Neutrophils, the most numerous leukocytes, play an important role in maintaining oral health through interactions with oral microbial biofilms. Both neutrophil hyperactivity and the bacterial subversion of neutrophil responses can cause inflammation-mediated tissue damage like that seen in periodontal disease.
KEYWORDS: biofilm, CD markers, commensal bacteria, immunology, microbiology, neutrophil, pathogens
ABSTRACT
Neutrophils, the most numerous leukocytes, play an important role in maintaining oral health through interactions with oral microbial biofilms. Both neutrophil hyperactivity and the bacterial subversion of neutrophil responses can cause inflammation-mediated tissue damage like that seen in periodontal disease. We describe here an assay that assesses neutrophil activation responses to monospecies biofilm bacteria in vitro based on the surface expression of cluster of differentiation (CD) markers associated with various neutrophil functions. Most of what we know about neutrophil responses to bacteria is based on in vitro assays that use planktonic bacteria and isolated/preactivated neutrophils, which makes interpretation of the neutrophil responses to bacteria a challenge. An understanding of how neutrophils differentially interact with and respond to commensal and pathogenic oral bacteria is necessary in order to further understand the neutrophil’s role in maintaining oral health and the pathogenesis of periodontal disease. In this study, a flow cytometry-based in vitro assay was developed to characterize neutrophil activation states based on CD marker expressions in response to oral monospecies bacterial biofilms. Using this approach, changes in CD marker expressions in response to specific prominent oral commensal and pathogenic bacteria were assayed. Several functional assays, including assays for phagocytosis, production of reactive oxygen species, activation of the transcription factor Nrf2, neutrophil extracellular trap formation, and myeloperoxidase release, were also performed to correlate neutrophil function with CD marker expression. Our results demonstrate that neutrophils display bacterial species-specific responses. This assay can be used to characterize how specific biofilms alter specific neutrophil pathways associated with their activation.
INTRODUCTION
Neutrophils are constitutively recruited to the oral cavity, where they play an important role in innate immune regulation and maintenance of oral health in response to the oral microbial biofilm (1–4). A recent study showed that the oral tissue neutrophil phenotypes associated with healthy individuals are distinct from those associated with individuals suffering from chronic periodontal (CP) disease (5). These phenotypes are defined by the surface expression signature of their cell surface pattern recognition receptors, which recognize the conserved motifs of offending pathogens (6). Via a mechanism called priming, induced by the cytokines and chemokines that bring the conserved motifs to the neutrophils, many of these receptors, resident in the cytoplasm’s secretory vesicles (7), are brought to the membrane surface. Neutrophils are also primed as they traverse the epithelial junctions while en route to the infected site (8). The contact of the pathogen or its products with the appropriate surface receptors triggers neutrophil activation responses, including phagocytosis, the production of reactive oxygen species (ROS), and the release of the antibacterial contents of its granules (6). A number of these receptors and other cell surface molecules have been categorized as cluster of differentiation (CD) markers, and certain of these have been associated with and reflect the activation of neutrophil functions, including phagocytosis, adhesion, migration, and degranulation. It has been shown (5) that oral neutrophils from CP patients, termed proinflammatory neutrophils, display elevated expression of an array of CD markers associated with phagocytosis, increased ROS production, and elevated neutrophil extracellular trap (NET) formation compared to those from healthy patients. Therefore, these markers reflect neutrophil activation states, and their relative magnitudes are indicative of the magnitude of the activation response, thus defining the phenotypes. In proinflammatory oral neutrophils, the overall CD marker signature (CD11b, CD66, CD63, CD64, CD55, CD14, CD18) displays higher surface expression, indicating that neutrophil phenotypes are changed from parainflammatory in the healthy state to proinflammatory in the diseased patient. We believe that this phenotype switch is due at least in part to the altered oral microbiome associated with periodontal disease.
Since the microbial community is altered in the transition from oral health to periodontal disease, we postulate that the microbiome, by inducing changes in cell surface receptor expression, plays a significant role in regulating the neutrophil phenotypes in oral health and disease. This role is significant, as neutrophil-mediated inflammation has been shown to play an important role in the pathogenesis of periodontitis (9).
In this study, we describe an in vitro assay that allows us to monitor neutrophil phenotype modifications, as determined by their CD marker signatures following their interaction with monospecies biofilms. This in vitro assay is based on a previously described flow cytometric assay looking at eight neutrophil CD markers associated with neutrophil function to characterize human neutrophil phenotypes in health and periodontal disease (5). The markers employed were CD66, CD11b, and CD18 for expression levels of adhesion, CD63 for degranulation, CD14 for lipopolysaccharide (LPS) binding, CD55 for complement inhibition, and CD16 and CD64 for Fcγ receptors (FcγR). In addition, phagocytosis, ROS production, NET formation, and myeloperoxidase (MPO) release were also assayed. Finally, activation of the nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor that promotes antioxidant pathways to protect neutrophils from ROS-mediated damage, was assessed (10). We hypothesized (i) that neutrophil phenotype changes from the baseline induced by specific oral bacteria can be detected in vitro via their CD marker expressions and (ii) that neutrophil functions are differentially activated by specific oral commensal and pathogenic bacteria, as shown by their CD marker expression patterns, phagocytosis, ROS production, Nrf2 activation, NETosis, and MPO release.
Up to now, little research has been done on the effects of bacterial biofilms on the modification of neutrophil phenotypes. We believe that we are the first to study the specific effects of major oral commensal bacterial biofilms and keystone pathogens on neutrophils through assessment of CD marker signatures. These results provide a gateway to further investigate the relevance of specific neutrophil CD marker changes associated with oral diseases, such as caries and periodontitis. This points to a new tool that can be used to further the understanding of host innate immune system-microbe dynamics during health-to-oral disease transitions.
RESULTS
It has been previously shown that not all neutrophils in the oral cavity display similar levels of cell surface CD marker expression (5, 11, 12). In the healthy oral cavity, neutrophils are in a parainflammatory state, whereas in a diseased cavity, neutrophil phenotypes are changed to a proinflammatory state, with neutrophils in the parainflammatory and proinflammatory states expressing low levels and high levels of CD markers, respectively (5). We hypothesized that this neutrophil phenotype switch is due to the altered oral microbiome in periodontal disease. Therefore, we tested the effect of several of the dominant oral bacteria affecting the neutrophil phenotypes in order to confirm this assumption.
Conventional isolation of neutrophils by density gradient centrifugation induced changes in cell surface CD marker expression.
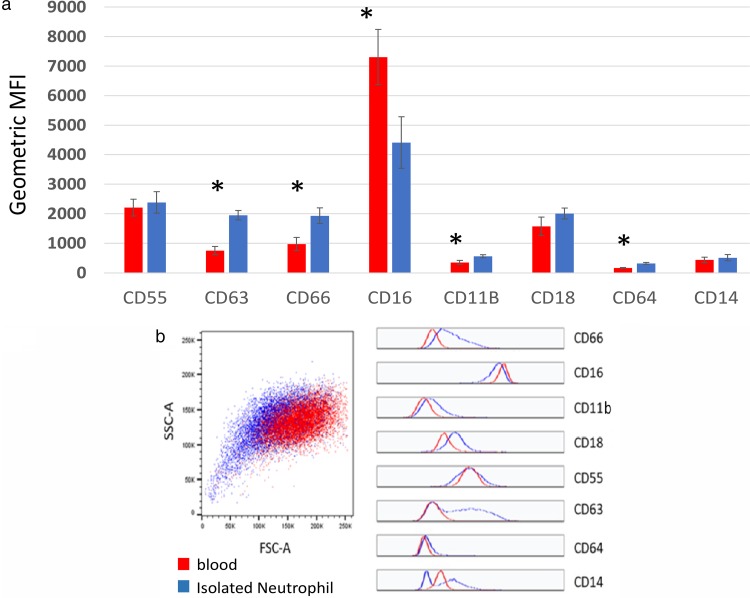
In order to determine whether neutrophils respond differently to specific oral commensal and pathogenic biofilms, we used a previously described assay (5) to assess the surface expression of specific CD markers on human neutrophils. Because neutrophils have been known to be highly sensitive to activation ex vivo and are thus easily activated by in vitro manipulation (13), we tested cell surface CD marker expression on whole-blood neutrophils and neutrophils isolated by standard density gradient centrifugation using 1-step Polymorphs solution (Fig. 1a). We found that neutrophil surface expressions of CD63, CD66, CD16, CD11b, and CD64 were significantly altered by the isolation procedure itself. Specifically, CD16 expression was reduced, while the other markers were upregulated, consistent with an activated neutrophil phenotype (5). In addition, it was noted (Fig. 1b) that the isolation procedures induced greater variation within the population for each CD marker and also in the forward scatter (FSC) and side scatter (SSC) compared to that for whole blood, confirming procedure-induced perturbations in cell activation. To avoid this artifact of ex vivo manipulation involved in neutrophil isolation, we used whole blood, which would also be more relevant to what is happening in the mouth, in subsequent experiments for the assessment of the effects of the biofilm on neutrophil CD marker expression. With our flow cytometry gating strategy and sorting methods, we ensured that neutrophils were the only leukocytes tested and that the expression levels from CD markers were only from neutrophils.
