Abstract
Background:
The 2017 American College of Cardiology/American Heart Association (ACC/AHA) blood pressure (BP) guideline provides updated recommendations for antihypertensive medication initiation and intensification.
Objective:
Determine the risk for cardiovascular disease (CVD) events among adults recommended and not recommended antihypertensive medication initiation or intensification by the 2017 ACC/AHA BP guideline.
Methods:
We analyzed data for black and white REasons for Geographic And Racial Differences in Stroke (REGARDS) study participants (age ≥45 years). Systolic BP (SBP) and diastolic BP (DBP) were measured twice at baseline (2003–2007) and averaged. Participants not taking (n=14,039) and taking (n=15,179) antihypertensive medication were categorized according to their recommendations for antihypertensive medication initiation and intensification by the 2017 ACC/AHA guideline. Overall, 4,094 CVD events (stroke, coronary heart disease and heart failure) occurred by December 31, 2014.
Results:
Among participants not taking antihypertensive medication, 34.4% were recommended pharmacological antihypertensive treatment initiation. The CVD event rate per 1,000 person-years among participants recommended antihypertensive medication initiation with SBP/DBP ≥140/90 mmHg was 22.7 (95%CI 20.3–25.0). Among participants with SBP/DBP 130–139/80–89 mmHg, the CVD event rate was 20.5 (95%CI 18.5–22.6) and 3.4 (95%CI 2.4–4.4) for those recommended and not recommended antihypertensive medication initiation, respectively. Among participants taking antihypertensive medication, 62.8% were recommended treatment intensification. The CVD event rate per 1,000 person-years among participants recommended treatment intensification was 33.6 (95%CI 31.5–35.6) and 22.4 (95%CI 20.8–23.9) for those with SBP/DBP ≥140/90 mmHg and 130–139/80–89 mmHg, respectively.
Conclusions:
Implementing the 2017 ACC/AHA guideline would direct antihypertensive medication initiation and intensification to adults with high CVD risk.
Keywords: Blood pressure, hypertension, antihypertensive agents, Practice guidelines, cardiovascular disease, Adult
Condensed abstract:
The 2017 American College of Cardiology/American Heart Association (ACC/AHA) blood pressure (BP) guideline provides updated recommendations for antihypertensive medication initiation and intensification. In the current analysis of black and white REasons for Geographic And Racial Differences in Stroke (REGARDS) study participants ≥45 years of age, those recommended antihypertensive medication initiation or intensification by the 2017 ACC/AHA BP guideline had high risk for cardiovascular events and all-cause mortality. These results indicate that implementing the 2017 ACC/AHA BP guideline would direct antihypertensive medication initiation and intensification to adults who will receive a substantial absolute risk reduction for cardiovascular events and all-cause mortality.
Introduction
In 2017, the American College of Cardiology (ACC) and the American Heart Association (AHA) published an evidence-based guideline for the prevention, detection, evaluation, and management of high blood pressure (BP) in adults (1). All adults with hypertension, defined by an average systolic BP (SBP) ≥130 mmHg or diastolic BP (DBP) ≥80 mmHg, are recommended nonpharmacological therapy according to this guideline. However, not all adults with hypertension are recommended to initiate antihypertensive medication. Antihypertensive medication initiation is recommended for adults with an SBP ≥140 mmHg or DBP ≥90 mmHg and those with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg who are at high risk for cardiovascular disease (CVD) events. This includes adults with a prior diagnosis of diabetes, chronic kidney disease (CKD) or CVD, a 10-year predicted CVD risk ≥10%, or those ≥65 years of age with SBP ≥130 mmHg (1). The recommendation for using CVD risk to guide the initiation of pharmacological therapy among adults with SBP between 130 and 139 mmHg or DBP between 80 to 89 mmHg was based on results of randomized trials which have consistently demonstrated that the absolute risk reduction for CVD events and all-cause mortality associated with antihypertensive medication increases with a higher predicted risk (2). Among adults taking antihypertensive medication, the 2017 ACC/AHA guideline recommend an SBP treatment target goal of <130 mmHg. Also, a DBP treatment target goal of <80 mmHg is recommended for adults <65 years of age and those ≥ 65 years of age with diabetes, CKD, a history of CVD or a 10-year predicted CVD risk ≥10%.
If the CVD event and all-cause mortality rates are high among adults recommended for antihypertensive medication initiation or intensification, this would indicate that the implementation of the 2017 ACC/AHA BP guideline is directing pharmacological treatment towards populations most likely to receive the largest absolute risk reduction. We used data from a large prospective population-based cohort, the REasons for Geographic And Racial Differences in Stroke (REGARDS) study (3), to determine the rates of CVD events and all-cause mortality among adults not taking antihypertensive medication who are recommended and not recommended for antihypertensive medication initiation according to the 2017 ACC/AHA BP guideline. Among REGARDS study participants taking antihypertensive medication, we also determined the rates of CVD events and all-cause mortality for those who are recommended and not recommended for antihypertensive medication intensification.
Methods
Study population
The REGARDS study included a total of 30,239 black and white men and women ≥45 years of age from all 48 contiguous US states and the District of Columbia between January 2003 and October 2007. For the present analysis, we excluded participants with missing data at baseline for BP (n=143), self-reported antihypertensive medication use (n=225) or variables needed to determine their recommendation for antihypertensive medication initiation or intensification according to the 2017 ACC/AHA guideline (n=195). After the exclusion of an additional 458 participants without follow-up data, a total of 29,218 REGARDS study participants were included in the current analysis (Online Figure 1). The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided written informed consent.
