Abstract
Hydrogen sulfide (H2S) is a gaseous mediator in various physiological and pathological processes, including neuroimmune modulation, metabolic pathways, cardiovascular system, tumour growth, inflammation and pain. Now the hydrogen polysulfides (H2Sn) have been recognised as signalling molecules modulating ion channels, transcription factors and protein kinases. Transient receptor potential (TRP) cation channels can be activated by mechanical, thermal or chemical triggers. Here, we review the current literature regarding the biological actions of sulfide and polysulfide compounds mediated by TRP channels with special emphasis on the role of TRPA1, best known as ion channels in nociceptors. However, the non‐neuronal TRPA1 channels should also be considered to play regulatory roles. Although sulfide and polysulfide effects in different pathological circumstances and TRPA1‐mediated processes have been investigated intensively, our review attempts to present the first comprehensive overview of the potential crosstalk between TRPA1 channels and sulfide‐activated signalling pathways.
Linked Articles
This article is part of a themed section on Chemical Biology of Reactive Sulfur Species. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.4/issuetoc
Abbreviations
- AITC
allyl isothiocyanate
- AMPK
AMP‐activated protein kinase
- apoE
apolipoprotein E
- ARDs
ankyrin repeat domains
- CBS
cystathionine β‐synthase
- CK2
casein kinase 2
- cryo‐EM
single‐particle electron cryomicroscopy
- DADS
diallyl disulfide
- DAS
diallyl sulfide
- DATS
diallyl trisulfide
- DMTS
dimethyl trisulfide
- DRG
dorsal root ganglion
- HIF‐1α
hypoxia inducible factor 1 alpha
- HNO
nitroxyl
- IKK
IκB kinase complex
- IP6
myo‐inositol‐1,2,3,4,5,6‐hexakisphosphate; phytic acid
- Nrf2
nuclear factor erythroid 2‐related factor 2
- SP
substance P
- SSNO‐
nitrosopersulfide
- SULFI/NO
dinitrososulfite
- TRG
trigeminal root ganglion
Introduction
The superfamily of the transient receptor potential (TRP) ion channels consists of six subfamilies: TRP canonical, TRP melastatin, TRP vanilloid (TRPV), TRP ankyrin (TRPA), TRP mucolipin and TRP polycystin (TRPP or PKD). TRP receptors form non‐selective cation channels, preferentially high calcium ion permeability. Calcium influx triggers several intracellular pathways. TRP channels are sensitive to a variety of stimuli including mechanical or thermal triggers, or chemical ligands. Based on such interactions, they are likely to be sensors for several physiological or pathophysiological stimuli. (Khalil et al., 2018). The main goal of this review paper is to discuss the TRP‐mediated effects of sulfide and polysulfide compounds, with particular emphasis on the role of TRPA1 channels.
H2S as a gaseous transmitter
At the beginning of the 1990s, H2S appeared as a new example of agaseous molecule modulating a number of biological functions, such as nociception, inflammation and vascular responses. Its potential biological effect on capsaicin‐sensitive sensory nerves was first suggested in the lung (Prior et al., 1990). In 2004, Patacchini et al. identified the H2S donor sodium hydrosulfide (NaHS) as the activator of the capsaicin‐sensitive neurons in the rat isolated urinary bladder. They concluded that H2S either acts on TRPV1 channels or stimulates a still unidentified TRP‐like channel co‐expressed with TRPV1 channels on sensory neurons (Patacchini et al., 2004). Four years later, Streng et al. (2008) suggested that NaHS acts on TRPA1 channels in the urinary bladder. The next key findings were published by Miyamoto et al. (2011) presenting evidence that the NaHS‐evoked increase in [Ca2+]i was inhibited by Ca2+ free condition and by a selective antagonist for TRPA1 channels, HC030031, suggesting that H2S stimulates sensory neurons via activation of these channels. Generation of Ca2+ signals in response to H2S were first described in astrocytes, but the authors could not conclude that TRPA1 channels were the mediators (Nagai et al., 2004). TRPA1 ion channels were first described as being sensitive to cold and later to be receptors for mustard oil (Story et al., 2003; Jordt et al., 2004). Several papers also suggest that TRPA1 channels may also be expressed by non‐neuronal cells (Chen and Hackos, 2015). Vasodilator effects of H2S were decreased in TRPA1 knockout (TRPA1−/−) mice or in wild‐type mice after treatment with HC030031 (Pozsgai et al., 2012), indicating a primary role of TRPA1 channels in mediating the vascular effects of H2S in the skin. Activation of TRPA1 receptors by sulfide‐released CGRP with consequent vasodilation (Pozsgai et al., 2012; Eberhardt et al., 2014; Hajna et al., 2016).
Structure and function of TRPA1 channels
TRPA1 channels were originally called ANKTM1 channels, as they were identified by a homology search for ankyrin repeats and six transmembrane domains. TRPA1 channels have a particularly long ankyrin repeat region within the N‐terminus and contain calcium‐sensitive regions in the N‐terminal EF‐hand motif and in the S4 transmembrane segment. The flexible ankyrin domains may serve the structural basis for protein–protein interactions (Brewster and Gaudet, 2015). In the TRP superfamily, TRPA1 channels have unique aspects and, reflecting a a wide range of ligands; it is a promiscuous receptor. Low and high temperature, osmotic changes, natural and synthetic irritants are all known to activate TRPA1 channels. Several TRPA1 channel agonists are reactive electrophilic ligands. Channel gating by these compounds is based on covalent modification of cysteine and lysine residues within the N‐terminus and the transmembrane domain. Regarding sulfide‐evoked TRPA1 channel activation, the formation of an intramolecular C422–C622 disulfide bond has been proposed (Kimura, 2015). TRPA1 channels can also be activated by non‐reactive compounds that bind with non‐covalent interactions as well as low or high pH or polyunsaturated fatty acids (Viana, 2016). Activated TRPA1 channels interact with signalling downstream mechanisms of several GPCRs, such as bradykinin B2 receptors or PAR2. TRPA1 channels behave as integrators of endogenous and exogenous activating stimuli, including temperature, light, bacterial toxins, mechanical and chemical stimuli (Baraldi et al., 2010). As TRPA1 channels can be triggered by endogenous compounds generated during tissue injury and inflammation, the main trend of their development treats these channels as novel potential drug targets for analgesics and anti‐inflammatory drug candidates. Recent papers have presented evidence that TRPA1 channels are also expressed in the brain and play pivotal roles in neurodegenerative disorders and neuroinflammation, such as multiple sclerosis and Alzheimer's disease, opening another potential benefit of TRPA1 channel antagonists (Sághy et al., 2016; Lee et al., 2016a).
Generally, TRPA1 channel subunits constitute homotetrameric complexes on the cell membrane, but there is evidence that these subunits also co‐localize with TRPV1 subunits into heterotetrameric complexes to form a single channel in sensory neurons (Garrison and Stucky, 2011). The structure of the TRPA1 channel at near‐atomic (4 A°) resolution has been determined by single‐particle electron cryomicroscopy (cryo‐EM) (Paulsen et al., 2015). This is essential for understanding TRPA1 channel function and determining the binding sites for drug interactions. Another comprehensive study of the structural characteristics and potential binding sites of agonists and antagonists of TRPA1 channels has been published recently (Brewster and Gaudet, 2015). The cryo‐EM analysis confirmed that TRPA1 channels are tetrameric proteins formed by the assembly of four subunits (1119 amino acids in humans) with six transmembrane α‐helices (S1–S6). Their structure includes a series (14–18 depending on species) of ankyrin repeat domains (ARDs) within the long intracellular N‐terminus (Zygmunt and Högestätt, 2014; Brewster and Gaudet, 2015). At the structural level, the permeation pathway shows two major constriction sites (Paulsen et al., 2015). The channel is highly Ca2+ permeable and the permeating divalent cations contribute to channel regulation, characterized by initial potentiation followed by desensitization (Kádková et al., 2017; Zimova et al., 2018). Recently, Brewster and Gaudet (2015) have edited a novel schematic map of TRPA1 channels demonstrating and proposing the functions of different parts of the receptor protein. They also reveal an unexpected novel ligand‐binding site and an unusual C‐terminal coiled coil stabilized by myo‐inositol‐1,2,3,4,5,6‐hexakisphosphate (phytic acid, IP6). They suggest that endogenous soluble intracellular ligands, such as IP6, are required to maintain the receptor in an agonist‐receptive state (Brewster and Gaudet, 2015). Following channel activation, removal of IP6 by the influx of Ca2+ could act a molecular kill switch to trigger inactivation. In spite of much experimental effort, the exact role of TRPA1 channels in thermo‐ and mechanosensation has not been elucidated yet (Chen and Hackos, 2015). On the basis of recent studies, these channels might not be primary cold sensors, but are associated with cold allodynia and mechanical hyperalgesia where endogenous activation mechanisms are present (Brewster and Gaudet, 2015; Viana, 2016) (Figure 1A, B).
Figure 1.
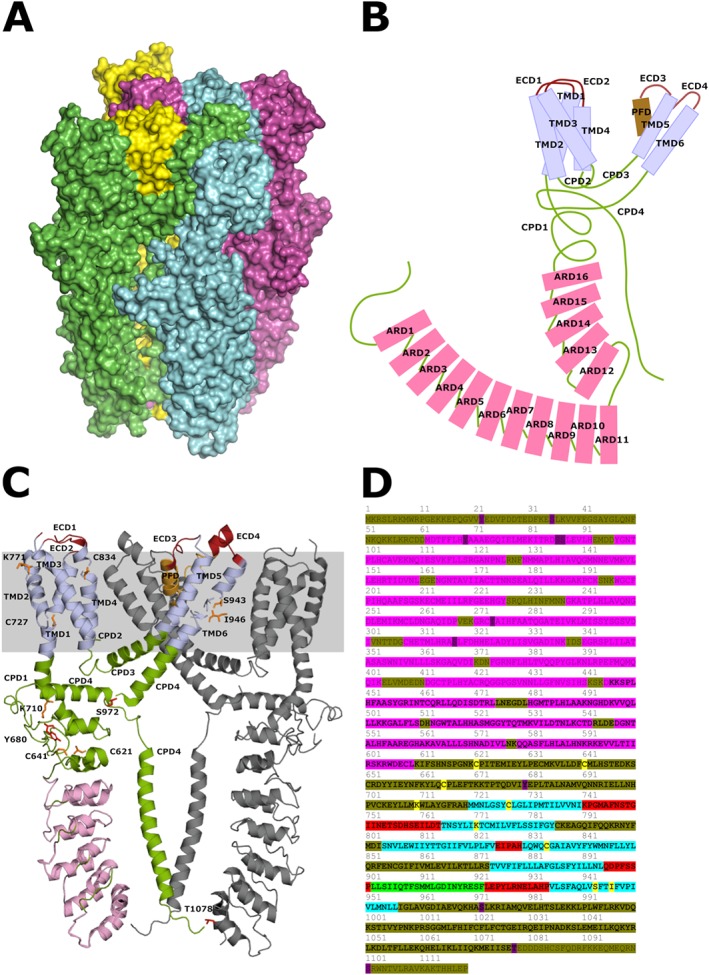
Panel (A) depicts the four protein units of the human TRPA1 ion channel. Panel (B) shows a diagram of the domains of a single protein unit of the channel. ARD1–16, ankyrin repeat domains 1–16 within cytoplasmic domain 1 shown as pink boxes; CPD1–4, cytoplasmic domains 1–4 shown as green thread; ECD1–4, extracellular domains 1–4 shown as red thread; and PFD, pore‐forming domain shown as brown box. Panel (C) represents the structure of human TRPA1 ion channel subunits as published by Paulsen et al. (2015). This model only contains residues 446–1078. Most of the N‐terminus including ankyrin repeats 1–11 (residues 1–445) and some of the C‐terminus (residues 1079–1119) are not included. The figure shows two out of four protein subunits of the ion channel to allow identification of key amino acid residues. The other two subunits not shown here would be located below and above the plane of the paper. One protein shows ankyrin repeats 12–16 in pink and the six transmembrane domains in light blue. Domains of the protein are colour coded and labelled as in the previous panel. The position of the plasma membrane is indicated by the grey stripe. Residues proposed to be involved in electrophilic activation are highlighted in orange and those phosphorylated by signalling kinases in red. Some serine, threonine and tyrosine amino acids targeted by such kinases are located in the proximal N‐terminus and are not shown in the figure. For an extensive review, see Kádková et al. (2017). Panel (D) shows target residues of electrophiles and signalling kinases in the human TRPA1 ion channel. Ankyrin repeats are highlighted in pink; transmembrane domains are highlighted in light blue. Cytoplasmic domains are olive, and extracellular domains are red. The pore‐forming domain is green. The bold section is included in the model of Paulsen and colleagues and is represented in panels (B) and (C). Amino acids participating in activation by electrophiles are highlighted in yellow, and those phosphorylated by signalling kinases are shown in purple.
