Abstract
Prenatal testosterone (T)-treated sheep, similar to women with polycystic ovary syndrome (PCOS), manifests reproductive defects that include multifollicular ovarian phenotype. Women with PCOS manifest increased ovarian matrix metalloproteinases (MMPs) activity. We tested the hypothesis that gestational T excess in sheep would alter ovarian expression of MMPs, tissue inhibitors of MMP (TIMP) and their target proteins laminin B (LAMB), collagen, tumor necrosis factor alpha (TNF), and connexin 43 (GJA1) consistent with increased MMP activity and that these changes are developmentally regulated. The ovarian content of these proteins was quantified by immunohistochemistry in fetal day 90, 140, and adult (21 months of age) ovaries. Prenatal T excess lowered GJA1 protein content in stroma and granulosa cells of primary follicles from fetal day 90 ovaries and decreased stromal MMP9, TIMP1, and LAMB in fetal day 140 ovaries. In the adult, prenatal T-treatment (1) increased MMP9 in theca cells of large preantral follicles and stroma, TNF in granulosa cells of small and large preantral follicles and theca cells of large preantral and antral follicles, and GJA1 in stroma, theca cells of large preantral follicles, and granulosa cells of antral follicles and (2) reduced TIMP1 in stroma, theca cells of large preantral and antral follicles, LAMB in stroma and small prenatral follicles, and collagen content in stroma and around antral follicles. These findings suggest a net increase in MMP activity and its target proteins TNF and GJA1 in prenatal T-treated adult but not in fetal ovaries and their potential involvement in the development of multifollicular morphology.
Keywords: female reproduction, PCOS, androgen, matrix metalloproteinases, ovary
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrinopathy that affects about 8% to 10% of the reproductive age women.1 Common reproductive defects observed in these women include oligo-/an-ovulation, hyperandrogenism, and multifollicular ovarian morphology.1,2 Because diagnosis of PCOS occurs in women after establishment of reproductive competency, and tissue-specific studies come from postmortem or elective surgical samples, the developmental ontogeny of ovarian factors contributing to the antral follicular accumulation in women with PCOS cannot be ascertained. Animal models of PCOS have pointed to the role of prenatal exposure of the female fetus to excess testosterone (T) in the development of disrupted ovarian morphology.3–6 For instance, studies with sheep treated prenatally with T recapitulate disruptions observed in women with PCOS with progressive loss in cyclicity, oligo-/an-ovulation, luteinizing hormone (LH) excess, functional hyperandrogenism, and multifollicular ovarian morphology.5,7 These animal models provide an opportunity to study the developmental ontogeny of polycystic ovarian morphology and the underlying mediators.
Studies in a sheep model of PCOS phenotype have found that both enhanced follicular recruitment and follicular persistence contribute to the antral follicular accumulation,8,9 features postulated to occur in women with PCOS.10,11 While the factors that program these ovarian phenotype are not known, endocrine, autocrine, and paracrine mechanisms are likely involved.12 In addition to disruptions in LH/follicle-stimulating hormone and insulin responses, that are endocrine signals to the ovary,7 controlled ovarian stimulation studies13 have pointed to the presence of intrinsic ovarian defects in prenatal T-treated sheep. Focused investigations at the ovarian level have found prenatal T excess perturbs ovarian steroid receptor balance favoring androgen receptor expression in antral follicles,14 disrupts expression levels of steroidogenic enzymes,15 decreases adiponectin and disrupts anti-mullerian hormone (AMH) levels—key mediators of preovulatory transition,16,17 and alters the balance between proapoptotic and procell survival proteins that contribute to follicular arrest.18
In view of the extensive tissue remodeling that occurs during the follicular development, changes in matrix proteins are likely contributors to the follicular arrest and persistence, an aspect not previously explored in prenatal T-treated sheep. Tissue remodeling in the ovary involves extracellular matrix turnover and is regulated by coordinated expression of proteases and their inhibitors.19 Members of the matrix metalloproteinases (MMPs) family are the main proteases involved in the remodeling of extracellular matrix.20 The proteolytic activities of MMPs are locally regulated by their specific inhibitors, the tissue inhibitors of MMPs (TIMP). In addition to regulating the extracellular matrix turnover through its action on matrix proteins, laminin and collagen, MMPs regulate cell function by cleaving cellular proteins such as tumor necrosis factor α (TNF) and connexin 43 (GJA1).20,21 Higher levels of MMP2 and 9 with unchanged/lower levels of TIMP1 or 2 were found in the circulation, follicular fluid, and granulosa cells of PCOS women22,23 indicative of increased MMP activity. This increase in protease activity likely disrupts the tissue remodeling process, growth factor availability, and gap junction communications contributing to the development of abnormal ovarian phenotype in women with PCOS. Studies in mouse cardiac cells have provided evidence that MMP9 and 7 cleave GJA1,24,25 a gap junction protein widely expressed in ovarian granulosa and theca cells.26,27 Attenuation of GJA1 function by changes in MMP activity can therefore impair transfer of hormones and nutrients across the gap junctions in granulosa cell layers impairing follicular development.28 Because MMP activity is required to process TNF into its mature form,29 elevated levels of MMP can also alter proteolytic processing of TNF, thus influencing follicular development and function.30,31
Because MMPs expression in ovarian cells is increased in women with PCOS,22 the reproductive attributes of whom prenatal T-treated sheep mimic,5 we tested the hypothesis that altered developmental balance of matrix proteins and their targets (laminin, collagen, TNF, and GJA1) are contributing factors in the development of follicular persistence in prenatal T-treated sheep.