Baseline assessment
Computer-assisted telephone interviews were administered by trained staff and used to collect information on each participant’s age, race, gender, home address, cigarette smoking, cognitive impairment, depressive symptoms, exhaustion, impaired mobility, history of falls, medical history (e.g., stroke, myocardial infarction [MI], coronary revascularization, diabetes, and dialysis therapy), and use of insulin and oral hypoglycemic and antihypertensive medications. Using the home address, participants were categorized into region of residence (i.e., stroke belt, stroke buckle, or other US regions), as defined in Online Table 1. After completion of the interview, trained health professionals conducted in-home examinations following standardized protocols. Procedures included an electrocardiogram, collection of blood and urine samples, an inventory of prescription and over the counter medications taken during the two-week period prior to each participant’s study visit, and measurements of height and weight, which were used to calculate the body mass index (BMI). Serum total and high-density lipoprotein cholesterol, triglycerides, glucose and creatinine were measured using the blood samples collected during the study visit (4). Estimated glomerular filtration rate was calculated using age, race, gender, serum creatinine and the Chronic Kidney Disease Epidemiology equation (5). Urinary albumin and creatinine were measured and used to calculate the albumin-to-creatinine ratio.
Definitions for a history of CVD, diabetes, CKD and frailty indicators, including low BMI, cognitive impairment, depressive symptoms, exhaustion, impaired mobility and history of falls are provided in Online Table 1. For participants without a history of CVD, the 10-year predicted CVD risk was calculated using the Pooled Cohort risk equations (6). Statin use was identified based on the medication inventory.
Blood pressure measurements
For each participant, BP was measured two times by a trained health professional during the in-home examination using the auscultatory method and an aneroid sphygmomanometer (American Diagnostic Corporation, Hauppauge, New York) with an appropriate sized cuff following a standardized protocol. Prior to their first BP measurement, participants rested for five minutes in a seated position with both feet on the floor. At least 30 seconds elapsed between each measurement. Quality control for the BP measurements included routine calibration of the aneroid device by the manufacturer and monitoring of the readings for digit preference. The two BP measurements from each participant were averaged for the current analyses. Participants were grouped into three mutually exclusive categories based on their BP: (1) SBP <130 mmHg and DBP <80 mmHg, (2) SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg, and (3) SBP ≥140 mmHg or DBP ≥90 mmHg. Participants whose SBP and DBP corresponded to two separate categories were assigned to the higher BP group (e.g., participants with an SBP of 136 mmHg and a DBP of 92 mmHg were assigned to the SBP ≥140 mmHg or DBP ≥90 mmHg category). Hypertension was defined as an SBP ≥130 mmHg or DBP ≥80 mmHg (1).
REGARDS study participants with SBP ≥140 mmHg or DBP ≥90 mmHg were advised to seek care from a healthcare professional (3). No recommendations on antihypertensive medication initiation or intensification were provided as part of the REGARDS study.
Identification of stroke, CHD, and heart failure hospitalization events
Living REGARDS participants or proxy respondents are contacted every six months via telephone to identify suspected stroke, CHD (i.e., MI or CHD death) and heart failure hospitalization events which are subsequently reviewed using medical records (3,7). Stroke events are confirmed by a panel of neurologists following the World Health Organization definition (8). Events not meeting this definition but characterized by symptoms lasting <24 hours with neuroimaging consistent with acute infarct or hemorrhage are also classified as strokes. MIs and heart failure hospitalizations are confirmed by trained clinicians following published guidelines (9,10). When deaths are identified, trained clinicians determine the main underlying cause of death based on interviews with next-of-kin, medical records, death certificates and autopsy reports (10,11). CVD events were defined as a non-fatal or fatal stroke, non-fatal or fatal MI, CHD death, or heart failure hospitalization. CVD events and all-cause mortality through December 31, 2014 were available for the current analysis. The attrition rate among REGARDS study participants was 2.9% per year.
Statistical analysis
All analyses described below were conducted among participants not taking and taking antihypertensive medication, separately. We calculated baseline characteristics by the three mutually exclusive BP categories (i.e., SBP <130 mmHg and DBP <80 mmHg, SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg, and SBP ≥140 mmHg or DBP ≥90 mmHg). Among participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg, we calculated baseline characteristics for those recommended and not recommended antihypertensive medication initiation or intensification by the 2017 ACC/AHA guideline (Online Table 2). We also calculated the distribution of REGARDS study participants across subgroups defined by the cross-tabulation between BP categories and recommendation for antihypertensive medication initiation or intensification by the 2017 ACC/AHA guideline.
We calculated CVD event rates as the number of participants who experienced a CVD event after baseline divided by the total person-years at risk. All-cause mortality rates were calculated as the number of participants who died divided by the total person-years at risk. For each participant, the time at risk for CVD was defined as the number of years from their in-home study visit (i.e., the end of the baseline assessment) through the occurrence of their first CVD event, death, last study contact or December 31, 2014, whichever occurred first. For the analysis of all-cause mortality, participants were followed from their in-home visit through their death, last study contact or December 31, 2014, whichever occurred first. We calculated the cumulative incidence of all-cause mortality using the Kaplan-Meier method. The Kaplan-Meier method overestimates the cumulative incidence in the presence of a competing risk event (12).
Therefore, we calculated the cumulative incidence of CVD events as described by Fine and Gray taking into account the competing risk for mortality (12). For the calculation of the cumulative incidence of CVD events and all-cause mortality, participants were censored on their last study contact or December 31, 2014, whichever occurred first. The cumulative incidence and rate of CVD events and all-cause mortality were calculated by the three mutually exclusive BP categories. Among participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg, we calculated the cumulative incidence and rate of CVD events and all-cause mortality for those recommended and not recommended pharmacological antihypertensive treatment initiation or intensification by the 2017 ACC/AHA guideline. Calculations of CVD event and all-cause mortality rates were also performed stratified by gender and race. All analyses were conducted using SAS v9.4 (SAS Institute, Cary North Carolina).
