The establishment of an animal model that closely approximates enterotoxigenic Escherichia coli (ETEC) disease in humans is critical for the development and evaluation of vaccines against this enteropathogen. Here, we evaluated the susceptibility of Aotus nancymaae, a New World monkey species, to ETEC infection.
Keywords: enterotoxigenic Escherichia coli, CfaE, vaccine, nonhuman primates
ABSTRACT
The establishment of an animal model that closely approximates enterotoxigenic Escherichia coli (ETEC) disease in humans is critical for the development and evaluation of vaccines against this enteropathogen. Here, we evaluated the susceptibility of Aotus nancymaae, a New World monkey species, to ETEC infection. Animals were challenged orogastrically with 109 to 1011 CFU of the human pathogenic CFA/I+ ETEC strain H10407 and examined for evidence of diarrhea and fecal shedding of bacteria. A clear dose-range effect was obtained, with diarrheal attack rates of 40% to 80%, validated in a follow-on study demonstrating an attack rate of 80% with 1011 CFU of H10407 ETEC. To determine whether this model is an effective approach for assessing ETEC vaccine candidates, we used it to evaluate the ability of the donor strand-complemented CFA/I adhesin CfaE (dscCfaE) to protect against H10407 challenge. In a series of experiments, animals were intranasally vaccinated with dscCfaE alone, dscCfaE with either cholera toxin B-subunit (CTB) or heat-labile toxin (LTB), or phosphate-buffered saline (PBS) alone and then challenged with 1011 CFU of H10407. Control animals vaccinated with PBS had attack rates of 70 to 90% on challenge. Vaccination with dscCfaE, or dscCfaE admixed with CTB or LTB, resulted in a reduction of attack rates, with vaccine efficacies of 66.7% (P = 0.02), 77.7% (P = 0.006), and 42.9% (P = 0.370) to 83.3% (P = 0.041), respectively. In conclusion, we have shown the H10407 ETEC challenge of A. nancymaae to be an effective, reproducible model of ETEC disease, and importantly, we have demonstrated that in this model, vaccination with the prototype vaccine candidate dscCfaE is protective against CF-homologous disease.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is one of the leading causes of secretory bacterial diarrheal disease worldwide among adults and children in and travelers to developing countries (1–3). Expression of colonization factors (CFs) and the elaboration of heat-labile (LT) and heat-stable (ST) enterotoxins contribute to complex disease pathogenesis, resulting in colonization of and fluid and electrolyte secretion into the intestinal lumen (4). Thus, these virulence factors are critical targets for vaccine development efforts. However, no ETEC vaccine candidate has achieved licensure to date (5, 6). Efforts to develop a broadly protective ETEC vaccine have been impeded by the absence of a reproducible animal model in which ETEC disease approximates that seen in humans. Several animal models of ETEC have been used to examine the pathogenesis of disease or immunogenicity and efficacy of ETEC vaccine candidates, including intranasal and orogastric adult mouse models (7–10), infant mouse challenge model (11), rat model (12), rabbit ileal loop (RIL) model (13), and reversible intestinal tie adult rabbit diarrhea (RITARD) model (14). These models do not adequately mimic the full spectrum of disease observed with human ETEC infection and are further limited in that they are difficult to reproduce or require surgery and/or death as an endpoint. An optimal preclinical model would, in the absence of antibiotic or surgical intervention, incorporate orogastric challenge of bacteria and demonstrate intestinal colonization, diarrheal disease, and the development of protective immunity. Despite several attempts, a reliable Old World ETEC monkey model that reproducibly demonstrates a ≥70% attack rate has not been developed (15, 16). Notably, oral challenge models have been successfully developed in Aotus nancymaae, a New World monkey species, for both Campylobacter jejuni and Shigella flexneri 2a, resulting in reproducible diarrheal attack rates of ≥70% (17, 18). Recent studies have also evaluated immune responses in A. nancymaae immunized intranasally (i.n.) and orogastrically (o.g.) with ETEC colonization factors. i.n. or o.g. immunization of A. nancymaae with coli surface antigen 6 (CS6), encapsulated in biodegradable poly-(dl-lactide-co-glycolide) microspheres, leads to robust anti-CS6 serum antibody titers (19). A. nancymaae animals immunized i.n. with donor strand-complemented CFA/I adhesin CfaE (dscCfaE), the in cis donor strand-complemented fimbrial tip adhesin of the class 5a CFA/I, exhibit robust systemic anti-adhesin immune responses (20). Many of the current approaches in ETEC vaccine development focus on stimulating antibodies against these and other colonization factors expressed by various ETEC strains, the aim of which is to inhibit ETEC attachment to the small intestine, thereby preventing disease onset. Comparative evolutionary analyses of the class 5 fimbrial major and minor subunits, which form the stalk and adhesin of the fimbriae, respectively, demonstrate that there is greater sequence conservation among the minor subunits than the major subunits (21). This suggests that utilization of the minor subunits in a multivalent ETEC vaccine would provide maximal coverage against the highly diverse serotypes observed in areas where the disease is endemic. Studies have shown that bovine colostral IgG antibodies against the CFA/I minor subunit CfaE provided significant protection against ETEC challenge in humans (22). This finding, and the demonstration of the immunogenicity of dscCfaE in A. nancymaae, supports the further evaluation of CfaE and other ETEC adhesins, especially in regard to protection against ETEC challenge. As such, we endeavored to develop a reproducible ETEC challenge model in A. nancymaae whereby ETEC vaccine candidates can be effectively evaluated prior to study in humans. The studies described here establish a model of ETEC disease in A. nancymaae for the CFA/I+ ETEC strain H10407 and, importantly, demonstrate the protective capacity of the prototype adhesin vaccine candidate dscCfaE.
RESULTS
Safety.
Fifty-five male and 54 female A. nancymaae animals, ranging in age from 11.4 to 73 months and in weight from 690 g to 1,890 g at study onset, were used in five separate studies. Ages, weights, and gender distributions for individual experiments are shown in Table 1. Weights were measured throughout the experiments and overall were not adversely affected by vaccination or challenge. Weights steadily increased across all groups and studies during the vaccination phase. During the challenge phase, there was minimal fluctuation in weights with no clear correlation to treatment, except in the initial dose-finding study, where decreases in weights were less than 10% (data not shown).
TABLE 1.