Methods
Institutional Animal Care and Use Committee of the University of Michigan approved all animal procedures used in this study, which are consistent with the National Research Council’s Guide for the Care and Use of Laboratory Animals. Housing, general husbandry, and diets of breeder sheep and lamb were as described in detail previously.9
Gestational T Programming
Prenatal T-treated animals were generated as described previously32 by intramuscular administration of 100 mg of 1.2 mg/kg T propionate (Sigma-Aldrich, St. Louis, Missouri) suspended in cottonseed oil to pregnant ewes twice weekly from days 30 to 90 of gestation. Ovaries were collected at 3 different ages, fetal days 90 (D90) and 140 (D140), and at postnatal 21 months of age (21-mo-old) at the end of second breeding season. The numbers of animals studied during each developmental time point were as follows: fetal D90: C = 6 and T = 5; fetal D140: C = 6 and T = 7, and adults at 21-mo-old: C = 5 and T = 8. Collection of ovaries from 21-month-old female sheep was performed during a presumptive follicular phase after synchronization with prostaglandin F2α (10 mg, intramuscular; Lutalyse, Pfizer Animal Health, Florham Park, New Jersey), given twice 11 days apart. Euthanasia, ovarian collection, and processing were performed as reported previously.33 Paraffin-embedded sections from 1 ovary from each animal were used in this study. Developmental changes in ovarian follicular distribution determined by ovarian morphometry and changes in expression of apoptotic factors, steroid receptors, steroidogenic enzymes, and ovarian vascular endothelial growth factor (VEGF) and AMH patterns as determined by immunohistochemistry in animals including those used in this study have been previously published.14–18,33,34
Changes in Key Proteins of the MMP System
Immunohistochemistry was performed using the immunoperoxidase method employing a commercially available kit, VECTASTAIN ABC kit (rabbit immunoglobulin G; cat no PK-4001) for GJA1 or Dako EnVision+ Dual Link System-HRP kit (cat no K4065) as per the manufacturers’ recommendations. Briefly, the sections were deparaffinized, and antigen retrieval was performed by heating in 1 M sodium citrate solution using a pressure cooker. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol, and nonspecific binding was blocked with 10% (v/v) normal goat serum. Sections were incubated with primary antibodies for 18 hours at 4°C, then with a secondary antibody supplied with the kit for 1 hour at room temperature, developed with the chromogen diaminobenzidine, counterstained with Mayer’s hematoxylin, dehydrated, and mounted. The primary antibodies used were MMP2 (cat no AVARP20016), MMP9 (cat no ARP33090), and LAMB (cat no ARP48567) from Aviva Systems Biology (San Diego, California), TIMP1 (cat no AB770) from EMD Millipore (Billerica, Massachusetts), TNF (cat no MCA2334) from Bio-Rad (Hercules, California), and GJA1 (cat no PA5-19115) from Thermo Scientific (Grand Island, New York).
Ovarian Collagen Content
Collagen content in ovarian sections was assessed by picrosirius red (PSR) staining.35 Briefly, deparaffinized and rehydrated ovarian sections were immersed in a PSR staining solution prepared by dissolving Sirius Red F3B (Sigma-Aldrich, St. Louis, Missouri) in a saturated aqueous solution of picric acid (Sigma-Aldrich) at 0.1% w/v for 60 minutes at room temperature. Thereafter, these slides were washed in acidified water containing 0.5% glacial acetic acid (Sigma-Aldrich) twice for 5 minutes. After which the excess water was wicked and sections dehydrated in 3 changes of 100% ethanol, cleared with xylene, and mounted with Permount (Fisher Scientific, Pittsburgh, Pennsylvania).
Image Analysis
To avoid the possibility of follicular overlap, 2 sections (the first section one-third into the ovary and the second two-thirds into the ovary) were used. Follicles (ranged between 4 and 15 for each follicular class) from all the stages of development from both sections were analyzed. Follicles that were healthy without any atretic signs (pyknotic nucleus and loss of cell adhesion in the granulosa layer) were only included for analysis. Follicle classes were distinguished using criteria previously established14 and included primordial, primary, small preantral, large preantral, and antral follicles. For antral follicles, granulosa from antrum to theca were evaluated to avoid subjectivity and differences in location of proteins. Also, for each ovary, stromal tissue was analyzed by obtaining 10 images in the central part of the ovarian section avoiding any vascular or follicular structures.
Images were obtained with Spot RT Slider digital camera (Diagnostic Instruments, Sterling Heights, Michigan) mounted on a conventional Leica DMR light microscope (Leica Microsystems, Wetzlar, Germany). The image analysis was performed using the Image Pro-Plus 3.0.1 system (Media Cybernetics, Rockville, Maryland) as described previously14 using a well-validated densitometrical methodology.36 The average density calculated as percentage of immunopositive area for brown stain (produced by the immunoperoxide reaction) relative to total area was obtained through color segmentation analysis. The percentage of immunopositive area was calculated separately for each follicular compartment (granulosa, theca interna, and theca externa for antral follicles, granulosa and theca cell layers for large preantral follicles, and granulosa cell layer for small preantral, primary, and primordial follicles) and stroma.
The area of ovarian tissue that was positive for PSR staining around antral and large preantral follicles and in ovarian stroma were assessed from bright-field images of PSR-stained ovarian sections using the ImageJ software (version 1.47; National Institutes of Health, Bethesda, Maryland) as per the protocol described on its website.37 Sections were analyzed with the observer blinded to treatment.
Statistical Analyses
The average density (percentage of immunopositive area) of each follicular compartment within a follicle class was first averaged and then a group mean across follicles was derived for each follicle type within an animal. Statistical tests were carried out using Prism software (version 7.0, GraphPad Software, La Jolla, California, USA). Changes in the granulosa cell layer across the different developmental age or follicular stages from the 21-mo-age control group were compared by analysis of variance, followed by Tukey post hoc test. A P < .05 value was considered significant. Considering the small sample size that reduced power, differences between control and prenatal T-treated groups were examined by effect size analysis.38–40 The computed statistics is Cohen d value, and values .8 and .5 and above were considered as large and medium effect sizes, respectively.38,39
Results
Developmental Changes in the Expression of MMPs and Their Target Proteins
Immunohistochemical localization of MMP2 and 9, TIMP1, LAMB, TNF, and GJA1 in the different follicular stages from 21-mo-old animals are shown in Figure 1. Developmental changes in intensity of their expression in granulosa cells and stromal cells including changes in stromal PSR staining across fetal D90 and D140 and postnatal 21-mo-old control animals are shown in Figure 2. The expression levels of both MMP2 (Figure 2A) and 9 (Figure 2B) were lower in granulosa cells from primordial and primary follicles of D140 and 21-mo-old females compared to that of D90. In the 21-mo-old female where advanced follicle classes were present, MMP9 expression was similar between primordial, primary, and small preantral, but significantly higher in antral follicles relative to the other 3 classes of follicles. Levels in large preantral follicles were intermediate and did not differ from any of the follicular classes. A similar direction of change was evident in TNF with levels being higher in antral follicles relative to primary, small, and large preantral follicles. The TNF protein in primary follicles from 21-mo-old animals was lower than that found in the fetal ages. In contrast, no changes were evident in the TIMP1, LAMB, and GJA1 protein expression within the different developmental stages of follicles from 21-mo-old animals nor were there differences in expression between primordial and primary across D90, D140, and 21-mo-old time points.