Results
Participants not taking antihypertensive medication
Participants in higher BP categories were older, more likely to be male, black, have less than a high school education, a history of CVD or a 10-year predicted CVD risk ≥10%, diabetes, CKD, cognitive impairment and impaired mobility (Table 1). Among participants not taking antihypertensive medication, 45.6% had hypertension and 34.4% were recommended to initiate antihypertensive medication according to the 2017 ACC/AHA BP guideline (Figure 1). Among participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg, those recommended versus not recommended antihypertensive medication initiation by the 2017 ACC/AHA guideline were older, more likely to be men, have less than a high school education, smoke cigarettes, be taking a statin and have cognitive impairment, exhaustion, impaired mobility and a history of falls (Online Table 3).
Table 1.
Baseline characteristics of REGARDS study participants not taking antihypertensive medication by blood pressure categories.
| Characteristics | SBP/DBP, mmHg * | ||
|---|---|---|---|
| <130 and <80 | 130–139 or 80–89 | ≥140 or ≥90 | |
| n | 7,637 | 4,199 | 2,203 |
| Age, years, mean (SD) | 62.8 (9.5) | 63.6 (9.5) | 65.7 (9.8) |
| Male, n (%) | 3,257 (42.6) | 2,202 (52.4) | 1,186 (53.8) |
| Black, n (%) | 1,935 (25.3) | 1,481 (35.3) | 945 (42.9) |
| Geographic region of residence, n (%) | |||
| Stroke belt† | 2,618 (34.3) | 1,372 (32.7) | 789 (35.8) |
| Stroke buckle‡ | 1,642 (21.5) | 806 (19.2) | 413 (18.7) |
| Other US regions§ | 3,377 (44.2) | 2,021 (48.1) | 1,001 (45.4) |
| Less than high school education, n (%) | 580 (7.6) | 418 (10.0) | 313 (14.2) |
| Current smoking, n (%) | 1,140 (15.0) | 593 (14.2) | 428 (19.5) |
| SBP, mmHg, mean (SD) | 113.8 (9.4) | 128.0 (7.9) | 148.3 (13.1) |
| DBP, mmHg, mean (SD) | 69.6 (6.5) | 79.8 (5.6) | 85.9 (9.9) |
| Total cholesterol, mg/dL, mean (SD) | 195.2 (39.0) | 198.2 (39.2) | 201.1 (41.9) |
| HDL cholesterol, mg/dL, mean (SD) | 54.0 (16.6) | 51.9 (15.8) | 52.3 (17.0) |
| Statin use, n (%) | 1,696 (22.3) | 879 (21.0) | 421 (19.2) |
| History of CVD or 10-year CVD risk ≥10%, n (%) | 3,073 (40.2) | 2,278 (54.3) | 1,611 (73.1) |
| Age ≥65 years with SBP ≥130 mmHg, n (%) | 0 (0.0) | 1,196 (28.5) | 1,147 (52.1) |
| Diabetes, n (%) | 782 (10.6) | 538 (13.0) | 347 (16.4) |
| Chronic kidney disease, n (%) | 800 (10.6) | 590 (14.1) | 495 (22.7) |
| Frailty indicators, n (%) | |||
| Depressive symptoms | 720 (9.5) | 356 (8.5) | 226 (10.3) |
| Low body mass index | 166 (2.2) | 28 (0.7) | 19 (0.9) |
| Cognitive impairment | 357 (5.6) | 244 (7.4) | 151 (8.9) |
| Exhaustion | 763 (10.0) | 406 (9.7) | 234 (10.6) |
| Impaired mobility | 810 (10.6) | 465 (11.1) | 297 (13.5) |
| History of falls | 480 (6.3) | 241 (5.7) | 142 (6.5) |
CVD: cardiovascular disease; DBP: diastolic blood pressure; HDL: high-density lipoprotein; REGARDS: REasons for Geographic and Racial Differences in Stroke; SBP: systolic blood pressure; SD: standard deviation; US: United States.
Blood pressure categories are mutually exclusive. Participants whose SBP and DBP correspond to two separate categories were assigned to the higher blood pressure group.
Stroke buckle includes coastal North Carolina, South Carolina and Georgia.
Stroke belt includes the remaining parts of the stroke buckle states and Tennessee, Mississippi, Alabama, Louisiana and Arkansas.
Other US regions includes the remaining 40 contiguous US states and the District of Columbia.
Figure 1. Distribution of REGARDS study participants not taking antihypertensive medication by blood pressure category and recommendation for pharmacological treatment initiation.