A. nancymaae demographicsa
| Study |
Vaccineb
(no. of doses) |
Primary challenge strain (dose, CFU) |
Demographic variable |
|||
|---|---|---|---|---|---|---|
| No. of animals | No. of males/no. of females | Mean age, moc (SD) | Mean weight, gc (SD) | |||
| Challenge (dose finding) | NA | H10407 (8.0 × 109) | 5 | 3/2 | 59.8 (7.97) | 1,338 (111.4) |
| H10407 (5.1 × 1010) | 5 | 2/3 | 60.1 (17.53) | 1,332 (272.9) | ||
| H10407 (5.8 × 1011) | 5 | 2/3 | 50.7 (5.605) | 1,482 (185.2) | ||
| Challenge (dose confirmation) | NA | H10407 (7.5 × 1011) | 10 | 5/5 | 38.8 (16.04) | 1,298 (139) |
| H10407-P (6.4 × 1011) | 5 | 4/1 | 53.4 (1.171) | 1,230 (70.71) | ||
| HS (7.9 × 1011) | 5 | 2/3 | 52.1 (0.6993) | 1,330 (201.2) | ||
| Vaccination/challenge (expt 1) |
dscCfaE (4) | H10407 (5.0 × 1011) | 10 | 4/6 | 19.91 (5.882) | 900 (146.4) |
| dscCfaE+CTB (4) | H10407 (5.0 × 1011) | 10 | 5/5 | 21.15 (5.305) | 828 (128.7) | |
| PBS (4) | H10407 (5.0 × 1011) | 10 | 5/5 | 22.24 (14.63) | 899 (183.8) | |
| Vaccination/challenge (expt 2) |
dscCfaE+LTB (4) | H10407 (5.4 × 1011) | 8 | 2/6 | 31.34 (5.761) | 917.5 (80.84) |
| dscCfaE+LTB (3) | H10407 (5.4 × 1011) | 8 | 5/3 | 30.31 (3.909) | 1,086d (242.5) | |
| PBS (4) | H10407 (5.4 × 1011) | 8 | 5/3 | 36.96 (13.38) | 943.8 (154.5) | |
| Vaccination/challengee
(expt 3) |
dscCfaE+LTB (4) | H10407 (5.3 × 1011) | 10 | 3/7 | 29.93 (10.55) | |
| PBS (4) | H10407 (5.3 × 1011) | 10 | 8/2 | 25.09 (4.415) | ||
Gender, age, and weight data are shown separately for each study.
All vaccine antigens were administered intranasally; for all vaccinations, doses of 0.2 mg dscCfaE and 0.29 mg adjuvant were given. NA, not applicable.
Age and weight data collected on day 0 except where noted.
Data for this group reflect weights collected prior to the first vaccination on day 56; data were collected on 5 of the 8 animals.
Weight data not available for these groups.
Challenge model development.
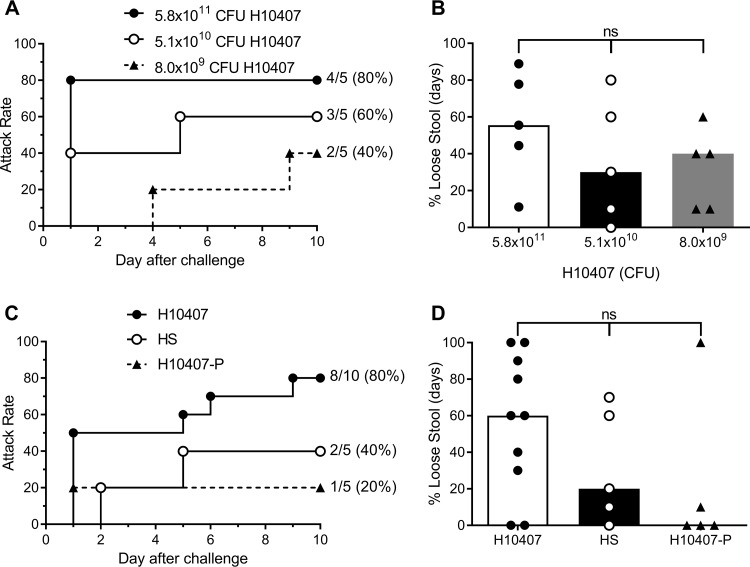
An initial experiment examining the ability to induce diarrhea in A. nancymaae subjects orally challenged with 8.0 × 109, 5.1 × 1010, and 5.8 × 1011 CFU of human CFA/I+ ETEC strain H10407 revealed a clear dose-dependent effect on attack rate, yielding attack rates of 40% (2/5), 60% (3/5), and 80% (4/5), respectively (Fig. 1A). However, this trend was not statistically significant (P = 0.197). Diarrheal onset and duration data are shown in Table S1 in the supplemental material. All animals shed H10407 ETEC by 2 days postchallenge (Table S1). Groups were also assessed for the comparative proportion of days on which a loose stool (grade 3 or higher) was recorded during the observation period (Fig. 1B). No significant difference was detected among the three dose groups (P = 0.346). Based on the 80% attack rate, the 5 × 1011 CFU dose was chosen for validation in a second set of monkeys. Animals were orogastrically challenged with 7.5 × 1011 CFU of CFA/I+ ETEC strain H10407, 6.4 × 1011 CFU of the nonpathogenic CFA/I− ETEC strain H10407-P, or 7.9 × 1011 CFU of the nontypeable E. coli strain HS, originally isolated from a healthy adult. Orogastric challenge with H10407 resulted in an attack rate of 80% (8/10), reproducing that seen in the initial dose-finding study (Fig. 1C). This attack rate was higher than that in animals challenged with H10407-P (1/5; 20%) (P = 0.089) or HS (2/5; 40%) (P = 0.251). Diarrheal onset and duration and fecal shedding data are shown in Table S1. The median proportion of days with loose stools for the H10407, HS, and H10407-P groups equaled 60%, 20%, and 0%, respectively. However, there was no significant difference in proportions among the three groups (P = 0.267) (Fig. 1D).
FIG 1.
Diarrhea attack rate (A and C) and percent total loose stool days (B and D) in A. nancymaae given a low, medium, and high dose of H10407 (A and B) and monkeys given a high dose of H10407 or control strains (C and D). Stool characteristics of monkeys challenged with H10407 ETEC, H10407-P, or E. coli HS were assessed as described in Materials and Methods. (B and D) Data from individual animals are shown as well as the median (bar) for the group. ns, not significant. (A and B) Attack rate of diarrhea (A) and percentage of loose stool days (B) in animals given 8.0 × 109, 5.1 × 1010, or 5.8 × 1011 CFU of H10407 ETEC. (C and D) Attack rate of diarrhea (C) and percentage of loose stool days (D) in animals given 7.5 × 1011 CFU of H10407, 6.4 × 1011 CFU of H10407-P, or 7.9 × 1011 CFU of HS.
Adhesin-based protection against H10407 ETEC challenge.
