Abstract
The purpose of the study was to examine the interrelationships among stress, eating behavior, and adiposity in a cohort of normal- and overweight individuals. Clinical markers of physiological stress (fasting serum cortisol) and adiposity (body mass index [BMI] and percent body fat) were obtained from participants selected for a natural history protocol (n = 107). Self-reported data on eating behavior (using the Three-Factor Eating Questionnaire subscales such as Cognitive Restraint, Disinhibition, and Hunger) and psychological stress (via the Perceived Stress Scale) were evaluated. Demographic information was incorporated using principal component analysis, which revealed sex- and weight-based differences in stress, adiposity, and eating behavior measures. Following a cross-sectional and descriptive analysis, significant correlations were found between the Disinhibition and Hunger eating behavior subscales and measures of adiposity including BMI (r = .30, p = .002 and r = .20, p = .036, respectively) and percent body fat (r = .43, p = .000 and r = .22, p = .022, respectively). Relationships between stress measures and eating behavior were also evident in the analysis. Disinhibition and Hunger correlated positively with perceived stress (r = .32, p .001 and r = .26, p = .008, respectively). However, Disinhibition varied inversely with serum cortisol levels (r = −.25, p = .009). Future studies are warranted to better understand this paradox underlying the effects of perceived and physiological stress on eating behavior.
Keywords: stress, cortisol, eating behavior, weight management, nutrition
Stress is characterized by a coordination of psychological and physiological responses to disruptive or threatening chemical, emotional, physiological, or social stimuli (Dinan & Cryan, 2012; Stephens, Mahon, McCaul, & Wand, 2016). Stress induces activation of the hypothalamic–pituitary–adrenal (HPA) axis and can cause negative effects on health and cognition (Michels et al., 2014; Muraven & Baumeister, 2000; Peace et al., 2012). Activation of the HPA axis results in the production of the glucocorticoid hormone cortisol, which mediates the physiological pathways underlying circadian rhythmicity, immunological responses, and cardiovascular system function (Hermoso & Cidlowski, 2003; Tomiyama et al., 2014).
Recent research has demonstrated potential relationships between stress and a variety of other conditions including obesity and eating behavior (Lemmens, Martens, Born, Martens, & Westerterp-Plantenga, 2011; Peters, Kubera, Hubold, & Langemann, 2011; Yau & Potenza, 2013). Driven by prolonged increases in circulating cortisol, stress intensifies an individual’s appetite for energy-dense, high-caloric foods (Chao, Jastreboff, White, Grilo, & Sinha, 2017; Järvelä-Reijonen et al., 2016; Lorig, Kießl, & Laessle, 2016; Pecoraro, Reyes, Gomez, Bhargava, & Dallman, 2004). Ingestion of such “comfort” foods can suppress the release of cortisol, constituting a feedback loop wherein the consumption of these foods signals a decrease in stress upon reaching satiety (Sominsky & Spencer, 2014). However, increased consumption of these foods often results in weight gain for individuals who face stress (Torres & Nowson, 2007).
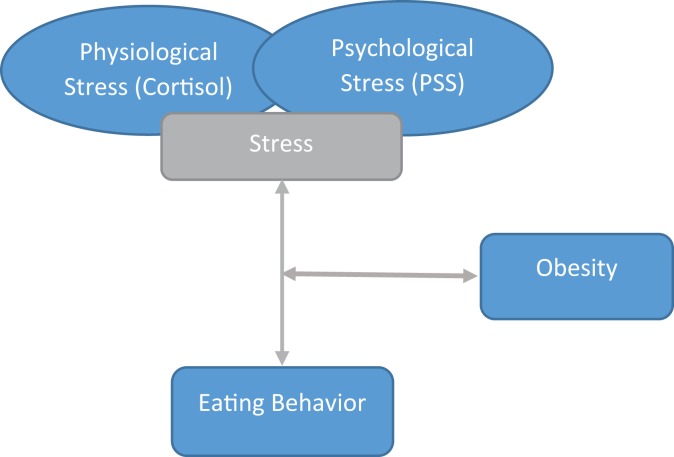
Insight into the physiological pathways underlying the effects of stress on eating behavior has paved the way for psychological research on the relationships between these variables. In these studies, researchers have often used the Three-Factor Eating Questionnaire (TFEQ), a tool that assesses hunger and an individual’s propensity for specific eating behaviors (Stunkard & Messick, 1985). Studies on stress and TFEQ-assessed eating behaviors have focused on disinhibition (characterized by a loss of control over food intake; Groesz et al., 2012) and cognitive restraint (characterized by deliberate food intake restriction; Lorig et al., 2016). In prior studies, researchers found disinhibited eating to be predictive of weight gain in women and correlated with excess body weight in both sexes (Hays et al., 2002; Järvelä-Reijonen et al., 2016; Provencher et al., 2005). However, studies that have focused on the stress and eating behavior relationship have tended to focus on such behaviors within cohorts that are largely homogenous with respect to weight; few existing studies have considered the interaction of obesity with the other variables in this relationship. Thus, in the present study, we aimed to examine this interaction by comparing the stress–eating behavior relationship between normal- and overweight individuals (Figure 1). To strengthen and validate our results, we assessed these relationships by including variables for each measure, examining both physiological and psychological stress (using circulating cortisol levels and Perceived Stress Scale [PSS] scores, respectively), eating behaviors (using the TFEQ), and measures of adiposity (including body mass index [BMI] and percent body fat).
Figure 1.
Conceptual model of biological and personal factors associated with obesity. PSS = Perceived Stress Scale.
Material and Method
Participants, Design and Setting, and Ethics
The parent study for the study we describe here aims to understand brain–gut interactions in normal- and overweight patients with chronic abdominal pain. Participants were recruited in the ongoing protocol (http://Clinicaltrial.gov #NCT00824941) at the National Institutes of Health (NIH) in Bethesda, MD, from January 2009 to December 2015 using flyers, online posts via http://clinicaltrials.gov, and referrals from health care or other NIH providers. Use of daily medication for treatment of any chronic medical condition as well as any known history of gastrointestinal, pulmonological, neurological, renal, endocrinal, or gynecological pathology excluded individuals from participation. Investigators obtained written informed consent from 127 healthy or overweight participants or their parents (participants ranged in age from 13 to 44 years). Children (ages 13–18) with the ability to read and understand assessment questionnaires provided assent in addition to parental consent. The Institutional Review Board and the Office of Human Subjects Research at the NIH approved the study.
