Abstract
Background:
A variety of methods and measures have been used to quantify oxidative stress in clinical studies related to preterm birth (PTB), and studies have reported conflicting findings. No integrative reviews have been conducted.
Objective:
To describe specific molecules used as markers of oxidative stress and methods to measure these molecules and to review the literature for associations between oxidative stress and PTB specific to these molecules.
Method:
Systematic literature searches were conducted in June 2015 and updated in 2017 in databases from the Biomedical Reference Collection: Basic Edition, including MEDLINE and clinicaltrials.gov. Articles were included if they described original research published after 2009 and compared PTB or preterm premature rupture of membranes with term birth (TB).
Results:
Abstracts (n = 3,107) were reviewed for inclusion/exclusion criteria. Of these, 308 were full-text reviewed, and 30 articles were included in this review. All were identified as nonexperimental. The most common measurements of oxidative stress were quantification of total oxidant or antioxidant status or lipid peroxidation. Studies measuring reactive oxygen species or by-products of oxidative stress reported higher levels of these molecules for preterm specimens compared to TB specimens. Studies measuring antioxidants reported lower levels for these molecules in PTB specimens. Few of the studies had inconclusive findings.
Discussion:
Findings suggest that an imbalance between oxidants and antioxidants may be associated with PTB. The measurements and findings to date limit interpretation and understanding. Research using multidimensional methods and multidisciplinary teams are necessary to advance research and practice.
Keywords: reactive oxygen species, premature birth, antioxidants
Preterm birth (PTB), defined as parturition prior to 37 weeks of gestational age, is the leading cause of morbidity and mortality in the neonatal population, affecting approximately 10% of newborns in the United States. In 2005, the economic burden of PTB was estimated at US$26.2 billion each year due to the acute care required in the newborn intensive care unit and the long-term health care and education needs in this population (Butler & Behrman, 2007). PTB occurring without a medical induction, referred to as spontaneous PTB, accounts for 80% of the PTB deliveries. The etiology of spontaneous PTB remains elusive despite advances in perinatal science and medicine.
One pathophysiologic mechanism associated with spontaneous PTB is oxidative stress, defined as the homeostatic imbalance within the reduction–oxidation (redox) environment that involves a dysregulation between oxidants and antioxidants (Sies & Jones, 2007). Reactive oxygen species (ROS) are oxygen-containing molecules and free radicals that act as oxidizing agents by removing an electron from or adding oxygen to other molecules. Endogenous ROS play a critical role in maintaining biological homeostasis and have been identified as signaling molecules in physiological and pathophysiological processes. Specifically, as Schieber and Chandel (2014) discussed, ROS can activate redox-sensitive transcription factors to regulate inflammatory cytokines or activate protein kinases to promote cell proliferation.
ROS are derived from molecular oxygen (O2) but are more reactive and damaging to cells compared to molecular O2. Excessive levels of intra- and extracellular ROS can lead to irreversible cellular damage, necrosis, and apoptosis from lipid peroxidation, protein alterations, DNA oxidation, and altered membrane ion channel activity (Burton & Jauniaux, 2011; Cross et al., 1987; Schieber & Chandel, 2014). Common ROS include superoxide (O2 • −), hydrogen peroxide (H2O2), hydroxyl radical (•OH), hydroperoxide (ROOH), and peroxyradical (ROO•). Nitric oxide (NO•) is often categorized as a ROS, but this free radical reacts with other ROS, particularly O2 • −, to form peroxynitrite (ONOO−), a reactive nitrogen species (RNS). Through nitration, or addition of a nitrogen molecule, RNS cause cellular damage comparable to that caused by oxidation. To attain equilibrium within the redox system, exogenous and endogenous antioxidants are present to counterbalance ROS/RNS. Antioxidants can prevent the formation of ROS/RNS by directly scavenging or removing ROS/RNS and/or repairing ROS/RNS-dependent cellular damage. Common antioxidants include endogenous cellular enzymes (e.g., superoxide dismutase [SOD], catalase [CAT]), small-molecule thiols (e.g., glutathione [GSH]), and dietary vitamins (e.g., vitamins C and E).
Oxidative stress has been implicated in many health complications during pregnancy, including PTB. Gaps exist in describing exact pathways in spontaneous PTB because several biological processes are likely involved, including infection, vascular placental disease, and medical indications for PTB. Identifying associations between oxidative stress measurements and perinatal outcomes has become popular, as scientists and clinicians strive to better understand the pathophysiology driving these complications associated with pregnancy. However, due to the large number of methods and measurements used to quantify oxidative stress, interpretation and synthesis of the research remains difficult. For this review, we examine the most common specific molecules described in oxidative stress mechanisms in spontaneous PTB and follow that discussion with a synthesis of findings related to those specific molecules. The purpose of this integrative review is to (1) describe specific molecules described in studies on oxidative stress (i.e., ROS/RNS, sources of ROS/RNS, antioxidants, and end products of oxidative stress used as biomarkers) and the measurements of the specific molecules in oxidative stress and (2) synthesize recent literature on associations between these specific molecules and spontaneous PTB compared to term birth (TB).
Method
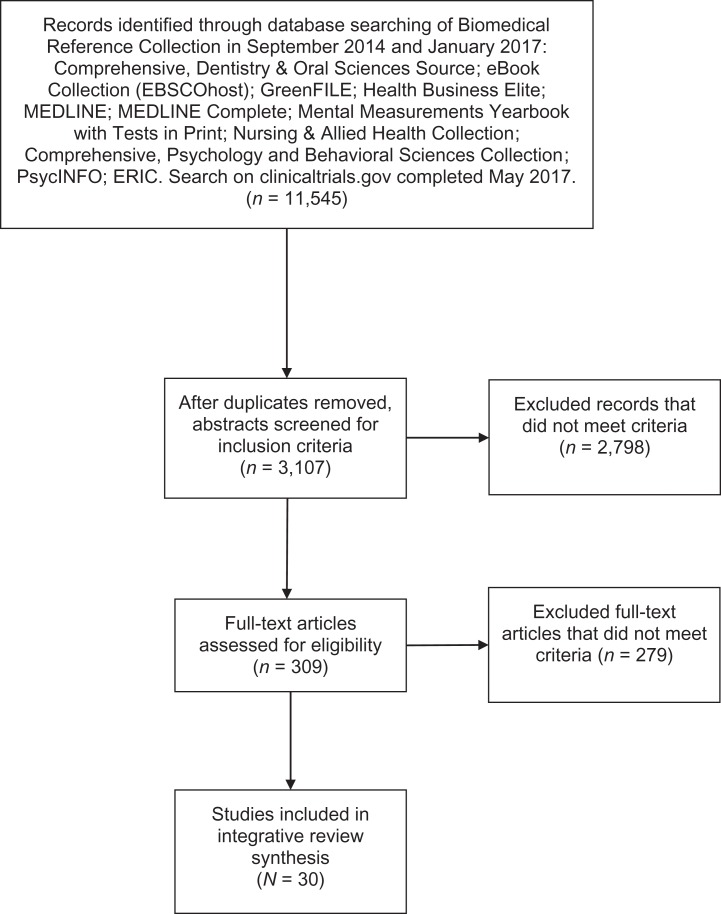
Figure 1 illustrates the process we used for selecting the articles to include in this integrative review. The search terms we used were oxidative stress, free radical, antioxidant, redox, reactive oxygen species, AND (preterm OR preterm birth OR preterm delivery OR prematurity OR premature birth OR premature delivery). Inclusion criteria were (1) original research, (2) comparison of preterm versus term delivery/birth or (3) preterm premature rupture of membranes (pPROM) compared to term delivery/birth, (4) published after 2009, and (5) written in English. Exclusion criteria were (1) neonatal complications/outcomes, (2) prenatal complications/outcomes, (3) intrauterine growth restriction, (4) animal models, (5) experiments performed ex vivo, (6) pregnancy failure/abortions/stillbirth, (7) preimplantation embryos, (8) cell lines, and (9) PTB from specific infections (e.g., malaria).
Figure 1.
Flow diagram of literature selection and review process.
We began the original review in 2014 and completed a repeat literature search in January 2017 using the same criteria but only for the years 2014–2017. In May 2017, we completed a search on clinicaltrials.gov using the search terms. The primary investigator completed abstract and full-text reviews and extracted data. A second team member independently reviewed the eligible full-text articles for inclusion/exclusion criteria. The primary investigator and the secondary reviewer independently scored the 30 eligible articles for quality assessment and risk of bias using the “Newcastle-Ottawa Quality Assessment Form for Cohort Studies” authored by Wells and colleagues (n.d.). We then converted scores to the Agency for Healthcare Research and Quality standards for “good,” “fair,” and “poor” (Hartling et al., 2012). For discrepancies concerning eligibility and/or quality rating, the research team met for final resolution.
Results
Figure 1 presents an adapted prefered reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram (Moher, Liberati, Tetzlaff, & Altman, 2009) for the current integrative review that originally began in June 2015. After removing duplicates, we reviewed abstracts for the inclusion and exclusion criteria listed above. We included 24 articles after full-text review of 181 eligible articles from the original search and an additional 6 articles after full-text review of 128 additional eligible articles from the updated 2017 search. None of the completed clinical trials fit the study criteria. Table 1 is an evidence table for the 30 articles that met study criteria. We gave a quality assessment score of “good” to seven of the articles because the control/term group was matched on a demographic variable. To the rest of the articles, we gave a quality assessment score of “poor.” All studies were Level IV evidence based on Melnyk and Fineout-Overholt’s (2011) hierarchy table of evidence indicating that the current research in this area uses nonexperimental, descriptive research designs, which limits the ability to produce conclusive evidence but is appropriate for establishing the foundation of knowledge in new areas of research. Most of the studies had sample sizes less than 100, specimen sources differed among studies (e.g., maternal venous blood, placental/membrane tissue), and researchers used varying methods and measurements to define oxidative stress. Table 2 provides further details on the variety of measurements used in the reviewed articles. We describe and qualitatively summarize the major ROS and RNS, sources of ROS and RNS, antioxidants, and end products of oxidative stress, referred to as oxidative stress biomarkers, relevant to the measurements used in PTB research below. For the purpose of this review, we defined PTB and pPROM as spontaneous events prior to 37 weeks of gestational age (GA) unless otherwise specified.
