Abstract
Fatigue, a commonly reported symptom, is defined as an overwhelming, debilitating, and sustained sense of exhaustion that decreases the ability to function and carry out daily activities. To date, cancer researchers have been in the forefront in investigating the possible biological mechanisms of fatigue, identifying inflammation, dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, and activation of the autonomic nervous system. The purpose of this systematic review is to describe fatigue and what is known about the biological mechanisms described in cancer in five chronic, noninfectious illnesses: heart failure, multiple sclerosis, chronic kidney disease, rheumatoid arthritis, and chronic obstructive pulmonary disease. We searched PubMed and EMBASE using fatigue as a major Medical subject headings (MeSH) heading with each individual disease added as a search term followed by each biological mechanism. We included only primary research articles published in English between 1996 and 2016 describing studies conducted in adult humans. We identified 26 relevant articles. While there is some evidence that the biological mechanisms causing fatigue in cancer are also associated with fatigue in other chronic illnesses, more research is needed to explore inflammation, the HPA axis, and the autonomic nervous system, and other mechanisms in relation to fatigue in a variety of chronic illnesses.
Keywords: fatigue, inflammation, hypothalamic–pituitary–adrenal axis, autonomic nervous system, heart failure, multiple sclerosis, chronic kidney disease, rheumatoid arthritis, chronic obstructive pulmonary disease
Fatigue has been defined as an overwhelming, debilitating, and sustained sense of exhaustion that decreases the ability to function and carry out daily activities (Cella et al., 2007). Up to 45% of the U.S. population has reported experiencing fatigue, which greatly reduces overall quality of life (Ricci, Chee, Lorandeau, & Berger, 2007). Chronic fatigue that is unresponsive to rest may be a sign of underlying pathology (Jorgensen, 2008) or chronic illness (Ricci et al., 2007). Much of the research on fatigue has been conducted in cancer patients, but fatigue is also common in persons with heart failure (HF), multiple sclerosis (MS), chronic kidney disease (CKD), rheumatoid arthritis (RA), and chronic obstructive pulmonary disease (COPD; Junghaenel, Christodoulou, Lai, & Stone, 2011). Fatigue may also be a prodromal symptom for acute myocardial infarction (McSweeney et al., 2003) or major depression (Fava, Grandi, Canestrari, & Molnar, 1990). It is not yet fully understood whether fatigue is a different entity in healthy and disease states, but in the case of chronic illness, fatigue may be a protective factor signaling the body to rest.
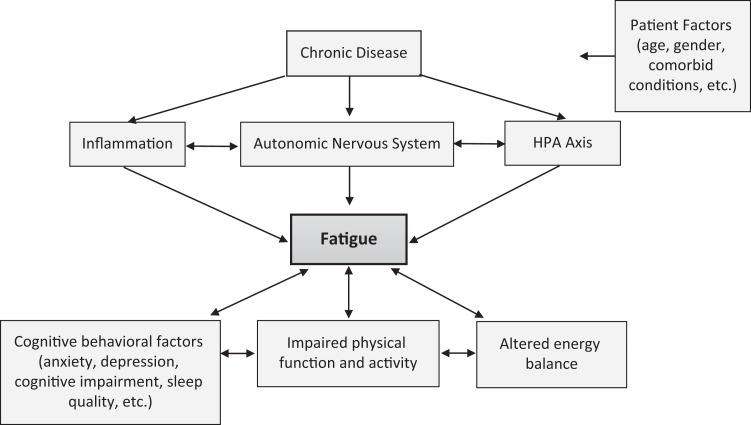
Despite the prevalence of fatigue and its negative impact on the human experience, research exploring mechanisms of fatigue in different disease states is limited. Cancer investigators have led the way in describing and exploring the underlying mechanisms of fatigue. Researchers have identified the causes of cancer-related fatigue as inflammation, dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, and/or activation of the autonomic nervous system (Bower, 2014; Ryan et al., 2007; Saligan et al., 2015). Although many studies investigating biological mechanisms of fatigue have focused on only one possible mechanism (e.g., inflammation), these biological pathways can influence each other and activate other systems (Figure 1). For example, activation of the sympathetic nervous system, a branch of the autonomic nervous system, can produce a pro-inflammatory response (Pongratz & Straub, 2014). Perhaps it is the complexity of these issues that explains the dearth of research into the mechanisms of fatigue.
Figure 1.
Chronic illnesses are associated with underlying biological changes (inflammation, autonomic nervous system activation, and hypothalamic–pituitary–adrenal axis dysregulation) that appear to be associated with fatigue. These biological changes can be influenced by patient characteristics and modifiable factors. The cascade of biological processes illustrated in this figure can impact cognitive behavioral factors and impair physical function, activity, and energy balance, which can likewise affect levels of fatigue.
The purpose of the present systematic review is to describe fatigue and explore findings regarding the biological mechanisms of fatigue in five chronic, noninfectious illnesses: HF, MS, CKD, RA, and COPD.
Method
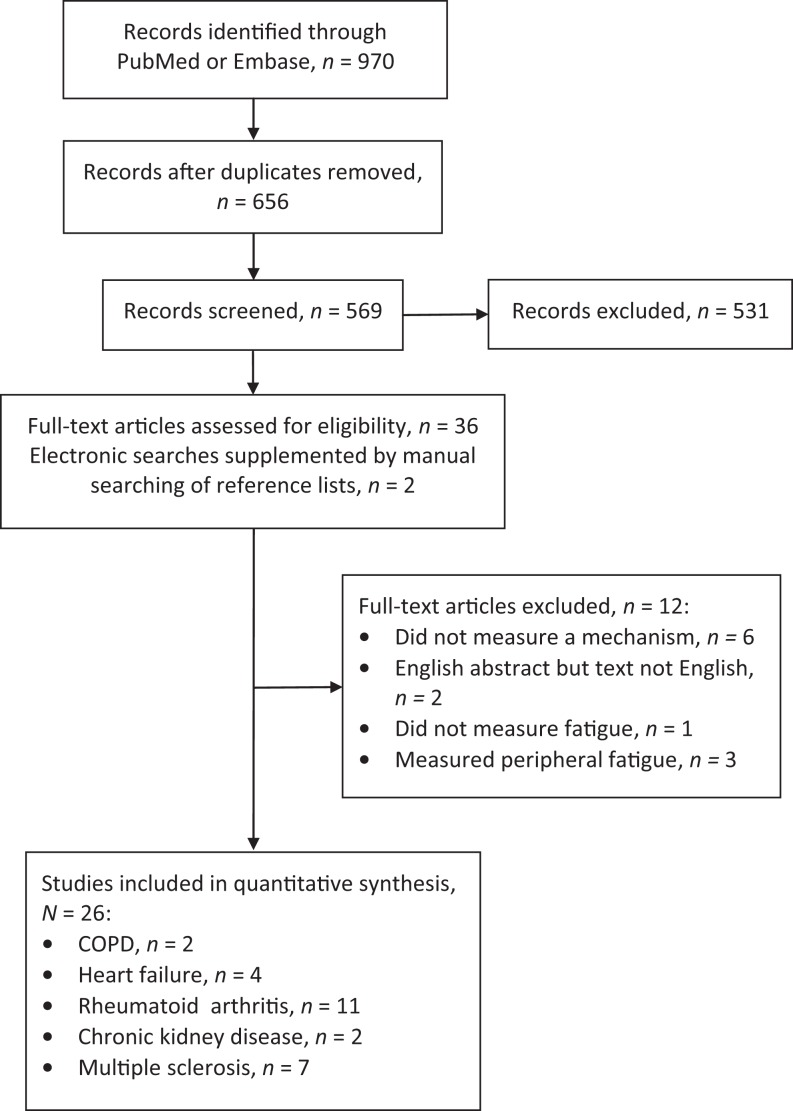
With the support of a medical librarian, we searched PubMed and EMBASE using fatigue as a major MESH heading with each individual disease (e.g., HF) added as a search term followed by each mechanism (e.g., inflammation). We included only primary research articles published in English from 1996 to 2016 that described studies conducted in adult humans. The initial search resulted in 970 articles (see the PRISMA diagram in Figure 2). After removing duplicates, we assessed 656 article titles and abstracts for relevance. We reviewed 36 full-text articles and excluded 12 because they did not describe a study that investigated fatigue in an illness of interest (i.e., HF, MS, CKD, RA, or COPD) with discussion of one of the three specific mechanisms (i.e., inflammation, HPA axis, or autonomic nervous system). We supplemented electronic searches with manual searching of reference lists and reviews, which produced another two articles on RA. A total of 26 articles fulfilled the inclusion criteria and were independently reviewed by the four authors. We discussed all potentially relevant articles as a group before including them in this systematic review.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. COPD = chronic obstructive pulmonary disease.
