Eimeria tenella can cause the disease coccidiosis in chickens. The direct and often detrimental impact of this parasite on chicken health, welfare, and productivity is well recognized; however, less is known about the secondary effects that infection may have on other gut pathogens.
KEYWORDS: Campylobacter jejuni, chicken, coinfection, Eimeria tenella
ABSTRACT
Eimeria tenella can cause the disease coccidiosis in chickens. The direct and often detrimental impact of this parasite on chicken health, welfare, and productivity is well recognized; however, less is known about the secondary effects that infection may have on other gut pathogens. Campylobacter jejuni is the leading cause of human bacterial foodborne disease in many countries and has been demonstrated to exert negative effects on poultry welfare and production in some broiler lines. Previous studies have shown that concurrent Eimeria infection can influence the colonization and replication of bacteria, such as Clostridium perfringens and Salmonella enterica serovar Typhimurium. Through a series of in vivo coinfection experiments, this study evaluated the impact that E. tenella infection had on C. jejuni colonization of chickens, including the influence of variations in parasite dose and sampling time after bacterial challenge. Coinfection with E. tenella resulted in a significant increase in C. jejuni colonization in the cecum in a parasite dose-dependent manner but a significant decrease in C. jejuni colonization in the spleen and liver of chickens. The results were reproducible at 3 and 10 days after bacterial infection. This work highlights that E. tenella not only has a direct impact on the health and well-being of chickens but can have secondary effects on important zoonotic pathogens.
INTRODUCTION
The commercial production of chickens has increased dramatically in recent decades, with further expansion predicted (1, 2), increasing their relevance to human food security and safety. Understanding the interactions between infectious agents within the chicken is important, as these can influence animal welfare, commercial success, and, potentially, public health. Interactions within the gut are of particular importance because the chicken intestinal microbiome influences performance parameters, such as feed conversion ratio and body weight gain (3, 4). Concurrent infections can influence the colonization and replication of pathogens in the chicken intestine, with a classic example being the enhanced growth of Clostridium perfringens potentiated by the high mucus production induced by coinfecting Eimeria species parasites (5). Recently, the translocation of Escherichia coli from the gut to internal organs was shown to be enhanced by coinfection with Campylobacter jejuni (6). Moreover, an extensive study of commercial broiler flocks showed a strong association between Campylobacter isolation and the rejection of carcasses due to unspecified microbial infections (7).
Eimeria tenella and C. jejuni are of considerable veterinary and medical significance, respectively. Eimeria species parasites are ubiquitous under intensive farming systems (8), have a huge economic impact (9), and can affect colonization by pathogenic bacteria, such as C. perfringens and Salmonella enterica serovar Typhimurium (5, 10). The use of live Eimeria vaccines in the poultry industry and the development of Eimeria as a vaccine vector (11, 12) prompted this investigation into the effects that Eimeria has on other pathogenic agents found in poultry, such as C. jejuni.
C. jejuni is the leading cause of human bacterial food poisoning in many countries, with an estimated global burden of 95 million illnesses and 21,000 deaths in 2010 and with 2.1 million disability-adjusted life years being lost in 2010 (13), and can induce severe sequelae, including inflammatory neuropathies, such as Guillain-Barré syndrome (14). Source attribution studies unequivocally identify chickens as the major reservoir of this zoonotic infection (15). Campylobacter is environmentally ubiquitous (16) and is commonly found in and around poultry houses, with horizontal transfer being the main route of infection for intensively reared broilers (15). The movement of humans in and out of poultry houses appears to be extremely important in the active carriage of the bacterium. Studies investigating transmission routes for Campylobacter on farms have isolated Campylobacter from multiple human sources, including the hands, boots, and clothes of farm workers, drivers, and managers. Molecular analysis found that in numerous cases these same isolates were subsequently recovered from the poultry (17). The bacterium is usually undetectable within chicken flocks during the first few weeks of life, and this is thought to be due to the presence of maternal anti-Campylobacter IgY antibodies, which gradually decrease and disappear after 2 to 3 weeks (18, 19). After this period, once the first bird becomes colonized, the infection spreads quickly throughout the flock via the fecal-oral route (20). C. jejuni replicates rapidly in the intestinal mucus of chickens and transiently invades epithelial cells to avoid mucosal clearance (21). Subsequently, C. jejuni can translocate across the intestinal epithelial barrier and disseminate into deeper tissues, including the liver and spleen, increasing its infectious potential, as internally located bacteria are less likely than fecal surface contaminants to be destroyed by cooking (22). Increasingly, outbreaks of human campylobacteriosis are linked to the consumption of undercooked chicken products, such as liver pâté (23).
The aim of this study was to investigate the influence of concurrent E. tenella infection on C. jejuni colonization in chickens, including investigation of the physical and immunological factors associated with the observed changes. E. tenella causes hemorrhagic enteritis in the chicken cecum, accompanied by the induction of strong proinflammatory immune responses that include the influx of heterophils, enhanced mucus production, increased T-cell proliferation, and a surge in the expression of a variety of immune effectors (5, 24–27). We postulated that the immune responses and/or the pathology induced by E. tenella may allow C. jejuni to flourish and breach the protective gut wall, increasing colonization and replication within the cecum, liver, and spleen.
RESULTS
E. tenella/C. jejuni coinfection.
For all 3 trials, at all sampling sites C. jejuni was not detected above the limit of detection in any of the unchallenged (C−) birds.
(i) Trial 1. In trial 1 (pilot study; Table 1), at 3 days after bacterial challenge, coinfection with nonattenuated or attenuated E. tenella caused a significant 2.5- or 1-log10 increase in the C. jejuni load in the cecum (P < 0.001 and P < 0.05), respectively, compared to that with infection with C. jejuni alone. A significant difference in cecal C. jejuni colonization was also detected between the nonattenuated and attenuated parasite-infected groups (P < 0.05). In the spleen, coinfection with either of the E. tenella lines caused a nonsignificant 1-log10 decrease in the C. jejuni load (P > 0.05) compared to that with infection with C. jejuni alone. Similarly, in the liver, coinfection with either parasite line caused a nonsignificant ∼1-log10 decrease in the C. jejuni load (P > 0.05).
TABLE 1.
Campylobacter jejuni and Eimeria tenella dose regimes and viable counts from single infection and coinfection of chickens in trial 1
| Groupa | E. tenella strain (oocyst dose; age at dosing [days]) | No. of C. jejuni log10 CFU at day 18 | Avg ± SD no. of log10 CFU/g at day 21b (3 days post C. jejuni) |
||
|---|---|---|---|---|---|
| Cecum | Liver | Spleen | |||
| nE+/C+ | Wis (4,000; 13) | 8.17 | 9.13 ± 0.19A | 2.03 ± 1.22A | 1.67 ± 1.51A |
| aE+/C+ | WisF96 (115,000; 15) | 8.17 | 7.55 ± 0.62B | 2.03 ± 1.23A | 1.35 ± 1.20A |
| E−/C+ | None | 8.17 | 6.61 ± 1.77C | 2.91 ± 1.53A | 2.70 ± 1.71A |
Eight birds were in each group. nE, nonattenuated E. tenella Wis; aE, attenuated E. tenella WisF96; C, C. jejuni; +, administered; −, not administered.
Day 21 was 3 days after C. jejuni inoculation. Averages that were significantly different within each column are identified by a different superscript letter (P < 0.05).