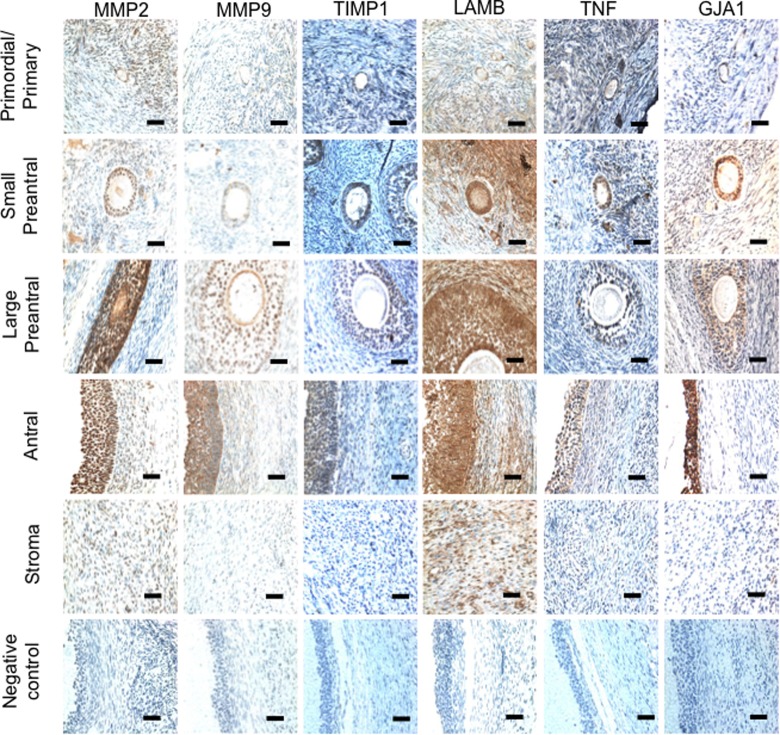
Figure 1.
Representative photomicrographs showing the immunolocalization of MMP2, MMP9, TIMP1, LAMB, TNF, and GJA1 in the different follicular developmental stages and stroma from sheep ovaries. Negative control represents the images obtained following immunolocalization performed by omitting the primary antibodies against the respective protein. Bar = 25 μm. MMP indicates matrix metalloproteinase; TNF, tumor necrosis factor alpha.
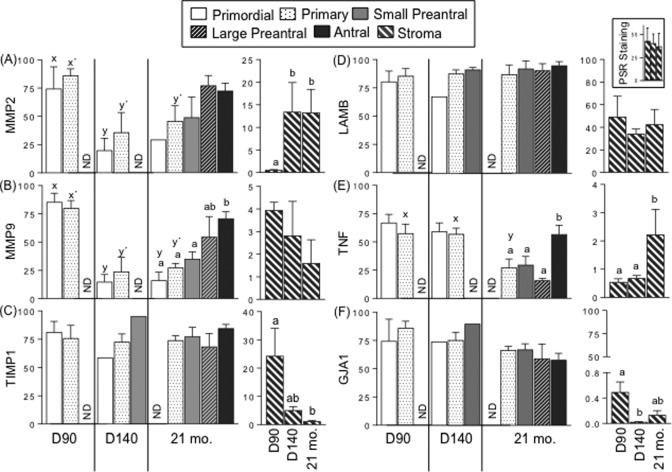
Figure 2.
Mean ± SEM of relative expression (measured as percentage of immunopositive area) of MMP2 (A), MMP9 (B), TIMP1 (C), LAMB (and stromal collagen as inset) (D), TNF (E), and GJA1 (F) in the granulosa cells of ovaries and stromal cells from control sheep of fetal D90 and D140 and adult 21-mo-old age are shown. Relative expression levels were measured in granulosa of primordial, primary, small preantral, large preantral and antral follicles, and in stromal cells. Relative collagen content was determined by the percent area positive for picrosirius red (PSR) staining. Different superscripts indicate significant differences as determined by analysis of variance followed by Tukey post hoc analysis; P < .05. Superscripts x and y are for comparison between ages in the same follicular class while superscripts a and b indicate comparison between the different follicular classes in 21-mo-old sheep. ND indicates not detectable; SEM, standard error of the mean; MMP, matrix metalloproteinase; TNF, tumor necrosis factor alpha; D90, fetal day 90; D140, fetal day 140.
The stromal expression of MMP2 was higher in D140 and 21-mo-ovaries compared to D90 ovaries (Figure 2A), while TIMP1 protein expression was significantly lower in ovaries from 21-mo-old animals relative to D90 animals (Figure 2C). The TIMP expression in D140 stroma was intermediate with expression not being different from D90 or 21-mo-old females. The TNF levels were higher in 21-mo-ovaries relative to fetal ages (Figure 2E). In contrast, GJA1 protein expression was significantly lower in D140 and tended to be lower in ovaries from 21-mo-old animals (21-mo-old not different from D90 or D140; Figure 2F) relative to D90. No changes were evident in the stromal expression of MMP9 (Figure 2B), LAMB (Figure 2D), and collagen (Figure 2D, inset) proteins across ages.
Effect of Prenatal T-Treatment on Expression of MMPs and Their Target Proteins
At fetal D90, no change in the expression of MMP2 and 9 proteins were found between granulosa cells of primary and primordial follicles or stromal cells (Figure 3); however, this comparison is limited in primary follicles, as sections from only 2 prenatal T-treated animals were available for analysis. In contrast, at fetal D140, prenatal T-treatment decreased MMP9 (d = 1.2) only in the ovarian stromal cells but not in follicular cells, while MMP2 showed no change in follicular as well as stromal cells. The impact of prenatal T was not evident in the expression level of MMP2 in any follicular classes or the stroma of 21-mo-old females. In contrast, prenatal T excess increased MMP9 protein (Figure 3) in theca cells from large preantral follicles (d = 1.2) and in the stroma (d = 1.1) of 21-mo-old animals.
Figure 3.

