Abstract
Treatments for endometriosis include pharmacological or surgical procedures that produce significant side effects. We aimed to determine how environmental enrichment (EE) could impact the progression of endometriosis using the autotransplantation rat model. Female rats were exposed to EE (endo-EE: toys and nesting materials, 4 rats per cage, larger area enclosure) or no enrichment (endo-NE: 2 rats per cage) starting on postnatal day 21. After 8 weeks, sham surgery or surgical endometriosis was induced by suturing uterine horn tissue next to the intestinal mesentery, then allowed to progress for 60 days during which EE or NE continued. At the time of killing, we measured anxiety behaviors, collected endometriotic vesicles and uterus, and processed for quantitative real-time polymerase chain reaction for corticotropin-releasing hormone (CRH), urocortin-1, CRH receptors type 1 and type 2, and glucocorticoid receptor (GR). Endometriosis did not affect anxiety-like behaviors, yet rats in enriched conditions showed lower basal anxiety behaviors than the nonenriched group. Importantly, the endo-EE group showed a 28% reduction in the number of endometriosis vesicles and the vesicles were significantly smaller compared to the endo-NE group. Endometriosis increased CRH and GR only in the vesicles of endo-NE, and this increase was dampened in the endo-EE. However, urocortin 1 was increased in the vesicles of the endo-EE group, suggesting different pathways of activation of CRH receptors in this group. Our results suggest that the use of multimodal complementary therapies that reduce stress in endometriosis could be an effective and safe treatment alternative, with minimal side effects.
Keywords: alternative medicine, environment, reduction, stress, HPA axis
Introduction
To date, most techniques used to treat endometriosis either employ invasive surgical procedures or pharmacological treatment such as contraceptive hormones or gonadotropin-releasing hormone suppressors.1,2 These treatment approaches do not result in long-term efficacy, and most are accompanied by substantial side effects that may be highly detrimental to women with endometriosis.3 Because the symptoms of endometriosis are disruptive of day-to-day activities, relationships, and work,4 the use of a multidisciplinary management approach has been suggested as the standard of care for women with this complex condition.5 Moreover, neuroendocrine disturbances have been reported in endometriosis, which would require a systemic management approach to the disease.6 Despite this, very little empirical data are found regarding the use of alternative or complementary treatments in endometriosis alone or in combination with standard medical treatment7,8
Endometriosis symptoms have a significant negative impact in a women’s life, causing high levels of stress.9 In a report by Toth, she summarizes that women with endometriosis are caught in a “vicious circle of high stress perception, inflammation, and disease progression.”10 Previous work from our group has demonstrated that stress can exacerbate the progression of endometriosis,11 but its controllability can have beneficial effects.12 In the clinical scenario, we and others have also demonstrated that endometriosis/dysmenorrhea causes an adaptive decrease in cortisol secretion once the disease has been established, pointing toward a malfunction of the hypothalamic–pituitary–adrenal (HPA) axis stress system.13,14 Consequently, women with endometriosis might greatly benefit from alternative interventions that target stress reduction and promote well-being.15
Environmental enrichment (EE) is a well-studied model in the field of behavioral neuroscience. Environmental enrichment has already been effectively used as a possible treatment for a myriad of disease symptoms including depression and anxiety-related behaviors in a Huntington disease model16,17 and behavioral and immune cell function in a model of Alzheimer disease.18 Moreover, EE has been shown to either prevent or block the effects of chronic stress on hippocampal integrity when EE was started before or after the stressor began.19 These studies demonstrate that EE can have beneficial effects at the neural and peripheral level, mostly targeting the HPA axis in response to either physical or physiological stressors.20 Environmental enrichment is not a single manipulation but is a “combination of complex inanimate and social stimulation.”21 We currently do not have a direct translational model for EE, but modalities such as mindfulness-based cognitive behavioral therapy (CBT) share some translational aspects of animal EE by reducing anxiety responses. In fact, CBT is currently under clinical trial as a complementary treatment for inflammatory bowel disease, which shares many symptomatology similarities with endometriosis.22 However, we do not fully understand, at the mechanistic level, how environmental manipulations that impact the stress system work in improving symptomatology.
We aimed at determining how EE could impact the progression of endometriosis using a well-validated autotransplantation rat model.23–26 This model has been shown to have multiple parallels with the human condition such as subfertility, inflammation, and hyperalgesia.27–29 Our research group and others have shown that endometriotic lesions in this rat model share gene expression profiles with human lesions.30,31 In the present study, we used this model of endometriosis to investigate if EE, composed of larger enclosures, increased social interaction and novelty (toys, chewing, and nesting materials changed weekly) could ameliorate the progression of endometriosis. We hypothesized that EE will produce a resilient phenotype in this model and reduce endometriosis progression by targeting the HPA axis stress system.
Materials and Methods
Enrichment Model
Female Sprague Dawley rats of 21 days old were used in the experiments. Rats were kept in a 12-hour light/dark cycle with food and water ad libitum. Rats were weighed twice per week to monitor their adequate development. All experimental procedures were approved by the Ponce Health Sciences University and University of Texas and Rio Grande Valley Institutional Animal Care and Use Committees and adhere to the NIH Guide for the Care and Use of Laboratory Animals. At postnatal day (PND) 21, rats were assigned to either EE or no enrichment (NE) conditions. Environmental enrichment consisted of 3 main elements as standardly described32: novelty, increased social contact, and a larger environment that allowed for more movement and exploration. Due to the additional variability that physical exercise may introduce and that we have observed in previous experiments,12 we decided not to use exercise wheels or any form of physical exercise. Environmental enrichment housing was a Plexiglas box (30.8 × 59.3 × 22.8 cm) containing standard bedding, 4 rats per box and 4 different toys and/or nesting materials that were changed weekly. Toys/nesting included plastic igloos where rats could hide, chewing bones, chewable thin cardboard tubes, wood sticks, fabric hammocks, wicker balls, pumice stone chewing blocks, hard plastic hanging rings, cotton rods, and soft paper that can be plucked for nesting. No enrichment consisted of a Plexiglas box (20.3 × 40.6 × 19.05 cm) with 2 rats per cage and standard bedding. Rats spent 8 consecutive weeks in either NE or EE conditions. At the end of the 8 weeks, surgical endometriosis or sham surgery occurred. After surgery, rats returned to their previous housing conditions and endometriosis was allowed to progress for 60 days. Overall, rats spent 56 days in EE or NE prior to any surgical manipulation and additional 60 days after endometriosis or sham surgery. If rats started on EE or NE at PND 21, they were continued on EE or NE after surgery; no change in conditions occurred. Therefore, rats were either in EE or NE for a total of 116 days starting on PND 21 until PND 137.
Endometriosis Induction
Endometriosis was surgically induced under isoflurane anesthesia by autotransplanting pieces of the right uterine horn to the intestinal mesentery. The protocol has been previously described.27,33 The control group were sham-operated animals for which the right uterine horn was massaged for 2 minutes and sutures were placed in the intestinal mesenteric area with no uterine implants. Similar to our previous report,33 endometriosis was allowed to progress for 60 days before killing.