Table 1.
Evidence Table for Articles Meeting Review Criteria.
| Citation | Sample | Specimen Source(s) | Oxidative Stress Measurement(s) | Conclusion(s) | Quality Assessment |
|---|---|---|---|---|---|
| Abiaka and Machado (2012) | n = 37 late PTB (34–37 weeks of GA) and n = 37 TB (>37 weeks of GA) | Maternal venous blood during labor; umbilical cord blood | NO•, GPx, and CAT | Dysregulation of RNS and the glutathione system may play a role in the redox imbalance. | Poor |
| Alexandre-Gouabau et al. (2013) | n = 7 VLBW PTB (<32 weeks of GA and birth weight < 1,500 g) and n = 8 TB | Maternal venous blood at delivery; venous and arterial umbilical cord blood | Metabolomics | Modifications in metabolites associated with fatty acid oxidation, NO• synthesis, and antioxidant pathways may play a role in the pathophysiology of PTB. | Poor |
| Chang et al. (2013) | n = 10 pPROM and n = 10 TB | Placental tissue | Proteomics | Thiol-specific antioxidant enzymes may play a role in PTB specific to pPROM. | Good |
| Cinkaya et al. (2010) | n = 25 PTB with intact membranes and n = 25 TB | Maternal venous blood at onset of labor (PTB) or at matched gestation (TB) | TAS | A maternal and systemic redox imbalance specific to antioxidants may be related to PTB. | Poor |
| Cipierre et al. (2013) | n = 31 PTB (28–36 weeks of GA without intensive care, oxygen therapy, or medication at birth) and n = 29 TB (> 37 weeks of GA) | Arterial cord blood at birth | MDA-hemoglobin | Lipid peroxidation of polyunsaturated fatty acids may be related to PTB. | Poor |
| Clerici et al. (2012) | n = 13 nonpathological PTB (25–32 weeks of GA) and n = 15 TB | Maternal venous blood at onset of labor (PTB) or at matched gestation (TB) | TAS and TOS | A systemic imbalance of oxidants/antioxidants may be associated with PTB. | Poor |
| Dhobale et al. (2012) | n = 96 spontaneous PTB (<37 weeks of GA) and n = 94 TB (>37 weeks of GA) | Maternal venous blood at birth; venous cord blood at birth | MDA | Oxidative stress from lipid peroxidation of polyunsaturated fats may be associated with PTB. | Poor |
| Ferguson et al. (2015) | n = 130 PTB (<37 weeks of GA; 56/130 were spontaneous PTB) and n = 352 TB | Maternal urine at four time points (10, 18, 26, and 35 weeks of gestation) | 8-OHdG and 8-isoprostane | Function of DNA repair mechanisms after oxidation and increased lipid peroxidation may be associated in the pathophysiology of PTB. | Poor |
| Gunko et al. (2016) | n = 10 PTB (34–37 weeks of GA from previous “threatened premature deliveries”) and n = 10 TB | Maternal venous blood at 16–17 weeks of gestation | Proteomics | Disruption of antioxidant proteins may be related to PTB. | Poor |
| Herway et al. (2013) | n = 48 PTB (24–26 weeks of GA) and n = 66 TB (>37 weeks of GA) | Amniotic fluid collected prior to delivery and rupture of membranes | Extracellular SOD | Psychosocial factors may influence antioxidant activity associated with PTB. | Poor |
| Ilhan, Celik, and Kumbak (2015) | n = 38 pPROM (24–24 weeks of GA) and n = 34 age-matched TB | Maternal venous blood at pPROM or at matched gestation during routine care (TB) | Vitamin A, vitamin C, vitamin E, 8-isoprostane, TAS, and TOS | Systemic dysregulation of nonspecific pathways within the redox environment may be associated with PTB. | Good |
| Khan et al. (2010) | n = 11 medically induced PTB (i.e., nonlabor); n = 8 spontaneous PTB (i.e., labor); n = 13 elective cesarean TB (i.e., nonlabor); n = 9 emergency cesarean TB (i.e., labor) | Myometrial tissue at birth from cesarean deliveries | Protein expression of Cu/Zn-SOD, protein expression of CAT, protein expression of GPx, MDA, and carbonyl groups | Decreased antioxidant activity from decreased protein expression may lead to protein damage associated with oxidative stress that remains specific to labor, suggesting that different pathways exist for spontaneous PTB and medically induced PTB. | Poor |
| Lázár, Orvos, Szőllősi, and Varga (2015) | n = 22 PTB twins (i.e., 11 sets of PTB twins; <37 weeks of GA) and n = 14 TB twins (i.e., 7 sets of TB twins; > 37 weeks of GA) | Blood from umbilical vein before birth of the placenta | Cu/Zn SOD; CAT, GPx, GSH, MDA, carbonyl groups, and TAS | Systemic dysregulation of multiple antioxidants may lead to increased lipid and protein damage from oxidative stress related to PTB. Specific ROS that play a role in this dysregulation remains unclear. | Poor |
| Menon et al. (2011) | n = 134 spontaneous PTB (<37 weeks of GA) and n = 193 TB (>37 weeks of GA) | Amniotic fluid during labor for vaginal deliveries and transabdominal amniocentesis of amniotic fluid for cesarean deliveries | F2-isoprostanes | Lipid peroxidation and oxidative stress may be associated with normal parturition but may play a role in a mechanistic pathway of PTB related to interactions between race and microbial invasion in the placenta. | Poor |
| Menon, Boldogh, et al. (2014) | n = 8 PTB with intact membranes (<34 weeks of GA), n = 8 pPROM (<34 weeks of GA), and n = 8 TB | Fetal membranes dissected from the placenta | Protein staining of 3-NT | Local protein damage from oxidative stress may differ according to whether membranes are intact or ruptured. | Poor |
| Menon, Polettini, Syed, Saade, and Boldogh (2014) | n = 8 PTB with intact membranes (<34 weeks of GA), n = 8 pPROM (<34 weeks of GA), and n = 8 TB | Primary amnion epithelia cells from placentas | 8-OGG1 | Repair enzymes used to remove oxidative-damaged DNA may be dysregulated in PTB specific to pPROM. | Poor |
| Micle et al. (2012) | N = 22 at risk for PTB (24–27 weeks of GA with uterine contractions and cervical changes); n = 14 healthy pregnant women matched at gestational age (TB) | Maternal venous blood at onset of labor for PTB or at matched gestation during routine care (TB) | MDA, ceruloplasmin, and uric acid | Systemic dysregulation in ceruloplasmin may be associated with PTB. | Good |
| Minghetti et al. (2011) | n = 18 PTB (twin birth < 34 weeks of GA), n = 42 late PTB (twin birth 34–37 weeks of GA), and n = 12 TB (twin birth >37 weeks of GA) | Umbilical cord blood at birth | F2-isoprostane; TAS | Systemic antioxidant activity may be dysregulated in PTB. Results also suggest that differing pathways exist for late PTB and PTB < 34 weeks of gestation. | Poor |
| Mustafa et al. (2010) | n = 60 PTB with intact membranes (< 37 weeks of GA) and n = 63 TB (matched at maternal age) | Maternal venous blood during labor | MDA, GSH, TAS, and 8-OHdG | Dysregulation of GSH and oxidative stress may be associated with PTB. | Good |
| Ozler et al. (2016) | n = 29 pPROM (< 37 weeks of GA) and n = 29 TB (>37 weeks of GA) | Amniotic membrane tissue during delivery | TAS and TOS | A local oxidant/antioxidant imbalance may be associated with PTB specific to pPROM. | Good |
| Pathak et al. (2010) | n = 30 PTB with intact membranes (<37 weeks of GA; primiparous) and n = 30 TB (>37 weeks of GA; matched on maternal age; primiparous) | Maternal venous blood and umbilical cord blood collected after delivery | MDA, carbonyl groups, TAS, and total GSH | Results suggest that dysregulation of antioxidants and oxidative stress may be associated with PTB. | Good |
| Picone et al. (2012) | n = 40 PTB (<37 weeks of GA) and n = 76 TB (>37 weeks of GA) | Arterial umbilical cord blood at birth | Lutein | Dysregulation of micronutrient antioxidants may be related to PTB. | Poor |
| Polettini et al. (2014) | n = 8 PTB with intact membranes (<34 weeks of GA), n = 8 pPROM (< 34 weeks of GA), and n = 8 TB | Fetal membranes dissected from the placenta | mRNA expression of NOX | Local dysregulation of NOX may be associated with PTB specific to pPROM. | Poor |
| Pressman et al. (2011) | n = 28 spontaneous PTB (<37 weeks of GA), n = 9 medically induced PTB, and n = 351 TB | Amniotic fluid during genetic amniocentesis between 14 and 25 weeks of gestation | Vitamin C and TAS | A local redox imbalance during the second trimester may not be associated with PTB. | Poor |
| Rejc, Kato, Karas-Kuzelicki, Osredkar, and Gersak (2016) | n = 13 PTB (<37 weeks of GA) and n = 101 TB (>37 weeks of GA) | Urine samples collected between 15 and 26 weeks of gestation | HEL, PRL, DiY, and 3-NT | Minimal evidence supports protein damage during the second trimester associated with oxidative stress may be related to PTB. | Poor |
| Sandal et al. (2013) | n = 92 PTB (mean GA = 35 weeks) and n = 92 TB (mean GA = 38 weeks) | Mixed venous and arterial umbilical cord blood | TAS, TOS, and OSI | Systemic dysregulation of the redox system at birth may be associated with PTB. | Poor |
| Song et al. (2012) | n = 29 PTB (29–37 weeks of GA) and n = 29 TB (>37 weeks of GA) | Placental tissue after delivery | TRX-1 mRNA and TRX-1 protein levels | Local dysregulation of the TRX system within the placenta may be associated with PTB. | Poor |
| Soydinç et al. (2012) | n = 50 pPROM (26–34 weeks of GA) and n = 50 TB | Maternal venous blood collected between 26 and 34 weeks of gestation upon admission after pPROM (PTB) or at matched gestation during routine care (TB) | TOS, TAS, GPx, CAT, and PON-1 | Systemic dysregulation within the redox system may be associated with PTB specific to pPROM. | Poor |
| Soydinç et al. (2013) | n = 50 pPROM (26–34 weeks of GA) and n = 50 TB | Vaginal washing fluid collected between 26 and 34 weeks of gestation upon admission after pPROM (PTB) or at matched gestation during routine care (TB) | TOS, TAS, GPx, CAT, and PON-1 | Local dysregulation leading to higher oxidant levels and lower antioxidant levels may be associated with PTB specific to pPROM. | Good |
| Weber et al. (2014) | n = 16 cord blood and n = 21 maternal blood PTB (<37 weeks of GA), n = 135 cord blood, and n = 179 maternal blood TB (>37 weeks of GA) | Umbilical cord blood obtained at birth; maternal venous blood obtained within 24 hr before/after birth | Carbonyl groups, 3-NT, MDA, retinol, lutein, carotene (α & β), and vitamin E (α & γ) | Damaged protein from oxidative stress may be associated with dysregulation of antioxidants in the cord blood with PTB compared to TB. Results do not support association of systemic dysregulation of the redox system with PTB; however, maternal samples were taken before and after parturition, which may affect oxidative stress measurements. | Poor |
Note. 3-NT = 3-nitrotyrosine; CAT = catalase; diY = dityrosine; GA = gestational age; GPx = gluthatione peroxidase; GSH = glutathione; HEL = hexanoyl-lysine; MDA = malonialdehyde; NO• = nitric oxide; NOX = nicotinamide adenine dinucleotide phosphate oxidase; OGG1 = oxoguanine glycosylase; OHdG = hydroxydeoxyguanosine; OSI = oxidative stress index; pPROM = preterm premature rupture of membranes; PRL = propanoyl-lysine; PON = paraoxonase; PTB = preterm birth; RNS = reactive nitrogen species; ROS = reactive oxygen species; SOD = superoxide dismutase; TAS = total antioxidant status; TB = term birth; TOS = total oxidant status; TRX-1 = thioredoxin-1; VLBW = very low birth weight.
