Abstract
Background/Aim
Presently, there are no therapeutic options for unresectable hepatocellular carcinoma (u‐HCC) patients who are intolerant to sorafenib or regorafenib failure. There have been no reports with detailed clinical findings of lenvatinib (LEN), a newly developed first‐line tyrosine kinase inhibitor (TKI), obtained in real‐world practice. We aimed to elucidate the therapeutic efficacy of LEN.
Materials/Methods
From March to August 2018, 105 u‐HCC patients were treated with LEN. Following exclusion of those who started with a reduced LEN dose and/or had a short observation period (<2 weeks), 77 patients (72.0 ± 8.9 years, 59 males, 8 mg/12 mg = 49/28, Liver Cancer Study Group of Japan 6th [LCSGJ]‐TNM stage II/III/IVa/IVb = 8/28/4/37, and American Joint Committee on Cancer/Union for International Cancer Control 8th [AJCC/UICC]‐TNM stage IB:II:IIIA:IIIB:IVA:IVB = 2:27:6:5:9:28) were divided into two groups (TKI naïve [n = 33] and TKI experienced [n = 44], including 11 with regorafenib history). Therapeutic response was evaluated using mRECIST. Clinical data were retrospectively evaluated.
Results
There were significant differences in age (74.6 ± 11.2 vs 70.0 ± 5.9 years, P = 0.040), LCSGJ‐TNM (II:III:IVa:IVb = 8:12:1:12 vs 0:16:3:25, P = 0.006), and AJCC/UICC‐TNM (IB:II:IIIA:IIIB:IVA:IVB = 2:17:1:1:4:8 vs 0:10:5:4:5:20, P = 0.028), while hepatic reserve function, adverse event (AE) profiles, and progression‐free survival (89.7%/80.4% vs 90.5%/80.1%, P = 0.499) and overall survival (96.7%/96.7% vs 100%/92.3%, P = 0.769) after 4 and 12 weeks were not significantly different between the TKI‐naïve and TKI‐experienced groups. Overall response rate and disease control rate at 4 weeks (n = 52) were 38.5% and 80.8%, respectively, and 32.4% and 70.3%, respectively, at 12 weeks (n = 37). A significant decline in log10 AFP from the baseline to 4 weeks after introducing LEN was observed in patients with PR and SD (2.047 ± 1.148 vs 1.796 ± 1.179, P < 0.001).
Conclusion
Regardless of past TKI therapy, therapeutic response and AEs after introducing LEN were similar. LEN may be an important treatment for the present unmet need regarding TKI treatment against u‐HCC.
Keywords: adverse event, albumin‐bilirubin grade, alpha‐fetoprotein, hand‐foot skin reaction, hepatocellular carcinoma, lenvatinib, muscle volume, regorafenib, sorafenib
1. INTRODUCTION
For treatment of unresectable hepatocellular carcinoma (u‐HCC), tyrosine kinase inhibitors (TKIs), such as sorafenib (SOR)1, 2 and regorafenib (REG),3, 4 have been introduced. SOR was developed as a first‐line agent for u‐HCC, while REG is used as second‐line therapy in patients who show good tolerability and progressive disease (PD) with SOR. Recently, lenvatinib (LEN), a new TKI,5, 6 has become available as a first‐line drug for u‐HCC. However, though the number of TKIs for u‐HCC has increased, there is presently no therapeutic option for patients with SOR failure or who show intolerability for SOR as well as failure with REG, indicating an important and practical unmet need. Following the introduction of LEN in clinical practice in Japan in March 2018, the drug has been used in real‐world practice not only for TKI‐naïve but also for TKI‐experienced patients as second‐ or third‐line treatment.7 On the other hand, therapeutic response and adverse events (AEs) associated with LEN treatment for u‐HCC patients with and without a past history of TKI have not been elucidated. In the present study, we analyzed clinical features of patients who received LEN treatment for u‐HCC for therapeutic response and AEs.
2. MATERIALS AND METHODS
From March to August 2018, LEN (Lenvima®, Eisai Co., Ltd., Tokyo, Japan) was given to 105 u‐HCC Japanese patients receiving treatment at 13 different institutions in Japan (Ehime Prefectural Central Hospital [n = 19], Okayama City Hospital [n = 12], Kagawa Prefectural Central Hospital [n = 12], Nippon Medical School Hospital [n = 12], Asahi General Hospital [n = 10], Teine Keijinkai Hospital [n = 7], Ogaki Municipal Hospital [n = 6], Toyama University Hospital [n = 6], Ehime University Hospital [n = 6], Otakanomori Hospital [n = 5], Tokushima Prefectural Central Hospital [n = 4], Saiseikai Niigata Daini Hospital [n = 3], Matsuyama Red Cross Hospital n = 3]). We retrospectively examined the records of those patients and collected clinical data obtained at the introduction of LEN, as well as after 2 weeks and every 4 weeks thereafter. Following exclusion of those who started with a reduced LEN dose and/or had a short observation period (<2 weeks), 77 patients were enrolled and divided into two groups, TKI naïve (n = 33) and TKI experienced (n = 44), according to their past history with TKI treatments. Flow diagram of enrolled patients is shown in Figure 1. Clinical characteristics, therapeutic response including progression‐free survival rate (PFSR) and overall survival rate (OSR), and AEs were analyzed in a retrospective manner.
Figure 1.
