Abstract
Problem:
Incorporating biomarkers of chronic stress into pediatric research studies may help to explicate the links between exposure to adversity and lifelong health, but there are currently very few parameters to guide nurse researchers in choosing appropriate biomarkers of chronic stress for use in research with children and adolescents.
Methods:
Biomarkers of chronic stress are described, including primary mediators (glucocorticoids, catecholamines, and cytokines) and secondary outcomes (neurologic, immune, metabolic, cardiovascular, respiratory, and anthropometric) of the chronic stress response.
Results:
Evidence of the use of each biomarker in pediatric research studies is reviewed. Recommendations for pediatric researchers, including selection of appropriate biomarkers, measurement considerations, potential moderators, and future directions for research, are presented.
Discussion:
A wide range of biomarkers is available for use in research studies with children. While primary mediators of chronic stress have been frequently measured in studies of children, measurement of secondary outcomes, particularly immune and metabolic biomarkers, has been limited. With thoughtful and theoretically based approaches to selection and measurement, these biomarkers present an important opportunity to further explore the physiologic pathways linking exposure to chronic stress with later health and disease.
Conclusion:
The incorporation of chronic stress biomarkers into pediatric research studies may provide valuable insight into the mechanisms through which stressful environments “get under the skin” and ultimately inform efforts to promote health and reduce inequities among children exposed to adversity.
Keywords: stress, allostasis, biomarkers, child health, vulnerable populations
Exposure to chronic stress can lead to brain alterations and physiological disruptions that impact health and developmental outcomes across the life course (S. B. Johnson, Riley, Granger, & Riis, 2013; McEwen, 2012; Shonkoff et al., 2012). This exposure can be particularly harmful for children because vulnerability to the effects of chronic stress is clearly heightened during sensitive and critical periods in the prenatal, early childhood, and adolescent stages of development (Andersen, 2003; Fox, Levitt, & Nelson, 2010). Understanding the physiological pathways through which the physical and social environments “get under the skin” is a crucial step toward promoting health and reducing health inequities among children exposed to stressful environments (Garner, Shonkoff, Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, & Section on Developmental and Behavioral Pediatrics, 2012; Hertzman & Boyce, 2010; McEwen, 2012). Integration of biological markers into pediatric research is one approach that may help to further explicate the pathways that link exposure to chronic stress and lifelong health.
Biological markers, or biomarkers, are objective measureable indicators of biological processes (Institute of Medicine, 2010). Researchers use biomarkers to evaluate normal biological processes, to evaluate biological responses to interventions, or as surrogates for clinical end points to assist with diagnosis and monitoring of disease (Colburn et al., 2001; Institute of Medicine, 2010). In pediatric research, biomarkers may be used to understand the pathogenic processes associated with exposure to chronic stress in childhood and adolescence. However, there are currently very few parameters to guide researchers in choosing appropriate biomarkers of chronic stress (Granger, Johnson, Szanton, Out, & Schumann, 2012; Juster, McEwen, & Lupien, 2010; Rodriguez et al., 2016). The purpose of this article is to describe biomarkers of chronic stress and review evidence of their use in pediatric research. I also discuss strategies and methods for biomarker collection, possible moderators for consideration, and future directions for biobehavioral research on chronic stress in childhood.
The Human Stress Response
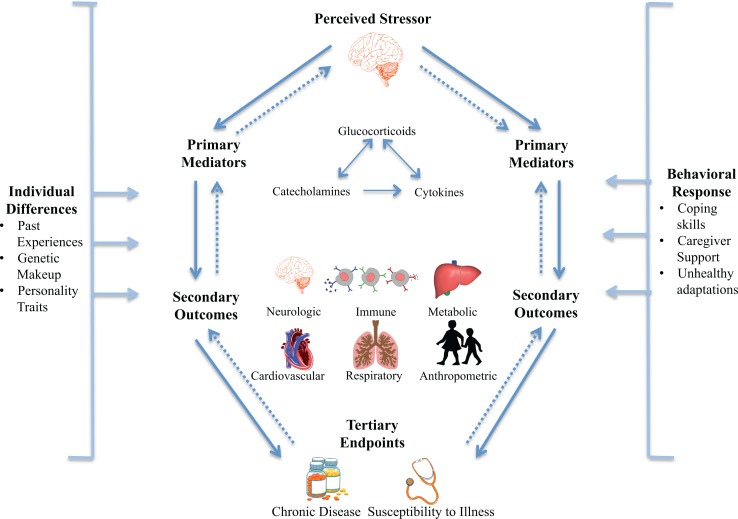
When faced with an acute stressor, the brain initiates behavioral and physiological adaptations to protect the body and prepares for a fight-or-flight response. These physiological adaptations are referred to as allostasis and include activation of the sympathetic nervous system (SNS) and hypothalamic–pituitary–adrenal (HPA) axis. This activation results in a release of glucocorticoids, catecholamines, and cytokines that interact as part of a complex, nonlinear network and act as primary mediators of the stress response, as depicted in Figure 1 (Juster et al., 2010; McEwen, 2003, 2012; Tottenham & Sheridan, 2009). As one mediator increases or decreases in response to stress, others compensate in an effort to regain homeostasis or the stability of physiological systems necessary to maintain life (Juster et al., 2010; McEwen & Wingfield, 2003). The parasympathetic nervous system also plays a regulatory role by reducing inflammation and cardiovascular response, thus contributing to the negative feedback loop intended to regain physiological stability (Juster et al., 2010).
Figure 1.
The physiological response to chronic stress. When the brain perceives a stressor, a complex, nonlinear network of primary mediators (glucocorticoids, catecholamines, and cytokines) is released in an effort to regain homeostasis. In response to chronic stressors, prolonged release of these mediators can lead to physiological disruptions and poor secondary outcomes across a range of systems, which ultimately lead to tertiary end points (chronic disease). Adapted from McEwen (2008) and McEwen and Wingfield (2003) with permission from Elsevier
In the short term, allostasis is adaptive, and physiological systems return to baseline in the absence of threat. However, repeated or chronic exposure to stressors can lead to allostatic load or overload, in which prolonged release of primary mediators (glucocorticoids, catecholamines, and cytokines) disrupts development and functioning of the brain and neuroendocrine, immune, metabolic, cardiovascular, and respiratory systems (McEwen, 2008; McEwen & Wingfield, 2003). These physiological disruptions, referred to as secondary outcomes of the stress response, can lead to diseased and disordered tertiary end points that affect mental and physical health across the life span (Garner et al., 2012; Juster et al., 2010). As displayed in Figure 1, this process does not occur in a linear fashion but as part of a complex cascade that is also influenced by environmental factors including individual differences and behavioral responses (McEwen, 2008). Table 1 describes concepts related to allostasis and allostatic load; for further review, see McEwen and Wingfield (2003). For an excellent review on the physiological response to chronic stress, see McEwen (2008).