Mean ± SEM of relative expression (measured as percentage of immunopositive area) of MMP2 and MMP9 in ovaries of control and prenatal T-treated fetal D90 and D140 and adult 21-mo-sheep. Relative expression levels were measured in granulosa cells from primary, primordial, small and large preantral, and antral follicles and stroma cells. Note scale bar difference between follicular and stromal compartments. ##Large effect as determined by Cohen effect size analysis. ND indicates not detectable; SEM indicates standard error of the mean; MMP, matrix metalloproteinase; D90, fetal day 90; D140, fetal day 140.
Gestational T-treatment had no effect on the expression of MMP inhibitor, TIMP, at fetal D90 but reduced its expression in the granulosa cells of primary follicles (d = .9; Figure 4) and in the ovarian stroma cells (d = .8) in fetal D140 ovaries, theca cells from large preantral follicles (d = 1.3), and granulosa cells from antral follicles (d = 1.0) of adult ovaries.
Figure 4.
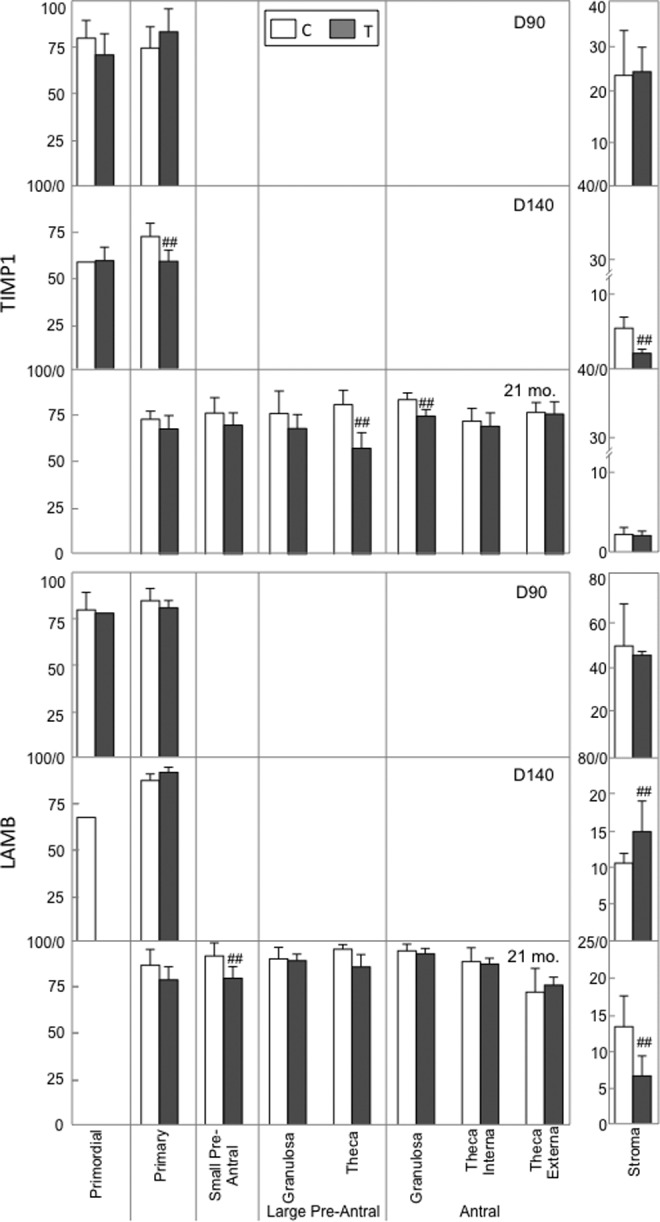
Mean ± SEM of relative expression (measured as percentage of immunopositive area) of TIMP1 and LAMB in ovaries of control and prenatal T-treated fetal D90 and D140 and adult 21-mo-sheep. Relative expression levels were measured in granulosa cells from primary, primordial, small and large preantral, and antral follicles and stroma cells. Note scale bar difference between follicular and stromal compartments. ##Large effects as determined by Cohen effect size analysis. ND indicates not detectable; SEM, standard error of the mean; D90, fetal day 90; D140, fetal day 140.
The effects of prenatal T-treatment on matrix protein LAMB at both fetal and adult ages are presented in Figure 4. The LAMB protein expression in both follicular and stroma cells of fetal D90 ovaries did not differ between C and those exposed to excess T. The LAMB protein expression increased moderately (d = .6) in stromal cells but not in primordial or primary follicles of fetal D140 prenatal T females. In contrast, prenatal T-treatment decreased LAMB protein content in granulosa cells from small preantral follicles (d = .8) and stromal cells (d = .8) from adult ovaries. Prenatal T-treatment also decreased collagen content (Figure 5) surrounding antral follicles (d = .9) and in the ovarian stroma (d = .6) of adult prenatal T females.
Figure 5.

Mean ± SEM of relative collagen content as determined by the percent area positive for picrosirius red (PSR) staining around large preantral and antral follicles and ovarian stroma are shown as histograms along with the respective PSR-stained representative photomicrographs from control and prenatal T-treated 21-mo-old sheep are shown. ##Large and #medium effects as determined by Cohen effect size analysis. Bar = 25 μm. SEM indicates standard error of the mean.
Among the cellular proteins that are targeted by MMP, TNF did not change with prenatal T-treatment in fetal D90 while GJA1 expression was reduced (d = .8) in primary follicles and stromal cells relative to control animals (Figure 6). At fetal D140, prenatal T-treatment had no effect on both proteins. In the adult ovary, TNF protein was found to be increased with prenatal T-treatment in granulosa cells from small (d = .8) and large preantral (d = .9) and theca cells from large preantral (d = 2.4) and antral follicles (d = .7 and .8 for theca interna and externa layers, respectively). Prenatal T-treatment also increased GJA1 protein content in theca cells from large preantral follicles (d = .9) and granulosa cells from antral follicles (d = .9) and stromal cells (d = 1.0).
Figure 6.

Mean ± SEM of relative expression (measured as percentage of immunopositive area) of TNF and GJA1 in ovaries of control and prenatal T-treated fetal D90 and D140 and adult 21-mo-sheep. Relative expression levels were measured in granulosa cells from primary, primordial, small and large preantral, and antral follicles and stroma cells. Note scale bar difference between follicular and stromal compartments. ##Large and #medium effects as determined by Cohen effect size analysis. ND indicates not detectable; SEM, standard error of the mean; TNF, tumor necrosis factor alpha; D90, fetal day 90; D140, fetal day 140.