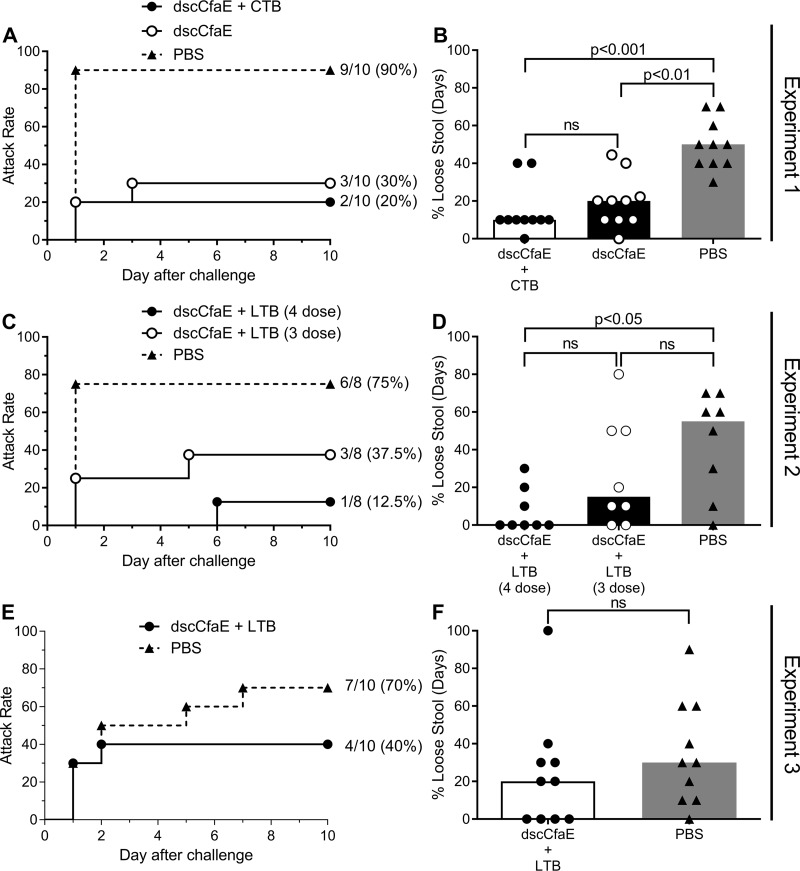
We used the model to test whether immunization with the prototypical ETEC adhesin vaccine candidate dscCfaE would protect against H10407 challenge in three independent experiments. In experiment 1, animals vaccinated with 0.2 mg dscCfaE or 0.2 mg dscCfaE plus 0.29 mg cholera toxin B-subunit (CTB) (termed dscCfaE+CTB) and subsequently challenged with 5.0 × 1011 CFU of H10407 had significantly lower diarrheal rates (30% and 20%, respectively) than mock-vaccinated animals (90%) (Fig. 2A). This corresponds to protective efficacies (PE) of 66.7% (P = 0.02) for dscCfaE and 77.7% (P = 0.006) for dscCfaE+CTB (Table 2). The proportion of loose stool days was significantly reduced in animals receiving dscCfaE or dscCfaE+CTB compared to that of animals receiving phosphate-buffered saline (PBS) (P < 0.01 and P < 0.001, respectively) (Fig. 2B). Fecal shedding of H10407 was observed in all animals beginning 1 day after challenge (Table S2).
FIG 2.
Diarrheal attack rate (A, C, and E) and percent total loose stool days (B, D, and F) in A. nancymaae vaccinated with PBS, 0.2 mg dscCfaE, 0.2 mg dscCfaE plus 0.29 mg CTB (dscCfaE+CTB) (A and B), or 0.2 mg dscCfaE plus 0.29 mg LTB (dscCfaE+LTB) (C to F) and challenged with H10407. Stool characteristics of monkeys challenged with H10407 ETEC were assessed as described in Materials and Methods. (A, C, and E) Day 0 on the x axis refers to the day of challenge, which corresponds to study day 98. (B, D, and F) Data from individual animals are shown as well as the median (bar) for the group. (A and B) Attack rate of diarrhea (A) and percentage of loose stool days (B) in animals vaccinated with 4 doses of dscCfaE, dscCfaE+CTB, or PBS and challenged with 5.0 × 1011 CFU of H10407 ETEC. (C and D) Attack rate of diarrhea (C) and percentage of loose stool days (D) in animals vaccinated with 3 or 4 doses of dscCfaE, dscCfaE+LTB, or PBS and challenged with 5.4 × 1011 CFU of H10407 ETEC. (E and F) Attack rate of diarrhea (E) and percentage of loose stool days (F) in animals vaccinated with 4 doses of dscCfaE+LTB or PBS and challenged with 5.3 × 1011 CFU of H10407 ETEC.
TABLE 2.
Protective efficacy in A. nancymaae
|
Expt and vaccine (no. of doses) |
No. with diarrhea/total no. (%) | Protective efficacy, % | P valuea |
|---|---|---|---|
| 1 | |||
| dscCfaE (4) | 3/10 (30) | 66.7 | 0.020 |
| dscCfaE+CTB (4) | 2/10 (20) | 77.7 | 0.006 |
| PBS (4) | 9/10 (90) | ||
| 2 | |||
| dscCfaE+LTB (4) | 1/8 (12.5) | 83.3 | 0.041 |
| dscCfaE+LTB (3) | 3/8 (37.5) | 50 | 0.315 |
| PBS (4) | 6/8 (75) | ||
| 3 | |||
| dscCfaE+LTB (4) | 4/10 (40) | 42.9 | 0.370 |
| PBS (4) | 7/10 (70) |
Fisher exact test, two-tailed, comparing frequency of diarrhea in test groups to that in PBS group.
To determine whether three doses of dscCfaE were sufficient to protect against ETEC challenge in this model, and to examine the influence of using the B-subunit of heat-labile toxin (LTB), in experiment 2, monkeys were vaccinated with either three or four doses of 0.2 mg dscCfaE plus 0.29 mg LTB (dscCfaE+LTB) prior to challenge with 5.4 × 1011 CFU H10407. The four-dose vaccination series significantly reduced the diarrhea rate (1/8; 12.5%) compared to that of the PBS control group (6/8; 75%) (Fig. 2C), yielding a PE of 83.3% (P = 0.041) (Table 2). Vaccination with only three doses of the same formulation resulted in a slightly higher attack rate of 37.5% (3/8) following challenge, with a PE of 50% (P = 0.315). The proportion of days with loose stool in animals receiving the four-dose regimen was significantly lower than that in animals receiving PBS (P < 0.05) (Fig. 2D). There was no significant difference between the three-dose group and either the four-dose or PBS group. The four-dose regimen was repeated in experiment 3, in which monkeys received either PBS alone or four doses of dscCfaE+LTB. A similar pattern of diarrheal incidence was observed, though at a reduced protective efficacy (42.9%; P = 0.370) (Fig. 2E and Table 2). The proportion of loose stool days was less in vaccinated animals than in the PBS-vaccinated animals; however, this effect was not significant (P = 0.2795) (Fig. 2F).
Serological responses after vaccination and challenge.