Neuronal and non‐neuronal TRPA1 channels
Expression of TRPA1 channels by somatic and visceral primary sensory neurones is well known. Peptidergic nociceptors, also known as capsaicin‐sensitive sensory neurones, co‐express TRPV1 and TRPA1 channels (Story et al., 2003). Activation of these channels induces membrane depolarization by Na+ influx, while the inward Ca2+ current increases [Ca2+]i, which releases neuropeptides such as CGRP, substance P (SP) or neurokinin A from large dense‐core vesicles via Ca2+‐dependent exocytosis (Kádková et al., 2017). Pro‐inflammatory neuropeptides amplify nociception and mediate neurogenic inflammation through the local efferent function of the capsaicin‐sensitive sensory nerve endings. Neurogenic inflammation results in vascular changes such as vasodilatation and plasma protein extravasation followed by recruitment of inflammatory and immune cells (Pintér et al., 2006; Bodkin and Brain, 2011; Szolcsányi, 2014; López‐Requena et al., 2017). Other events involving TRPA1 channels modulate a number of chronic pain conditions associated with inflammation. Thus , pro‐inflammatory and algogenic mediators released within the injured or inflamed tissue, such as PGs, bradykinin, 5‐HT and proteases, modulate TRPA1 channels via GPCRs and phospholipase C‐coupled signalling cascades. These mediators, including lipid peroxidation products such as 4‐oxo‐2‐nonenal, 4‐hydroxy‐2‐nonenal, 4‐hydroxyhexenal as well as 15‐deoxy‐12,14‐prostaglandin J2, stimulate PKA, PKC and phospholipase C (PLC) pathways to induce phosphorylation of TRPA1 channels (Chen and Hackos, 2015; Viana, 2016; Kádková et al., 2017). The putative roles of the PKA‐ and PLC‐dependent signalling pathways in TRPA1 channel‐mediated nociception have been very well reviewed (Zygmunt and Högestätt, 2014; Viana, 2016).
Following the activation of TRPA1 channels, not only pro‐inflammatory but anti‐inflammatory peptides are also released from the nerve ending, such as somatostatin (Pozsgai et al., 2017). Somatostatin is a cyclic peptide found in the CNS and peripheral tissues. Neuronally derived somatostatin could exert systemic antinociceptive and anti‐inflammatory effects (Pintér et al., 2006), mediated by sst4 receptors (Pintér et al., 2002; Helyes et al., 2009). This could explain why activation of TRPA1 channels in wild‐type mouse diminishes mild heat injury‐induced thermal hyperalgesia; nevertheless, it cannot be observed in TRPA1 receptor knock out animals. Alkylated polysulfide compounds, such as dimethyl trisulfide (DMTS), activate TRPA1 channels of primary sensory neurons inducing release of the neuropeptide somatostatin that might elicit analgesic effect in animal models of nociception via sst4 receptors (Pozsgai et al., 2017).
Several non‐neuronal cell types express TRPA1 channels. In human and murine lung, apart from the sensory fibres, non‐neuronal cells including fibroblasts, alveolar epithelial cells, and smooth muscle cells express these channels. TRPA1 channels were also detected in melanocytes, keratinocytes and fibroblasts and, therefore, these channels have been suggested to regulate keratinocyte differentiation and inflammation in the skin. In addition to sensory fibres innervating the urinary tract, TRPA1 channels have been found in epithelial cells and might play a role in urinary micturition (Khalil et al., 2018). Activation of TRPA1 channels in insulin producing beta cells of the pancreatic islet results in insulin secretion (Cao et al., 2012). Astrocytes also express these channels in different brain structures, which may play a role in calcium homeostasis, influence GABA transporter GAT‐3 and regulate the oligodendrocyte apoptosis with consequent exacerbation of demyelination (Shigetomi et al., 2012; Sághy et al., 2016). Hydrogen polysulfides were first reported to activate TRPA1 channels of astrocytes more potently than H2S (Kimura et al., 2013). There are few data on the expression of these channels in immune cells. The functional role of TRPA1 channels in macrophages was recently investigated in the pathogenesis of atherosclerosis by Zhao et al., 2016. Expression of these channels was increased in macrophage foam cells in atherosclerotic aortas of apolipoprotein E (apoE)‐deficient mice. Atherosclerotic lesions, hyperlipidaemia and systemic inflammation were worsened with chronic administration of the channel antagonist HC030031 or genetic ablation of TRPA1 channels in apoE knockout mice. These findings suggest that TRPA1 channels may serve to regulate the pathogenesis of atherosclerosis and cholesterol metabolism of macrophage foam cells (Zhao et al., 2016). TRPA1 immuno‐positive macrophages have also been identified by merged fluorescent double staining in the human oral submucosa. The number of TRPA1 positive macrophages was significantly elevated in samples from patients suffering oral lichen planus (Kun et al., 2017). Expression of TRPA1 channels was also detected by Northern blot, Western blot and immunohistochemical methods in Jurkat T cells and in human splenocytes. Expression of these channels has been confirmed in murine and human T cells at mRNA and protein level (Bertin et al., 2017). While the pro‐inflammatory effects of the activation of TRPA1 channels are well established, there is emerging evidence for its protective effects. Deletion of TRPA1 channels enhanced inflammatory responses in various colitis animal models (Kun et al., 2014; Bertin et al., 2017). Kemény et al. (2018) provided clear evidence that psoriasiform dermatitis induced by topically applied imiquimod was enhanced in TRPA1 KO mice and after treatment with the TRPA1 channel antagonist A967079, compared to the wild‐type controls. Their immunohistochemical data revealed that CD4+ T helper cells expressed TRPA1 channels in murine skin. They concluded that protective effects in psoriasiform dermatitis could be mediated by the activation of neuronal and non‐neuronal TRPA1 channels (Kemény et al., 2018). Although the best known site of TRPA1 channels is the nociceptive primary sensory neuron, these channels in non‐neuronal sites should also be considered when assessing the regulatory role of TRPA1 channel activation. As TRPA1 channel antagonists are under active development for pain and inflammation, it is important to allow for the possibility of on‐target side effects mediated by non‐neuronal TRPA1 channels.
Activators of TRPA1 channels
In contrast with other chemoreceptors, which are usually stimulated by ligands with rather conserved structures, a unique feature of TRPA1 channels is that they appear to be activated by a variety of structurally unrelated compounds.
Exogenous compounds
Plant‐derived organosulfur compounds
Allium sativum is commonly known as garlic and its active compounds include thiosulfinates that are responsible for the distinctive pungent and spicy aroma of garlic. Allicin is an unstable organosulfur component generated from alliin by the vacuolar enzyme alliinase after the clove has been cracked. Like other highly reactive thiosulfinates, allicin is converted to more stable organosulfur compounds such as diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulfide (DATS). While alliin is odourless, these derivatives are volatile compounds responsible for the pervasive garlic aroma and flavour. Thiosulfinates have structural similarities with allyl isothiocyanate (AITC), the pungent agent of wasabi, horseradish and mustard oil. These compounds activate TRPA1 channels on primary sensory neurons causing the release of pro‐inflammatory neuropeptides with consequent pain sensation and neurogenic inflammation (Bautista et al., 2005). Highly reactive isothiocyanates such as propargyl isothiocyanate, benzyl isothiocyanate and phenethyl isothiocyanate also act on TRPA1 channels, but their experimental use is strongly limited due to their toxicity. AITC activates other TRPs (including TRPV1). These organosulfur compounds could be conjugated with cysteines in the TRPA1 channel structure to form disulfide bridges (Baraldi et al., 2010; Viana, 2016).
Irritant chemicals from air pollution or cigarette smoke
TRPA1 channels are activated by almost all oxidizing industrial electrophiles, including aldehydes (e.g. formaldehyde and acetaldehyde), alkenals (e.g. acrolein and crotonaldehyde), hypochlorites, toluene diisocyanate and tear gases, most likely through covalent protein modification (Bessac and Jordt, 2008).
Endogenous substances
TRPA1 channels act as sensors of toxic signals and molecular integrators of cellular stress, including ROS. Algogenic activators of these channels are released from the sites of inflammation or tissue injury. They are lipid peroxidation products such as 4‐oxo‐2‐nonenal, 4‐hydroxy‐2‐nonenal and 4‐hydroxyhexenal, and oxidized lipids such as the cyclopentenone PG (PGA1, PGA2, 8‐isoPGA2, 15‐deoxy‐Δ12,14‐PGJ2 and Δ12‐PGJ2, formed by non‐enzymic dehydration of the respective PGs (PGD2, PGE2 and PGE1). These compounds are TRPA1 channel ligands and directly gate the channel to cause acute nociception (Chen and Hackos, 2015). Activators of the inflammasome, such as monosodium urate crystals, stimulate TRPA1 channels by an indirect mechanism involving production of H2O2 (Trevisan et al., 2014).
Neuronal and non‐neuronal expression patterns of TRPA1 channels allow a broad activation profile and enable it to be a versatile sensor of tissue injury. Inflammatory mediators and reactive electrophilic agents bind to TRPA1 channels covalently as electrophilic attack can damage cellular components by Michael addition. Nitrated fatty acids such as nitro‐oleic acid that are generated during inflammation by phospholipids and NO are other examples endogenous lipidergic TRPA1 activators. DAG and arachidonic acid, generated through bradykinin B2‐receptor/PLA2/PLC pathways, are also activators of these channels and may represent a downstream mechanism of bradykinin‐induced pain (Brewster and Gaudet, 2015) (Table 1).
Table 1.