Discussion
Results from this study indicate that MMP and their target proteins are expressed in the fetal and adult sheep ovaries, undergo developmental and age-specific changes, and prenatal T-treatment leads to modest changes in the members of MMP system that are consistent with increased MMP activity. The altered ovarian proteases and TNF content resulting from prenatal T excess could potentially contribute to increased follicular recruitment and/or arrest. The significance of these findings is discussed below.
Developmental Changes
Although not directly implicated, correlative data have suggested that proteases including MMP family members play an important role in extracellular matrix remodeling allowing follicular growth to about 400-fold larger in size from the primordial to the antral stage.19,20,41,42 The increase in MMP9 evidenced during the sheep follicular developmental progression in this study parallels increased MMP expression or activity found in other species. For instance, MMP2 and 9 are either low or absent in the neonatal rat ovary with expression of both proteins increasing upon stimulation of follicular growth with equine chorionic gonadotropin.43 Analogous to this, collagenase activity in the goat44 and the expression of MMP2 and 14 protein in the humans45 have been found to increase with follicular development. As opposed to findings in other species of increased TIMP expression that parallel those of the MMPs,20 no changes in TIMP1 expression were evident across follicular development in this study. Irrespective of this, the increased MMP expression with no change in TIMP1 suggests a net increase in MMP activity during follicular development in the sheep.
This is the first study that has evaluated age-specific changes in ovarian MMP expression in any species. Age-specific changes in the MMP system were manifested as decreased expression of MMP2 and 9 in primordial and primary follicles in sheep. The increased MMP activity at fetal D90 relative to D140 and 21-mo-old animals may be related to increased primordial follicle formation that occurs during gestational days 90 to 100 in the sheep.46 The age-specific decline of MMP in the primordial and primary follicles might also be reflective of the difference in hormonal milieu at D90 compared D140 or in the adult ovary. For instance, the levels of estradiol are higher in fetal D140 compared to fetal D9047 along with low concentrations of progesterone48 during this time. The late follicular phase of 21-mo-old animals from which adult ovaries were collected is also characterized by higher estradiol and lower progesterone levels.49 While the age-specific decline in MMP2 and 9 in primordial and primary follicles may be a function of elevated estradiol and/or reduced progesterone, paradoxically similar endocrine milieu promotes expression of MMP3 and 7 in the endometrium,50 suggestive of tissue-specific regulation. Because the MMP family consists of multiple members with redundant functions, it needs to be recognized that a signal that regulates an MMP to increase its expression may differentially regulate another MMP in the same or opposite direction.20 In contrast to the decrease in MMPs in primordial and primary follicular cells with age, there was increased stromal expression of MMPs accompanied by decrease in TIMP1 suggestive of increasing stromal MMP activity with age. Whether these changes relate to the follicular and stromal turnover or the paracrine environment within these tissue compartments remains unclear.
Although it is well known that collagen and laminin are the major extracellular matrix proteins in the ovary, developmental changes in the expression of these proteins across follicular development are not available for any species. The changes in MMPs and TIMP1 observed in sheep (this study) during follicular development were not reflected as a change in the expression of LAMB or stromal collagen. Because the extracellular matrix is constantly being degraded and reformed, a rapid turnover of the extracellular matrix within the ovary would be expected. As such, a single time point assessment may not be meaningful.
Apart from its action on matrix proteins, MMPs are known to process TNF to its mature form.29 In this study, an age-dependent decline in TNF was also evident from fetal to adult life analogous to what was seen with the MMPs. In contrast, parallel increases in MMPs and TNF expression were also evident in the granulosa cells with advancement in follicular development. Since TNF can promote MMP expression,51 the similar direction of age-specific and follicular development–specific changes insinuate that TNF could be regulating MMP expression in the ovary. Ovarian expression of TNF has been documented in oocytes and granulosa cells of most species52 and all follicular stages in adult rat ovaries,53,54 and increased expression has been found in the human granulosa and theca cells as the follicles mature.55,56 Because TNF has pleiotropic actions31 influencing both follicular atresia30 and steroidogenesis,52 it is possible that increased TNF content in granulosa cells may contribute to the growth of follicles.
The GJA1, a member of gap junctions that facilitate intracellular communication and a target protein of MMP action, did not show any changes across follicular development in the adult sheep ovary. Because assessment of GJA1 protein was restricted to the follicular phase, it is hard to relate these findings to estrous cycle stage-dependent changes in GJA1 expression that have been reported in sheep57 and pigs.58,59 The reason for the age-specific decrease in GJA1 protein content in the stroma of D140 and 21-mo-old females compared to the fetal D90 stage is not clear. Because GJA1 is also expressed in stromal blood vessels,57 the higher amounts detected in D90 fetal ovary may stem from contribution from stromal blood vessels that are hard to partition during image analysis (vascular elements were avoided during assessment), thus contributing to artificially elevated levels in fetal ovaries.
Impact of Prenatal T Excess
The modest changes observed in the MMP system with increased MMP9 protein in theca cells from large preantral follicles and stroma and reduction in TIMP1 and matrix proteins LAMB and collagen are suggestive of moderate increase in ovarian MMP activity in prenatal T-treated females. An increase in MMP2 and 9 protein expression has also been reported in atretic cystic follicles of hyperandrogenic guinea pig60 and atretic follicles of hypophysectomized sheep.61 These findings are not comparable to the findings in the present study, as expression of MMP members was assessed only in healthy follicles. The processes by which prenatal T excess increases levels of MMP are not known and may be related to increased androgen receptor expression.14 In support of this, the increase in MMP9 activity in rat prostate cells that follows exposure to environmental endocrine disruptor di-n-butyl-phthalate62 has been found to be associated with elevated androgen receptor expression. The low progesterone and high estradiol milieu49 of prenatal T-treated sheep may be another contributing factor for the altered MMP activity of prenatal T-treated sheep. In the uterus, progesterone represses the expression of multiple MMPs,63 therefore a low progesterone endocrine milieu in prenatal T animals64 may allow selective increase in expression of ovarian MMPs. Considering increased theca cell MMP9 expression as well as gelatinolytic activity underlies follicular development in rats,65 the increased MMP9 protein in theca cells of preantral follicles may contribute to the multifollicular appearance of prenatal T-treated females.