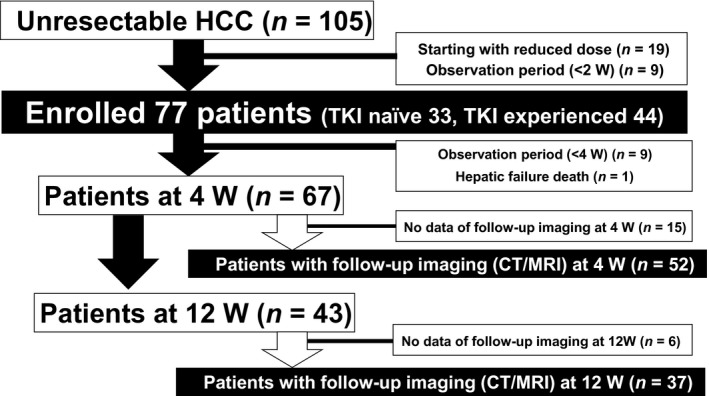
Flow diagram of enrolled patients. HCC, hepatocellular carcinoma; TKI, tyrosine kinase inhibitor; W, weeks
Patients positive for anti‐hepatitis C virus (HCV) were judged to have HCC due to HCV, while those positive for hepatitis B virus surface antigen (HBsAg) were judged to have HCC due to hepatitis B virus (HBV).
2.1. Assessment of hepatic reserve function and prognosis.
Child‐Pugh classification8 and ALBI grade were used to assess hepatic reserve function. ALBI grade was calculated based on serum albumin and total bilirubin values using the following formula: [ALBI score = (log10 bilirubin (µmol/L) × 0.66) + (albumin (g/L) × −0.085)], and defined by the following scores: ≤−2.60 = Grade 1,>−2.60 to ≤−1.39 = Grade 2,>−1.39 = Grade 3.9, 10 For a more detailed evaluation of the middle grade of ALBI (grade 2), we used a modified ALBI (mALBI) grade by creating 4 grades, including sub‐grading for the middle grade (2a and 2b), with an ALBI score of −2.270, which was reported as the cutoff value for indocyanine green retention 15 minutes 30%, used as the value for dividing 2a and 2b.12, 13
2.2. Assessment of muscle volume
Muscle volume loss (MVL) was determined using the following previously reported index: [psoas index (PI): bilateral psoas muscle area of middle L3 level (cm2)/height (m)2; cutoff values for MVL of males = 4.24 cm2/m2, females = 2.50 cm2/m2], based on CT findings.14 PI was manually calculated using psoas muscle area at the middle L3 level in CT findings with personal computer software (Centricity Web DX ver.3.7.3.6417: GE Healthcare Japan, Tokyo, Japan, or OsiriX DICOM Viewer MD 9.5: https://www.osirix-viewer.com). Muscle volume was calculated using computed tomography (CT) findings obtained at the start of LEN and again after 4 and 12 weeks in patients for whom CT imaging data were available.
2.3. Diagnosis and treatment of HCC
HCC was diagnosed based on an increasing course of alpha‐fetoprotein (AFP), as well as dynamic CT,15 magnetic resonance imaging (MRI),16, 17 contrast‐enhanced ultrasonography (CEUS) with perflubutane (Sonazoid®, Daiichi Sankyo Co., Ltd. Tokyo, Japan),18, 19 and/or pathological findings. American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) tumor node metastasis (TNM) stage was used for evaluation of tumor progression as well as TNM stage, which was determined as previously reported in studies for staging of HCC conducted by the Liver Cancer Study Group of Japan (LCSGJ).20 The present study protocol was approved by the Institutional Ethics Committee of Ehime Prefectural Central Hospital (No. 29‐75).
2.4. LEN treatment and assessment of AEs
After obtaining written informed consent, LEN was orally administered at 8 mg/d to patients weighing <60 kg and 12 mg/d to those ≥60 kg, and discontinued when any unacceptable or serious AE or clinical tumor progression was observed. According to the guidelines for administration of LEN, the drug dose should be reduced or treatment interrupted when a patient develops any grade 3 or more severe AE or if any unacceptable grade 2 drug‐related AE occurs. AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.21 The worst grade for each AE during the present observation period was recorded. If a drug‐related AE occurred, dose reduction or temporary interruption was maintained until the symptom was resolved to grade 1 or 2, according to the guidelines provided by the manufacturer.
2.5. Evaluation of therapeutic response
Local physicians at each institution evaluated tumors using enhanced CT or MRI results obtained at 4, 8, or 12 weeks after introducing LEN, in accordance with the modified RECIST guidelines.22, 23
2.6. Statistical analysis
Data are expressed as the mean and standard deviation (SD). Statistical analyses were performed using Welch's t test, Fischer's exact test, Mann‐Whitney's U test, a paired t test, Wilcoxon signed‐rank test, Kaplan‐Meyer method, and a log‐rank test. A P value less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using Easy R (EZR), version 1.29 (Saitama Medical Center, Jichi Medical University, Saitama, Japan),24 a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
Following exclusion of patients whose initial LEN dose was reduced or those with a short observation period <2 weeks, a total of 77 were analyzed and their characteristics are shown in Table 1. The average observation period for all subjects after introduction of LEN was 79.3 ± 40.7 days. Forty‐four (57.1%) had a past history of SOR treatment (TKI‐experienced group), including 11 (14.4%) who were also treated with REG as second‐line therapy.