Publications on TRPA1 channel‐mediated effects of inorganic sulfide donors
| Sulfide source | Cell type, organ, in vivo setting | Readout | References |
|---|---|---|---|
| Hydrogen sulfide donors | |||
| NaHS | Rat TRG neurons | Electrophysiology activation inhibited by HC030031 | Koroleva et al. (2017) |
| NaHS | Chicken thoracic aorta epitheloid cells | 5‐HT release by HPLC inhibited by TRPA1 antagonist | Delgermurun et al. (2016) |
| NaHS | Human ureter | Precontraction by electrical stimulation was relaxed. | Weinhold et al. (2017) |
| NaHS | RIN14B cells | Ca2+ imaging and 5‐HT release activation inhibited by HC030031 | Ujike et al. (2015) |
| NaHS | Murine cerulein‐induced pancreatitis | Elevated spinal cFOS expression by immunohistochemistry inhibited by AP18 mechanical abdominal allodynia inhibited by AP18 and CaV3.2 channel inhibitor | Terada et al. (2015) |
| NaHS | CHO hTRPA1 | Ca2+ imaging | Hajna et al. (2016) |
| NaHS |
CHO hTRPA1 Murine TRG neurons |
Ca2+ imaging activation inhibited by HC030031 and TRPA1 knockout | Hajna et al. (2016) |
| NaHS | Murine skin | Increased blood flow by laser Doppler imaging inhibited by RTX pretreatment, CGRP and NK1 antagonists, glibenclamide | Hajna et al. (2016) |
| NaHS | RIN14B cells | Ca2+ imaging and 5‐HT release response enhanced by acidosis | Takahashi and Ohta (2013) |
| NaHS | Rat lung vagal afferents | Ca2+ imaging and respiratory changes respiratory effect inhibited by HC030031 | Hsu et al. (2013) |
| NaHS | Murine DRG neurons | Ca2+ imaging and electrophysiology inhibited by TRPA1 knockout potentiated by acidosis | Andersson et al. (2012) |
| NaHS | CHO mTRPA1 | Electrophysiology | Andersson et al. (2012) |
| NaHS | Murine hind paw | Mechanical hyperalgesia inhibited by AP18 and TRPA1 knockout | Andersson et al. (2012) |
| NaHS | Murine colon | Nocifensive behaviour present in TRPA1 knockout | Andersson et al. (2012) |
| NaHS | Rat mesenteric artery | Dilatation prevented by capsaicin pretreatment, HC030031, CGRP receptor antagonist and Cl− channel inhibitor | White et al. (2013) |
| NaHS | Murine colon | Nocifensive behaviour inhibited by AP18 and CaV3.2 channel inhibitor | Tsubota‐Matsunami et al. (2012) |
| NaHS | Rat trachea nerve endings | CGRP release by RIA inhibited by HC030031 | Pozsgai et al. (2012) |
| NaHS | Murine skin | Elevated blood flow by laser Doppler imaging inhibited by HC030031 and TRPA1 knockout | Pozsgai et al. (2012) |
| NaHS | Murine DRG neurons | Ca2+ imaging activation inhibited by HC030031 and TRPA1 knockout | Ogawa et al. (2012) |
| NaHS | HEK293 mTRPA1 | Ca2+ imaging activation inhibited by mutation of key cysteines potentiated by acidosis | Ogawa et al. (2012) |
| NaHS | Murine hind paw | Nocifensive behaviour inhibited by TRPA1 knockout potentiated by acidosis | Ogawa et al. (2012) |
| NaHS | Murine hind paw | Nocifensive behaviour inhibited by AP18, gene silencing of TRPA1, inhibition and silencing of CaV3.2 channels | Okubo et al. (2012) |
| NaHS | Rat DRG neurons | Ca2+ imaging activation inhibited by HC030031 | Miyamoto et al. (2011) |
| NaHS | Human prostate | Relaxation of precontracted smooth muscle | Gratzke et al. (2010) |
| NaHS | Rat urinary bladder | Intravesicular NaHS increased micturition frequency and lowered voiding volume | Streng et al. (2008) |
| NaHS | CHO m/hTRPA1 | Ca2+ imaging | Streng et al. (2008) |
| Na2S | RIN14B cells | Ca2+ imaging activation inhibited by HC030031 | Ujike et al. (2018) |
| Na2S | CHO hTRPA1 | Ca2+ imaging | Hajna et al. (2016) |
| Na2S | Rat TRG neuron, brainstem slice, dura mater | CGRP release by ELISA | Wild et al. (2015) |
| GYY4137 | Porcine bronchioles | Relaxation electrical stimulation‐induced relaxation inhibited by CSE‐inhibitor and TRPA1 antagonist | Fernandes et al. (2014) |
| GYY4137 | Porcine intravesical ureter | Relaxation of precontracted smooth muscle prevented by HC030031, CGRP and PACAP receptor antagonists | Fernandes et al. (2014) |
CaV3.2, a member of voltage‐gated T‐type calcium channels; CSE, cystathionine‐γ‐lyase; NK1, neurokinin 1 receptor; PACAP, pituitary adenylate cyclase‐activating polypeptide; RTX, resiniferatoxin.
Sulfide (H2S)‐NO, polysulfides
Several papers provided evidence suggesting the signalling pathways that are shared by NO and hydrogen sulfide (H2S) (Takahashi et al., 2012). The synergistic effect of the two gasotransmitters was first identified in the late 1990s (Hosoki et al., 1997). The chemical basis of this interaction has not been elucidated properly. Moreover, polysulfides (H2Sn) recently emerged as potential mediators of H2S/sulfide signalling, but their biosynthesis and relationship to NO are still under intensive investigation (Kimura, 2017). Some recent papers have suggested that the reaction between sulfide and NO leads to formation of different bioactive intermediates [including nitrosopersulfide (SSNO), H2Sn and dinitrososulfite (SULFI/NO)] capable of scavenging, transporting and releasing NO and generating its redox congeners, nitroxyl (HNO), nitrous oxide (N2O) and sulfane sulfur (Eberhardt et al., 2014; Cortese‐Krott et al., 2015; Kimura, 2017; Miyamoto et al., 2017). These reports conclude that SSNO is a potent NO donor, resistant to the reducing milieu of the cell, and able to release both NO and H2Sn. SULFI/NO is a weak combined NO/HNO donor and generator of N2O with potent effects on the heart. Formation of its precursor sulfite and generation of sulfur and/or oxygen‐centred free radicals may be responsible for the scavenging effects of sulfide on NO bioavailability. Polysulfides may be formed secondary to the reaction of sulfide with NO, either through HSNO or after decomposition of SSNO, and may also contribute to NO scavenging and sulfane sulfur signalling. These findings open new areas of research with SSNO, HSn and SULFI/NO as biologically important mediators of both the NO and H2S transduction pathways. These interactions could be important in the cardiovascular, neuronal and immune systems (Cortese‐Krott et al., 2015) (Tables 2 and 3). Endogenous polysulfides were produced in the mouse brain by 3‐mercaptopyruvate sulfurtransferase, making this pathway physiologically highly relevant (Kimura et al., 2015, 2017). Reports of the effects of various products of the sulfide/NO interaction are shown in Tables 2 and 3 and Figure 2.
Table 2.
Publications on TRPA1‐mediated effects of reaction products of sulfide and NO
| Products of sulfide and NO interactions | |||
|---|---|---|---|
| Na2S | Rat middle meningeal artery | Vasodilatation inhibited by HC030031, l‐NMMA and CGRP receptor antagonist | Dux et al. (2016) |
| Na2S | Rat dura mater | CGRP release by ELISA potentiation by DEA‐NONOate inhibition by l‐NMMA | Dux et al. (2016) |
| Na2S | Rat meningeal artery | Vasodilatation inhibited by HC030031, l‐NMMA, l‐NAME and CGRP receptor antagonist | Eberhardt et al. (2014) |
| Na2S | Murine BP | Hypotensive effect inhibited by knockout of TRPA1, CGRP and l‐NMMA | Eberhardt et al. (2014) |
| Na2S | Murine mesentery | CGRP release by ELISA inhibited by l‐NMMA | Eberhardt et al. (2014) |
| Na2S | Rat mesenteric artery | Vasodilatation inhibited by l‐NMMA, capsaicin pretreatment, CGRP receptor antagonist and HC030031 | Eberhardt et al. (2014) |
| DEA‐NONOate | Rat middle meningeal artery | Vasodilatation inhibited by oxamic acid and ODQ | Dux et al. (2016) |
| Na2S + DEA‐NONOate | Rat spinal trigeminal nucleus | Electrophysiology potentiating effect | Teicher et al. (2017) |
| Na2S + DEA‐NONOate | Rat DRG neurons | Ca2+ imaging potentiation activation inhibited by HC030031 | Miyamoto et al. (2017) |
| Na2S + DEA‐NONOate | Murine DRG neurons | Ca2+ imaging activation inhibited by HC030031, DTT and mutation of key cysteines | Eberhardt et al. (2014) |
| Na2S + DEA‐NONOate | Murine heart | CGRP release by ELISA inhibited by knockout of TRPA1 | Eberhardt et al. (2014) |
| Na2S + DEA‐NONOate | Human skin | Flare by laser Doppler imaging pain and itch potentiation | Eberhardt et al. (2014) |
| Na2S + DEA‐NONOate | Rat TRG neuron, brainstem slice, dura mater | CGRP release by ELISA potentiation | Wild et al. (2015) |
| HNO | Murine DRG neurons | Ca2+ imaging activation inhibited by HC030031, TRPA1 knockout and mutation of key cysteines | Eberhardt et al. (2014) |
| HNO | Rat dura mater | CGRP release by ELISA inhibited by HC030031 | Eberhardt et al. (2014) |
| HNO | Murine dura mater | CGRP release by ELISA inhibited by HC030031 and knockout of TRPA1 | Eberhardt et al. (2014) |
| HNO | Murine sciatic nerve | CGRP release by ELISA inhibited by HC030031 and knockout of TRPA1 | Eberhardt et al. (2014) |
| HNO | Rat meningeal artery | Vasodilatation inhibited by HC030031 and CGRP receptor antagonist | Eberhardt et al. (2014) |
| HNO | Mouse BP | Hypotensive effect inhibited by knockout of TRPA1 | Eberhardt et al. (2014) |
| HNO | Human skin | Vasodilatation by laser Doppler imaging | Eberhardt et al. (2014) |
DEA‐NONOate, diethylamine NONOate; l‐NMMA, N G‐monomethyl‐l‐arginine; ODQ, 1H‐[1,2,4]oxadiazolo[4,3‐a]quinoxalin‐1‐one.
Table 3.
Publications on TRPA1 channel‐mediated effects of inorganic and organic polysulfides
| Inorganic polysulfide | |||
|---|---|---|---|
| Na2S2 and Na2S3 | Rat DRG neurons | Ca2+ imaging | Miyamoto et al. (2017) |
| Na2S3 | Chicken thoracic aorta epitheloid cells | 5‐HT release by HPLC inhibited by TRPA1 antagonist | Delgermurun et al. (2016) |
| Na2S3 | RIN14B cells | Ca2+ imaging activation inhibited by HC030031 | Ujike et al. (2018) |
| Na2S3 | HEK293 mTRPA1 | Ca2+ imaging and electrophysiology activation prevented by mutation of key cysteines and DTT | Hatakeyama et al. (2015) |
| Na2S3 | Murine hind paw | Nocifensive behaviour and oedema formation inhibited by TRPA1 knockout | Hatakeyama et al. (2015) |
| Na2S3 | RIN14B cells | Ca2+ imaging and electrophysiology | Hatakeyama et al. (2015) |
| Na2S3 and Na2S4 | Murine DRG neurons | Ca2+ imaging activation inhibited by HC030031 and TRPA1 knockout | Hatakeyama et al. (2015) |
| Na2S3 and Na2S4 | Rat astrocytes | Ca2+ imaging inhibition by AP18 and HC030031 | Kimura et al. (2013) |
| Sodium polysulfide | Murine hind paw carrageenan‐induced inflammation | Antihyperalgesic effect inhibited by TRPA1 and sst4 knockout | Bátai et al. (2018) |
| Organic polysulfides | |||
| Ajoene | Xenopus oocyte | Electrophysiology enhances activation by TRPA1 agonists | Yassaka et al. (2010) |
| Allicin | Rat TRG neurons | Ca2+ imaging | Bautista et al. (2005) |
| Allicin | HEK293 hTRPA1 | Ca2+ imaging | Bautista et al. (2005) |
| Allicin | Xenopus oocyte hTRPA1 | Ca2+ imaging | Bautista et al. (2005) |
| Allicin | Rat mesenteric artery | Relaxation prevented by capsaicin pretreatment and CGRP receptor antagonist | Bautista et al. (2005) |
| Asadisulfide | HEK293 rTRPA1 | Ca2+ imaging | Shokoohinia et al. (2013) |
| DAS | CHO hTRPA1 | Ca2+ imaging activation inhibited by HC030031 | Koizumi et al. (2009) |
| DADS | CHO hTRPA1 | Ca2+ imaging activation inhibited by HC030031 | Koizumi et al. (2009) |
| DADS | Rat TRG neurons | Ca2+ imaging | Bautista et al. (2005) |
| DADS | HEK293 hTRPA1 | Ca2+ imaging | Bautista et al. (2005) |
| DADS | Xenopus oocyte hTRPA1 | Ca2+ imaging | Bautista et al. (2005) |
| DADS | Rat mesenteric artery | Relaxation prevented by capsaicin pretreatment and CGRP receptor antagonist | Bautista et al. (2005) |
| DATS | CHO hTRPA1 | Ca2+ imaging activation inhibited by HC030031 | Koizumi et al. (2009) |
| DMTS | CHO hTRPA1 | Ca2+ imaging, automated patch‐clamp activation inhibited by HC030031 | Pozsgai et al. (2017) |
| DMTS | Murine TRG neurons | Ca2+ imaging activation inhibited by HC030031 and TRPA1 knockout | Pozsgai et al. (2017) |
| DMTS | Murine skin | Somatostatin release by RIA inhibited by HC030031 | Pozsgai et al. (2017) |
| DMTS | Murine hind paw heat injury | Anti‐hyperalgesic effect inhibited by TRPA1 and sst4 knockout | Pozsgai et al. (2017) |
| DMTS | Murine hind paw carrageenan‐induced inflammation | Anti‐hyperalgesic effect inhibited by sst4 knockout; lowered oedema formation inhibited by sst4 knockout; decreased MPO activity | Bátai et al. (2018) |
MPO, myeloperoxidase.