FIG 1.
Upregulation of neutrophil CD markers is induced by isolation procedures. Whole blood or neutrophils isolated by conventional gradient centrifugation procedures were fixed and analyzed by multicolor flow cytometry. (a) The mean MFI ± SEM is shown for each marker. Significant differences were determined by paired Student's t test (n = 5). *, P ≤ 0.05. (b) The isolation procedures induced a greater variation within the population for each CD marker and also in the forward scatter and side scatter than it did with whole blood, confirming procedure-induced perturbations in cell activation.
Neutrophil CD marker expression levels differed in response to specific commensal and pathogenic bacterial biofilms.
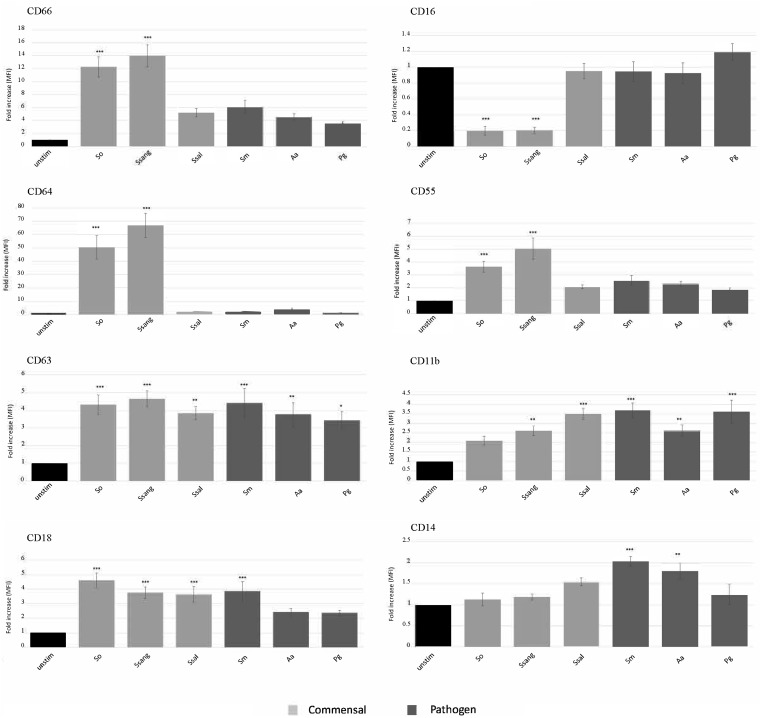
To determine the effect of infection by the various bacteria, using our in vitro assay, we incubated 100 μl of whole blood with six different monospecies biofilms (Streptococcus oralis, S. sanguinis, S. salivarius, S. mutans, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis). Samples were then prepared for flow cytometry as described in Materials and Methods, neutrophils were gated to ensure that all other cells were excluded, and the mean fluorescent intensity (MFI) of each CD marker was measured using flow cytometry. Of the three commensal strains, S. oralis and S. sanguinis induced the surface upregulation of CD66, CD64, CD55, CD63, and CD18 and the downregulation of CD16 compared to their regulation in the unstimulated controls. CD63 and CD18 were upregulated by S. salivarius, while CD11b was significantly upregulated by S. sanguinis and S. salivarius. None of the commensal strains induced neutrophil upregulation of the LPS receptor, CD14 (Fig. 2). The surface expressions of CD66, CD64, CD55, and CD16 were not significantly altered from that at the baseline by the oral bacterial pathogens. However, the neutrophil surface expression of CD14 was upregulated by the pathogens S. mutans and A. actinomycetemcomitans, and all three pathogenic bacteria induced neutrophil upregulation of CD63 and CD11b. Lastly, S. mutans upregulated CD18 expression (Fig. 2).
FIG 2.
Neutrophil CD markers are upregulated differentially by monospecies biofilms of commensal and pathogenic bacteria. Human blood was incubated with commensal and pathogenic bacterial biofilms for 1 h at 37°C. The fold increase of surface CD marker expressions of gated neutrophil populations relative to that for unstimulated controls was determined. The mean fold increase ± SEM is shown for each CD marker (n = 9). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. Abbreviations: unstim, unstimulated; So, S. oralis; Ssang, S. sanguinis; Ssal, S. salivarius; Sm, S. mutans; Aa, A. actinomycetemcomitans; Pg, P. gingivalis.
Neutrophil CD marker expression response to planktonic bacteria.
The purpose of this study was to determine the effects of bacterial biofilms on neutrophils. However, since much of the literature deals with planktonic bacteria, for comparison purposes, the responses of S. oralis, A. actinomycetemcomitans, and P. gingivalis at a multiplicity of infection (MOI) of 20 were compared to the monospecies biofilm responses of these bacteria under the same conditions. All bacteria were grown on agar plates. S. oralis was grown at 37°C with 5% CO2, whereas the other two bacterial species were grown under anaerobic conditions. S. oralis planktonic bacteria induced increased expression of CD64 and decreased expression CD11b and CD16 compared with their expression by the biofilm form (see Fig. S2a in the supplemental material). Planktonic A. actinomycetemcomitans increased CD64 to a greater extent than the biofilm form (Fig. S2b). There was greater neutrophil activation of CD63, CD66, and CD64 by planktonic P. gingivalis than by its biofilm form (Fig. S2c).
Neutrophil CD marker expression levels in response to mixed biofilms.
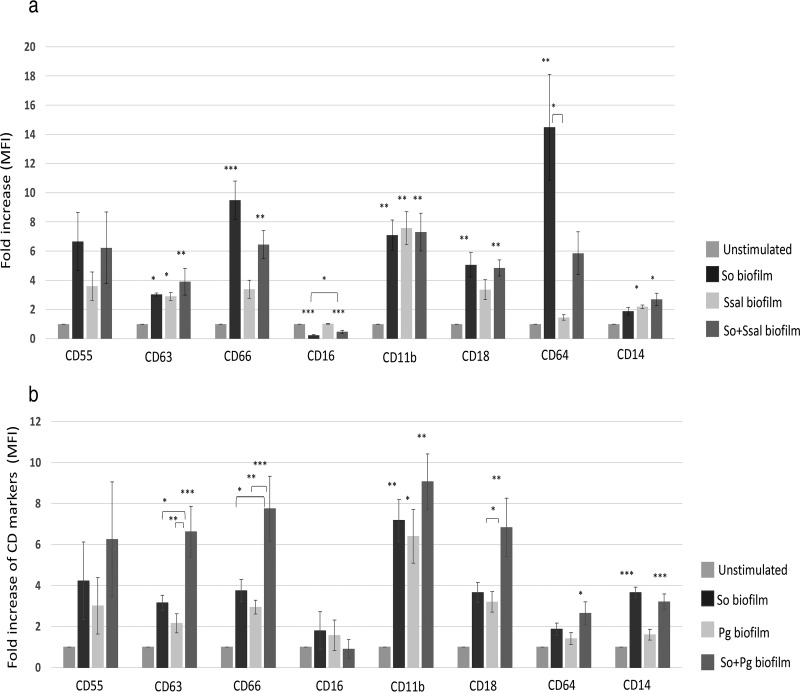
Mixed biofilms of S. oralis and S. salivarius as well as mixtures of S. oralis and P. gingivalis were grown aerobically and anaerobically, respectively, as described previously. All the bacteria were grown individually, and for the mixed biofilms, bacteria were grown in equal proportions. The results for each bacterial mixture were compared to those for the monospecies grown under the same conditions. The results showed that although S. oralis activated neutrophils more than S. salivarius as monospecies biofilms, the mixed biofilm resulted in less upregulation of CD64 and more upregulation of CD14 and CD16 than the S. oralis biofilm (Fig. 3a). However, the mixed biofilm of S. oralis and P. gingivalis showed more activation of neutrophils than the monospecies P. gingivalis biofilm, as indicated by the regulation of the CD63, CD66, and CD18 markers (Fig. 3b).