Table 1.
Concepts Related to the Human Stress Response.
| Concept | Definition |
|---|---|
| Homeostasis |
|
| Allostasis |
|
| Allostatic state |
|
| Allostatic load |
|
| Allostatic overload |
|
Source. Adapted from McEwen & Wingfield (2003); HPA axis = hypothalamic–pituitary–adrenal axis.
For the purposes of this article, I define chronic stress as the process by which any stressor leads to a prolonged release of primary mediators and places children at risk of secondary outcomes and tertiary end points associated with allostatic load and overload. This concept is distinct from that of acute stress, which includes a temporary allostatic response with a return to homeostasis after the resolution of a single psychologically or physically threatening event (McEwen & Wingfield, 2003). In childhood and adolescence, chronic stressors may include extreme experiences, such as abuse, neglect, or institutionalization, as well as more prevalent stressors such as exposure to poverty, food insecurity, interpersonal violence, parental mental illness, racism, discrimination, unstable foster care placement, or unsafe neighborhoods and community violence (Shonkoff et al., 2012). In this review, I focus specifically on the primary mediators (Table 2) and secondary outcomes (Table 3) that investigators commonly measure in studies of children experiencing chronic stress. I provide an overview of the physiology and research evidence for each biomarker, with a focus on studies examining chronic stress in children or adolescents. Although I generally summarize the information, I do report statistical information where possible. I encourage the reader to review each primary source for further detail on the methods and findings.
Table 2.
Primary Mediators of the Stress Response.
| Biomarker | Measurement | Overview of Biomarker Function |
|---|---|---|
| Glucocorticoids | ||
| Cortisol | Serum, saliva, urine, and hair | Produced by the adrenal glands in response to HPA-axis activation; involved in fluid regulation, inflammation, immune system functioning, metabolism, glucose transport, appetite, cognition, and reproduction |
| Catecholamines | ||
| Epinephrine | Serum and urine | Released by the adrenal medulla in SNS response to stress; acts on skeletal muscle, increases heart rate and glucose levels |
| Norepinephrine | Serum and urine | Released by sympathetic neurons in SNS response to stress; regulates blood vessel restriction and blood flow to organs with sympathetic nerve innervation |
| Dopamine | Serum and urine | Released by the adrenal medulla and peripheral sympathetic nerves in SNS response; involved in cognitive, behavioral, and cardiovascular responses to stress |
| Cytokines | ||
| IL-6, IL-1β, TNF-α, cytokine panels | Serum and saliva | Intercellular protein messengers of the immune system produced locally by immune cells and organs such as the brain and liver; regulate pro- and anti-inflammatory responses to stress |
Note. HPA axis = hypothalamic–pituitary–adrenal axis; IL = interleukin; SNS = sympathetic nervous system; TNF-α = tumor necrosis factor-α.
Table 3.
Secondary Outcomes Related to Chronic Stress.
| Biomarker | Measurement | Overview of Biomarker Function |
|---|---|---|
| Neurologic | ||
| Hippocampal volume/activity | MRI and fMRI | Involved in learning and memory formation; also regulates negative feedback of glucocorticoids in HPA-axis response to stress |
| Amygdala volume/activity | MRI and fMRI | Involved in fear conditioning and emotional processing |
| Immune | ||
| C-reactive protein | Serum, saliva, and urine | Protein synthesized in the liver; stimulated by inflammatory cytokines and altered by glucocorticoid levels |
| Fibrinogen | Serum and urine | Glycoprotein produced by the liver; involved in platelet and erythrocyte aggregation, determines plasma viscosity |
| Secretory IgA | Serum, saliva, and urine | Antibody primarily produced in mucous membranes of the intestinal tract; protects epithelium from harmful toxins and microorganisms |
| Metabolic | ||
| Insulin | Serum, saliva, and urine | Hormone produced by the pancreas; involved in glucose metabolism |
| Glucose | Serum, saliva, and urine | Monosaccharide synthesized in the liver and kidneys; body’s main energy source |
| Leptin | Serum, saliva, and urine | Hormone secreted from adipose tissue; regulates energy homeostasis |
| α-Amylase | Serum, saliva, and urine | Enzyme produced by the salivary glands; initiates digestion of carbohydrates and starches. |
| Lipids | Serum, saliva | Lipoproteins (HDL and LDL) and triglycerides responsible for transport of cholesterol and dietary fat |
| Cardiovascular and respiratory | ||
| Blood pressure | Sphygmomanometer | Reflects force exerted by blood against the blood vessel walls during systole and diastole; elevated in SNS response to stress |
| Heart rate | Arterial pulsations | Number of palpations made by the heart within a specified period of time; elevated in SNS response to stress |
| Heart rate variability | Electrocardiogram | Variation in the time interval between palpations of the heart; reflects ANS activity |
| RSA | Electrocardiogram | Variation in heart rate that occurs during a respiratory cycle; indicator of cardiac vagal tone |
| Anthropometric | ||
| BMI | Height and weight | Measure of weight relative to height; estimation of body fat percentage based on age and gender-specific standards |
| Waist circumference | Waist circumference | Measure of distance around the abdomen; estimation of abdominal adiposity |
| Waist-to-hip ratio | Waist and hip circumferences | Measure of waist circumference divided by hip circumference; measure of central adiposity |
| Waist-to-height ratio | Waist circumference and height | Measure of waist circumference relative to height; estimates distribution of body fat |
| Growth and stature | Height | Measure of growth/stature relative to age and gender-specific standards |
Note. ANS = autonomic nervous system; BMI = body mass index; fMRA = functional magnetic resonance imaging; HDL = high-density lipoproteins; HPA = hypothalamic-pituitary-adrenal; IgA = immunoglobulin; LDL = low-density lipoproteins; MRI = magnetic resonance imaging; RSA = respiratory sinus arrhythmia; SNS = sympathetic nervous system.