Table 2.
Methods of Measurement Used in the Reviewed Articles to Determine Oxidative Stress in Preterm Birth.
| Oxidative Stress Measurement | Method or Measurement | |
|---|---|---|
| I. ROS, RNS and sources of ROS/RNS | ||
| O• − |
|
|
| NO• |
|
|
| NOS |
|
|
| II. Antioxidants | ||
| Catalase |
|
|
| Ceruloplasmin |
|
|
| GSH |
|
|
| GPx |
|
|
| Peroxiredoxins |
|
|
| PON-1 |
|
|
| SOD |
|
|
| TRX |
|
|
| Uric acid |
|
|
| Vitamins |
|
|
| III. End products of oxidative stress damage/biomarkers | ||
| Lipids | ||
| Metabolites of lipid peroxidation |
|
|
| HEL |
|
|
| PRL |
|
|
| MDA |
|
|
| Isoprostanes |
|
|
| Proteins and oxidatively modified proteins | ||
| Dityrosine |
|
|
| 3-NT |
|
|
| DNA | ||
| 8-OHdG | ||
| OGG1 |
|
|
| TAS and TAC |
|
|
| TOS |
|
|
| OSI |
|
|
Note. 2DE MALDI-TOF MS = 2-dimensional polyacrylamide gel electrophoresis with matrix-assisted laser desorption/ionization time of flight mass spectrometry; 3-NT = 3-nitrotyrosine; 8-OHdG = 8-hydroxydeoxyguanasine; ELISA = enzyme-linked immunosorbent assay; FRAP = ferric-reducing ability of plasma; GPx = glutathione peroxidase; GSH = glutathione; HEL = hexanoyl-lysine; IHC = immunohistochemistry; LC-HRMS = liquid chromatography and high-resolution mass spectrometry; HPLC = high-performance liquid chromatography; LC-MS/MS = liquid chromatography-tandem mass spectrometer; MALDI = matrix-assisted laser desorption/ionization; MB-WCS = magnetic beads with weak cation-exchange; MDA = malondialdehyde; NO• = nitric oxide; NOS = nitric oxide synthase; O• − = superoxide; OGG1 = 8-oxoguanine glycosylase; OSI = oxidative stress index; PON-1 = paraoxonase 1; PRL = propanoyl-lysine; qRT-PCR = quantitative reverse transcriptase-polymerase chain reaction; RNS = reactive nitrogen species; ROS = reactive oxygen species; SOD = superoxide dismutase; TAC = total antioxidant capacity; TAS = total antioxidant status; TBARS = thiobarbituric acid reactive substances; TOS = total oxidant status; TRX = thioredoxin.
ROS and Sources of ROS
H2O2
H2O2 is an ROS, which is diffusible within and between cells and is more stable than free radicals because it does not have an unpaired electron. The main source of endogenous H2O2 is derived from the dismutation of O2 • − either spontaneously or through a reaction catalyzed by SOD. Enzymes such as xanthine oxidase and monoamine and D-amino acid oxidases, glucose, and ascorbate can generate H2O2. H2O2 plays an important role as a signaling molecule in common biological pathways such as vascular remodeling and immune cell activation (Bretón-Romero & Lamas, 2014). Excessive levels of H2O2, however, can be toxic to cells when it reacts with metal ions to form the •OH via the Haber–Weiss reaction and Fenton chemistry. •OH is highly reactive and is believed to be the most damaging free radical for surrounding cellular components (Burton & Jauniaux, 2011; Cross et al., 1987; Kehrer, 2000). We found no studies that specifically measured H2O2 or •OH levels; however, we do discuss studies that indirectly measured H2O2 or •OH.
ROOHs
A common ROOH discussed in the literature is lipid hydroperoxide (LOOH), a nonradical ROS intermediate derived from lipid peroxidation. Polyunsaturated fatty acids (PUFAs) are most susceptible to lipid peroxidation because of their highly reactive hydrogen atoms in the carbon–carbon double bond. Lipid peroxidation involves three distinct steps: initiation, propagation, and termination. The initiation step occurs when ROS react with lipids to form a lipid radical (L•). During the propagation phase, this unstable radical quickly reacts with O2 to yield a lipid peroxyradical (LOO•) that continues to propagate to form a new LOOH and LOO•. In excess, products of the propagation phase are believed to be cytotoxic and mutagenic to cells. LOOH also can react with metal ions to form LOO• and/or alkoxyl radical (LO•), which further exaggerates the propagation phase and exacerbates the damage from lipid peroxidation. The termination phase only occurs when two radicals conjugate and/or LOO• reacts with a chain-breaking antioxidant (e.g., vitamin E; Ayala, Muñoz, & Argüelles, 2014; Chamulitrat & Mason, 1989). We found no studies that specifically measured LOOH or LOO• levels; however, we do discuss studies that indirectly measured LOOH or LOO•.
O2 • −
O2 • − is a one-electron reduction of molecular O2 generated by cellular enzymes, particularly xanthine oxidase and nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase, or NOX. The mitochondrial electron transport chain (ETC) is a common source of O2 • −, as electrons can leak off the ETC complexes onto molecular O2. O2 • − is considered one of the most abundant ROS under physiologic conditions because of the inefficient leakage of electrons along the mitochondrial ETC (Burton & Jauniaux, 2011).
In one of the studies we reviewed, researchers measured O2 • − levels in the mitochondria but did not compare levels between PTB and TB (Polettini et al., 2014). In this same study, researchers examined the messenger RNA (mRNA) expression of NOX isoforms and found that the expression of NOX 1–5 did not differ between PTB and TB membrane tissue. However, when looking at PTB with intact membranes versus pPROM, they found that higher mRNA levels of NOX-2 were expressed in PTB membrane tissue compared to pPROM membrane tissue, while lower mRNA levels of NOX-3 were expressed in PTB membrane tissue compared to pPROM membrane tissue. Although inconclusive, these results suggest that local dysregulation of NOX may be associated with PTB specific to pPROM.
RNS and Sources of RNS
NO•
NO• is a highly diffusible messenger molecule that affects vasodilation, neurotransmission, immune responses, and genetic alterations. NO• is produced by the enzyme nitric oxide synthase (NOS). With three isoforms, NOS catalyzes the generation of endogenous NO• from L-arginine. NO• and O2 • − can react to produce peroxynitrite (ONOO−), an oxidant and nitrate that alters cellular function via nitration of tyrosine and GSH, causes lipid peroxidation, and damages DNA (Buetler, Krauskopf, & Ruegg, 2004; Burton & Jauniaux, 2011).
In one study that we reviewed, researchers found alterations in metabolite levels of NO• synthesis in maternal blood, venous cord blood, and arterial cord blood at birth in PTB versus TB (Alexandre-Gouabau et al., 2013). In another study, investigators found NO• levels measured in maternal blood during labor to be higher in mothers delivering late preterm infants (34–37 weeks of GA) compared to TB (>37 weeks of GA; Abiaka & Machado, 2012). However, they found no differences in the cord blood of PTB compared to TB, suggesting a potential role of dysregulated maternal RNS in the mechanism of late PTB. It is unclear from this single study, however, whether dysregulated systemic NO• levels were generated from mechanisms related to PTB or dysregulation of NOS.