FIG 3.
Mixed biofilms of S. oralis and S. salivarius result in a lower level of neutrophil activation than the monospecies biofilm of S. oralis, whereas the biofilm mixture of S. oralis and P. gingivalis results in a higher level of neutrophil activation than the monospecies biofilm of S. oralis. Each sample was incubated with 100 μl human whole blood for 1 h. Flow cytometry was performed, and the mean fold increase ± SEM is shown for each CD marker (n = 3). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. (a) The activation of neutrophils through CD16 and CD64 is higher by S. oralis than by S. salivarius. However, in mixed biofilms, this expression is reduced. (b) There is greater neutrophil activation through CD63, CD66, and CD18 by a mixed biofilm of S. oralis and P. gingivalis than by the anaerobically grown monospecies S. oralis. Abbreviations: So, S. oralis; Ssal, S. salivarius; Pg, P. gingivalis.
S. oralis induced neutrophil ROS production and Nrf2 activation.
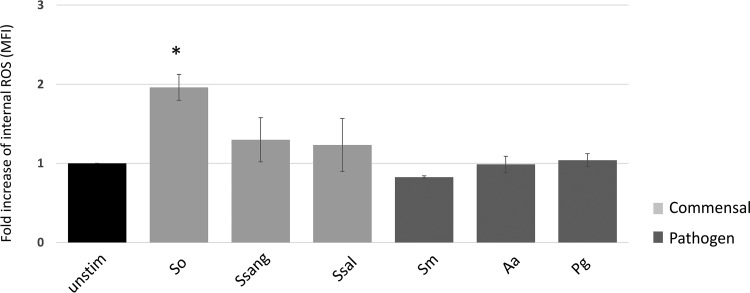
In order to test the functional parameters of human blood neutrophils in response to commensal and pathogenic oral biofilms, we assessed reactive oxygen species (ROS) production and activation of the nuclear antioxidant factor Nrf2. Monospecies biofilms of bacteria were made as described in the Materials and Methods section, and flow cytometry and Western blotting were performed to detect ROS and Nrf2 activation, respectively. We found that the S. oralis biofilm induced a 2-fold increase in ROS production in neutrophils, while the other bacteria had no significant effect (Fig. 4). Assays for activation of the neutrophil antioxidant transcription factor Nrf2 in response to S. oralis, S. salivarius, and A. actinomycetemcomitans confirmed that only S. oralis caused a significant increase in Nrf2 activation through its translocation to the nucleus (Fig. 5).
FIG 4.
Only the S. oralis biofilm induces neutrophil ROS production. Isolated human blood neutrophils were incubated with the biofilms for 1 h at 37°C. The fold increase in the level of intracellular ROS production relative to that for unstimulated controls was determined (n = 4). *, P ≤ 0.05. Abbreviations: unstim, unstimulated; So, S. oralis; Ssang, S. sanguinis; Ssal, S. salivarius; Sm, S. mutans; Aa, A. actinomycetemcomitans; Pg, P. gingivalis.
FIG 5.
S. oralis biofilm induces nuclear translocation/activation of Nrf2. Isolated human blood neutrophils were incubated with biofilms for 1 h at 37°C. The densitometry of nuclear Nrf2 was determined by Western blotting. Cytoplasmic Nrf2 was also detected, and there were no significant differences between the samples. Actin was used as a protein control (n = 4). **, P ≤ 0.01. Abbreviations: unstim, unstimulated; So, S. oralis; Ssal or Sal, S. salivarius; Aa, A. actinomycetemcomitans.
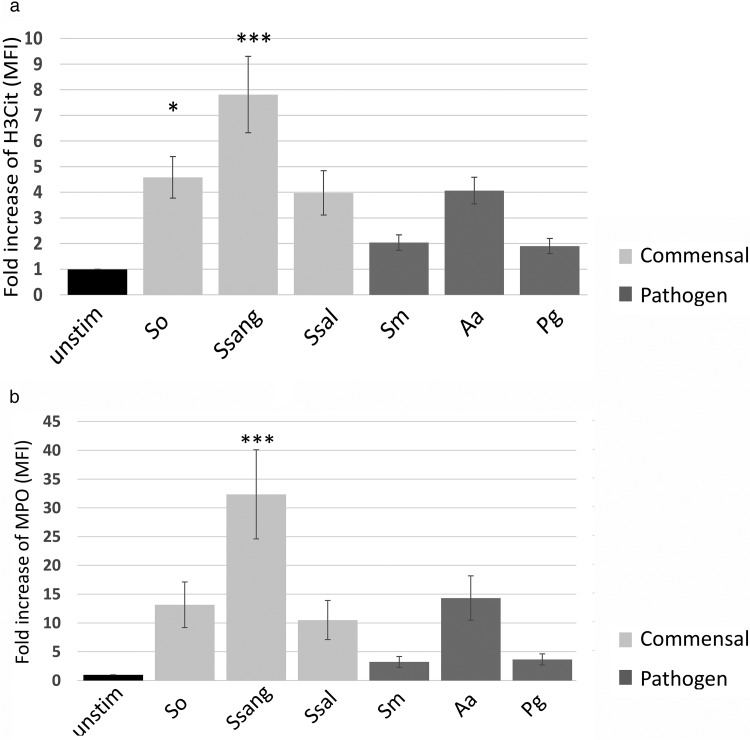
S. oralis and S. sanguinis induced elevated NET formation.
To further assess the functional responses to oral biofilms, we assessed neutrophil extracellular trap (NET) formation and MPO release. Neutrophil-associated histone H3 citrullination (H3Cit) and myeloperoxidase (MPO) release to the neutrophil surface were assessed by flow cytometry. We found that two of the commensal biofilms, those of S. oralis and S. sanguinis, induced a significant upregulation of H3Cit on the neutrophils, consistent with NET production (5) (Fig. 6a). Only S. sanguinis induced the upregulation of neutrophil-associated MPO compared to that in the unstimulated controls, consistent with degranulation (Fig. 6b).
FIG 6.
S. oralis and S. sanguinis induce expression of the NETosis marker H3Cit. Only S. sanguinis results in a significant release of MPO. Human blood neutrophils were incubated with biofilms for 1 h at 37°C. Using flow cytometry, the fold increase in the levels of surface H3Cit (a) and MPO (b) on gated neutrophil populations relative to that for unstimulated controls was determined (n = 6). *, P ≤ 0.05; ***, P ≤ 0.001. Abbreviations: unstim, unstimulated; So, S. oralis; Ssang, S. sanguinis; Ssal, S. salivarius; Sm, S. mutans; Aa, A. actinomycetemcomitans; Pg, P. gingivalis.
DISCUSSION
In this study, we describe a newly developed flow cytometric assay which is based on assessment of the upregulation of CD markers that define neutrophil phenotypes in response to oral monobacterial biofilms. Based on neutrophil surface expression and functional assays, such as assays for ROS production, Nrf2 activation, NETosis, and MPO release, we show that S. oralis and S. sanguinis biofilms induce increased neutrophil cell surface CD marker expression compared to the biofilms of pathogens.
This novel assay allowed us to focus on the different responses of neutrophil phenotypes to specific bacterial biofilms, and we selected clinically relevant and commonly studied oral commensal and pathogenic bacteria. As this is a study on oral biofilms implicated in periodontal health and disease, our rationale for the choice of bacteria was as follows: we chose two predominant commensal bacteria in health-associated oral biofilms, S. oralis and S. sanguinis (14), plus the commensal S. salivarius, because it is a major constituent of the microflora of the oral cavity and is a known inhibitor of the opportunistic pathogen S. mutans (15). Since we are interested in the pathogenesis of periodontal disease, we chose the keystone pathogen P. gingivalis, which is associated with chronic periodontal disease (16). A. actinomycetemcomitans was selected as it is strongly associated with aggressive periodontitis (17) and produces a leukotoxin that specifically targets human neutrophils (18). The opportunistic pathogen S. mutans was included because of its role in dental caries (19) and because it has the ability to induce neutrophil chemotaxis (20), utilize its virulence factors to develop a formidable biofilm (21), and evade neutrophil killing (22).
Our assays utilized whole blood.