Primary Mediators
Glucocorticoids
Glucocorticoids play a complex role in the stress response, including functions that mediate current responses to stress, suppress ongoing stress responses, and prepare the body for exposure to subsequent stressors (Sapolsky, Romero, & Munck, 2000). Glucocorticoid receptors are present in almost every bodily tissue, and glucocorticoids exert effects on fluid volume, cardiovascular effects, inflammation and immune system functioning, metabolism, glucose transport, appetite, cognition, and reproduction (McEwen, 2003; Sapolsky et al., 2000). Cortisol is a naturally occurring glucocorticoid produced by the adrenal glands in response to HPA-axis activation (Jessop & Turner-Cobb, 2008). Cortisol can be measured in blood serum, saliva, urine, or hair. However, authors have documented the benefits of measuring salivary cortisol over serum cortisol from as early as the 1980s, as salivary cortisol is noninvasive and levels are directly proportional to those found in serum (Laudat et al., 1988; Vining, McGinley, Maksvytis, & Ho, 1983). In recent published studies, researchers have used serum cortisol to measure adrenal function in acute or chronically ill children but have rarely measured it to understand the impacts of chronic stress (Landstra, Postma, Boezen, & Van Aalderen, 2012; Malakasioti et al., 2013; Nakavachara & Viprakasit, 2013). Thus, I have reviewed only research including salivary, urinary, or hair cortisol measurement.
Salivary cortisol
Salivary cortisol is a sensitive and specific measure of biologically active, unbound cortisol that has been widely measured in pediatric research studies for over 20 years (Aardal & Holm, 1995; Granger, Kivlighan, Fortunato, Harmon, Hibel, Schwartz, & Whembolua, 2007; Hanrahan, McCarthy, Kleiber, Lutgendorf, & Tsalikian, 2006; Jessop & Turner-Cobb, 2008; Keil, 2012). As healthy HPA-axis functioning follows a strong diurnal rhythm, salivary cortisol is best measured by capturing this diurnal rhythm, which requires collecting multiple samples over time. Adam and Kumari (2009) outline diurnal measures commonly used in field-based research, including a cortisol awakening response (surge in cortisol that occurs 30–45 min after waking), diurnal cortisol slope (degree of change in cortisol levels from morning to evening), and area under the daytime cortisol curve (area under all cortisol data points measured across the day). Salivary cortisol levels can also be measured in reactivity to daily or momentary stressors (Adam & Kumari, 2009). In studies of children, elevated salivary cortisol levels have been associated with increased maternal stress in children aged 4.5 years, low-socioeconomic status in 6- to 10-year-olds, and a history of institutionalization in 6- to 12-year-olds (Essex, Klein, Cho, & Kalin, 2002; Gunnar, Morison, Chisholm, & Schuder, 2001; Lupien, King, Meaney, & McEwen, 2000). However, chronic exposure to stress can also lead to hypocortisolism, and reduced cortisol levels in children have been associated with family conflict in adolescents, maternal depression in 6-year-olds, and social deprivation in 9- to 11-year-olds (Apter-Levi et al., 2016; Hostinar, Johnson, & Gunnar, 2015; Kushner, Barrios, Smith, & Dougherty, 2015; Zhang et al., 2016). This variability is related to a number of factors that require consideration, including the timing of exposure, nature of the stressor, and individual coping mechanisms (G. E. Miller, Chen, & Zhou, 2007).
Urinary cortisol
Measurement of urinary free cortisol (UFC) offers an advantage over serum and salivary cortisol as it provides an index of adrenocortical activity over a 24-hr period. While cortisol measured at a single time point may reflect momentary stressors or fluctuations in circadian rhythm, UFC collected over 24 hr provides a more stable assessment of adrenocortical activity over time (Curcio et al., 2016; Gomez, Malozowski, Winterer, Vamvakopoulos, & Chrousos, 1991). UFC is commonly measured to diagnose conditions associated with abnormal adrenocortical activity, such as Cushing syndrome, congenital adrenal hyperplasia, or adrenal insufficiency (Taylor, Machacek, & Singh, 2002). However, researchers have also measured UFC to assess adrenocortical activity in response to chronic stress in studies of children and adolescents. For example, in a study of noise as a source of chronic stress, Evans (2013) found that higher noise levels were positively associated with overnight UFC levels (R 2 = 0.03, p = .014) in a 9-year-old children, even when controlling for gender, poverty status, family structure, and maternal education. Authors have also reported elevated UFC levels in young children with a history of severe neglect (M age = 53.7 ± 4.4 months) and in 13- to 18-year-olds with a history of depression (Fries, Shirtcliff, & Pollak, 2008; Rao, Hammen, & Poland, 2010). UFC levels may also respond to stress-reducing interventions. In a study of 7- to 11-year-old children hospitalized with respiratory illnesses, a greater number of the children demonstrated a decrease in UFC levels following a play intervention compared with those who did not receive the intervention (R 2 = 1.73, p = .04; Potasz, Varela, Carvalho, Prado, & Prado, 2013).
Hair cortisol
In the past decade, researchers have begun using hair cortisol as a noninvasive biomarker of long-term HPA-axis functioning (Sauvé, Koren, Walsh, Tokmakejian, & Van Uum, 2007). In the collection of hair cortisol, 1 cm of hair represents approximately 1 month of systemic cortisol exposure. This measure offers a major advantage over serum, salivary, and urinary cortisol as it allows researchers to evaluate HPA-axis functioning over time (Russell, Koren, Rieder, & Van Uum, 2012; Sauvé et al., 2007). The exact mechanism through which cortisol is incorporated into hair remains unknown, but the primary hypothesis is that cortisol is incorporated through blood circulation during the formation of the hair shaft (Meyer & Novak, 2012; Russell et al., 2012; Sauvé et al., 2007). In studies of children, increased hair cortisol levels have been associated with exposure to parenting stress and maternal depression in infants, lower parental education in 6-year-olds, increased negative life events in 5- to 11-year-olds, cumulative negative psychosocial exposures in 10-year-olds, and lower socioeconomic status in 4- to 18-year-olds (Karlen, Frostell, Theodorsson, Faresjo, & Ludvigsson, 2013; Karlen et al., 2015; C. H. Liu, Snidman, Leonard, Meyer, & Tronick, 2016; Palmer et al., 2013; Rippe et al., 2016; Simmons et al., 2016; Vaghri et al., 2013; Vanaelst et al., 2012; Vanaelst et al., 2013; Vliegenthart et al., 2016). Hair cortisol may also be used to measure a change in physiological stress over time (Groeneveld et al., 2013; Smy et al., 2015). In a study of stress in children at school entry (M age = 50.1 ± 0.42 months), hair cortisol levels were significantly higher in the 2 months following school entry than the 2 months prior to school entry (t = −4.21, p = .002; Groeneveld et al., 2013). Elevated hair cortisol levels have also been associated with elevated body mass index (BMI; r = .407, p < .01) and increased waist circumference (r = .430, p < .01) in children aged 8–12 years, reflecting a link with secondary outcomes of the chronic stress response (Veldhorst et al., 2013).