Figure 2.
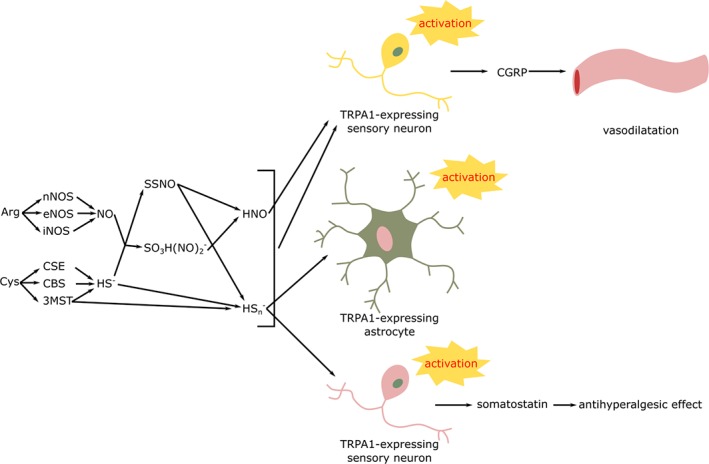
Products of the interaction of sulfide and NO and their documented effects in neurons and astrocytes. Related publications are listed in Tables 2 and 3. 3MST, 3‐mercaptopyruvate sulfurtransferase; Arg, l‐arginine; CSE, cystathionine‐γ‐lyse; Cys, l‐cysteine; HS−, sulfide; HSn −, hydrogen polysulfide; n/e/iNOS, neuronal/endothelial/inducible NO synthase; SO3H(NO)2 −, dinitrososulfite.
Effects of sulfur species mediated by TRPA1 channels
As neurons are the main cell type expressing TRPA1 channels, apart from astrocytes, numerous scientific accounts describe activation of the ion channel by sulfide in these cells (Kimura et al., 2013). The most abundantly investigated expression systems are Xenopus oocytes, RIN14B rat pancreatic islet cells, CHO and HEK293 cells. Murine and rat trigeminal and dorsal root ganglion (TRG and DRG) neurons as well as isolated rat vagal afferents and neurons were also investigated. NaHS and sodium sulfide nonahydrate (Na2S) were mostly used as sulfide donors. Ion channel opening was either detected as a Ca2+ signal indicated by Ca2+‐sensitive fluorescent dyes, electrophysiology in case of nerve fibres, patch‐clamp or serotonin release from RIN14B pancreatic islet cells. Involvement of TRPA1 channels in the increased [Ca2+]i and cation currents was confirmed with selective receptor antagonists AP18, HC030031 or genetic deletion of the gene encoding the ion channel (Miyamoto et al., 2011; Ujike et al., 2015; Hajna et al., 2016). Some papers report a potentiating effect of acidic pH on sulfide‐evoked responses of TRPA1 channels (Andersson et al., 2012; Ogawa et al., 2012; Takahashi and Ohta, 2017). Others identified critical cysteine residues involved in activation of TRPA1 channels by sulfide, using cells expressing mutated ion channel (Ogawa et al., 2012). A relatively recent publication detected activation of TRPV1 channels in rat TRG neurons by NaHS based on inhibition by capsazepine. However, the majority of investigators in the sulfide field consider the gasotransmitter as selective for TRPA1 channels and evidence for activation of TRPV1 channels is scarce (Trevisani et al., 2005; Ang et al., 2010; Medeiros et al., 2012; Lu et al., 2014; Koroleva et al., 2017; Yu et al., 2017). No individual excitatory action but potentiation of the capsaicin‐induced response by NaHS was reported in isolated vagal neurons from rat lungs . Even in this model, respiratory effects of sulfide could be abolished by HC030031 (Hsu et al., 2013). CGRP release from peptidergic nerves of rat tracheae was reported in response to stimulation with NaHS. Peptide release was prevented by the TRPA1 channel antagonist (Pozsgai et al., 2012).
Contribution of TRPA1 channels to sulfide‐induced smooth muscle relaxation was examined in rat mesenteric arteries, pig bronchioles and intravesical ureter, as well as human ureter and prostate. Besides the obvious inhibition by defunctionalization of peptidergic nociceptors or HC030031, dilator effects of sulfide in rat mesenteric arteries was diminished by a CGRP receptor antagonist or a Cl− channel inhibitor (White et al., 2013). Precontracted porcine bronchiole rings were relaxed by a slow‐release sulfide donor, GYY4137. Sulfide‐induced relaxation was ameliorated by elevated extracellular K+ concentration and inhibition of large conductance Ca2+‐activated K+ channels. Endogenous sulfide and TRPA1 channel activation were involved in electrical stimulation‐evoked relaxation (Fernandes et al., 2016). Porcine intravesical ureter precontracted with a TXA2 analogue was relaxed by GYY4137. The response was prevented by the selective antagonist HC030031. Release and participation of neuropeptides is indicated by inhibitory effect of either pituitary adenylate cyclase‐activating peptide or CGRP receptor antagonists. In this model, relaxation produced by GYY4137 was also inhibited by a TRPV1 channel antagonist (Fernandes et al., 2014). NaHS prevented contractions evoked by electrical stimulation in human ureter and prostate smooth muscle (Weinhold et al., 2017). In vivo studies on TRPA1 channel‐mediated effects of sulfide focused on vasodilatation, somatic and visceral nociception, as well as bladder function. Vasorelaxant effect of NaHS was investigated in the mouse ear. The sulfide donor was applied topically, and blood flow changes were detected by laser Doppler imaging. Sulfide‐evoked elevated blood flow could be lowered by the TRPA1 channel antagonist, desensitization of peptidergic nerve endings by resiniferatoxin pretreatment and genetic lack of the ion channel. The role of vasodilator peptides CGRP and SP was implied by the inhibitory action of the corresponding receptor antagonists. Inhibition of ATP‐dependent K+ channels reversed vasodilatation (Pozsgai et al., 2012; Hajna et al., 2016). Intraplantar administration of NaHS leads to nocifensive behaviour in mice shown as mechanical and cold hyperalgesia. Such responses are weakened by trpa1‐gene deficiency, gene silencing of TRPA1 channels and a TRPA1 channel antagonist. Several papers claim relieving activity of inhibitors or gene silencing of T‐type Ca2+ channels, especially CaV3.2 channels. A contribution of endogenous sulfide to intraplantar LPS‐evoked pain was indicated by amelioration after the TRPA1 channel antagonist AP18 or inhibition of sulfide synthesis. Acidic pH potentiated nocifensive reaction to sulfide (Andersson et al., 2012; Ogawa et al., 2012; Okubo et al., 2012). Application of NaHS into the pancreatic duct in mice undergoing cerulein‐induced pancreatitis led to elevated cFos expression in the spinal cord and mechanical alldoynia. Visceral pain was relieved by a TRPA1 channel antagonist and a CaV3.2 channel inhibitor (Terada et al., 2015). Intracolonic administration of NaHS induced visceral nociception, which was similarly inhibited by a TRPA1 channel antagonist and Ca2+ channel blocker (Tsubota‐Matsunami et al., 2012). However, another study detected visceral pain in TRPA1 knockout mice after intracolonic sulfide treatment (Andersson et al., 2012; Tsubota‐Matsunami et al., 2012). Intravesical administration of NaHS in rats produced increased micturition frequency and decreased voiding volume as observed with known TRPA1 channel agonists. Expression of TRPA1 channels was demonstrated by immunohistochemistry in the urothelium (Streng et al., 2008) (Table 1).
An emerging field of sulfide research proposes that products of the reaction of sulfide with NO are responsible for various biological actions, including those mediated by TRPA1 channels. The most investigated derivatives are HNO, polysulfides, SSNO and SULFI/NO (Cortese‐Krott et al., 2015). Combined application of sulfide and NO activated TRPA1 channels in rat TRG and DRG neurons, astrocytes and brainstem slices, as well as murine DRG cells. Responses were abolished by TRPA1 channel antagonists or reciprocal inhibition of sulfide or NO synthesis or downstream signalling. CGRP release in response to the sulfide/NO combination was detected in rat neurons and dura mater. A functional role of HNO in elevated dura skin blood flow, as well as its effect on murine BP was demonstrated by Eberhardt and colleagues in very elegant experiments. They also detected CGRP release in response to HNO from various tissues including murine heart, mesentery, sciatic nerve and rat dura (Eberhardt et al., 2014; Wild et al., 2015; Dux et al., 2016; Miyamoto et al., 2017; Teicher et al., 2017). Interaction of inorganic polysulfide with TRPA1 ion channels was examined in RIN14B, HEK293, chicken thoracic aorta epitheloid cells and murine DRG neurons. Calcium signal readouts were inhibited by a TRPA1 channel antagonist. In some reports, inhibitors of L‐ and N‐type Ca2+ channels as well as NO synthesis ameliorated activation of TRPA1 channels. Critical cysteine residues of these channels involved in the activation by polysulfide were identified using cells expressing mutant receptor protein (Hatakeyama et al., 2015; Delgermurun et al., 2016; Ujike et al., 2018). Intraplantar injection of sodium polysulfide evoked reduced pain and oedema formation in TRPA1 gene deficient mice compared to wild‐type controls (Hatakeyama et al., 2015). Repeated i.p. administration of sodium polysulfide was reported to relieve mechanical hyperalgesia due to carrageenan‐induced paw inflammation in mice. The effect was inhibited if TRPA1 channels or somatostatin sst4 receptors were knocked out (Bátai et al., 2018) (Table 2).
Plant‐derived asadisulfide produced Ca2+ signals in TRPA1‐expressing HEK293 cells. Ajoene from garlic did not produce membrane currents in Xenopus oocytes alone but potentiated the action of various TRPA1 channel agonists. Organic sulfide compounds from garlic – DAS, DADS, DATS and DMTS – activated TRPA1 channels in CHO cells. Effects of diallyl compounds were not only diminished by a TRPA1 channel antagonist but also by capsazepine. Similar activity of allicin and DADS was found in rat TRG neurons. DMTS activated TTRPA1 channels in murine TRG cells but did not have any effect on neurons from knockout animals. DMTS activated nerve endings in mouse isolated skin and provoked somatostatin release. This effect was abolished in skin samples dissected from TRPA1 channel knockout mice. Allicin and DADS dilate rat mesenteric arteriesand this vasodilatation was inhibited either by desensitization of peptidergic nerve fibres by capsaicin pretreatment or a CGRP receptor antagonist. In vitro, DMTS reduced the mechanical hyperalgesia due to mild heat injury in a manner dependent on TRPA1 channels and sst4 receptors. In contrast, repeated i.p. administration of DMTS inhibited nociception, oedema formation and myeloperoxidase activity in murine carrageenan‐evoked paw inflammation. These inhibitory effects were still present in TRPA1 knockout mice, indicating actions independent of TRPA1 channels (Bautista et al., 2005; Koizumi et al., 2009; Yassaka et al., 2010; Shokoohinia et al., 2013; Pozsgai et al., 2017; Bátai et al., 2018) (Table 3).
Putative mechanisms of sulfide‐TRPA1 channel interactions
Functional modulation of TRP channels – including TRPA1 channels – by phosphorylation is a well‐known phenomenon (Kádková et al., 2017). In this section, we attempt to give a summary of indirect sulfide–TRPA1 channel interactions based on the effect of sulfide on kinases. Reports of the sensitization or activation of TRPA1 channels by signalling kinases should be assessed cautiously, as elevated neuronal [Ca2+]i can also reflect PLC activation or simply being included in external patch‐clamp solutions could activate the channel (Figure 3).