Eligible participants fasted and attended two outpatient visits typically scheduled 24 or 48 hr apart. Female participants completed the protocol between Days 3 and 10 of their menstrual cycle to control for hormonal variation. The participants attended early morning visits (i.e., 8 a.m.) to account for cortisol, a circadian-dependent biomarker. Demographic data were assessed using the Sociodemographic Questionnaire, a 25-item instrument developed in 1999 by the Center for Research in Chronic Disorders located at the University of Pittsburgh School of Nursing, which asks participants to report their sex, age, marital status, and race. Cross-sectional analyses were conducted on data collected from biological samples and questionnaires over the two outpatient visits.
Stress
Given that elevated circulating cortisol levels are understood to be a key physiological marker of stress (Karthikeyan & Aswath, 2016; Kirschbaum & Hellhammer, 1989), we used levels of serum cortisol as a proxy for physiological stress in the present study. A phlebotomist obtained venous blood samples via fasting peripheral venipuncture on the same day participants completed the TFEQ and PSS questionnaire. Staff in the NIH Clinical Laboratory, Bethesda, MD, processed samples to evaluate fasting serum cortisol. The normal range of cortisol detected in our laboratories is 5–25 mcg/dl.
Participants also completed the PSS, which assesses the degree to which an individual appraises situations as stressful (Cohen & Janicki-Deverts, 2012). The PSS consists of 14 items, each of which respondents rank from 0 (never) to 4 (very often). Of the 14 items, 7—questions 4, 5, 6, 7, 9, 10, and 13—are “positive” and are reverse-scored accordingly (4 for 0, 3 for 1, etc.). PSS scores range from 0 to 56; higher overall scores indicate increased perceived stress. The Cronbach’s α for the scale was .84–.86 (Cohen, Kamarck, & Mermelstein, 1983), which is above the acceptable Cronbach’s (1951) cutoff of .70.
Eating Behavior
Participants completed the TFEQ—a frequently used, 51-item questionnaire that measures cognitive restraint (Factor 1), disinhibition (Factor 2), and hunger (Factor 3; Stunkard & Messick, 1985)—during Visit 1 for assessment of eating behaviors. Responses on this instrument are scored 0 or 1 and summed, with higher scores indicating greater levels of restrained eating (Cognitive Restraint), disinhibited eating (Disinhibition), and feelings of hunger (Hunger). The Cronbach’s αs reported for all three subscales range from .74 to .87 (Stunkard & Messick, 1985).
Adiposity
Clinical data were collected from NIH’s medical record system (Clinical Research Information System, NIH Clinical Center). Weight was measured in triplicate and then averaged, while height was measured in duplicate and then averaged. The averages of both height and weight were used to determine BMI (calculated as weight in kilograms divided by height in meters squared). Weight status was classified as follows: normal weight (BMI 18.5–24.9) and overweight ( BMI ≥25.0). A trained nurse completed a whole-body air displacement plethysmography (BOD POD™ Body Composition System; Life Measurement, Inc., Concord, CA) on all patients, which determined percent body fat. The BOD POD is a noninvasive body composition measure, and its results are comparable to body density measurements obtained using underwater weighing (Fields, Goran, & McCrory, 2002). The nurse tested each participants in a tight-fitting bathing suit or underwear with a swim cap using the standard recommended BOD POD protocol. Participants were asked to empty their bladder prior to testing to avoid error measures due to excess water volume. The BOD POD generates good, reliable, and reproducible measures compared to dual X-ray absorptiometry (DXA). The reported within-subjects coefficient of variation ranges between 2.0% and 3.3% for adults tested on the same day (Sardinha, Lohman, Teixeira, Guedes, & Going, 1998) or different days (Nunez et al., 1999). Researchers have compared BOD POD to other methods of body composition analysis in several studies (Ginde et al., 2005; Hames, Anthony, Thornton, Gallagher, & Goodpaster, 2014; Wagner, Heyward, & Gibson, 2000). In addition, systolic and diastolic blood pressure levels were collected both days and then averaged.
Statistical Analysis
We analyzed clinical and demographic data with Statistica Version 12.0 (StatSoft, Tulsa, OK), with p values significant at α ≤ .05. We performed exploratory analyses of the data using principal component analysis (PCA) of percent body fat, eating behaviors (Restraint, Disinhibition, and Hunger), weight, and sex. PCA is a nonparametric statistical analysis technique used to examine the interrelations among variables in a group in order to identify the underlying structure of those variables and reduce the number of variables in the data set by transforming highly correlated variables into a two-dimensional plane. This technique is independent of any hypothesis about data distribution. We then overlaid the grouping variables (weight group and sex) onto the PCA plots to further examine the association between patterns in the data and body weight and sex. For the TFEQ, we used the raw scores because they are defined as continuous variables before being transformed into categories of eating behavior.
We measured the association of eating behavior subscale measures with indices of physiological (cortisol) and psychological (PSS) stress and between PSS scores and cortisol levels using raw scores of Pearson correlations. To test whether mean eating behavior scores differed between PSS category and weight groups, we performed analysis of variance (ANOVA) and multivariate analysis of variance (MANOVA). We used a multiple regression analysis to identify the factors associated with BMI and percent body fat. We also assessed group differences (sex, weight group, and stress group) using Students t test or Mann–Whitney U test. Data are presented as mean ± standard deviation (SD) for continuous variables and counts and percentages for categorical variables. We tested for normality of data using the Kolmogorov–Smirnov test. We included the outcome measures related to stress (cortisol and PSS scores) in a general linear (multivariate) model.
Results
We included data from 107 of the 127 participants (48.6% male, mean age = 27.8 ± 7.4 years, mean BMI = 26.6 ± 5.8 kg/m2) in the analysis. For the remaining 20 participants, greater than or equal to 10% of their data were missing. Table 1 displays demographic, stress, and eating behavior statistics for the overall cohort as well as for each weight-category group. Compared to the normal-weight group (n = 52, 42.31% male, mean age = 25.03 ± 5.50 years, mean BMI = 22.08 ± 1.68 kg/m2), participants in the overweight group (n = 55, 57.69% male, mean age = 30.49 ± 8.06 years, mean BMI = 30.85 ± 5.04 kg/m2) were significantly older, t(105) = 4.07, p .001, but we found no significant differences in sex or race between weight-category groups. PSS scores and cortisol levels were negatively associated (r = −.21, p = .032). There was a positive association between Disinhibition and PSS score for both males (r = .35, p = .017) and females (r = .30, p = .025). However, there was no significant relationship between PSS score and either Cognitive Restraint or Hunger in males or females alone or when weight group was included in the analysis.