Cancer-Related Fatigue
Prior literature reviews have focused on possible biological mechanisms or etiologies of cancer-related fatigue (Bower, 2014; Ryan et al., 2007; Saligan et al., 2015). Patients commonly report fatigue in relation to cancer treatments; however, fatigue may be present before treatment and may increase during therapy. It may limit the receipt of optimal courses of treatment due to early discontinuation and may be a prognostic factor in cancer survival (Bower, 2007). Research on possible biological mechanisms of cancer-related fatigue has been robust because of its potential to lead to targeted interventions to improve fatigue and ultimately quality of life in cancer patients and survivors.
Possible biological mechanisms of cancer-related fatigue explored in the literature include inflammation, dysregulation of the HPA axis, and activation of the autonomic nervous system (Bower, 2014; Saligan et al., 2015). Inflammation related to the release of inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α has been implicated in cancer-related fatigue prior to treatment, during treatment and after treatment (Bower, 2014). Dysregulation in the HPA axis, with alterations in cortisol levels (Saligan et al., 2015) or cytokine production (Saligan et al., 2015) resulting in increased inflammation, has also been implicated in cancer-related fatigue. In addition, activation of the autonomic nervous system, with increased sympathetic activity and reduced parasympathetic activity, may contribute to cancer-related fatigue (Bower, 2014).
Inflammation
Inflammation is a component of immune function. Patients with cancer have increased levels of inflammatory markers (e.g., TNF-α, IL-6) that are associated with fatigue levels (Ryan et al., 2007; Saligan et al., 2015). For example, Meyers, Albitar, and Estey (2005) found that, prior to treatment, patients with acute myelogenous leukemia had elevated inflammatory markers that were associated with fatigue levels. Similarly, in another study, patients with ovarian cancer had elevated levels of IL-6 that were associated with fatigue prior to surgery (Lutgendorf et al., 2008).
Investigators have also found cancer treatments to increase fatigue and inflammation both during and after treatment (Bower, 2014). Bower et al. (2009) found that, during radiation treatment for breast or prostate cancer, fatigue was associated with C-reactive protein (CRP) and IL-1 levels. Researchers found a similar result in breast cancer patients undergoing chemotherapy, with fatigue associated with IL-6 levels (Liu et al., 2012). In another study, in patients with advanced colorectal, esophageal, and nonsmall cell lung cancer who were receiving both radiation and chemotherapy, fatigue levels were, once again, associated with increased levels of IL-6 (Bower, 2014; Wang et al., 2010). After treatment for cancer, fatigue can persist for 5–10 years or even longer (Bower et al., 2006). Both TNF receptor II (Bower et al., 2011) and CRP (Alexander, Minton, Andrews, & Stone, 2009) levels have been associated with fatigue 1 month after the completion of treatment in patients with breast cancer.
HPA Axis
Dysregulation of the HPA axis may be a mechanism in cancer-related fatigue or may cause inflammation that ultimately produces fatigue (Bower, 2014; Saligan et al., 2015). Cytokine production can be altered by production of glucocorticoid by the HPA axis (McEwen et al., 1997). In one study, cortisol levels were higher in breast cancer patients reporting fatigue compared to their counterparts without fatigue (Bower et al., 2005). In another, cortisol levels were also higher and cortisol variability was reduced in patients with ovarian cancer reporting fatigue before cancer treatment (Weinrib et al., 2010).
Autonomic Nervous System
Researchers have posited that the autonomic nervous system, both the sympathetic and parasympathetic branches, plays a role in cancer-related fatigue (Bower, 2014; Ryan et al., 2007) because an increased sympathetic response can increase inflammation. There may also be a vagal response to cancer treatments that induces “sickness behavior” (Ryan et al., 2007). Fagundes et al. (2011) found that, in patients with breast cancer, fatigue was associated with elevated levels of norepinephrine (sympathetic response) and lower heart rate variability (parasympathetic response).
Fatigue in Other Chronic Noninfectious Diseases
Using cancer-related fatigue as the gold standard for comparison, we report below the results of our review of the literature to describe fatigue in five noninfectious illnesses and explore the similarities between mechanisms of action of fatigue across these illnesses. Table 1 outlines the measures researchers used to study inflammation, the HPA axis and the autonomic nervous system for each disease. Table 2 lists the measures of fatigue used in each study.
Table 1.
Measures of Potential Mechanisms of Fatigue Used in Studies of Cancer and Five Other Noninfectious Chronic Diseases—Heart Failure (HF), Multiple Sclerosis (MS), Chronic Obstructive Pulmonary Disease (COPD), Chronic Kidney Disease (CKD), and Rheumatoid Arthritis (RA).
| Study | Mechanism Measure (Disease) | ||
|---|---|---|---|
| Inflammation | HPA Axis Function | Autonomic Nervous System Function | |
| Alexander et al. (2009) | CRP (cancer), IL-1β (cancer) | ||
| Al-Shair et al. (2011) | CRP (COPD), IL-6 (COPD), TNF-α (COPD) | ||
| Bergman et al. (2009) | DAS28 (RA) | ||
| Bossola et al. (2015) | IL-6 (CKD) | ||
| Bower et al. (2005) | Cortisol (cancer) | ||
| Bower et al. (2009) | CRP (cancer), IL-1β (cancer) | ||
| Bower et al. (2011) | TNFR2 (cancer) | ||
| Bower (2014) | IL-6 (cancer) | ||
| Bunevicius et al. (2012) | Cortisol (HF) | ||
| Davis et al. (2008) | IL-6 (RA) | ||
| Druce et al. (2016) | DAS28 (RA) | ||
| Druce, Jones, MacFarlane, and Basu (2015a) | CRP (RA), DAS28 (RA), and erythrocyte sedimentation rate (RA) | ||
| Druce, Jones, MacFarlane, and Basu (2015b) | DAS28 (RA) | ||
| Druce, Jones, Macfarlane, Verstappen, et al. (2015) | DAS28 (RA) | ||
| Fagundes et al. (2011) | HRV (cancer), norepinephrine (cancer) | ||
| Feldthusen et al. (2016) | DAS28 (RA) | ||
| Fink et al. (2012) | CRP (cancer, HF), IL-6 (cancer, HF), IL-10 (HF), and TNF-α (cancer, HF) | ||
| Fujii et al. (2013) | HRV (CKD) | ||
| Giovannoni, Thompson, Miller, and Thompson (2001) | CRP (MS), sICAM-1 (MS), and urinary neopterin excretion (MS) | ||
| Goebel et al. (2005) | IFNγ (MS), IL-10 (MS), and TNF-α (MS) | ||
| Gold et al. (2011) | CD8+ T cells (MS) | Cortisol (MS) | |
| Heesen et al. (2006) | IFNγ (MS), TNF-α | ||
| Keselbrener et al. (2000) | Fluctuations in HR, BP, blood flow supine, and standing (MS) | ||
| Kumar et al. (2007) | IL-6 (HF), IL-10 (HF), and TNF-α (HF) | ||
| Lee et al. (2014) | CRP (RA) | ||
| Lennie et al. (2013) | IL-10 (HF), sTNFR1 and 2 (HF), TNF-α (HF) | ||
| Liu et al. (2012) | IL-6 (cancer) | ||
| Lutgendorf et al. (2008) | IL-6 (cancer) | ||
| Merkelbach, Dillmann, Kolmel, Holz, and Muller (2001) | Battery of testsa (MS) | ||
| Powell et al. (2015) | Cortisol (MS) | ||
| Repping-Wuts et al. (2007) | DAS28 (RA) | ||
| Ryan et al. (2007) | IL-6 (cancer), TNF-α (cancer) | ||
| Saligan et al. (2015) | IL-6 (cancer), TNF-α (cancer) | ||
| Schuetz et al. (2015) | Cortisol (COPD) | ||
| Treharne et al. (2008) | Erythrocyte sedimentation rate (RA) | ||
| Van Hoogmoed et al. (2010) | DAS28 (RA) | ||
| Wang et al. (2010) | IL-6 (cancer) | ||
| Weinrib et al. (2010) | Cortisol (cancer) | ||
Note. BP = blood pressure; CD = cluster of differentiation; CRP = C-reactive protein; DAS28 = disease activity score in 28 joints; HPA = hypothalamic–pituitary–adrenal; HR = heart rate; HRV = heart rate variability; IFN = interferon; IL = interleukin; sICAM = soluble intercellular adhesion molecule; sTNFR = soluble tumor necrosis factor receptor; TNF = tumor necrosis factor; TNFR = tumor necrosis factor receptor.