Figure 3.

Putative mechanisms of the modulation of TRPA1 function or expression by sulfide. Modulation of the activation or expression of TRPA1 ion channel by the signalling kinases, transcription factors, hormones and reactive species is documented in the literature. Effect of sulfide on these signalling mechanisms was proven independently. Modulation of TRPA1 channels via these pathways has never been confirmed directly. In case of PKA persulfidation and disulfide formation in the are indicated. In case of IκB‐α, p38, PKC, Src and TRPA1 phosphorylation or the lack thereof are shown. Arrows mean activation, and capped lines mean inhibition. CR, cytokine receptor; RTK, receptor TK.
Protein kinase A
The activation of TRPA1 channels by agonists is potentiated by bradykinin. This effect is partly mediated via PKA, as it is reproduced by PKA activators and prevented by its inhibitors (Kádková et al., 2017). The most likely serine and threonine phosphorylation sites of human TRPA1 channels are located in ARD 1 (S86 and S87), 2 (T274), 8 (S317) and C‐terminal cytoplasmic domain (S972 and S1101) (Kádková et al., 2017). A wide range of reports claim modulation of PKA by sulfide or polysulfides. Sulfide treatment increases cAMP concentration in neuronal and glial cell lines, as well as in Xenopus oocytes (Kimura, 2000). The sulfide donor NaHS leads to persulfidation and activation of PKA in rat hippocampal neurons (Li et al., 2016). NaHS raised [Ca2+]i in human neuroblastoma SH‐SY5Y cells partly by PKA‐mediated phosphorylation of plasma membrane Ca2+ channels. The effect was blocked by a PKA inhibitor (Yong et al., 2010). Sulfide‐induced PKA activation was also described in microglial cells (Lee et al., 2006). Apart from neuronal cells, similar effects were observed in hepatocytes (Untereiner et al., 2016). Cysteine persulfides created in reactions with polysulfides might be reduced in a reaction with thiols of the same or other proteins leading to disulfide formation. Disulfide bond formation between two regulatory subunits of PKA involving cysteines C17 and C38 leads to enzyme activation in rat tissues (Burgoyne and Eaton, 2009). Sulfide might not only activate PKA by direct interaction but by inhibition of PDE enzymes. Sulfide‐induced relaxation of rat aortic smooth muscle is mediated by elevated cGMP and is prevented by a pretreatment with a PDE inhibitor (Bucci et al., 2010). Besides being an alternative electron acceptor in oxidative phosphorylation in a certain concentration range, inhibition of PDE2A in the mitochondrial matrix contributes to protective effects of sulfide, too (Módis et al., 2013). Dimethyl disulfide, an organic disulfide compound, leads to pulmonary vasodilatation in rats by activating NOS via phosphorylation by PKA (Han et al., 2017).
PKC
The interaction of PKC and TRPA1 channels is only supported by indirect data and still awaits direct evidence. PLC inhibitors – preventing PKC activation – ameliorate TRPA1 channel sensitization in response to activation of bradykinin or protease‐activated receptors (Dai et al., 2007; Pethő and Reeh, 2012). This effect might be simply mediated by lower Ca2+ efflux from the endoplasmic reticulum and consequent lower activation of TRPA1 channels. The A kinase anchor protein (AKAP79/150) that is able to bind PKA and PKC was reported to attach to TRPA1 channels. Several possible phosphorylation sites of PKC were identified in the sequence of TRPA1 protein (Kádková et al., 2017). In order to provide a realistic view of the interaction of sulfide and PKC, it has to be noted that at least as many papers report inhibitory effect of the gasotransmitter on enzyme activity as those proposing activation. Activation of PKC by sulfide was documented in various cell types and tissues. Chronic pancreatitis leads to increased expression of cystathionine β‐synthase (CBS) – the key enzyme of sulfide synthesis – and PKCγ in the arcuate nucleus. (Zheng et al., 2016). Inhalation of H2S gas activated PKC in the affected brain area and ameliorated the damage (Wei et al., 2015). The protective effect of NaHS in an in vitro model of Parkinson's disease was mediated by the induction of PKCα and PKCε in SH‐SY5Y neuronal cells (Yong et al., 2008). NaHS induced PKC phosphorylation and activation in the rat hippocampus (Li et al., 2016). The PKC inhibitor chelerythrine blunted Ca2+ responses to NaHS in SH‐SY5Y cells (Yong et al., 2010). The protective effect of NaHS against hypoxic damage in SH‐SY5Y cells was prevented by various PKC inhibitors (Tay et al., 2010). Outside the nervous system, murine bone marrow mesenchymal stem cells heterozygous for CBS or with the enzyme being inhibited showed smaller PKC activity. PKC activity was elevated by NaHS treatment (Liu et al., 2014). Beneficial effect of ischaemic postconditioning on rat cardiac myocytes was blunted by the inhibition of endogenous H2S synthesis due to diminished activity of PKCα and PKCε (Yong et al., 2008). Preconditioning of rat cardiac cells with NaHS produced translocation of PKCε and PKCδ into the plasma membrane (Hu et al., 2008; Pan et al., 2008).
Src kinase
Unlike the signalling kinases discussed above, Src kinase is a non‐receptor, protein‐tyrosine kinase (TK). Src integrates growth factor signalling, including that from nerve growth factor, as it can either switch on Ras/Raf/MEK/ERK or the PI3K/Akt signalling routes (Roskoski, 2015). Brain‐derived neurotrophic factor and glial cell‐derived neurotrophic factor were reported to induce TRPA1 channels in rat DRG neurons and increase the responsiveness to agonists (Ciobanu et al., 2009). There is no direct evidence of phosphorylation of the TRPA1 channel by Src. On the other hand, sensitivity of neuronal SH‐SY5Y cells to a TRPA1 channel agonist – lost during several passages of the cells – could be restored by an Src kinase inhibitor (Kádková et al., 2017). The site of interaction might be tyrosine 69 (Y69) in the first ARD (Morgan et al., 2015). Based on in silico models, there are two other tyrosines in TRPA1 channels proposed for phosphorylation by Src: number 22 and 680 in the N‐terminal cytoplasmic domain. Few accounts on the interaction of sulfide and Src are available. NaHS increased neuritogenesis in NG108‐15 cells. The effect was blocked by Src inhibition. It has to be mentioned that supraphysiological concentrations of NaHS were applied (Tarui et al., 2010). Interaction of sulfide and Src was also reported in non‐neuronal cells. Phosphorylation of Src due to NaHS treatment contributes to migration of RAW264.7 murine macrophages (Miao et al., 2016). Activation of Src upon NaHS treatment was described in murine pancreas acini (Tamizhselvi et al., 2010).
p38 MAPKs
Odontoblast cells respond to stimulation by TNF‐α with increased reaction of TRPA1 channels to agonist ligands and membrane stretch. The effects were mediated by MAPKs 38 (p38)‐evoked induction of TRPA1 channels (El Karim et al., 2015). There is limited evidence that sulfur compounds are able to increase phosphorylation and activation of p38 kinase. NaHS increases glucagon‐like peptide‐1 (GLP‐1) secretion in mice via activation of p38 (Pichette et al., 2017). Activation of p38 in response to NaHS was detected in human monocytes (Sulen et al., 2016). Garlic‐derived DATS ameliorates cisplatin‐induced oxidative injury in NCI‐H460 human lung carcinoma cells in a similar manner (Jiang et al., 2017).
Inhibitory effects of sulfide on p38 kinase is more widely accepted. Inhibition of p38 by sulfide was detected in human, murine, rat neuronal tissues and cell lines (Li et al., 2016; Lee et al., 2016b). Inhibitory action of sulfide on p38 MAPK was reported in non‐neuronal cells, (Wu et al., 2017). The organic compound DADS inhibited p38 in C28/I2 human chondrocyte cell line (Hosseinzadeh et al., 2017).
Casein kinase 2 (CK2)
CK2 is a highly conserved and ubiquitous serine/threonine protein kinase. CK2 is currently gaining significance in the regulation of Th17 immune response and inflammatory processes involving glomerulonephritis, intestinal inflammation, pulmonary disease, multiple sclerosis and contact dermatitis (Gibson and Benveniste, 2018). One suggested phosphorylation site of TRPA1 channels by CK2 is threonine number 1078 in the C‐terminal cytoplasmic part of the ion channel. Replacement of threonine 1078 with aspartate, mimicking phosphorylation, strongly influences electrophysiological properties of the channel in response to Ca2+ (Kádková et al., 2017). Protection of rat myocardial cells from ischaemia‐reperfusion injury and amelioration of apoptosis by NaHS involved increased CK2 activity (Yao et al., 2012). The activity of CK2 is increased when intramolecular disulfides are reduced to the corresponding thiols (Zhang et al., 1998). Recently, sulfide has been demonstrated to participate in the reduction of disulfides under physiological conditions, offering another possible mechanism for the interaction with CK2 (Vasas et al., 2015).
AMP‐activated protein kinase (AMPK)
AMPK functions as an energetic sensor of cells being activated by increased AMP (and therefore decreased ATP) concentrations. AMPK is either activated by phosphorylation or a Ca2+ signal via Ca2+/calmodulin‐activated protein kinase kinase β. Recently, the role of AMPK has been demonstrated in the regulation of cell autophagy, atherosclerosis, inflammatory disease and cancer. AMPK down‐regulated TRPA1 channels in DRG neurons, as shown by a lowered amount of membrane‐bound TRPA1 protein in response to metformin and elevated level of TRPA1 protein when AMPK activity was decreased (Wang et al., 2018). Altogether the link between TRPA1 channels and AMPK is rather weak and is included to provide a full view of the area. Wang et al. (2017) provide an excellent review on the modulation of AMPK by sulfide. No evidence on the interaction of sulfide with AMPK is available in neurons. However, microglia‐mediated neuroinflammation was ameliorated by various donors of sulfide via activation of AMPK (Wang et al., 2017). Na2S increased AMPK activity and attenuated myocardial damage due to smoking, high fat diet, ischaemia or cardiac arrest (Minamishima et al., 2009; Wang et al., 2017). Similar positive effects were recorded in rat aortic endothelial cells following challenge by high glucose and palmitic acid. Sulfide elicited this action by activating nuclear factor erythroid 2‐related factor 2 (Nrf2), a transcription factor involved in the response to oxidants and electrophiles (Wang et al., 2017). Activation of Nrf2 involves thiol modification and inhibition of a regulatory protein – Kelch‐like ECH‐associated protein 1 (Keap1) – that normally promotes ubiquitination and degradation of Nrf2 (Taguchi et al., 2011). Nrf2‐mediated protection against oxidative stress was detected in mouse neuroblastoma cells and glomerular endothelial cells in response to NaHS and polysulfide (Koike et al., 2013; Wang et al., 2017). Viability of rat embryonic cardiac cells in a high glucose environment was enhanced by the slow‐release sulfide donor GYY4137 due to AMPK phosphorylation (Wang et al., 2017). Elevated phosphorylation of AMPK in response to sulfide was detected in hepatic cells, colonic epithelial cells, osteoblasts and human monocyte cell line (Wang et al., 2017). Garlic‐derived organic disulfide ajoene initiated similar signalling mechanisms in hepatocytes (Wang et al., 2017).
IκB kinase complex (IKK)
Activation of the pivotal and ubiquitous transcription factor NF‐κB might occur via phosphorylation and consequent degradation of its inhibitory protein IκB‐α by the IKK. Functional data on nociceptor neurons genetically lacking IKK suggest that the kinase complex suppresses expression of TRPA1 channels (Bockhart et al., 2009). There is evidence that both inorganic sulfide and organic polysulfides modulate IKK and in this way could affect the expression of TRPA1 channels. Regulation of the IKK complex by sulfide was only investigated in non‐neuronal tissues. Garlic‐derived DATS promoted apoptosis in primary effusion lymphoma cells by inhibiting IKK activity, preventing proteasome‐driven degradation of IκB‐α and consequently suppressing NF‐κB (Shigemi et al., 2016). Similar effect of DADS was detected in carbon tetrachloride‐exposed rat hepatocytes (Lee et al., 2014). Inhibition of NF‐κB signalling by modulation of IKK by inorganic sulfide was described in rat cardiomyocytes exposed to haemorrhagic shock (Gao et al., 2012). Sulfide‐releasing diclofenac could prevent breast cancer‐induced osteoclast formation via a comparable mechanism (Frantzias et al., 2012).