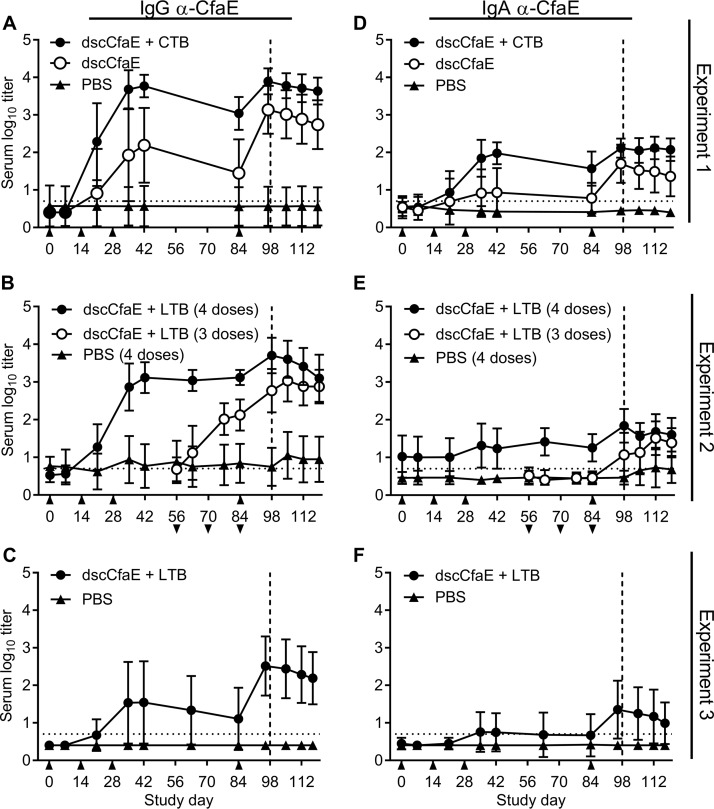
Anti-adhesin and anti-toxoid serological responses generally peaked 2 to 3 weeks after the third i.n. vaccination with dscCfaE with or without CTB or LTB (Fig. 3A to F and 4A to C). Anti-CfaE IgG antibody titers 14 days after the third dose (day 42) in animals vaccinated with dscCfaE were significantly greater than those of the PBS controls (P < 0.0001), and addition of CTB led to significantly greater titers than those for dscCfaE alone (P < 0.0001) (Fig. 3A). Two weeks following the boost on day 84 and just prior to challenge on day 98, anti-CfaE IgG titers in the groups given dscCfaE or dscCfaE+CTB were significantly greater than those in the PBS group (P values were <0.0001 in all cases). Further, titers in the dscCfaE+CTB group were significantly greater than those of the dscCfaE only group (P < 0.01). A similar pattern of responses was observed for anti-CfaE IgA titers, although at overall lower titers (P values were <0.0001 for all comparisons, except comparison of dscCfaE and PBS, day 42, and dscCfaE+CTB and dscCfaE, day 98, for which P values were <0.05) (Fig. 3D). For both days 42 and 98, anti-CTB IgG antibody titers in the dscCfaE+CTB group were significantly greater than those of the PBS group or CfaE only group (P values were <0.0001 in all cases) (Fig. 4A).
FIG 3.
Serum antibody responses over time in A. nancymaae animals vaccinated with 0.2 mg dscCfaE, 0.2 mg dscCfaE plus 0.29 mg CTB or 0.29 mg LTB, or PBS and challenged with 5.0 × 1011 CFU (A and D), 5.4 × 1011 CFU (B and E), or 5.3 × 1011 CFU (C and F) H10407. (A to C) Anti-dscCfaE IgG serum responses. (D to F) Anti-dscCfaE IgA serum responses. All values are the mean log10 titers ± standard deviations (SD). Days of vaccination in four dose groups are indicated by upward arrows (A to F), and days of vaccination in three dose groups are indicated by downward arrows below the x axis scale (B and E). Day of challenge is indicated by a vertical dotted line. The horizontal dotted line denotes the lowest dilution tested.
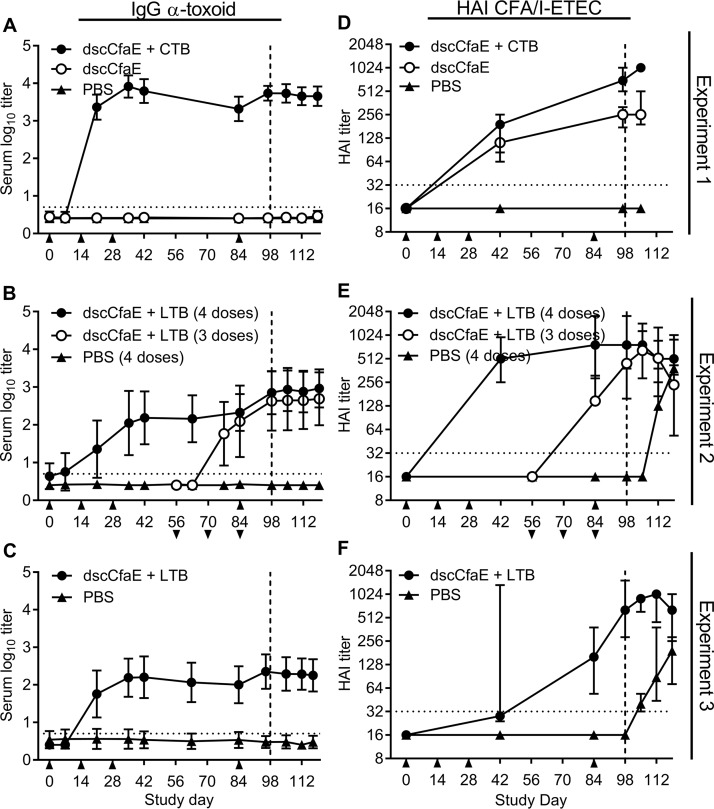
FIG 4.
Serum and functional antibody titers over time are shown in A. nancymaae animals vaccinated with 0.2 mg dscCfaE, 0.2 mg dscCfaE plus 0.29 mg CTB or 0.29 mg LTB, or PBS and challenged with 5.0 × 1011 CFU (A and D), 5.4 × 1011 CFU (B and E), or 5.3 × 1011 CFU (C and F) H10407. (A to C) Anti-CTB or anti-LTB IgG serum responses. Values are the mean log10 titers ± SD. (D to F) Functional antibody titers over time, as measured by the HAI assay using CFA/I+ ETEC (H10407) and bovine erythrocytes. Values are the median and interquartile ranges of data from each group. Days of vaccination in the four dose groups are indicated by upward arrows (A to F), and days of vaccination in three dose groups are indicated by downward arrows below the x axis scale (B and E). Day of challenge is indicated by a vertical dotted line. The horizontal dotted line denotes the lowest dilution tested.
Coadministration of LTB with dscCfaE yielded anti-adhesin IgG antibody titers significantly greater in both three-dose and four-dose vaccine groups than in the PBS group 2 weeks after the final dose (day 98) (P < 0.0001) (Fig. 3B). Additionally, significantly greater titers were elicited by day 98 after animals received four rather than three doses of vaccine (P < 0.01). Anti-adhesin IgA responses were lower, although differences among groups mirrored those in the IgG analysis, with titers in the four- and three-dose groups both being significantly greater than those of the PBS group on day 98 (P < 0.0001 and P < 0.05, respectively) and with the four-dose series inducing a more robust response than the three-dose series (P < 0.01) (Fig. 3E). As expected, anti-LTB responses by day 98 in both vaccine groups were significantly greater than those in the PBS group (P < 0.0001) and not different from each other (Fig. 4B). A repetition of the four-dose regimen in a follow-on study led to a similar pattern of responses, although at overall lower titers (Fig. 3C and F and 4C). Specifically, anti-adhesin IgG and IgA antibody titers were significantly greater in the dscCfaE+LTB group than in the PBS group on day 42 (P < 0.005 and P < 0.05, respectively) and on day 98 (P < 0.0001 and P < 0.005, respectively). Anti-toxoid titers were greater in the dscCfaE+LTB group than the PBS group on both days (P values were <0.0001 in all cases).