Behavioral Assessment
On the day of killing, rats were tested in 2 anxiety behavioral tasks: the open field and the elevated zero maze. The open field test measures exploratory and locomotor activity of a rodent in an open arena. Time spent at the walls versus the center can be measured as an index of anxiety. The testing apparatus consisted of a square wood arena (91 ×91 × 38 cm, fabricated in house) with overhead light illumination and video monitoring to record animal activity using Any-Maze software (Stoelting, Wood Dale, Illinois). Animals were placed at the center of the field and activity was recorded for 20 minutes, quantifying the following parameters: (1) total distance moved, (2) time spent moving, (3) time spent at the center of the arena, (4) time spent near the walls of the arena (defined by the 15 cm of floor arena closest to the walls), and (5) total fecal pellets. Data were analyzed in 5-minute intervals. At the end of the testing period, animals were returned to the home cage and, after a 5-minute break, were tested in the elevated zero maze. Only the first 5 minutes in the open field are presented in Figure 1 to maintain a comparable amount of behavioral time in both the open field and the zero maze. Sequential testing of animals in behavioral tests, using the open field as the first test, has been shown to have no effect on subsequent testing.34
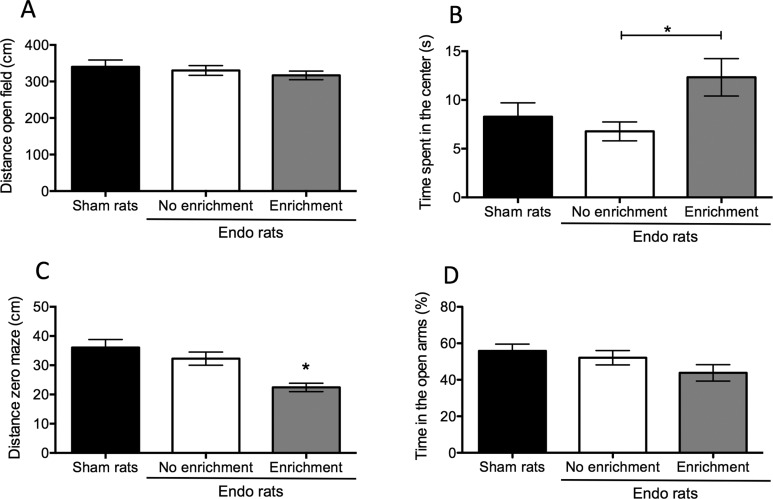
Figure 1.
Behavioral assessment of anxiety in rats with endometriosis and sham controls. A, There was no difference in locomotor activity for the rats with endometriosis or sham controls in the open field test. B, Rats belonging to the endo-enrichment group (EE) spent significantly more time in the center of the arena compared to the endo-no enrichment (NE) group suggesting decreased anxiety. C, In the zero maze, rats belonging to the endo-EE group had decreased locomotor activity compared to the sham and the endo-NE groups. D, Despite the difference in locomotor activity, there was no difference between groups in the percentage of time spent in the open arms of the zero maze. In this and subsequent graphs, bars represent average ± standard error of the mean (SEM). * P < .05.
The elevated zero maze is very similar to the more classically used elevated plus maze, with the advantage of not having a center zone, thus eliminated the ambiguity the center zone causes. The zero maze measures the unconditioned anxiety caused in response to an elevated platform. The apparatus consisted of a circle with an arm width of 10 cm and elevated 40 cm from floor. Two sections of the circle were open without walls and 2 enclosed by 40-cm high walls. The zero maze measures the general aversion of rodents to open and elevated spaces. The more time that the animal spends on the open arms was interpreted as having less anxiety. The maze was purchased from Stoelting Co. A description and picture of the specific maze used can be accessed in the website, http://www.anymaze.co.uk/plus-zero-y-t-radial-mazes.htm. Rats were placed in the intersection of an open arm, facing the closed arm and opposite to the experimenter. Behavior was videotaped for 5 consecutive minutes using the Any-Maze software recording the following parameters: time spent in the open/closed arms and the number of entries made by the rodent onto the open/closed arms. An arm entry is considered when 60% of the animal body is on the arm. At the end of the 5-minute testing period, the animal was returned to the home cage, total fecal pellets are counted, and the maze is cleaned with bactericide solution. Rats were immediately anesthetized with an overdose of 65% sodium pentobarbital to proceed with laparotomy.
Sample Collection and Processing
At the time of killing, all animals had a cytological smear taken to verify the stage of the estrous cycle. The peritoneal cavity was opened and examined for the presence of vesicles and the original sutures. Vesicles were defined as cystic structures, round or oval in shape around the original implantation site of uterine tissue. In most vesicles, the original piece of suture could be identified (see panel F, Figure 2). We first collected peritoneal fluid using a sterile plastic pipette. The endometriotic vesicles that developed were weighed, and the longest length and width were measured with a digital caliper. Classification of vesicles was carried out as previously described.12,35 Vesicles were assigned the following grades: grade 1 = disappeared; grade 2 = 0.01-1.99 mm; grade 3 = 2-4.49 mm; grade 4 = 4.5-5.99 mm; and grade 5 = 6.0 mm or larger. We collected the adrenal glands, removed all surrounding fatty tissue, and weighed them. Tissue segments from the colon, uterus, and vesicles were flash frozen and stored at −80° until further processing.
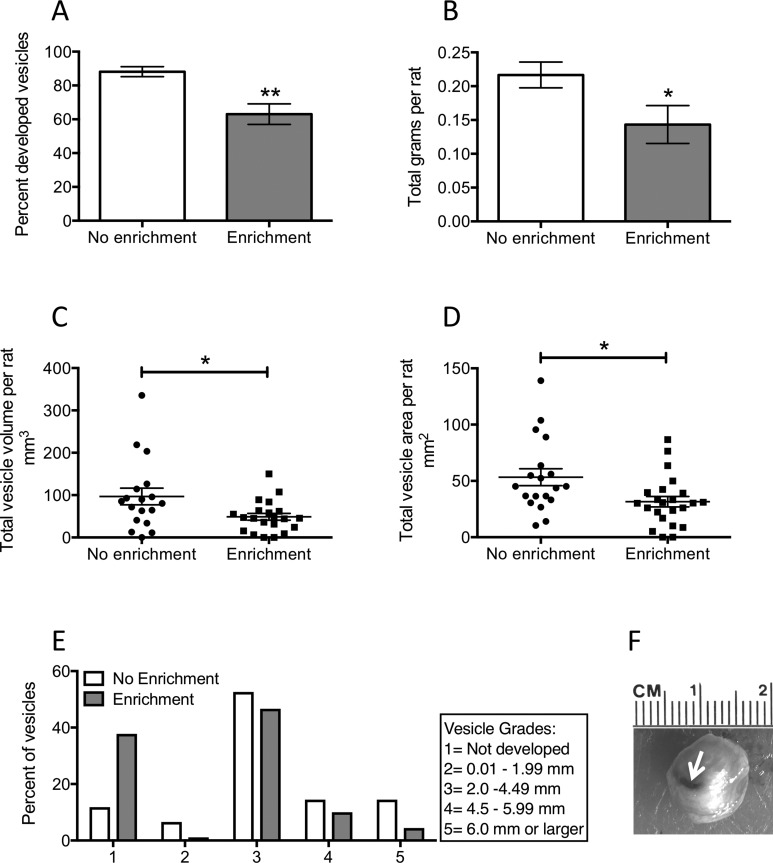
Figure 2.
Morphological characteristics of endometriosis vesicles. A, The percentage of implants that developed into vesicles was significantly lower in the endo-enrichment (EE) group compared to the endo-no enrichment (NE). B, The total weight of all vesicles per rat was smaller for the endo-EE group. C, The total vesicle volume per rat was significantly smaller for the endo-EE group compared to the endo-NE group. D, Similar to volume, the total vesicle area per rat was significantly smaller in the endo-EE compared to the endo-NE group. E, Vesicles that developed were classified by grade based on a scale by size. F, Example of a grade 5 vesicle. The white arrow is pointing toward the silk suture used to attach the uterine tissue to the mesentery, which got completely enveloped in the vesicle as it developed. *P < .05; **P < 0.01.
RNA Isolation and Complementary DNA Synthesis
Collected endometrial implants (vesicle) and uterine horn tissue, from endo or sham rats, were lysed by mortar and pestle in RLT buffer (Qiagen, Germantown, Maryland). Extractions of the total RNA from the lysates were extracted according to RNAeasy Mini Kit manufacturer’s protocol (Qiagen). RNA concentration and purity were measured on a NanoDrop 2000 UV spectrophotometer (Thermo Scientific, Wilmington, Delaware). Concentration and quality of RNA samples were acquired based on the ratio of absorbance at 260/280 nm in the spectrophotometer. Synthesis of complementary DNA (cDNA) from RNA samples was done using iScript cDNA Synthesis Kit according to the manufacturer’s protocol (Bio-Rad, Hercules, California). Total reaction volume was 20 μL including 0.1 μg of total RNA concentration and synthesis reagents. Reactions were carried out in PTC-100 thermal cycler (Bio-Rad). Reverse transcription polymerase chain reaction (RT-PCR) running method was as follows: 25°C for 5 minutes, 42°C for 30 minutes, 85°C for 5 minutes. Samples were stored at −80°C for later experimentation or quantitative RT-PCR (qRT-PCR).