Antioxidants
CAT
CAT is an endogenous, intracellular antioxidant enzyme expressed in most cells. It is a tetrameric ferriheme that decomposes H2O2 to form H2O and O2 (Kodydková, Vávrová, Kocík, & Žák, 2014; Pisoschi & Pop, 2015). In the studies we reviewed, researchers found no differences in CAT levels in maternal or cord blood specimens (Abiaka & Machado, 2012; Lázár, Orvos, Szőllősi, & Varga, 2015; Soydinç et al., 2012, 2013) and no changes in CAT protein expression in the myometrium tissue (Khan, Matharoo-Ball, & Shaw, 2010) between PTB and TB. However, Soydinc et al. (2013) did find lower CAT levels in the vaginal wash fluid in pPROM compared to a matched control group. Although inconclusive, combined results suggest that CAT dysregulation associated with PTB may be specific to the local vaginal redox environment.
Ceruloplasmin
Ceruloplasmin, an extracellular copper-transport protein, is categorized as an antioxidant because it functions as a ferroxidase enzyme and catalyzes the oxidation of ferrous ions (i.e., iron II) to the ferric state (i.e., iron III), thereby decreasing the potential for Haber–Weiss reactions and Fenton chemistry. Ceruloplasmin also is believed to inactivate extracellular O2 • − and maintains plasma nitrite. However, dysfunction of ceruloplasmin, speculated to be caused by ROS, leads to the accumulation of iron and copper, thus increasing the potential for Haber–Weiss reactions and Fenton chemistry (Cross et al., 1987; Jeremy & Shukla, 2014). Micle, Muresan, Antal, Bodog, and Bodog (2012) found, in one of the studies we reviewed, that ceruloplasmin levels were lower in PTB maternal blood compared to TB maternal blood. Results, however, remain inconclusive and lack insight into the source and significance of low systemic levels of ceruloplasmin.
GSH system
Glutathione peroxidase (GPx) is an intracellular selenoprotein enzyme that also reduces H2O2, yielding H2O and glutathione disulfide (GSSG). Another important role of GPx is in the detoxification of ROOH/LOOH. In conditions of excessive ROOH/LOOH, GPx utilizes reduced GSH to generate H2O, GSSG, and a corresponding stable alcohol, which is then reduced back to GSH by glutathione reductase. GSH, an intracellular cysteine tripeptide produced in the cytosol, is primarily present in cells in the reduced form (GSH) but can also be present in the oxidized form (GSSG). It is a nonenzymatic antioxidant that helps maintain active exogenous antioxidants and primarily scavenges ROOH/LOOH as described above (Deneke & Fanburg, 1989; Kemp, Go, & Jones, 2008). The ratio GSH/GSSG is often used as a marker of oxidative stress.
Three studies in the present review measured GSH in cord blood. In two of the studies, researchers reported lower levels of GSH in PTB cord blood versus TB cord blood (Abiaka & Machado, 2012; Pathak et al., 2010). In contrast, in the third study, investigators found a trend toward higher GSH levels in PTB cord blood compared to TB cord blood (Lázár et al., 2015). In a study using maternal blood during labor and cord blood at birth from late preterm infants (34–37 weeks of GA), GPx expression was lower in the maternal blood and cord blood of PTB compared to TB (Abiaka & Machado, 2012). However, GPx protein expression during labor collected in maternal blood (Soydinç et al., 2012), vaginal wash fluid (Soydinc et al., 2013), and myometrium tissue (Khan et al., 2010) did not differ between PTB and TB. Although inconclusive, results suggest that the global GSH system may be linked to PTB.
Peroxiredoxins (Prxs)
Prxs are thiol-specific antioxidant enzymes found throughout the body in six different isoforms. Prxs are believed to be essential in protecting cells against ROOH/LOOH, H2O2, and ONOO− and may be important as a signaling modulator for peroxides (Perkins, Nelson, Parsonage, Poole, & Karplus, 2015).
In one of the reviewed studies, researchers used proteomic analysis (Gunko, Pogorelova, & Linde, 2016) on maternal serum collected in the second trimester and found altered expressions of Prx-2 and Prx-3 in women with PTB compared to TB. However, in another study, Chang et al. (2013) used a different proteomic approach on placental tissue at birth and found altered expression of Prx-3 in women with pPROM compared to TB. Results suggest a potential local and/or systemic role of Prx in PTB that may be specific to pPROM. More research using consistent measurements is needed to understand the regulation of Prx expression and to identify the specific ROS involved.
Paraoxonases (PONs)
PONs are endogenous enzymes with three different subtypes based on location: PON-1 is the most abundant PON and is produced in the liver, PON-2 is an intracellular enzyme expressed in many tissues, and PON-3 is also expressed in the liver but in lesser amounts compared to PON-1. The primary role of PONs is believed to be working with high-density lipoproteins to protect against the oxidation of low-density lipoproteins, and thus, they are considered to be antiatherogenic (Draganov et al., 2005).
In the studies we reviewed, researchers found that levels of PON-1 were lower in maternal blood and the vaginal washing fluid of women with pPROM compared to a matched control group (Soydinç et al., 2012, 2013). Initial findings suggest a local and/or systemic role of PON-1 in PTB specific to pPROM.
SOD
SODs are endogenous antioxidant enzymes that catalyze the dismutation of O2 • − to H2O2 and O2. There are three forms of SOD in humans: (1) copper/zinc SOD (CuZnSOD; aka SOD1), which is primarily localized in the cytoplasm but is also expressed in the intermembrane space of mitochondria; (2) manganese SOD (MnSOD; aka SOD2), which is specifically targeted to the mitochondria matrix; and (3) extracellular SOD (EcSOD; aka SOD3), which is secreted into the extracellular space. SOD also is believed to play a major role in cell signaling, interacting with O2 • − and H2O2, and thus is critical in the redox biology of tissues and cells (Buetler et al., 2004; Cross et al., 1987).
In four of the reviewed studies, researchers measured SOD in the PTB population. Herway et al. (2013) measured EcSOD in maternal blood and did not find significant differences between PTB and TB. However, the authors reported significantly higher levels of EcSOD in African American women with PTB compared to African American women with TB, suggesting that psychosocial and/or genetic factors may influence antioxidant activity in PTB. Gunko, Pogorelova, and Linde (2016) used proteomic analysis on maternal serum collected in the second trimester and found lower expressions of Cu/Zn-SOD in women with PTB compared to women with TB. In the remaining two studies, researchers did not find differences in EcSOD levels between PTB and TB in cord blood (Lázár et al., 2015) or in the protein expression of Cu/ZnSOD in the myometrium tissue (Khan et al., 2010). These contradicting results remain inconclusive but indicate that a dysregulation of EcSOD may be associated with race/ethnicity.
Thioredoxin (Trx) system
The Trx system consists of NADPH, thioredoxin (Trx), and thioredoxin reductase (TrxR). Electrons from NADPH are removed by TrxR and transferred to Trx, a thiol-dependent antioxidant enzyme that facilitates reduction of other proteins by a cysteine thiol–disulfide exchange. Trx1 is located in the nuclei and cytoplasm, while Trx2 is primarily located in the mitochondria. TrxR alone can act as an antioxidant by providing electrons to smaller molecules (e.g., lipoic acid). The Trx system is believed to have cross talk with the GSH system such that the Trx system can reduce oxidized GSH and the GSH system can reduce oxidized Trx (Lu & Holmgren, 2014).
In one study that we reviewed, researchers reported higher TRX-1 mRNA and protein levels in PTB placental tissue compared to TB placental tissue, suggesting that dysregulation of the Trx system within the placenta may be associated with PTB (Song et al., 2012).
Uric acid
Uric acid is a nonenzymatic, low molecular weight antioxidant formed as an end product of purine metabolism. It can act as a scavenger for a variety of oxidants including O2 • −, ONOO−, NO•, and •OH and ROO•/ LOO•. Although it is an antioxidant, uric acid can cause problems when present in excess because it triggers inflammatory mechanisms via chemokine and cytokine messaging and has been associated with conditions of gout, metabolic syndrome, and diabetes (Kanbay et al., 2013).
In one study we reviewed, Micle et al. (2012) found no differences in uric acid levels in maternal blood between PTB and TB. In a different study, researchers found altered levels of uric acid metabolites in arterial cord blood of PTB compared to TB but no differences in venous cord blood or maternal blood at delivery (Alexandre-Gouabau et al., 2013). Results are inconclusive but may suggest that uric acid mechanisms may develop later in gestation and thus may be different in fetal samples.
Vitamins
Vitamins are a well-established micronutrient antioxidant source. Therefore, many vitamin supplements and combinations of vitamin supplements have been given in an effort to reduce oxidative stress and improve perinatal outcomes (Asemi, Samimi, Tabassi, Shakeri, & Esmaillzadeh, 2013; Conde-Agudelo, Romero, Kusanovic, & Hassan, 2011). The most common water-soluble vitamin discussed in the literature is vitamin C, or ascorbic acid. Levels of vitamin C are maintained exogenously through dietary consumption with little endogenous regeneration. Vitamin C exhibits antitumor mechanisms primarily because it can interact with O2 to produce H2O2 (Klingelhoeffer et al., 2012). Vitamin C has many roles in the redox environment because it is a reducing agent to (1) ROS, (2) α-tocopheroxyl radical for regeneration of α-tocopherol, and (3) transition metal reactions (e.g., Fenton chemistry). The resulting molecules from these reactions form an ascorbyl radical, which is reduced by cellular reductants (Lane & Richardson, 2014). Other water-soluble vitamins acting as antioxidants include riboflavin, thiamin-B1, riboflavin-B3, niacin, pyridoxine/vitamin B6, and folacin. Riboflavin, pyridoxine, and niacin are important in the maintenance of GSH.