(ii) Trial 2. In the cecum, at 3 days after bacterial challenge, coinfection with nonattenuated or attenuated E. tenella caused a significant 2.9- or 1.35-log10 increase in the C. jejuni load, respectively, compared to that with infection with C. jejuni alone (P < 0.001; Fig. 1A). A significant difference in C. jejuni colonization was again detected in the cecum between the nonattenuated and attenuated parasite-infected groups (P < 0.001). Here, the C. jejuni load was positively correlated with parasite replication, measured in terms of total oocyst output (r = 0.893, P < 0.001; Fig. 1E). In the liver, coinfection with nonattenuated and attenuated E. tenella caused a significant ∼1-log10 decrease in the C. jejuni load (P < 0.05; Fig. 1B), although no difference between the parasite lines was detected (P > 0.05). Similarly, in the spleen, coinfection with the nonattenuated and attenuated E. tenella line caused significant 1.8- and 1.1-log10 decreases in the C. jejuni load, respectively (P < 0.05; Fig. 1C), with no difference between the parasite lines being detected. In both the liver and spleen, no association between the C. jejuni load and the level of fecal oocyst output was detected (P > 0.05; Fig. 1F and G). The total oocyst output was higher in chickens infected with nonattenuated E. tenella than in those infected with the attenuated line (Fig. 1D).
FIG 1.
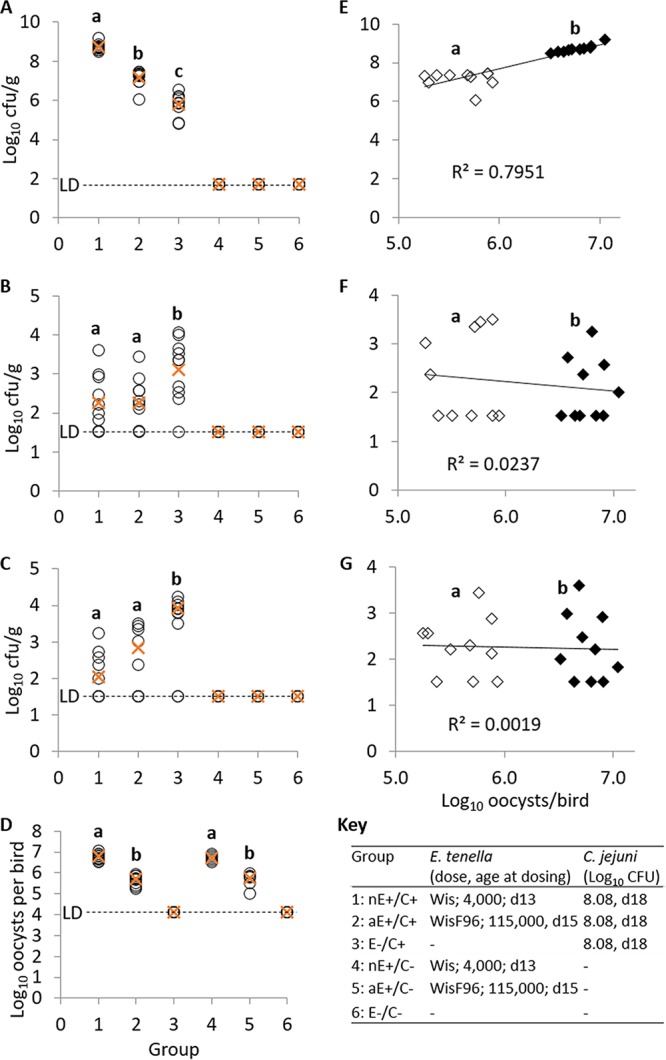
(A to C) C. jejuni load in single or coinfected Light Sussex chickens (trial 2). Circles, count per bird (log10); ×, average count per treatment group (log10). (A) Cecal contents; (B) liver; (C) spleen. (D) Total log10 E. tenella oocyst output per bird (circles) and average per group (×). (E to G) Relationship between C. jejuni load and E. tenella oocyst output. Solid symbols, nonattenuated E. tenella; hollow symbols, attenuated E. tenella. (E) Cecal contents; (F) liver; (G) spleen. Group identifiers and experimental schedule are abbreviated as follows: nE, nonattenuated E. tenella Wisconsin; aE, attenuated E. tenella WisF96; C, C. jejuni 81-176; +, administered; −, not administered (mock-infected control); LD, limit of detection. Different superscript letters within a plot indicate significant statistically significant differences between groups.
(iii) Trial 3. In trial 3 (Table 2), cloacal swab specimens were collected from all groups at 3 days after C. jejuni infection. Coinfection initiated with a high nonattenuated E. tenella dose caused a significant, 1.6-log10 increase in the cloacal C. jejuni load (P < 0.001) compared to that with infection with C. jejuni alone. In the coinfected group infected with a low parasite dose, no difference in the C. jejuni load from that in the group infected with C. jejuni alone was observed (P > 0.05). A significant difference in the cloacal C. jejuni load was noted between the groups coinfected with high and low parasite doses (P < 0.001).
TABLE 2.
Campylobacter jejuni and Eimeria tenella dose regimes and viable counts from single and coinfection of chickens in trial 3a
| Groupb | E. tenella strain (oocyst dose; age at dosing [days]) | No. of C. jejuni log10 CFU at day 18 | Output (no. of log10 oocysts/bird)c | Avg ± SD no. of log10 CFU/gc |
|||
|---|---|---|---|---|---|---|---|
| Day 21,d cloacal swab | Day 28e |
||||||
| Cecum | Liver | Spleen | |||||
| nEh+/C+ | Wis (4,000; 13) | 8.27 | 7.28 ± 0.06A | 9.16 ± 0.51A | 8.47 ± 0.51A | 1.99 ± 0.19A | 2.42 ± 0.50A |
| nEl+/C+ | Wis (400; 13) | 8.27 | 6.75 ± 0.09B | 7.64 ± 0.49B | 7.05 ± 0.93B | 2.72 ± 0.26AB | 2.60 ± 0.47A |
| E−/C+ | None | 8.27 | ND | 7.56 ± 0.54B | 6.97 ± 1.03B | 3.06 ± 0.32B | 3.27 ± 0.82A |
| nEh+/C− | Wis (4,000; 13) | Mock | 7.28 ± 0.04A | ND | ND | ND | ND |
| nEl+/C− | Wis (400; 13) | Mock | 6.73 ± 0.07B | ND | ND | ND | ND |
| E−/C− | None | Mock | ND | ND | ND | ND | ND |
nE, nonattenuated E. tenella Wis; C, C. jejuni; h, high dose; l, low dose; +, administered; −, not administered; Mock, no bacterial control; ND, none detected.
Eight birds were in each group.
Averages that were significantly different within each column are identified by a different superscript letter (P < 0.05).
Sampled 3 days after C. jejuni inoculation.
Sampled 10 days after C. jejuni inoculation.
In the cecum, at 10 days after C. jejuni infection, coinfection initiated with a high E. tenella dose caused a significant 1.5-log10 increase in C. jejuni colonization compared to that with infection with C. jejuni alone (P < 0.01). There was a significant association with oocyst output (r = 0.682, P = 0.001). Coinfection with the low parasite dose did not cause a significant change in C. jejuni colonization compared to that with C. jejuni alone (P < 0.05). A significant variation in the level of C. jejuni colonization was noted between the groups infected with high and low doses of E. tenella (P < 0.01).
In the spleen, at 10 days after C. jejuni infection, no significant difference in the levels of C. jejuni in the presence and in the absence of E. tenella was detected; however, a nonsignificant (P > 0.05) decreasing trend in C. jejuni colonization was observed. No association between C. jejuni colonization in the spleen and the level of fecal oocyst output was detected (r = −0.44, P > 0.05).