aBattery of tests included postural changes, pressure tests, the Valsalva maneuver, deep breathing, and hyperventilation.
Table 2.
Measures of Fatigue Used in Reviewed Studies on Chronic Kidney Disease (CKD), Chronic Obstructive Pulmonary Disease (COPD), Heart Failure (HF), Multiple Sclerosis (MS), and Rheumatoid Arthritis (RA).
| Measure of Fatigue | Disease | Study |
|---|---|---|
| Bristol Rheumatoid Arthritis Fatigue Multidimensional Questionnaire | RA | Feldthusen et al. (2016) |
| Fatigue Questionnaire Scale | MS | Giovannoni et al. (2001) |
| Fatigue Scale | MS | Powell et al. (2015) |
| Fatigue Severity Scale | MS | Keselbrener et al. (2000) and Merkelbach et al. (2001) |
| Fatigue severity subscale of the checklist individual strength (CIS20r) | RA | Van Hoogmoed et al. (2010) and Repping-Wuts et al. (2007) |
| Italian version of the Short-Form (short form [SF]-36Ò) Vitality Scale | CKD | Bossola et al. (2015) |
| Kuratsume and Yumaguti New Fatigue Scale | CKD | Fuji et al. (2013) |
| Manchester COPD Fatigue Scale | COPD | Al-shair et al. (2011) |
| Memorial Symptom Assessment Scale-Heart Failure | HF | Lennie et al. (2013) |
| Modified Fatigue Impact Scale | MS | Gold et al. (2011) and Heesen et al. (2006) |
| Modified Performance Score for COPD | COPD | Schuetz et al. (2015) |
| Multidimensional Fatigue Inventory -20 + Dutch Exertion Fatigue Scale + standardized cardiopulmonary exercise testing | HF | Bunevicius et al. (2012) |
| Multidimensional Health Assessment Questionnaire | RA | Lee et al. (2014) |
| Numerical Rating Scale | RA | Davis et al. (2008) |
| Profile of mood states | HF, MS | Fink et al. (2012; HF) and Goebel et al. (2005; MS) |
| SF-36 vitality scale | RA | Druce, Jones, McFarlane, & Basu (2015), Druce, Jones, McFarlane, and Basu (2015b), and Druce et al. (2016) |
| Visual Analog Scale | HF | Kumar et al. (2007) |
| Visual Analog Scale (0−100 mm) | RA | Druce, Jones, Macfarlane, Verstappen, et al. (2015) and Treharne et al. (2008) |
| Visual Analog Scale (unspecified) | RA | Bergman et al. (2009) |
HF
Fatigue and dyspnea are the hallmark symptoms of chronic HF, but there is surprisingly little research on fatigue in HF. In a qualitative study, the authors characterized HF fatigue as having both bodily and mental aspects (Falk, Granger, Swedberg, & Ekman, 2007). Patients described the bodily experience of fatigue as a feeling of sleepiness and lacking strength and energy. They described the mental aspects of fatigue as demoralization and intellectual deficiency. Restorative activites (e.g., social interaction) alleviated fatigue in this small sample of patients with chronic HF.
Fatigue in HF may reflect interacting peripheral and central factors (Witte & Clark, 2004). Peripheral, or muscle, fatigue is typically described as a deficiency in peripheral blood flow and skeletal muscle function leading to exercise-induced fatigue (Coats, 1998; Kränkel et al., 2003; Piepoli, Scott, Capucci, & Coats, 2001). Exercise training may be an appropriate treatment for peripheral fatigue. Some authors speak about peripheral fatigue as a contributor to central fatigue in HF (Marzilli, 2014). The manner in which peripheral and central factors interact in HF needs further study, but for the purposes of this review, we focused on central fatigue. In HF, greater fatigue is associated with worse clinical outcomes (Perez-Moreno et al., 2014). Smith, Kupper, de Jonge, and Denollet (2010) found that both severe exertional fatigue and severe general fatigue significantly predicted an increased mortality rate.
Studies of mechanisms of fatigue in HF are limited, and it is clear that more research is needed. Researchers have attributed fatigue in HF to illness severity (Jasiukeviciene et al., 2008), chronic hyponatremia (Rai, Whaley-Connell, McFarlane, & Sowers, 2006), sleep apnea (Köhnlein, Klante, Elliott, & Welte, 2001), and depression (Fink et al., 2012; Iasiukiavichene & Vasiliauskas, 2006; Smith, Gidron, Kupper, Winter, & Denollet, 2009). Others have discussed fatigue in HF in terms of vital exhaustion, a psychological state characterized by unusual fatigue and depression, which has been linked to elevated levels of pro-inflammatory cytokines (Herrmann-Lingen et al., 2003).
Inflammation
Several investigative teams have studied the link between fatigue in HF and inflammation. Fink and colleagues (2012) found that fatigue was associated with HF severity as measured by the Seattle HF Model but also that cytokine levels were not associated with fatigue. Two teams have proposed dietary treatments to reduce inflammation in HF patients. One team improved fatigue in HF patients by supplementing with ubiquinol + l-carnitine to reduce pro-inflammatory cytokines (Kumar et al., 2007). Another team is currently studying the influence of supplemental lycopene and omega-3 fatty acids on fatigue in HF patients (Lennie et al., 2013). These nutritional approaches address components in the mitochondrial electron transport chain pathway, but a deficiency in this pathway is rare except in liver disease (Paradies, Paradies, Ruggiero, & Petrosillo, 2014). Such dietary approaches to influencing fatigue in HF represent a unique approach to a problem that is poorly understood.
HPA axis
Few investigators have studied the influence of the HPA axis on fatigue in HF. In a study of patients with coronary artery disease (48 of the 83 participants had HF), decreased thyroid hormone concentrations were independently associated with higher levels of physical fatigue, and lower morning cortisol levels were independently associated with higher levels of mental fatigue (Bunevicius et al., 2012).
Autonomic nervous system
The effect of the autonomic nervous system on the symptom of fatigue has not been studied in HF.