Oestrogen
The mechanism of modulating TRPA1 channels by oestrogen shown below is highly debatable and rests upon relatively limited evidence, but the authors feel obliged to include it in order to provide a full picture. There is a single report of ovariectomy‐related apoptosis of rat hippocampal and DRG neurons partly relies on Ca2+ influx mediated by TRPA1 channels (Yazğan and Nazıroğlu, 2017). It is well known that oestrogen increases the expression of sulfide‐synthesizing enzymes (Li et al., 2017) and some data suggest that sulfide might reciprocally affect oestrogen synthesis or metabolism. Treatment with the sulfide precursor cysteine for 8 weeks elevated the serum concentration of 17β‐oestradiol in rats (Han et al., 2016). A garlic‐derived organic sulfide, DAS, was reported to interact with intracellular constitutive androstane receptor and induce oestrogen metabolizing sulfotransferases. However, this did not affect the serum oestrogen concentration but only that of exogenously applied hormone (Green et al., 2007; Sueyoshi et al., 2011).
Hypoxia inducible factor 1α (HIF‐1α)
Cigarette smoke extract induces expression of TRPA1 channels via HIF‐1α activation in A549 human alveolar adenocarcinoma cells (Nie et al., 2016). The influence of sulfide on HIF‐1α activity and expression is still debatable. Some authors have reported destabilization and lowered expression of the transcription factor in various cell lines (Wu et al., 2012). Others found the opposite, with activation and stabilization of HIF‐1α by sulfide in human THP‐1 macrophages and rat endothelial cells (Liu et al., 2010; Flannigan et al., 2015; Lohninger et al., 2015).
Mitochondrial dysfunction and oxygen radicals
Inhibition of complex III of the mitochondrial electron transport chain by antimycin A leads to the activation of TRPA1 channels in murine bronchopulmonary C fibres. The effect was mediated by ROS resulting from mitochondrial dysfunction (Nesuashvili et al., 2013). Large amounts of sulfide are well known to inhibit complex IV (cytochrome c oxidase) and open the way for the production of ROS and depletion of GSH. The effect of sulfide on oxidative phosphorylation offers an indirect way of activating TRPA1 channels (Figure 3).
Concluding remarks
The biological importance of H2S and polysulfides has been extensively investigated in vitro and in vivo. The role of TRPA1 channels and the signalling effects of sulfide compounds have been demonstrated separately in different organs either in the CNS or on the periphery in numerous physiological and pathological processes, including neuroimmune modulation, metabolic pathways, cardiovascular system, tumour growth, inflammation and pain. The present review provides a comprehensive overview about potential interactions between TRPA1 channel‐ and sulfide‐activated signal transduction pathways. There are several sets of experimental data demonstrating direct binding and agonist effect of sulfide containing ligands on TRPA1 channels. They open the ion channel presumably by covalent modification of cysteine and lysine residues within the N‐terminus and transmembrane domain. Analysing the list of recently published data, it is very likely that, apart from direct activation, sulfides modulate TRPA1 channels indirectly, via signalling kinases, as well. The key link to reveal the molecular mechanism is that sensitization or activation of TRPA1 channels has followed the phosphorylation of the protein. There is substantial evidence that sulfides can activate several kinases (PKA, PKC, Src, p38 MAPK, CK2, AMPK and IkB) in the signal transduction pathways. Modulation of TRPA1 channels by transcription factors, hormones and reactive species is also documented in the literature. TRPA1‐independent and TRPA1‐dependent effects – resulting from either by direct or indirect mechanisms – of sulfides play pivotal roles in regulation of cellular functions. Understanding interactions between TRPA1 channels and sulfides may contribute to the discovery of novel drug targets for the treatment of a range of diseases.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c, 2017d, 2017e, 2017f).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was funded by the following grants of the National Research, Development and Innovation Office – NKFIH, Hungary: OTKA PD 112171, OTKA NN 114458. GINOP‐2.3.2.‐15‐2016‐00050 ‘PEPSYS’, EFOP‐3.6.3‐VEKOP‐16‐2017‐00009 and EFOP‐3.6.2‐16‐2017‐00009 ‘HECRIN’ from the European Regional Development Fund. This project was supported by the János Bolyai Research Scholarship (G.P.) and by János Szentágothai Fellowship A2‐SZJÖ‐TOK‐13‐0149 (E.P.) of the Hungarian Academy of Sciences (Magyar Tudományos Akadémia) and by the ÚNKP‐18‐4 New National Excellence Program of the Ministry of Human Capacities.
Pozsgai, G. , Bátai, I. Z. , and Pintér, E. (2019) Effects of sulfide and polysulfides transmitted by direct or signal transduction‐mediated activation of TRPA1 channels. British Journal of Pharmacology, 176: 628–645. 10.1111/bph.14514.
References
- Alexander SP, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017e). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br J Pharmacol 174: S208–S224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017f). The Concise Guide to PHARMACOLOGY 2017/18: Other proteins. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Bevan S (2012). TRPA1 has a key role in the somatic pro‐nociceptive actions of hydrogen sulfide. PLoS One 7: e46917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang S, Moochhala SM, Bhatia M (2010). Hydrogen sulfide promotes transient receptor potential vanilloid 1‐mediated neurogenic inflammation in polymicrobial sepsis*. Crit Care Med 38: 619–628. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Preti D, Materazzi S, Geppetti P (2010). Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti‐inflammatory agents. J Med Chem 53: 5085–5107. [DOI] [PubMed] [Google Scholar]
- Bátai IZ, Horváth Á, Pintér E, Helyes Z, Pozsgai G (2018). Role of transient receptor potential ankyrin 1 Ion channel and somatostatin sst4 receptor in the antinociceptive and anti‐inflammatory effects of sodium polysulfide and dimethyl trisulfide. Front Endocrinol (Lausanne) 9: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED et al (2005). Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A 102: 12248–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin S, Aoki‐Nonaka Y, Lee J, de Jong PR, Kim P, Han T et al (2017). The TRPA1 ion channel is expressed in CD4+ T cells and restrains T‐cell‐mediated colitis through inhibition of TRPV1. Gut 66: 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Jordt S‐E (2008). Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23: 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockhart V, Constantin CE, Haussler A, Wijnvoord N, Kanngiesser M, Myrczek T et al (2009). Inhibitor B kinase deficiency in primary nociceptive neurons increases TRP channel sensitivity. J Neurosci 29: 12919–12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin JV, Brain SD (2011). Transient receptor potential ankyrin 1: emerging pharmacology and indications for cardiovascular biology. Acta Physiol 203: 87–98. [DOI] [PubMed] [Google Scholar]
- Brewster MSJ, Gaudet R (2015). How the TRPA1 receptor transmits painful stimuli: inner workings revealed by electron cryomicroscopy. Bioessays 37: 1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C et al (2010). Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 30: 1998–2004. [DOI] [PubMed] [Google Scholar]
- Burgoyne JR, Eaton P (2009). Transnitrosylating nitric oxide species directly activate type I protein kinase A, providing a novel adenylate cyclase‐independent cross‐talk to beta‐adrenergic‐like signaling. J Biol Chem 284: 29260–29268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D‐S, Zhong L, Hsieh T‐H, Abooj M, Bishnoi M, Hughes L et al (2012). Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells. PLoS One 7: e38005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hackos DH (2015). TRPA1 as a drug target – promise and challenges. Naunyn Schmiedebergs Arch Pharmacol 388: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciobanu C, Reid G, Babes A (2009). Acute and chronic effects of neurotrophic factors BDNF and GDNF on responses mediated by thermo‐sensitive TRP channels in cultured rat dorsal root ganglion neurons. Brain Res 1284: 54–67. [DOI] [PubMed] [Google Scholar]
- Cortese‐Krott MM, Kuhnle GGC, Dyson A, Fernandez BO, Grman M, DuMond JF et al (2015). Key bioactive reaction products of the NO/H2S interaction are S/N‐hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci U S A 112: E4651–E4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T et al (2007). Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest 117: 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgermurun D, Yamaguchi S, Ichii O, Kon Y, Ito S, Otsuguro K (2016). Hydrogen sulfide activates TRPA1 and releases 5‐HT from epithelioid cells of the chicken thoracic aorta. Comp Biochem Physiol Part C Toxicol Pharmacol 187: 43–49. [DOI] [PubMed] [Google Scholar]
- Dux M, Will C, Vogler B, Filipovic MR, Messlinger K (2016). Meningeal blood flow is controlled by H2 S‐NO crosstalk activating a HNO‐TRPA1‐CGRP signalling pathway. Br J Pharmacol 173: 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt M, Dux M, Namer B, Miljkovic J, Cordasic N, Will C et al (2014). H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO–TRPA1–CGRP signalling pathway. Nat Commun 5: 4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Karim I, McCrudden MTC, Linden GJ, Abdullah H, Curtis TM, McGahon M et al (2015). TNF‐α‐induced p38MAPK activation regulates TRPA1 and TRPV4 activity in odontoblast‐like cells. Am J Pathol 185: 2994–3002. [DOI] [PubMed] [Google Scholar]
- Fernandes VS, Ribeiro ASF, Martínez P, López‐Oliva ME, Barahona MV, Orensanz LM et al (2014). Hydrogen sulfide plays a key role in the inhibitory neurotransmission to the pig intravesical ureter. PLoS One 9: e113580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes VS, Recio P, López‐Oliva E, Martínez MP, Ribeiro AS, Barahona MV et al (2016). Role of endogenous hydrogen sulfide in nerve‐evoked relaxation of pig terminal bronchioles. Pulm Pharmacol Ther 41: 1–10. [DOI] [PubMed] [Google Scholar]
- Flannigan KL, Agbor TA, Motta J‐P, Ferraz JGP, Wang R, Buret AG et al (2015). Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia‐inducible factor‐1α. FASEB J 29: 1591–1602. [DOI] [PubMed] [Google Scholar]
- Frantzias J, Logan JG, Mollat P, Sparatore A, Del Soldato P, Ralston SH et al (2012). Hydrogen sulphide‐releasing diclofenac derivatives inhibit breast cancer‐induced osteoclastogenesis in vitro and prevent osteolysis ex vivo. Br J Pharmacol 165: 1914–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Xu D‐Q, Gao C‐J, Ding Q, Yao L‐N, Li Z‐C et al (2012). An exogenous hydrogen sulphide donor, NaHS, inhibits the nuclear factor κB inhibitor kinase/nuclear factor κB inhibitor/nuclear factor‐κB signaling pathway and exerts cardioprotective effects in a rat hemorrhagic shock model. Biol Pharm Bull 35: 1029–1034. [DOI] [PubMed] [Google Scholar]
- Garrison SR, Stucky CL (2011). The dynamic TRPA1 channel: a suitable pharmacological pain target? Curr Pharm Biotechnol 12: 1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SA, Benveniste EN (2018). Protein kinase CK2: an emerging regulator of immunity. Trends Immunol 39: 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzke C, Weinhold P, Reich O, Seitz M, Schlenker B, Stief CG et al (2010). Transient receptor potential A1 and cannabinoid receptor activity in human normal and hyperplastic prostate: relation to nerves and interstitial cells. Eur Urol 57: 902–910. [DOI] [PubMed] [Google Scholar]