Additionally, as prenatal T-treatment elevates TNF content in ovaries from 21-mo-old animals, increased MMP expression may be related, as TNF can promote MMP expression.51 While direct effects of T in inducing MMP expression in prostate cells are known,66 such an effect was not evident in fetal D90 ovaries obtained from gestational T-treated animals. However, the reduction in MMP9 and TIMP1 in D140 fetuses of gestational T-treated animals suggests that prenatal T excess could alter the developmental trajectory of MMP9 and TIMP1 expression.
The impact of prenatal T-treatment in increasing ovarian TNF protein content in granulosa and theca cells from various follicular classes in the adult ovary may be a function of the compromised intraovarian milieu. Our previous studies have found disruption of steroidogenic and steroid receptor balance resulting in functional hyperandrogenism in the ovary.14,15 That the elevated TNF expression may be the function of increased androgen action is supported by similar findings in women with PCOS.67 Apart from its role in promoting cell growth and differentiation, TNF also functions as a pro-inflammatory cytokine.68 The elevated TNF in the prenatal T-treated animals could therefore indicate an inflammatory state in the prenatal T-treated sheep. Interestingly, elevated granulosa cell expression of pro-inflammatory cytokines is also a feature of women with PCOS,69,70 the phenotype of whom prenatal T-treated animals recapitulates. The inflammatory status is also consistent with the reduced expression of adiponectin in granulosa cells16 of prenatal T-treated sheep.71
Considering that GJA1 is a target protein for MMP degradation,25,61 we expected a reduction in GJA1 expression in follicles with higher MMP9 or lower TIMP1 expression in the adult animals. Contrary to our expectation, prenatal T excess increased GJA1 expression in 21-mo-old animals. In support of these findings, pigs exposed prenatally to androgen receptor antagonist manifested decreased GJA1 expression.72 In stark contrast to these findings, the reduction in GJA1 protein in fetal D90 ovaries may be the result of direct action of T. For instance, T-treatment was shown to decrease GJA1 expression in human granulosa lutein cells and mouse ovary.73,74 These opposing outcomes of GJA1 expression in the fetal and adult ovaries of gestational T-treated sheep are paradoxical, since an increase in androgen receptor expression/action is also evident in granulosa cells of adult females.
Translational Relevance
The findings from this study are likely to be of translational relevance, as prenatal T-treated female sheep manifest reproductive defects similar to that observed in the women with PCOS.5 Akin to the observations in the prenatal T-treated sheep, women with PCOS appear to have increased follicular fluid MMP activity; one study reported elevated MMP2 and 9 with unchanged TIMP1,22,75 while another study found no changes in MMP2 or 9, but reduced TIMP1 protein.23 Irrespective of whether regulation was at MMP or TIMP level, our studies, as well as studies in women with PCOS, point to increased MMP activity in hyperandrogenic states. It is suggested that increase in MMP activity might be a compensatory process to overcome thickening of ovarian capsule in women with PCOS.23 Another parallel finding between prenatal T-treated sheep and women with PCOS67,76 is the increase in the levels of TNF, supportive of a role in the development of ovarian defects.
There are some limitations that should be considered in interpreting our findings. The changes in protein expression reported are modest. Considering that MMP family is comprised of multiple proteins with redundant functions,20 it is possible that other MMPs not measured here may each show modest increases adding up to a robust net increase in MMP activity. In spite of this limitation, the findings from this study point to an overall increase in MMP activity along with increased expression of its target proteins TNF and GJA1 in adult animals. These changes, albeit modest, may contribute to the increased follicular recruitment and arrest seen in these animals7 leading to multifollicular appearance. Additionally, these findings are consistent with observations in women with PCOS underlining the usefulness of prenatal T-treated sheep model in the study of PCOS pathogenesis.
Acknowledgments
We are grateful to Mr Douglas Doop for his expert animal care, facility management, and help with generation of the experimental lambs. Drs Mohan Manikkam and Teresa Steckler, Ms Olga Astapova, Ms Carol Herkimer, and Mr James Lee for their assistance with prenatal steroid treatment and help during collection of ovaries.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (grant no P01 HD44232).
References
- 1. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conway G, Dewailly D, Diamanti-Kandarakis E, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4):P1–P29. [DOI] [PubMed] [Google Scholar]
- 3. Abbott DH, Barnett DK, Levine JE, et al. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008;79(1):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78(8):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1-2):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maliqueo M, Benrick A, Stener-Victorin E. Rodent models of polycystic ovary syndrome: phenotypic presentation, pathophysiology, and the effects of different interventions. Semin Reprod Med. 2014;32(3):183–193. [DOI] [PubMed] [Google Scholar]
- 7. Cardoso RC, Puttabyatappa M, Padmanabhan V. Steroidogenic versus metabolic programming of reproductive neuroendocrine, ovarian and metabolic dysfunctions. Neuroendocrinology. 2015;102(3):226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146(7):3185–3193. [DOI] [PubMed] [Google Scholar]
- 9. Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects: partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147(4):1997–2007. [DOI] [PubMed] [Google Scholar]
- 10. Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10(2):107–117. [DOI] [PubMed] [Google Scholar]
- 11. Franks S, Mason H, Willis D. Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol. 2000;163(1-2):49–52. [DOI] [PubMed] [Google Scholar]