In the liver, at 10 days after C. jejuni infection, there was a significant decrease in C. jejuni colonization in the group infected with a high dose of E. tenella compared to that in the group infected with C. jejuni alone (P < 0.05). No significant changes from the group infected with a low parasite dose were observed. No association between the C. jejuni load in the liver and the level of fecal oocyst output was detected (r = −0.31, P > 0.05).
Cytokine response to E. tenella/C. jejuni challenge.
E. tenella infection induces a strong immune response, and it was postulated that the changes in the C. jejuni load noted in the coinfection models could be due to an associated bystander immune response. Cecal tissues collected during trial 2 at 21 days of age were used to investigate the transcription of a variety of cytokines (i.e., at a single time point, equivalent to 7, 5, and 3 days after challenge by the Wisconsin [Wis] strain, the WisF96 strain, and C. jejuni, respectively). The fold change in the level of transcription in each group compared to that in the uninfected control group is summarized in Table 3, along with the fold change in the coinfected groups from that in the group infected with C. jejuni only. Infection with C. jejuni alone significantly increased the transcription of interleukin-1β (IL-1β) and inducible nitric oxide synthase (iNOS) (P ≤ 0.001 for both), as well as IL-13 (P ≤ 0.01). Infection with nonattenuated or attenuated E. tenella increased the cecal transcription of IL-1β, IL-2, IL-6, IL-10, iNOS, and gamma interferon (IFN-γ) significantly compared to that in the uninfected and C. jejuni-only-infected groups, irrespective of C. jejuni coinfection. Transcription of IL-13 was significantly decreased in all Eimeria-infected groups. The accompanying P values are indicated in Table 3.
TABLE 3.
Transcriptional fold change of cytokines and mucins in cecal tissue collected during trial 2a
| Target gene | Fold change ± SEM versusb: |
||||||
|---|---|---|---|---|---|---|---|
| Uninfected birds |
C. jejuni only-infected group |
||||||
| nE+/C+ | aE+/C+ | E−/C+ | nE+/C− | aE+/C− | nE+/C+ | aE+/C+ | |
| IL-1β | 11.88*** ± 0.55 | 11.33** ± 0.71 | 8.4*** ± 0.40 | 10.6*** ± 0.62 | 11.1*** ± 0.97 | 1.42*** ± 0.06 | 1.35** ± 0.08 |
| IL-2 | 11.87*** ± 0.88 | 10.37*** ± 1.01 | 3.07 (ns) ± 0.17 | 10.03*** ± 0.73 | 7.97*** ± 0.79 | 3.87*** ± 0.29 | 3.38*** ± 0.33 |
| IL-6 | 18.86*** ± 1.36 | 20.24*** ± 1.15 | 3.83 (ns) ± 0.20 | 18.12*** ± 1.66 | 14.37*** ± 1.27 | 4.92*** ± 0.35 | 5.28*** ± 0.30 |
| IL-10 | 9.89*** ± 0.78 | 9.06*** ± 0.61 | 2.09 (ns) ± 0.15 | 8.18*** ± 1.13 | 8.97*** ± 0.91 | 4.74*** ± 0.37 | 4.34*** ± 0.29 |
| IL-13 | −20*** ± 0.003 | −16.67*** ± 0.004 | 1.34** ± 0.09 | −25*** ± 0.004 | −16.67*** ± 0.006 | −27.03*** ± 0.003 | −21.01*** ± 0.003 |
| iNOS | 8.72*** ± 0.43 | 6.33*** ± 0.31 | 4.56*** ± 0.26 | 8.73*** ± 0.60 | 5.94*** ± 0.32 | 1.91*** ± 0.09 | 1.39** ± 0.06 |
| IFN-γ | 34.60*** ± 1.84 | 29.96*** ± 1.42 | 5.02 (ns) ± 0.18 | 35.37*** ± 1.54 | 32.84*** ± 1.16 | 6.89*** ± 0.37 | 5.96*** ± 0.28 |
| Muc2 | 1.41*** ± 0.06 | 1.19 (ns) ± 0.05 | 1.00 (ns) ± 0.04 | 1.41** ± 0.06 | 1.16 (ns) ± 0.04 | 1.41*** ± 0.06 | 1.19 (ns) ± 0.06 |
| Muc5ac | 3.27*** ± 0.23 | 2.75*** ± 0.15 | 1.22 (ns) ± 0.10 | 3.16*** ± 0.19 | 2.69*** ± 0.15 | 2.68*** ± 0.18 | 2.25*** ± 0.12 |
| Muc13 | 1.83*** ± 0.11 | 1.33 (ns) ± 0.09 | 1.20 (ns) ± 0.08 | 1.82*** ± 0.06 | 1.34 (ns) ± 0.07 | 1.53*** ± 0.10 | 1.11 (ns) ± 0.08 |
Samples were collected 3 days after C. jejuni challenge. nE, nonattenuated E. tenella Wis; aE, attenuated E. tenella WisF96; C, C. jejuni; +, administered; −, not administered.
Fold change data that were significantly different are identified by asterisks, as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. ns, not significant.
Mucin gene transcription in response to E. tenella/C. jejuni challenge.
Cecal transcription of the mucin genes muc2, muc5ac, and muc13 was assessed to explore the consequences of infection. C. jejuni infection alone resulted in no difference in muc gene transcription at 3 days postchallenge (Table 3). Infection with nonattenuated E. tenella resulted in the upregulation of muc2, muc5ac, and muc13 transcription, most notably, the transcription of muc5ac, which was the only muc gene significantly upregulated during attenuated E. tenella infection.
DISCUSSION
In vivo trials were carried out to analyze the impact of parasite coinfection on C. jejuni colonization of the cecum, spleen, and liver of chickens. The local transcription of selected cytokine and mucin genes was assessed in an effort to explain the differences detected. It was hypothesized that damage to the cecal epithelial barrier induced by the hemorrhagic parasite E. tenella and/or the consequential proinflammatory immune response would facilitate increased bacterial colonization in the cecum, liver, and spleen. Quantification of C. jejuni colonization at these three sites revealed significant variation in the presence or absence of concurrent E. tenella infection, disproving the hypothesis for the liver and spleen. Parasite coinfection was associated with elevated C. jejuni loads within the cecal contents but reduced loads in the liver and spleen. Thus, while fecal shedding of C. jejuni was increased by concomitant E. tenella infection, deep tissue bacterial contamination was decreased. This is in direct contrast to what has been observed when chickens are coinfected with Eimeria parasites and either C. perfringens or S. enterica serovar Typhimurium (5, 10). It has been shown that E. tenella infection can influence the cecal microflora in a manner that has been reported by some (28, 29) to potentially benefit C. jejuni colonization, which demonstrates that E. tenella-induced dysbiosis may increase susceptibility to enteric pathogens, such as C. jejuni. Further analysis of the microbiome of coinfected poultry is needed to investigate this hypothesis. An increased bacterial load in the gut but not the internal organs due to coinfection with globally enzootic Eimeria parasites (8) is relevant to the food safety risk posed by C. jejuni. Furthermore, these results are pertinent to the development of Eimeria as a novel vaccine vector system. This approach aims to utilize transgenic attenuated strains of the parasite to deliver vaccine antigens to chickens. Live attenuated vaccines are currently used to vaccinate over 1 billion birds each year (11), and results from this study suggest that attenuated strains have the potential to reduce C. jejuni colonization in the liver of poultry, which could limit human cases of campylobacteriosis. Paradoxically, increases in C. jejuni colonization in the cecum are of concern, although improvements in abattoir protocols have been associated with a shift from the importance of surface contamination by feces to the importance of deep tissue colonization by C. jejuni, exacerbated by the deliberate undercooking or sautéing of chicken liver due to the belief that this will enhance the flavor and appearance of the end product (30).