A comparison of the upregulation of neutrophil CD marker expressions using whole blood and neutrophils isolated from blood was made. Consistent with the findings of many studies (23–25), isolation procedures were shown to induce the activation of neutrophils in terms of both the mean fluorescent intensity (MFI) and the mean expression distribution for each CD marker (Fig. 1). In addition, we confirmed here that neutrophils are highly sensitive cells that are easily activated by the in vitro manipulation associated with their isolation from blood. Thus, whole blood instead of the initially collected neutrophils was used in our assays to avoid minimizing the absolute increase in activation that would occur in cells due to their preactivation during isolation. To ensure that our CD marker assay results were specific to neutrophils, we ensured that we restricted our data acquisition to granulocytes using the SSC area (SSC-A) by FSC area (FSC-A) and then gated on neutrophils by using CD16hi and CD66hi in our gating strategy. To confirm this, the cells were sorted using a BD FACSAria cell sorter on medium pressure, and it was confirmed that only neutrophils were selected.
Some more than other commensal bacteria upregulate CD expression.
Most reports studying neutrophil-bacterium interactions use experiments with planktonic bacteria (26–32). For example, Hirschfeld et al. (28) assayed neutrophil responses to 19 planktonic bacteria. They found that only a subset of bacteria induced increased ROS production and NET release. This is consistent with our results that neutrophils respond differently to specific bacterial species. However, considering that 65% of microbial infections are related to biofilms (33), not enough study has been done on neutrophil-biofilm interactions. We therefore focused on neutrophil-biofilm interactions which would be more relevant to periodontal disease. There have been some studies on neutrophil interactions with Staphylococcus and Pseudomonas aeruginosa biofilms (34, 35) but not on those with the most prevalent oral bacteria in healthy and diseased oral cavities.
To our knowledge, this is the first study of neutrophil-oral biofilm interactions using neutrophil CD marker expression levels to determine neutrophil phenotypes. This assists in determining the differences in neutrophil responses to the various biofilms. This study demonstrates that specific commensal bacterial biofilms possess the ability to induce higher neutrophil CD marker expressions and functional outputs than the biofilms of key oral pathogens. These findings support the hypothesis that neutrophils are activated by specific oral commensal bacteria as part of a normal healthy innate immune response. The neutrophil phenotype determined by the lower expression of the CD marker signature in healthy oral cavities, termed the parainflammatory neutrophil phenotype, likely facilitates efficient management of the oral biofilm without causing excessive collateral tissue damage (5).
One of the explanations for the greater upregulation of neutrophil cell surface receptors by S. sanguinis and S. oralis than by S. salivarius may be their increased production of H2O2, which stimulates phagocytosis and the subsequent upregulation of surface receptors (36). In contrast, S. salivarius inhibits the neutrophil response by the downregulation of the proinflammatory NF-κB pathway (37). Regarding the difference between S. sanguinis and S. oralis, S. sanguinis exhibits slightly greater upregulation of some markers, possibly due to its expression of a unique cell wall nuclease, SWAN, which digests the DNA scaffolding of NETs, thus attenuating the bactericidal activity of NETs and decreased bacterial killing (38).
Pathogenic bacteria do not upregulate the cell surface markers, and the oral pathogens tend to inhibit neutrophil FcγR-dependent phagocytosis.
CD64 and CD16 are members of the Fcγ receptor family of receptors for phagocytosis (39). Based on the findings of previous studies, the expression of CD64 on the surface of neutrophils is an indicator of phagocytic activity (40). It has also been shown that CD16 sheds off neutrophil membranes when cells are activated (41). Our results show that, as opposed to the commensals, the pathogens did not alter the FcγR-dependent phagocytosis, based on CD16 and CD64 expression. This is consistent with recent studies showing that these pathogens inhibit phagocytosis (2, 42–44). For example, P. gingivalis gingipains cleave complement C5, producing high levels of C5a, which coactivate the C5a receptor and Toll-like receptor 2 (TLR2), leading to the activation of phosphatidylinositol 3-kinase and the inhibition of neutrophil phagocytosis (2). Additionally, Kgp, a Lys-specific gingipain, can degrade IgG1 and IgG3 to inhibit opsonization and, consequently, phagocytosis (42). While its effect on neutrophils has not yet been tested, the virulence factor known as cytolethal distending toxin in A. actinomycetemcomitans has been shown to inhibit phagocytosis in macrophages (43). Another study on macrophages has shown that the surface lipoprotein PpiA of S. mutans also results in a reduction of phagocytosis (44). The inhibition of phagocytosis by these bacteria is reflected in our FcγR-mediated phagocytosis markers, CD16 and CD64, which indicate the activation of neutrophil phagocytosis (45). To confirm that the phagocytosis activity is greater with S. oralis and S. sanguinis, we performed a phagocytosis assay by labeling bacteria with pHrodo Red. The result is consistent with what was shown with CD16 and CD64, confirming that there is a significant increase in neutrophil phagocytosis when neutrophils are incubated with S. oralis and S. sanguinis (Fig. 7). The inhibition of the neutrophil response to pathogenic bacteria is consistent with the findings of previous studies which have shown that the virulence factors of S. mutans, A. actinomycetemcomitans, and P. gingivalis interfere with neutrophil bactericidal activities (46, 47), chemotaxis (48), and other neutrophil responses to bacteria (49).
FIG 7.
S. oralis and S. sanguinis induce neutrophil phagocytosis. Biofilms of monospecies were labeled with pHrodo Red for 1 h. Neutrophils were then incubated with labeled bacteria for 1 h at 37°C. The mean fluorescent intensity of pHrodo Red was measured using flow cytometry. S. oralis and S. sanguinis resulted in more neutrophil phagocytosis (n = 3). *, P ≤ 0.05; **, P ≤ 0.01. Abbreviations: unstim, unstimulated; So, S. oralis; Ssang, S. sanguinis; Ssal, S. salivarius; Sm, S. mutans; Aa, A. actinomycetemcomitans; Pg, P. gingivalis.
The LPS marker (CD14) was upregulated by some pathogens.
We found that pathogenic bacterial strains, including S. mutans, A. actinomycetemcomitans, and P. gingivalis, displayed different patterns of neutrophil cell surface marker expression. The LPS receptor CD14 was upregulated only by A. actinomycetemcomitans and S. mutans. Surface upregulation of CD14 by A. actinomycetemcomitans is expected, as it is a Gram-negative bacterium, which therefore binds LPS to its outer membrane. Although S. mutans is a Gram-positive bacterium, its upregulation of CD14 can be attributed to the presence of surface lipoteichoic acid (LTA), which binds to CD14/TLR2 in macrophages and may have a similar interaction with neutrophils (50). On the other hand, although P. gingivalis is a Gram-negative bacterium, it expresses gingipains, virulence factors that selectively cleave CD14 (51). This may have inhibited the upregulation of the LPS marker, CD14, and supports our finding. This is also in agreement with the findings of several studies involving monocytes/macrophages as well as neutrophils (51–53). Two of the studies used purified gingipains, while the third employed whole bacteria, with similar results. We also confirmed this in our system using two P. gingivalis mutants lacking gingipain expression: KDP131 (Arg-gingipain A) and KDP132 (Arg-gingipain B). The results showed the upregulation of CD14 with these two mutants lacking gingipain but not with wild-type P. gingivalis (Fig. 8).
FIG 8.
P. gingivalis lacking gingipains activates neutrophils through CD14, whereas P. gingivalis does not. This suggests that gingipains play an important role in cleaving CD14. Human blood was incubated with P. gingivalis and P. gingivalis gingipain mutant (KDP131 and KDP132) biofilms for 1 h at 37°C. The fold increase in the level of surface CD14 expressions of gated neutrophil populations relative to that for unstimulated controls was determined. The mean fold increase ± SEM is shown for CD14 (n = 4). ***, P ≤ 0.001. Abbreviations: unstim, unstimulated; Pg, P. gingivalis; KDP131, P. gingivalis Arg-gingipain A mutant; KDP132, P. gingivalis Arg-gingipain B mutant.
Planktonic bacteria induce greater CD marker expression on neutrophils than on biofilms.