MS
Fatigue is common in people with MS and is similar to the fatigue reported by individuals with other chronic illnesses as well as healthy individuals (Krupp, 2003). Fatigue in MS is disabling, as assessed by self-report and by performance-based measures of motor and cognitive function. Motor fatigue is significantly greater in patients with MS than in healthy individuals (Djaldetti, Ziv, Achiron, & Melamed, 1996). Memory and conceptual thinking are decreased in patients with MS compared to healthy controls, and both decline over the time course of the disease (Krupp & Elkins, 2000). Fatigue in patients with MS is associated with psychological distress and depressed mood along with neurological impairment. Researchers found that depressed mood influenced the experience of fatigue in MS (Krupp, LaRocca, Muir-Nash, & Steinberg, 1989). Additional authors reported that patients with MS who were distressed and depressed had higher levels of fatigue (Kroencke, Lynch, & Denney, 2000; Krupp et al., 2002). Cadden and Arnett (2015) also found that fatigue is a common feature in patients with MS and is associated with unemployment.
Few researchers have investigated possible mechanisms of fatigue in MS, perhaps believing that fatigue is a physiological adaptation to the pathology of MS (Krupp & Elkins, 2000). One possible explanation of fatigue in MS that may be unique to this disease is involvement of the central nervous system. There may be changes in the premotor, limbic, basal ganglia, or brainstem areas that decrease motivation or motor readiness resulting in fatigue (Krupp, 2003). Research has implicated immune function, specifically the action of inflammatory cytokines (Iriarte, Subirá, & de Castro, 2000), as a possible cause of fatigue in MS as well as alterations in the neuroendocrine system (Powell, Moss-Morris, Liossi, & Schlotz, 2015).
Inflammation
One study on fatigue in MS focused solely on inflammation. Investigators found that markers of systemic inflammation were not associated with self-reported fatigue levels (Giovannoni, Thompson, Miller, & Thompson, 2001). Injection of interferon β (INF-β), a type of cytokine therapy used in MS to balance pro- and anti-inflammatory agents in the brain, can reduce the rate of MS relapses. However, 4 hr after injection of INF-β, patients reported higher levels of fatigue than before the injection, which were associated with the percentage of granulocytes relative to total leukocytes, suggesting an increased inflammatory response (Goebel et al., 2005). In another study, increased CD8+ T cells and cortisol levels were associated with fatigue in MS suggesting a possible contribution of both inflammation and the HPA axis to fatigue (Gold et al., 2011). Finally, TNF-α and INFγ levels were higher in patients with MS who reported fatigue than in those who did not (Heesen et al., 2006).
HPA axis
Cortisol, a common marker of stress, regulates the immune system and energy metabolism. In patients with MS, fatigue was associated with lower waking cortisol levels and larger awakening cortisol responses (Powell et al., 2015). MS patients with fatigue had higher levels of adrenocorticotropic hormone, a compound that can increase cortisol production and release, than those who did not report fatigue.
Autonomic nervous system
There is also some evidence of a role for the autonomic nervous system in fatigue in MS. In one study, hand grip (one test of autonomic function) was associated with self-reported fatigue scores in patients with MS (Merkelbach, Dillmann, Kölmel, Holz, & Müller, 2001). In another, increased vagal activity occurred in younger patients with MS (less than 45 years old) who reported fatigue compared to those who did not (Keselbrener et al., 2000).
COPD
Fatigue is common, often severe and disabling in COPD (Baltzan et al., 2011). Patients identify fatigue as second only to dyspnea as the most common symptom that interferes with their lives (Paddison, Effing, Quinn, & Frith, 2013). Dyspnea and fatigue scores have been associated with impaired physical functioning (Woo, 2000) and reduced ability to perform daily activities (Kapella, Larson, Patel, Covey, & Berry, 2006). Fatigue can increase COPD progression, negatively affect health status and quality of life, and lead to disability in these patients (Baghai-Ravary et al., 2009; Paddison et al., 2013). Increasing fatigue levels also predict future hospitalizations (Paddison et al., 2013).
Although fatigue is common in COPD, there is little research on the mechanisms of fatigue. Some have suggested an association between fatigue and inflammation (Al-Shair et al., 2011) and between fatigue and the HPA axis in patients with COPD (Schuetz et al., 2015). Research studying the association between fatigue and the autonomic nervous system in patients with COPD is lacking, however.
Inflammation
We identified only one study that assessed the association between fatigue and inflammatory markers in patients with COPD (Al-Shair et al., 2011). Of the three markers examined—TNF-α, CRP, and IL-6—only higher levels of TNF-α were associated with fatigue in patients with COPD.
HPA axis
Likewise, we identified only one study that investigated the association between fatigue and adrenal function in COPD. These investigators did not find an association between fatigue and cortisol levels (Schuetz et al., 2015).
Autonomic nervous system
We did not find any studies investigating the association of the autonomic nervous system and fatigue in COPD.
CKD
Fatigue is one of the most common symptoms people living with CKD report, with an average prevalence of 71% (Murtagh, Addington-Hall, & Higginson, 2007). Patients with CKD have described fatigue as a constant lack of energy (Horigan, 2012), which investigators have attributed to both physical and mental domains. Fatigue is especially prevalent in those with end-stage renal disease receiving hemodialysis (CKD5; Caplin, Kumar, & Davenport, 2011; Jablonski, 2007; Weisbord et al., 2005). One study found that 14.7% of the patients with CKD5 had fatigue scores higher than twice the standard deviation of the mean for healthy volunteers (Koyama et al., 2010). Fatigue in these patients is associated with reduced quality of life, increased risk of cardiovascular events, and reduced survival (Bossola, Vulpio, & Tazza, 2011; Horigan, 2012; Horigan, Rocchiccioli, & Trimm, 2012; Koyama et al., 2010). Fatigue predicts cardiovascular events in CKD5, independent of the accepted risk factors of age, diabetes, malnutrition, and a history of cardiovascular disease (Koyama et al., 2010).
Mechanisms of fatigue in CKD are largely understudied. However, existing studies suggest that inflammation and the autonomic nervous system may be associated with fatigue in CKD (Bossola, Di Stasio, Giungi, Rosa, & Tazza, 2015; Cruz, Mahnensmith, & Perazella, 1997; Fujii et al., 2013).
Inflammation
CKD is thought to be a pro-inflammatory state; however, little is known regarding the inflammatory pathways involving fatigue. One study revealed that fatigue in CKD5 was significantly associated with increased levels of IL-6 (Bossola et al., 2015). We found no other studies exploring inflammatory pathways in relation to fatigue in CKD.
HPA axis
We found no studies investigating the relationship between the HPA axis and fatigue in CKD.
Autonomic nervous system
One study compared autonomic function in patients with CKD to that of healthy people and found that there was a positive association between impaired autonomic function and fatigue in CKD patients (Fujii et al., 2013; Fukuda et al., 2008).
RA
RA is an autoimmune disease characterized by joint pain, tenderness, and inflammation. It is accompanied by several factors that impact quality of life including sleep disturbances, pain, depression, and fatigue (Jump, Fifield, Tennen, Reisine, & Giuliano, 2004; Pollard, Choy, Gonzalez, Khoshaba, & Scott, 2006). About 80% of persons with RA report experiencing fatigue, with 50% rating the fatigue as severe (Pollard et al., 2006). Individuals with RA describe fatigue as a loss of physical and mental energy, or weariness and lack of motivation, that is overwhelming (Hewlett et al., 2005). While patients consider management of their fatigue to be a treatment priority, health-care providers address it infrequently, and researchers rarely consider it to be a primary outcome in RA research (Hewlett et al., 2005; Hewlett, Nicklin, & Treharne, 2008).
Several treatments targeting improvement in RA disease activity have also reduced fatigue. For example, pharmacologic agents given to reduce inflammation caused by disease activity reduced fatigue (Almeida et al., 2016; Chauffier, Salliot, Berenbaum, & Sellam, 2012). Increasing physical activity to limit physical deconditioning (Cramp et al., 2013) and psychosocial interventions used to improve coping strategies and promote behavior change (Cramp et al., 2013) also reduced fatigue. Because fatigue was not the focus of these studies, researchers made no effort to elucidate the mechanisms underlying RA-related fatigue. However, the success of these varied approaches in reducing RA-related fatigue, albeit limited, suggests that multiple mechanisms lead to RA-related fatigue (Cramp et al., 2013).