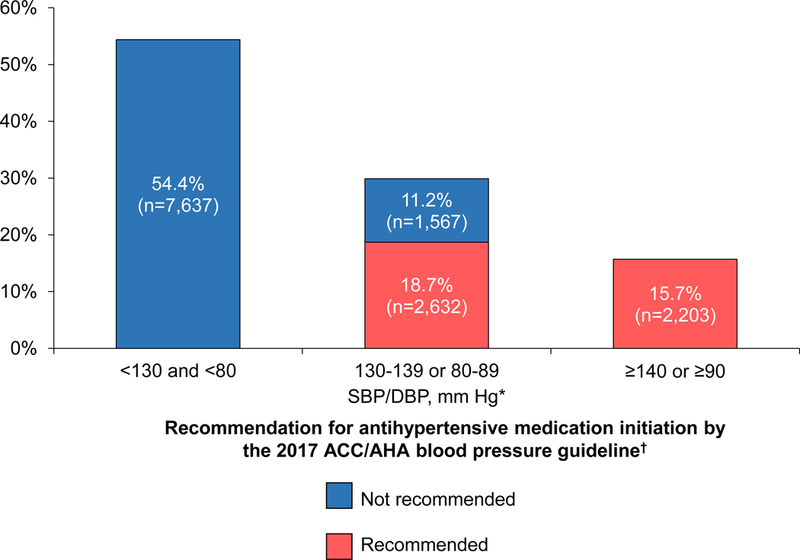
ACC: American College of Cardiology; AHA: American Heart Association; CVD: cardiovascular disease; DBP: diastolic blood pressure; REGARDS: REasons for Geographic and Racial Differences in Stroke; SBP: systolic blood pressure.* Blood pressure categories are mutually exclusive. Participants whose SBP and DBP correspond to two separate categories were assigned to the higher blood pressure group† Recommendations for antihypertensive medication initiation according to the 2017 ACC/AHA blood pressure guideline are shown in Online Table 2.
The cumulative incidence and rate of CVD events and all-cause mortality were higher at higher BP levels (Figure 2, top panel, and Table 2, top panel). Among participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg, the rate of CVD events and allcause mortality was six-fold higher for those recommended versus not recommended antihypertensive medication by the 2017 ACC/AHA guideline (Figure 2, bottom panel and Table 2, bottom panel). In subgroups defined by gender and race, the rates of CVD events and all-cause mortality were higher with higher BP levels and among participants recommended versus not recommended antihypertensive medication initiation by the 2017 ACC/AHA guideline (Online Table 4).
Figure 2. Cumulative incidence of CVD events and all-cause mortality among 14,039 REGARDS study participants not taking antihypertensive medication by blood pressure category (top panel) and recommendation for pharmacological antihypertensive treatment initiation (bottom panel).
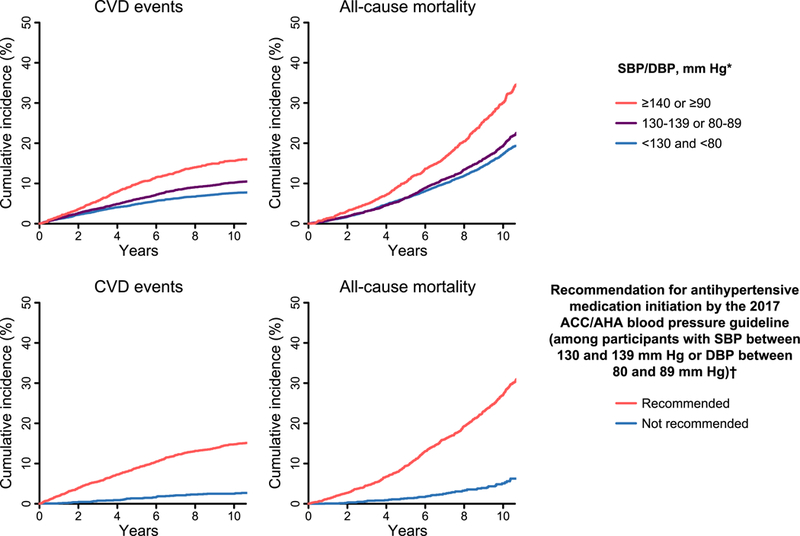
ACC: American College of Cardiology; AHA: American Heart Association; CVD: cardiovascular disease; DBP: diastolic blood pressure; REGARDS: REasons for Geographic and Racial Differences in Stroke; SBP: systolic blood pressure.* Blood pressure categories are mutually exclusive. Participants whose SBP and DBP correspond to two separate categories were assigned to the higher blood pressure group† Analyses of the recommendation for antihypertensive medication initiation were restricted to participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg. Recommendations for antihypertensivemedication initiation according to the 2017 ACC/AHA blood pressure guideline are shown in Online Table 2. The median follow-up for CVD events was 8.3 years (maximum 11.9 years). The median follow-up for all-cause mortality was 8.5 years (maximum 11.9 years). On the X-axis, time 0 represents the date of the in-home baseline study visit for each participant.
Table 2.
Rates of CVD events and all-cause mortality among 14,039 REGARDS study participants not taking antihypertensive medication by blood pressure category (top panel) and recommendation for pharmacological antihypertensive treatment initiation (bottom panel).
| CVD events | All-cause mortality | |||
|---|---|---|---|---|
| Events / Person-years | Rate (95% CI) per 1,000 person-years | Events / Person-years | Rate (95% CI) per 1,000 person-years | |
| SBP/DBP, mmHg* | ||||
| <130 and <80 | 599 / 58,581 | 10.2 (9.4, 11.0) | 1,059 / 60,400 | 17.5 (16.5, 18.6) |
| 130–139 or 80–89 | 444 / 32,133 | 13.8 (12.5, 15.1) | 674 / 33,465 | 20.1 (18.6, 21.7) |
| ≥140 or ≥90 | 356 / 15,711 | 22.7 (20.3, 25.0) | 551 / 16,729 | 32.9 (30.2, 35.7) |
| Recommendation for antihypertensive medication initiation by the 2017 ACC/AHA blood pressure guideline (among participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg)† | ||||
| Not recommended | 43 / 12,615 | 3.4 (2.4, 4.4) | 61 / 12,739 | 4.8 (3.6, 6.0) |
| Recommended | 401 / 19,518 | 20.5 (18.5, 22.6) | 613 / 20,726 | 29.6 (27.2, 31.9) |
ACC: American College of Cardiology; AHA: American Heart Association; CVD: cardiovascular disease; REGARDS: REasons for Geographic and Racial Differences in Stroke.