Quantitative RT-PCR (Corticotropin-Releasing Hormone, Urocortin-1, Corticotropin-Releasing Hormone Receptor Type 1, Corticotropin-Releasing Hormone Receptor Type 2, and Glucocorticoid Receptor)
To evaluate gene expression, qRT-PCR was performed in a Mastercycler Realplex 2 System (Eppendorf, Hauppauge, New York) using 25 µL of a total volume of reaction assay with a 1:10 dilution of cDNA with IQ SYBR Green Supermix (Bio-Rad) in a 96 well-plate according to the manufacturer’s protocol. Commercial primers for corticotropin-releasing hormone (CRH), urocortin 1, CRH-receptor type 1 (CRHR1), CRH-receptor type 2 (CRHR2), and glucocorticoid receptor (GR) were used (Qiagen). Real-time PCR cycles consisted of 95°C for 10 minutes for enzyme activation followed by 40 cycles of denaturing at 95°C for 15 seconds and annealing at 60°C for 1 minute. Fold change expression were normalized with the levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) of each sample. Cycle threshold (CT) values and changes per gene expression level were automatically analyzed by the Realplex v.15 software (Bio-Rad). All samples were run in duplicate.
Corticosterone Stress Hormone Assay
Serum and peritoneal fluid corticosterone were analyzed using commercially available enzyme-linked immunosorbent assay (ELISA) kits for rat corticosterone (IBL-America, Spring Lake Park, Minnesota) following the manufacturer’s instructions.
Statistical Analyses
GraphPad Prism 6.0 (Graph-Pad Software, San Diego, California) was used to prepare graphs and presented as mean difference ± standard error of the mean (SEM). We used SPSS version 21 for statistical analyses. A P value <.05 was considered statistically significant. A 1-way analysis of variance was used for normally distributed variables (assessed by Shapiro-Wilk normality test), for comparisons of 3 or more groups. A Student t test was used for comparisons between 2 groups. When the variability between groups was significantly different, a Welch corrected t test was used. A test for outlier values was also conducted. qRT-PCR results were analyzed using a 1-sample t test against the sham rats value normalized to 1.0.
Results
Anxiety Behaviors Are Altered by Endometriosis and EE
Environmental enrichment in this experiment consisted of 3 main factors: increased social contact, a larger environment that allowed for more movement and exploration, and novelty provided by weekly change of toys, chewing, and nesting materials. Rats started enrichment immediately after weaning and remained in enrichment for 8 consecutive weeks. Rats received surgical implantation of 4 uterine pieces on their mesentery (endometriosis) based on the model of Vernon and Wilson27 after 8 weeks of enrichment or standard housing. Sham rats remained in standard housing and only received sutures (n = 11). A group of 20 rats were used for NE, whereas 24 rats were used for the EE group. One rat from the NE group died during surgery due to anesthesia overdose, whereas 1 rat from the EE group was eliminated due to a subcutaneous abscess in the abdominal wall resulting in final group numbers of 19 and 23 animals, respectively.
After 60 days of endometriosis progression, we deeply anesthetized the rats and performed a laparotomy to examine whether endometriotic vesicles formed, then measured and excised the vesicles. On the morning of laparotomy, we assessed anxiety behaviors using 2 frequently used behavioral tasks: the open field and the elevated zero maze. We observed a dichotomized response in anxiety behaviors. First, in the open field, we observed equal locomotor activity for all 3 groups (F2,52 = 1.41; P > .05; Figure 1A), suggesting that neither endometriosis nor enrichment conditions changed exploratory activity in this task. We observed the endo-EE rats with less anxiety behaviors spend significantly more time at the center of the field as compared to the endo-NE group (main effect F2,49 = 3.44; P < .05; post hoc, P < .05; Figure 1B). On the other hand, the endo-EE rats showed a decrease in the total locomotor activity in the zero maze as measured by the total distance traveled in the whole maze (F2,52 = 12.34, P < .001; post hoc, P < .01 compared to sham and endo-NE; Figure 1C). When we quantified the percentage of time that the rats spent in the open arms, we did not find any difference between groups (F2,51 = 2.53; P > .05; Figure 1D).
Endometriotic Vesicles Are Decreased in Number by EE
We consistently recorded a significantly decreased number of implant sites that developed into endometriosis vesicles in the enrichment group (t = 3.46; df = 40; P < .01; Figure 2A). Within the vesicles that developed in the 2 groups, the total weight of vesicles averaged per rat was lower for the endo-EE group than for the endo-NE group (Welch corrected t test: t = 2.16; df = 36; P < .05; Figure 2B). The lower weight was also related to a smaller total volume (Welch corrected t test: t = 2.10; df = 24, P < .05; Figure 2C) and area (t = 2.47; df = 40; P < .05; Figure 2D) of developed vesicles per rat. Consistent with our previous reports,12,33 we also quantified the grade of the vesicles based on a length scale for each vesicle, where 1 denotes an implant that disappeared and 5 an implant that developed into a vesicle equal or larger than 6 mm (Figure 2E). We observed a larger percentage of implants that did not develop into endometriosis vesicles in the endo-EE group. The percentage of vesicles assigned a grade 3 or 4 was similar for both groups, with a smaller number of vesicles reaching a grade of 5 in the endo-EE group compared to the endo-NE group. Vesicles that did develop showed a pattern of characteristics such as infiltrate color, some being yellowish and others transparent, while yet others showed a brownish coloration (Figure 2F), similar to what is observed in women with endometriosis.36,37
Endometriosis Changes the HPA Axis Regardless of EE
At the time of killing, we collected the left and right adrenal glands, peritoneal fluid, and blood to quantify corticosterone content by ELISA. Since adrenal weight can vary depending on the size of the animal, we normalized for the rat body weight measured right before killing. We found a significant decrease in adrenal size for the rats that had endo-EE and endo-NE as compared to the sham group (F2,49 = 4.08; P <.05; Figure 3A). In addition, we found a significant hypocortisolism in both endo-EE and endo-NE groups compared to the sham control group. Main effect analysis revealed that this change was significantly different for both the corticosterone content in peritoneal fluid (F2,34 = 8.21; P <.01; Figure 3B) and in serum (F2,34 = 4.25, P <.05; Figure 3C). Post hoc analysis revealed that the sham group was significantly different from both groups of endo in the peritoneal fluid (P < .01 both comparisons) and in serum (P < .05, both comparisons). However, EE did not produce any change in corticosterone content in rats with endometriosis.
Figure 3.
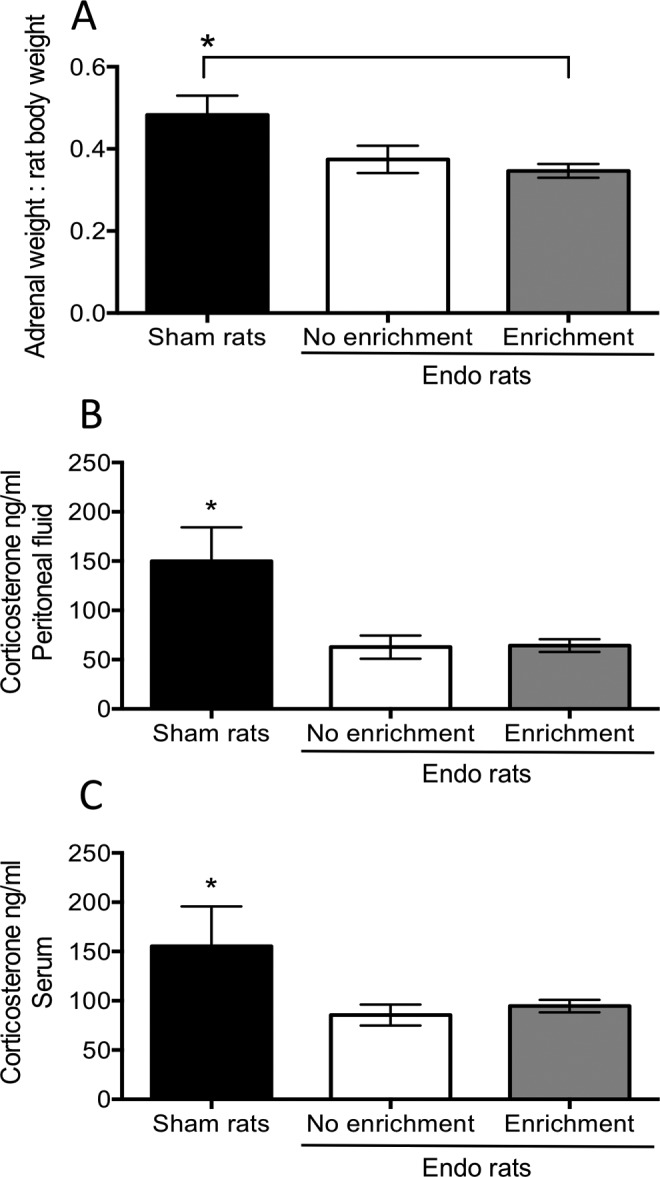
Adrenal weight and corticosterone measurements in peritoneal fluid and plasma. A, The weight of both adrenal glands normalized to the total body weight at killing. The size for the adrenal glands in endo-enrichment (EE) group was significantly smaller than that of the sham group. B, Corticosterone levels in peritoneal fluid measured by enzyme-linked immunosorbent assay (ELISA) showed a significant decrease for both groups with endometriosis compared to sham control group. C, Similar to peritoneal fluid, corticosterone in plasma was also decreased for both groups of rats with endometriosis compared to sham control rats. *P < .05.