Effects of H10407 challenge on antibody titers following vaccination were ascertained by comparing anti-adhesin IgG and IgA and anti-toxoid IgG responses prior to challenge (day 98) and postchallenge (day 119). We found that challenge with H10407 did not lead to any increase in responses (Fig. 3A to F and 4A to C), including the PBS control group. Instead, we observed a decrease in anti-CfaE- and anti-toxoid-specific antibody titers in select groups as immune responses waned.
Analysis of hemagglutination inhibition (HAI) responses prior to challenge on day 98 demonstrated that titers were significantly greater in all vaccine groups than in the PBS controls (P values were <0.0001 in all cases) (Fig. 4D to F). Addition of CTB elicited significantly higher HAI titers than CfaE alone (P < 0.0001) (Fig. 4D), and there was no significant difference in titers between three and four doses of CfaE+LTB (Fig. 4E). The repeat of the four-dose regimen in experiment 3 demonstrated an approximation of those HAI titers observed in experiment 2 on study day 98, although there was a noticeable difference in the kinetics of the response (Fig. 4F). Finally, animals in the PBS control group demonstrated a positive HAI response following H10407 challenge, in contrast to the absence of anti-CfaE or anti-toxoid responses in these animals.
DISCUSSION
ETEC vaccine development, much of which targets disrupting intestinal adherence, has been long hindered by the existence of a diverse array of pathogenic strains and their associated enterotoxins, colonization factors, and serotypes (23). As such, an effective vaccine would represent a broad set of CFs in a multivalent formulation. The recent push toward development of such a vaccine and the associated costs of clinical trials have prompted the need for an animal model in which diarrheal disease mirroring human ETEC infection can be successfully and reliably induced. The experiments described here are the first demonstration that ETEC diarrheal disease can be induced in a species of the New World owl monkey, Aotus nancymaae, through orogastric challenge with the CFA/I+ ETEC strain H10407. When three groups of animals were challenged in a preliminary dose-finding study, a targeted dose of 5.8 × 1011 CFU was identified as sufficient for inducing diarrheal disease in 80% of animals. A subsequent study in which a larger group of animals was challenged with 7.5 × 1011 CFU H10407 confirmed the initial findings. Fecal shedding of H10407 was observed in all challenged animals, indicating intestinal colonization. Building on our previously published work demonstrating that the donor strand-complemented CFA/I adhesin dscCfaE is immunogenic in both mice and A. nancymaae and protective in neonatal mice (11, 20), we next sought to determine if vaccination with dscCfaE can reduce the observed attack rate. Here, we show that four doses of dscCfaE with or without CTB significantly reduce the diarrheal attack rate compared to that of vaccination with PBS alone. We conducted two follow-on studies to determine if dscCfaE coadministered with the homologous B subunit of the heat-labile toxin (LTB) produced by ETEC would provide similar protection. These studies revealed that vaccination with dscCfaE in conjunction with LTB also confers protection against H10407 challenge and that the number of vaccine doses is important in the induction of these protective responses in this model. Our results confirm those of a prior study demonstrating that i.n. vaccination with dscCfaE+CTB is highly immunogenic in A. nancymaae, inducing both IgG and IgA anti-adhesin antibodies as well as strong HAI titers (20). Here, we have further shown that coadministration with the homologous LTB toxoid also leads to strong anti-adhesin, anti-toxoid, and HAI antibody responses. Taken together, the data presented here, as well as evidence published previously, demonstrate the protective capacity of dscCfaE against ETEC challenge and support the further evaluation of dscCfaE as a vaccine candidate in humans.
It is worth discussing some limitations specific to the studies presented here. First, as the enzyme-linked immunosorbent assay (ELISA) methodology for A. nancymaae was not fully optimized at the time of this study, we are limited in the ability to present serological data for the model. Our analysis of immunogenicity in the vaccination challenge studies was limited to serological measures of induced immunity; however, methods to assess the intestinal immune response in future studies are under development. Despite these limitations, the primary measure of protection in this model is diarrheal output, for which reduction was significantly associated with dscCfaE vaccination. Second, variability in attack rates and anti-dscCfaE titers was observed between experiments 2 and 3, in which animals were vaccinated with either PBS alone or dscCfaE+LTB. The groups of animals presented in experiment 3 were control groups in a larger, unrelated study and were presented here as a comparator. The variability observed here may be due, in part, to innate variability between animals combined with the small numbers of animals used in each study or possibly differences in technical application of antigen and/or inoculum. We have since replicated the dscCfaE+LTB group as a control in a follow-on study and observed an 11% attack rate (PE, 84%; P <0.05; unpublished data), suggesting that the results in experiment 3 are an outlier and/or we have since optimized our technique. Finally, it is worth noting that challenge with the nonpathogenic E. coli strain HS in the dose confirmation study resulted in an attack rate of 40%. The small number of animals in this group may have led to an imprecise estimate of diarrhea (2/5 animals), and in fact use of this control strain in subsequent studies has demonstrated routinely low or no diarrhea in the animals (6%, 3/54 animals; data not shown).
As with many experimental models, there are certain strengths and limitations to the A. nancymaae model. An optimal animal model would employ an orogastric challenge of ETEC bacteria, demonstrate intestinal colonization and diarrheal disease, and have the capacity to develop protective immunity. Our model is able to incorporate these key elements and has several advantages over existing models. The monkey model, though more costly, is superior to most small-animal models, as there is minimal manipulation needed and challenge methodology more closely approximates that of human models. However, experimental work with nonhuman primates has some inherent complexities not present in smaller animal models, and this, in addition to greater intersubject differences, may lead to greater variability. With the A. nancymaae model, we are able to directly measure frequency and grade of diarrhea, while small-animal models are often limited to measurements of survival rate or intestinal volume. Although rhesus macaques are a common nonhuman primate species for infectious disease research, previous work has demonstrated inconsistent diarrheal disease rates, with a maximum attack rate of 60% for a 5 × 1012-CFU inoculum of ETEC strain H10407 (16). The ability of the A. nancymaae model to consistently achieve greater than or equal to 70% attack rate is important, as this allows for the use of fewer animals while still powering the study appropriately. Another important advantage of our model is that, relative to rhesus, the A. nancymaae model is a more cost-effective preclinical approach for assessing safety, immunogenicity, and protective efficacy of candidate enteric vaccines prior to costly human clinical trials. In contrast to the human model, however, we are unable to measure stool volume due to animal husbandry logistics. Further, an important limitation to this model is that while the goal is to accurately mimic human disease, human ETEC infection is characterized by not only diarrhea but also other symptoms, such as cramping, fever, nausea, headache, and vomiting. Our definition of disease for the A. nancymaae model is limited to diarrheal incidence, while human studies can incorporate this symptomatology in calculating disease severity (24). The necessity for patient reporting prevents the use of these subjective symptomologies for disease characterization in the A. nancymaae model. Additionally, as a predictor of ETEC colonization, fecal shedding of H10407 was observed across all animals, regardless of disease state or vaccine dose. This has been similarly observed in the human challenge model (25), indicating that the A. nancymaae model more closely mimics the disease seen in human challenge volunteers. Finally, while key physiological differences may exist between the two species, the A. nancymaae model allows for initial experimental inquiries into immunological and cellular processes (e.g., relative to different antigens, adjuvants, and routes), allowing the more complex and costly human experiments to be better informed and directed in design.