Table 1.
Characteristics of patients without a reduced lenvatinib dose starting and with observation period more than 2 wk
| All (n = 77) | TKI naïve (n = 33) | TKI experienced (n = 44) | P value | |
|---|---|---|---|---|
| Age (y) | 72.0 ± 8.9 | 74.6 ± 11.2 | 70.0 ± 5.9 | 0.040 |
| Gender (male:female) | 59:18 | 26:7 | 33:11 | 0.705 |
| BMI (kg/m2) | 22.2 ± 4.2 | 22.6 ± 3.4 | 21.9 ± 4.8 | 0.439 |
| ECOG PS (0:1:2) | 67:8:2 | 28:4:1 | 39:4:1 | 0.634 |
| Etiology (HCV:HBV:alcohol:others) | 38:14:12:13 | 14:6:5:8 | 24:8:7:5 | 0.188 |
| AST (IU/L) | 57.2 ± 67.7 | 48.8 ± 26.0 | 63.6 ± 86.6 | 0.289 |
| ALT (IU/L) | 43.9 ± 50.3 | 42.7 ± 36.3 | 44.8 ± 59.2 | 0.853 |
| Platelets (×104/µL) | 14.7 ± 5.5 | 14.1 ± 5.8 | 15.1 ± 5.4 | 0.434 |
| Total bilirubin (mg/dL) | 0.8 ± 0.4 | 0.8 ± 0.3 | 0.9 ± 0.5 | 0.732 |
| Albumin (g/dL) | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.5 | 0.498 |
| Prothrombin time (%) | 90.6 ± 13.3 | 89.9 ± 13.5 | 91.2 ± 13.3 | 0.673 |
| Child‐Pugh score (5:6:7:8) | 42:25:9:1 | 19:11:3:0 | 23:14:6:1 | 0.512 |
| ALBI grade (1:2:3) | 26:49:2 | 11:21:1 | 15:28:1 | 0.917 |
| [mALBI grade (1:2a:2b:3)] | [26:19:30:2] | [11:7:14:1] | [15:12:16:1] | 0.690 |
| (ALBI score) | (−2.32 ± 0.49) | (−2.36 ± 0.49) | (−2.30 ± 0.49) | 0.577 |
| AFP (ng/mL) | 3248.2 ± 15406.1 | 2515.9 ± 8252.5 | 3797.4 ± 19185.9 | 0.693 |
| Intrahepatic tumor size (cm) | 4.1 ± 4.5 | 2.8 ± 2.3 | 5.1 ± 5.5 | 0.019 |
| Number of intrahepatic tumors (none:single:multiple) | 9:4:64 | 5:3:25 | 4:1:39 | 0.326 |
| TNM stage, AJCC/UICC 8th (IB:II:IIIA:IIIB:IVA:IVB) | 2:27:6:5:9:28 | 2:17:1:1:4:8 | 0:10:5:4:5:20 | 0.028 |
| TNM stage, LCSGJ 6th (II:III:IV) [IVa:IVb] |
8:28:41 [4:37] |
8:12:13 [1:12] |
0:16:28 [3:25] |
0.006 |
| Positive for MVI (Vp1:Vp2:Vp3:Vp4:Vv1:Vv2:Vv3)a |
17 (22.1%) (2:5:4:3:1:2:4) |
2 (6.1%) (0:1:1:0:0:0) |
15 (34.1%) (2:4:3:3:1:2:4) |
0.005 |
| Positive for EHM (lung:LN: bone:peritoneum:adrenal gland:others)a |
37 (48.1%) 11:15:9:5:2:2 |
12 (36.4%) (1:5:1:4:0:2) |
25 (56.8%) (10:10:8:1:2:0) |
0.107 |
| naïve:recurrence | 2:75 (median number of past treatments 5) | 2:31 | 0:44 | 0.180 |
| Past history of hypertension | 33 (42.9%) | 9(27.3%) | 24 (54.5%) | 0.021 |
| Past history of diabetes mellitus | 21 (27.3%) | 11 (33.3%) | 10 (22.7%) | 0.316 |
| Past history of SOR and REG | 44 (57.1%) | — | 11 (14.4%) (REG) | — |
| Initial dose of LEN (8:12 mg/d) | 49:28 | 22:11 | 27:17 | 0.811 |
| Average observation period after starting LEN (d) | 79.3 ± 40.7 | 67.1 ± 41.9 | 88.4 ± 37.7 | 0.025 |
AFP, alpha‐fetoprotein; AJCC/UICC 8th, American Joint Committee on Cancer/Union for International Cancer Control, 8th edition; ALBI grade, albumin‐bilirubin grade; ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status; EHM, extra‐hepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; LCSGJ 6th, the Liver Cancer Study Group of Japan, 6th edition; LEN, lenvatinib; LN, lymph node; MVI, major venous invasion; REG, regorafenib; SOR, sorafenib; TNM stage, tumor node metastasis stage.
Overlapping cases.