Catecholamines
Catecholamines, including epinephrine, norepinephrine, and dopamine, are also released in response to a perceived threat. Like glucocorticoids, catecholamines exert effects on cardiovascular function, fluid and electrolyte balance, inflammation and immunity, metabolism, body temperature, and functioning of the central nervous system (Charmandari, Tsigos, & Chrousos, 2005; McEwen, 2003). Catecholamine levels offer potentially useful indices of chronic stress in children, especially if levels are elevated overnight when SNS activity is typically decreased (McEwen, 2003). Although catecholamine levels are most commonly measured to evaluate or diagnose hormone-secreting tumors, researchers have also occasionally measured urinary catecholamines to assess chronic stress in studies of children (Peaston & Weinkove, 2004).
Epinephrine and norepinephrine
As part of the SNS response to threat, epinephrine is released by the adrenal medulla, and norepinephrine is released by sympathetic neurons (McEwen, 2003; Sapolsky, 2002). Epinephrine acts on skeletal muscles, while norepinephrine regulates blood vessel restriction and blood flow to organs with sympathetic nerve innervation, such as the heart, spleen, and pancreas (McEwen, 2003). Epinephrine release is closely associated with emotional distress, while norepinephrine release is typically associated with physical activity. Although epinephrine and norepinephrine represent independent indices of SNS activity, they are often measured together as part of a composite index of chronic stress in studies of children. For example, Evans (2003) included urinary epinephrine and norepinephrine as part of an allostatic load index that also included measures of resting blood pressure, urinary cortisol, and BMI. In that and other studies, the allostatic load index and independent measures of urinary epinephrine and norepinephrine were significantly associated with childhood cumulative risk as measured by physical (crowding, noise, and housing quality) and psychosocial (child separation, turmoil, and violence) aspects of the home environment and personal characteristics (poverty, single parenthood, and maternal high school dropout status) in childhood and adulthood (Doan, Dich, & Evans, 2014; Doan & Evans, 2011; Evans, 2003; Evans & Fuller-Rowell, 2013; Evans & Kim, 2012; Evans, Kim, Ting, Tesher, & Shannis, 2007). Researchers also measured urinary catecholamines in a randomized controlled trial of the Strong African American Families program, a family-centered preventive intervention designed to reduce substance use, antisocial behavior, and early sexual involvement (Brody, Yu, Chen, & Miller, 2014). In that study, parental psychological dysfunction and nonsupportive parenting (lack of nurturance, communication, and family rules) measured in children at age 11 predicted an increase in overnight catecholamine levels at age 20 for participants in the control group (βs = 0.175–0.203, ps < .05) but not for those in the intervention group. These findings suggest both a protective effect of the intervention and the potential for urinary catecholamine levels to accurately capture the biological effects of a behavioral intervention.
Dopamine
Dopamine is released by the adrenal medulla and peripheral sympathetic nerves in response to stressful stimuli and plays an important role in reward-system processing and behavioral responses to perceived stressors (Belujon & Grace, 2015; Snider & Kuchel, 1983; Trainor, 2011). In studies of chronic stress in children, dopamine has occasionally been measured with other urinary catecholamines. In a case–control study of 8- to 15-year-old girls, urinary dopamine, epinephrine, and norepinephrine were included in a composite measure of catecholamine synthesis, and investigators reported significantly higher total catecholamine synthesis in sexually abused girls compared with controls (t = 2.18, p < .05; De Bellis, Lefter, Trickett, & Putnam, 1994). In a study of 6- to 8-year-old children, urinary catecholamines were measured as an indication of emotional stress, and investigators found that elevated epinephrine and dopamine levels were associated with an increased risk of developing bruxism (rs = .007 to .21, ps = .01 to .03; Vanderas, Menenakou, Kouimtzis, & Papagiannoulis, 1999). In a study of 9- and 10-year-old children, those with a history of preterm birth had significantly higher urinary epinephrine, norepinephrine, and dopamine levels when compared with controls (ps = .02 to .11), while children who were small for gestational age at birth had increased dopamine levels only (p = .03; Johansson et al., 2007). However, in a study of children diagnosed with posttraumatic stress disorder (PTSD; M age = 10.8 ± 2.0 years), children with PTSD had higher urinary epinephrine levels when compared with controls (F = 9.18, p < .001), but there was no difference between groups for urinary norepinephrine or dopamine levels (De Bellis, Baum, Birmaher, & Ryan, 1997).
Inflammatory Cytokines
Cytokines consist of a diverse group of molecules that serve as the primary intercellular protein messengers of the immune system (McEwen, 2003; Riis, Granger, DiPietro, Bandeen-Roche, & Johnson, 2015). Cytokines are produced locally by immune cells as well as by other organs including the brain and liver (McEwen, 2003). These molecules have multiple mechanisms of action and are influenced by stress-related activity in the SNS and HPA axis. Cytokines also act as part of a negative feedback loop between the immune and central nervous systems: Release of norepinephrine in response to acute stress results in an increase in inflammatory cytokines, which in turn stimulates the HPA axis to release cortisol and inhibit inflammatory cytokine production. However, dysregulation of this feedback loop due to chronic stress over time can result in chronic inflammation, autoimmune and neurologic diseases, and insufficient immune functioning (Morey, Boggero, Scott, & Segerstrom, 2015; Riis et al., 2015).
Pro-inflammatory cytokines initiate inflammation and are activated in response to stressors or pathogens. They include interleukin (IL)-1, IL-2, IL-6, tumor necrosis factors (TNFs), fibroblast growth factors, and interferons (IFNs). Anti-inflammatory cytokines, which inhibit pro-inflammatory cytokine production, include IL-4 and IL-10 (McEwen, 2003). Both pro- and anti-inflammatory cytokines can be detected in serum or saliva, and recent development of multiplex immunoassays has allowed for rapid detection of multiple cytokines in a single sample (Vignali, 2000). To date, however, most research in children has focused on analysis of single cytokines, primarily the pro-inflammatory cytokines IL-6, IL-1β, and TNF-α.