Environmental Enrichment Decreases CRH and GR Messenger RNA in Endometriosis but Does Not Impact CRH Receptors
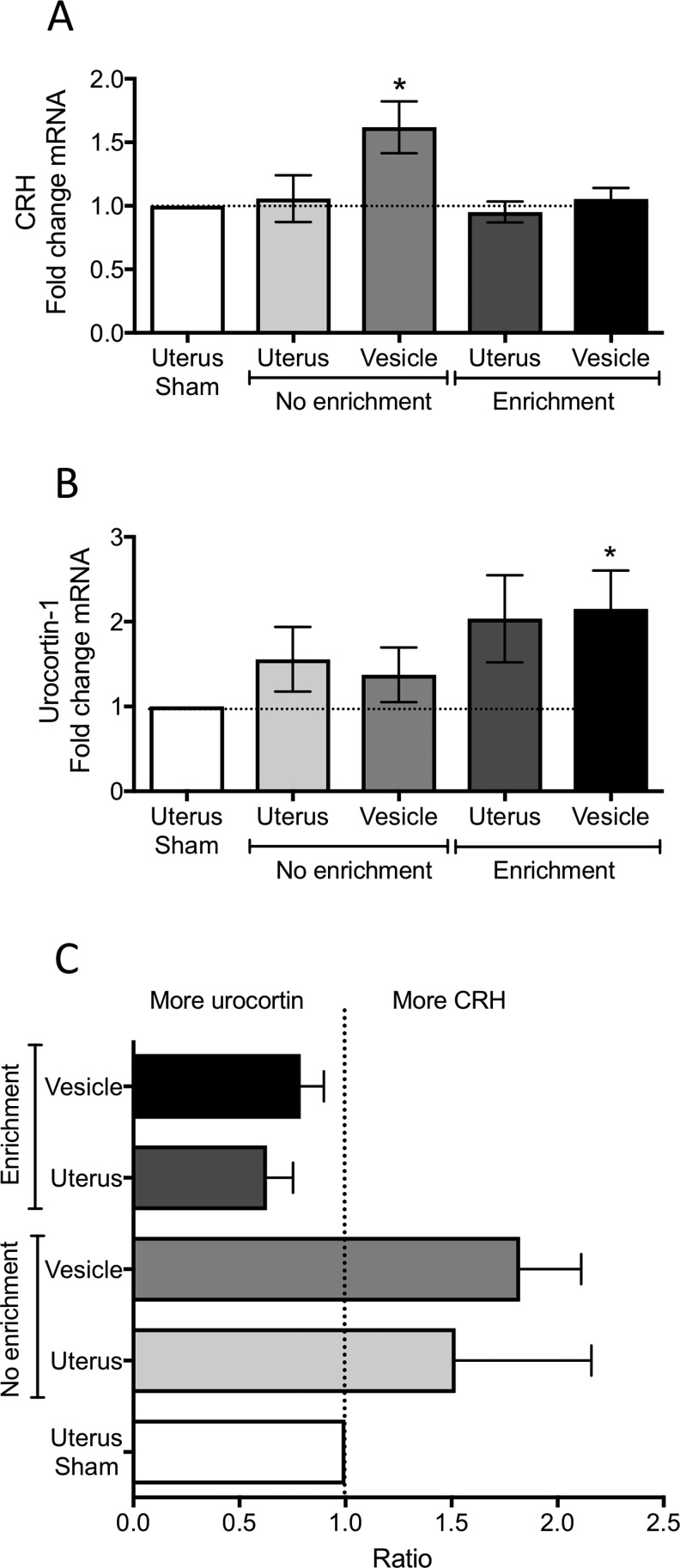
At the time of laparotomy, the developed endometriosis vesicles were collected, flash frozen, and later used for the analysis of messenger RNA (mRNA) content of GR, CRH, urocortin-1, CRHR1, and CRHR2 using qRT-PCR. We selected these markers since previous reports have shown abnormalities in CRH and CRH receptors in endometriosis samples from women.38–40 All vesicles and uterus were compared against the housekeeping gene (GAPDH). By having the normal uterus of the same rats in the comparison, we controlled for individual variabilities between rats and for the possible effects of the estrous cycle on the parameters measured. We also quantified mRNA in the uterus from the sham group. We observed no changes in the uterus of sham rats for any of the 3 genes measured (fold change: GR = 1.02; CRH = 1.09; CRHR1 = 1.04; CRHR2 = 1.01). As such, all values from the uterus and vesicles from endo rats were normalized and statistically compared to sham. We observed a significant increase in CRH mRNA within the endometriotic vesicles of the endo-NE group compared to sham uterus (1 sample t test: t = 2.59; df = 13; P <.05; Figure 4A). No changes in CRH were observed for the uterus or vesicles of the endo-EE group. For urocortin-1, a significant increase in the vesicles of the endo-EE group was observed (1 sample t test: t = 2.54; df = 7; P < .05; Figure 4B). Similarly, the uterus of the endo-EE group showed an increase in urocortin-1 mRNA of 50%, which fell close to significance (1 sample t test: t = 2.02; df = 7; P = .08; Figure 4B). However, it is notable that even when we did not observe significance, there was an increase of 30% in urocortin mRNA in both uterus and vesicles of the endo-NE rats. We contrasted the relative amount of CRH to urocortin-1 in uterus and vesicles of the NE group to the EE group, given that CRH preferentially binds to CRHR1 but urocortin-1 has equal selectivity to both CRHR1 and CRHR2. The ratio of CRH to urocortin-1 within animals revealed that the EE group has relatively more urocortin-1 than CRH in uterus and vesicles, while the opposite is true for the NE group (Figure 4C).
Figure 4.
Messenger RNA (mRNA) levels of corticotropin-releasing hormone (CRH) and urocortin 1 measured by qRT-PCR from the uterus and endometriosis vesicles. A, Corticotropin releasing hormone. B, Urocortin 1. C, Ratio of CRH to urocortin 1 in uterus and vesicles, illustrating the comparative amount of peptides in each group. Data normalized to the uterus of sham rats. *P < .05 from sham levels.