The first evaluation of the therapeutic effect of LEN using an imaging modality was performed in 52 patients at 4 weeks after introduction of the drug. Complete response (CR) was noted in none, partial response (PR) in 20, stable disease (SD) in 22, and PD in 10 (overall response rate [ORR]: 38.5%, disease control rate [DCR]: 80.8%). In results of CT/MRI imaging of 37 patients evaluated at 12 weeks, CR was noted in 1, PR in 11, SD in 14, and PD in 11 (ORR: 32.4% and DCR: 70.3%). At 4, 8, and 12 weeks, PFSR was 90.1%, 82.1%, and 80.1%, respectively (Figure 2A), while OSR was 98.6%, 96.9%, and 93.4%, respectively (Figure 2B). Although older age, shorter observation period, lower frequency of past history of hypertension, and reduced tumor burden shown by lower TNM stage with both systems were noted in the TKI‐naïve group as compared to the experienced group, there were no significant differences between them in regard to clinical characteristics, especially hepatic reserve function (Child‐Pugh score, ALBI score/grade, mALBI grade) (Table 1). As a result, PFSR and OSR at 4, 8, and 12 weeks were not significantly different between the TKI‐naïve and TKI‐experienced groups (PFSR: naïve group, 89.7%, 80.4%, 80.4% vs experienced group, 90.5%, 83.0%, 80.1%, respectively, P = 0.499; OSR: 96.7%, 96.7%, 96.7% vs 100%, 97.4%, 92.3%, respectively, P = 0.769) (Figure 3A,B). A significant decline in log10 AFP from the baseline to 4 weeks after introducing LEN was observed in all patients who obtained disease control (PR:SD = 20:22) (baseline vs 4 weeks: 2.047 ± 1.148 vs 1.796 ± 1.179, P < 0.001). After exclusion of those with normal AFP level (<10 ng/mL) (n = 13), the significant relative decline remained (baseline vs 4 weeks: 2.662 ± 0.798 vs 2.393 ± 0.946, P = 0.002) (n = 29).
Figure 2.
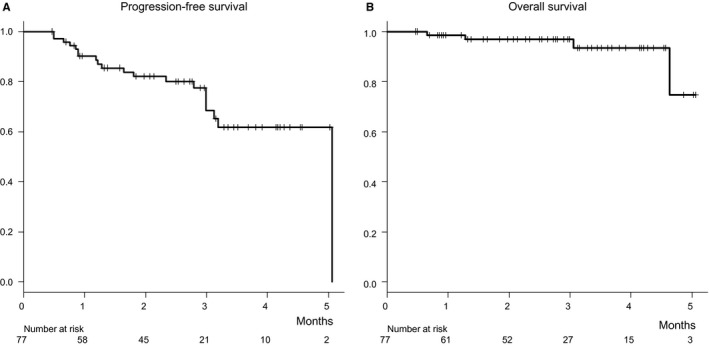
Progression‐free and overall survivals. After 4, 8, and 12 wk of treatment, the progression‐free survival rate (PFSR) was 90.1%, 82.1%, and 80.1%, respectively (A), while overall survival rate (OSR) was 98.6%, 96.9%, and 93.4%, respectively (B)
Figure 3.
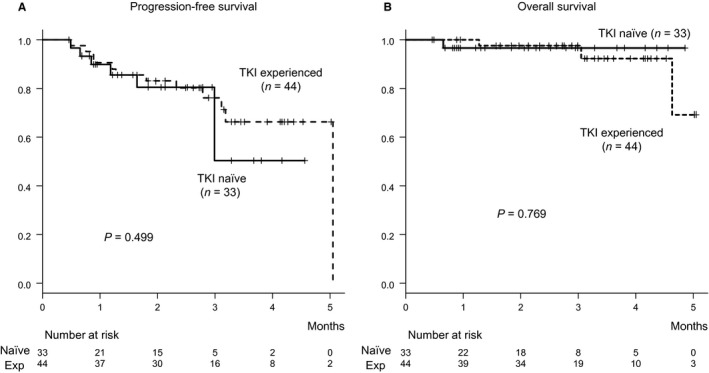
Progression‐free and overall survivals for tyrosine kinase inhibitor‐naïve and TKI‐experienced groups. After 4, 8, and 12 wk of treatment, the progression‐free survival rate (PFSR) and overall survival (OSR) were not significantly different between the tyrosine kinase inhibitor (TKI)‐naïve (solid line) and TKI‐experienced groups (broken line) (PFSR: 89.7%/80.4%/80.4% vs 90.5%/83.0%/80.1%, P = 0.499; OSR: 96.7%/96.7%/96.7% vs 100%/97.4%/92.3%, P = 0.769)
After starting LEN treatment, patients with Child‐Pugh class A had worsened to Child‐Pugh B or C in 23.4% of patients at 4 (P < 0.001) and in 23.7% at 12 weeks (P = 0.002) (Figure 4). Furthermore, a significant decline in ALBI score from the baseline (−2.32 ± 0.49) was observed at 4 (−2.18 ± 0.54 [n = 67], P < 0.001) and 12 (−2.10 ± 0.55 [n = 43], P < 0.001) weeks after the start of LEN treatment. AEs that occurred following the start of LEN in these patients are presented in Table 2. HFSR was the most common in the present cohort (all grades: n = 31 [40.3%], grade 3: n = 7 [9.1%]), followed by general fatigue (grades 1/2: n = 26 [33.8%]), appetite loss (all grades: n = 22 [28.6%], grade 3: n = 5 [6.5%]), hoarseness (all grades: n = 17 [22.1%], grade 3: none), and hypothyroidism (all grades: n = 15 [19.5%], grade 3: none). In 3 (3.9%) patients, destructive thyroiditis was observed (grade 2, n = 1; grade 3, n = 2).25 Although there was no significant relative change in regard to the level of FT4 (baseline vs 4 weeks: 2.0 ± 4.1 vs 1.1 ± 0.5 ng/dL, P = 0.107), TSH level was significantly elevated from the baseline after the first 4 weeks of LEN treatment (baseline vs 4 weeks: 5.9 ± 12.5 vs 15.2 ± 29.7 µIU/mL, P = 0.003). Also, the levels of NH3, serum amylase, and estimated glomerular filtration rate (eGFR) did not show significant changes within the first 4 weeks (NH3: 47.7 ± 27.1 vs 47.9 ± 32.7 µg/dL, P = 0.548; serum amylase: 88.6 ± 38.4 vs 101.1 ± 19.9 U/L, P = 0.809; eGFR: 75.7 ± 19.6 vs 73.0 vs 21.2 mL/min/1.73 m2, P = 0.279). Among 45 patients who experienced down‐dosing or a pause in LEN treatment due to an AE during the present observation period, LEN was abandoned in 17 (PD, n = 5; AEs, n = 12). The median period to initial dose reduction or pause of LEN was 40 days. Of 8 with an HFSR with a grade of 3 in previous treatments with a TKI, 87.5% also had an HFSR (grade 3, 2, 1; n = 2, 3, 2, respectively) with LEN treatment, while 44.4% of 18 with an HFSR with a grade of 2 during previous TKI treatments had an HFSR with LEN (grade 3, 2, 1; n = 3, 3, 2, respectively) (Figure 5).