For this study, our focus was on the effect of bacterial biofilms on neutrophils. A dose-response is not possible with biofilms. However, most studies of neutrophils and bacteria have been conducted with planktonic bacteria. So, for comparison, planktonic bacteria of S. oralis, A. actinomycetemcomitans, and P. gingivalis were compared to their biofilm equivalents. Planktonic bacteria showed greater neutrophil CD marker expression than the biofilm bacteria. Prior studies have shown that neutrophils respond differentially to planktonic and biofilm bacteria and that biofilm bacteria can evade host defenses more than planktonic bacteria (54). In addition, the dose-responses to S. oralis and A. actinomycetemcomitans were assayed, and the results showed that the CD marker expression dosage increased (data not shown). These results highlight the importance of studying neutrophil responses to bacteria in biofilms, which are closer to the oral format that exists in the oral cavity.
The response of neutrophils to mixed biofilms is varied.
The mixed biofilm of S. oralis with S. salivarius resulted in less CD64 expression and more CD14 and CD16 expression, whereas the mixture of S. oralis with P. gingivalis showed greater neutrophil expression of the CD markers CD63, CD66, and CD18. This can be explained by the monospecies P. gingivalis biofilm inhibiting neutrophil activation, while under the dysbiotic condition with S. oralis and P. gingivalis interacting, P. gingivalis inhibition was reduced. These results highlight the value of using this assay in the future to study the complex nature of mixed biofilms in activating neutrophils.
The commensal S. oralis increased ROS production but limited its damage by activation of Nrf2.
The infection by S. oralis induced increased intracellular ROS production in neutrophils. However, neutrophils can limit excessive exposure of healthy biofilms and tissues to ROS by the activation of Nrf2, a transcription factor that regulates antioxidant expression. Neutrophils are known to express elevated levels of several antioxidant genes in a healthy periodontium (55). This characteristic may explain the enhanced tolerance of neutrophils to commensal biofilms (56). On the other hand, in the setting of periodontal disease, it has been shown that there is less activation of Nrf2 and, therefore, an excessive net release of ROS (10). Our in vitro results, determined by utilizing one representative commensal and one representative pathogen, confirmed that Nrf2 is activated by S. oralis but not by A. actinomycetemcomitans. A previous study (57) showed that the upregulation of ROS by S. oralis can cause a TLR2-dependent oxidative response. This agrees with studies that showed that neutrophils activated by S. oralis can generate the cytokine interleukin 8 (IL-8), activating additional neutrophils (58) to generate more ROS (59), which is consistent with the observed increase in ROS production. In our study, as expected, there was increased localization of the transcription factor Nrf2 to the nucleus when neutrophils were infected by S. oralis. In the setting of periodontal disease, it has been shown that there is less activation of Nrf2 and, therefore, an excessive net release of ROS (10, 56).
Because S. oralis infection causes the production of intracellular ROS from neutrophils while other mitis group commensals do not, we wanted to confirm that higher intracellular ROS results in higher Nrf2 activation. Therefore, we tested only three bacteria: one shown to yield high levels of ROS production (S. oralis) and two shown to yield low levels of ROS production (S. salivarius and A. actinomycetemcomitans). Our results demonstrated that Nrf2 is activated by S. oralis but not by S. salivarius or A. actinomycetemcomitans, consistent with the observed increase in ROS production. The upregulation of ROS by S. oralis is consistent with the work of Xu et al. (57), which showed that a TLR2-dependent oxidative response can be caused by S. oralis.
Our results demonstrate that neutrophils have a higher response to two of the commensal monospecies biofilms (S. oralis and S. sanguinis), based on the expression of specific CD markers and functional assays, and therefore have the potential to produce ROS and NETs in destructive amounts. However, we have shown that in healthy oral situations, these bacteria also inhibit this damage by inducing the upregulation of CD55 and Nrf2. Upregulation of the decay-accelerating factor (CD55) protects the neutrophils from complement-mediated destruction, while Nrf2 promotes antioxidant pathways and, thus, increases neutrophil survival in the inflamed oral cavity. This response to S. oralis and S. sanguinis indicates a parainflammatory response where neutrophils eliminate bacteria without causing inflammation (5). We believe that this is the normal state when the neutrophils have a response to a healthy plaque in which the number of Gram-negative pathogens is low.
This study has several limitations that must be kept in mind: (i) this was an in vitro-based study employing the conditions of bacterial growth recommended by the ATCC website, which does not mimic the exact oral conditions, (ii) while the oral cavity has a great many bacteria, in this study, we used only six monospecies biofilms and three bacteria for mixed biofilms, and (iii) whole blood was used, since the collection of neutrophils prior to infection with conventional isolation procedures activates the neutrophils. Our current work describes a new assay that will allow us to gain new insights and a better understanding of the complex nature of biofilm-neutrophil interactions.
MATERIALS AND METHODS
Ethics statement.
Human peripheral blood was drawn from healthy volunteers. The study was approved by the University of Toronto’s Research Ethics Board (30044, 29410). Signed consent was obtained from all participants.
Bacterial strains and culture conditions.
S. oralis (ATCC 10577), S. sanguinis (ATCC 10556), S. salivarius (ATCC 13419), and S. mutans (UA159) were grown aerobically for 24 h. A. actinomycetemcomitans (SUNY465) and P. gingivalis (ATCC 33277) were grown in an anaerobic chamber containing 90% nitrogen, 5% hydrogen, 5% carbon dioxide for 24 h and 48 h, respectively. The growth conditions and media for all these wild-type strains are listed in Table S1 in the supplemental material. We confirmed biofilm growth and thickness through the crystal violet staining method. All bacterial strains except P. gingivalis were obtained from the D. Cvitkovich lab at the Faculty of Dentistry, University of Toronto. P. gingivalis was purchased from ATCC.
Biofilm formation.
Bacterial cultures of S. oralis, S. sanguinis, and S. mutans were grown overnight at 37°C in 5% CO2. S. salivarius was grown overnight at 37°C without CO2. The cultures of A. actinomycetemcomitans and P. gingivalis were grown in the anaerobic chamber at 37°C for 24 h and 48 h, respectively. Different growth conditions for each bacterial biofilm were tested, and the most suitable ones for each strain were chosen, as follows. For biofilm formation of S. oralis, S. sanguinis, S. mutans, and A. actinomycetemcomitans, overnight cultures of each bacterium were diluted 1:100 in its own fresh medium. To ensure that the biofilm was normalized to bacterial growth, the optical densities were measured at 600 nm and subsequently adjusted to 0.01 for each bacterial suspension. Then, 100 μl of each suspension was added to 100 µl of fresh warm medium appropriate to each bacterium in a 96-well polystyrene microtiter plate (1). To develop S. salivarius and P. gingivalis biofilms, a modification of the method described above including the following changes was used: 20 μl of overnight bacterial cell suspensions was added to 180 μl of fresh warm medium in a 96-well polystyrene microtiter plate and incubated under the conditions described above. The A. actinomycetemcomitans and P. gingivalis biofilms were grown in an anaerobic chamber at 37°C for 24 h and 48 h, respectively. Biofilms of S. oralis, S. sanguinis, S. salivarius, and S. mutans were grown at 37°C in 5% CO2. To confirm that we began with approximately the same number of bacteria within each biofilm, the number of viable cells in each biofilm was detected by counting the number of CFU for each bacterium using the Miles and Misra method. Briefly, each biofilm was resuspended in its own medium, serial dilution was performed, and the number of CFU for each biofilm was determined by plating serially diluted bacterial suspensions on appropriate agar plates before counting the bacterial colonies (Table S2).
The formation of each biofilm was measured using 0.1% crystal violet, which was incubated with each biofilm well for 15 min. The crystal violet was washed with distilled H2O. Acetic acid (30%) was used to solubilize the crystal violet, and a FLUOstar plate reader was used to measure the absorbance at 600 nm. Confocal microscopy was used to confirm biofilm formation (Table S2 and Fig. S4).
Confocal microscopy.
Each biofilm was grown in an 8-chamber polystyrene vessel as described above. The supernatant was discarded, and the cells were stained with LIVE/DEAD BacLight Green and Red bacterial stains (Molecular Probes) containing SYTO 9 and propidium iodide at final concentrations of 3.32 μM and 20 μM, respectively. The cells were then incubated in the dark for 15 min. The stain was discarded, and the biofilm was washed with phosphate-buffered saline (PBS). The chamber was carefully removed and mounted with BacLight mounting oil, and a coverslip was applied. Biofilm formation was examined using a Leica TCS SP8 confocal microscope. The SYTO 9 signals were detected with an excitation wavelength of 488 nm. For propidium iodide, excitation was 561 nm. Image stacks were obtained using a 1,024- by 1,024-pixel area. Images were obtained using a 100× oil objective. For each bacterium, three independent biofilms were used, and for each biofilm, at least three images were saved. Images were processed using LASX software, and ImageJ 1.46r software was used to measure the thickness of each biofilm (Table S2 and Fig. S4).