The best characterized of the CFs are the genetically related and serologically distinct class 5 fimbriae, the archetype of which is CFA/I (21, 26). The H10407 strain used in the present study not only represents the widely studied CFA/I fimbriae, which are expressed in strains isolated in areas of ETEC endemicity worldwide, but also has been evaluated extensively and used in a well-developed human challenge model (25). However, to accommodate the diversity of ETEC, successful establishment of an animal CFA/I+ ETEC challenge model in the present study has led to further development of models representing other class 5 fimbria-expressing ETEC strains, including CS19+, CS17+, and CS14+ strains, as well as the afimbrial CS6+ strain, B7A (unpublished data). Moreover, having successfully demonstrated here that the i.n. vaccination of A. nancymaae with dscCfaE is protective against oral challenge with a homologous ETEC strain, we will be able to test and down-select additional adhesin-based vaccine candidates. This model will also allow for the evaluation of the efficacy of other adjuvants, dose schedules, and more clinically relevant routes of administration. Ideally inclusion of an LT derivative would provide adjuvanticity as well as antitoxin immunogenicity.
The establishment of a challenge model of ETEC in A. nancymaae is an important development in ETEC vaccine research. When contrasted with existing preclinical models of ETEC, the A. nancymaae model more accurately reproduces human-like infection and, importantly, does not require surgical procedures or death as an endpoint. Importantly, Campylobacter jejuni and Shigella flexneri 2a vaccination and challenge models in A. nancymaae have been developed, opening the door for preclinical evaluation of multipathogen vaccine platforms in a single diarrheal disease model (17, 18, 27). The establishment of a model of ETEC in Aotus nancymaae that more closely mirrors human disease is a powerful tool for vaccine development and testing, with the opportunity to accelerate the development of enteric vaccines.
MATERIALS AND METHODS
Animal use and welfare.
All studies were approved by the IACUC at the U.S. Naval Medical Research Unit No. 6 (NAMRU-6), Lima, Peru (protocol numbers 1009-99 and NMRCD 04-1, 06-2, 09-03, and 10-08). Aotus nancymaae animals were purchased from the Instituto Veterinario de Investigaciones Tropicales y de Altura (IVITA), University of San Marcos, San Marcos, Peru. Animals were housed in the NAMRU-6 Primate Facility, Lima, Peru, fed a standard monkey diet supplemented with fruit, and provided water ad libitum. Animals were pair housed during the vaccination periods but caged individually immediately prior to and during the challenge period. Animals were not previously used in an ETEC study, and any animal with a baseline reciprocal anti-CFA/I IgG titer of >200 was excluded from the study. Animals were randomly assigned to study groups for either model development or vaccination challenge studies as outlined in Table 1. Temperatures, complete blood counts, and blood chemistry were monitored by the veterinary staff throughout the experiments and were evaluated by both published and internally produced reference values (28). Overall, the values observed during the study did not deviate from accepted standards.
Challenge inoculum preparations.
The CFA/I+ H10407 strain of ETEC, serotype O78:K80:H11 LT+ STh+ STp+, was used as the challenge strain in all studies (29). Seed stocks of H10407 (lots C022504 and B111604) were prepared at the Walter Reed Army Institute of Research, Silver Spring, MD. Two nonpathogenic control E. coli strains, HS and H10407-P, were also used. The HS strain of E. coli is a nontypeable E. coli strain isolated from the stool of a healthy adult (15). H10407-P is a CFA/I-negative, avirulent, spontaneous derivative of H10407 lacking the pCS1 62-MDa plasmid that encodes the CFA/I and STh virulence factors (29–31). Challenge inocula were prepared by harvesting bacteria after incubation on CFA agar for 14 to 28 h at 37°C, suspending in sterile saline, and adjusting to an appropriate optical density at 600 nm (OD600) to yield the experimental dose. The most probable number of bacteria (mean value of pre- and postinoculum counts) administered to animals was determined retrospectively.
Antigen and adjuvant preparations.
The recombinant CFA/I fimbrial tip adhesin dsc19CfaE[His]6, used in the vaccination experiment (referred to as dscCfaE), was produced and purified using previously described expression clone and production methods (32). The dscCfaE lots BB-011 and BB-048 (≥95% purity) used here were low in endotoxin content (9.4 and 927 endotoxin units [EU] per mg, respectively). Cholera toxin B-subunit (CTB), purified from native cholera toxin, was purchased from Sigma (lot 085K4142; St. Louis, MO), and recombinant heat-labile toxin B-subunit lot BB-050 was purified and released by the Biochemistry Laboratory, Enteric Diseases Department, Naval Medical Research Center (≥95% purity), and was low in endotoxin content (178 EU per mg).
Challenge model development: dose-finding and dose confirmation study designs.
Animals (n = 5 to 10/group) were orogastrically (o.g.) challenged with various doses of CFA/I+ ETEC strain H10407 (Table 1). Control animals were challenged with either H10407-P or HS. On the day of ETEC challenge, ranitidine (1.5 mg kg−1 of body weight) was administered by intramuscular (i.m.) injection 90 min before anesthesia to increase gastric pH. Animals were anaesthetized by i.m. injection of ketamine hydrochloride (10 mg kg−1). Thirty min before challenge, 5.0 ml of CeraVacx was administered via gastric tube to further neutralize gastric acid. Inoculum was administered via gastric tube in 5 ml of 1× phosphate-buffered saline (PBS). Animals were observed for 10 days following inoculation for symptoms of illness (described below) and then treated with enrofloxacin (5 mg kg−1) administered i.m. once daily for 5 days.
Vaccination challenge study designs.
Three subsets of animals were vaccinated intranasally (i.n.) prior to challenge (Table 1). Briefly, animals received either a four-dose immunization regimen on days 0, 14, 28, and 84 or a three-dose immunization regimen on days 56, 70, and 84, followed by CFA/I+ ETEC strain H10407 challenge occurring on day 98 for all groups. Vaccine preparations were adjusted to a total volume of 100 µl in PBS. Fifty µl of the vaccine preparation was administered into each nare. For vaccination and blood sampling, animals were anaesthetized by i.m. injection of ketamine hydrochloride (10 mg kg−1). In the first vaccination experiment, groups of 10 A. nancymaae animals were immunized via the four-dose immunization regimen with either 0.2 mg dscCfaE with or without 0.29 mg CTB or PBS alone and challenged with 5.0 × 1011 CFU of H10407. In the second vaccination experiment, groups of eight A. nancymaae animals were immunized with 0.2 mg dscCfaE+LTB or PBS alone via the four-dose immunization regimen. A third group of eight animals was immunized via the three-dose immunization regimen with 0.2 mg dscCfaE+LTB. All animals were challenged on study day 98 with 5.4 × 1011 CFU of H10407 as described above. In the third vaccination experiment, groups of 10 animals were immunized with 0.2 mg CfaE plus 0.29 mg LTB or PBS alone via the four-dose immunization regimen and challenged with 5.3 × 1011 CFU of H10407.