Figure 4.
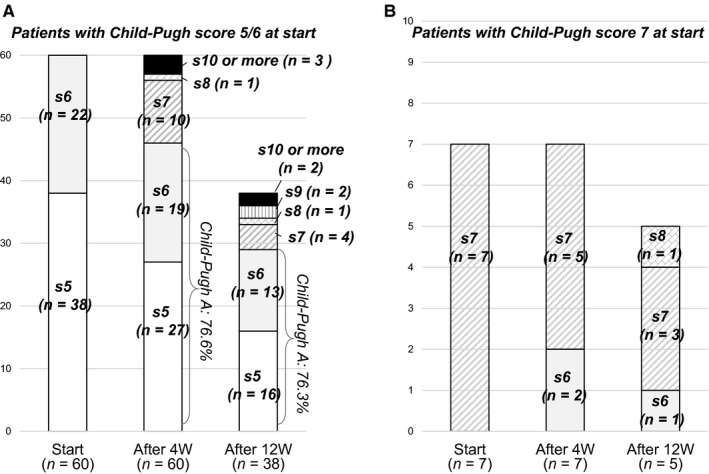
Child‐Pugh score at start, then 4 and 12 weeks after introducing lenvatinib. After starting LEN treatment, Child‐Pugh class A had worsened to Child‐Pugh B or C 23.4% patients at 4 wk (P < 0.001) and 23.7% at 12 wk (P = 0.002)
Table 2.
Adverse events of lenvatinib treatment
| All patients (n = 77) | TKI naïve (n = 33) | TKI experienced (n = 44) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| G1/2 | G3/4 | Any grade (%) | G1/2 | G3/4 | G1/2 | G3/4 | ||
| HFSRa | 24 | 7 | 31 (40.3) | 6/4 | 1/0 | 6/8 | 6/0 | 0.288 |
| General fatiguea | 26 | — | 26 (33.8) | 3/6 | — | 7/8 | — | 0.727 |
| Appetite lossa | 17 | 5 | 22 (28.6) | 1/4 | 2/0 | 6/6 | 3/0 | 0.451 |
| Hoarsenessa | 17 | 0 | 17 (22.1) | 9/2 | 0/— | 5/1 | 0/— | 0.104 |
| Hypothyroidisma | 15 | 0 | 15 (19.5) | 2/4 | 0/0 | 0/9 | 0/0 | 0.199 |
| Destructive thyroiditisa | 1 | 2 | 3 (3.9) | 0/0 | 1/0 | 0/1 | 1/0 | 1.000 |
| Hypertensiona | 9 | 5 | 14 (18.2) | 2/3 | 2/0 | 0/4 | 3/0 | 0.825 |
| Diarrheaa | 12 | 1 | 13 (16.7) | 2/2 | 0/0 | 6/2 | 1/0 | 0.735 |
| Urine proteina | 8 | 3 | 11 (14.3) | 1/3 | 2/— | 2/2 | 1/— | 0.675 |
| Hyperammonemia/hepatic comaa | 3 | 2 | 5 (6.5) | 1/1 | 1/0 | 0/1 | 1/0 | 0.788 |
| Fever within 2 wka | 2 | 1 | 3 (3.9) | 0/0 | 0/0 | 1/1 | 1/0 | 0.132 |
| Othersa | 24 | 9 | 33 | 2/2 | 5/1b | 13/7 | 3/0 | |
G, grade; HFSR, hand‐foot skin reaction; TKI, tyrosine kinase inhibitor; —: no setting for the applicable grade in the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.0.
Some events are overlapped.
Case of hypoglycemia due to appetite loss.
Figure 5.