Blood pressure categories are mutually exclusive. Participants whose SBP and DBP correspond to two separate categories were assigned to the higher blood pressure group.
Analyses of the recommendation for antihypertensive medication initiation were restricted to participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg. Recommendations for antihypertensive medication initiation according to the 2017 ACC/AHA blood pressure guideline include SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg with diabetes, chronic kidney disease, a history of CVD or a 10-year predicted CVD risk ≥10%, or age ≥65 years with SBP ≥130 mmHg. All participants with SBP ≥140 mmHg or DBP ≥90 mmHg are recommended antihypertensive medication initiation by the 2017 ACC/AHA blood pressure guideline.
The median follow-up for CVD events was 8.3 years (maximum 11.9 years). The median follow-up for all-cause mortality was 8.5 years (maximum 11.9 years).
Participants taking antihypertensive medication
Participants taking antihypertensive medication with higher BP levels were more likely to be men, black, have less than a high school education, a history of CVD or a 10-year predicted CVD risk ≥10%, diabetes, CKD, depressive symptoms, cognitive impairment and exhaustion, and less likely to be taking a statin (Online Table 5). Overall, 62.8% of participants were recommended intensification of their antihypertensive medication to meet the BP target goals in the 2017 ACC/AHA guideline (Online Figure 2). Among participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg, 5,023 (98.7%) were recommended antihypertensive medication intensification. The 66 participants not recommended antihypertensive medication intensification were women, non-smokers, without diabetes, CKD, or a history of CVD and with a 10-year predicted CVD risk <10% (Online Table 6).
The risk for CVD events and all-cause mortality was higher among participants with SBP ≥140 mmHg or DBP ≥90 mmHg compared to their counterparts with lower BP levels (Figure 3, top panel and Table 3, top panel). Among participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg, the risk for CVD and all-cause mortality was higher for those recommended versus not recommended antihypertensive intensification by the 2017 ACC/AHA guideline (Figure 3, bottom panel and Table 3, bottom panel). The rates of CVD events and allcause mortality were higher among participants with SBP ≥140 mmHg or DBP ≥90 mmHg versus their counterparts with lower BP levels, and among those recommended versus not recommended antihypertensive medication intensification when analyses were stratified by gender and race (Online Table 7).
Figure 3. Cumulative incidence of CVD events and all-cause mortality among 15,179 REGARDS study participants taking antihypertensive medication by blood pressure category (top panel) and recommendation for pharmacological antihypertensive treatment intensification (bottom panel).
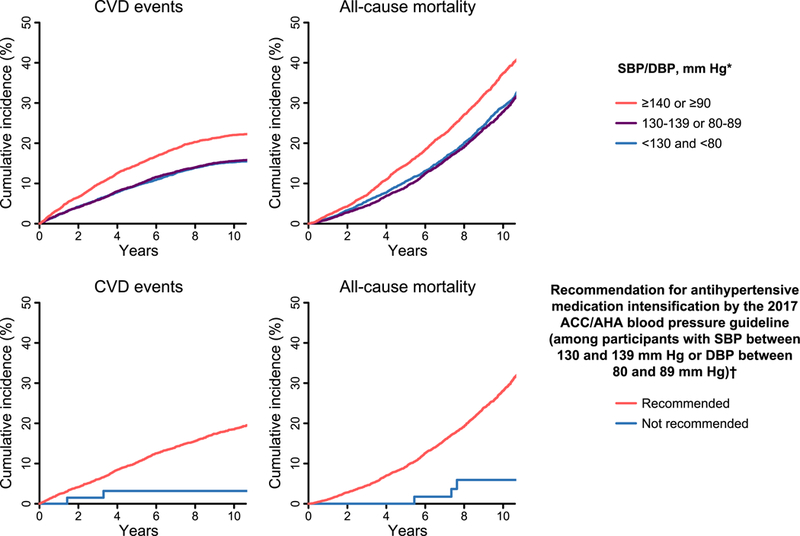
ACC: American College of Cardiology; AHA: American Heart Association; CVD: cardiovascular disease; DBP: diastolic blood pressure; REGARDS: REasons for Geographic and Racial Differences in Stroke; SBP: systolic blood pressure.* Blood pressure categories are mutually exclusive. Participants whose SBP and DBP correspond to two separate categories were assigned to the higher blood pressure group† Analyses of the recommendation for antihypertensive medication intensification were restricted to participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg. Recommendations for antihypertensive medication intensification according to the 2017 ACC/AHA blood pressure guideline are shown in Online Table 2. The median follow-up for CVD events was 7.8 years (maximum 11.8 years). The median follow-up for all-cause mortality was 8.2 years (maximum 11.9 years). On the X-axis, time 0 represents the date of the in-home baseline study visit for each participant.
Table 3.