Disease assessment.
Following challenge, monkeys were observed twice daily for 10 days for the development of diarrhea according to the following stool grading system: grade 1, hard (normal); grade 2, soft (normal); grade 3, thick liquid (diarrhea); grade 4, opaque-watery (diarrhea); and grade 5, clear/watery (diarrhea). A diarrhea episode was defined as a period that begins with two or more consecutive days of grade 3 or higher stool consistency and ends the day prior to two or more consecutive days of grade 2 or lower stool consistency. Percent total loose stool days was calculated by determining the number of days a stool of grade 3 or higher was observed divided by the total number of observation days. Animals observed to meet the case definition of diarrhea 2 days prior to challenge were excluded from the study.
Fecal excretion of H10407 ETEC was monitored daily for 10 days after challenge by streaking fresh stool and serial dilutions of stool directly onto MacConkey agar. Presumptive H10407 isolates (lactose positive) were confirmed by colony blotting using rabbit antisera against CFA/I. Stool was considered negative for H10407 if no lactose-positive E. coli cells were isolated or if 10 presumptive colonies were negative by immunoblotting. A period of fecal shedding was defined as isolation of H10407 from stool collected after challenge that begins (onset) as early as day one after challenge and ends (duration) on the last day, up to day 10, that H10407 organisms are shed in the stool. Due to limitations in the ability to differentiate them from other intestinal commensal E. coli strains, fecal shedding of HS and H10407-P strains in control groups was not assessed.
Analysis of antibodies in serum samples by ELISA.
Sera were tested by ELISA for the presence of antibodies against vaccine antigens. Coating antigens dsc19CfaE (lot numbers BB-011 and BB-048), GM1, CTB (lot number 085KR4153; Sigma), and LTB (lot number BB-050) were diluted in sterile PBS to 2 µg/ml, 2 µg/ml, 0.5 µg/ml, and 0.5 µg/ml, respectively, and 96-well ELISA plates were coated with 100 µl of the appropriate antigen. The plates were blocked with 0.1% bovine serum albumin-PBS, pH 7.2, 0.05% Tween 20. Serum samples were diluted in the blocking buffer. Binding of IgG and IgA antibodies was detected with rabbit anti-A. nancymaae IgG-horseradish peroxidase (HRP) conjugate (lot number 043063739; Lampire Biological Laboratories, Pipersville, PA) and rabbit anti-A. nancymaae IgA-HRP conjugate (lot number 043063740; Lampire Biological Laboratories), respectively. Both conjugates were diluted 1:4,000 in the blocking buffer solution. The HRP-specific substrate used was orthophenylenediamine. The serum samples were serially diluted, and the endpoint titers were assigned as the interpolated dilutions of the samples giving an absorbance value at 450 nm of 0.4 above background. The antibody titer ascribed to each sample represented the geometric mean of duplicate determinations. Serum samples with undetectable titers (i.e., reciprocal endpoint titer of <5) were assigned a value of 2.5 for computational purposes.
HAI.
The hemagglutination inhibition (HAI) assay was used to approximate the neutralization of fimbria-mediated adhesion. Serum samples were assayed using bovine erythrocytes and CFA/I+ ETEC strain H10407, as previously described (21). The HAI titer was defined as the reciprocal of the highest dilution of serum that inhibited agglutination. Samples with undetectable hemagglutination at the 1:32 dilution were assigned a reciprocal titer of 16 for computational purposes.
Statistical analyses.
Diarrheal data in the initial dose-range study in the model development phase were analyzed using a chi-square test for trend. For all other experiments, the proportion of animals experiencing diarrhea in each test group was compared to that in the control group with a Fisher exact test. Frequency analyses were not adjusted for multiple comparisons. The proportion of days with loose stool was compared between groups using a Kruskal-Wallis or Mann-Whitney U test, followed by Dunn’s multiple-comparison tests. ELISA and HAI titers were log10 transformed for statistical analysis. Group titers were compared using a one-way analysis of variance (ANOVA), or t test, and over time using the repeated-measures ANOVA or paired t test. A Tukey’s post hoc test was used for pairwise comparisons. P values of <0.05 were considered significant, and tests were interpreted in a two-tailed fashion. GraphPad Prism, version 6.07 for Windows (GraphPad Software, San Diego, CA), was used for all statistical analyses.
Supplementary Material
ACKNOWLEDGMENTS
We thank the veterinary staff of Naval Medical Research Unit 6 for their technical support, Joseph Royal for input regarding health and safety parameters, and Ramiro Gutierrez, Sabrina Joseph, Yang Liu, and Chad Porter for editorial input.
This research was supported by U.S. Army Military Infectious Diseases Research Program Work Unit Number 6000.RAD1.DA2.A0307 (to S.J.S.), the National Institutes of Health (grant number AI070638), and the Henry M. Jackson Foundation for the Advancement of Military Medicine.
All studies were performed in compliance with all applicable federal regulations governing the protection of animals in research. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. E.H., R.M., M.G.P. and S.J.S. served as military service members over the course of this work, which was prepared as part of their official duties.
We declare that there are no financial, institutional, or other relationships that might lead to bias or a conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00634-18.