Table 1.
Demographic, Stress, and Eating Behavior Characteristics Overall and by Weight-Category Group.
| Measure | Overall Group (n = 107) | Normal Weight (n = 52) | Overweight (n = 55) | p Value |
|---|---|---|---|---|
| Demographic | ||||
| Adiposity, mean ± SD | ||||
| BMI, kg/m2 | 26.59 ± 7.42 | 22.08 ± 1.68 | 30.85 ± 5.04 | |
| Percent body fata | 28.22 ± 10.46 | 22.42 ± 7.65 | 33.79 ± 9.78 | |
| Intra-abdominal fatb, cm | 9.76 ± 2.45 | 8.21 ± 1.52 | 11.19 ± 2.28 | |
| Gender, female, n (%) | 55 (51.4) | 30 (54.55) | 25 (44.45) | |
| Race, n (%) | ||||
| Asian | 16 (14.95) | 13 (81.25) | 3 (18.75) | |
| African American | 31 (28.97) | 8 (25.81) | 23 (74.19) | |
| Caucasian | 49 (45.80) | 26 (53.01) | 23 (46.99) | |
| Mixed race/other | 11 (10.28) | 5 (41.67) | 6 (58.33) | |
| Age, years, mean ± SD | 27.84 ± 7.42 | 25.03 ± 5.50 | 30.49 ± 8.06 | .000*** |
| Physiological stress, mean ± SD (range) | ||||
| Serum cortisol | 10.38 ± 4.14 (3.30–21.70) | 11.80 ± 4.55 | 8.94 ± 3.10 | .000*** |
| Systolic BP | 117.32 ± 11.83 (93.00–143.00) | 114.48 ± 11.31 | 120.00 ± 11.78 | .013** |
| Diastolic BP | 69.62 ± 8.12 (56.00–98.00) | 68.59 ± 7.49 | 70.58 ± 8.63 | .172 |
| Psychological stress, mean ± SD | ||||
| PSS total scorec | 12.24 ± 6.56 (0.00–30.00) | 11.26 ± 6.33 | 13.20 ± 6.70 | .110 |
| Eating behavior, mean ± SD (range) | ||||
| TFEQ Restraint subscale | 8.87 ± 5.19 (0.00–21.00) | 8.58 ± 5.44 | 9.15 ± 4.98 | .462 |
| TFEQ Disinhibition subscale | 5.44 ± 3.40 (0.00–14.00) | 4.63 ± 3.20 | 6.20 ± 3.44 | .010** |
| TFEQ Hunger subscale | 4.67 ± 3.54 (0.00–14) | 4.31 ± 3.05 | 5.01 ± 3.94 | .611 |
Note. Household income is not reported here because data were missing for 14% (n = 16) of participants. BMI = body mass index; BP = blood pressure; PSS = Perceived Stress Scale; SD = standard deviation; TFEQ = Three-Factor Eating Questionnaire.
a Missing data for percent body fat: normal weight, n = 50; overweight, n = 52. bMissing data for intra-abdominal fat: normal weight, n = 47; overweight, n = 51. cMissing data for PSS total score: normal weight, n = 50; overweight, n = 51. *p ≤ .05. **p ≤ .01. ***p ≤ .001.
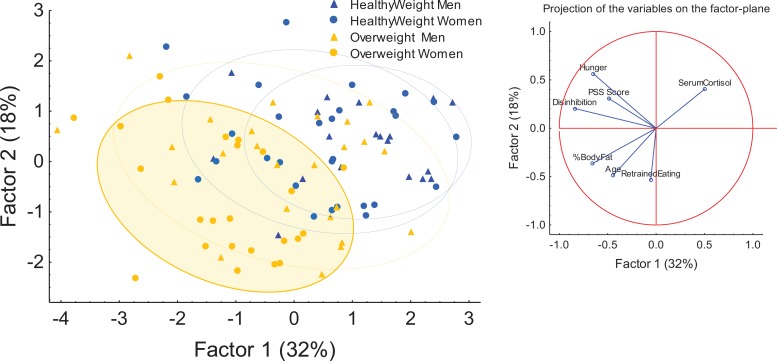
PCA
We used PCA (Figure 2) to compress the following variables: PSS score, cortisol, percent body fat, age, and TFEQ categories (cognitive restraint, disinhibition, and hunger) represented by Factors 1 and 2. This analysis demonstrated moderate separation between healthy weight and overweight individuals (Factor 1 = 18% and Factor 2 = 32%). The projection to the right of the figure denotes the variables driving the movement of different points across the factor plane of the figure. Overweight female participants were strongly polarized along the Factors 1 and 2 axis. The demonstrated effect of different variables on the pattern of distributions along the two-factor axes indicates that the variables percent body fat, Disinhibition, and Hunger drive polarization of overweight female participants along the first factor, while the variables age and cognitive restraint influenced polarization along the Factor 2 axis. Overweight female participants appeared to exhibit a unique profile for the variables used in the model, a trend indicated by the shaded yellow oval in Figure 2. For example, overweight female participants were older (M = 31.08, SD = 6.45 years) and had lower cortisol levels (M = 8.75 mcg/dl, SD = 2.95) and higher PSS (M = 13.64, SD = 5.05), Cognitive Restraint (M = 10.84, SD = 5.62), and Disinhibition scores (M = 7.04, SD = 3.36) compared to normal-weight women and all men.
Figure 2.
Principal component analysis of clinical variables in the study. Factors 1 and 2 represent condensed versions of the variables. Ovals denote patterns by gender and weight differences. The shaded oval with the solid outline represents a trend in the overweight females.