It is well recognized that, individually, both E. tenella and C. jejuni generate an immune response of varying levels in chickens following infection (24, 27, 31–33). The impact of E. tenella coinfection on C. jejuni colonization and the concurrent effect on cytokine production have not been reported. Previously wild-type (nonattenuated) strains of E. tenella have been shown to induce a significant immune response in chickens (24, 27) which is far greater than that induced by C. jejuni alone (31, 32). These findings were replicated in this study, where the transcription of all but one of the cytokines tested, IL-13, was increased in nonattenuated E. tenella Wis-infected, C. jejuni-uninfected (nE+/C−) chickens compared to E. tenella-uninfected, C. jejuni-infected (E−/C+) chickens. Additionally, in this study it is notable that there was a significant increase in the transcription of the majority of cytokines investigated in attenuated E. tenella WisF96-infected, C. jejuni-uninfected (aE+/C+) chickens compared to E−/C+ chickens, despite the considerable attenuation of the WisF96 parasite line. To the best of our knowledge, this is the first report of immune responses associated with in vivo WisF96 infection. The induction of immune responses in the absence of significant pathology is relevant to the efficacy of attenuated anticoccidial vaccines. It is postulated that the reduction in C. jejuni colonization in the liver and spleen in the coinfection model could be due to an associated, bystander immune response induced by the parasite. E. tenella infection stimulates a strong proinflammatory immune response, including significant increases in IFN-γ and iNOS transcription (34). iNOS has also been directly linked to the control of C. jejuni (35). The level of cecal iNOS transcription was increased 6- or 8-fold during infection with attenuated or nonattenuated E. tenella. The upregulation of immune factors linked to the control of C. jejuni as a consequence of an ongoing E. tenella infection may explain, at least in part, the reduced translocation of C. jejuni to the liver and spleen in coinfected chickens. IFN-γ levels are balanced by anti-inflammatory cytokines, such as IL-10 (36). Humphrey et al. (2014) reported that the regulation of IL-10 is important in controlling intestinal pathology in C. jejuni-infected chickens, where lower levels are associated with prolonged inflammation and diarrhea (31). In support of that finding, Vaezirad et al. (2017) demonstrated that using glucocorticoids to dampen the immune system of chickens reduced the expression of proinflammatory genes and increased the colonization of C. jejuni in the cecum as well as translocation to and colonization of the liver (37). The work of Vaezirad et al. (37) supports the hypothesis that the increase in C. jejuni cecal colonization may also be influenced by physical damage. E. tenella infection causes the sloughing of cells which form the epithelial barrier, and this damage may facilitate enhanced C. jejuni colonization in the cecum, akin to the mechanism utilized by C. perfringens to invade the gut in the presence of Eimeria (38, 39).
Increased transcription of the majority of cytokines in the cecal tissue in coinfected birds did not appear to impede C. jejuni colonization of the cecal contents, although it is not clear if this was a cause or an effect. These results suggest that the mechanism(s) responsible for the increase in C. jejuni colonization detected within the cecal lumen is distinct from translocation through the cecal wall and/or deep tissue colonization. E. tenella can cause a hemorrhagic form of coccidiosis characterized by large volumes of blood in the cecum (40). Iron is an essential nutrient for the colonization of C. jejuni; however, bioavailability is limited within many host environments (41). Bacteria can take up iron via environmental sources, such as hemin and hemoglobin (42). It is hypothesized that the increased availability of hemoglobin in the cecum, due to the epithelial damage caused by E. tenella, may have provided C. jejuni with an increased source of iron, facilitating enhanced growth and replication. The apparent pathology-dependent effect between nonattenuated and attenuated parasite infections supports such a hypothesis, and it is noted that the attenuated line was expected to induce little or no hemorrhage. Attenuated E. tenella parasites are less pathogenic than nonattenuated parasites (43) and cause less damage to the intestinal epithelium, but they still induce an immune response equivalent to that induced by nonattenuated parasites (44). The subsequent comparison of high and low nonattenuated parasite doses confirmed a dose-effect of Eimeria on C. jejuni colonization within the cecal contents but not the liver or spleen, supporting the association between pathological severity and dose in the former but not the latter. While the parasite crowding effect is expected to have reduced the scale of the difference between the high and low doses by the time of oocyst excretion (45), it is clear that pathology (lesion score) does associate with dose level (46). Variations in unidentified immune factors may contribute to this effect and could influence the increased cecal C. jejuni load in chickens coinfected with the attenuated parasite, where cecal pathology would have been minimal.
Trials 1 and 2 explored the impact of an ongoing infection with nonattenuated or attenuated E. tenella parasites on C. jejuni colonization of chickens 3 days after bacterial challenge. The healthy chicken cecum empties several times per day, suggesting that the figures recorded represent true bacterial colonization (47). However, to confirm the association, the study was repeated using a later sampling point, revealing results at 10 days similar to those obtained at 3 days after bacterial challenge. Once C. jejuni-contaminated food or fecal material is ingested by the chicken, the transit time through the upper gastrointestinal tract is ∼2.5 h (48). Work by Shaughnessy et al. (2009), using an inoculating dose similar to the doses used in this study, showed high levels of persistent cecal colonization at 6, 20, and 48 h after C. jejuni infection, indicating rapid colonization of the bacteria in the cecum (33). Meade et al. (2009) showed that the liver and spleen of the majority of birds were colonized by C. jejuni at 48 h postinfection (49). These studies support analysis of C. jejuni colonization in the E. tenella coinfection model at 3 days after bacterial infection, and the results should be confirmed at 10 days postinfection. Practically, these results are also relevant to the field situation, where anticoccidial drugs are commonly withdrawn from broiler diets 3 to 5 days prior to slaughter, indicating a risk of a parasite and associated C. jejuni surge at the time of transportation and carcass processing.
In addition to hemorrhage, several Eimeria species have been associated with enteric mucogenesis in chickens (5). C. jejuni has been shown to replicate rapidly in intestinal mucus from chickens (21), suggesting that a mucogenic response may encourage Campylobacter proliferation within the mucus layer. Bacterial proteins required for motility and colonization, including flagellin A and Campylobacter invasion antigens, are known to be secreted in the presence of chicken mucus (50, 51). Chicken mucus has also been shown to enhance C. jejuni motility and expression of the flagellar protein FlgR (52), to protect C. jejuni from some short- and medium-chain fatty acids (53, 54), and the viscous environment might aid binding and invasion of mammalian cells (55). However, enteric mucus from chickens has also been reported to attenuate C. jejuni 81-176 invasion of both avian and human epithelial cells (56), possibly contributing to reduced translocation away from the cecum. Mucins are a major component of mucus, and in this study, the transcription of muc2 and muc5ac (both of which are secreted, mucus-forming mucins [57]) and muc13 (a transmembrane mucin) increased in the presence of nonattenuated E. tenella. The transcription of muc5ac was also increased during attenuated E. tenella infection. It was therefore postulated that intestinal mucus could play a key role in the enteric colonization of C. jejuni in chickens and the interaction with E. tenella. A pilot study investigating the impact of the mucus-thinning dietary supplement N-acetylcysteine (NAC; Sigma-Aldrich) (58, 59) was carried out during an in vivo coinfection trial to test this theory (summarized in the Methods section and Table S2 in the supplemental material). It was hypothesized that inclusion of a mucus-thinning agent in the feed of chickens would balance E. tenella-induced mucus secretion, directly reducing nutrient availability in the cecal lumen and indirectly reducing C. jejuni replication and colonization. Further, depleting the secreted mucus layer might be expected to facilitate increased translocation to extraintestinal sites, such as the liver and spleen. In mucin 2-deficient mice presenting with a diminished intestinal barrier, the levels of infection and mortality caused by S. enterica serovar Typhimurium were increased (60). Here, using periodic acid-Schiff (PAS) staining, it was not possible to detect any consistent variation in the thickness or consistency of the intestinal mucus layer with NAC supplementation. As a consequence, no direct functional conclusions can be drawn. However, NAC supplementation did abrogate the E. tenella-associated increase in the cecal C. jejuni load, with a further nonsignificant reduction in treated compared to untreated single C. jejuni-infected chickens being seen. These results support the view that chicken mucus may aid C. jejuni colonization and/or replication, possibly via the provision of nutrients required for sustained growth (61), but further work will be required for confirmation. NAC supplementation is also likely to have exerted other profound effects on the broader enteric microbiome, the influence of which is not currently known. Interestingly, the significant decreases in C. jejuni colonization of the liver and spleen detected in the coinfection model were maintained in the presence of NAC, suggesting either a limited role for mucus in this aspect of the parasite-bacterium interaction or the inefficacy of the NAC protocol.