Inflammation
The inflammatory activity that characterizes RA, as measured by swollen/tender joint counts, erythrocyte sedimentation rates, CRP, and the disease activity score 28 (a composite score), has an inconsistent relationship with RA-related fatigue (Bergman et al., 2009; van Hoogmoed, Fransen, Bleijenberg, & van Riel, 2010). In one study, patients reporting either the least or the greatest level of fatigue had minimal evidence of inflammation (low swollen joint counts and low CRP levels), whereas patients reporting moderate levels of fatigue had high swollen joint counts and CRP (Lee et al., 2014). Few measures of inflammation have been associated with RA-related levels of fatigue in cross-sectional (Bergman et al., 2009; van Hoogmoed et al., 2010) or longitudinal studies (Druce, Jones, Macfarlane, Verstappen, & Basu, 2015; Feldthusen, Grimby-Ekman, Forsblad-d’Elia, Jacobsson, & Mannerkorpi, 2016; Repping-Wuts, Fransen, van Achterberg, Bleijenberg, & van Riel, 2007; Treharne et al., 2008). There have been mixed results regarding an association between RA-related fatigue and IL-6 in chronically stressed patients: In vivo plasma levels of IL-6 have not been associated with RA-related fatigue but increased in vitro stimulated cellular production of IL-6 and reduced glucocorticoid inhibition of IL-6 have (Davis et al., 2008).
Capitalizing on the use of pharmacological interventions for RA that reduce joint inflammation and inhibit pro-inflammatory cytokines, several investigative teams sought to determine whether fatigue was reduced following commencement of these treatments. In one study, after beginning anti-TNF treatment, 70% of patients who were nonresponsive to disease-modifying anti-rheumatoid drugs reported reduced fatigue levels; effects were sustained in 80% of participants 1 year later (Druce, Jones, Macfarlane, & Basu, 2015b). Responders were more likely to be nonhypertensive, nondepressed, and nonsteroid using seropositive females reporting good mental health and low disability levels. Changes in fatigue were not directly related to inflammation or disease activity but rather were related to changes in pain, mental health, depression, and disability (Druce, Jones, Macfarlane, & Basu, 2015a). Evidence that patients continue to report RA-related fatigue even after disease remission confounds our understanding of the possible role of inflammation in this fatigue (Druce, Bhattacharya, Jones, Macfarlane, & Basu, 2016). Collectively, this limited evidence is inconclusive regarding a precise role for inflammation in RA-related fatigue.
HPA axis
We found no studies investigating the relationship between the HPA axis and fatigue in RA.
Autonomic nervous system
Likewise, we found no studies investigating the relationship between the autonomic nervous system and fatigue in RA.
Discussion
While investigators of cancer have led the way in defining possible mechanisms of fatigue, little research has been done investigating possible biological mechanisms of the common symptom of fatigue in other chronic illnesses. In this review, we synthesized the current state of the literature describing inflammation, HPA axis, and the autonomic nervous system as possible mechanisms of fatigue in patients with HF, MS, COPD, CKD, and RA—common chronic conditions associated with persistent and disabling fatigue.
We found studies investigating the association between fatigue and inflammation in HF, MS, COPD, CKD, and RA. However, there were conflicting reports in studies on MS and RA, where increased inflammation was not consistently associated with elevated levels of inflammatory markers. Possible reasons for these discrepancies could be related to the procurement and processing of the blood samples, disease severity, small sample sizes, or other treatment effects. In addition, researchers tested the inflammatory markers of IL-6, TNF-α, and CRP, but perhaps there are other pro-inflammatory and anti-inflammatory markers associated with fatigue that investigators have not yet explored in this context.
We found studies investigating fatigue and the HPA axis only in patients with HF, MS, and COPD. Levels of cortisol, a commonly used measure of HPA axis activity, were associated with mental fatigue in HF and fatigue levels in MS but were not associated with fatigue in patients with COPD. However, in several illnesses, there were only single studies, so we can draw few conclusions. There may be other mechanisms that are unique to specific disease processes.
We also identified research on the autonomic nervous system and fatigue in patients with HF, MS, and CKD. The autonomic nervous system was implicated in peripheral fatigue in patients with HF; notably, only HF research distinguished between peripheral and central fatigue. Impaired autonomic function was also associated with fatigue in patients with MS and CKD. Typically, a battery of tests is used to evoke both sympathetic and parasympathetic responses. There was variability in the measurement of autonomic function across the HF, MS, and CKD studies. Without consistent measurements across disease states, it is difficult to discern whether these mechanisms of fatigue are the same among the diseases.
We found few studies testing the HPA axis or the autonomic nervous system as mechanisms for fatigue in the chronic diseases we examined. Further, even in studies of inflammation and fatigue, the methods used to collect specimens varied significantly across studies (e.g., time of day when samples were collected). There is a circadian effect for inflammatory markers, the HPA axis, and the autonomic nervous system (Kalsbeek et al., 2012; Li et al., 2011). In cases where investigators did not account for this circadian effect in the studies, results may be suspect.
Another limitation of the studies we reviewed is that researchers measured self-reported fatigue using variety of different instruments. Some of the fatigue measures were subscales of more general instruments. For example, the Profile of Mood States measures vigor and depression along with fatigue (Curran, Andrykowski, & Studts, 1995). As investigators continue to study fatigue, use of a more uniform measure such as the Patient-Reported Outcomes Measurement Information System (PROMIS) fatigue measure would allow for comparisons across studies and diseases. Finally, there is the possibility that negative studies were not published. Absence of these findings impacts our conclusions regarding possible mechanisms of fatigue.
Other factors that may contribute to fatigue in chronic illnesses include anemia, depression, sleep disorders (Ryan et al., 2007), and genetic risk factors (Bower, 2014; e.g., IL-6 single-nucleotide polymorphisms). These factors should also be explored in future studies.
Conclusions and Future Directions
While there is evidence of some underlying biological mechanisms of fatigue in cancer and the other chronic illnesses we explored, there are still gaps and areas of limited knowledge. Inflammation, the HPA axis, and the autonomic nervous system may interact to cause or accentuate fatigue, but more research is greatly needed to explore these mechanisms and others. Consistent use of measures of self-reported fatigue and biological mechanisms would help investigators to compare and contrast potential mechanisms across different disease states. Because fatigue is a commonly reported symptom that can be debilitating, understanding biological mechanisms is essential in order to test targeted interventions.
The National Institutes of Health/National Institute of Nursing Research (NINR) recommends the use of common data elements for measuring fatigue (https://cde.nlm.nih.gov/formView?tinyId=QktmQ9BqM). At the time of this writing, NINR was seeking input from stakeholders regarding common data elements for measuring biomarkers such as cytokines (IL-1β, IL-6, and IL-10) for inflammation and free cortisol for HPA axis activity. Researchers should consider using standardized measurements of both patient-reported outcomes and biomarkers when designing future research in order to facilitate comparisons across disease states and patient populations.
Acknowledgments
The authors gratefully acknowledge reviews of a previous version of this article by Lakeetra Josey and Charlene Compher.
Footnotes
Author Contributions: L.A. Matura, S. Malone, R. Jaime-Lara, and B. Riegel contributed to conception and design, drafted the manuscript, critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was partially funded by Grants from the National Institutes of Health: K23 NR014885 and T32 HL07953.