Rates of CVD events and all-cause mortality among 15,179 REGARDS study participants taking antihypertensive medication by blood pressure category (top panel) and recommendation for pharmacological antihypertensive treatment intensification (bottom panel).
| SBP/DBP, mmHg* | CVD events | All-cause mortality | ||
|---|---|---|---|---|
| Events / Person-years | Rate (95% CI) per 1,000 person-years | Events / Person-years | Rate (95% CI) per 1,000 person-years | |
| <130 and <80 | 873 / 39,821 | 21.9 (20.5, 23.4) | 1,289 / 42,168 | 30.6 (28.9, 32.2) |
| 130–139 or 80–89 | 808 / 36,566 | 22.1 (20.6, 23.6) | 1,151 / 38,984 | 29.5 (27.8, 31.2) |
| ≥140 or ≥90 | 1,014 / 30,201 | 33.6 (31.5, 35.6) | 1,407 / 33,108 | 42.5 (40.3, 44.7) |
| Recommendation for antihypertensive medication intensification by the 2017 ACC/AHA blood pressure guideline (among participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg)† | ||||
| Not recommended | 2 / 523 | 3.8 (0.0, 9.1) | 3 / 538 | 5.6 (0.0, 11.9) |
| Recommended | 806 / 36,043 | 22.4 (20.8, 23.9) | 1,148 / 38,446 | 29.9 (28.1, 31.6) |
ACC: American College of Cardiology; AHA: American Heart Association; CVD: cardiovascular disease; REGARDS: REasons for Geographic and Racial Differences in Stroke.
Blood pressure categories are mutually exclusive. Participants whose SBP and DBP correspond to two separate categories were assigned to the higher blood pressure group.
Analyses of the recommendation for antihypertensive medication intensification were restricted to participants with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg. Recommendations for antihypertensive medication intensification according to the 2017 ACC/AHA blood pressure guideline include SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg with diabetes, chronic kidney disease, a history of CVD or a 10-year predicted CVD risk ≥10%, age <65 years, or age ≥65 years with SBP ≥130 mmHg. Participants ≥65 years of age with SBP <130 mmHg, DBP between 80 and 89 mmHg and a 10-year predicted CVD risk <10% who do not have diabetes, chronic kidney disease or a history of CVD are not recommended antihypertensive medication intensification according to the 2017 ACC/AHA blood pressure guideline.
The median follow-up for CVD events was 7.8 years (maximum 11.8 years). The median follow-up for all-cause mortality was 8.2 years (maximum 11.9 years).
Discussion
In the current study, the rate of CVD events and all-cause mortality among participants not taking antihypertensive medication with an average SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg was 6-fold higher for those recommended versus not recommended pharmacological treatment initiation by the 2017 ACC/AHA BP guideline (Central illustration).CVD event and all-cause mortality rates were also high among participants taking antihypertensive medication who are recommended treatment intensification. Results from the current study indicate that the implementation of the 2017 ACC/AHA BP guideline would direct pharmacological antihypertensive treatment initiation and intensification to adults with high risk for CVD events and all-cause mortality while avoiding treatment initiation and intensification for adults with low CVD risk.
Central Illustration. Rates of CVD events and all-cause mortality.
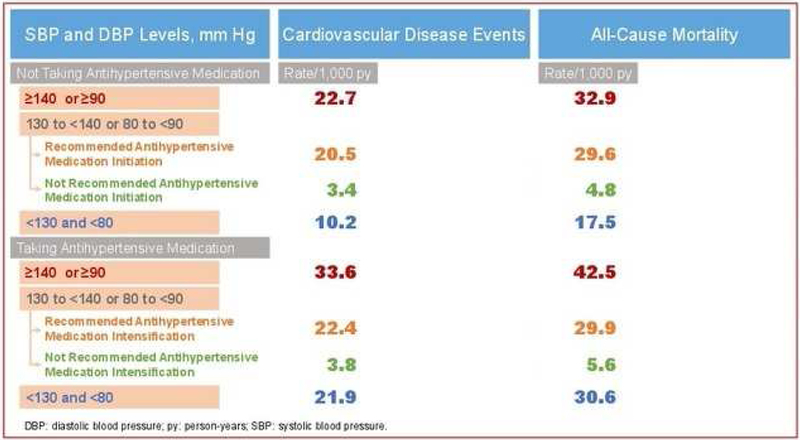
CVD: cardiovascular disease; DBP: diastolic blood pressure; py: person-years; REGARDS: REasons for Geographic and Racial Differences in Stroke; SBP: systolic blood pressure.
Adults ≥45 years of age recommended antihypertensive medication initiation or intensification by the 2017 American College of Cardiology/American Heart Association blood pressure guideline had high risk for cardiovascular disease events and all-cause mortality
The 2017 ACC/AHA BP guideline recommends considering BP levels and CVD risk in the decision to initiate pharmacological antihypertensive treatment (1). This approach was taken to direct pharmacological treatment to adults more likely to have CVD events and, therefore, maximize absolute risk reduction and quality-adjusted life-years saved from initiation of antihypertensive medication (13). Implementation of the 2017 ACC/AHA guideline has been projected to increase the number of U.S. adults ≥20 years of age recommended antihypertensive medication initiation by 4.2 million (14). In the current study, the CVD event rate among participants recommended initiation of pharmacological antihypertensive treatment by the 2017 ACC/AHA guideline was 22.7 per 1,000 person-years for those with SBP ≥140 mmHg or DBP ≥90 mmHg, and 20.5 per 1,000 person-years for those with SBP between 130 and 139 mmHg or DBP between 80 and 89 mmHg. These high CVD rates suggest that a large absolute CVD risk reduction would occur with pharmacological antihypertensive treatment in these populations. Among U.S. adults ≥20 years of age not taking antihypertensive mediation, 21.4 million have hypertension according to the 2017 ACC/AHA BP guideline but are not recommended pharmacological treatment initiation (14). In the current analysis, the CVD event rate was only 3.4 per 1,000 person-years among REGARDS study participants with hypertension who were not recommended to initiate pharmacological antihypertensive treatment by the 2017 ACC/AHA guideline. The low CVD event rate suggests a small absolute CVD risk reduction with antihypertensive medication in this population, supporting the recommendation to not initiate pharmacological treatment.