IL-6
IL-6 has been widely investigated in studies of chronic stress due to its known involvement in inflammation, infection response, and regulation of metabolic and neural processes (Scheller, Chalaris, Schmidt-Arras, & Rose-John, 2011). In children, elevated serum IL-6 concentrations have been associated with social and environmental stressors (socioeconomic status at 13–16 years, cumulative adversity from birth to 8 years, and sexual abuse at 6–12 years), child behavior and mental health (internalizing behaviors, externalizing behaviors, and psychopathology at 6–13 years), and child resilience and protective factors (self-efficacy at 7–13 years, optimism at 13–16 years, and presence of supportive role models at 13–16 years; Caserta, Wyman, Wang, Moynihan, & O’Connor, 2011; E. Chen, Lee, Cavey, & Ho, 2013; Cunha et al., 2016; Muller, Errington, Szabo, Pitts, & Jacklin, 2014; Pervanidou, Margeli, Lazaropoulou, Papassotiriou, & Chrousos, 2008; Slopen, Kubzansky, & Koenen, 2013; Slopen, Kubzansky, McLaughlin, & Koenen, 2013). Authors have criticized the measurement of cytokines in saliva, as salivary cytokine levels have been associated with indicators of oral inflammation such as loose teeth, bleeding gums, or untreated cavities (Riis et al., 2015). However, salivary IL-6 levels have also been correlated with maternal distress in 5-year-olds, sleep disruption in 8- to 9-year-olds, and depression, anxiety, and behavioral problems in 7- to 11-year-olds, suggesting that salivary cytokine levels may also be indicative of systemic inflammation (El-Sheikh, Buckhalt, Granger, Erath, & Acebo, 2007; Keller, El-Sheikh, Vaughn, & Granger, 2010; Riis et al., 2016).
TNF-α, IL-1β, and cytokine panels
Other mediators of the inflammatory response, including TNF-α and IL-1β, have also been independently measured in studies of children (E. Chen, Fisher, Bacharier, & Strunk, 2003; E. Chen et al., 2011; Lopez-Castejon & Brough, 2011; Mills, Scott, Wray, Cohen-Woods, & Baune, 2013). TNF-α and IL-1β are pro-inflammatory cytokines that play a key role in the innate immune response, and TNF-α also has important metabolic effects (Calcagni & Elenkov, 2006; Hotamisligil, Shargill, & Spiegelman, 1993). In a study of Latino children aged 5–10 years, elevated plasma TNF-α levels were associated with stressful life events (r = .26, p = .01), and this relationship was not modified by child sex or family history of type 2 diabetes mellitus (Dixon, Meng, Goldberg, Schneiderman, & Delamater, 2009). In a study of children aged 3–5 years, salivary IL-1β was associated with the number of past-month contextual stressors (F = 6.07, p = .018), lifetime contextual stressors (F = 4.67, p = .037), and traumatic life events (F = 4.73, p = .036; Tyrka, Parade, Valentine, Eslinger, & Seifer, 2015).
Researchers have also used multiplex immunoassays in a few studies to measure panels of multiple pro- and anti-inflammatory cytokines (Bücker et al., 2015; Carlsson, Frostell, Ludvigsson, & Faresjo, 2014; Gariup et al., 2015; Sesso et al., 2014). In an analysis of salivary cytokine levels in a 5-year-old children, Riis and colleagues reported a significant interaction between salivary cytokines (IL-1β, IL-6, IL-8, and TNF-α) and a composite score for maternal psychological distress (depressive symptoms, anxiety, and parenting stress) among girls in the sample (βs = 0.18–0.27, p = <.001 to .009; Riis et al., 2016). In a study of salivary cytokines (IL-6, IL-10, IL-12, and IFN-γ) collected in neonates, mean salivary cytokine levels at birth were significantly higher in infants born preterm compared to those born full term (ps = < .05; Sesso et al., 2014). In a study of 3- to 12-year-old children with and without childhood trauma, investigators reported increased plasma TNF-α levels in the trauma group (F = 9.23, p = .004), while IL-12p70, IL-6, IL-8, IL-10, and IL-1 β levels were not significantly different between groups (Bücker et al., 2015).
Secondary Outcomes
Neurologic
While the brain plays a critical role in assessing and responding to stressful environmental stimuli, it is also structurally and chemically affected by the HPA-axis response to stress (Fox et al., 2010; McEwen, 2008). As glucocorticoids can cross the blood–brain barrier, HPA-axis activation has the greatest impact on cortisol receptor-rich areas of the brain, particularly the amygdala and hippocampus (Tottenham & Sheridan, 2009).
Hippocampus
The hippocampus plays an important role in learning and memory formation and also regulates negative feedback of glucocorticoids as part of the HPA-axis response to stress (Kim & Yoon, 1998; Tottenham & Sheridan, 2009). However, prolonged exposure to glucocorticoids results in apoptosis of hippocampal neurons, which in turn alters hippocampal volume and function in response to chronic stress (Kim & Yoon, 1998; Sapolsky, 1996; Tottenham & Sheridan, 2009). In a recent review, S. B. Johnson, Riis, and Noble (2016) outlined a number of studies that have examined the relationship between exposure to poverty and hippocampal volume in childhood. For example, in longitudinal studies, smaller hippocampal volumes measured by magnetic resonance imaging (MRI) have been associated with lower family income at age 12 and lower income-to-needs ratios at age 10 (Hair, Hanson, Wolfe, & Pollak, 2015; J. Luby et al., 2013). In cross-sectional studies of children ranging in age from 3 to 17 years, smaller hippocampal volumes have been associated with poverty as measured by parent education and income level (Hanson, Chandra, Wolfe, & Pollak, 2011; Hanson et al., 2015; Jednoróg et al., 2012; Noble et al., 2015; Noble, Houston, Kan, & Sowell, 2012).
Smaller hippocampal volumes have also been reported in studies of children with a history of institutionalization (age range = 12–14 years), traumatic life events in early childhood (age range = 7–12 years), and adolescents exposed to parental depression (age range = 12–20 years; Hodel et al., 2015; Pagliaccio et al., 2014; Rao, Chen, Bidesi, Shad, Thomas & Hammen, 2010). The hippocampus is also amenable to protective factors, as maternal support at age 3–5 years has been associated with larger hippocampal volume at age 7–13 years (F= 18.58, p < .001; J. L. Luby et al., 2012). In addition to impacts on hippocampal volume, chronic stress can affect hippocampal activity. In a study of 10- to 17-year-olds with posttraumatic stress symptoms, adolescents with a history of interpersonal trauma had decreased hippocampal activity on functional MRI (fMRI) in response to a memory task when compared with healthy controls (t = 2.92, p = .008; Carrión, Haas, Garrett, Song, & Reiss, 2010).