References
- Alexander S., Minton O., Andrews P., Stone P. (2009). A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. European Journal of Cancer, 45, 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida C., Choy E. H., Hewlett S., Kirwan J. R., Cramp F., Chalder T.…Christensen R. (2016). Biologic interventions for fatigue in rheumatoid arthritis. Cochrane Database of Systemic Reviews, 6, Cd008334 doi:10.1002/14651858.CD008334.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shair K., Kolsum U., Dockry R., Morris J., Singh D., Vestbo J. (2011). Biomarkers of systemic inflammation and depression and fatigue in moderate clinically stable COPD. Respiratory Research, 12, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghai-Ravary R., Quint J. K., Goldring J. J. P., Hurst J. R., Donaldson G. C., Wedzicha J. A. (2009). Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respiratory Medicine, 103, 216–223. [DOI] [PubMed] [Google Scholar]
- Baltzan M. A., Scott A. S., Wolkove N., Bailes S., Bernard S., Bourbeau J., Maltais F. (2011). Fatigue in COPD: Prevalence and effect on outcomes in pulmonary rehabilitation. Chronic Respiratory Disease, 8, 119–128. [DOI] [PubMed] [Google Scholar]
- Bergman M. J., Shahouri S. H., Shaver T. S., Anderson J. D., Weidensaul D. N., Busch R. E.…Wolfe F. (2009). Is fatigue an inflammatory variable in rheumatoid arthritis (RA)? Analyses of fatigue in RA, osteoarthritis, and fibromyalgia. Journal of Rheumatology, 36, 2788–2794. [DOI] [PubMed] [Google Scholar]
- Bossola M., Di Stasio E., Giungi S., Rosa F., Tazza L. (2015). Fatigue is associated with serum interleukin-6 levels and symptoms of depression in patients on chronic hemodialysis. Journal of Pain and Symptom Management, 49, 578–585. [DOI] [PubMed] [Google Scholar]
- Bossola M., Vulpio C., Tazza L. (2011). Fatigue in chronic dialysis patients. Seminars in Dialysis, 24, 550–555. [DOI] [PubMed] [Google Scholar]
- Bower J. E. (2007). Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain, Behavior, and Immunity, 21, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J. E. (2014). Cancer-related fatigue: Mechanisms, risk factors, and treatments. Nature Reviews. Clinical Oncology, 11, 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Desmond K. A., Bernaards C., Rowland J. H., Meyerowitz B. E., Belin T. R. (2006). Fatigue in long-term breast carcinoma survivors. Cancer, 106, 751–758. [DOI] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Dickerson S. S., Petersen L., Aziz N., Fahey J. L. (2005). Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology, 30, 92–100. [DOI] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Irwin M. R., Kwan L., Breen E. C., Cole S. W. (2011). Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology, 29, 3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Tao M. L., Hu W., Belin T. R., Sepah S.…Aziz N. (2009). Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clinical Cancer Research, 15, 5534–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunevicius A., Gintauskiene V., Podlipskyte A., Zaliunas R., Brozaitiene J., Prange A. J., Jr, Bunevicius R. (2012). Fatigue in patients with coronary artery disease: Association with thyroid axis hormones and cortisol. Psychosomatic Medicine, 74, 848–853. [DOI] [PubMed] [Google Scholar]
- Cadden M., Arnett P. (2015). Factors associated with employment status in individuals with multiple sclerosis. International Journal of MS Care, 17, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplin B., Kumar S., Davenport A. (2011). Patients’ perspective of haemodialysis-associated symptoms. Nephrology Dialysis Transplantation, 26, 2656–2663. [DOI] [PubMed] [Google Scholar]
- Cella D., Yount S., Rothrock N., Gershon R., Cook K., Reeve B.…Rose M. (2007). The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH roadmap cooperative group during its first two years. Medical Care, 45, S3–S11. doi:10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauffier K., Salliot C., Berenbaum F., Sellam J. (2012). Effect of biotherapies on fatigue in rheumatoid arthritis: A systematic review of the literature and meta-analysis. Rheumatology (Oxford), 51, 60–68. [DOI] [PubMed] [Google Scholar]
- Coats A. J. S. (1998). Optimizing exercise training for subgroups of patients with chronic heart failure. European Heart Journal, 19, O29–O34. [PubMed] [Google Scholar]
- Cramp F., Hewlett S., Almeida C., Kirwan J. R., Choy E. H., Chalder T.…Christensen R. (2013). Non-pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database of Systematic Reviews, 8, Cd008322 doi:10.1002/14651858.CD008322.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz D. N., Mahnensmith R. L., Perazella M. A. (1997). Intradialytic hypotension: Is midodrine beneficial in symptomatic hemodialysis patients? American Journal of Kidney Disease, 30, 772–779. [DOI] [PubMed] [Google Scholar]
- Curran S. L., Andrykowski M. A., Studts J. L. (1995). Short form of the Profile of Mood States (POMS-SF): Psychometric information. Psychological Assessment, 7, 80. [Google Scholar]
- Davis M. C., Zautra A. J., Younger J., Motivala S. J., Attrep J., Irwin M. R. (2008). Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: Implications for fatigue. Brain, Behavior, and Immunity, 22, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djaldetti R., Ziv I., Achiron A., Melamed E. (1996). Fatigue in multiple sclerosis compared with chronic fatigue syndrome: A quantitative assessment. Neurology, 46, 632–635. [DOI] [PubMed] [Google Scholar]
- Druce K. L., Bhattacharya Y., Jones G. T., Macfarlane G. J., Basu N. (2016). Most patients who reach disease remission following anti-TNF therapy continue to report fatigue: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford), 55, 1786–1790. [DOI] [PubMed] [Google Scholar]
- Druce K. L., Jones G. T., Macfarlane G. J., Basu N. (2015. a). Determining pathways to improvements in fatigue in rheumatoid arthritis: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Arthritis and Rheumatology, 67, 2303–2310. [DOI] [PubMed] [Google Scholar]
- Druce K. L., Jones G. T., Macfarlane G. J., Basu N. (2015. b). Patients receiving anti-TNF therapies experience clinically important improvements in RA-related fatigue: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford), 54, 964–971. [DOI] [PubMed] [Google Scholar]
- Druce K. L., Jones G. T., Macfarlane G. J., Verstappen S. M., Basu N. (2015). The longitudinal course of fatigue in rheumatoid arthritis: Results from the Norfolk Arthritis Register. Journal of Rheumatology, 42, 2059–2065. [DOI] [PubMed] [Google Scholar]
- Fagundes C. P., Murray D. M., Hwang B. S., Gouin J.-P., Thayer J. F., Sollers J. J.…Kiecolt-Glaser J. K. (2011). Sympathetic and parasympathetic activity in cancer-related fatigue: More evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology, 36, 1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Granger B. B., Swedberg K., Ekman I. (2007). Breaking the vicious circle of fatigue in patients with chronic heart failure. Qualitative Health Research, 17, 1020–1027. [DOI] [PubMed] [Google Scholar]
- Fava G. A., Grandi S., Canestrari R., Molnar G. (1990). Prodromal symptoms in primary major depressive disorder. Journal of Affective Disorders, 19, 149–152. [DOI] [PubMed] [Google Scholar]
- Feldthusen C., Grimby-Ekman A., Forsblad-d’Elia H., Jacobsson L., Mannerkorpi K. (2016). Explanatory factors and predictors of fatigue in persons with rheumatoid arthritis: A longitudinal study. Journal of Rehabilitation Medicine, 48, 469–476. [DOI] [PubMed] [Google Scholar]