Conclusion.
The current study has demonstrated that E. tenella coinfection exerts a significant impact on colonization by C. jejuni in Light Sussex chickens, while it upregulates several relevant immune factors. Coinfection caused a significant increase in C. jejuni colonization in the cecal contents in a parasite pathology- and dose-dependent manner but a decrease in the liver and spleen. The results were reproducible on days 3 and 10 after bacterial challenge, highlighting the stability of the effect. Investigation into the levels of mucin transcription suggested that the presence of a depleted intestinal mucosal barrier may contribute. Similar coinfection studies with broiler chickens raised under intensive farming conditions are required to assess if these results are reproducible in a commercial setting. Building on these studies, the influence of eimerian infection on C. jejuni colonization of poultry may impact both the use of live anticoccidial vaccines and the development of Eimeria as a novel vaccine vector.
MATERIALS AND METHODS
Ethics statement.
The work described here was conducted in accordance with UK Home Office regulations under the Animals (Scientific Procedures) Act 1986 (ASPA), with the protocols being approved by the Institute for Animal Health and the Royal Veterinary College (RVC) Animal Welfare and Ethical Review Bodies (AWERB). Study birds were observed daily for signs of illness and/or welfare impairment and were sacrificed under Home Office license by cervical dislocation.
Animals.
Light Sussex chickens, purchased from the Institute for Animal Health Poultry Production Unit (IAH PPU; Compton, UK), were used for all experiments. All chickens were certified to be specific pathogen free (SPF). Throughout the study, all chickens had access to food and water ad libitum and were fed a standard commercial poultry grower diet including 20% protein and 55% wheat (LBS-Biotech, UK).
Parasites and propagation.
The E. tenella Wisconsin (Wis) strain and its derivative, the attenuated WisF96 line, were used throughout these studies (62, 63). The Wis strain is a wild-type (nonattenuated) E. tenella isolate with a standard prepatent period of ∼132 h. The WisF96 line has been attenuated by selection for precocious development, resulting in a single round of schizogony with a reduced prepatent period of ∼96 h and much reduced pathology due to the loss of the second-generation schizont, which is responsible for deep tissue damage and hemorrhage (63). Nonetheless, the WisF96 line retains the ability to induce a fully protective immune response during natural infection that is comparable to that induced by the nonattenuated Wis strain (62). These parasites are phenotypically stable, were passaged through chickens at the Institute for Animal Health and then the Royal Veterinary College through dosing and recovery as previously described (64), and were used in these studies less than 1 month after sporulation.
Bacterial propagation.
C. jejuni strain 81-176 was used due to its proven ability to efficiently colonize the chicken gastrointestinal tract (65). Bacteria were routinely cultured in Mueller-Hinton (MH) broth and on sheep blood agar plates at 37°C for 48 h in a microaerophilic atmosphere created using the CampyGen system (all from Oxoid, Basingstoke, UK). Charcoal cefoperazone deoxycholate agar (CCDA; Oxoid) was used to retrospectively enumerate the CFU of C. jejuni administered per animal by directly plating 10-fold serial dilutions of the inoculum in phosphate-buffered saline (PBS; Oxoid). CCDA was also used to enumerate the C. jejuni bacteria recovered from chickens by directly plating 10-fold serial dilutions of homogenates of cecal contents, liver, and spleen (as described below). The plates were incubated at 37°C for 48 h in a microaerophilic atmosphere, as detailed above. Animals not challenged using C. jejuni were screened for exposure to Campylobacter by enrichment of cecal contents using modified Exeter broth as described previously (65), followed by plating on CCDA plates.
Experimental design. (i) E. tenella/C. jejuni coinfection.
Three in vivo trials were undertaken to investigate the influence of the presence and severity of ongoing E. tenella infection on the outcome of oral C. jejuni challenge.
In trial 1 (pilot study, conducted at the Institute for Animal Health), 24 SPF Light Sussex chickens were caged in 3 groups of eight. Chickens in group 1 received 4,000 sporulated E. tenella Wis (nonattenuated) oocysts by oral gavage at 13 days of age (nE+). Chickens in group 2 received 115,000 sporulated E. tenella WisF96 (attenuated) oocysts by oral gavage at 15 days of age (aE+). Chickens in group 3 were not infected with E. tenella. Chickens in all 3 groups received ∼108 CFU of C. jejuni by oral gavage at 18 days of age (C+). The differential dosing schedule of nE+/C+ chickens and aE+/C+ chickens was to adjust for the different prepatent periods of these parasites, to ensure peak parasitemia in the ceca at the time of C. jejuni challenge in both groups. The nonattenuated and attenuated parasite lines were used to compare the severity of the pathology (i.e., the presence or absence of the second-generation schizont), and the dose sizes were designed to reduce the confounding effect of differential parasite replication, although it should be noted that an equivalent oocyst output was not expected (62). Parasite-associated pathology was anticipated only for the nonattenuated Wis-infected groups. At 3 days after C. jejuni challenge (21 days of age), all birds were culled. Postmortem cecal contents and liver and spleen tissue were collected immediately.
Trial 2 followed an experimental outline similar to that for trial 1, with groups 1 to 3 (nE+/C+, aE+/C+, and E. tenella-uninfected and C. jejuni-infected [E−/C+] chickens, respectively) receiving identical treatment (undertaken at RVC). In addition, to directly compare the effect of C. jejuni challenge on parasite replication, control groups received E. tenella treatment without C. jejuni challenge, using sterile MH broth in place of C. jejuni (groups 4 to 6; E. tenella Wis only-treated [nE+/C−], E. tenella WisF96 only-treated [aE+/C−], and E. tenella- and C. jejuni-untreated [E−/C−] chickens, respectively). Groups 1 to 3 (all C+) comprised 10 Light Sussex chickens per group, while groups 4 to 6 (all C−) comprised six chickens per group, reflecting the greater bird-to-bird variation in C. jejuni enumeration than in E. tenella enumeration. All birds were caged separately to facilitate collection of individual bird feces and enumeration of the total daily oocyst output between 18 and 21 days of age, as described previously (66). All birds were culled at 3 days after C. jejuni challenge (21 days of age), and samples were collected as described above for trial 1.