Amygdala
The amygdala plays an important role in fear conditioning and emotional processing (Phelps & LeDoux, 2005). In response to chronic stress, increased circulation of glucocorticoids and corticotropin-releasing hormone (CRH) leads to changes in amygdala structure and functioning. The result is an initial growth and hyperactivity of the amygdala, which over time leads to cellular atrophy and apoptosis (Tottenham & Sheridan, 2009). S. B. Johnson et al. (2016) also reviewed studies on the relationship between poverty and amygdala volume in childhood. Unlike the consistent findings noted in MRI studies of hippocampal volume, the relationship between chronic stress and amygdala volume in children is less clear. Smaller amygdala volume in children has been associated with a history of traumatic life events in 7- to 12-year-olds and with low-socioeconomic status, history of abuse, and history of neglect in 9- to 14-year-olds (Hanson et al., 2015; Pagliaccio et al., 2014). However, low family income and traumatic life events have also been associated with increased amygdala activity on fMRI in 6- to 12-year-olds, and larger amygdala volumes have been noted in 10-year-old children of depressed mothers (J. Luby et al., 2013; Lupien et al., 2011; Suzuki et al., 2014). Further, in a study with participants ranging from 3 to 20 years old, researchers found no association between amygdala volume and child socioeconomic status or parental education (Noble et al., 2015).
Immune
The systemic inflammation caused by prolonged release of glucocorticoids and pro-inflammatory cytokines can result in the dysregulation of the immune system and increased risk for chronic diseases (Morey et al., 2015). Glucocorticoids also play an important role in regulating innate immune responses to bacterial and viral infections and in inhibiting the production of pro-inflammatory cytokines (Kemeny & Schedlowski, 2007). Thus, the interaction of these primary mediators over time can lead to a number of inflammatory- and immune-related secondary outcomes.
C-reactive protein (CRP)
CRP is widely considered to be an important nonspecific marker of inflammation (Danesh et al., 2004). CRP synthesis, which primarily occurs in the liver, is stimulated by inflammatory cytokines and can be altered by glucocorticoid levels (Du Clos & Mold, 2004). In studies of adolescents, elevated CRP levels have been associated with daily interpersonal stress in 12th graders and unpleasant stressful life events and interpersonal conflict in 14- to 19-year-olds (Fuligni et al., 2009; Low, Matthews, & Hall, 2013). In a study of children aged 5–18 years, Broyles and colleagues (2012) found that children living in neighborhoods with high levels of crime or poverty had 2.7 times higher odds of having elevated CRP levels compared to children from other neighborhoods (95% CI [1.2,6.2]) even after controlling for adiposity, demographics, and behavioral factors. Associations between chronic stress and CRP levels in children have also been demonstrated prospectively; Slopen and colleagues (2013) found that cumulative adversity from birth to 8 years of age was associated with higher levels of CRP at both 10 (β = 0.06, p = .01) and 15 (β = 0.05, p = .04) years of age.
Fibrinogen
Fibrinogen is a glycoprotein produced by the liver and is upregulated by IL-6 in response to stress. Fibrinogen plays an important role in platelet aggregation and erythrocyte aggregation and is a key determinant of plasma viscosity (Reinhart, 2003). Child maltreatment has been linked with adult fibrinogen levels in prospective studies, but research on chronic stress and child fibrinogen levels is limited (Coelho, Viola, Walss-Bass, Brietzke, & Grassi-Oliveira, 2014). In one study of young adults aged 16–34 years, psychological distress was positively associated with fibrinogen levels (β = 0.024, p < .01), including when controlling for demographic and health factors (Goldman-Mellor, Brydon, & Steptoe, 2010). However, while fibrinogen has been frequently measured in studies of acutely ill children, the relationship between exposure to chronic stress and fibrinogen levels in community samples of children and adolescents has not otherwise been examined.
Secretory immunoglobulin A (sIgA)
sIgA is an antibody primarily produced in the mucous membranes of the intestinal tract. As the body is exposed to most pathogens through mucosal exposure, sIgA serves as the first line of defense in protecting the epithelium from harmful toxins and microorganisms (Brandtzaeg, 2009; Mantis, Rol, & Corthésy, 2011; Pabst, 2012). Release of sIgA is controlled by the neuroendocrine system, and evidence suggests that chronic stress can lead to decreased sIgA levels in adults (Engeland et al., 2016). In children, low sIgA levels have been associated with low caregiver sensitivity in toddlers, exposure to stressful experiences in 8- to 12-year-olds, and elevated salivary cortisol levels in 3- to 5-year-olds (Drummond & Hewson-Bower, 1997; Vermeer, van IJzendoorn, Groeneveld, & Granger, 2012; Watamura, Coe, Laudenslager, & Robertson, 2010). However, in a study of children aged 3–8 years, sIgA levels were not associated with time spent in childcare, an experience the researcher had hypothesized to be stressful (Waynforth, 2007). Researchers have also examined secretory IgA levels in association with interventions. In a study of 5- to 14-year-olds undergoing forensic interviews for alleged sexual abuse, sIgA levels tended to be lower when a therapy dog was present in the room (t = 1.986, p = .055; Krause-Parello & Friedmann, 2014).
Metabolic
In the presence of prolonged HPA-axis activity, chronic secretion of glucocorticoids and catecholamines leads to hypersecretion of insulin and hyposecretion of growth and sex hormones (McEwen, 2008; Pervanidou & Chrousos, 2012). Over time, this combination leads to the accumulation of fat in visceral adipose tissue and loss of muscle and ultimately contributes to the development of central obesity and metabolic alterations (Pervanidou & Chrousos, 2012). In addition to changes in insulin levels, other secondary outcomes related to these metabolic processes include changes in glucose, leptin, α-Amylase, and cholesterol levels.