- Fink A. M., Gonzalez R. C., Lisowski T., Pini M., Fantuzzi G., Levy W. C., Piano M. R. (2012). Fatigue, inflammation, and projected mortality in heart failure. Journal of Cardiac Failure, 18, 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Koyama H., Fukuda S., Tokai H., Tajima S., Koizumi J.…Nishizawa Y. (2013). Autonomic function is associated with health-related quality of life in patients with end-stage renal disease: A case-control study. Journal of Renal Nutrition, 23, 340–347. [DOI] [PubMed] [Google Scholar]
- Fukuda S., Takashima S., Iwase M., Yamaguchi K., Kuratsune H., Watanabe Y. (2008). Development and validation of a new fatigue scale for fatigued subjects with and without chronic fatigue syndrome In Watanabe Y., Evengard B., Natelson B. H., Jason L. A., Kuratsune H. (Eds.), Fatigue science for human health (pp. 89–102). New York, NY: Springer. [Google Scholar]
- Giovannoni G., Thompson A. J., Miller D. H., Thompson E. J. (2001). Fatigue is not associated with raised inflammatory markers in multiple sclerosis. Neurology, 57, 676–681. [DOI] [PubMed] [Google Scholar]
- Goebel M. U., Czolbe F., Becker H., Janssen O. E., Schedlowski M., Limmroth V. (2005). Effects of interferon-β1a on the hypothalamic-pituitary-adrenal axis, leukocyte distribution and mood states in multiple sclerosis patients. European Neurology, 53, 182–187. [DOI] [PubMed] [Google Scholar]
- Gold S. M., Krüger S., Ziegler K. J., Krieger T., Schulz K.-H., Otte C., Heesen C. (2011). Endocrine and immune substrates of depressive symptoms and fatigue in multiple sclerosis patients with comorbid major depression. Journal of Neurology, Neurosurgery & Psychiatry, 82, 814–818. [DOI] [PubMed] [Google Scholar]
- Heesen C., Nawrath L., Reich C., Bauer N., Schulz K. H., Gold S. M. (2006). Fatigue in multiple sclerosis: An example of cytokine mediated sickness behaviour? Journal of Neurology, Neurosurgery, and Psychiatry, 77, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann-Lingen C., Binder L., Klinge M., Sander J., Schenker W., Beyermann B.…Pieske B. (2003). High plasma levels of N-terminal pro-atrial natriuretic peptide associated with low anxiety in severe heart failure. Psychosomatic Medicine, 65, 517–522. [DOI] [PubMed] [Google Scholar]
- Hewlett S., Cockshott Z., Byron M., Kitchen K., Tipler S., Pope D., Hehir M. (2005). Patients’ perceptions of fatigue in rheumatoid arthritis: Overwhelming, uncontrollable, ignored. Arthritis & Rheumatology, 53, 697–702. [DOI] [PubMed] [Google Scholar]
- Hewlett S., Nicklin J., Treharne G. J. (2008). Fatigue in musculoskeletal conditions (Topical Reviews: Reports on the Rheumatic Diseases, series 6, no. 1). Retrieved from Arthritis Research UK at http://www.arthritisresearchuk.org/health-professionals-and-students/reports/topical-reviews/topical-reviews-autumn-2008.aspx
- Horigan A. E. (2012). Fatigue in hemodialysis patients: A review of current knowledge. Journal of Pain and Symptom Management, 44, 715–724. [DOI] [PubMed] [Google Scholar]
- Horigan A., Rocchiccioli J., Trimm D. (2012). Dialysis and fatigue: Implications for nurses—A case study analysis. MEDSURG Nursing, 21, 158–163, 175. [PMC free article] [PubMed] [Google Scholar]
- Iasiukiavichene L., Vasiliauskas D. (2006). Chronic fatigue syndrome in cardiology neurohumoral changes. Kardiologiia, 46, 58–64. [PubMed] [Google Scholar]
- Iriarte J., Subirá M. L., de Castro P. (2000). Modalities of fatigue in multiple sclerosis: Correlation with clinical and biological factors. Multiple Sclerosis Journal, 6, 124–130. [DOI] [PubMed] [Google Scholar]
- Jablonski A. (2007). The multidimensional characteristics of symptoms reported by patients on hemodialysis. Nephrology Nursing Journal, 34, 29–37. [PubMed] [Google Scholar]
- Jasiukeviciene L., Vasiliauskas D., Kavoliuniene A., Marcinkeviciene J., Grybauskiene R., Grizas V., Tumyniene V. (2008). Evaluation of a chronic fatigue in patients with moderate-to-severe chronic heart failure. Medicina (Kaunas, Lithuania), 44, 366–372. [PubMed] [Google Scholar]
- Jorgensen R. (2008). Chronic fatigue: An evolutionary concept analysis. Journal of Advanced Nursing, 63, 199–207. [DOI] [PubMed] [Google Scholar]
- Jump R. L., Fifield J., Tennen H., Reisine S., Giuliano A. J. (2004). History of affective disorder and the experience of fatigue in rheumatoid arthritis. Arthritis & Rheumatology, 51, 239–245. [DOI] [PubMed] [Google Scholar]
- Junghaenel D. U., Christodoulou C., Lai J.-S., Stone A. A. (2011). Demographic correlates of fatigue in the US general population: Results from the patient-reported outcomes measurement information system (PROMIS) initiative. Journal of Psychosomatic Research, 71, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A., Van der Spek R., Lei J., Endert E., Buijs R., Fliers E. (2012). Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Molecular and Cellular Endocrinology, 349, 20–29. [DOI] [PubMed] [Google Scholar]
- Kapella M. C., Larson J. L., Patel M. K., Covey M. K., Berry J. K. (2006). Subjective fatigue, influencing variables, and consequences in chronic obstructive pulmonary disease. Nursing Research, 55, 10–17. [DOI] [PubMed] [Google Scholar]
- Keselbrener L., Akselrod S., Ahiron A., Eldar M., Barak Y., Rotstein Z. (2000). Is fatigue in patients with multiple sclerosis related to autonomic dysfunction? Clinical Autonomic Research, 10, 169–175. [DOI] [PubMed] [Google Scholar]
- Köhnlein T., Klante T., Elliott M. W., Welte T. (2001). Cardiac insufficiency and disturbed central respiratory regulation: Cheyne-stokes respiration during sleep in advanced left heart insufficiency. Pneumologie, 55, 13–20. [DOI] [PubMed] [Google Scholar]
- Koyama H., Fukuda S., Shoji T., Inaba M., Tsujimoto Y., Tabata T.…Nishizawa Y. (2010). Fatigue is a predictor for cardiovascular outcomes in patients undergoing hemodialysis. Clinical Journal of the American Society of Nephrology, 5, 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kränkel N., Adams V., Gielen S., Linke A., Erbs S., Schuler G., Hambrecht R. (2003). Differential gene expression in skeletal muscle after induction of heart failure: Impact of cytokines on protein phosphatase 2A expression. Molecular Genetics and Metabolism, 80, 262–271. [DOI] [PubMed] [Google Scholar]
- Kroencke D. C., Lynch S. G., Denney D. R. (2000). Fatigue in multiple sclerosis: Relationship to depression, disability, and disease pattern. Multiple Sclerosis Journal, 6, 131–136. [DOI] [PubMed] [Google Scholar]
- Krupp L. B. (2003). Fatigue in multiple sclerosis: Definition, pathophysiology and treatment. CNS Drugs, 17, 225–234. [DOI] [PubMed] [Google Scholar]
- Krupp L. B., Christodoulou C., Madigan D., Morgan T., Scherl W., Melville P., McIlree C. (2002). The use of inteferon-beta-1a (Avonex) and modafinil to evaluate and treat cytokine-induced fatigue in multiple sclerosis. Annals of Neurology, 52, s87. [Google Scholar]
- Krupp L. B., Elkins L. E. (2000). Fatigue and declines in cognitive functioning in multiple sclerosis. Neurology, 55, 934–939. [DOI] [PubMed] [Google Scholar]
- Krupp L. B., LaRocca N. G., Muir-Nash J., Steinberg A. D. (1989). The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology, 46, 1121–1123. [DOI] [PubMed] [Google Scholar]