Lipid-soluble vitamins include vitamins A, D, E, and certain carotenoids. Vitamin A (i.e., retinols) plays an indirect role in the redox environment through resistance and reparation during oxidative stress. The primary functions of retinols are to maintain epithelial integrity, regulate cell proliferation and growth, react with peroxyl radicals, and modulate the levels of the extracellular antioxidant ceruloplasmin (Chow, 1991; Hovdenak & Haram, 2012). Vitamin D also indirectly affects the redox environment by maintaining calcium homeostasis, thereby reducing oxidative damage. Although low levels of calcium stimulate lipid peroxidation from the release of bound ferrous ions, higher levels of calcium ions are thought to inhibit lipid peroxidation (Babizhayev, 1988; Wiseman, 1993). Vitamin E (i.e., tocopherol) is considered to be the most abundant of the antioxidants. It is lipophilic and accumulates within the lipid membranes, making it an effective scavenger of lipid peroxyl radicals (LOO•) because it can break the lipid peroxidation cycle. The reaction between vitamin E and LOO• creates a LOOH and a tocopherol radical, which may be involved in secondary reactions such as the regeneration of vitamin E from vitamin C or ubiquinol (Niki, 2014a, 2014b). Carotenoids are colorful fat-soluble pigments found in many vegetables. One specific carotenoid discussed in the reviewed literature is lutein, an exogenous nonprovitamin A, oxygenated xanthophyll. Lutein is believed to have antioxidant mechanisms that scavenge ROS and intermediates of ROS (Kaulmann & Bohn, 2014).
In the articles we reviewed, researchers found vitamin C levels to be lower in the maternal blood of women with pPROM compared to a matched control group (Ilhan, Celik, & Kumbak, 2015); however, another group of researchers reported that they found no differences in vitamin C levels in the amniotic fluid collected during the second trimester between PTB and TB (Pressman et al., 2011). Authors reported finding no differences in maternal blood levels of vitamins A and E or carotenoids between PTB and TB (Ilhan et al., 2015; Weber et al., 2014). In the cord blood, however, researchers found higher lutein levels with PTB compared to TB (Picone et al., 2012) and lower retinal levels with PTB compared to TB (Weber et al., 2014). Findings suggest that systemic dysregulation of micronutrient antioxidants may be related to PTB and warrants further investigation.
End Products of Oxidative Stress/Oxidative Stress Biomarkers
Many of the oxidative stress measurements found in the literature are categorized as biomarkers, which, as described by the National Institutes of Health, are objective measurements and methods of evaluation for biological processes (Biomarkers Definitions Working Group 2001). As previously mentioned, an imbalance in the redox environment causes damage to lipids, proteins, and DNA. Thus, researchers use oxidative stress biomarkers to attempt to evaluate and quantify oxidative stress based on the levels of by-products from lipid, protein, and DNA damage.
Lipids
The lipids found in cellular membranes are the most susceptible to oxidation and damage, termed lipid peroxidation. Molecules generated during the multiple-step process of nonenzymatic lipid peroxidation are commonly measured biomarkers of oxidative stress. As previously discussed, products of lipid peroxidation form unstable lipid hydroperoxides (LOO• and LOOH) that may become a lipid peroxide–derived modified protein or decompose to a more stable secondary product. The lipid peroxide–derived modified proteins that researchers have used to measure differences in PTB and TB include an amide adduct from the unstable lipid hydroperoxide from oxidation of an omega-6 PUFA called hexanoyl-lysine (HEL) and an amide adduct from the unstable lipid hydroperoxide from oxidation of an omega-3 PUFA called propionyl-lysine (PRL; Kato & Osawa, 2010). The more common measures of lipid peroxidation validated throughout the literature include malondialdehyde (MDA), a secondary aldehyde formed from lipid peroxidation of PUFAs, and F2-isoprostanes, prostaglandin-like compounds formed during the later stages of lipid peroxidation of PUFAs. Both MDA and F2-isoprostanes are believed to be specific to arachidonic acid peroxidation (Niki, 2014a, 2014b).
Among the articles we reviewed, researchers who conducted a metabolomics study reported alterations in metabolites related to fatty acid oxidation in maternal blood, venous cord blood, and arterial cord blood of PTB compared to TB (Alexandre-Gouabau et al., 2013). In a different study, researchers measured urinary HEL and PRL during the second trimester and did not report any significant differences in levels between PTB and TB (Rejc, Kato, Karas-Kuzelicki, Osredkar, & Gersak, 2016).
In four studies, researchers found higher levels of MDA in maternal blood of PTB compared to TB (Cipierre, Haÿs, Maucort-Boulch, Steghens, & Picaud, 2013; Dhobale, Mehendale, Pisal, Nimbargi, & Joshi, 2012; Mustafa et al., 2010; Pathak et al., 2010); however, in one, researchers did not find a significant difference between PTB and TB levels of MDA (Micle, Muresan, Antal, Bodog, & Bodog, 2012). In two of these studies, researchers also reported higher levels of MDA in cord blood of PTB compared to TB (Dhobale et al., 2012; Pathak et al., 2010). Khan, Matharoo-Ball, and Shaw (2010) found no differences in protein expression of MDA in the myometrium of PTB compared to TB tissue.
8-isoprostanes (an F2-isoprostane) were higher in the urine (Ferguson et al., 2015) and amniotic fluid (Menon et al., 2011) of women with PTB compared to women with TB, while women with pPROM (compared to a matched control group) had lower levels of 8-isoprostane in maternal blood (Ilhan et al., 2015). Interestingly, cord blood levels of F2-isoprostane in late PTB twins (34–37 weeks of gestation) were lower compared to PTB twins (<34 weeks of gestation) and TB twins, but levels did not differ between the PTB and TB twins (Minghetti et al., 2011). These inconclusive findings suggest that various oxidative stress pathways may exist for PTB based on pPROM status, local redox environment, and GA. Specific ROS and antioxidants involved in these mechanisms remain elusive.
Proteins
Biomarkers used to detect protein damage from oxidative stress are modified proteins or the amino acids themselves. One measurement of protein damage from oxidative stress that researchers used in the reviewed literature was carbonyl groups. Oxidation-damaged, or carbonylated, proteins of amino acid side chains (e.g., lysine, cysteine) form carbonyl groups, specifically ketones (-CO-) and aldehydes (-COH), upon oxidative cleavage of damaged proteins. These carbonyl groups also can form secondary to lipid peroxidation and thus may reflect lipid damage as well. Researchers consider carbonylated proteins to be a reliable measure of oxidative damage because these are stable modifications believed to be irreversible and unrepairable (Thanan et al., 2014). By-products from protein damage specific to aromatic amino acids (e.g., tyrosine) identified in the reviewed literature include (1) dityrosine, derived from the oxidation of tyrosine with HO•, and (2) 3-nitrotyrosine (3-NT), derived from tyrosine nitration where ONOO− reacts with tyrosine (Pisoschi & Pop, 2015).
In the reviewed studies, carbonyl protein levels were higher in PTB cord blood (Lázár et al., 2015; Pathak et al., 2010; Weber et al., 2014) and maternal blood (Pathak et al., 2010; Weber et al., 2014) compared to TB blood. Researchers found no differences in protein expression of carbonyl, however, in the myometrium between PTB and TB tissue (Khan et al., 2010), suggesting that protein damage from nonspecific oxidative stress may be systemic not local. Urinary dityrosine levels obtained during the second trimester were higher for PTB compared to TB (Rejc et al., 2016), but urinary 3-NT levels were not significantly different between the two groups, suggesting that a systemic dysregulation may involve •HO. However, in tissue membranes, 3-NT protein expression was higher in PTB with pPROM than PTB with intact membranes, but the differences were not significant (Menon et al., 2014). The authors also noted the pPROM and term membrane tissue displayed similar phenotypes, which differed from that of the PTB tissue with intact membranes, suggesting that nitration damage associated with PTB may be specific to membrane tissue and significant in pPROM.
DNA
The biomarker that researchers in the reviewed literature used to quantify DNA damage from oxidative stress is 8-hyroxydeoxyguanisine (8-OHdG). This commonly used, reliable biomarker measures the amount of an oxidized nucleoside that is released upon repair of the DNA by 8-oxoguanine glycosylase (OGG1). Specifically, the eighth position of guanine is most susceptible to oxidation by •HO. Upon repair of the DNA, 8-OHdG is removed and excreted out of the cell. This excised adduct of DNA then can be quantified in the blood or urine to determine the amount of DNA damage from oxidative stress (Barnes & Lindahl, 2004).
Researchers in the reviewed literature reported mixed results regarding 8-OHdG levels. In one study, researchers reported higher levels of 8-OHdG in maternal blood of PTB compared to TB (Mustafa et al., 2010); however, in another, they reported lower 8-OHdG levels in maternal urine of PTB compared to TB (Ferguson et al., 2015). In relation to repair enzymes, researchers in one study found that OGG1 expression in amnion cells did not differ between PTB and TB; however, in another, expression was higher in PTB with intact membranes compared to PTB with pPROM (Menon et al., 2014). These inconclusive findings may suggest that dysregulation of the redox environment is specific to location (i.e., membrane tissue) and may be related to PTB specific to pPROM.
Total antioxidant status (TAS)
Several studies measured TAS, also referred to as total antioxidant capacity (TAC), in maternal and neonatal specimens. TAS is considered a sum total of all of the antioxidants in the specimen. Table 2 provides the measurement methods used to quantify TAS.
