. Free Radic Biol Med 113: 355–362. [DOI] [PubMed] [Google Scholar]
- Koike S, Ogasawara Y, Shibuya N, Kimura H, Ishii K (2013). Polysulfide exerts a protective effect against cytotoxicity caused by t‐buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett 587: 3548–3555. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dejanovic B, Hetsh F, Semtner M, Fusca D, Arjune S et al (2018). S‐sulfocysteine/NMDA receptor‐dependent signaling underlies neurodegeneration in molybdenum cofactor deficiency. J Clin Invest 127: 4365–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsett MN, Norton JS, Peterkofsky A (1967). A requirement for beta‐mercaptopyruvate in the in vitro thiolation of transfer ribonucleic acid. Biochemistry 6: 855–860. [DOI] [PubMed] [Google Scholar]
- Maher P, Davis J (1996). The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci 16: 6394–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark LP, Prost RW, Ulmer JL, Smith MM, Daniels DL, Strottmann JM et al (2001). Pictorial review of glutamate excitotoxicity: Fundamental concepts for neuroimaging. AJNR Am J Neuroradiol 22: 1813–1824. [PMC free article] [PubMed] [Google Scholar]
- Marutani E, Yamada M, Ida T, Tokuda K, Ikeda K, Kai S et al (2015). Thiosulfate mediates cytoprotective effects of hydrogen sulfide against neuronal ischemia. J Am Heart Assoc 4: e002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Li R, Zhang X (2005). Levels of sulfite in three organs from mice exposed to sulfur [corrected] dioxide. Inhal Toxicol 17: 309–313. [DOI] [PubMed] [Google Scholar]
- Miyamoto R, Koike S, Takano Y, Shibuya N, Kimura Y, Hanaoka K et al (2017). Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Sci Rep 7: 45995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT (1989). Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2: 1547–1558. [DOI] [PubMed] [Google Scholar]
- Nagahara N, Koike S, Nirasawa T, Kimura H, Ogasawara Y (2018). Alternative pathway of H2S and polysulfides production from sulfurated catalytic‐cysteine of reaction intermediates of 3‐mercaptopyruvate sulfurtransferase. Biochem Biophys Res Commun 496: 648–653. [DOI] [PubMed] [Google Scholar]
- Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL et al (2006). Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol 209: 4011–4023. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, Souvannakitt D, Gadalla MM, Kumar GK et al (2010). H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci U S A 107: 10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Piasecki D (2001). Oxidative glutamate toxicity can be a component of the excitotoxicity cascade. J Neurosci 21: 7455–7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K et al (2009). 3‐Mercaptopyruvate sulfurtransferease produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714. [DOI] [PubMed] [Google Scholar]
- Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R (2009). Relative contributions of cystathionine beta‐synthase and gamma‐cystathionase to H2S biogenesis via alternative trans‐sulfuration reactions. J Biol Chem 284: 22457–22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbert D, Prysyazhna O, Rudyk O, Scotcher J, Burgoyne JR, Eaton P (2014). Protein kinase G Ialpha oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension 64: 1344–1351. [DOI] [PubMed] [Google Scholar]
- Sun YP, Cotgreave IA, Lindeke B, Moldeus P (1990). The protective effect of sulfite on menadione‐ and diquat‐induced cytotoxicity in isolated rat hepatocytes. Pharmacol Toxicol 66: 393–398. [DOI] [PubMed] [Google Scholar]
- Tan S, Schubert D, Maher P (2001). Oxytosis: a novel form of programmed cell death. Curr Top Med Chem 1: 497–506. [DOI] [PubMed] [Google Scholar]
- Togawa T, Ogawa M, Nawata M, Ogasawara Y, Kawanabe K, Tanabe S (1992). High performance liquid chromatographic determination of bound sulfide and sulfite and thiosulfate at their low levels in human serum by pre‐column fluorescence derivatization with monobromobimane. Chem Pharm Bull 40: 3000–3004. [DOI] [PubMed] [Google Scholar]
- Umemura K, Kimura H (2007). Hydrogen sulfide enhances reducing activity in neurons: neurotrophic role of H2S in the brain? Antioxid Redox Signal 9: 2035–2041. [DOI] [PubMed] [Google Scholar]
- Vally H, Misso NL (2012). Adverse reactions to the sulphite additives. Gastroenterol Hepatol Bed Bench 5: 16–23. [PMC free article] [PubMed] [Google Scholar]
- Wong TW, Harris MA, Jankowicz CA (1974). Transfer ribonucleic acid sulfurtransferase isolated from rat cerebral hemispheres. Biochemistry 13: 2805–2812. [DOI] [PubMed] [Google Scholar]
- Zanardo RCO, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL (2006). Hydrogen sulphide is an endogenous modulator of leukocyte‐mediated inflammation. FASEB J 20: 2118–2120. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R (2001). The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]