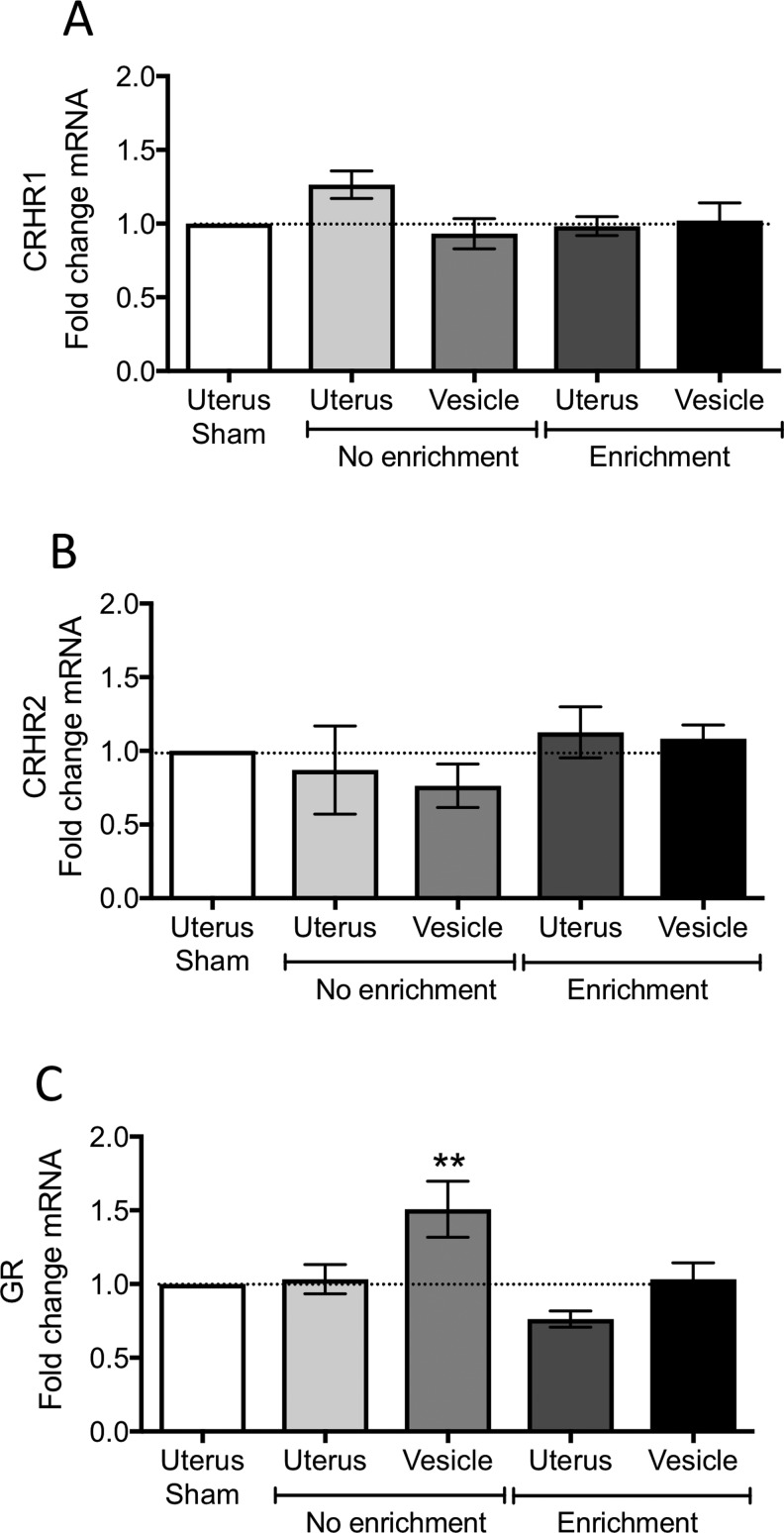
The CRHR1 or CRHR2 did not show any changes between endometriosis vesicles and normal uterus compared to the sham group (Figure 5A and B). We measured a significant increase in GR mRNA in the vesicles from the endo-NE group compared to sham uterus (1 sample t test: t = 2.56; df = 14; P <.05; Figure 5C). This increase was not observed for either the uterus or vesicles of the endo-EE group, suggesting that EE directly impacts the levels of GR in endometriotic vesicles.
Figure 5.
Messenger RNA (mRNA) levels of corticotropin-releasing hormone (CRH) receptors and glucocorticoid receptor measured by qRT-PCR from the uterus and endometriosis vesicles. A, Corticotropin-releasing hormone receptor type 1 (CRHR1). B, Corticotropin-releasing hormone receptor type 2 (CRHR2). C, Glucocorticoid receptor (GR). Data normalized to the uterus of sham rats. *P < .05 from sham levels.
Discussion
Our data demonstrate that a noninvasive manipulation of the environmental conditions under which the individual lives can have a significant impact on decreasing certain aspects of endometriosis progression. Environmental enrichment has been shown to have beneficial effects on symptoms in multiple diseases including, but not limited to, anxiety, addictive phenotype, depression-like behaviors, and neurodegenerative diseases such as Alzheimer and Huntington.20 In the present study, we adhere to the most frequently used protocol of EE in the field20 by starting interventions at weaning and continuing enrichment throughout the duration of the disease. In our endometriosis model, EE was effective in decreasing vesicle number and size and preventing an increase in CRH and GR expression within the vesicles. We propose that a potential model to explain how EE helps chronic disease presentation is through an increased ability of the HPA axis to achieve stability, referred to as allostasis.41 As endometriosis progresses, it creates a state of increased demands by several physiological systems, consequently resulting in increased allostatic load.42 Allostatic load is better defined as the “wear and tear that results from too much stress”.42 Our results suggest that rats without enrichment were more vulnerable to the increased allostatic load generated by endometriosis and presented with a more pronounced pathological state compared to those animals receiving enrichment. On the other hand, rats exposed to EE seemed better able to mount an adequate allostatic response when endometriosis was induced, resulting in a milder expression of the disease. In addition, social interaction—since the subjects were exposed to larger groups of individuals during EE—might have further helped the animals learn adaptive responses to the environmental changes leading to resilience32 of the HPA axis. In fact, EE has been shown to increase the resilience of animals to social defeat stress by enhancing infralimbic cortical connections with downstream limbic areas and this is done via strengthening the cortical control that the infralimbic prefrontal cortex has over the HPA axis.43,44
In our study, EE produced an increase in the amount of time spent at the center of the open field, indicating less anxiety and replicating what many previous studies have found regarding the positive behavioral sequelae of EE. On the other hand, we interpret the decreased activity observed in the zero maze for the endo-EE group as a decreased drive to explore because these animals already have a significantly stimulating environment. In our current study, the zero maze seems to be innocuous for unconditioned anxiety responses. This concurs with prior studies which have shown decreased activity due to enrichment and no changes in the time spent in the open arms of an elevated zero maze in response to enrichment.45,46 On the other hand, reduced locomotor activity in EE rats might suggest a faster habituation to mild stressors that exposure to a novel environment might produce, since similar observations have been reported in previous studies.43,47 Endometriosis did not produce an anxiety profile in the rats, meaning that rats with endometriosis but without enrichment were not more anxious than the sham control group. This is a behavioral parameter that has not been replicated from the clinical scenario because women with endometriosis tend to show a higher incidence of introversion anxiety.48 This indicates that the behavioral tests used in this study might not be sensitive enough to measure certain aspects of anxiety that can be present in the animal, since our previous work has demonstrated that administered external stress can produce increased anxiety as assessed by increased fecal pellet counts.11
Our data parallel the clinical observations found in women with endometriosis,13,14 showing a decrease in cortisol basal state, even when controlling for treatment status of the patients. Cortisol is a key regulator of inflammation, thus a decrease in cortisol content, both in the peritoneal compartment and serum, can have negative consequences in terms of immune responses in women with endometriosis. Endometriosis, regardless of enrichment, produced a significant decrease in corticosterone compared to sham animals. Therefore, both endo-NE and endo-EE had a blunted corticosterone response, but this was not the case for sham rats (which were housed in similar conditions and underwent the same behavioral tests as the endo-NE rats). Environmental enrichment did not affect the levels of corticosterone either in blood or in peritoneal fluid. Previous studies have shown that baseline plasma corticosterone levels do differ between enriched and nonenriched female rats after 12 weeks of housing conditions,49 and others have shown inconsistent results specifically in female rodents (reviewed in Girbovan and Plamondon).50 It is important to note that our endo-NE group and sham group were always paired housed, and not isolated, which in female rats seems to have a larger effect in increasing corticosterone concentrations.51 Our data point toward the fact that a blunted HPA response is due to the presence of endometriosis. We were unable to obtain reliable measurement of adrenocorticotropic hormone (ACTH) due to the limited amounts of peritoneal fluid. Glucocorticoid independent anti-inflammatory effects of ACTH have been identified.52,53 Therefore, in future studies, levels of peritoneal ACTH could help identify if during endometriosis, other mechanisms of anti-inflammatory action are taking place or are also disrupted as the disease progresses.