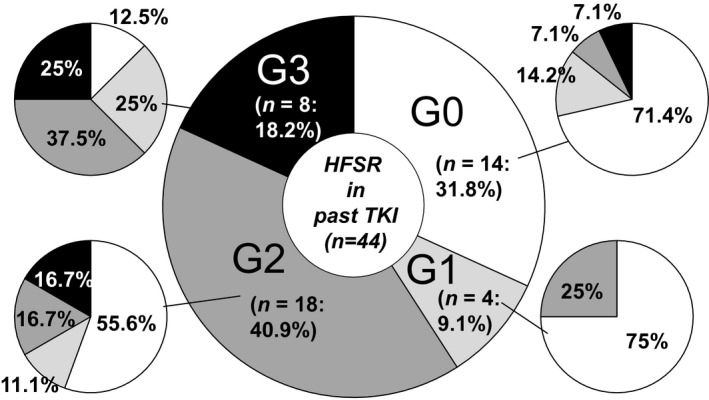
Hand‐foot skin reaction occurrence in tyrosine kinase inhibitor‐experienced group. Of 8 patients who had undergone past tyrosine kinase inhibitor (TKI) treatments and developed a grade 3 hand‐foot skin reaction (HFSR), 87.5% had an HFSR with lenvatinib (LEN) therapy (G3, 2, 1; n = 2, 3, 2, respectively). Furthermore of 18 with a grade 2 HFSR in past TKI treatments, 44.4% had an HFSR with LEN (G3, 2, 1; n = 3, 3, 2, respectively). Of 4 with a grade 1 HFSR in previous TKI treatments, grade 2 occurred in 1 and no HFSR was seen in 3 with LEN treatment. In 14 without an HFSR in past TKI treatments, 28.6% developed HFSR with LEN treatment (G3, 2, 1; n = 3, 3, 2, respectively)
The data of CT imaging findings at introducing LEN were obtained for 51 patients, of whom 18 (35.3%) had MVL. As a sub‐analysis, relative changes in muscle volume at 4 and 12 weeks after introducing LEN were evaluated in 41 and 25 patients, respectively, whose data of CT imaging findings obtained at the start of and approximately 4 and 12 weeks after beginning treatment. In those, the relative change in PI with total psoas muscle area at L3 level in the 41 patients after 4 weeks was −0.210 ± 0.315 cm2/m2, while that in the 25 patients after 12 weeks was −0.275 ± 0.372 cm2/m2.
4. DISCUSSION
Development of TKI treatment for u‐HCC has improved the prognosis of affected patients. Following the introduction of SOR, global trials of sunitinib,26 brivanib,27 linifanib,28 and erlotinib plus SOR29 were conducted, though none of those drugs showed superiority and each of the trials ended in failure. Thus, there was no additional first‐ or second‐line TKI treatment for u‐HCC developed until the introductions of REG3, 4 and LEN.6 Recently, REG was developed as a second‐line option for SOR in patients who met the RESORCE trial criteria,3 after which LEN became available as a first‐line oral TKI targeting VEGF receptors 1‐4, PDGF receptor α, RET, and KIT.6
In the present study, early therapeutic response with LEN was favorable and patients obtained disease control within 4 weeks, as follow‐up imaging showed a significant decline in log10 AFP from the baseline. Kuzuya et al30 have reported that a decreased AFP ratio from the baseline at 4 weeks after introduction of SOR was a predictor of good therapeutic response (PR, SD). Thus, a decline of log10 AFP from the baseline within the first 4 weeks might also have predictive value for therapeutic response in patients receiving LEN treatment. In addition, a previous study noted that fever within 2 weeks after the start of SOR therapy may be useful as a predictor of favorable treatment response in patients with advanced HCC and receiving SOR treatment.31 Three of our patients developed a fever within 2 weeks after the start of LEN (grade 1, 2, 3: each 1 patient), in whom therapeutic response was PD in 2 and SD in 1. It would be important to examine the clinical importance of fever in patients receiving LEN therapy as a useful predictor of a favorable treatment response in a future study with a larger number of patients.
Not all patients treated with SOR in clinical practice meet the RESORCE trial criteria; thus, the frequency of u‐HCC patients indicated for REG has been reported to only range from 30.6% to 37.0% of those confirmed by radiological findings to have PD with SOR therapy.32, 33 Absence of a therapeutic option for second‐line treatment after SOR other than REG in u‐HCC patients who do not meet the RESORCE trial eligibility criteria (tolerability for SOR) is an important clinical issue. Our previous study examined the use of LEN for u‐HCC in both TKI‐naïve and TKI‐experienced patients in real‐world clinical situations in Japan,7 as accurate determination of clinical profile differences including detailed therapeutic response and AE occurrence between TKI‐naïve and TKI‐experienced patients has become an urgent clinical issue. In the present results, PFSR and OSR were not significantly different between the TKI‐naïve and TKI‐experienced groups, and similar AE profiles were observed in both. We consider that LEN may have potential to satisfy the unmet need of clinical TKI treatment for u‐HCC. On the other hand, the cohort in this retrospective study included u‐HCC patients classified as Child‐Pugh B and/or with major vein tumor thrombosis. However, indication for LEN treatment in such patients should be determined with caution, because no evidence has been presented showing the safety or effects of LEN in u‐HCC patients classified as Child‐Pugh B and/or with major vein tumor thrombosis.