PTB, compared to TB, was associated with lower TAS in maternal blood (Cinkaya et al., 2010; Clerici et al., 2012; Mustafa et al., 2010; Pathak et al., 2010; Soydinç et al., 2012, 2013) and vaginal washing fluid (Soydinc et al., 2013) in the reviewed studies. Although researchers in two studies did not find differences in cord blood TAS between PTB and TB (Lázár et al., 2015; Sandal et al., 2013), those in two other studies reported that cord blood TAS was lower with PTB compared to TB (Minghetti et al., 2011; Pathak et al., 2010). Pressman et al. (2011) found no differences in TAS in amniotic fluid between PTB and TB specimens. Ozler et al. (2016), however, found that TAS trended lower in amniotic tissue obtained at birth for women with pPROM compared to the term control group. Together these inconclusive findings suggest that a dysregulated redox environment may be associated with PTB and is location specific. It is unclear, however, whether TAS is developmental and influenced by age and/or weight of the infant.
Total oxidant status (TOS)
Similar to TAC, TOS quantifies all oxidants in a specimen using methods described in Table 2. Five studies in the reviewed literature used TOS as the oxidative stress measurement. Researchers found TOS to be higher in the maternal blood (Clerici et al., 2012; Ilhan et al., 2015; Soydinç et al., 2012, 2013) and vaginal washing fluid (Soydinc et al., 2013) in PTB compared to TB. TOS levels were significantly higher in amniotic tissue obtained at birth for women with pPROM compared to the term control group (Ozler et al., 2016). Findings collectively suggest that a local and systemic dysregulated redox environment may be associated with PTB. The specific ROS associated with this dysregulation, however, remains elusive.
Oxidative stress index (OSI)
The OSI is calculated as the ratio TOS/TAC. Researchers in one of the reviewed studies calculated the OSI (Soydinc et al., 2013) and found it to be higher in the vaginal washing fluid of women with PTB compared to those with TB, suggesting that a local, nonspecific dysregulated redox environment may be associated with PTB.
Discussion
We included 30 articles in this integrative review on oxidative stress and PTB. Studies measuring ROS or RNS or measuring by-products of oxidative stress (i.e., biomarkers) often reported higher levels in PTB specimens compared to TB specimens, while studies measuring antioxidants reported lower levels in PTB specimens compared to TB specimens. Findings suggest that an imbalance in oxidants and antioxidants exists in PTB; thus, a dysregulated redox environment may be associated with the pathophysiology of PTB. While some of the studies support a relationship between oxidative stress and PTB, other studies were inconsistent and contradicting. The variety of measures used further limits the interpretation of this body of work and the potential for understanding the oxidative stress mechanisms associated with PTB. The research designs were all nonexperimental and descriptive, meaning that the implications for clinical research and practice remain inconclusive.
The variety of different methods and protocols used to measure oxidative stress is reflected in Table 2. As Marrocco, Altieri, and Peluso (2017) discussed in a recent review, measuring specific molecules associated with oxidative stress is difficult because these molecules are extremely reactive and have very short half-lives. Thus, accurate measurement is highly dependent on the method used and how quickly the measurement is performed once the sample is obtained. Variations or errors in methodology are likely a major explanation for the differences in results between studies and groups. For example, researchers in the reviewed studies used different methods and instruments to measure the same specific molecule (i.e., SOD). Validity of these measurements is difficult to determine because the literature does not clearly identify a gold standard for most methods.
The most common methods that researchers used to identify a relationship between oxidative stress and PTB were ELISAs that quantified total oxidant/antioxidant status and other methods that quantified lipid peroxidation. Although these methods are affordable and accessible, results are very nonspecific and extremely difficult to interpret. For example, the methods used in assay kits for TAS/TAC and TOS simply measure the ability of the specimen to react to an excess of antioxidants or oxidants, respectively, without considering several background reactions that may occur during processing. These methods also do not identify specific ROS or RNS or antioxidants involved in the processes for mechanistic interpretation.
Another common measure in the reviewed studies was antioxidant levels/activity. Antioxidant measures are more helpful than the oxidative stress biomarker measures because results allow for further understanding of potential mechanisms and therapeutic interventions. It is essential, however, to measure multiple antioxidants and to include measures identifying specific ROS or RNS that may be associated with the change in antioxidant levels or activity. Including these additional measures provides a more comprehensive understanding of the biologic processes that involve the antioxidants.
In some of the reviewed studies, researchers did not find significant relationships between oxidative stress and PTB, but their findings did warrant further investigation. For example, NOX expression did not differ between PTB and TB in Polettini et al.’s study (2014), which may suggest that a different source is involved in the generation of O2 • − in this pathophysiological process. Researchers in these studies found only minimally significant differences between PTB and TB specimens for some of the scavengers/antioxidants such as the GSH system and SOD. Interpretation of these findings is challenging without the identification of levels of the ROS or RNS counterparts to further explain the dysregulation. Other considerations when interpreting the results are the specimen source (e.g., blood and placental tissue), timing of the specimen collection (e.g., first trimester and at birth), and method used to measure the specimen. For example, researchers did not find CAT to be significantly associated with PTB in blood (Abiaka & Machado, 2012; Lázár et al., 2015; Soydinç et al., 2012, 2013), but it did display dysregulation associated with PTB in the vaginal wash (Soydinç et al., 2013), suggesting a difference between local and systemic dysregulation of the redox environment.
The present review demonstrates that further classification of spontaneous PTB (i.e., pPROM vs. preterm labor) is an important consideration for analyzing and interpreting data. For example, protein expression of NOX in tissue membranes did not differ between PTB and TB, yet researchers did note differences between PTB with pPROM and PTB with intact membranes (Polettini et al., 2014). In a different study, the phenotype of pPROM membrane tissue was similar to TB membrane tissue but differed from membrane tissue from PTB with intact membranes (Menon et al., 2014). Together, these findings emphasize the point that multiple mechanistic pathways exist for PTB. Future research should examine PTB according to additional classification such as medically induced PTB versus spontaneous PTB and further categorize spontaneous PTB into pPROM versus preterm labor.
The summarized results of the reviewed studies suggest that relationships exist between oxidative stress and PTB. Mechanistic interpretation of the specific ROS or RNS and antioxidants in the multifactorial pathways leading to PTB, however, remains difficult. One major question to consider is whether a dysregulated redox environment is the result of PTB or is associated with the etiology of PTB. Understanding the complexity of PTB requires scientific rigor and transparency and advanced technologies. A major limitation of the current literature is that none of the reviewed studies used what many consider to be the gold standard for measuring free radicals, that is, electron paramagnetic resonance (EPR) spectroscopy, also known as electron spin resonance. Researchers have used this method in animal models to identify specific free radicals in a biological sample using EPR spin traps and spin probes (Hawkins & Davies, 2014). For example, biological samples can be incubated with free radical–sensitive cyclic hydroxylamine spin probes. Oxidation of these spin probes results in the formation of stable nitroxide radicals that can be detected by EPR spectroscopy. The amount of nitroxide formed is directly proportional to the concentration of the free radical in the reaction. Adding antioxidants to scavenge specific free radicals during the sample processing provides greater ability to identify the specific free radicals being measured. The concentration of free radicals in a biological sample can be determined by analyzing the amplitude of the EPR spectra.
Other advanced measurement techniques that allow for a greater understanding of mechanisms involved in PTB include those using high-throughput technologies, or omics. Omics methods are newer, relevant, and essential to advance the science and knowledge of PTB, as these techniques provide expansive and comprehensive data for interpretation. Among the articles we reviewed, Chang and colleagues (2013) used proteomics to identify differences in protein levels between women with pPROM and women with TB. They identified several proteins involved in oxidative stress and energy metabolism that were altered between the two groups of women. Alexandre-Gouabau and colleagues (2013) used metabolomics to differentiate metabolite expressions between PTB and TB. Their results suggest involvement of GSH pathways as well other pathways involving tryptophan, phenylalanine, and corticosteroids in PTB. Further research using omics techniques is warranted to identify physiologic mechanisms involved in PTB.
We conducted this integrative review to synthesize findings from recent clinical studies exploring associations between oxidative stress and PTB. One limitation of this review is our inclusion criteria. We included only recent literature (nothing older than 5 years prior to 2014, when we initiated the review), which could have biased the review toward modern technology and caused us to miss classic research published prior to 2009. Limiting studies to human studies only reduced the range of measurements included and restricted the inclusion of mechanistic findings from basic science. However, we wanted to focus the review on current research that is relevant to practitioners and researchers working in this clinical population. Another limitation of the review is the lack of statistical analyses to identify quantitative relationships. Instead, our synthesis of the literature was a qualitative review of the findings because the knowledge in this area is developing and studies are descriptive and comparative at this stage and cannot be analyzed using complex meta-analysis methods.
Future studies in this area should involve multidisciplinary teams that include basic and clinical scientists. Researchers should consider gold standard methods, such as EPR spectroscopy, to measure ROS in the clinical population. Science is advancing in the field of redox biology, with new tools and methods, such as omics technologies, being used in animal models that could be incorporated into clinical research. Multiple measurements assessing a variety of specific ROS and antioxidants involved in the imbalanced redox environment associated with PTB are needed to progress the science in this area. Other disciplines, including epidemiology and nursing, may provide further insight into the pathophysiology of PTB by examining associations with stress and psychosocial factors. Chronic stress, such as that association with race/ethnicity or socioeconomic status, is an influential factor affecting biological mechanisms and pathophysiological phenomena including PTB (Olson et al., 2015). Future research should thus incorporate a multidimensional approach for data collection and interpretation to encompass the multifactorial phenomenon of PTB.