Stress and Adiposity
Significant differences in the measures of physiological stress were evident between weight-category groups: Overweight individuals had lower cortisol levels, t(105) = −3.94, p .001, and higher systolic blood pressure, t(105) = 2.47, p = .015, compared to the normal-weight group. We found no significant differences between weight-category groups with respect to PSS scores.
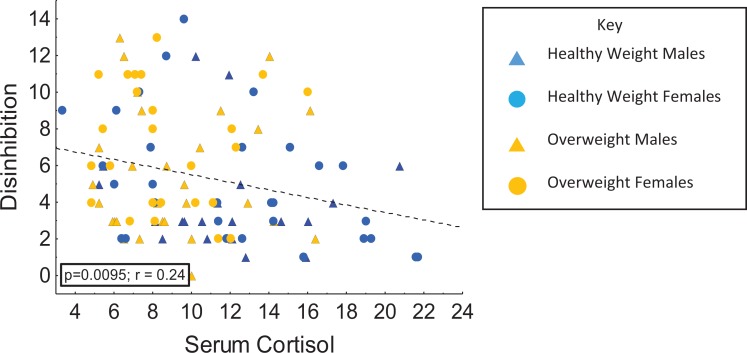
We ran multiple correlations to assess the associations among the measures of physiological and psychological stress in the overall, normal- and overweight groups. In the overall group, serum cortisol was inversely correlated with Disinhibition (r = −.25, p = .009; Figure 3) but not with the other eating behavior measures. We found similar results in the normal-weight group: a negative correlation between cortisol levels and Disinhibition (r = −.33, p = .018) but no associations between serum cortisol and other TFEQ subscales.
Figure 3.
Relationships between disinhibition and serum cortisol levels in overweight and normal-weight males and females.
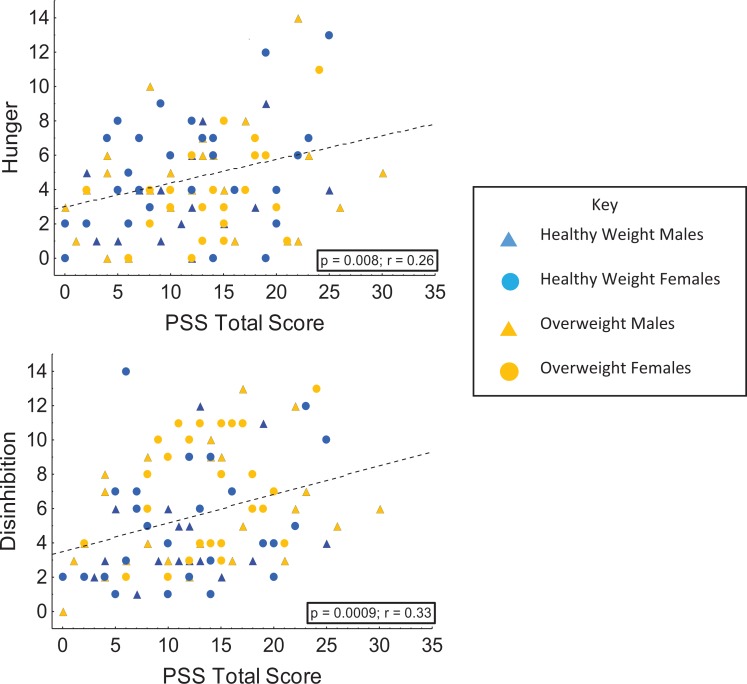
We ran multiple correlations to assess psychological stress, which was proxied by PSS scores. For the overall group, PSS was correlated with Disinhibition (r = .32, p = .00; Figure 4) and Hunger (r = .26, p = .008; Figure 4) but not with Cognitive Restraint (r = −.03, p = .786). Likewise, for the normal-weight group, PSS was correlated with Disinhibition (r = 0.32, p = .020) and Hunger (r = .33, p = .020) but not with Cognitive Restraint. For the overweight group, PSS was correlated with Disinhibition (r = .32, p = .020), but neither Cognitive Restraint nor Hunger was significantly associated with PSS. Disinhibition was a significant predictor of both BMI and percent body fat.
Figure 4.
Relationships between total score on the perceived stress scale (PSS) and both hunger and disinhibition in overweight and normal-weight males and females.
Eating Behavior and Adiposity
To examine the relationship between adiposity and eating behavior, we used two methods: (1) Student’s t tests to compare weight-category groups with respect to eating behaviors and (2) multivariate regressions run between continuous measures of adiposity (BMI and percent body fat) and eating behavior subscales. Disinhibition was positively associated with both BMI (r = .30, p = .002) and percent body fat (r = .43, p = .000). Likewise, the overweight group had significantly higher Disinhibition scores compared to the normal-weight group, F(1, 105) = 5.92, p = .017. We found no associations between Cognitive Restraint and measures of adiposity and no significant differences between normal- and overweight individuals for this subscale. Hunger was positively associated with BMI (r = .20, p = .036) and percent body fat (r = .22, p = .022); however, we found no significant differences in Hunger between the normal- and overweight groups.
We used multivariate regression models to predict BMI and percent body fat by eating behavior (TFEQ subscale scores). Disinhibition was most predictive of adiposity in both BMI and percent body fat analyses (Table 2).
Table 2.
Multiple Regression of TFEQ Factors for BMI and Percent Body Fat.
| Model for BMI | B | Standard Error β | t(103) | p Value |
|---|---|---|---|---|
| Intercept | 21.27 | 2.45 | 17.24 | .000*** |
| Cognitive Restraint | −0.53 | 0.11 | −0.50 | .620 |
| Disinhibition | 0.52 | 0.21 | 2.45 | .016** |
| Hunger | 0.00 | 0.21 | 0.00 | .100 |
| F(3, 103) = 3.57, p < .017, adjusted R 2 = .068 | ||||
| Model for Percent Body Fat | B | Standard Error β | t(103) | p Value |
| Intercept | 21.27 | 2.45 | 8.67 | .000*** |
| Cognitive Restraint | −0.53 | 0.00 | 0.19 | .100 |
| Disinhibition | 1.49 | 0.37 | 4.02 | .000*** |
| Hunger | −0.03 | 0.36 | −0.71 | .482 |
| F(3, 98) = 7.68, p < .000, adjusted R 2 = .166 | ||||
Note. BMI = body mass index; TFEQ = Three-Factor Eating Questionnaire.