Our data add to an increasing body of evidence showing that endometriosis is associated with alterations in GR and CRH signaling. We observed both increased GR and CRH mRNA within the endometriosis vesicles of the endo-NE group, but EE was sufficient to normalize this change in the mRNA and bring the levels similar to sham rats. Within normal uterus GR signaling is responsible for maintaining fertility and reduced inflammation in response to decidualization.54 On the other hand, CRH induces decidualization of endometrial stromal cells55 and in rat uterus appears to be a paracrine/autocrine pro-inflammatory mediator.56 It is very likely then that an increased CRH activates secondary cascades that in turn allows for ectopic endometrium to undergo morphological changes and increase proliferation at ectopic places, similar to the signaling that has been observed in colon cancer57 and endometrial carcinoma.58 Although the same mechanisms have not been demonstrated for endometriosis, we believe this could be taken into consideration for future studies.
An increased GR might be a compensatory response to try to decrease inflammation at the ectopic implantation site, thus initiating a tug of war between CRH and GR signaling cascades. Although the CRHR1 was not altered in any of the groups examined, the relative ratio of increased amount of CRH within the endometriotic vesicles and a relative ratio of lower amount of urocortin-1 (see panel C in Figure 4) could engage/activate the available CRHR1 chronically, leading to receptor desensitization and alteration in signaling. On the other hand, a relatively higher ratio of urocortin-1 compared to CRH in the vesicles and uterus of the endo-EE group could shift mechanisms that favor CRHR2 signaling cascades. A deficiency in CRHR2 signaling in colorectal cancer promotes tumor expansion and spread via a pro-inflammatory mechanisms.59 It is possible that similarly, lower activation of CRHR2 signaling relative to CRHR1 could perpetuate endometriosis progression. In addition, CRH induces decidualization of endometrial stromal cells and appears to be a pro-inflammatory mediator in the uterus,55,56 while GR signaling is responsible for maintaining fertility and reduce inflammation in response to decidualization54; hence, it is possible that perpetuation of GR and CRH signaling may lead to infertility via abnormally sustained signaling of these 2 receptors.
Translational Significance
In the clinical scenario, several groups have found increases in GR, CRH, and urocortin in tissues from women with endometriosis. Specifically, within primary endometrial stromal cells (proliferative phase) from women with endometriosis, GR mRNA was increased with a concomitant decrease in the progesterone receptor.60 The glucocorticoid-regulated kinase, which mediates cell responses to environmental stress and has been shown to have antiapoptotic properties, is upregulated in tissue from ovarian endometriosis.61 Similar to GR, CRH and urocortin are upregulated at the mRNA and protein levels in lesions compared to eutopic endometrium in women with endometriosis.40 In this same study, an increase in the CRHR1 subtype beta was also observed. Corticotropin-releasing hormone receptor type 1 splice variants can be differentially regulated by estradiol or progesterone,62 suggesting that splice variant analysis of the CRHR1 might give some additional clues as to the role they play in the pathophysiology of endometriosis. Additional support on the alterations in CRH comes from evidence, showing that in the endometrium (secretory phase) of women with endometriosis there is a lack of increase in CRH and urocortin neuropeptides as is normally observed in healthy women.38 It has been observed that the CRH increase in endometriotic lesions most likely comes from activated mast cells within the lesions.63 There is an increase in CRH-binding protein in the peritoneal fluid of patients with advanced endometriosis, which seems to be an attempt for the system to control the abnormal expression of CRH.64 Taken together, our current data support the clinical observations of anomalous expression of CRH, urocortin-1, and GR in endometriotic tissues. It is proposed that CRH stimulates the secretion of vascular endothelial growth factor (VEGF),65 leading to increased angiogenesis and perpetuation of the disease. Therefore, increased stress leading to the activation of CRH-dependent pathways could aggravate the disease, while controlling the stress with interventions such as environmental manipulations can be effective on decreasing disease progression via this mechanism.
Despite the multiple beneficial effects that EE has demonstrated in the animal models of disease, to our knowledge, a translational approach has not been tested in the clinical scenario. We therefore propose that EE interventions can be effectively adapted and modified for women with endometriosis resulting in reduced stress, reduced inflammation, and increased outcomes related to quality of life. To mimic the elements of EE, we could implement a multimodal intervention that features the 3 hallmarks of EE: increased social interaction (more animals per cage = support group meetings, online support via social media), novelty (toys and activities = exposure to new hands-on activities and learning activities), and larger enclosures (larger cages = meetings in open environments). Although the proposed intervention has not been tested yet, there are 2 theories that support the use of more naturalistic, open environments in reducing disease. The Stress Recovery Theory postulates that humans evolved over a long period in natural environments; therefore, people are to some extent physiologically and perhaps psychologically adapted to natural (open), as opposed to urban (enclosed), settings.66 The second theory is the Attention Restoration Theory. It postulates that humans have an unlearned predisposition to pay attention and respond positively to natural content (eg, vegetation, water) favorable to survival during evolution.67 These 2 theories highlight the relevance of manipulating the environment as a complementary method to treat endometriosis and similar inflammatory chronic disorders resulting in benefit to patients.
Study Limitations
Environmental enrichment frequently includes a component of physical exercise. In our enrichment conditions, we decided not to include a running wheel or similar type of enrichment in the cage as it would have been difficult to monitor the amount of exercise each animal was getting since multiple animals were housed in the same cage (social enrichment). Although some behavioral equipment can be purchased for individual monitoring of voluntary exercise, that type of equipment may be bulky and limit the inclusion of other elements that we thought were important in the enrichment condition such as the toys and nesting materials. The selected enrichment conditions without exercise have been previously shown to have beneficial effects in spatial and working memory of rodents.68 Previous studies from our laboratory has demonstrated that physical activity in the form of swimming provides certain beneficial effects for improving endometriosis.12 However, the effects of exercise in improving endometriosis outcomes can be explored on its own given the multiple factors that play a role in exercise physiology. It is plausible that exercise plus the enrichment conditions proposed in this study can have improved beneficial effects than either one on its own, but this can be tested in future experiments.
One of the clinical landmarks of endometriosis is pelvic pain. We did not assess pain modality in the current study as it could have interfered with the anxiety behavioral measurements. Enrichment has been shown to alleviate neuropathic and inflammatory pain in rodents.69,70 Future experiments should address the impact of EE and pain perception in endometriosis as this is an important relevant factor for the clinical setting.
The effects of EE in the brain are well documented (see recent review by Gelfo et al71). Although the current article focused on anxiety behaviors and peripheral markers of disease severity, we strongly believe that under enriched conditions, there was a direct impact at the level of the hypothalamus and hippocampus in these animals. Therefore, future studies may combine direct manipulations at the level of the hypothalamus, in addition to the possible involvement of other stress-related brain areas, such as the amygdala, in endometriosis progression and pain perception.
Conclusion
By using EE, we demonstrated a stabilization of GR and CRH in endometriotic tissues, but urocortin-1 remained unaffected. Therefore, within the clinical scenario, noninvasive environmental manipulations that replicate the elements of EE: novelty, increased social interaction, and open natural spaces can be of significant benefit for impacting abnormal signaling and reducing endometriosis development and/or progression. Translationally, our results suggest that multimodal interventions that reduce stress in endometriosis and that target the neuroendocrine–immune disequilibrium6 could impact disease development and limit the use of invasive treatments that compromise reproductive abilities with considerable side effects. We propose that combining social support, novelty, and open spaces will have a significant positive effect on endometriosis progression. These combined therapeutic modalities can be easily applied to younger women with endometriosis who might not want to deal with the side effects of pharmacological interventions but would like a complementary or alternate treatment modality to help slow disease progression resulting in improved quality of life.