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steffen R, Hill DR, DuPont HL. 2015. Traveler's diarrhea: a clinical review. JAMA 313:71–80. doi: 10.1001/jama.2014.17006. [DOI] [PubMed] [Google Scholar]
- 4.Fleckenstein JM, Hardwidge PR, Munson GP, Rasko DA, Sommerfelt H, Steinsland H. 2010. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect 12:89–98. doi: 10.1016/j.micinf.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed T, Bhuiyan TR, Zaman K, Sinclair D, Qadri F. 2013. Vaccines for preventing enterotoxigenic Escherichia coli (ETEC) diarrhoea. Cochrane Database Syst Rev 7:CD009029. doi: 10.1002/14651858.CD009029.pub2:Cd009029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker RI. 2015. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine 33:954–965. doi: 10.1016/j.vaccine.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 7.Allen KP, Randolph MM, Fleckenstein JM. 2006. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect Immun 74:869–875. doi: 10.1128/IAI.74.2.869-875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrd W, Cassels FJ. 2003. Mucosal immunization of BALB/c mice using enterotoxigenic Escherichia coli colonization factors CFA/I and CS6 administered with and without a mutant heat-labile enterotoxin. Vaccine 21:1884–1893. doi: 10.1016/S0264-410X(03)00014-8. [DOI] [PubMed] [Google Scholar]
- 9.Byrd W, Mog SR, Cassels FJ. 2003. Pathogenicity and immune response measured in mice following intranasal challenge with enterotoxigenic Escherichia coli strains H10407 and B7A. Infect Immun 71:13–21. doi: 10.1128/IAI.71.1.13-21.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolick DT, Medeiros P, Ledwaba SE, Lima AAM, Nataro JP, Barry EM, Guerrant RL. 2018. The critical role of zinc in a new murine model of enterotoxigenic E. coli (ETEC) diarrhea. Infect Immun 86:e00183-18. doi: 10.1128/IAI.00183-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luiz WB, Rodrigues JF, Crabb JH, Savarino SJ, Ferreira LC. 2015. Maternal vaccination with a fimbrial tip adhesin and passive protection of neonatal mice against lethal human enterotoxigenic Escherichia coli challenge. Infect Immun 83:4555–4564. doi: 10.1128/IAI.00858-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klipstein FA, Engert RF, Clements JD. 1981. Protection in rats immunized with Escherichia coli heat-stable enterotoxin. Infect Immun 34:637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De SN, Bhattacharya K, Sarkar JK. 1956. A study of the pathogenicity of strains of Bacterium coli from acute and chronic enteritis. J Pathol Bacteriol 71:201–209. doi: 10.1002/path.1700710126. [DOI] [PubMed] [Google Scholar]
- 14.Spira WM, Sack RB, Froehlich JL. 1981. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun 32:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DuPont HL, Formal SB, Hornick RB, Snyder MJ, Libonati JP, Sheahan DG, LaBrec EH, Kalas JP. 1971. Pathogenesis of Escherichia coli diarrhea. N Engl J Med 285:1–9. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- 16.Hall ER, Cassels FJ, Jones FR, Diaz-Mayoral CM, Wolf MK, Scott DA, Savarino SJ. 2003. Development of non-human primate animal models for enterotoxigenic Escherichia coli (ETEC) diarrhea and vaccine testing, abstr D-173, p 233 Abstr 103rd Gen Meet Am Soc Microbiol. American Society for Microbiology, Washington, DC. [Google Scholar]
- 17.Gregory M, Kaminski RW, Lugo-Roman LA, Galvez Carrillo H, Tilley DH, Baldeviano C, Simons MP, Reynolds ND, Ranallo RT, Suvarnapunya AE, Venkatesan MM, Oaks EV. 2014. Development of an Aotus nancymaae model for Shigella vaccine immunogenicity and efficacy studies. Infect Immun 82:2027–2036. doi: 10.1128/IAI.01665-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones FR, Baqar S, Gozalo A, Nunez G, Espinoza N, Reyes SM, Salazar M, Meza R, Porter CK, Walz SE. 2006. New World monkey Aotus nancymae as a model for Campylobacter jejuni infection and immunity. Infect Immun 74:790–793. doi: 10.1128/IAI.74.1.790-793.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones FR, Hall ER, Tribble D, Savarino SJ, Cassels FJ, Porter C, Meza R, Nunez G, Espinoza N, Salazar M, Luckett R, Scott D. 2006. The New World primate, Aotus nancymaae, as a model for examining the immunogenicity of a prototype enterotoxigenic Escherichia coli subunit vaccine. Vaccine 24:3786–3792. doi: 10.1016/j.vaccine.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Sincock SA, Hall ER, Woods CM, O’Dowd A, Poole ST, McVeigh AL, Nunez G, Espinoza N, Miller M, Savarino SJ. 2016. Immunogenicity of a prototype enterotoxigenic Escherichia coli adhesin vaccine in mice and nonhuman primates. Vaccine 34:284–291. doi: 10.1016/j.vaccine.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Anantha RP, McVeigh AL, Lee LH, Agnew MK, Cassels FJ, Scott DA, Whittam TS, Savarino SJ. 2004. Evolutionary and functional relationships of colonization factor antigen i and other class 5 adhesive fimbriae of enterotoxigenic Escherichia coli. Infect Immun 72:7190–7201. doi: 10.1128/IAI.72.12.7190-7201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savarino SJ, McKenzie R, Tribble DR, Porter CK, O’Dowd A, Cantrell JA, Sincock SA, Poole ST, DeNearing B, Woods CM, Kim H, Grahek SL, Brinkley C, Crabb JH, Bourgeois AL. 2017. Prophylactic efficacy of hyperimmune bovine colostral antiadhesin antibodies against enterotoxigenic Escherichia coli diarrhea: a randomized, double-blind, placebo-controlled, phase 1 trial. J Infect Dis 216:7–13. doi: 10.1093/infdis/jix144. [DOI] [PubMed] [Google Scholar]
- 23.Wolf MK. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol Rev 10:569–584. doi: 10.1128/CMR.10.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter CK, Riddle MS, Alcala AN, Sack DA, Harro C, Chakraborty S, Gutierrez RL, Savarino SJ, Darsley M, McKenzie R, DeNearing B, Steinsland H, Tribble DR, Bourgeois AL. 2016. An evidenced-based scale of disease severity following human challenge with enteroxigenic Escherichia coli. PLoS One 11:e0149358. doi: 10.1371/journal.pone.0149358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter CK, Riddle MS, Tribble DR, Louis Bougeois A, McKenzie R, Isidean SD, Sebeny P, Savarino SJ. 2011. A systematic review of experimental infections with enterotoxigenic Escherichia coli (ETEC). Vaccine 29:5869–5885. doi: 10.1016/j.vaccine.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 26.Gaastra W, Sommerfelt H, van Dijk L, Kusters JG, Svennerholm AM, Grewal HM. 2002. Antigenic variation within the subunit protein of members of the colonization factor antigen I group of fimbrial proteins in human enterotoxigenic Escherichia coli. Int J Med Microbiol 292:43–50. doi: 10.1078/1438-4221-00189. [DOI] [PubMed] [Google Scholar]
- 27.Monteiro MA, Baqar S, Hall ER, Chen YH, Porter CK, Bentzel DE, Applebee L, Guerry P. 2009. Capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infect Immun 77:1128–1136. doi: 10.1128/IAI.01056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baer J, Weller R, Kakoma I (ed). 1994. Aotus: the owl monkey. Academic Press, San Diego, CA. [Google Scholar]
- 29.Evans DG, Silver RP, Evans DJ Jr, Chase DG, Gorbach SL. 1975. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect Immun 12:656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto T, Yokota T. 1983. Plasmids of enterotoxigenic Escherichia coli H10407 evidence for two heat-stable enterotoxin genes and a conjugal transfer system. J Bacteriol 153:1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crossman LC, Chaudhuri RR, Beatson SA, Wells TJ, Desvaux M, Cunningham AF, Petty NK, Mahon V, Brinkley C, Hobman JL, Savarino SJ, Turner SM, Pallen MJ, Penn CW, Parkhill J, Turner AK, Johnson TJ, Thomson NR, Smith SG, Henderson IR. 2010. A commensal gone bad: complete genome sequence of the prototypical enterotoxigenic Escherichia coli strain H10407. J Bacteriol 192:5822–5831. doi: 10.1128/JB.00710-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole ST, McVeigh AL, Anantha RP, Lee LH, Akay YM, Pontzer EA, Scott DA, Bullitt E, Savarino SJ. 2007. Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol Microbiol 63:1372–1384. doi: 10.1111/j.1365-2958.2007.05612.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.