In the REFLECT trial reported by Kudo,6 the frequency of HFSR was less in patients that received SOR (all grades: 26.9% vs 52.4%), whereas all grades of HFSR were observed more frequently in the present analysis (40.3%). Although there was no statistical difference in the frequency of HFSR between our TKI‐naïve and TKI‐experienced groups (33.3% vs 45.5%, P = 0.350), past history of high‐grade HFSR might be an important risk factor of it developing during LEN therapy. Thus, HFSR should be kept in mind as a commonly occurring AE in patients with a history of that condition during previous SOR and/or REG treatments. HFSR in association with SOR has been reported as a factor predicting a better therapeutic effect of SOR for HCC.36, 37 Based on these findings, the relationship between HFSR and therapeutic response with LEN treatment should be analyzed in the future. Nevertheless, countermeasures against HFSR are most important for patients with a past history of HFSR in order to maintain TKI adherence for enhancing the anti‐cancer effect.
In the present cohort, Child‐Pugh class and ALBI score became worse at 4 and 12 weeks after starting LEN treatment. In addition to HFSR, general fatigue and appetite loss were the second and third most frequent AEs seen in the present analysis, and it is thought that LEN and these AEs have direct effects on liver function and nutritional status. To maintain adherence to LEN therapy, it is important not only to monitor and treat as carefully as possible AEs but also to keep in mind to introduce LEN, as well as SOR, as possible as in better liver function, if necessary for LEN treatment.
HCC is often seen in patients with chronic liver disease (CLD). Additionally, MVL is not rare in affected patients14 and its frequency was shown to increase in association with the progression of CLD stage.38 Moreover, it has been reported that MVL is a more significant prognostic factor than portal hypertension in liver cirrhosis (LC) patients (Child‐Pugh class A/B),39 and also an important prognostic factor following not only surgical resection40 but also SOR treatment41 in patients with HCC. Recently, a meta‐analysis study reported that MVL was a prognostic factor regardless of therapeutic modality given for HCC by Chang et al.42 Furthermore, in addition to LC and HCC, muscle volume has been reported to be an important prognostic factor in patients receiving treatments for other types of cancers.43 In the present study, a fair percentage of our patients (35.3%) had MVL and a relative decline in muscle volume at 4 and 12 weeks after starting LEN was observed in patients who had data of CT imaging findings available, similar to our past study of patients treated with SOR.41 It will be important to analyze the relationships among therapeutic response, OSR, and muscle volume in patients undergoing LEN therapy in a future study. Although some trials of intervention with nutrition and exercise CLD patients have been reported,44, 45 effective intervention methods have yet to be reported. Establishment of strategies for preventing and improving muscle wasting in CLD patients with and without HCC is needed for improving prognosis.
The present study has some limitations, including its retrospective nature. Additionally, though this was a multicenter study, the number of analyzed patients was not large and the observation period was limited. In a future study, we hope to examine the relationship between prognosis and clinical features including AEs and muscle volume with a larger number of patients receiving LEN therapy.
In summary, regardless of previous TKI treatments, therapeutic response and AE occurrence following introduction of LEN treatments were similar. We consider that LEN might complement the unmet need for TKI therapy for patients with u‐HCC.
CONFLICT OF INTEREST
None declared.
Hiraoka A, Kumada T, Kariyama K, et al. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real‐world conditions: Multicenter analysis. Cancer Med. 2019;8:137–146. 10.1002/cam4.1909
REFERENCES
- 1. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378‐390. [DOI] [PubMed] [Google Scholar]
- 2. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia‐Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double‐blind, placebo‐controlled trial. Lancet Oncol. 2009;10:25‐34. [DOI] [PubMed] [Google Scholar]
- 3. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;389:56‐66. [DOI] [PubMed] [Google Scholar]
- 4. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase 3 RESORCE trial. J Hepatol. 2018;69:353‐358. [DOI] [PubMed] [Google Scholar]
- 5. Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet. 2018;391:1163‐1173. [DOI] [PubMed] [Google Scholar]
- 7. Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Itobayashi E, Shimada N. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: multicenter analysis. Hepatol Res. 2018. 10.1111/hepr.13243 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646‐649. [DOI] [PubMed] [Google Scholar]
- 9. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence‐based approach‐the ALBI grade. J Clin Oncol. 2015;33:550‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiraoka A, Kumada T, Michitaka K, et al. Usefulness of albumin‐bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1031‐1036. [DOI] [PubMed] [Google Scholar]
- 11. Hiraoka A, Kumada T, Kudo M, et al. Albumin‐Bilirubin (ALBI) grade as part of the Evidence‐Based Clinical Practice Guideline for HCC of the Japan Society of Hepatology: a comparison with the liver damage and child‐pugh classifications. Liver Cancer. 2017;6:204‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiraoka A, Michitaka K, Kumada T, et al. Validation and potential of albumin‐bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6:325‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hiraoka A, Kumada T, Tsuji K, et al. Validation of modified ALBI grade for more detailed assessing hepatic function of hepatocellular carcinoma ‐ multicenter analysis. Liver Cancer. 2018. 10.1159/000488778 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hiraoka A, Aibiki T, Okudaira T, et al. Muscle atrophy as pre‐sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. J Gastroenterol. 2015;50:1206‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208‐1236. [DOI] [PubMed] [Google Scholar]
- 16. Di Martino M, Marin D, Guerrisi A, et al. Intraindividual comparison of gadoxetate disodium‐enhanced MR imaging and 64‐section multidetector CT in the Detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010;256:806‐816. [DOI] [PubMed] [Google Scholar]
- 17. Sano K, Ichikawa T, Motosugi U, et al. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid‐enhanced MR imaging. Radiology. 2011;261:834‐844. [DOI] [PubMed] [Google Scholar]
- 18. Hiraoka A, Ichiryu M, Tazuya N, et al. Clinical translation in the treatment of hepatocellular carcinoma following the introduction of contrast‐enhanced ultrasonography with Sonazoid. Oncol Lett. 2010;1:57‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hiraoka A, Hiasa Y, Onji M, Michitaka K. New contrast enhanced ultrasonography agent: impact of Sonazoid on radiofrequency ablation. J Gastroenterol Hepatol. 2011;26:616‐618. [DOI] [PubMed] [Google Scholar]
- 20. The Liver Cancer Study Group of Japan . The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 6th edn Kanehara: Tokyo; 2015:26. [Google Scholar]
- 21. National Cancer Institute , Protocol Development Cancer Therapy . https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm-ctc_40. Accessed May 31, 2018.