Insulin and glucose
The pancreas produces the hormone insulin and regulates glucose metabolism through a balance of insulin action and secretion (Stumvoll, Tataranni, Stefan, Vozarova, & Bogardus, 2003). However, prolonged release of cortisol in response to chronic stress can result in a decrease in hepatic and extrahepatic sensitivity to insulin and lead to insulin resistance (Rizza, Mandarino, & Gerich, 1982). Insulin resistance leads to hypersecretion of insulin, which can result in elevated glucose levels over time (Stumvoll et al., 2003). While researchers have commonly measured insulin and glucose levels as indicators of altered metabolism due to chronic stress in adults, use of insulin and glucose as indicators of chronic stress in children has been limited, and their utility remains unclear (Juster et al., 2010). In a recent study, Kepper et al. (2016) found that chronic stress, as measured by concentrated neighborhood disadvantage, was not significantly associated with insulin resistance in prepubescent children aged 7–9 years. Similarly, in a large study (N = 1,952) by van Dijk, van Eijsden, Stronks, Gemke, and Vrijkotte (2015), prenatal psychosocial stress in mothers was not associated with fasting glucose or insulin resistance in children at age 5–6 years. However, in other studies, researchers found that low parental education and socioeconomic position were associated with altered glucose and insulin levels in both children (age 5–6 years) and adolescents (age 12–20 years; Goodman, Daniels, & Dolan, 2007; Goodman, Must, Daniels, & Dolan, 2010; Thomas et al., 2012; van Den Berg, Van Eijsden, Vrijkotte, & Gemke, 2012).
Leptin
Leptin is a hormone secreted from adipose tissue that helps to regulate energy homeostasis by suppressing appetite (Pervanidou & Chrousos, 2012). The hormone also plays important roles in glucose metabolism, insulin secretion and sensitivity, neuroendocrine function, immune function, and bone metabolism (Mantzoros et al., 2011). Leptin interacts with the HPA axis and follows a circadian rhythm in an inverse relationship with cortisol levels. While cortisol levels peak in the morning and reach nadir around midnight, leptin concentrations are highest after midnight and lowest in the early to midafternoon (Houseknecht, Baile, Matteri, & Spurlock, 1998; Pervanidou & Chrousos, 2012). While elevated leptin levels have been associated with early life adversity in retrospective studies of adults (Farr et al., 2015; Joung et al., 2014), only two studies have investigated the association between leptin and chronic stress in children. In a study of 10-year-old children, Kohlboeck et al. (2014) reported that increased emotional symptoms (β = 1.03, p < .04) and peer problems (β = 1.05, p = .0001) were significantly associated with higher serum leptin levels, including when controlling for BMI and sociodemographic factors. However, in a prospective study, Danese et al. (2014) found that 12-year-old children with a history of maltreatment exhibited blunted leptin levels in response to increasing adiposity (β = −1.37, p = .001) and inflammation (β = −0.57, p < .001) compared with nonmaltreated children.
α-Amylase
α-Amylase, an enzyme produced by the salivary glands, is primarily responsible for initiating digestion of carbohydrates and starch. Salivary α-Amylase (sAA) release is regulated by autonomic nervous system (ANS) activity and thus may be considered an indirect indicator of the stress response (Granger, Kivlighan, El-Sheikh, Gordis, & Stroud, 2007; Nater & Rohleder, 2009). In a number of studies, researchers have demonstrated that sAA levels increase in response to acute stressors (Granger, Kivlighan, El-Sheikh, et al., 2007). However, altered sAA levels have also been demonstrated in studies of children experiencing chronic stress. For example, PTSD severity has been associated with elevated morning sAA levels in 12- to 17-year-old girls with a history of sexual abuse (r = .51, p = .02), and altered diurnal sAA patterns have been associated with child adiposity in low-income preschool-age children (β = −0.12, p < .03; Keeshin, Strawn, Out, Granger, & Putnam, 2015; A. L. Miller et al., 2015). sAA levels have also been measured in association with cortisol levels in school-age children, as researchers have hypothesized that asymmetrical ANS and HPA-axis responses to stress indicate altered regulation of the stress-response system (F. R. Chen, Raine, Soyfer, & Granger, 2015; Koss et al., 2014; Ursache & Blair, 2015).
Lipids
Lipid levels and other cardiovascular risk factors are linked to chronic stress through both physiological and behavioral pathways (Holman, 2015). Lipids include low-density lipoproteins, which transport cholesterol to tissues, high-density lipoproteins, which transport cholesterol from tissues to the liver, and triglycerides, which transport dietary fat (Juster et al., 2010). In adults, various forms of chronic stress have been associated with increased cholesterol levels and metabolic risk including workplace stress, caregiver stress, and experiencing major life events (Chandola, Brunner, & Marmot, 2006; Pedersen et al., 2016; Vitaliano et al., 2002). In prospective studies, researchers have also detected a relationship between adversity in childhood and cardiometabolic risk in adulthood (Friedman, Karlamangla, Gruenewald, Koretz, & Seeman, 2015; Winning, Glymour, McCormick, Gilsanz, & Kubzansky, 2015). However, to our knowledge, only one study has examined the relationship between chronic stress and lipid levels in children. In that study, Kepper et al. (2016) found no significant association in 7- to 9-year-old children between neighborhood disadvantage and either intrahepatic or intramyocellular lipid levels.
Cardiovascular and Respiratory
In response to stress, cardiovascular tone, and respirations increase, pro-inflammatory cytokines are released, endothelial function is temporarily impaired, and platelets are activated (Steptoe & Kivimäki, 2013). As a consequence, secondary outcomes related to the cardiovascular and respiratory systems may represent important indicators of chronic stress in children.
Systolic and diastolic blood pressure
Activation of the SNS results in a release of norepinephrine, which subsequently leads to vasoconstriction and blood pressure elevation. Thus, exposure to chronic stress can lead to systemic vascular resistance and risk of hypertension over time (Grassi & Ram, 2016; Sorota, 2014; Spruill, 2010). Blood pressure levels are also affected by other secondary outcomes of the stress response, including insulin resistance and adiposity (Addison, Stas, Hayden, & Sowers, 2008). Studies of blood pressure and chronic stress have primarily been conducted with adolescents, and elevated blood pressure levels have been associated with experiencing chronic, negative life events in 14- to 16-year-olds, greater relative household income deprivation in 13-year-olds, and family conflict in 11- to 15-year-olds (Brady & Matthews, 2006; Clark & Armstead, 2000; Kwok, Subramanian, Leung, & Schooling, 2015). Although studies of chronic stress and blood pressure among young children are limited, there is evidence to suggest that blood pressure elevations in relation to chronic stress can be seen as early as school age (Hollar et al., 2010; Martinovic et al., 2014; Rogosch, Dackis, & Cicchetti, 2011).