Neutrophil isolation.
Human peripheral blood was drawn from healthy volunteers using Vacutainers containing 0.1 volume of sodium citrate as an anticoagulant. Neutrophils were isolated from blood by density centrifugation using 1-step Polymorphs solution (Accurate Chemical & Scientific Corporation) according to the manufacturer’s instructions. After centrifugation at 500 × g, using a Beckman Coulter centrifuge, for 35 min at room temperature, the neutrophils were collected from the lower band. The cells were washed with 1× PBS and lysed with 1× lysing buffer (BD Pharm Lyse), and an automated counter was used to count the cells.
Neutrophil activation assay with biofilms.
To be able to detect the neutrophil phenotype changes associated with different bacteria, blood was taken from healthy individuals without periodontal disease. One hundred microliters of whole blood was infected with monospecies biofilms of S. oralis, S. sanguinis, S. salivarius, S. mutans, A. actinomycetemcomitans, and P. gingivalis in 96-well plates for 1 h at 37°C. Samples were fixed with 1.6% paraformaldehyde for 15 min at 4°C. Red blood cells (RBCs) were lysed using BD Pharm Lyse solution by repeated 5-min incubations on ice. Leukocytes were resuspended in fluorescent-activated cell sorting buffer (Hanks’ balanced salt solution, 1% bovine serum albumin, 2 mM EDTA) and blocked with mouse IgG (2 µg; Sigma) and rat serum (60 to 80 µg; Sigma) for 20 min on ice. Antibody labeling was performed for 30 min at 4°C as follows: CD66-allophycocyanin (APC) (eBioscience), CD16-Alexa Fluor 700 (BioLegend), CD11-APC-Cy7 (BioLegend), CD18-brilliant violet 421 (BV421) (BD), CD55-fluorescein isothiocyanate (BD), CD63-peridinin chlorophyll protein (PerCP)-Cy5.5 (BioLegend), CD64-phycoerythrin (PE) (BD), and CD14 PE-Cy7 (BioLegend). This assay was based on previous studies which used a similar approach to assess neutrophil phenotypes in blood from patients with periodontal disease (5, 60).
Neutrophil activation assay with planktonic bacteria.
S. oralis (ATCC 10577), A. actinomycetemcomitans (SUNY465), and P. gingivalis (ATCC 33277) were grown on an agar plate. S. oralis was grown at 37°C in 5% CO2, while the other two bacterial species were grown under anaerobic conditions with 90% nitrogen, 5% hydrogen, and 5% carbon dioxide. The agar plate used for each bacterium was prepared according to the ATCC recipe. Bacteria were then scraped from the plate and resuspended in PBS. MOIs of 1, 5, 10, and 20 were calculated for each bacterium using the standard curve. Fifty microliters of bacterial culture at an MOI of 1, 5, 10, or 20 was incubated with 100 µl of whole blood for 20 min at 37°C (n = 3).
Neutrophil activation assay with mixed biofilm.
Additionally, mixed biofilms of S. oralis and S. salivarius, as well as mixtures of S. oralis and P. gingivalis biofilms, were grown aerobically and anaerobically, respectively. The results for each bacterial biofilm mixture were compared to those for the monospecies grown under the same conditions. Overnight cultures were diluted 1:100 in medium for S. oralis (ATCC 10577) and 1:10 in prewarmed fresh medium for S. salivarius (ATCC 13419) and P. gingivalis (ATCC 33277). The medium used was prepared by following ATCC protocols. For the mixed biofilm of S. oralis and S. salivarius, the culture of diluted bacteria was mixed equally (100 µl of each bacterium) and incubated at 37°C in 5% CO2 overnight. For P. gingivalis and S. oralis, the mixed biofilm was diluted 1:10 in medium. P. gingivalis was grown overnight in a 96-well plate, and on the next day 1:100-diluted S. oralis was added. The plate was then incubated for another day at 37°C under anaerobic conditions. For growing mixed biofilms, we followed the procedures of Kommerein et al. with some modification (61). The biofilm growth was confirmed with crystal violet staining (n = 4) (Table S3).
Effect of gingipains.
To confirm that the P. gingivalis results were due to its gingipains, we obtained two gingipain mutants of P. gingivalis, KDP131 (Arg-gingipain A) and KDP132 (Arg-gingipain B). The mutant bacteria were kindly provided by Danial Grenier from the University of Laval. We used P. gingivalis ATCC 33277 and the two P. gingivalis mutants, KDP131, an rgpA (Arg-gingipain A) mutant, and KDP132, an rgpB (Arg-gingipain B) mutant. These bacteria were grown in Todd-Hewitt broth with yeast extract (THYE broth) supplemented with 1 µg/ml vitamin K and 5 µg/ml hemin. For the overnight growth of each mutant, bacterium-specific antibiotics were added. For the KDP131 mutant, we used erythromycin at 10 µg/ml, and for the KDP132 mutant, we used tetracycline at 0.7 µg/ml. Each bacterial culture was grown for 2 days. Biofilms of monospecies bacteria were made by diluting the culture 1:10 using the same medium but with no antibiotics, as we did not want to have the effects of antibiotics on the experiments (62). The neutrophil phenotype assays were performed as described above (n = 4).
Multicolor flow cytometry.
An LSR Fortessa (BD) flow cytometer was used to acquire the data. Rainbow beads were used to ensure similar detector sensitivities between experimental repeats. Single-stained One Comp eBeads (Bioscience) were used for automated compensation setup. At least 20,000 events were acquired per sample. Gating was performed by use of an initial FSC-A and SSC-A gate for granulocytes followed by a CD16hi and CD66hi gate for neutrophils and an SSC width (SSC-W)-by-SSC height (SSC-H) gate as well as an FSC width (FSC-W)-by-FSC height (FSC-H) gate to remove doublets (Fig. S1). FMO (fluorescence minus one) controls were used to confirm our gating strategy and antibody labeling. Data were analyzed by FlowJo software (v10; Tree Star), and mean fluorescence intensities (MFIs) were determined for each CD marker. Significant differences (P < 0.05) were determined by analysis of variance (ANOVA) with the post hoc Tukey test (n = 9).
Phagocytosis assay.
pHrodo Red succinimidyl ester (pHrodo; Life Sciences) was used to measure phagocytosis. Bacteria were labeled following the Jason C. Lenzo protocol with slight modifications (1). Monospecies biofilms were grown as described above. The supernatant was removed. Fifty microliters of 100 mM sodium bicarbonate, pH 8.5, was added to each well. pHrodo Red was then added to the biofilm at a concentration of 0.5 mM, and the biofilm was incubated at room temperature for 60 min with protection from light and gently mixed. The labeled biofilms were then washed with PBS to remove free dye. Flow cytometry preparation was performed using CD66-APC (eBioscience), CD16-Alexa Fluor 700 (BioLegend), and CD63-PerCP-Cy5.5 (BioLegend). The controls used were unstimulated neutrophils and neutrophils stimulated with unstained biofilm. For a compensation control, an unstained sample was mixed with a single-stained pHrodo Red sample. Samples were then run on a flow cytometer, and the mean fluorescent intensity of the samples was measured. Significant differences (P < 0.05) were determined by ANOVA with the post hoc Tukey test (n = 3).
ROS assay.
Whole blood was incubated with the biofilms for 30 min at 37°C and supplemented with CellRox reagent (Invitrogen) for an additional 30 min. Samples were fixed, lysed, blocked, and labeled with CD66-APC (eBioscience), CD16-Alexa Fluor 700 (BioLegend), or CD63-PerCP-Cy5.5 (BioLegend) for flow cytometry as described above. As a compensation control, an unstained sample was mixed with a single-stained CellRox sample. Samples were then run on a flow cytometer, and the mean fluorescent intensity of the samples was measured. Significant differences (P < 0.05) were determined by ANOVA with the post hoc Tukey test (n = 4).
Nrf2 Western blotting.