In conclusion, in this integrative review, we described common specific molecules involved in oxidative stress and recent literature identifying relationships between oxidative stress and PTB. Researchers used a variety of measures and methods to define oxidative stress. Most of the measures used were nonspecific because they measure end products of cellular damage believed to be caused by oxidative stress. Conclusions that can be drawn from this body of research are limited, but qualitative results of the reviewed studies showed an overall increase in oxidative stress in relation to PTB. This increase in oxidative stress suggests that an altered redox environment plays a role in PTB. Descriptive and comparative studies provide the foundation for scientific evidence and are essential in the early understanding of a phenomenon, but mechanistic interpretations of the results are difficult in this early stage of research. The lack of research designs with higher levels of evidence illustrates the limited knowledge in this area of research. More studies are needed to advance knowledge and understanding of oxidative stress and PTB. Future studies in clinical research in this population should involve a multidimensional approach using a variety of methods and advanced measures for further understanding of the multiple pathways leading to PTB.
Footnotes
Author Contributions: Tiffany A. Moore contributed to conception, design, acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Iman M. Ahmad contributed to design and interpretation; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Matthew C. Zimmerman contributed to conception, design, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was supported by NIH/NINR 1K01NR014474-01 (April 15, 2014 to March 31, 2017) and the University of Nebraska Medical Center Edna Ittner grant awarded to Tiffany A Moore.
References
- Abiaka C., Machado L. (2012). Nitric oxide and antioxidant enzymes in venous and cord blood of late preterm and term Omani mothers. Sultan Qaboos University Medical Journal, 12, 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre-Gouabau M., Courant F., Moyon T., Küster A., Le Gall G., Tea I.…Darmaun D. (2013). Maternal and cord blood LC-HRMS metabolomics reveal alterations in energy and polyamine metabolism, and oxidative stress in very-low birth weight infants. Journal of Proteome Research, 12, 2764–2778. doi:10.1021/pr400122v [DOI] [PubMed] [Google Scholar]
- Asemi Z., Samimi M., Tabassi Z., Shakeri H., Esmaillzadeh A. (2013). Vitamin D supplementation affects serum high-sensitivity C-reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. Journal of Nutrition, 143, 1432–1438. doi:10.3945/jn.113.177550 [DOI] [PubMed] [Google Scholar]
- Ayala A., Muñoz M. F., Argüelles S. (2014). Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity, 2014, 360438 doi:10.1155/2014/360438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babizhayev M. A. (1988). The biphasic effect of calcium on lipid peroxidation. Archives of Biochemistry and Biophysics, 266, 446–451. [DOI] [PubMed] [Google Scholar]
- Barnes D. E., Lindahl T. (2004). Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annual Review of Genetics, 38, 445–476. doi:10.1146/annurev.genet.38.072902.092448 [DOI] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group. (2001). Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology and Therapeutics, 69, 89–95. [DOI] [PubMed] [Google Scholar]
- Bretón-Romero R., Lamas S. (2014). Hydrogen peroxide signaling in vascular endothelial cells. Redox Biology, 2, 529–534. doi:10.1016/j.redox.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetler T. M., Krauskopf A., Ruegg U. T. (2004). Role of superoxide as a signaling molecule. News in Physiological Sciences, 19, 120–123. [DOI] [PubMed] [Google Scholar]
- Burton G. J., Jauniaux E. (2011). Oxidative stress. Best Practice & Research. Clinical Obstetrics & Gynaecology, 25, 287–299. doi:10.1016/j.bpobgyn.2010.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A. S., Behrman R. E. (Eds.). (2007). Preterm birth: Causes, consequences, and prevention. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Chamulitrat W., Mason R. P. (1989). Lipid peroxyl radical intermediates in the peroxidation of polyunsaturated fatty acids by lipoxygenase. Direct electron spin resonance investigations. Journal of Biological Chemistry, 264, 20968–20973. [PubMed] [Google Scholar]
- Chang A., Zhang Z., Zhang L., Gao Y., Zhang L., Jia L.…Wang P. (2013). Proteomic analysis of preterm premature rupture of membranes in placental tissue. Archives of Gynecology and Obstetrics, 288, 775–784. doi:10.1007/s00404-013-2837–5 [DOI] [PubMed] [Google Scholar]
- Chow C. K. (1991). Vitamin E and oxidative stress. Free Radical Biology & Medicine, 11, 215–232. [DOI] [PubMed] [Google Scholar]
- Cinkaya A., Keskin H., Buyukkagnici U., Gungor T., Keskin E. A., Avsar A. F., Bilge U. (2010). Maternal plasma total antioxidant status in preterm labor. Journal of Obstetrics and Gynaecology Research, 36, 1185–1188. doi:10.1111/j.1447-0756.2010.01300.x [DOI] [PubMed] [Google Scholar]
- Cipierre C., Haÿs S., Maucort-Boulch D., Steghens J., Picaud J. (2013). Malondialdehyde adduct to hemoglobin: A new marker of oxidative stress suitable for full-term and preterm neonates. Oxidative Medicine and Cellular Longevity, 2013, 694014 doi:10.1155/2013/694014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici G., Slavescu C., Fiengo S., Kanninen T. T., Romanelli M., Biondi R., Di Renzo G. C. (2012). Oxidative stress in pathological pregnancies. Journal of Obstetrics and Gynaecology: Journal of the Institute of Obstetrics and Gynaecology, 32, 124–127. doi:10.3109/01443615.2011.637139 [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A., Romero R., Kusanovic J. P., Hassan S. S. (2011). Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. American Journal of Obstetrics and Gynecology, 204, 503–512. doi:10.1016/j.ajog.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L.…Harman D. (1987). Oxygen radicals and human disease. Annals of Internal Medicine, 107, 526–545. doi:10.7326/0003-4819-107-4-526 [DOI] [PubMed] [Google Scholar]
- Deneke S. M., Fanburg B. L. (1989). Regulation of cellular glutathione. American Journal of Physiology-Lung Cellular and Molecular Physiology, 257, 163–173. [DOI] [PubMed] [Google Scholar]
- Dhobale M., Mehendale S., Pisal H., Nimbargi V., Joshi S. (2012). Reduced maternal and cord nerve growth factor levels in preterm deliveries. International Journal of Developmental Neuroscience, 30, 99–103. doi:10.1016/j.ijdevneu.2011.12.007 [DOI] [PubMed] [Google Scholar]
- Draganov D. I., Teiber J. F., Speelman A., Osawa Y., Sunahara R., La Du B. N. (2005). Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. Journal of Lipid Research, 46, 1239–1247. [DOI] [PubMed] [Google Scholar]
- Ferguson K. K., McElrath T. F., Chen Y., Loch-Caruso R., Mukherjee B., Meeker J. D. (2015). Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. American Journal of Obstetrics and Gynecology, 212, 208, e1–e8. doi:10.1016/j.ajog.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunko V. O., Pogorelova T. N., Linde V. A. (2016). Proteomic profiling of the blood serum for prediction of premature delivery. Bulletin of Experimental Biology and Medicine, 161, 829–832. [DOI] [PubMed] [Google Scholar]
- Hartling L., Hamm M., Milne A., Vandermeer B., Santaguida P. L., Ansari M.…Dryden D. M. (2012). Validity and inter-rater reliability testing of quality assessment instruments. Rockville, MD: Agency for Healthcare Research and Quality; Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK92293/ [PubMed] [Google Scholar]
- Hawkins C. L., Davies M. J. (2014). Detection and characterisation of radicals in biological materials using EPR methodology. Biochimica Et Biophysica Acta, 1840, 708–721. doi:10.1016/j.bbagen.2013.03.034 [DOI] [PubMed] [Google Scholar]
- Herway C., Kanninen T., Witkin S. S., Saade G., Fortunato S. J., Menon R. (2013). Ethnic disparity in amniotic fluid levels of hyaluronan, histone H2B and superoxide dismutase in spontaneous preterm birth. Journal of Perinatal Medicine, 41, 277–282. doi:10.1515/jpm-2012-0189 [DOI] [PubMed] [Google Scholar]
- Hovdenak N., Haram K. (2012). Influence of mineral and vitamin supplements on pregnancy outcome. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 164, 127–132. doi:10.1016/j.ejogrb.2012.06.020 [DOI] [PubMed] [Google Scholar]
- Ilhan N., Celik E., Kumbak B. (2015). Maternal plasma levels of interleukin-6, C-reactive protein, vitamins C, E and A, 8-isoprostane and oxidative status in women with preterm premature rupture of membranes. Journal of Maternal-Fetal & Neonatal Medicine, 28, 316–319. doi:10.3109/14767058.2014.916674 [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Shukla N. (2014). Ceruloplasmin dysfunction: A key factor in the pathophysiology of atrial fibrillation? Journal of Internal Medicine, 275, 191–194. doi:10.1111/joim.12156 [DOI] [PubMed] [Google Scholar]
- Kanbay M., Segal M., Afsar B., Kang D., Rodriguez-Iturbe B., Johnson R. J. (2013). The role of uric acid in the pathogenesis of human cardiovascular disease. Heart (British Cardiac Society), 99, 759–766. doi:10.1136/heartjnl-2012-302535 [DOI] [PubMed] [Google Scholar]
- Kato Y., Osawa T. (2010). Detection of lipid-lysine amide-type adduct as a marker of PUFA oxidation and its applications. Archives of Biochemistry and Biophysics, 501, 182–187. doi:10.1016/j.abb.2010.06.010 [DOI] [PubMed] [Google Scholar]