Several meta-analyses of randomized controlled trials have shown that intensification of pharmacological antihypertensive treatment to lower BP goals reduces the risk for CVD and mortality (15–18). Using data from 42 randomized trials, Bundy and colleagues reported hazard ratios for CVD events and all-cause mortality comparing an achieved SBP of 120–124 versus 130–134 mmHg of 0.71 (95% confidence interval [CI] 0.60, 0.83) and 0.73 (95% CI 0.58, 0.93), respectively (16). The 2017 ACC/AHA BP guideline recommends SBP and DBP treatment goals of <130 mmHg and <80 mmHg, respectively for the vast majority of adults taking antihypertensive medication (1). In the current study, participants taking antihypertensive medication who were recommended pharmacological treatment intensification had high rates of CVD events and all-cause mortality. These results suggest that intensification of antihypertensive medication following the 2017 ACC/AHA guideline recommendations has the potential to result in a substantial absolute risk reduction for CVD events and all-cause mortality.
Participants taking antihypertensive medication in the current analysis who had an SBP <130 mmHg and DBP <80 mmHg had high CVD and mortality event rates. This result should not be interpreted as a lack of efficacy of pharmacological antihypertensive treatment, which has been demonstrated in clinical trials (2). Prior studies have shown that antihypertensive medication does not return CVD risk to the level of someone with normal or elevated BP not taking antihypertensive medication (19–23). Comprehensive risk reduction interventions including statin use and nonpharmacological therapies may be useful adjunctive therapies to further reduce the residual CVD risk among adults taking antihypertensive medication who are not recommended pharmacological treatment intensification according to the 2017 ACC/AHA BP guideline.
All adults with hypertension are recommended nonpharmacological therapy by the 2017 ACC/AHA BP guideline (1). Nonpharmacological therapies, including weight loss (24), healthy diet (25,26), reduced intake of dietary sodium (27,28), enhanced intake of dietary potassium (29), physical activity (30–33) and moderate alcohol intake (34,35) have been shown to reduce BP levels in randomized trials. Therefore, nonpharmacological therapy may lower BP below thresholds used to recommend antihypertensive medication initiation or intensification, reducing the need for initiating or intensifying pharmacological antihypertensive treatment (36–38). Future studies are needed to determine the potential impact of nonpharmacological therapies on CVD events among adults with hypertension according to the 2017 ACC/AHA guideline.
The current analysis has several strengths. We used data from the REGARDS study, a large population-based cohort that enrolled adults in all 48 contiguous US states and the District of Columbia. Baseline data collection in the REGARDS study was conducted by trained health professionals and included two BP measurements following a standardized protocol. Stroke, CHD and heart failure events were adjudicated by trained personnel using published guidelines. Despite these strengths, the current results should be interpreted in the context of known and potential limitations. Some REGARDS study participants may have initiated or intensified antihypertensive medication treatment after their baseline assessment if recommended by a health care professional following prior BP guidelines (e.g., the Joint National Committee Seventh Report [JNC7]). Therefore, the current results may underestimate the risk for CVD events and all-cause mortality among adults recommended antihypertensive medication initiation or intensification by the 2017 ACC/AHA BP guideline. The REGARDS study enrolled community-dwelling blacks and whites ≥45 years of age. Therefore, the results may not be generalizable to other race groups, those living in nursing homes or adults <45 years of age. Also, the REGARDS study oversampled older adults, blacks and residents of the stroke belt and stroke buckle regions of the United States (3). However, we have no reason to believe that the oversampling of these populations had a substantial effect on the main findings from the current analysis. BP was measured during a single visit and using an aneroid sphygmomanometer. The 2017 ACC/AHA BP guideline recommends making the diagnosis of hypertension based on two or more BP measurements taken on two or more separate occasions. Also, aneroid sphygmomanometers are susceptible to calibration issues. Some individuals who initiate or intensify antihypertensive medication may experience side effects (39). However, data on side effects associated with antihypertensive medication are not available in the REGARDS study. Despite the low annual attrition rate in the REGARDS study, loss to follow-up may not have been at random, which may result in biased estimations.
Conclusions
In the current study, participants with hypertension recommended pharmacological antihypertensive treatment initiation by the 2017 ACC/AHA BP guideline had a 6-fold higher rate of CVD events and all-cause mortality compared with their counterparts who had hypertension but are not recommended pharmacological antihypertensive treatment initiation. Among participants taking pharmacological antihypertensive medication, the risk for CVD events and all-cause mortality was high for those recommended to intensify their treatment to achieve the BP goals by the 2017 ACC/AHA guideline. These results support the implementation of the 2017 ACC/AHA BP guideline to identify populations who should receive substantial CVD and all-cause mortality risk reduction benefit through antihypertensive medication initiation or intensification.
Supplementary Material
Clinical perspectives
Competency in patient care:
The 2017 American College of Cardiology/American Heart Association blood pressure guideline directs antihypertensive medication initiation and intensification to adults who have high risk for cardiovascular disease events and all-cause mortality. These individuals should receive substantial cardiovascular and all-cause mortality risk reduction benefit through antihypertensive medication initiation or intensification.
Translational outlook:
Efforts are needed to implement the 2017 American College of Cardiology/American Heart Association blood pressure guideline to reduce cardiovascular disease events and mortality associated with high blood pressure.
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions and further information about the study can be found at http://www.regardsstudy.org.
Funding: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional support was provided by grants R01 HL080477, 1K01HL133468, K24 HL125704, and K24 HL111154 from the National Heart, Lung, and Blood Institute, 15SFRN2390002 from the American Heart Association, and P20GM109036 (Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases) from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Disclosures: JNB receives support from American Heart Association Strategically Focused Research Network grant 15SFRN2390002. AB has received research support to his institution from Novartis not related to the current project. EBL, MMS and PM have received research support from Amgen, Inc. unrelated to this paper. EBL also has served on advisory boards for Amgen, Inc. and has been a consultant for a research project funded by Novartis unrelated to this paper. PM also has received research honoraria from Amgen, Inc. unrelated to this paper. LDC, PKW, DS and GH have no disclosures.