**p < .01. ***p < .001.
Discussion
The aim of this study was to examine interrelationships of behavioral and physiologic measures of stress (perceived stress and serum cortisol) with eating behaviors, adiposity, and sex in a subset consisting of both normal- and overweight individuals. Our results show relationships among scores on the TFEQ subscales of Hunger and Disinhibition, perceived stress, and BMI that demonstrate differences between sexes. Our findings are consistent with preexisting research on stress and obesity where researchers did not measure eating behavior (Isasi et al., 2015; Tom & Berenson, 2013) and contribute to existing knowledge on the relationship between stress and subsequent adiposity. In the overall sample in the present study, individuals with higher perceived stress scores also had higher scores on the Disinhibition subscale of the TFEQ, indicating that their eating behaviors were more disinhibited than those of participants with lower perceived stress scores. Disinhibition was associated with BMI, percent body fat, and predicted percent body fat. Previous studies also found disinhibited eating behavior to be associated with BMI (Dykes, Brunner, Martikainen, & Wardle, 2004; Kruger, De Bray, Beck, Conlon, & Stonehouse, 2016) and percent body fat in normal- and overweight individuals (Kruger et al., 2016). Disinhibition has also been associated with high levels of energy intake (Emery, Levine, & Jakicic, 2016; Lindroos et al., 1997), which may lead to excess weight gain. Therefore, disinhibited eating behavior may be an important factor for researchers to consider when developing behavioral interventions for weight management.
Hunger also predicted BMI in our cohort and was associated with higher perceived stress scores. A closer examination of coordinated hormone response in appetite regulation (i.e., leptin and ghrelin) and stress (cortisol) is warranted. Previous studies have demonstrated that ghrelin is associated with self-reported perception of hunger in normal-weight adults (Langlois et al., 2011; Peters et al., 2011) and that individuals eat foods with higher fat and sugar content during periods of increased stress because they perceive these foods as being more rewarding (Adam & Epel, 2007; Boggiano et al., 2015; Lowe et al., 2016; Oliver & Wardle, 1999; Wallis & Hetherington, 2009; Winter et al., 2016). Thus, conditions perceived to be highly stressful may produce the sensation of hedonic hunger, which refers to eating for pleasure in the absence of current energy needs (Lowe & Butryn, 2007).
Insights on eating behavior from our data are particularly interesting for binge eating and food addiction paradigms, where disinhibited eating behavior is a key characteristic of the food addiction phenotype (Carlier, Marshe, Cmorejova, Davis, & Müller, 2015). This finding also aligns with research on the effects of cognitive load on restrained eaters (Ward & Mann, 2000), where increased cognitive load results in a shift to disinhibited eating. The perceived stress of cognitive demand (or cognitive load) thus has an effect on eating behavior. Although lower Cognitive Restraint does not necessarily imply a shift from restrained eating to disinhibited eating in our subjects, the observation that higher perceived stress scores were associated with greater tendencies toward disinhibited eating behavior prompts questions about the relationships among psychological and physical measures of stress, cognitive load, and eating behaviors (restrained, disinhibited, and hunger).
Variations among sex and weight groups also provide insight into the nature and nuance of the relationship between stress and eating behavior. Our findings demonstrate notable patterns such as healthy weight females having a positive relationship between hunger and perceived stress and overweight males having a positive association between disinhibited eating behavior and perceived stress. These results indicate the need for further research to examine differences in eating behaviors among sex and weight groups in relation to stress.
We hypothesized that individuals’ higher levels of stress would have a disinhibition phenotype for eating behavior. Our findings, however, show discordance between cortisol levels and eating behavior constructs (Disinhibition vs. physiological stress; Disinhibition vs. perceived stress). Disinhibition was inversely related to physiological stress (fasting serum cortisol levels) by positively related to psychological stress (PSS scores). This discord may be due to blunting of the HPA axis. In states of high stress, the HPA axis protects the immune system by preventing chronically high cortisol levels leading to hypocortisolism. Other studies have found low cortisol levels in patients chronically exposed to stressful environments (Dallman et al., 2003; Gunnar & Vazquez, 2001). These findings also suggest that cortisol may not be an accurate stand-alone measure for the relationship between stress and eating behavior. Cortisol is a hormone involved in the regulation of several systems beyond the coordinated stress response. Given the complexities of the physiological systems on which cortisol, eating behavior, and stress act, the discordance in our findings suggest the need to consider additional variables in order to elucidate a physical context for the conceptual framework of eating behavior as it relates to stress. While we did not study it in this experiment due to unavailability, ghrelin has been well researched in conjunction with cortisol in obese subjects as a potential mediator between stress and eating behavior (Chao et al., 2017; Geliebter, Carnell, & Gluck, 2013; Labarthe et al., 2014). In future research, investigators should study additional clinical variables such as adrenocorticotropic hormone, ghrelin, leptin, and insulin alongside cortisol to derive a clearer assessment of the physiological relationship between stress and eating behavior.
Taken as a whole, our findings indicate that nuanced frameworks for understanding the stress response have important implications for a personalized approach to nutrition. Possibilities include the incorporation of stress management techniques into nutritional and dietary support, identification of existing eating behavior type, and clinical examination of patients’ relationship to food, eating, and hunger. Existing research demonstrates that the gut and brain are involved in dialogue with one another through multiple mechanisms in both the neurological and gastrointestinal systems; however, little research incorporates the component of eating behaviors into this paradigm (Filaretova & Bagaeva, 2016). Our current findings on associations between perceived stress and eating behavior suggest a behavioral component to the brain–gut axis paradigm. Researchers should thus consider clinical presentations of stress alongside stress “symptoms” or concurrent biobehavioral manifestations of the stress response that are not conventionally associated with the stressed state. The relationship between stress and eating behaviors demands further study, particularly in the biological realm.