- Kaulmann A., Bohn T. (2014). Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutrition Research, 34, 907–929. doi:10.1016/j.nutres.2014.07.010 [DOI] [PubMed] [Google Scholar]
- Kehrer J. P. (2000). The Haber–Weiss reaction and mechanisms of toxicity. Toxicology, 149, 43–50. [DOI] [PubMed] [Google Scholar]
- Kemp M., Go Y., Jones D. P. (2008). Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radical Biology & Medicine, 44, 921–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R. N., Matharoo-Ball B., Shaw R. W. (2010). Antioxidant enzyme expression, lipid peroxidation, and protein oxidation in human myometrium with parturition. Reproductive Sciences (Thousand Oaks, Calif.), 17, 78–84. doi:10.1177/1933719109348027 [DOI] [PubMed] [Google Scholar]
- Klingelhoeffer C., Kämmerer U., Koospal M., Mühling B., Schneider M., Kapp M.…Otto C. (2012). Natural resistance to ascorbic acid induced oxidative stress is mainly mediated by catalase activity in human cancer cells and catalase-silencing sensitizes to oxidative stress. BMC Complementary and Alternative Medicine, 12, 61–61. doi:10.1186/1472-6882-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodydková J., Vávrová L., Kocík M., Žák A. (2014). Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biologica, 60, 153–167. [DOI] [PubMed] [Google Scholar]
- Lane D. J. R., Richardson D. R. (2014). The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption! Free Radical Biology & Medicine, 75, 69–83. doi:10.1016/j.freeradbiomed.2014.07.007 [DOI] [PubMed] [Google Scholar]
- Lázár R., Orvos H., Szőllősi R., Varga I. S. (2015). The quality of the antioxidant defence system in term and preterm twin neonates. Redox Report: Communications in Free Radical Research, 20, 103–108. doi:10.1179/1351000214Y.0000000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Holmgren A. (2014). The thioredoxin superfamily in oxidative protein folding. Antioxidants & Redox Signaling, 21, 457–470. doi:10.1089/ars.2014.5849 [DOI] [PubMed] [Google Scholar]
- Marrocco I., Altieri F., Peluso I. (2017). Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxidative Medicine and Cellular Longevity, 2017, 6501046 doi:10.1155/2017/6501046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk B. M., Fineout-Overholt E. (2011). Evidence-based practice in nursing and healthcare: A guide to best practice. Philadelphia, PA: Lippincott, Williams & Wilkins. [Google Scholar]
- Menon R., Boldogh I., Hawkins H. K., Woodson M., Polettini J., Syed T. A.…Taylor R. N. (2014). Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. The American Journal of Pathology, 184, 1740–1751. doi:10.1016/j.ajpath.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Menon R., Fortunato S. J., Milne G. L., Brou L., Carnevale C., Sanchez S. C.…Taylor R. N. (2011). Amniotic fluid eicosanoids in preterm and term births: Effects of risk factors for spontaneous preterm labor. Obstetrics and Gynecology, 118, 121–134. doi:10.1097/AOG.0b013e3182204eaa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R., Polettini J., Syed T. A., Saade G. R., Boldogh I. (2014). Expression of 8-oxoguanine glycosylase in human fetal membranes. American Journal of Reproductive Immunology (New York, N.Y.: 1989), 72, 75–84. doi:10.1111/aji.12220 [DOI] [PubMed] [Google Scholar]
- Micle O., Muresan M., Antal L., Bodog F., Bodog A. (2012). The influence of homocysteine and oxidative stress on pregnancy outcome. Journal of Medicine and Life, 5, 68–73. [PMC free article] [PubMed] [Google Scholar]
- Minghetti L., Suppiej A., Greco A., Franzoi M., Pascoli I., Zanardo V. (2011). Oxidative stress in twin neonates is influenced by birth weight and weight discordance. Clinical Biochemistry, 44, 654–658. doi:10.1016/j.clinbiochem.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151, 264. [DOI] [PubMed] [Google Scholar]
- Mustafa M. D., Pathak R., Ahmed T., Ahmed R. S., Tripathi A. K., Guleria K., Banerjee B. D. (2010). Association of glutathione S-transferase M1 and T1 gene polymorphisms and oxidative stress markers in preterm labor. Clinical Biochemistry, 43, 1124–1128. doi:10.1016/j.clinbiochem.2010.06.018 [DOI] [PubMed] [Google Scholar]
- Niki E. (2014. a). Antioxidants: Basic principles, emerging concepts, and problems. Biomedical Journal, 37, 106–111. doi:10.4103/2319-4170.128727 [DOI] [PubMed] [Google Scholar]
- Niki E. (2014. b). Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radical Biology & Medicine, 66, 3–12. doi:10.1016/j.freeradbiomed.2013.03.022 [DOI] [PubMed] [Google Scholar]
- Olson D. M., Severson E. M., Verstraeten B. S. E., Ng J. W. Y., McCreary J. K., Metz G. A. S. (2015). Allostatic load and preterm birth. International Journal of Molecular Sciences, 16, 29856–29874. doi:10.3390/ijms161226209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozler S., Oztas E., Guler B. G., Ergin M., Uygur D., Yucel A.…Danisman N. (2016). ADAMTS4 and oxidative/antioxidative status in preterm premature rupture of membranes. Fetal and Pediatric Pathology, 35, 239–250. doi:10.1080/15513815.2016.1175529 [DOI] [PubMed] [Google Scholar]
- Pathak R., Suke S. G., Ahmed T., Ahmed R. S., Tripathi A. K., Guleria K.…Banerjee B. D. (2010). Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Human & Experimental Toxicology, 29, 351–358. doi:10.1177/0748233710363334 [DOI] [PubMed] [Google Scholar]
- Perkins A., Nelson K. J., Parsonage D., Poole L. B., Karplus P. A. (2015). Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends in Biochemical Sciences, 40, 435–445. doi:10.1016/j.tibs.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone S., Ritieni A., Fabiano A., Troise A. D., Graziani G., Paolillo P.…Gazzolo D. (2012). Arterial cord blood lutein levels in preterm and term healthy newborns are sex and gestational age dependent. Clinical Biochemistry, 45, 1558–1563. doi:10.1016/j.clinbiochem.2012.07.109 [DOI] [PubMed] [Google Scholar]
- Pisoschi A. M., Pop A. (2015). The role of antioxidants in the chemistry of oxidative stress: A review. European Journal of Medicinal Chemistry, 97, 55–74. doi:10.1016/j.ejmech.2015.04.040 [DOI] [PubMed] [Google Scholar]
- Polettini J., Silva M. G., Kacerovsky M., Syed T. A., Saade G., Menon R. (2014). Expression profiles of fetal membrane nicotinamide adenine dinucleotide phosphate oxidases (NOX) 2 and 3 differentiates spontaneous preterm birth and pPROM pathophysiologies. Placenta, 35, 188–194. doi:10.1016/j.placenta.2013.12.012 [DOI] [PubMed] [Google Scholar]
- Pressman E. K., Thornburg L. L., Glantz J. C., Earhart A., Wall P. D., Ashraf M.…Woods J. R., Jr (2011). Inflammatory cytokines and antioxidants in midtrimester amniotic fluid: Correlation with pregnancy outcome. American Journal of Obstetrics and Gynecology, 204, 155.e1–e7. doi:10.1016/j.ajog.2010.08.064 [DOI] [PubMed] [Google Scholar]
- Rejc B., Kato Y., Karas-Kuzelicki N., Osredkar J., Gersak K. (2016). Lipid-lysine adducts and modified tyrosines as markers of oxidative stress in the second trimester of pregnancy and their association with infant characteristics. Experimental and Therapeutic Medicine, 11, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandal G., Uras N., Gokmen T., Oguz S. S., Erdeve O., Dilmen U. (2013). Assessment of oxidant/antioxidant system in newborns and their breast milks. Journal of Maternal-Fetal & Neonatal Medicine, 26, 540–543. doi:10.3109/14767058.2012.717998 [DOI] [PubMed] [Google Scholar]
- Schieber M., Chandel N. S. (2014). ROS function in redox signaling and oxidative stress. Current Biology: CB, 24, R453–R462. doi:10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H., Jones D. (2007). Oxidative stress In Fink G. (Ed.), Encyclopedia of stress (2nd ed). London, England: Academic Press. [Google Scholar]
- Song J., Dong X., Chen Y., Chen G., Liang H., Nakamura H.…Bai J. (2012). The expression of thioredoxin-1 in preterm delivery placenta. Redox Report: Communications in Free Radical Research, 17, 187–193. doi:10.1179/1351000212Y.0000000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soydinç H. E., Sak M. E., Evliyaoğlu O., Evsen M. S., Turgut A., Ozler A.…Gül T. (2012). Maternal plasma prolidase, matrix metalloproteinases 1 and 13, and oxidative stress levels in pregnancies complicated by preterm premature rupture of the membranes and chorioamnionitis. Journal of the Turkish German Gynecological Association, 13, 172–177. doi:10.5152/jtgga.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soydinç H. E., Sak M. E., Evliyaoglu O., Evsen M. S., Turgut A., Özler A.…Gul T. (2013). Prolidase, matrix metalloproteinases 1 and 13 activity, oxidative-antioxidative status as a marker of preterm premature rupture of membranes and chorioamnionitis in maternal vaginal washing fluids. International Journal of Medical Sciences, 10, 1344–1351. doi:10.7150/ijms.4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanan R., Oikawa S., Hiraku Y., Ohnishi S., Ma N., Pinlaor S.…Murata M. (2014). Oxidative stress and its significant roles in neurodegenerative diseases and cancer. International Journal of Molecular Sciences, 16, 193–217. doi:10.3390/ijms16010193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D., Stuetz W., Bernhard W., Franz A., Raith M., Grune T., Breusing N. (2014). Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. European Journal of Clinical Nutrition, 68, 215–222. doi:10.1038/ejcn.2013.263 [DOI] [PubMed] [Google Scholar]
- Wells G. A, Shea B., O’Connel D., Peterson J., Welch V., Losos M., Tugwell P. (n.d.). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Retrieved from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Wiseman H. (1993). Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Letters, 326, 285–288. [DOI] [PubMed] [Google Scholar]