Neutrophils were isolated as mentioned above. Cells were washed and resuspended in PBS, 5 × 106 neutrophils were added to the biofilms, and the mixture was incubated at 37°C for 1 h. The cells were then centrifuged at 800 × g using an Eppendorf 5415R centrifuge, and the pellets were resuspended in hypotonic buffer solution (based on the Thermo Fisher nuclear extraction protocol). After additional centrifugation at 16,000 × g, the remaining pellets were resuspended in cell extraction buffer (based on the Thermo Fisher nuclear extraction protocol) for extraction of nuclear Nrf2, while cytoplasmic Nrf2 was from the supernatant. Cytoplasmic Nrf2 and nuclear Nrf2 were analyzed as described above using a primary antibody directed against Nrf2 (1:200; Santa Cruz) and IRDye 800 CW secondary goat anti-rabbit immunoglobulin (1:10,000; Odyssey). Significant differences (P < 0.05) were determined by ANOVA with the post hoc Tukey test (n = 4).
NETosis and MPO production.
Whole blood was incubated with the various biofilms for 3 h. Samples were then fixed with 1.6% paraformaldehyde for 15 min at 4°C. Red blood cells were lysed, and the samples were blocked. Primary antibody anti-histone H3 (Abcam) was added to the samples, which were then incubated on ice for 30 min. The cells were then stained with goat anti-rabbit immunoglobulin-Alexa Fluor 488 (Abcam) antibodies as a secondary antibody, CD66-APC (eBioscience), CD16-Alexa Fluor 700 (BioLegend), CD63-PerCP-Cy5.5 (BioLegend), and MPO-PE (Origen) for another 30 min. Samples were then run on a flow cytometer, and the mean fluorescent intensity of the samples was measured (5). Significant differences (P < 0.05) were determined by ANOVA with the post hoc Tukey test (n = 6).
Statistical analysis.
One-way ANOVA with the post hoc Tukey test was performed to determine statistical significance. Student’s t test was used to compare neutrophil activation by whole blood and that by isolated neutrophils. A P value of <0.05 was considered statistically significant. Data were analyzed by IBM SPSS Statistics (version 24) statistical software.
Supplementary Material
ACKNOWLEDGMENTS
We give special thanks to Anca Serbanescu for her input and assistance in bacterial growth and biofilm formation and to Dhaarmini Rajshankar for her assistance in confocal imaging. We also thank Danial Grenier for providing P. gingivalis mutant strains.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We declare no conflict of interest in this study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00790-18.
REFERENCES
- 1.Kobayashi SD, DeLeo FR. 2009. Role of neutrophils in innate immunity: a systems biology‐level approach. Wires Syst Biol Med 1:309–333. doi: 10.1002/wsbm.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen I, Hajishengallis G. 2016. Major neutrophil functions subverted by Porphyromonas gingivalis. J Oral Microbiol 8:30936. doi: 10.3402/jom.v8.30936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostanci N, Thurnheer T, Aduse-Opoku J, Curtis MA, Zinkernagel AS, Belibasakis GN. 2013. Porphyromonas gingivalis regulates TREM-1 in human polymorphonuclear neutrophils via its gingipains. PLoS One 8:e75784. doi: 10.1371/journal.pone.0075784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Z, Weinberg A. 2006. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000 40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 5.Fine N, Hassanpour S, Borenstein A, Sima C, Oveisi M, Scholey J, Cherney D, Glogauer M. 2016. Distinct oral neutrophil subsets define health and periodontal disease states. J Dent Res 95:931–938. doi: 10.1177/0022034516645564. [DOI] [PubMed] [Google Scholar]
- 6.El-Benna J, Dang PM-C, Gougerot-Pocidalo M-A. 2008. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol 30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 7.Faurschou M, Borregaard N. 2003. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Condliffe A, Kitchen E, Chilvers E. 1998. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Lond) 94:461–471. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- 9.Kornman KS. 2008. Mapping the pathogenesis of periodontitis: a new look. J Periodontol 79:1560–1568. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- 10.Hybertson B, Gao B. 2014. Role of the Nrf2 signaling system in health and disease. Clin Genet 86:447–452. doi: 10.1111/cge.12474. [DOI] [PubMed] [Google Scholar]
- 11.Borenstein A, Fine N, Hassanpour S, Sun C, Oveisi M, Tenenbaum HMG. 2018. Morphological characterization of para- and proinflammatory neutrophil phenotypes using transmission electron microscopy. J Periodontal Res 53:972–982. doi: 10.1111/jre.12595. [DOI] [PubMed] [Google Scholar]
- 12.Lakschevitz FS, Aboodi GM, Glogauer M. 2013. Oral neutrophil transcriptome changes result in a pro-survival phenotype in periodontal diseases. PLoS One 8:e68983. doi: 10.1371/journal.pone.0068983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fives-Taylor P, Meyer D, Mintz K. 1996. Virulence factors of the periodontopathogen Actinobacillus actinomycetemcomitans. J Periodontol 67:291–297. doi: 10.1902/jop.1996.67.3s.291. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Helmerhorst E, Leone CW, Troxler R, Yaskell T, Haffajee A, Socransky S, Oppenheim F. 2004. Identification of early microbial colonizers in human dental biofilm. J Appl Microbiol 97:1311–1318. doi: 10.1111/j.1365-2672.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa A, Furukawa S, Fujita S, Mitobe J, Kawarai T, Narisawa N, Sekizuka T, Kuroda M, Ochiai K, Ogihara H, Kosono S, Yoneda S, Watanabe H, Morinaga Y, Uematsu H, Senpuku H. 2011. Inhibition of Streptococcus mutans biofilm formation by Streptococcus salivarius FruA. Appl Environ Microbiol 77:1572–1580. doi: 10.1128/AEM.02066-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajishengallis G, Darveau RP, Curtis MA. 2012. The keystone-pathogen hypothesis. Nat Rev Microbiol 10:717. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson B, Ward JM, Ready D. 2010. Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen? Periodontol 2000 54:78–105. doi: 10.1111/j.1600-0757.2009.00331.x. [DOI] [PubMed] [Google Scholar]
- 18.Curtis MA, Zenobia C, Darveau RP. 2011. The relationship of the oral microbiotia to periodontal health and disease. Cell Host Microbe 10:302–306. doi: 10.1016/j.chom.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemos JA, Quivey RG Jr, Koo H, Abranches J. 2013. Streptococcus mutans: a new Gram-positive paradigm? Microbiology 159:436–445. doi: 10.1099/mic.0.066134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haniastuti T. 2013. Chemotactic activity on human neutrophils to Streptococcus mutans. J Dent Indonesia 16:149–162. [Google Scholar]
- 21.Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A. 2014. The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis 33:499–515. doi: 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg D, Poran S, Shapira L. 1999. The effect of extracellular polysaccharides from Streptococcus mutans on the bactericidal activity of human neutrophils. Arch Oral Biol 44:437–444. doi: 10.1016/S0003-9969(99)00014-X. [DOI] [PubMed] [Google Scholar]
- 23.Haslett C, Guthrie LA, Kopaniak MM, Johnston RB Jr, Henson PM. 1985. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol 119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 24.Kuijpers T, Tool A, Van der Schoot C, Ginsel L, Onderwater J, Roos D, Verhoeven A. 1991. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood 78:1105–1111. [PubMed] [Google Scholar]
- 25.Berends C, Dijkhuizen B, de Monchy JG, Gerritsen J, Kauffman HF. 1994. Induction of low density and up-regulation of CD11b expression of neutrophils and eosinophils by dextran sedimentation and centrifugation. J Immunol Methods 167:183–193. doi: 10.1016/0022-1759(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 26.Fossati G, Moots RJ, Bucknall RC, Edwards SW. 2002. Differential role of neutrophil Fcγ receptor IIIB (CD16) in phagocytosis, bacterial killing, and responses to immune complexes. Arthritis Rheum 46:1351–1361. doi: 10.1002/art.10230. [DOI] [PubMed] [Google Scholar]