- 22. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 23. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52‐60. [DOI] [PubMed] [Google Scholar]
- 24. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hirooka M, Ochi H, Hiraoka A, et al. Destructive thyroiditis induced by lenvatinib in three patients with hepatocellular carcinoma. Internal Med. 2018. 10.2169/internalmedicine.1874-18 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067‐4075. [DOI] [PubMed] [Google Scholar]
- 27. Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first‐line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK‐FL study. J Clin Oncol. 2013;31:3517‐3524. [DOI] [PubMed] [Google Scholar]
- 28. Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a phase III, randomized, double‐blind, placebo‐controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33:559‐566. [DOI] [PubMed] [Google Scholar]
- 30. Kuzuya T, Asahina Y, Tsuchiya K, et al. Early decrease in alpha‐fetoprotein, but not des‐gamma‐carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:251‐258. [DOI] [PubMed] [Google Scholar]
- 31. Kuzuya T, Ishigami M, Ishizu Y, et al. Fever within 2 weeks of Sorafenib therapy predicts favorable treatment efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2016;91:261‐266. [DOI] [PubMed] [Google Scholar]
- 32. Kuzuya T, Ishigami M, Ishizu Y, et al. Prognostic factors associated with postprogression survival in advanced hepatocellular carcinoma patients treated with Sorafenib not eligible for second‐line regorafenib treatment. Oncology. 2018;95:91‐99. [DOI] [PubMed] [Google Scholar]
- 33. Shinsuke U, Kawaoka T, Aikata H, et al. Clinical outcomes of sorafenib treatment failure for advanced hepatocellular carcinoma and candidates for regorafenib treatment in real‐world practice. Hepatol Res. 2018. [DOI] [PubMed] [Google Scholar]
- 34. Terashima T, Yamashita T, Sunagozaka H, et al. Analysis of the liver functional reserve of patients with advanced hepatocellular carcinoma undergoing sorafenib treatment: prospects for regorafenib therapy. Hepatol Res. 2018;48:956‐966. [DOI] [PubMed] [Google Scholar]
- 35. Ogasawara S, Chiba T, Ooka Y, et al. Characteristics of patients with sorafenib‐treated advanced hepatocellular carcinoma eligible for second‐line treatment. Invest New Drugs. 2018;36:332‐339. [DOI] [PubMed] [Google Scholar]
- 36. Ogawa C, Morita M, Omura A, et al. Hand‐foot syndrome and post‐progression treatment are the good predictors of better survival in advanced hepatocellular carcinoma treated with sorafenib: a multicenter study. Oncology. 2017;93(Suppl 1):113‐119. [DOI] [PubMed] [Google Scholar]
- 37. Wang P, Tan G, Zhu M, Li W, Zhai B, Sun X. Hand‐foot skin reaction is a beneficial indicator of sorafenib therapy for patients with hepatocellular carcinoma: a systemic review and meta‐analysis. Expert Rev Gastroenterol Hepatol. 2018;12:1‐8. [DOI] [PubMed] [Google Scholar]
- 38. Hiraoka A, Michitaka K, Ueki H, et al. Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2016;28:940‐947. [DOI] [PubMed] [Google Scholar]
- 39. Hiraoka A, Kitahata S, Izumoto H, et al. Muscle volume loss a prognostic factor for death in liver cirrhosis patients and special relationship to portal hypertension. Hepatol Res. 2018;48:E354‐E359. [DOI] [PubMed] [Google Scholar]
- 40. Hiraoka A, Otsuka Y, Kawasaki H, et al. Impact of muscle volume and muscle function decline in patients undergoing surgical resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33:1271‐1276. [DOI] [PubMed] [Google Scholar]
- 41. Hiraoka A, Hirooka M, Koizumi Y, et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. 2017;47:558‐565. [DOI] [PubMed] [Google Scholar]
- 42. Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta‐analysis. Liver Cancer. 2018;7:90‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539‐1547. [DOI] [PubMed] [Google Scholar]
- 44. Hiraoka A, Michitaka K, Kiguchi D, et al. Efficacy of branched‐chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1416‐1423. [DOI] [PubMed] [Google Scholar]
- 45. Koya S, Kawaguchi T, Hashida R, et al. Effects of in‐hospital exercise on liver function, physical ability, and muscle mass during treatment of hepatoma in patients with chronic liver disease. Hepatol Res. 2017;47:E22‐E34. [DOI] [PubMed] [Google Scholar]