Abbreviations
- BP
Blood pressure
- CHD
Coronary heart disease
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- MI
Myocardial infarction
- SBP
Systolic blood pressuremmHg
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–248. [DOI] [PubMed] [Google Scholar]
- 2.Blood Pressure Lowering Treatment Trialists Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet 2014;384:591–598. [DOI] [PubMed] [Google Scholar]
- 3.Howard VJ, Cushman M, Pulley L et al. The REasons for Geographic And Racial Differences in Stroke Study: objectives and design. Neuroepidemiology 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 4.Kurella Tamura M, Wadley V, Yaffe K et al. Kidney function and cognitive impairment in US adults: the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 2008;52:227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goff DC Jr., Lloyd-Jones DM, Bennett G et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safford MM, Brown TM, Muntner PM et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA 2012;308:1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroke−−1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 1989;20:1407–31. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, White HD et al. Universal definition of myocardial infarction. Circulation 2007;116:2634–53. [DOI] [PubMed] [Google Scholar]
- 10.Luepker RV, Apple FS, Christenson RH et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003;108:2543–9. [DOI] [PubMed] [Google Scholar]
- 11.Soliman EZ, Howard G, Cushman M et al. Prolongation of QTc and risk of stroke: The REGARDS (REasons for Geographic And Racial Differences in Stroke) study. J Am Coll Cardiol 2012;59:1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 13.Muntner P, Whelton PK. Using Predicted Cardiovascular Disease Risk in Conjunction With Blood Pressure to Guide Antihypertensive Medication Treatment. J Am Coll Cardiol 2017;69:2446–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muntner P, Carey RM, Gidding S et al. Potential U.S. Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71(2):109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bangalore S, Toklu B, Gianos E et al. Optimal Systolic Blood Pressure Target After SPRINT: Insights from a Network Meta-Analysis of Randomized Trials. Am J Med 2017;130:707–719.e8. [DOI] [PubMed] [Google Scholar]
- 16.Bundy JD, Li C, Stuchlik P et al. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol 2017;2:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verdecchia P, Angeli F, Gentile G, Reboldi G. More Versus Less Intensive Blood Pressure-Lowering Strategy: Cumulative Evidence and Trial Sequential Analysis. Hypertension 2016;68:642–53. [DOI] [PubMed] [Google Scholar]
- 18.Xie X, Atkins E, Lv J et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016;387:435–43. [DOI] [PubMed] [Google Scholar]
- 19.Asayama K, Ohkubo T, Yoshida S et al. Stroke risk and antihypertensive drug treatment in the general population: the Japan arteriosclerosis longitudinal study. J Hypertens 2009;27:357–64. [DOI] [PubMed] [Google Scholar]
- 20.Clausen J, Jensen G. Blood pressure and mortality: an epidemiological survey with 10 years follow-up. J Hum Hypertens 1992;6:53–9. [PubMed] [Google Scholar]
- 21.Lindholm L, Ejlertsson G, Schersten B. High risk of cerebro-cardiovascular morbidity in well treated male hypertensives. A retrospective study of 40–59-year-old hypertensives in a Swedish primary care district. Acta Med Scand 1984;216:251–9. [DOI] [PubMed] [Google Scholar]
- 22.Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: a critical reappraisal. Circ Res 2015;116:1058–73. [DOI] [PubMed] [Google Scholar]
- 23.Ibsen H Antihypertensive treatment and risk of cardiovascular complications: is the cure worse than the disease? J Hypertens 2009;27:221–3. [DOI] [PubMed] [Google Scholar]
- 24.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2003;42:878–884. [DOI] [PubMed] [Google Scholar]
- 25.Appel LJ, Champagne CM, Harsha DW et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003;289:2083–2093. [DOI] [PubMed] [Google Scholar]
- 26.Appel LJ, Moore TJ, Obarzanek E et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 27.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ (Clinical research ed) 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He FJ, Li J, MacGregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ (Clinical research ed) 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 29.Whelton PK, He J, Cutler JA et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 1997;277:1624–1632. [DOI] [PubMed] [Google Scholar]
- 30.Carlson DJ, Dieberg G, Hess NC, Millar PJ, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis. Mayo Clin Proc 2014;89:327–34. [DOI] [PubMed] [Google Scholar]
- 31.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res 2016;39:88–94. [DOI] [PubMed] [Google Scholar]
- 33.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002;136:493–503. [DOI] [PubMed] [Google Scholar]
- 34.Roerecke M, Kaczorowski J, Tobe SW, Gmel G, Hasan OSM, Rehm J. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. Lancet Public Health 2017;2:e108–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2001;38:1112–1117. [DOI] [PubMed] [Google Scholar]
- 36.Eckel RH, Jakicic JM, Ard JD et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2960–84. [DOI] [PubMed] [Google Scholar]
- 37.The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA 1992;267:1213–20. [DOI] [PubMed] [Google Scholar]
- 38.Whelton PK, Appel LJ, Espeland MA et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 1998;279:839–46. [DOI] [PubMed] [Google Scholar]
- 39.Phillips RA, Xu J, Peterson LE, Arnold RM, Diamond JA, Schussheim AE. Impact of cardiovascular risk on the relative benefit and harm of intensive treatment of hypertension. J Am Coll Cardiol 2018;71:1601–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