Our study is not without limitations. Data we obtained from the behavioral and sociodemographic questionnaires were self-reported and carry the potential for reporting bias. Participants were self-referred to the protocol used in this study. We did not have dietary data or reports of physical activity for these participants. We also did not screen participants for self-reported behaviors that could reflect increased cognitive restraint or hunger such as dieting to lose weight, disordered eating, or special dieting for food allergies. Cultural and ethnic limitations to the eating behavior tool and the PSS also need to be considered. Participants were mainly from the Washington, DC, metropolitan area, and findings are not necessarily generalizable to the general population. We did not study the effects of the immune system on stress in the study. While prior researchers have used differences in levels of a preliminary, early day measure of cortisol (typically, the time of day where diurnal cortisol is highest) as an indicator for stress (Epel et al., 2000; Pruessner et al., 2013; van Rossum, 2017), differences in the slope of changes in the levels of diurnal cortisol across the day are an ideal clinical measurement for perceived stress (Geiger, Kirschbaum, & Wolf, 2017). Further, hair cortisol analysis, a newer method for measuring long-term cortisol levels, may prove best for measuring the physiology of stress (Jackson, Kirschbaum, & Steptoe, 2017; O’Brien, Tronick, & Moore, 2013; Staufenbiel, Penninx, Spijker, Elzinga, & van Rossum, 2013). Due to the details of the natural history protocol we used in this cross-sectional study, we did not collect a second measure of serum cortisol or hair cortisol from participants. Further, the TFEQ, the self-reported measure of eating behavior we used, describes the frequency, propensity, and behaviors around the decision to eat and not the types of food consumed. Further studies examining types of food consumed—including those that involve self-reported data on food decisions—should be pursued in order to relate disinhibition and HPA-axis dysregulation to the drive for nutrient-dense foods.
Strengths of this study include use of the combination of a stress biomarker and a subjective measure of perceived stress in real time to assess their relationships with eating behaviors and their influences in adiposity. The finding that cortisol levels were negatively associated with disinhibited eating behavior scores in overweight participants is novel and interesting and raises questions about HPA axis response to stress and different eating behavior categories across normal- and overweight individuals. Future studies are warranted to investigate the associations among stress, the immune system, and gut hormones.
Conclusion
The relationships among perceived stress, eating behaviors of hunger and disinhibition, and BMI demonstrate the need for stress management in patient support, particularly in nutritional and weight loss programs. Researchers should consider incorporation of other endocrine biomarkers into future study designs, particularly ghrelin and leptin. While these hormones are not individually associated with the stress response, examination of the relationships between additional hormones and cortisol would better contextualize the physiological mechanisms of eating and stress behaviors, thus elucidating the discordance found in our results between physiological and psychological measures of stress. Understanding the relationships among these variables across normal- and overweight individuals will illuminate areas for the development of effective clinical interventions for obesity- and stress-related conditions.
Acknowledgments
The authors would like to thank the participants in the study. In addition, authors gratefully thank the staff of the Clinical Center of NIH and Dr. Kong Chen, director of Human Energy & Body Weight Regulation Core, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The authors acknowledge the support and maintenance of the Clinical Trials Database at NIH. We appreciate the comments on this article from Drs. Joan Austin and Ann Cashion.
Authors’ Note: The opinions expressed herein and the interpretation and reporting of these data are the responsibility of the author(s) and should not be seen as an official recommendation or interpretation of the National Institutes of Health. The protocol was approved by the Institutional Review Board at the National Institutes of Health. http://Clinicaltrial.gov # NCT00824941.
Author Contributions: Paule Joseph contributed to conception, design, acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Hannah Davidson contributed to analysis and interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Christina Boulineaux contributed to interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Nicolaas Fourie contributed to acquisition and analysis; drafted manuscript; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Alexis Franks contributed to interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Sarah Abey contributed to conception, design, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Wendy Henderson contributed to conception, design, acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support provided by the National Institute of Nursing Research (to WAH, 1ZIANR000018-01-07 and to PVJ, CMB, ATF, SKA, NHF, NINR, NIH, Intramural Research Training Award); Office of Workforce Diversity, NIH to PVJ and Intramural Training Awards Community College Summer Internship Program Award to HRD, NIH, DHHS, Bethesda, MD.
ORCID iD: Paule V. Joseph  http://orcid.org/0000-0002-1198-9622
http://orcid.org/0000-0002-1198-9622
Christina M. Boulineaux  http://orcid.org/0000-0002-2202-3401
http://orcid.org/0000-0002-2202-3401
Wendy A. Henderson  http://orcid.org/0000-0003-3924-7118
http://orcid.org/0000-0003-3924-7118
References
- Adam T. C., Epel E. S. (2007). Stress, eating and the reward system. Physiology & Behavior, 91, 449–458. doi:10.1016/j.physbeh.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Boggiano M. M., Wenger L. E., Turan B., Tatum M. M., Sylvester M. D., Morgan P. R.…Burgess E. E. (2015). Real-time sampling of reasons for hedonic food consumption: Further validation of the Palatable Eating Motives Scale. Frontiers in Psychology, 6, 744 doi:10.3389/fpsyg.2015.00744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier N., Marshe V. S., Cmorejova J., Davis C., Müller D. J. (2015). Genetic similarities between compulsive overeating and addiction phenotypes: A case for “food addiction”? Current Psychiatry Reports, 17, 96 doi:10.1007/s11920-015-0634-5 [DOI] [PubMed] [Google Scholar]