- 12. Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steckler TL, Lee JS, Ye W, Inskeep EK, Padmanabhan V. Developmental programming: exogenous gonadotropin treatment rescues ovulatory function but does not completely normalize ovarian function in sheep treated prenatally with testosterone. Biol Reprod. 2008;79(4):686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137(5):865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Padmanabhan V, Salvetti NR, Matiller V, Ortega HH. Developmental programming: prenatal steroid excess disrupts key members of intraovarian steroidogenic pathway in sheep. Endocrinology. 2014;155(9):3649–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ortega HH, Rey F, Velazquez MM, Padmanabhan V. Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep. Biol Reprod. 2010;82(6):1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Veiga-Lopez A, Ye W, Padmanabhan V. Developmental programming: prenatal testosterone excess disrupts anti-Müllerian hormone expression in preantral and antral follicles. Fertil Steril. 2012;97(3):748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salvetti NR, Ortega HH, Veiga-Lopez A, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on ovarian cell proliferation and apoptotic factors in sheep. Biol Reprod. 2012;87(1):22, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curry TE, Jr, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med. 2006;24(4):228–241. [DOI] [PubMed] [Google Scholar]
- 20. Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24(4):428–465. [DOI] [PubMed] [Google Scholar]
- 21. Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–21494. [DOI] [PubMed] [Google Scholar]
- 22. Baka S, Zourla K, Kouskouni E, et al. Matrix metalloproteinases 2 and 9 and their tissue inhibitors in the follicular fluid of patients with polycystic ovaries undergoing in vitro fertilisation. In Vivo. 2010;24(3):293–296. [PubMed] [Google Scholar]
- 23. Lahav-Baratz S, Kraiem Z, Shiloh H, Koifman M, Ishai D, Dirnfeld M. Decreased expression of tissue inhibitor of matrix metalloproteinases in follicular fluid from women with polycystic ovaries compared with normally ovulating patients undergoing in vitro fertilization. Fertil Steril. 2003;79(3):567–571. [DOI] [PubMed] [Google Scholar]
- 24. Peng HJ, Dai DZ, Ji H, Dai Y. The separate roles of endothelin receptors participate in remodeling of matrix metalloproteinase and connexin 43 of cardiac fibroblasts in maladaptive response to isoproterenol. Eur J Pharmacol. 2010;634(1-3):101–106. [DOI] [PubMed] [Google Scholar]
- 25. Wu X, Huang W, Luo G, Alain LA. Hypoxia induces connexin 43 dysregulation by modulating matrix metalloproteinases via MAPK signaling. Mol Cell Biochem. 2013;384(1-2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gittens JE, Mhawi AA, Lidington D, Ouellette Y, Kidder GM. Functional analysis of gap junctions in ovarian granulosa cells: distinct role for connexin43 in early stages of folliculogenesis. Am J Physiol Cell Physiol. 2003;284(4):C880–C887. [DOI] [PubMed] [Google Scholar]
- 27. Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123(5):613–620. [DOI] [PubMed] [Google Scholar]
- 28. Winterhager E, Kidder GM. Gap junction connexins in female reproductive organs: implications for women’s reproductive health. Hum Reprod Update. 2015;21(3):340–352. [DOI] [PubMed] [Google Scholar]
- 29. Gearing AJ, Beckett P, Christodoulou M, et al. Matrix metalloproteinases and processing of pro-TNF-alpha. J Leukoc Biol. 1995;57(5):774–777. [DOI] [PubMed] [Google Scholar]
- 30. Jiang JY, Cheung CK, Wang Y, Tsang BK. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front Biosci. 2003;8: d222–d237. [DOI] [PubMed] [Google Scholar]
- 31. Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991;88(20):9292–9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148(7):3532–3540. [DOI] [PubMed] [Google Scholar]
- 33. Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod. 2009;80(4):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ortega HH, Veiga-Lopez A, Sreedharan S, et al. Developmental programming: does prenatal steroid excess disrupt the ovarian VEGF system in sheep? Biol Reprod. 2015;93(3):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89(5):397–410. [DOI] [PubMed] [Google Scholar]
- 36. Lejeune M, Jaen J, Pons L, et al. Quantification of diverse subcellular immunohistochemical markers with clinicobiological relevancies: validation of a new computer-assisted image analysis procedure. J Anat. 2008;212(6):868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rasband W. Quantifying stained liver tissue. 2016; https://imagej.nih.gov/ij/docs/examples/stained-sections/index.html. Updated March 16, 2011; Accessed November 03, 2016.
- 38. Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- 39. Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82(4):591–605. [DOI] [PubMed] [Google Scholar]
- 40. Padmanabhan V, Veiga-Lopez A, Herkimer C, et al. Developmental programming: prenatal and postnatal androgen antagonist and insulin sensitizer interventions prevent advancement of puberty and improve LH surge dynamics in prenatal testosterone-treated sheep. Endocrinology. 2015;156(7):2678–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Monniaux D, Huet C, Besnard N, et al. Follicular growth and ovarian dynamics in mammals. J Reprod Fertil Suppl. 1997;51:3–23. [PubMed] [Google Scholar]
- 42. Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction. 2003;126(4):415–424. [DOI] [PubMed] [Google Scholar]
- 43. Bagavandoss P. Differential distribution of gelatinases and tissue inhibitor of metalloproteinase-1 in the rat ovary. J Endocrinol. 1998;158(2):221–228. [DOI] [PubMed] [Google Scholar]
- 44. Garcia R, Ballesteros LM, Hernandez-Perez O, et al. Metalloproteinase activity during growth, maturation and atresia in the ovarian follicles of the goat. Anim Reprod Sci. 1997;47(3):211–228. [DOI] [PubMed] [Google Scholar]
- 45. Vos MC, van der Wurff AA, Last JT, et al. Immunohistochemical expression of MMP-14 and MMP-2, and MMP-2 activity during human ovarian follicular development. Reprod Biol Endocrinol. 2014;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66(4):1134–1150. [DOI] [PubMed] [Google Scholar]
- 47. Veiga-Lopez A, Steckler TL, Abbott DH, et al. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84(1):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strott CA, Sundel H, Stahlman MT. Maternal and fetal plasma progesterone, cortisol, testosterone and 17beta-estradiol in preparturient sheep: response to fetal ACTH infusion. Endocrinology. 1974;95(5):1327–1339. [DOI] [PubMed] [Google Scholar]
- 49. Hauger RL, Karsch FJ, Foster DL. A new concept for control of the estrous cycle of the ewe based on the temporal relationships between luteinizing hormone, estradiol and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology. 1977;101(3):807–817. [DOI] [PubMed] [Google Scholar]