Trial 3 was similar to trial 2, except that instead of using the attenuated E. tenella WisF96 line, a low dose (400 oocysts) of nonattenuated E. tenella Wis was used to assess the effect of parasite dose/replication rather than the effect of reduced pathogenicity on the outcome of C. jejuni infection. In this trial, the culling of birds was delayed to 10 days after C. jejuni challenge to assess if the changes observed in the C. jejuni load at 3 days (trials 1 and 2) were stable over a longer period. Additionally, to provide a semiquantitative comparison of the bacterial load between trials 1, 2, and 3, birds were swabbed cloacally at 3 days after C. jejuni challenge, as described previously (11). At 13 days of age, groups 1 and 4 received a high dose of 4,000 sporulated E. tenella Wis oocysts (nEh+/C+ and nEh+/C− chickens, respectively), while groups 2 and 5 received a low dose of 400 sporulated E. tenella Wis oocysts (nEl+/C+ and nEl+/C− chickens, respectively). Chickens in groups 3 and 6 were not infected with the parasite (E−/C+ and E−/C− chickens, respectively). At 18 days of age, groups 1, 3, and 5 were challenged with ∼108 CFU of C. jejuni, while groups 2, 4, and 6 were mock challenged with sterile MH broth. The daily oocyst output was assessed for each chicken at between 18 and 22 days of age. Chickens were culled at 10 days after bacterial challenge (28 days of age), and samples were collected as described above for trial 1.
(ii) Sample collection. Postmortem, 0.2 to 1.0 g of cecal contents and liver and spleen tissue was collected aseptically from the same approximately central part of each tissue/organ, placed into universal tubes, and stored separately on ice prior to homogenization in all trials. On the day of collection, all samples were weighed and homogenized in an equal volume (wt/vol) of sterile PBS using a TissueRuptor homogenizer (Qiagen, Hilden, Germany), followed by serial 10-fold dilution in PBS. Additionally, an ∼3-cm tissue section from the midpoint of one cecum, half the spleen, and an ∼1-cm3 section of the mid-liver were recovered from chickens in trial 2 and stored in RNAlater stabilization solution (Sigma), as recommended by the manufacturer, for subsequent RNA extraction and real-time quantitative PCR (RT-qPCR).
RNA extraction and integrity.
Total RNA was extracted from thawed tissue samples after storage at −20°C in RNAlater stabilization solution using an RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The optional DNase digestion step was included to remove contaminating genomic DNA. The RNA concentration was determined using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA), and samples were diluted in nuclease-free water to produce a final concentration of 40 ng/μl. The quality of a subset of samples (∼5%) was confirmed using an Agilent RNA 6000 Nano kit (Agilent Technologies, Waldbronn, Germany) following the manufacturer’s instructions, confirming RNA integrity number results in excess of 6 for further analysis.
RT-qPCR.
SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) was used to make cDNA, using total RNA purified from the samples collected, following the manufacturer’s instructions. Oligo(dT)12–18 (Invitrogen, Carlsbad, CA, USA) was used along with the optional RNaseOut recombinant ribonuclease inhibitor (Invitrogen) step. cDNA was used as the template in all RT-PCRs.
The oligonucleotide primer sequences used to target the cDNA copies of each of the mRNA transcripts investigated, including those for the genes for Muc2, Muc5ac, Muc13, IL-1β, IL-6, IFN-γ, IL-2, IL-10, IL-13, and inducible nitric oxide synthase (iNOS), and three reference transcripts are summarized in Table S1 in the supplemental material. The final reaction volumes for RT-qPCR consisted of 10 µl SsoFast EvaGreen super mix containing Sybr Green dye (Bio-Rad) and 70 nM each forward and reverse primer (Sigma-Aldrich) and were made up to 19 µl using RNase- and DNase-free water (Invitrogen, Paisley, UK). To 1 volume of this master mix, 1 µl of cDNA was added. As a negative control, 1 µl of water was used in place of cDNA. DNA was amplified on a Bio-Rad CFX (v2.0) cycler (Bio-Rad) in triplicate for every sample using the following conditions: 1 cycle at 95°C for 60 s, followed by 40 cycles of 95°C for 15 s and the appropriate annealing temperature (as indicated in Table S1) for 30 s. After completion, a melt curve was generated by running one cycle at 65°C for 0.05 s and 95°C for 0.5 s. The levels of the individual transcripts were normalized individually to those of the transcripts of the 3 reference genes and used to calculate a mean value for each replicate. Briefly, quantification cycle (Cq) values were generated for each sample using Bio-Rad CFX (v2.0) software and enabled quantification of cDNA when normalized to the values for the reference genes, consisting of the genes for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), TATA-binding protein (TATA-BP), and 28S rRNA.
Statistical analysis.
Statistical analyses, including calculation of arithmetic means, the associated standard deviation or standard error of the mean, analysis of variance, and associated post hoc Tukey tests, were performed using SPSS Statistics (v24) software (IBM). Bacterial counts were logarithmically transformed. Differences were considered significant when P was <0.05.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tom Jeffers for the provision of the E. tenella WisF96 parasite line.
This study was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) through grants BB/L004046/1 and BB/L00478X/1 and the London Interdisciplinary Doctoral Program (LIDo). S.E.M. was also supported by a Bloomsbury College Ph.D. scholarship.
The funding bodies had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00772-18.
The Royal Veterinary College has assigned this manuscript the reference number PPS_01844.
REFERENCES
- 1.Clark EL, Tomley FM, Blake DP. 2017. Are Eimeria genetically diverse, and does it matter? Trends Parasitol 33:231–241. doi: 10.1016/j.pt.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations. 2018. Food and Agriculture Organization of the United Nations FAOstat database. http://faostat3.fao.org/home/E. Accessed 15 October 2015.