- Chao A. M., Jastreboff A. M., White M. A., Grilo C. M., Sinha R. (2017). Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity, 25, 713–720. doi:10.1002/oby.21790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Janicki-Deverts D. (2012). Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. Journal of Applied Social Psychology, 42, 1320–1334. [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Cronbach L. J. (1951). Coefficient alpha and the internal structure of tests. Psychometrika, 16, 297–334. doi:10.1007/bf02310555 [Google Scholar]
- Dallman M. F., Pecoraro N., Akana S. F., La Fleur S. E., Gomez F., Houshyar H.…Manalo S. (2003). Chronic stress and obesity: A new view of “comfort food.” Proceedings of the National Academy of Sciences of the U S A, 100, 11696–11701. doi:10.1073/pnas.1934666100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T. G., Cryan J. F. (2012). Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology, 37, 1369–1378. doi:10.1016/j.psyneuen.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Dykes J., Brunner E. J., Martikainen P. T., Wardle J. (2004). Socioeconomic gradient in body size and obesity among women: The role of dietary restraint, disinhibition and hunger in the Whitehall II study. International Journal of Obesity and Related Metabolic Disorders, 28, 262–268. doi:10.1038/sj.ijo.0802523 [DOI] [PubMed] [Google Scholar]
- Emery R. L., Levine M. D., Jakicic J. M. (2016). Examining the effect of binge eating and disinhibition on compensatory changes in energy balance following exercise among overweight and obese women. Eating Behaviors, 22, 10–15. doi:10.1016/j.eatbeh.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E. S., McEwen B., Seeman T., Matthews K., Castellazzo G., Brownell K. D.…Ickovics J. R. (2000). Stress and body shape: Stress-induced cortisol secretion is consistently greater among women with central fat. Psychosomatic Medicine, 62, 623–632. [DOI] [PubMed] [Google Scholar]
- Fields D. A., Goran M. I., McCrory M. A. (2002). Body-composition assessment via air-displacement plethysmography in adults and children: A review. American Journal of Clinical Nutrition, 75, 453–467. [DOI] [PubMed] [Google Scholar]
- Filaretova L., Bagaeva T. (2016). The realization of the brain-gut interactions with corticotropin-releasing factor and glucocorticoids. Current Neuropharmacology, 14, 876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger A. M., Kirschbaum C., Wolf J. M. (2017). Comparison group matters for chronic stress effects of subjective social status. Journal of Health Psychology, 1359105317709511 Advance online publication. doi:10.1177/1359105317709511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter A., Carnell S., Gluck M. E. (2013). Cortisol and ghrelin concentrations following a cold pressor stress test in overweight individuals with and without night eating. International Journal of Obesity, 37, 1104–1108. doi:10.1038/ijo.2012.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginde S. R., Geliebter A., Rubiano F., Silva A. M., Wang J., Heshka S., Heymsfield S. B. (2005). Air displacement plethysmography: Validation in overweight and obese subjects. Obesity Research, 13, 1232–1237. doi:10.1038/oby.2005.146 [DOI] [PubMed] [Google Scholar]
- Groesz L. M., McCoy S., Carl J., Saslow L., Stewart J., Adler N.…Epel E. (2012). What is eating you? Stress and the drive to eat. Appetite, 58, 717–721. doi:10.1016/j.appet.2011.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M. R., Vazquez D. M. (2001). Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology, 13, 515–538. [DOI] [PubMed] [Google Scholar]
- Hames K. C., Anthony S. J., Thornton J. C., Gallagher D., Goodpaster B. H. (2014). Body composition analysis by air displacement plethysmography in normal weight to extremely obese adults. Obesity, 22, 1078–1084. doi:10.1002/oby.20655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays N. P., Bathalon G. P., McCrory M. A., Roubenoff R., Lipman R., Roberts S. B. (2002). Eating behavior correlates of adult weight gain and obesity in healthy women aged 55–65 y. American Journal of Clinical Nutrition, 75, 476–483. [DOI] [PubMed] [Google Scholar]
- Hermoso M. A., Cidlowski J. A. (2003). Putting the brake on inflammatory responses: The role of glucocorticoids. IUBMB Life, 55, 497–504. doi:10.1080/15216540310001642072 [DOI] [PubMed] [Google Scholar]
- Isasi C. R., Parrinello C. M., Jung M. M., Carnethon M. R., Birnbaum-Weitzman O., Espinoza R. A.…Gallo L. C. (2015). Psychosocial stress is associated with obesity and diet quality in Hispanic/Latino adults. Annals of Epidemiology, 25, 84–89. doi:10.1016/j.annepidem.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. E., Kirschbaum C., Steptoe A. (2017). Hair cortisol and adiposity in a population-based sample of 2,527 men and women aged 54 to 87 years. Obesity (Silver Spring, Md.), 25, 539–544. doi:10.1002/oby.21733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvelä-Reijonen E., Karhunen L., Sairanen E., Rantala S., Laitinen J., Puttonen S.…Kolehmainen M. (2016). High perceived stress is associated with unfavorable eating behavior in overweight and obese Finns of working age. Appetite, 103, 249–258. doi:10.1016/j.appet.2016.04.023 [DOI] [PubMed] [Google Scholar]
- Karthikeyan P., Aswath N. (2016). Stress as an etiologic co-factor in recurrent aphthous ulcers and oral lichen planus. Journal of Oral Science, 58, 237–240. doi:10.2334/josnusd.15-0610 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Hellhammer D. H. (1989). Salivary cortisol in psychobiological research: An overview. Neuropsychobiology, 22, 150–169. [DOI] [PubMed] [Google Scholar]
- Kruger R., De Bray J. G., Beck K. L., Conlon C. A., Stonehouse W. (2016). Exploring the relationship between body composition and eating behavior using the Three Factor Eating Questionnaire (TFEQ) in young New Zealand women. Nutrients, 8, 386 doi:10.3390/nu8070386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarthe A., Fiquet O., Hassouna R., Zizzari P., Lanfumey L., Ramoz N.…Tolle V. (2014). Ghrelin-derived peptides: A link between appetite/reward, GH axis, and psychiatric disorders? Frontiers in Endocrinology, 5, 163 doi:10.3389/fendo.2014.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois F., Langlois M. F., Carpentier A. C., Brown C., Lemieux S., Hivert M. F. (2011). Ghrelin levels are associated with hunger as measured by the Three-Factor Eating Questionnaire in healthy young adults. Physiology & Behavior, 104, 373–377. doi:10.1016/j.physbeh.2011.04.013 [DOI] [PubMed] [Google Scholar]