- 50. Osteen KG, Keller NR, Feltus FA, Melner MH. Paracrine regulation of matrix metalloproteinase expression in the normal human endometrium. Gynecol Obstet Invest. 1999;48(suppl 1):2–13. [DOI] [PubMed] [Google Scholar]
- 51. Rawdanowicz TJ, Hampton AL, Nagase H, Woolley DE, Salamonsen LA. Matrix metalloproteinase production by cultured human endometrial stromal cells: identification of interstitial collagenase, gelatinase-A, gelatinase-B, and stromelysin-1 and their differential regulation by interleukin-1 alpha and tumor necrosis factor-alpha. J Clin Endocrinol Metab. 1994;79(2):530–536. [DOI] [PubMed] [Google Scholar]
- 52. Terranova PF. Potential roles of tumor necrosis factor-alpha in follicular development, ovulation, and the life span of the corpus luteum. Domest Anim Endocrinol. 1997;14(1):1–15. [DOI] [PubMed] [Google Scholar]
- 53. Marcinkiewicz JL, Krishna A, Cheung CM, Terranova PF. Oocytic tumor necrosis factor alpha: localization in the neonatal ovary and throughout follicular development in the adult rat. Biol Reprod. 1994;50(6):1251–1260. [DOI] [PubMed] [Google Scholar]
- 54. Sancho-Tello M, Tash JS, Roby KF, Terranova PF. Effects of lipopolysaccharide on ovarian function in the pregnant mare serum gonadotropin-treated immature rat. Endocrine. 1993;1:503–511. [Google Scholar]
- 55. Kondo H, Maruo T, Mochizuki M. Immunohistochemical evidence for the presence of tumor necrosis factor-alpha in the infant and adult human ovary. Endocr J. 1995;42(6):771–780. [DOI] [PubMed] [Google Scholar]
- 56. Li S, Maruo T, Ladines-Llave CA, Samoto T, Kondo H, Mochizuki M. Expression of transforming growth factor-alpha in the human ovary during follicular growth, regression and atresia. Endocr J. 1994;41(6):693–701. [DOI] [PubMed] [Google Scholar]
- 57. Grazul-Bilska AT, Redmer DA, Bilski JJ, Jablonka-Shariff A, Doraiswamy V, Reynolds LP. Gap junctional proteins, connexin 26, 32, and 43 in sheep ovaries throughout the estrous cycle. Endocrine. 1998;8(3):269–279. [DOI] [PubMed] [Google Scholar]
- 58. Lenhart JA, Downey BR, Bagnell CA. Connexin 43 gap junction protein expression during follicular development in the porcine ovary. Biol Reprod. 1998;58(2):583–590. [DOI] [PubMed] [Google Scholar]
- 59. Melton CM, Zaunbrecher GM, Yoshizaki G, et al. Expression of connexin 43 mRNA and protein in developing follicles of prepubertal porcine ovaries. Comp Biochem Physiol B Biochem Mol Biol. 2001;130(1):43–55. [DOI] [PubMed] [Google Scholar]
- 60. Li JR, Shen T, Wang YL, Wei QW, Shi FX. Expression of matrix metalloproteinases and ovarian morphological changes in androgenized cyclic female guinea pigs. Tissue Cell. 2016;48(1):72–80. [DOI] [PubMed] [Google Scholar]
- 61. Huet C, Monget P, Pisselet C, Hennequet C, Locatelli A, Monniaux D. Chronology of events accompanying follicular atresia in hypophysectomized ewes. Changes in levels of steroidogenic enzymes, connexin 43, insulin-like growth factor II/mannose 6 phosphate receptor, extracellular matrix components, and matrix metalloproteinases. Biol Reprod. 1998;58(1):175–185. [DOI] [PubMed] [Google Scholar]
- 62. Scarano WR, Toledo FC, Guerra MT, et al. Long-term effects of developmental exposure to di-n-butyl-phthalate (DBP) on rat prostate: proliferative and inflammatory disorders and a possible role of androgens. Toxicology. 2009;262(3):215–223. [DOI] [PubMed] [Google Scholar]
- 63. Gaide Chevronnay HP, Selvais C, Emonard H, Galant C, Marbaix E, Henriet P. Regulation of matrix metalloproteinases activity studied in human endometrium as a paradigm of cyclic tissue breakdown and regeneration. Biochim Biophys Acta. 2012;1824(1):146–156. [DOI] [PubMed] [Google Scholar]
- 64. Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod. 2009;80(4):718–725.19122183 [Google Scholar]
- 65. Curry TE, Jr, Song L, Wheeler SE. Cellular localization of gelatinases and tissue inhibitors of metalloproteinases during follicular growth, ovulation, and early luteal formation in the rat. Biol Reprod. 2001;65(3):855–865. [DOI] [PubMed] [Google Scholar]
- 66. Liao X, Thrasher JB, Pelling J, Holzbeierlein J, Sang QX, Li B. Androgen stimulates matrix metalloproteinase-2 expression in human prostate cancer. Endocrinology. 2003;144(5):1656–1663. [DOI] [PubMed] [Google Scholar]
- 67. Thathapudi S, Kodati V, Erukkambattu J, Katragadda A, Addepally U, Hasan Q. Tumor necrosis factor-alpha and polycystic ovarian syndrome: a clinical, biochemical, and molecular genetic study. Genet Test Mol Biomarkers. 2014;18(9):605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gruss HJ. Molecular, structural, and biological characteristics of the tumor necrosis factor ligand superfamily. Int J Clin Lab Res. 1996;26(3):143–159. [DOI] [PubMed] [Google Scholar]
- 69. Adams J, Liu Z, Ren YA, et al. Enhanced inflammatory transcriptome in the granulosa cells of women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2016;101(9):3459–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schmidt J, Weijdegard B, Mikkelsen AL, Lindenberg S, Nilsson L, Brannstrom M. Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Mol Hum Reprod. 2014;20(1):49–58. [DOI] [PubMed] [Google Scholar]
- 71. Caselli C. Role of adiponectin system in insulin resistance. Mol Genet Metab. 2014;113(3):155–160. [DOI] [PubMed] [Google Scholar]
- 72. Durlej M, Knapczyk-Stwora K, Duda M, et al. Prenatal and neonatal exposure to the antiandrogen flutamide alters connexin 43 gene expression in adult porcine ovary. Domest Anim Endocrinol. 2011;40(1):19–29. [DOI] [PubMed] [Google Scholar]
- 73. Wu CH, Yang JG, Yang JJ, et al. Androgen excess down-regulates connexin43 in a human granulosa cell line. Fertil Steril. 2010;94(7):2938–2941. [DOI] [PubMed] [Google Scholar]
- 74. Yang M, Li J, An Y, Zhang S. Effects of androgen on immunohistochemical localization of androgen receptor and connexin 43 in mouse ovary. Tissue Cell. 2015;47(5):526–532. [DOI] [PubMed] [Google Scholar]
- 75. Shalev E, Goldman S, Ben-Shlomo I. The balance between MMP-9 and MMP-2 and their tissue inhibitor (TIMP)-1 in luteinized granulosa cells: comparison between women with PCOS and normal ovulatory women. Mol Hum Reprod. 2001;7(4):325–331. [DOI] [PubMed] [Google Scholar]
- 76. Wu H, Yu K, Yang Z. Associations between TNF-alpha and interleukin gene polymorphisms with polycystic ovary syndrome risk: a systematic review and meta-analysis. J Assist Reprod Genet. 2015;32(4):625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]