- 27.Galicia JC, Benakanakere MR, Stathopoulou PG, Kinane DF. 2009. Neutrophils rescue gingival epithelial cells from bacterial‐induced apoptosis. J Leukoc Biol 86:181–186. doi: 10.1189/jlb.0109003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirschfeld J, White PC, Milward MR, Cooper PR, Chapple IL. 2017. Modulation of neutrophil extracellular trap and reactive oxygen species release by periodontal bacteria. Infect Immun 85:e00297-17. doi: 10.1128/IAI.00297-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji S, Hyun J, Park E, Lee BL, Kim KK, Choi Y. 2007. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res 42:410–419. doi: 10.1111/j.1600-0765.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 30.Johansson A, Sandström G, Claesson R, Hänström L, Kalfas S. 2000. Anaerobic neutrophil‐dependent killing of Actinobacillus actinomycetemcomitans in relation to the bacterial leukotoxicity. Eur J Oral Sci 108:136–146. doi: 10.1034/j.1600-0722.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi SD, Braughton KR, Whitney AR, Voyich JM, Schwan TG, Musser JM, DeLeo FR. 2003. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc Natl Acad Sci U S A 100:10948–10953. doi: 10.1073/pnas.1833375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsuragi H, Ohtake M, Kurasawa I, Saito K. 2003. Intracellular production and extracellular release of oxygen radicals by PMNs and oxidative stress on PMNs during phagocytosis of periodontopathic bacteria. Odontology 91:13–18. doi: 10.1007/s10266-003-0022-1. [DOI] [PubMed] [Google Scholar]
- 33.Hirschfeld J. 2014. Dynamic interactions of neutrophils and biofilms. J Oral Microbiol 6:26102. doi: 10.3402/jom.v6.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghafoor A, Hay ID, Rehm BH. 2011. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl Environ Microbiol 77:5238–5246. doi: 10.1128/AEM.00637-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Günther F, Wabnitz GH, Stroh P, Prior B, Obst U, Samstag Y, Wagner C, Hänsch GM. 2009. Host defence against Staphylococcus aureus biofilms infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN). Mol Immunol 46:1805–1813. doi: 10.1016/j.molimm.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Bejarano I, Terrón M, Paredes S, Barriga C, Rodríguez A, Pariente J. 2007. Hydrogen peroxide increases the phagocytic function of human neutrophils by calcium mobilisation. Mol Cell Biochem 296:77–84. doi: 10.1007/s11010-006-9301-9. [DOI] [PubMed] [Google Scholar]
- 37.Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, Yu LL, Pistolic J, Falsafi R, Tagg J, Hancock REW. 2008. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun 76:4163–4175. doi: 10.1128/IAI.00188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morita C, Sumioka R, Nakata M, Okahashi N, Wada S, Yamashiro T, Hayashi M, Hamada S, Sumitomo T, Kawabata S. 2014. Cell wall-anchored nuclease of Streptococcus sanguinis contributes to escape from neutrophil extracellular trap-mediated bacteriocidal activity. PLoS One 9:e103125. doi: 10.1371/journal.pone.0103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fanger NA, Wardwell K, Shen L, Tedder TF, Guyre PM. 1996. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J Immunol 157:541–548. [PubMed] [Google Scholar]
- 40.Danikas D, Karakantza M, Theodorou G, Sakellaropoulos G, Gogos C. 2008. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol 154:87–97. doi: 10.1111/j.1365-2249.2008.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Middelhoven P, Van Buul J, Hordijk P, Roos D. 2001. Different proteolytic mechanisms involved in FcγRIIIB shedding from human neutrophils. Clin Exp Immunol 125:169–175. doi: 10.1046/j.1365-2249.2001.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincents B, Guentsch A, Kostolowska D, von Pawel-Rammingen U, Eick S, Potempa J, Abrahamson M. 2011. Cleavage of IgG1 and IgG3 by gingipain K from Porphyromonas gingivalis may compromise host defense in progressive periodontitis. FASEB J 25:3741–3750. doi: 10.1096/fj.11-187799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ando-Suguimoto ES, da Silva MP, Kawamoto D, Chen C, DiRienzo JM, Mayer MPA. 2014. The cytolethal distending toxin of Aggregatibacter actinomycetemcomitans inhibits macrophage phagocytosis and subverts cytokine production. Cytokine 66:46–53. doi: 10.1016/j.cyto.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Mukouhara T, Arimoto T, Cho K, Yamamoto M, Igarashi T. 2011. Surface lipoprotein PpiA of Streptococcus mutans suppresses scavenger receptor MARCO-dependent phagocytosis by macrophages. Infect Immun 79:4933–4940. doi: 10.1128/IAI.05693-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freeman SA, Grinstein S. 2014. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev 262:193–215. doi: 10.1111/imr.12212. [DOI] [PubMed] [Google Scholar]
- 46.Kreth J, Merritt J, Qi F. 2009. Bacterial and host interactions of oral streptococci. DNA Cell Biol 28:397–403. doi: 10.1089/dna.2009.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh A, Wyant T, Anaya-Bergman C, Aduse-Opoku J, Brunner J, Laine ML, Curtis MA, Lewis JP. 2011. The capsule of Porphyromonas gingivalis leads to a reduction in the host inflammatory response, evasion of phagocytosis, and increase in virulence. Infect Immun 79:4533–4542. doi: 10.1128/IAI.05016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiwari RK. 2017. Bacterial fMLP modulates both ROS and chemotaxis. Scand J Immunol 86:118. doi: 10.1111/sji.12569. [DOI] [PubMed] [Google Scholar]
- 49.Hajishengallis G, Lamont RJ. 2016. Dancing with the stars: how choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol 24:477–489. doi: 10.1016/j.tim.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong SW, Baik JE, Kang S-S, Yun C-H, Seo D-G, Han SH. 2014. Lipoteichoic acid of Streptococcus mutans interacts with Toll-like receptor 2 through the lipid moiety for induction of inflammatory mediators in murine macrophages. Mol Immunol 57:284–291. doi: 10.1016/j.molimm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Wilensky A, Tzach-Nahman R, Potempa J, Shapira L, Nussbaum G. 2015. Porphyromonas gingivalis gingipains selectively reduce CD14 expression, leading to macrophage hyporesponsiveness to bacterial infection. J Innate Immun 7:127–135. doi: 10.1159/000365970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haziot A, Tsuberi B-Z, Goyert SM. 1993. Neutrophil CD14: biochemical properties and role in the secretion of tumor necrosis factor-alpha in response to lipopolysaccharide. J Immunol 150:5556–5565. [PubMed] [Google Scholar]
- 53.Nicu E, Van Der Velden U, Everts V, Loos B. 2008. Expression of FcγRs and mCD14 on polymorphonuclear neutrophils and monocytes may determine periodontal infection. Clin Exp Immunol 154:177–186. doi: 10.1111/j.1365-2249.2008.03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLaughlin RA, Hoogewerf AJ. 2006. Interleukin-1β-induced growth enhancement of Staphylococcus aureus occurs in biofilm but not planktonic cultures. Microb Pathog 41:67–79. doi: 10.1016/j.micpath.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Sima C, Aboodi GM, Lakschevitz FS, Sun C, Goldberg MB, Glogauer M. 2016. Nuclear factor erythroid 2-related factor 2 down-regulation in oral neutrophils is associated with periodontal oxidative damage and severe chronic periodontitis. Am J Pathol 186:1417–1426. doi: 10.1016/j.ajpath.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiu A, Saigh MA, McCulloch C, Glogauer M. 2017. The role of NrF2 in the regulation of periodontal health and disease. J Dent Res 96:975–983. doi: 10.1177/0022034517715007. [DOI] [PubMed] [Google Scholar]
- 57.Xu H, Sobue T, Thompson A, Xie Z, Poon K, Ricker A, Cervantes J, Diaz P, Dongari-Bagtzoglou A. 2014. Streptococcal co‐infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol 16:214–231. doi: 10.1111/cmi.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strieter RM, Kasahara K, Allen RM, Standiford TJ, Rolfe MW, Becker FS, Chensue SW, Kunkel SL. 1992. Cytokine-induced neutrophil-derived interleukin-8. Am J Pathol 141:397. [PMC free article] [PubMed] [Google Scholar]
- 59.Baggiolini M, Walz A, Kunkel S. 1989. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest 84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lakschevitz FS, Hassanpour S, Rubin A, Fine N, Sun C, Glogauer M. 2016. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp Cell Res 342:200–209. doi: 10.1016/j.yexcr.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Kommerein N, Stumpp SN, Müsken M, Ehlert N, Winkel A, Häussler S, Behrens P, Buettner FF, Stiesch M. 2017. An oral multispecies biofilm model for high content screening applications. PLoS One 12:e0173973. doi: 10.1371/journal.pone.0173973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grenier D, Roy S, Chandad F, Plamondon P, Yoshioka M, Nakayama K, Mayrand D. 2003. Effect of inactivation of the Arg- and/or Lys-gingipain gene on selected virulence and physiological properties of Porphyromonas gingivalis. Infect Immun 71:4742–4748. doi: 10.1128/IAI.71.8.4742-4748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.