- Lemmens S. G., Martens E. A., Born J. M., Martens M. J., Westerterp-Plantenga M. S. (2011). Lack of effect of high-protein vs. high-carbohydrate meal intake on stress-related mood and eating behavior. Nutrition Journal, 10, 136 doi:10.1186/1475-2891-10-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroos A. K., Lissner L., Mathiassen M. E., Karlsson J., Sullivan M., Bengtsson C., Sjostrom L. (1997). Dietary intake in relation to restrained eating, disinhibition, and hunger in obese and nonobese Swedish women. Obesity Research, 5, 175–182. [DOI] [PubMed] [Google Scholar]
- Lorig F., Kießl G. R. R., Laessle R. G. (2016). Stress-related cortisol response and laboratory eating behavior in obese women. Eating and Weight Disorders—Studies on Anorexia, Bulimia and Obesity, 21, 237–243. doi:10.1007/s40519-015-0190-3 [DOI] [PubMed] [Google Scholar]
- Lowe M. R., Arigo D., Butryn M. L., Gilbert J. R., Sarwer D., Stice E. (2016). Hedonic hunger prospectively predicts onset and maintenance of loss of control eating among college women. Health Psychology, 35, 238–244. doi:10.1037/hea0000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M. R., Butryn M. L. (2007). Hedonic hunger: A new dimension of appetite? Physiology & Behavior, 91, 432–439. doi:10.1016/j.physbeh.2007.04.006 [DOI] [PubMed] [Google Scholar]
- Michels N., Sioen I., Boone L., Clays E., Vanaelst B., Huybrechts I., De Henauw S. (2014). Cross-lagged associations between children’s stress and adiposity: The Children’s Body Composition and Stress study. Psychosomatic Medicine, 77, 50–58. [DOI] [PubMed] [Google Scholar]
- Muraven M., Baumeister R. F. (2000). Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin, 126, 247–259. [DOI] [PubMed] [Google Scholar]
- Nunez C., Kovera A. J., Pietrobelli A., Heshka S., Horlick M., Kehayias J. J.…Heymsfield S. B. (1999). Body composition in children and adults by air displacement plethysmography. European Journal of Clinical Nutrition, 53, 382–387. [DOI] [PubMed] [Google Scholar]
- O’Brien K. M., Tronick E. Z., Moore C. L. (2013). Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress and Health, 29, 337–344. doi:10.1002/smi.2475 [DOI] [PubMed] [Google Scholar]
- Oliver G., Wardle J. (1999). Perceived effects of stress on food choice. Physiology & Behavior, 66, 511–515. [DOI] [PubMed] [Google Scholar]
- Peace R. M., Majors B. L., Patel N. S., Wang D., Valle-Pinero A. Y., Martino A. C., Henderson W. A. (2012). Stress and gene expression of individuals with chronic abdominal pain. Biological Research for Nursing, 14, 405–411. doi:10.1177/1099800412458350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro N., Reyes F., Gomez F., Bhargava A., Dallman M. F. (2004). Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology, 145, 3754–3762. doi:10.1210/en.2004-0305 [DOI] [PubMed] [Google Scholar]
- Peters A., Kubera B., Hubold C., Langemann D. (2011). The selfish brain: Stress and eating behavior. Frontiers in Neuroscience, 5, 74 doi:10.3389/fnins.2011.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher V., Perusse L., Bouchard L., Drapeau V., Bouchard C., Rice T.…Lemieux S. (2005). Familial resemblance in eating behaviors in men and women from the Quebec Family Study. Obesity Research, 13, 1624–1629. doi:10.1038/oby.2005.199 [DOI] [PubMed] [Google Scholar]
- Pruessner M., Béchard-Evans L., Boekestyn L., Iyer S. N., Pruessner J. C., Malla A. K. (2013). Attenuated cortisol response to acute psychosocial stress in individuals at ultra-high risk for psychosis. Schizophrenia Research, 146, 79–86. doi:10.1016/j.schres.2013.02.019 [DOI] [PubMed] [Google Scholar]
- Sardinha L. B., Lohman T. G., Teixeira P. J., Guedes D. P., Going S. B. (1998). Comparison of air displacement plethysmography with dual-energy X-ray absorptiometry and 3 field methods for estimating body composition in middle-aged men. American Journal of Clinical Nutrition, 68, 786–793. [DOI] [PubMed] [Google Scholar]
- Sominsky L., Spencer S. J. (2014). Eating behavior and stress: A pathway to obesity. Frontiers in Psychology, 5, 434 doi:10.3389/fpsyg.2014.00434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel S. M., Penninx B. W. J. H., Spijker A. T., Elzinga B. M., van Rossum E. F. C. (2013). Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology, 38, 1220–1235. doi:10.1016/j.psyneuen.2012.11.015 [DOI] [PubMed] [Google Scholar]
- Stephens M. A. C., Mahon P. B., McCaul M. E., Wand G. S. (2016). Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology, 66, 47–55. doi:10.1016/j.psyneuen.2015.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard A. J., Messick S. (1985). The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research, 29, 71–83. [DOI] [PubMed] [Google Scholar]
- Tom S. E., Berenson A. B. (2013). Associations between poor sleep quality and psychosocial stress with obesity in reproductive-age women of lower socioeconomic status. Women’s Health Issues, 23, e295–e300. doi:10.1016/j.whi.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama A. J., Epel E. S., McClatchey T. M., Poelke G., Kemeny M. E., McCoy S. K., Daubenmier J. (2014). Associations of weight stigma with cortisol and oxidative stress independent of adiposity. Health Psychology, 33, 862–867. doi:10.1037/hea0000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres S. J., Nowson C. A. (2007). Relationship between stress, eating behavior, and obesity. Nutrition, 23, 887–894. doi:10.1016/j.nut.2007.08.008 [DOI] [PubMed] [Google Scholar]
- van Rossum E. F. C. (2017). Obesity and cortisol: New perspectives on an old theme. Obesity (Silver Spring, Md.), 25, 500–501. doi:10.1002/oby.21774 [DOI] [PubMed] [Google Scholar]
- Wagner D. R., Heyward V. H., Gibson A. L. (2000). Validation of air displacement plethysmography for assessing body composition. Medicine and Science in Sports and Exercise, 32, 1339–1344. [DOI] [PubMed] [Google Scholar]
- Wallis D. J., Hetherington M. M. (2009). Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite, 52, 355–362. doi:10.1016/j.appet.2008.11.007 [DOI] [PubMed] [Google Scholar]
- Ward A., Mann T. (2000). Don’t mind if I do: Disinhibited eating under cognitive load. Journal of Personality and Social Psychology, 78, 753–763. doi:10.1037/0022-3514.78.4.753 [DOI] [PubMed] [Google Scholar]
- Winter S. R., Feig E. H., Kounios J., Erickson B., Berkowitz S., Lowe M. R. (2016). The relation of hedonic hunger and restrained eating to lateralized frontal activation. Physiology & Behavior, 163, 64–69. doi:10.1016/j.physbeh.2016.04.050 [DOI] [PubMed] [Google Scholar]
- Yau Y. H., Potenza M. N. (2013). Stress and eating behaviors. Minerva Endocrinologica, 38, 255–267. [PMC free article] [PubMed] [Google Scholar]