- Green M, Newell O, Aboyade‐Cole A, Darling‐Reed S, Thomas RD (2007). Diallyl sulfide induces the expression of estrogen metabolizing genes in the presence and/or absence of diethylstilbestrol in the breast of female ACI rats. Toxicol Lett 168: 7–12. [DOI] [PubMed] [Google Scholar]
- Hajna Z, Sághy É, Payrits M, Aubdool AA, Szőke É, Pozsgai G et al (2016). Capsaicin‐sensitive sensory nerves mediate the cellular and microvascular effects of H2S via TRPA1 receptor activation and neuropeptide release. J Mol Neurosci 60: 157–170. [DOI] [PubMed] [Google Scholar]
- Han N‐R, Kim N‐R, Kim H‐M, Jeong H‐J (2016). Cysteine prevents menopausal syndromes in ovariectomized mouse. Reprod Sci 23: 670–679. [DOI] [PubMed] [Google Scholar]
- Han C, Qi J, Gao S, Li C, Ma Y, Wang J et al (2017). Vasodilation effect of volatile oil from Allium macrostemon Bunge are mediated by PKA/NO pathway and its constituent dimethyl disulfide in isolated rat pulmonary arterials. Fitoterapia 120: 52–57. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama Y, Takahashi K, Tominaga M, Kimura H, Ohta T (2015). Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons. Mol Pain 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyes Z, Pintér E, Sándor K, Elekes K, Bánvölgyi A, Keszthelyi D et al (2009). Impaired defense mechanism against inflammation, hyperalgesia, and airway hyperreactivity in somatostatin 4 receptor gene‐deleted mice. Proc Natl Acad Sci U S A 106: 13088–13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H (1997). The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh A, Jafari D, Kamarul T, Bagheri A, Sharifi AM (2017). Evaluating the protective effects and mechanisms of diallyl disulfide on interlukin‐1β‐induced oxidative stress and mitochondrial apoptotic signaling pathways in cultured chondrocytes. J Cell Biochem 118: 1879–1888. [DOI] [PubMed] [Google Scholar]
- Hsu C‐C, Lin R‐L, Lee L‐Y, Lin YS (2013). Hydrogen sulfide induces hypersensitivity of rat capsaicin‐sensitive lung vagal neurons: role of TRPA1 receptors. Am J Physiol Regul Integr Comp Physiol 305: R769–R779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L‐F, Pan T‐T, Neo KL, Yong QC, Bian J‐S (2008). Cyclooxygenase‐2 mediates the delayed cardioprotection induced by hydrogen sulfide preconditioning in isolated rat cardiomyocytes. Pflügers Arch ‐ Eur J Physiol 455: 971–978. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhu X, Liu N, Xu H, Zhao Z, Li S et al (2017). Diallyl trisulfide inhibits growth of NCI‐H460 in vitro and in vivo, and ameliorates cisplatin‐induced oxidative injury in the treatment of lung carcinoma in xenograft mice. Int J Biol Sci 13: 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt S‐E, Bautista DM, Chuang H‐H, McKemy DD, Zygmunt PM, Högestätt ED et al (2004). Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265. [DOI] [PubMed] [Google Scholar]
- Kádková A, Synytsya V, Krusek J, Zímová L, Vlachová V (2017). Molecular basis of TRPA1 regulation in nociceptive neurons. A review Physiol Res 66: 425–439. [DOI] [PubMed] [Google Scholar]
- Kemény Á, Kodji X, Horváth S, Komlódi R, Szőke É, Sándor Z et al (2018). TRPA1 acts in a protective manner in imiquimod‐induced psoriasiform dermatitis in mice. J Invest Dermatol 138: 1774–1784. [DOI] [PubMed] [Google Scholar]
- Khalil M, Alliger K, Weidinger C, Yerinde C, Wirtz S, Becker C et al (2018). Functional role of transient receptor potential channels in immune cells and epithelia. Front Immunol 9: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H (2000). Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun 267: 129–133. [DOI] [PubMed] [Google Scholar]
- Kimura H (2015). Signaling molecules: hydrogen sulfide and polysulfide. Antioxid Redox Signal 22: 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H (2017). Hydrogen sulfide and polysulfide signaling. Antioxid Redox Signal 27: 619–621. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka J, Kimura H (2013). Polysulfides are possible H2S‐derived signaling molecules in rat brain. FASEB J 27: 2451–2457. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Toyofuku Y, Koike S, Shibuya N, Nagahara N, Lefer D et al (2015). Identification of H2S3 and H2S produced by 3‐mercaptopyruvate sulfurtransferase in the brain. Sci Rep 5: 14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Koike S, Shibuya N, Lefer D, Ogasawara Y, Kimura H (2017). 3‐Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine‐ and glutathione‐persulfide (Cys‐SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Sci Rep 7: 10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Ogasawara Y, Shibuya N, Kimura H, Ishii K (2013). Polysulfide exerts a protective effect against cytotoxicity caused by t‐buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett 587: 3548–3555. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Iwasaki Y, Narukawa M, Iitsuka Y, Fukao T, Seki T et al (2009). Diallyl sulfides in garlic activate both TRPA1 and TRPV1. Biochem Biophys Res Commun 382: 545–548. [DOI] [PubMed] [Google Scholar]
- Koroleva K, Mustafina A, Yakovlev A, Hermann A, Giniatullin R, Sitdikova G (2017). Receptor mechanisms mediating the pro‐nociceptive action of hydrogen sulfide in rat trigeminal neurons and meningeal afferents. Front Cell Neurosci 11: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kun J, Szitter I, Kemény Á, Perkecz A, Kereskai L, Pohóczky K et al (2014). Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS One 9: e108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kun J, Perkecz A, Knie L, Sétáló G, Tornóczki T, Pintér E et al (2017). TRPA1 receptor is upregulated in human oral lichen planus. Oral Dis 23: 189–198. [DOI] [PubMed] [Google Scholar]
- Lee SW, Hu Y‐S, Hu L‐F, Lu Q, Dawe GS, Moore PK et al (2006). Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia 54: 116–124. [DOI] [PubMed] [Google Scholar]
- Lee I‐C, Kim S‐H, Baek H‐S, Moon C, Kang S‐S, Kim S‐H et al (2014). The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride‐induced hepatic oxidative damage and inflammatory response in rats. Food Chem Toxicol 63: 174–185. [DOI] [PubMed] [Google Scholar]
- Lee K‐I, Lee H‐T, Lin H‐C, Tsay H‐J, Tsai F‐C, Shyue S‐K et al (2016a). Role of transient receptor potential ankyrin 1 channels in Alzheimer's disease. J Neuroinflammation 13: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, McGeer EG, McGeer PL (2016b). Sodium thiosulfate attenuates glial‐mediated neuroinflammation in degenerative neurological diseases. J Neuroinflammation 13: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y‐L, Zhou J, Zhang H, Luo Y, Long L‐H, Hu Z‐L et al (2016). Hydrogen sulfide promotes surface insertion of hippocampal AMPA receptor GluR1 subunit via phosphorylating at Serine‐831/Serine‐845 sites through a sulfhydration‐dependent mechanism. CNS Neurosci Ther 22: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mani S, Wu L, Fu M, Shuang T, Xu C et al (2017). The interaction of estrogen and CSE/H2S pathway in the development of atherosclerosis. Am J Physiol Circ Physiol 312: H406–H414. [DOI] [PubMed] [Google Scholar]
- Liu X, Pan L, Zhuo Y, Gong Q, Rose P, Zhu Y (2010). Hypoxia‐inducible factor‐1α is involved in the pro‐angiogenic effect of hydrogen sulfide under hypoxic stress. Biol Pharm Bull 33: 1550–1554. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T et al (2014). Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell 15: 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohninger L, Tomasova L, Praschberger M, Hintersteininger M, Erker T, Gmeiner BMK et al (2015). Hydrogen sulphide induces HIF‐1α and Nrf2 in THP‐1 macrophages. Biochimie 112: 187–195. [DOI] [PubMed] [Google Scholar]
- López‐Requena, A. , Boonen, B. , Van Gerven, L. , Hellings, P.W. , Alpizar, Y.A. , and Talavera, K. (2017). Roles of neuronal TRP channels in neuroimmune interactions. In: Emir TLR. (ed.). Neurobiology of TRP Channels. 2nd edition Chapter 15. CRC Press/Taylor & Francis: Boca Raton (FL). [PubMed] [Google Scholar]
- Lu W, Li J, Gong L, Xu X, Han T, Ye Y et al (2014). H2 S modulates duodenal motility in male rats via activating TRPV1 and K (ATP) channels. Br J Pharmacol 171: 1534–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros JVR, Bezerra VH, Lucetti LT, Lima‐Júnior RCP, Barbosa ALR, Tavares BM et al (2012). Role of KATP channels and TRPV1 receptors in hydrogen sulfide‐enhanced gastric emptying of liquid in awake mice. Eur J Pharmacol 693: 57–63. [DOI] [PubMed] [Google Scholar]
- Miao L, Xin X, Xin H, Shen X, Zhu Y‐Z (2016). Hydrogen sulfide recruits macrophage migration by integrin β1‐Src‐FAK/Pyk2‐Rac pathway in myocardial infarction. Sci Rep 6: 22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamishima S, Bougaki M, Sips PY, De Yu J, Minamishima YA, Elrod JW et al (2009). Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3‐dependent mechanism in mice. Circulation 120: 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto R, Otsuguro K, Ito S (2011). Time‐ and concentration‐dependent activation of TRPA1 by hydrogen sulfide in rat DRG neurons. Neurosci Lett 499: 137–142. [DOI] [PubMed] [Google Scholar]
- Miyamoto R, Koike S, Takano Y, Shibuya N, Kimura Y, Hanaoka K et al (2017). Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Sci Rep 7: 45995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Módis K, Panopoulos P, Coletta C, Papapetropoulos A, Szabo C (2013). Hydrogen sulfide‐mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem Pharmacol 86: 1311–1319. [DOI] [PubMed] [Google Scholar]
- Morgan K, Sadofsky LR, Morice AH (2015). Genetic variants affecting human TRPA1 or TRPM8 structure can be classified in vitro as ‘well expressed’, ‘poorly expressed’ or ‘salvageable. Biosci Rep 35: e00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Tsugane M, Oka J‐I, Kimura H (2004). Hydrogen sulfide induces calcium waves in astrocytes. FASEB J 18: 557–559. [DOI] [PubMed] [Google Scholar]
- Nesuashvili L, Hadley SH, Bahia PK, Taylor‐Clark TE (2013). Sensory nerve terminal mitochondrial dysfunction activates airway sensory nerves via transient receptor potential (TRP) channels. Mol Pharmacol 83: 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Huang C, Zhong S, Wortley MA, Luo Y, Luo W et al (2016). Cigarette smoke extract (CSE) induces transient receptor potential ankyrin 1(TRPA1) expression via activation of HIF1αin A549 cells. Free Radic Biol Med 99: 498–507. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Takahashi K, Miura S, Imagawa T, Saito S, Tominaga M et al (2012). H2S functions as a nociceptive messenger through transient receptor potential ankyrin 1 (TRPA1) activation. Neuroscience 218: 335–343. [DOI] [PubMed] [Google Scholar]
- Okubo K, Matsumura M, Kawaishi Y, Aoki Y, Matsunami M, Okawa Y et al (2012). Hydrogen sulfide‐induced mechanical hyperalgesia and allodynia require activation of both Cav3.2 and TRPA1 channels in mice. Br J Pharmacol 166: 1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T‐T, Neo KL, Hu L‐F, Yong QC, Bian J‐S (2008). H2S preconditioning‐induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am J Physiol Physiol 294: C169–C177. [DOI] [PubMed] [Google Scholar]
- Patacchini R, Santicioli P, Giuliani S, Maggi CA (2004). Hydrogen sulfide (H2S) stimulates capsaicin‐sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol 142: 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen CE, Armache J‐P, Gao Y, Cheng Y, Julius D (2015). Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520: 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethő G, Reeh PW (2012). Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet‐activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev 92: 1699–1775. [DOI] [PubMed] [Google Scholar]