- 3.Stanley D, Denman SE, Hughes RJ, Geier MS, Crowley TM, Chen HL, Haring VR, Moore RJ. 2012. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl Microbiol Biotechnol 96:1361–1369. doi: 10.1007/s00253-011-3847-5. [DOI] [PubMed] [Google Scholar]
- 4.Rubio LA, Peinado MJ, Ruiz R, Suárez-Pereira E, Ortiz Mellet C, García Fernández JM. 2015. Correlations between changes in intestinal microbiota composition and performance parameters in broiler chickens. J Anim Physiol Anim Nutr 99:418–423. doi: 10.1111/jpn.12256. [DOI] [PubMed] [Google Scholar]
- 5.Collier CT, Hofacre CL, Payne AM, Anderson DB, Kaiser P, Mackie RI, Gaskins HR. 2008. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol 122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Awad WA, Dublecz F, Hess C, Dublecz K, Khayal B, Aschenbach JR, Hess M. 2016. Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult Sci 95:2259–2265. doi: 10.3382/ps/pew151. [DOI] [PubMed] [Google Scholar]
- 7.Bull SA, Thomas A, Humphrey T, Ellis-Iversen J, Cook AJ, Lovell R, Jorgensen F. 2008. Flock health indicators and Campylobacter spp. in commercial housed broilers reared in Great Britain. Appl Environ Microbiol 74:5408–5413. doi: 10.1128/AEM.00462-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Kumar S, Ayoade S, Fornace KM, Jatau ID, Moftah A, Nolan MJ, Sudhakar NR, Adebambo AO, Lawal IA, Alvarez Zapata R, Awuni JA, Chapman HD, Karimuribo E, Mugasa CM, Namangala B, Rushton J, Suo X, Thangaraj K, Srinivasa Rao AS, Tewari AK, Banerjee PS, Dhinakar Raj G, Raman M, Tomley FM, Blake DP. 2016. Cryptic Eimeria genotypes are common across the Southern but not Northern Hemisphere. Int J Parasitol 46:537–544. doi: 10.1016/j.ijpara.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake DP, Tomley FM. 2014. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol 30:12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Baba E, Fukata T, Arakawa A. 1982. Establishment and persistence of Salmonella typhimurium infection stimulated by Eimeria tenella in chickens. Res Vet Sci 33:95–98. doi: 10.1016/S0034-5288(18)32366-X. [DOI] [PubMed] [Google Scholar]
- 11.Clark JD, Oakes RD, Redhead K, Crouch CF, Francis MJ, Tomley FM, Blake DP. 2012. Eimeria species parasites as novel vaccine delivery vectors: anti-Campylobacter jejuni protective immunity induced by Eimeria tenella-delivered CjaA. Vaccine 30:2683–2688. doi: 10.1016/j.vaccine.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Marugan-Hernandez V, Cockle C, Macdonald S, Pegg E, Crouch C, Blake DP, Tomley FM. 2016. Viral proteins expressed in the protozoan parasite Eimeria tenella are detected by the chicken immune system. Parasit Vectors 9:463. doi: 10.1186/s13071-016-1756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, Praet N, Bellinger DC, de Silva NR, Gargouri N, Speybroeck N, Cawthorne A, Mathers C, Stein C, Angulo FJ, Devleesschauwer B, World Health Organization Foodborne Disease Burden Epidemiology Reference Group. 2015. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med 12:e1001923. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen LN, Sheppard SK, McCarthy ND, Maiden MC, Ingmer H, Krogfelt KA. 2010. MLST clustering of Campylobacter jejuni isolates from patients with gastroenteritis, reactive arthritis and Guillain-Barre syndrome. J Appl Microbiol 108:591–599. doi: 10.1111/j.1365-2672.2009.04444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skarp CPA, Hänninen M-L, Rautelin HIK. 2016. Campylobacteriosis: the role of poultry meat. Clin Microbiol Infect 22:103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Newell DG, Fearnley C. 2003. Sources of Campylobacter colonization in broiler chickens. Appl Environ Microbiol 69:4343–4351. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnsen G, Kruse H, Hofshagen M. 2006. Genetic diversity and description of transmission routes for Campylobacter on broiler farms by amplified-fragment length polymorphism. J Appl Microbiol 101:1130–1139. doi: 10.1111/j.1365-2672.2006.02995.x. [DOI] [PubMed] [Google Scholar]
- 18.Cawthraw SA, Newell DG. 2010. Investigation of the presence and protective effects of maternal antibodies against Campylobacter jejuni in chickens. Avian Dis 54:86–93. doi: 10.1637/9004-072709-Reg.1. [DOI] [PubMed] [Google Scholar]
- 19.Young KT, Davis LM, Dirita VJ. 2007. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol 5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 20.Hermans D, Van Deun K, Martel A, Van Immerseel F, Messens W, Heyndrickx M, Haesebrouck F, Pasmans F. 2011. Colonization factors of Campylobacter jejuni in the chicken gut. Vet Res 42:82. doi: 10.1186/1297-9716-42-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Deun K, Pasmans F, Ducatelle R, Flahou B, Vissenberg K, Martel A, Van den Broeck W, Van Immerseel F, Haesebrouck F. 2008. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet Microbiol 130:285–297. doi: 10.1016/j.vetmic.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison M, Harrison D, Richardson I, Tchorzewska M. 2015. A method for the preparation of chicken liver pate that reliably destroys campylobacters. Int J Environ Res Public Health 12:4652–4669. doi: 10.3390/ijerph120504652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little CL, Gormley FJ, Rawal N, Richardson JF. 2010. A recipe for disaster: outbreaks of campylobacteriosis associated with poultry liver pate in England and Wales. Epidemiol Infect 138:1691–1694. doi: 10.1017/S0950268810001974. [DOI] [PubMed] [Google Scholar]
- 24.Hong YH, Lillehoj HS, Lee SH, Dalloul RA, Lillehoj EP. 2006. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet Immunol Immunopathol 114:209–223. doi: 10.1016/j.vetimm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Rose ME, Hesketh P, Wakelin D. 1992. Immune control of murine coccidiosis: CD4+ and CD8+ T lymphocytes contribute differentially in resistance to primary and secondary infections. Parasitology 105:349–354. doi: 10.1017/S0031182000074515. [DOI] [PubMed] [Google Scholar]
- 26.Vervelde L, Vermeulen AN, Jeurissen SH. 1996. In situ characterization of leucocyte subpopulations after infection with Eimeria tenella in chickens. Parasite Immunol 18:247–256. doi: 10.1046/j.1365-3024.1996.d01-94.x. [DOI] [PubMed] [Google Scholar]
- 27.Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, Smith AL, Kaiser P. 2004. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol 173:2675–2682. doi: 10.4049/jimmunol.173.4.2675. [DOI] [PubMed] [Google Scholar]
- 28.Bereswill S, Plickert R, Fischer A, Kuhl AA, Loddenkemper C, Batra A, Siegmund B, Gobel UB, Heimesaat MM. 2011. What you eat is what you get: novel Campylobacter models in the quadrangle relationship between nutrition, obesity, microbiota and susceptibility to infection. Eur J Microbiol Immunol (Bp) 1:237–248. doi: 10.1556/EuJMI.1.2011.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdonald SE, Nolan MJ, Harman K, Boulton K, Hume DA, Tomley FM, Stabler RA, Blake DP. 2017. Effects of Eimeria tenella infection on chicken caecal microbiome diversity, exploring variation associated with severity of pathology. PLoS One 12:e0184890. doi: 10.1371/journal.pone.0184890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones AK, Rigby D, Burton M, Millman C, Williams NJ, Jones TR, Wigley P, O'Brien SJ, Cross P, ENIGMA Consortium. 2016. Restaurant cooking trends and increased risk for Campylobacter infection. Emerg Infect Dis 22:1208–1215. doi: 10.3201/eid2207.151775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphrey S, Chaloner G, Kemmett K, Davidson N, Williams N, Kipar A, Humphrey T, Wigley P. 2014. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio 5:e01364-14. doi: 10.1128/mBio.01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith CK, Abuoun M, Cawthraw SA, Humphrey TJ, Rothwell L, Kaiser P, Barrow PA, Jones MA. 2008. Campylobacter colonization of the chicken induces a proinflammatory response in mucosal tissues. FEMS Immunol Med Microbiol 54:114–121. doi: 10.1111/j.1574-695X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- 33.Shaughnessy RG, Meade KG, Cahalane S, Allan B, Reiman C, Callanan JJ, O'Farrelly C. 2009. Innate immune gene expression differentiates the early avian intestinal response between Salmonella and Campylobacter. Vet Immunol Immunopathol 132:191–198. doi: 10.1016/j.vetimm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Laurent F, Mancassola R, Lacroix S, Menezes R, Naciri M. 2001. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect Immun 69:2527–2534. doi: 10.1128/IAI.69.4.2527-2534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iovine NM, Pursnani S, Voldman A, Wasserman G, Blaser MJ, Weinrauch Y. 2008. Reactive nitrogen species contribute to innate host defense against Campylobacter jejuni. Infect Immun 76:986–993. doi: 10.1128/IAI.01063-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Hu T, Rothwell L, Vervelde L, Kaiser P, Boulton K, Nolan MJ, Tomley FM, Blake DP, Hume DA. 2016. Analysis of the function of IL-10 in chickens using specific neutralising antibodies and a sensitive capture ELISA. Dev Comp Immunol 63:206–212. doi: 10.1016/j.dci.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaezirad MM, Keestra-Gounder AM, de Zoete MR, Koene MG, Wagenaar JA, van Putten JPM. 2017. Invasive behavior of Campylobacter jejuni in immunosuppressed chicken. Virulence 8:248–260. doi: 10.1080/21505594.2016.1221559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F. 2011. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol 40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- 39.Witlock DR, Lushbaugh WB, Danforth HD, Ruff MD. 1975. Scanning electron microscopy of the cecal mucosa in Eimeria-tenella-infected and uninfected chickens. Avian Dis 19:293–304. doi: 10.2307/1588983. [DOI] [PubMed] [Google Scholar]
- 40.Witlock DR. 1983. Physiologic basis of blood loss during Eimeria tenella infection. Avian Dis 27:1043–1050. doi: 10.2307/1590205. [DOI] [PubMed] [Google Scholar]
- 41.Palyada K, Threadgill D, Stintzi A. 2004. Iron acquisition and regulation in Campylobacter jejuni. J Bacteriol 186:4714–4729. doi: 10.1128/JB.186.14.4714-4729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pickett CL, Auffenberg T, Pesci EC, Sheen VL, Jusuf SSD. 1992. Iron acquisition and hemolysin production by Campylobacter jejuni. Infect Immun 60:3872–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirley MW, Millard BJ. 1986. Studies on the immunogenicity of 7 attenuated lines of Eimeria given as a mixture to chickens. Avian Pathol 15:629–638. doi: 10.1080/03079458608436326. [DOI] [PubMed] [Google Scholar]
- 44.McDonald V, Shirley MW, Millard BJ. 1986. A comparative study of two lines of Eimeria tenella attenuated either by selection for precocious development in the chicken or by growth in chicken embryos. Avian Pathol 15:323–335. doi: 10.1080/03079458608436296. [DOI] [PubMed] [Google Scholar]
- 45.Williams RB. 2001. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int J Parasitol 31:1056–1069. doi: 10.1016/S0020-7519(01)00235-1. [DOI] [PubMed] [Google Scholar]
- 46.Nolan MJ, Tomley FM, Kaiser P, Blake DP. 2015. Quantitative real-time PCR (qPCR) for Eimeria tenella replication—implications for experimental refinement and animal welfare. Parasitol Int 64:464–470. doi: 10.1016/j.parint.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanley D, Geier MS, Chen H, Hughes RJ, Moore RJ. 2015. Comparison of fecal and cecal microbiotas reveals qualitative similarities but quantitative differences. BMC Microbiol 15:51. doi: 10.1186/s12866-015-0388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ. 2014. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One 9:e91941. doi: 10.1371/journal.pone.0091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meade KG, Narciandi F, Cahalane S, Reiman C, Allan B, O'Farrelly C. 2009. Comparative in vivo infection models yield insights on early host immune response to Campylobacter in chickens. Immunogenetics 61:101–110. doi: 10.1007/s00251-008-0346-7. [DOI] [PubMed] [Google Scholar]
- 50.Biswas D, Fernando UM, Reiman CD, Willson PJ, Townsend HGG, Potter AA, Allan BJ. 2007. Correlation between in vitro secretion of virulence-associated proteins of Campylobacter jejuni and colonization of chickens. Curr Microbiol 54:207–212. doi: 10.1007/s00284-006-0295-z. [DOI] [PubMed] [Google Scholar]
- 51.Wassenaar TM, van der Zeijst BAM, Ayling R, Newell DG. 1993. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J Gen Microbiol 139:1171–1175. doi: 10.1099/00221287-139-6-1171. [DOI] [PubMed] [Google Scholar]
- 52.Shortt C, Scanlan E, Hilliard A, Cotroneo CE, Bourke B, Croinin TO. 2016. DNA supercoiling regulates the motility of Campylobacter jejuni and is altered by growth in the presence of chicken mucus. mBio 7:e01227-16. doi: 10.1128/mBio.01227-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermans D, Martel A, Van Deun K, Verlinden M, Van Immerseel F, Garmyn A, Messens W, Heyndrickx M, Haesebrouck F, Pasmans F. 2010. Intestinal mucus protects Campylobacter jejuni in the ceca of colonized broiler chickens against the bactericidal effects of medium-chain fatty acids. Poult Sci 89:1144–1155. doi: 10.3382/ps.2010-00717. [DOI] [PubMed] [Google Scholar]
- 54.Van Deun K, Haesebrouck F, Van Immerseel F, Ducatelle R, Pasmans F. 2008. Short-chain fatty acids and l-lactate as feed additives to control Campylobacter jejuni infections in broilers. Avian Pathol 37:379–383. doi: 10.1080/03079450802216603. [DOI] [PubMed] [Google Scholar]
- 55.Szymanski CM, King M, Haardt M, Armstrong GD. 1995. Campylobacter jejuni motility and invasion of Caco-2 cells. Infect Immun 63:4295–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Byrne CM, Clyne M, Bourke B. 2007. Campylobacter jejuni adhere to and invade chicken intestinal epithelial cells in vitro. Microbiology 153:561–569. doi: 10.1099/mic.0.2006/000711-0. [DOI] [PubMed] [Google Scholar]
- 57.Lang T, Hansson GC, Samuelsson T. 2006. An inventory of mucin genes in the chicken genome shows that the mucin domain of Muc13 is encoded by multiple exons and that ovomucin is part of a locus of related gel-forming mucins. BMC Genomics 7:197. doi: 10.1186/1471-2164-7-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hyman HC, Levisohn S, Yogev D, Razin S. 1989. DNA probes for Mycoplasma gallisepticum and Mycoplasma synoviae: application in experimentally infected chickens. Vet Microbiol 20:323–337. doi: 10.1016/0378-1135(89)90057-6. [DOI] [PubMed] [Google Scholar]
- 59.De Lisle RC, Roach E, Jansson K. 2007. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol 293:G577–G584. doi: 10.1152/ajpgi.00195.2007. [DOI] [PubMed] [Google Scholar]
- 60.Zarepour M, Bhullar K, Montero M, Ma C, Huang T, Velcich A, Xia L, Vallance BA. 2013. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect Immun 81:3672–3683. doi: 10.1128/IAI.00854-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stahl M, Vallance BA. 2015. Insights into Campylobacter jejuni colonization of the mammalian intestinal tract using a novel mouse model of infection. Gut Microbes 6:143–148. doi: 10.1080/19490976.2015.1016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDougald LR, Jeffers TK. 1976. Comparative in vitro development of precocious and normal strains of Eimeria tenella (Coccidia). J Protozool 23:530–534. doi: 10.1111/j.1550-7408.1976.tb03834.x. [DOI] [PubMed] [Google Scholar]
- 63.McDougald LR, Jeffers TK. 1976. Eimeria tenella (Sporozoa, Coccidia): gametogony following a single asexual generation. Science 192:258–259. doi: 10.1126/science.1257765. [DOI] [PubMed] [Google Scholar]
- 64.Long P, Joyner L, Millard B, Norton C. 1976. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Latina 6:201–217. [PubMed] [Google Scholar]
- 65.Guccione E, Leon-Kempis MDR, Pearson BM, Hitchin E, Mulholland F, van Diemen PM, Stevens MP, Kelly DJ. 2008. Amino acid-dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol Microbiol 69:77–93. doi: 10.1111/j.1365-2958.2008.06263.x. [DOI] [PubMed] [Google Scholar]
- 66.Blake DP, Hesketh P, Archer A, Shirley MW, Smith AL. 2006. Eimeria maxima: the influence of host genotype on parasite reproduction as revealed by quantitative real-time PCR. Int J Parasitol 36:97–105. doi: 10.1016/j.ijpara.2005.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