Supplemental Material
Supp-Fig for Environmental Manipulations as an Effective Alternative Treatment to Reduce Endometriosis Progression by Annelyn Torres-Reverón, Leslie L. Rivera, Idhaliz Flores, and Caroline B. Appleyard in Reproductive Sciences
Acknowledgments
The authors acknowledge the participation of the graduate students Inevy Seguinot and Raura Doreste and the undergraduate students Dayanira Morales and Anaiz Santana. The helpful discussions of Dr Karla Martinez and Dr Emily Perez are greatly valued. The authors would like to acknowledge the technical assistance of Maria del C. Colon and Myrella L. Cruz. The helpful comments on the manuscript from Dr Siomara Hernandez, Dr Raymond Isidro, and Dr Bruce McEwen are also greatly appreciated.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Torres-Reveron and Dr Appleyard declare that part of the work presented herein is under review for a patent in the United States.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the following NIH grants: K07AT008027 to A.T.R. and the RCMI BRAIN (behavioral) and MAGIC (molecular) cores MD007579 and by RISE grant 2R25GM082406.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Abu Hashim H. Gonadotrophin-releasing hormone analogues and endometriosis: current strategies and new insights. Gynecol Endocrinol. 2012;28(4):314–321. [DOI] [PubMed] [Google Scholar]
- 2. Raffi F, Metwally M, Amer S. The impact of excision of ovarian endometrioma on ovarian reserve: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97(9):3146–3154. [DOI] [PubMed] [Google Scholar]
- 3. Schrager S, Falleroni J, Edgoose J. Evaluation and treatment of endometriosis. Am Fam Physician. 2013;87(2):107–113. [PubMed] [Google Scholar]
- 4. Fourquet J, Baez L, Figueroa M, Iriarte RI, Flores I. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril. 2011;96(1):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Graaff AA, D’Hooghe TM, Dunselman GAJ, Dirksen CD, Hummelshoj L, Simoens S. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677–2685. [DOI] [PubMed] [Google Scholar]
- 6. Tariverdian N, Theoharides TC, Siedentopf F, et al. Neuroendocrine-immune disequilibrium and endometriosis: an interdisciplinary approach. Semin Immunopathol. 2007;29(2):193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kong S, Zhang YH, Liu CF, et al. The complementary and alternative medicine for endometriosis: a review of utilization and mechanism. Evid Based Complement Alternat Med. 2014;2014:146383 doi:10.1155/2014/146383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goncalves AV, Barros NF, Bahamondes L. The practice of hatha yoga for the treatment of pain associated with endometriosis. J Altern Complement Med. 2017;23(1):45–52. [DOI] [PubMed] [Google Scholar]
- 9. Lemaire GS. More than just menstrual cramps: symptoms and uncertainty among women with endometriosis. J Obstet Gynecol Neonatal Nurs. 2004;33(1):71–79. [DOI] [PubMed] [Google Scholar]
- 10. Toth B. Stress, inflammation and endometriosis: are patients stuck between a rock and a hard place? J Mol Med (Berl). 2010;88(3):223–225. [DOI] [PubMed] [Google Scholar]
- 11. Cuevas M, Flores I, Thompson KJ, Ramos-Ortolaza DL, Torres-Reveron A, Appleyard CB. Stress exacerbates endometriosis manifestations and inflammatory parameters in an animal model. Reprod Sci. 2012;19(8):851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hernandez S, Cruz M, Torres-Reveron A, Appleyard C. Impact of physical activity on pain perception in an animal model of endometriosis. J endometr. 2015;7(3):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quinones M, Urrutia R, Torres-Reveron A, Vincent K, Flores I. Anxiety, coping skills and hypothalamus-pituitary-adrenal (HPA) axis in patients with endometriosis. J Reprod Biol Health. 2015; 3(2) doi:10.7243/2054-0841-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petrelluzzi KFS, Garcia MC, Petta CA, Grassi-Kassisse DM, Spadari-Bratfisch RC. Salivary cortisol concentrations, stress and quality of life in women with endometriosis and chronic pelvic pain. Stress. 2008;11(5):390–397. [DOI] [PubMed] [Google Scholar]
- 15. Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du X, Leang L, Mustafa T, Renoir T, Pang TY, Hannan AJ. Environmental enrichment rescues female-specific hyperactivity of the hypothalamic-pituitary-adrenal axis in a model of Huntington’s disease. Transl Psychiatry. 2012;2:e133 doi:10.1038/tp.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Renoir T, Pang TYC, Mo C, et al. Differential effects of early environmental enrichment on emotionality related behaviours in Huntington’s disease transgenic mice. J Physiol. 2013;591(1):41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gimenez-Llort L, Mate I, Manassra R, Vida C, De la Fuente M. Peripheral immune system and neuroimmune communication impairment in a mouse model of Alzheimer’s disease. Ann N Y Acad Sci. 2012;1262:74–84. [DOI] [PubMed] [Google Scholar]
- 19. Hutchinson KM, McLaughlin KJ, Wright RL, et al. Environmental enrichment protects against the effects of chronic stress on cognitive and morphological measures of hippocampal integrity. Neurobiol Learn Mem. 2012;97(2):250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simpson J, Kelly JP. The impact of environmental enrichment in laboratory rats—behavioural and neurochemical aspects. Behav Brain Res. 2011;222(1):246–264. [DOI] [PubMed] [Google Scholar]
- 21. Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 1978;153(3):563–576. [DOI] [PubMed] [Google Scholar]
- 22. Schoultz M, Atherton I, Watson A. Mindfulness-based cognitive therapy for inflammatory bowel disease patients: findings from an exploratory pilot randomised controlled trial. Trials. 2015;16:379 doi:10.1186/s13063-015-0909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McAllister SL, Dmitrieva N, Berkley KJ. Sprouted innervation into uterine transplants contributes to the development of hyperalgesia in a rat model of endometriosis. PLoS One. 2012;7(2):e31758 doi:10.1371/journal.pone.0031758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharpe-Timms KL. Using rats as a research model for the study of endometriosis. Ann N Y Acad Sci. 2002;955:318–327. 312–318-406 http://www.ncbi.nlm.nih.gov/pubmed/11949958. [DOI] [PubMed] [Google Scholar]
- 25. Torres-Reveron A, Palermo K, Hernandez-Lopez A, et al. Endometriosis is associated with a shift in MU opioid and NMDA receptor expression in the brain periaqueductal gray. Reprod Sci. 2016;23(9):1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vernon MW. Experimental endometriosis in laboratory animals as a research model. Prog Clin Biol Res. 1990;323:49–60. http://www.ncbi.nlm.nih.gov/pubmed/2406756. [PubMed] [Google Scholar]
- 27. Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44(5):684–694. [PubMed] [Google Scholar]
- 28. Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm Behav. 2003;44(2):123–131. [DOI] [PubMed] [Google Scholar]
- 29. Grummer R. Translational animal models to study endometriosis-associated infertility. Semin Reprod Med. 2013;31(2):125–132. [DOI] [PubMed] [Google Scholar]
- 30. Konno R, Fujiwara H, Netsu S, et al. Gene expression profiling of the rat endometriosis model. Am J Reprod Immunol. 2007;58(4):330–343. [DOI] [PubMed] [Google Scholar]
- 31. Fourquet J, Gao X, Zavala D, et al. Patients’ report on how endometriosis affects health, work, and daily life. Fertil Steril. 2010;93(7):2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crofton EJ, Zhang Y, Green TA. Inoculation stress hypothesis of environmental enrichment. Neurosci Biobehav Rev. 2015;49:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Appleyard CB, Cruz ML, Hernandez S, Thompson KJ, Bayona M, Flores I. Stress management affects outcomes in the pathophysiology of an endometriosis model. Reprod Sci. 2015;22(4):431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30(7):1288–1301. [DOI] [PubMed] [Google Scholar]
- 35. Hernandez S, Cruz ML, Seguinot II, Torres-Reveron A, Appleyard CB. Impact of psychological stress on pain perception in an animal model of endometriosis. Reprod Sci. 2017;24(10):1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strehl JD, Hackl J, Wachter DL, et al. Correlation of histological and macroscopic findings in peritoneal endometriosis. Int J Clin Exp Pathol. 2014;7(1):152–162. [PMC free article] [PubMed] [Google Scholar]