- Pichette J, Fynn‐Sackey N, Gagnon J (2017). Hydrogen sulfide and sulfate prebiotic stimulates the secretion of GLP‐1 and improves glycemia in male mice. Endocrinology 158: 3416–3425. [DOI] [PubMed] [Google Scholar]
- Pintér E, Helyes Z, Németh J, Pórszász R, Pethö G, Thán M et al (2002). Pharmacological characterisation of the somatostatin analogue TT‐232: effects on neurogenic and non‐neurogenic inflammation and neuropathic hyperalgesia. Naunyn Schmiedebergs Arch Pharmacol 366: 142–150. [DOI] [PubMed] [Google Scholar]
- Pintér E, Helyes Z, Szolcsányi J (2006). Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther 112: 440–456. [DOI] [PubMed] [Google Scholar]
- Pozsgai G, Hajna Z, Bagoly T, Boros M, Kemény Á, Materazzi S et al (2012). The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen‐sulphide‐induced CGRP‐release and vasodilation. Eur J Pharmacol 689: 56–64. [DOI] [PubMed] [Google Scholar]
- Pozsgai G, Payrits M, Sághy É, Sebestyén‐Bátai R, Steen E, Szőke É et al (2017). Analgesic effect of dimethyl trisulfide in mice is mediated by TRPA1 and sst4 receptors. Nitric Oxide 65: 10–21. [DOI] [PubMed] [Google Scholar]
- Prior M, Green F, Lopez A, Balu A, DeSanctis GT, Fick G (1990). Capsaicin pretreatment modifies hydrogen sulphide‐induced pulmonary injury in rats. Toxicol Pathol 18: 279–288. [DOI] [PubMed] [Google Scholar]
- Roskoski R (2015). Src protein‐tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res 94: 9–25. [DOI] [PubMed] [Google Scholar]
- Sághy É, Sipos É, Ács P, Bölcskei K, Pohóczky K, Kemény Á et al (2016). TRPA1 deficiency is protective in cuprizone‐induced demyelination – a new target against oligodendrocyte apoptosis. Glia 64: 2166–2180. [DOI] [PubMed] [Google Scholar]
- Shigemi Z, Furukawa Y, Hosokawa K, Minami S, Matsuhiro J, Nakata S et al (2016). Diallyl trisulfide induces apoptosis by suppressing NF‐κB signaling through destabilization of TRAF6 in primary effusion lymphoma. Int J Oncol 48: 293–304. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS (2012). TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT‐3. Nat Neurosci 15: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokoohinia Y, Chianese G, Appendino G, Di Marzo V, De Petrocellis L, Ghannadi A et al (2013). Some like it pungent and vile. TRPA1 as a molecular target for the malodorous vinyl disulfides from asafoetida. Fitoterapia 90: 247–251. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR et al (2003). ANKTM1, a TRP‐like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829. [DOI] [PubMed] [Google Scholar]
- Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt S‐E, Bevan S et al (2008). Distribution and function of the hydrogen sulfide–sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53: 391–400. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Green WD, Vinal K, Woodrum TS, Moore R, Negishi M (2011). Garlic extract diallyl sulfide (DAS) activates nuclear receptor CAR to induce the Sult1e1 gene in mouse liver. PLoS One 6: e21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulen A, Gullaksen S‐E, Bader L, McClymont DW, Skavland J, Gavasso S et al (2016). Signaling effects of sodium hydrosulfide in healthy donor peripheral blood mononuclear cells. Pharmacol Res 113: 216–227. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J (2014). Capsaicin and sensory neurones: a historical perspective. Prog Drug Res 68: 1–37. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Motohashi H, Yamamoto M (2011). Molecular mechanisms of the Keap1‐Nrf2 pathway in stress response and cancer evolution. Genes Cells 16: 123–140. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ohta T (2013). Inflammatory acidic pH enhances hydrogen sulfide‐induced transient receptor potential ankyrin 1 activation in RIN‐14B cells. J Neurosci Res 91: 1322–1327. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ohta T (2017). Membrane translocation of transient receptor potential ankyrin 1 induced by inflammatory cytokines in lung cancer cells. Biochem Biophys Res Commun 490: 587–593. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kozai D, Mori Y (2012). TRP channels: sensors and transducers of gasotransmitter signals. Front Physiol 3: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamizhselvi R, Koh Y‐H, Sun J, Zhang H, Bhatia M (2010). Hydrogen sulfide induces ICAM‐1 expression and neutrophil adhesion to caerulein‐treated pancreatic acinar cells through NF‐κB and Src‐family kinases pathway. Exp Cell Res 316: 1625–1636. [DOI] [PubMed] [Google Scholar]
- Tarui T, Fukami K, Nagasawa K, Yoshida S, Sekiguchi F, Kawabata A (2010). Involvement of Src kinase in T‐type calcium channel‐dependent neuronal differentiation of NG108‐15 cells by hydrogen sulfide. J Neurochem 114: 512–519. [DOI] [PubMed] [Google Scholar]
- Tay AS, Hu LF, Lu M, Wong PTH, Bian JS (2010). Hydrogen sulfide protects neurons against hypoxic injury via stimulation of ATP‐sensitive potassium channel/protein kinase C/extracellular signal‐regulated kinase/heat shock protein90 pathway. Neuroscience 167: 277–286. [DOI] [PubMed] [Google Scholar]
- Teicher C, De Col R, Messlinger K (2017). Hydrogen sulfide mediating both excitatory and inhibitory effects in a rat model of meningeal nociception and headache generation. Front Neurol 8: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y, Fujimura M, Nishimura S, Tsubota M, Sekiguchi F, Kawabata A (2015). Roles of Cav3.2 and TRPA1 channels targeted by hydrogen sulfide in pancreatic nociceptive processing in mice with or without acute pancreatitis. J Neurosci Res 93: 361–369. [DOI] [PubMed] [Google Scholar]
- Trevisan G, Hoffmeister C, Fortes Rossato M, Marchesan Oliveira S, Arnoldi Silva M, Regina Silva C et al (2014). TRPA1 receptor stimulation by hydrogen peroxide is critical to trigger hyperalgesia and inflammation in a model of acute gout. Free Radic Biol Med 72: 200–209. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N et al (2005). Hydrogen sulfide causes vanilloid receptor 1‐mediated neurogenic inflammation in the airways. Br J Pharmacol 145: 1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota‐Matsunami M, Noguchi Y, Okawa Y, Sekiguchi F, Kawabata A (2012). Colonic hydrogen sulfide‐induced visceral pain and referred hyperalgesia involve activation of both Cav3.2 and TRPA1 channels in mice. J Pharmacol Sci 119: 293–296. [DOI] [PubMed] [Google Scholar]
- Ujike A, Otsuguro K, Miyamoto R, Yamaguchi S, Ito S (2015). Bidirectional effects of hydrogen sulfide via ATP‐sensitive K+ channels and transient receptor potential A1 channels in RIN14B cells. Eur J Pharmacol 764: 463–470. [DOI] [PubMed] [Google Scholar]
- Ujike A, Kuraishi T, Yamaguchi S, Eguchi R, Kitano T, Kamise J et al (2018). IL‐1β augments H2S‐induced increase in intracellular Ca2+ through polysulfides generated from H2S/NO interaction. Eur J Pharmacol 821: 88–96. [DOI] [PubMed] [Google Scholar]
- Untereiner AA, Wang R, Ju Y, Wu L (2016). Decreased gluconeogenesis in the absence of cystathionine gamma‐lyase and the underlying mechanisms. Antioxid Redox Signal 24: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasas A, Dóka É, Fábián I, Nagy P (2015). Kinetic and thermodynamic studies on the disulfide‐bond reducing potential of hydrogen sulfide. Nitric Oxide 46: 93–101. [DOI] [PubMed] [Google Scholar]
- Viana F (2016). TRPA1 channels: molecular sentinels of cellular stress and tissue damage. J Physiol 594: 4151–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Tang W, Zhu YZ (2017). An update on AMPK in hydrogen sulfide pharmacology. Front Pharmacol 8: 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kobayashi K, Kogure Y, Yamanaka H, Yamamoto S, Yagi H et al (2018). Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic neuropathy. Diabetes 67: 98–109. [DOI] [PubMed] [Google Scholar]
- Wei X, Zhang B, Cheng L, Chi M, Deng L, Pan H et al (2015). Hydrogen sulfide induces neuroprotection against experimental stroke in rats by down‐regulation of AQP4 via activating PKC. Brain Res 1622: 292–299. [DOI] [PubMed] [Google Scholar]
- Weinhold P, Hennenberg M, Strittmatter F, Stief CG, Gratzke C, Hedlund P (2017). Transient receptor potential a1 (TRPA1) agonists inhibit contractions of the isolated human ureter. NeurourolUrodyn 37: 600–608. [DOI] [PubMed] [Google Scholar]
- White BJO, Smith PA, Dunn WR (2013). Hydrogen sulphide‐mediated vasodilatation involves the release of neurotransmitters from sensory nerves in pressurized mesenteric small arteries isolated from rats. Br J Pharmacol 168: 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild V, Messlinger K, Fischer MJM (2015). Hydrogen sulfide determines HNO‐induced stimulation of trigeminal afferents. Neurosci Lett 602: 104–109. [DOI] [PubMed] [Google Scholar]
- Wu B, Teng H, Yang G, Wu L, Wang R (2012). Hydrogen sulfide inhibits the translational expression of hypoxia‐inducible factor‐1α. Br J Pharmacol 167: 1492–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Luo N, Wang L, Zhao Z, Bu H, Xu G et al (2017). Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF‐κB signaling pathways. Sci Rep 7: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Tan G, He C, Gao Y, Pan S, Jiang H et al (2012). Hydrogen sulfide protects cardiomyocytes from myocardial ischemia‐reperfusion injury by enhancing phosphorylation of apoptosis repressor with caspase recruitment domain. Tohoku J Exp Med 226: 275–285. [DOI] [PubMed] [Google Scholar]
- Yassaka RT, Inagaki H, Fujino T, Nakatani K, Kubo T (2010). Enhanced activation of the transient receptor potential channel TRPA1 by ajoene, an allicin derivative. Neurosci Res 66: 99–105. [DOI] [PubMed] [Google Scholar]
- Yazğan Y, Nazıroğlu M (2017). Ovariectomy‐induced mitochondrial oxidative stress, apoptosis, and calcium ion influx through TRPA1, TRPM2, and TRPV1 are prevented by 17β‐estradiol, tamoxifen, and raloxifene in the hippocampus and dorsal root ganglion of rats. Mol Neurobiol 54: 7620–7638. [DOI] [PubMed] [Google Scholar]
- Yong QC, Lee SW, Foo CS, Neo KL, Chen X, Bian J‐S (2008). Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Circ Physiol 295: H1330–H1340. [DOI] [PubMed] [Google Scholar]
- Yong QC, Choo CH, Tan BH, Low C‐M, Bian J‐S (2010). Effect of hydrogen sulfide on intracellular calcium homeostasis in neuronal cells. Neurochem Int 56: 508–515. [DOI] [PubMed] [Google Scholar]
- Yu W, Liao Y, Huang Y, Chen SY, Sun Y, Sun C et al (2017). Endogenous hydrogen sulfide enhances carotid sinus baroreceptor Sensitivity by activating the transient receptor potential cation channel subfamily V member 1 (TRPV1) channel. J Am Heart Assoc 6: e004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Davis AT, Ahmed K (1998). Mechanism of protein kinase CK2 association with nuclear matrix: role of disulfide bond formation. J Cell Biochem 69: 211–220. [PubMed] [Google Scholar]
- Zhao J‐F, Shyue S‐K, Kou YR, Lu T‐M, Lee T‐S (2016). Transient receptor potential ankyrin 1 channel involved in atherosclerosis and macrophage‐foam cell formation. Int J Biol Sci 12: 812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zhu H‐Y, Zhang X‐Y, Wang M, Xiao Y, Xu G‐Y et al (2016). Upregulation of cystathionine β‐synthetase in the arcuate nucleus produces pain hypersensitivity via PKC upregulation and GluN2B phosphorylation in rats with chronic pancreatitis. Sheng Li Xue Bao 68: 575–584. [PubMed] [Google Scholar]
- Zimova L, Sinica V, Kadkova A, Vyklicka L, Zima V, Barvik I et al (2018). Intracellular cavity of sensor domain controls allosteric gating of TRPA1 channel. Sci Signal 11: eaan8621. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Högestätt ED (2014). TRPA1. Handb Exp Pharmacol 222: 583–630. [DOI] [PubMed] [Google Scholar]