- Kumar A., Singh R. B., Saxena M., Niaz M. A., Joshi S. R., Chattopadhyay P.…Fedacko J. (2007). Effect of carni Q-gel (ubiquinol and carnitine) on cytokines in patients with heart failure in the Tishcon study. Acta Cardiologica, 62, 349–354. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Frits M. L., Iannaccone C. K., Weinblatt M. E., Shadick N. A., Williams D. A., Cui J. (2014). Subgrouping of patients with rheumatoid arthritis based on pain, fatigue, inflammation, and psychosocial factors. Arthritis & Rheumatology, 66, 2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie T. A., Moser D. K., Biddle M. J., Welsh D., Bruckner G. G., Thomas D. T.…Bailey A. L. (2013). Nutrition intervention to decrease symptoms in patients with advanced heart failure. Research in Nursing and Health, 36, 120–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Shaffer M. L., Rodríguez-Colón S. M., He F., Bixler E. O., Vgontzas A. N.,…Liao D. (2011). Systemic inflammation and circadian rhythm of cardiac autonomic modulation. Autonomic Neuroscience, 162, 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Mills P. J., Rissling M., Fiorentino L., Natarajan L., Dimsdale J. E.…Ancoli-Israel S. (2012). Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain, Behavior, and Immunity, 26, 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf S. K., Weinrib A. Z., Penedo F., Russell D., DeGeest K., Costanzo E. S.…Lucci J. A., III (2008). Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. Journal of Clinical Oncology, 26, 4820–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzilli M. (2014). Heart failure: A cardiac or a systemic disease? Heart and Metabolism, 64, 8–12. [Google Scholar]
- McEwen B. S., Biron C. A., Brunson K. W., Bulloch K., Chambers W. H., Dhabhar F. S.…Spencer R. L. (1997). The role of adrenocorticoids as modulators of immune function in health and disease: Neural, endocrine and immune interactions. Brain Research Reviews, 23, 79–133. [DOI] [PubMed] [Google Scholar]
- McSweeney J. C., Cody M., O’Sullivan P., Elberson K., Moser D. K., Garvin B. J. (2003). Women’s early warning symptoms of acute myocardial infarction. Circulation, 108, 2619–2623. [DOI] [PubMed] [Google Scholar]
- Merkelbach S., Dillmann U., Kölmel C., Holz J., Müller M. (2001). Cardiovascular autonomic dysregulation and fatigue in multiple sclerosis. Multiple Sclerosis Journal, 7, 320–326. [DOI] [PubMed] [Google Scholar]
- Meyers C. A., Albitar M., Estey E. (2005). Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer, 104, 788–793. [DOI] [PubMed] [Google Scholar]
- Murtagh F. E., Addington-Hall J., Higginson I. J. (2007). The prevalence of symptoms in end-stage renal disease: A systematic review. Advances in Chronic Kidney Disease, 14, 82–99. [DOI] [PubMed] [Google Scholar]
- Paddison J. S., Effing T. W., Quinn S., Frith P. A. (2013). Fatigue in COPD: Association with functional status and hospitalisations. European Respiratory Journal, 41, 565–570. [DOI] [PubMed] [Google Scholar]
- Paradies G., Paradies V., Ruggiero F. M., Petrosillo G. (2014). Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World Journal of Gastroenterology: WJG, 20, 14205–14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno A. C., Jhund P. S., Macdonald M. R., Petrie M. C., Cleland J. G., Bohm M.…McMurray J. J. (2014). Fatigue as a predictor of outcome in patients with heart failure: Analysis of CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure). JACC. Heart Failure, 2, 187–197. [DOI] [PubMed] [Google Scholar]
- Piepoli M. F., Scott A. C., Capucci A., Coats A. J. S. (2001). Skeletal muscle training in chronic heart failure. Acta Physiologica Scandinavica, 171, 295–303. [DOI] [PubMed] [Google Scholar]
- Pollard L. C., Choy E. H., Gonzalez J., Khoshaba B., Scott D. L. (2006). Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford), 45, 885–889. [DOI] [PubMed] [Google Scholar]
- Pongratz G., Straub R. H. (2014). The sympathetic nervous response in inflammation. Arthritis Research & Therapy, 16, 504 doi:10.1186/s13075-014-0504-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. J., Moss-Morris R., Liossi C., Schlotz W. (2015). Circadian cortisol and fatigue severity in relapsing-remitting multiple sclerosis. Psychoneuroendocrinology, 56, 120–131. [DOI] [PubMed] [Google Scholar]
- Rai A., Whaley-Connell A., McFarlane S., Sowers J. R. (2006). Hyponatremia, arginine vasopressin dysregulation, and vasopressin receptor antagonism. American Journal of Nephrology, 26, 579–589. [DOI] [PubMed] [Google Scholar]
- Repping-Wuts H., Fransen J., van Achterberg T., Bleijenberg G., van Riel P. (2007). Persistent severe fatigue in patients with rheumatoid arthritis. Journal of Clinical Nursing, 16, 377–383. [DOI] [PubMed] [Google Scholar]
- Ricci J. A., Chee E., Lorandeau A. L., Berger J. (2007). Fatigue in the U.S. workforce: Prevalence and implications for lost productive work time. Journal of Occupational and Environmental Medicine, 49, 1–10. [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Carroll J. K., Ryan E. P., Mustian K. M., Fiscella K., Morrow G. R. (2007). Mechanisms of cancer-related fatigue. Oncologist, 12, 22–34. [DOI] [PubMed] [Google Scholar]
- Saligan L. N., Olson K., Filler K., Larkin D., Cramp F., Sriram Y.…Mustian K. (2015). The biology of cancer-related fatigue: A review of the literature. Supportive Care in Cancer, 23, 2461–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz P., Leuppi J. D., Bingisser R., Bodmer M., Briel M., Drescher T.…Maier S. (2015). Prospective analysis of adrenal function in patients with acute exacerbations of COPD: The Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial. European Journal of Endocrinology, 173, 19–27. [DOI] [PubMed] [Google Scholar]
- Smith O. R., Gidron Y., Kupper N., Winter J. B., Denollet J. (2009). Vital exhaustion in chronic heart failure: Symptom profiles and clinical outcome. Journal of Psychosomatic Research, 66, 195–201. [DOI] [PubMed] [Google Scholar]
- Smith O. R., Kupper N., de Jonge P., Denollet J. (2010). Distinct trajectories of fatigue in chronic heart failure and their association with prognosis. European Journal of Heart Failure, 12, 841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne G. J., Lyons A. C., Hale E. D., Goodchild C. E., Booth D. A., Kitas G. D. (2008). Predictors of fatigue over 1 year among people with rheumatoid arthritis. Psychology, Health, and Medicine, 13, 494–504. [DOI] [PubMed] [Google Scholar]
- van Hoogmoed D., Fransen J., Bleijenberg G., van Riel P. (2010). Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford), 49, 1294–1302. [DOI] [PubMed] [Google Scholar]
- Wang X. S., Shi Q., Williams L. A., Mao L., Cleeland C. S., Komaki R. R.…Liao Z. (2010). Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain, Behavior, and Immunity, 24, 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrib A. Z., Sephton S. E., DeGeest K., Penedo F., Bender D., Zimmerman B.…Lutgendorf S. K. (2010). Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer, 116, 4410–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbord S. D., Fried L. F., Arnold R. M., Fine M. J., Levenson D. J., Peterson R. A., Switzer G. E. (2005). Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. Journal of the American Society of Nephrology, 16, 2487–2494. [DOI] [PubMed] [Google Scholar]
- Witte K. K. A., Clark A. L. (2004). The effect of aspirin on the ventilatory response to exercise in chronic heart failure. European Journal of Heart Failure, 6, 745–748. [DOI] [PubMed] [Google Scholar]
- Woo K. (2000). A pilot study to examine the relationships of dyspnoea, physical activity and fatigue in patients with chronic obstructive pulmonary disease. Journal of Clinical Nursing, 9, 526–533. [DOI] [PubMed] [Google Scholar]