- 37. Agarwal N, Subramanian A. Endometriosis—morphology, clinical presentations and molecular pathology. J Lab Physicians. 2010;2(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Novembri R, Borges LE, Carrarelli P, et al. Impaired CRH and urocortin expression and function in eutopic endometrium of women with endometriosis. J Clin Endocrinol Metab. 2011;96(4):1145–1150. [DOI] [PubMed] [Google Scholar]
- 39. Tariverdian N, Rucke M, Szekeres-Bartho J, et al. Neuroendocrine circuitry and endometriosis: progesterone derivative dampens corticotropin-releasing hormone-induced inflammation by peritoneal cells in vitro. J Mol Med (Berl). 2010;88(3):267–278. [DOI] [PubMed] [Google Scholar]
- 40. Vergetaki A, Jeschke U, Vrekoussis T, et al. Differential expression of CRH, UCN, CRHR1 and CRHR2 in eutopic and ectopic endometrium of women with endometriosis. PLoS One. 2013;8(4):e62313 doi:10.1371/journal.pone.0062313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McEwen BS. Protective and damaging effects of stress mediators. Semin Med Beth Isr Deaconess Med Cent. 2004;338(3):171–179. [DOI] [PubMed] [Google Scholar]
- 42. McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8(4):283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ronzoni G, Anton M, Mora F, Segovia G, Del Arco A. Infralimbic cortex controls the activity of the hypothalamus-pituitary-adrenal axis and the formation of aversive memory: effects of environmental enrichment. Behav Brain Res. 2016;297:338–344. [DOI] [PubMed] [Google Scholar]
- 44. Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci. 2011;31(16):6159–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brenes JC, Padilla M, Fornaguera J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav Brain Res. 2009;197(1):125–137. [DOI] [PubMed] [Google Scholar]
- 46. Mann PE, Gervais KJ. Environmental enrichment delays pup-induced maternal behavior in rats. Dev Psychobiol. 2011;53(4):371–382. [DOI] [PubMed] [Google Scholar]
- 47. Del Arco A, Segovia G, Garrido P, de Blas M, Mora F. Stress, prefrontal cortex and environmental enrichment: studies on dopamine and acetylcholine release and working memory performance in rats. Behav Brain Res. 2007;176(2):267–273. [DOI] [PubMed] [Google Scholar]
- 48. Luisi S, Pinzauti S, Regini C, Petraglia F. Serum markers for the noninvasive diagnosis of endometriosis. Womens Health (Lond). 2015;11(5):603–610. [DOI] [PubMed] [Google Scholar]
- 49. Pena Y, Prunell M, Rotllant D, Armario A, Escorihuela RM. Enduring effects of environmental enrichment from weaning to adulthood on pituitary-adrenal function, pre-pulse inhibition and learning in male and female rats. Psychoneuroendocrinology. 2009;34(9):1390–1404. [DOI] [PubMed] [Google Scholar]
- 50. Girbovan C, Plamondon H. Environmental enrichment in female rodents: considerations in the effects on behavior and biochemical markers. Behav Brain Res. 2013;253:178–190. [DOI] [PubMed] [Google Scholar]
- 51. Brown KJ, Grunberg NE. Effects of housing on male and female rats: crowding stresses male but calm females. Physiol Behav. 1995;58(6):1085–1089. [DOI] [PubMed] [Google Scholar]
- 52. Arnason BG, Berkovich R, Catania A, Lisak RP, Zaidi M. Mechanisms of action of adrenocorticotropic hormone and other melanocortins relevant to the clinical management of patients with multiple sclerosis. Mult Scler. 2013;19(2):130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Montero-Melendez T. ACTH: the forgotten therapy. Semin Immunol. 2015;27(3):216–226. [DOI] [PubMed] [Google Scholar]
- 54. Whirledge SD, Oakley RH, Myers PH, Lydon JP, DeMayo F, Cidlowski JA. Uterine glucocorticoid receptors are critical for fertility in mice through control of embryo implantation and decidualization. Proc Natl Acad Sci U S A. 2015;112(49):15166–15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zoumakis E, Margioris AN, Stournaras C, et al. Corticotrophin-releasing hormone (CRH) interacts with inflammatory prostaglandins and interleukins and affects the decidualization of human endometrial stroma. Mol Hum Reprod. 2000;6(4):344–351. [DOI] [PubMed] [Google Scholar]
- 56. Makrigiannakis A, Zoumakis E, Kalantaridou S, et al. Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat Immunol. 2001;2(11):1018–1024. [DOI] [PubMed] [Google Scholar]
- 57. Fang X, Hong Y, Dai L, et al. CRH promotes human colon cancer cell proliferation via IL-6/JAK2/STAT3 signaling pathway and VEGF-induced tumor angiogenesis. Mol Carcinog. 2017;56(11):2434–2445. [DOI] [PubMed] [Google Scholar]
- 58. Sato N, Takagi K, Suzuki T, et al. Immunolocalization of corticotropin-releasing hormone (CRH) and its receptors (CRHR1 and CRHR2) in human endometrial carcinoma: CRHR1 as a potent prognostic factor. Int J Gynecol Cancer. 2014;24(9):1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodriguez JA, Huerta-Yepez S, Law IKM, et al. Diminished expression of CRHR2 in human colon cancer promotes tumor growth and EMT via persistent IL-6/Stat3 signaling. Cell Mol Gastroenterol Hepatol. 2015;1(6):610–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grandi G, Mueller MD, Papadia A, et al. Inflammation influences steroid hormone receptors targeted by progestins in endometrial stromal cells from women with endometriosis. J Reprod Immunol. 2016;117:30–38. [DOI] [PubMed] [Google Scholar]
- 61. Monsivais D, Dyson MT, Yin P, et al. Estrogen receptor beta regulates endometriotic cell survival through serum and glucocorticoid-regulated kinase activation. Fertil Steril. 2016;105(5):1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Karteris E, Markovic D, Chen J, Hillhouse EW, Grammatopoulos DK. Identification of a novel corticotropin-releasing hormone type 1beta-like receptor variant lacking Exon 13 in human pregnant myometrium regulated by estradiol-17beta and progesterone. Endocrinology. 2010;151(10):4959–4968. [DOI] [PubMed] [Google Scholar]
- 63. Kempuraj D, Papadopoulou N, Stanford EJ, et al. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am J Reprod Immunol. 2004;52(4):267–275. [DOI] [PubMed] [Google Scholar]
- 64. Florio P, Busacca M, Vignali M, et al. Peritoneal fluid levels of immunoreactive corticotropin-releasing factor (CRF) and CRF-binding protein (CRF-BP) in healthy and endometriosic women. J Endocrinol Invest. 1998;21(1):37–42. [DOI] [PubMed] [Google Scholar]
- 65. Cao J, Papadopoulou N, Kempuraj D, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174(12):7665–7675. [DOI] [PubMed] [Google Scholar]
- 66. Ulrich RS. Effects of interior design on wellness: theory and recent scientific research. J Health Care Inter Des. 1991;3:97–109. [PubMed] [Google Scholar]
- 67. Kaplan R, Kaplan S. The Experience of Nature: A Psychological Perspective. Cambridge University Press, New York, NY; 1989. [Google Scholar]
- 68. Birch AM, McGarry NB, Kelly AM. Short-term environmental enrichment, in the absence of exercise, improves memory, and increases NGF concentration, early neuronal survival, and synaptogenesis in the dentate gyrus in a time-dependent manner. Hippocampus. 2013;23(6):437–450. [DOI] [PubMed] [Google Scholar]
- 69. Vachon P, Millecamps M, Low L, et al. Alleviation of chronic neuropathic pain by environmental enrichment in mice well after the establishment of chronic pain. Behav Brain Funct. 2013;9:22 doi:10.1186/1744-9081-9-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zheng J, Jiang YY, Xu LC, et al. Adult hippocampal neurogenesis along the dorsoventral axis contributes differentially to environmental enrichment combined with voluntary exercise in alleviating chronic inflammatory pain in mice. J Neurosci. 2017;37(15):4145–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gelfo F, Mandolesi L, Serra L, Sorrentino G, Caltagirone C. The neuroprotective effects of experience on cognitive functions: evidence from animal studies on the neurobiological bases of brain reserve [published online August 4, 2017.]. Neuroscience. 2017. pii:S0306–4522(17)30551–1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp-Fig for Environmental Manipulations as an Effective Alternative Treatment to Reduce Endometriosis Progression by Annelyn Torres-Reverón, Leslie L. Rivera, Idhaliz Flores, and Caroline B. Appleyard in Reproductive Sciences