Chronic stress may also impact blood pressure reactivity, an important indicator of early cardiovascular dysfunction (Evans, Exner-Cortens, Kim, & Bartholomew, 2013). For example, in a study of middle-school-aged children, those with a history of higher psychosocial and physical risk factors had slower, less efficient recovery in blood pressure in response to an acute stressor (b = .13, p < .01) compared to those with no such history (Evans, Kim, Ting, Tesher, & Shannis, 2007). In adolescents, low blood pressure reactivity has also been associated with childhood exposure to family conflict in 17- and 18-year-olds and residence in a low-income neighborhood in 13- to 16-year-olds (Evans, Exner-Cortens, et al., 2013; Wilson, Kliewer, Plybon, & Sica, 2000).
Heart rate and heart rate variability (HRV)
The SNS response to stress includes an increase in heart rate, and thus, measures of resting heart rate or HRV may provide important insight into the functioning of the ANS (Rozanski, Blumenthal, & Kaplan, 1999). High variability in heart rate indicates a healthy and adaptive autonomic response system, while low variability may be an early indicator of abnormal ANS functioning (Pumprla, Howorka, Groves, Chester, & Nolan, 2002). In pediatric studies, higher resting heart rates have been associated with exposure to marital violence in 5- to 13-year-olds, harsh parenting in 7- to 12-year-olds, and low parental education levels in 7- to 12-year-olds as well as low-socioeconomic status (range = 14–16 years) and parental military deployment (M age = 15.8 ± 1.1 years) in adolescents (Davis & Treiber, 2007; Krenichyn, Saegert, & Evans, 2001; McGrath, Matthews, & Brady, 2006; Saltzman, Holden, & Holahan, 2005). Low HRV in children has been associated with peer problems in 5- to 10-year-olds and experienced violence in 16- to 19-year-olds (Michels et al., 2013; Murali & Chen, 2005). Measures of HRV may also be responsive to stress-reducing interventions. In a study of a stress-management intervention for third-grade children, researchers saw significant improvements in HRV for children in the intervention group immediately following the intervention and at 1-year follow-up (effect size = .906, p = .003), but HRV did not improve significantly for children in the control group (effect size = .15, p = .07; Bothe, Grignon, & Olness, 2014).
Respiratory sinus arrhythmia (RSA)
RSA is a measure of synchronization between HRV and respirations. In healthy individuals, heart rate increases during inspiration and decreases during expiration, thus improving coordination between the cardiovascular and respiratory systems and efficacy of gas exchange in the lung (Yasuma & Hayano, 2004). RSA is considered an indicator of cardiac vagal tone, and an altered or abnormal RSA may reflect dysfunction of the respiratory, cardiovascular, or ANSs (Grossman & Taylor, 2007). RSA is often measured in response to a stressful laboratory task, and suppressed RSA responses in children have been associated with lower socioeconomic status in 7- to 12-year-olds, harsh parenting history in 8- to 16-year-olds, poor parent–child relationship quality in 2-year-olds and 10- to 17-year-olds, disorganized attachment in 2- to 7-year-olds, exposure to prenatal maternal stress in infants, and exposure to parent conflict in infants and in 8- to 12-year-olds (Calkins, Graziano, Berdan, Keane, & Degnan, 2008; El-Sheikh & Hinnant, 2011; Evans et al., 2013; Hinnant, El-Sheikh, Keiley, & Buckhalt, 2013; Hinnant, Erath, & El-Sheikh, 2015; Moore, 2010; Oosterman, De Schipper, Fisher, Dozier, & Schuengel, 2010; Rash, Campbell, Letourneau, & Giesbrecht, 2015; Willemen, Schuengel, & Koot, 2009). However, prospective studies of infants and young children have indicated that childhood RSA is not associated with prenatal exposure to maternal stress or adversity (Alkon et al., 2014; van Dijk, van Eijsden, Stronks, Gemke, & Vrijkotte, 2012).
Anthropometric Measures
Anthropometric measures, including body size and stature, are also affected by exposure to chronic stress. Prolonged release of primary mediators leads to metabolic outcomes such as insulin and leptin resistance that promote storage of fat in adipose tissue (Pervanidou & Chrousos, 2012). Chronic activation of the HPA axis also results in hyposecretion of growth hormone, and circulating glucocorticoids inhibit the effects of growth factors on target tissues (Charmandari et al., 2005).
BMI
BMI is a measure of weight relative to height and does not distinguish between body fatness, muscle mass, and skeletal mass (Freedman & Sherry, 2009). However, research in children suggests that BMI is a good indicator of excess adiposity in children with a high BMI (>85%) according to standard CDC growth charts by gender and age. Elevated BMI in children and adolescents has been associated with a number of chronic stressors including low-socioeconomic status and exposure to parental stress (Carroll-Scott et al., 2013; Carter, Dellucci, Turek, & Mir, 2015; Evans, Fuller-Rowell, & Doan, 2012; Powell, Wada, Krauss, & Wang, 2012; Tate, Wood, Liao, & Dunton, 2015). Measures of BMI have also been correlated with physiological measures related to chronic stress, including salivary cortisol and sAA levels, in preschool, school-age, and adolescent children (Francis, Granger, & Susman, 2013; A. L. Miller et al., 2013, 2015; Ruttle et al., 2014). However, in a recent study of families living in socioeconomically disadvantaged neighborhoods, hair cortisol levels were not associated with BMI z-scores (standard deviation scores accounting for age and gender) in the 10- to 17-year-old children studied (Olstad et al., 2016).
Central adiposity
Measures of central adiposity, including waist circumference, waist-to-hip ratio, and waist-to-height ratio, may represent a more accurate method for assessing body fatness than BMI. However, these methods are prone to measurement error, and recommendations for use in children are limited (Freedman & Sherry, 2009; Mushtaq et al., 2011; Neovius, Linne, & Rossner, 2005). As such, studies on the relationship between chronic stressors and measures of central adiposity in children have produced mixed results. In a study of adverse childhood experiences (ACEs) and child health, Pretty, O’Leary, Cairney, and Wade (2013) found a dose–response relationship between accumulation of ACEs and increased waist circumference in 11- to 14-year-old children (R 2 = 0.06, p < .01). In prospective studies, high levels of prenatal maternal stress predicted increased waist-to-height ratio in children at 11, 13, and 15 years of age (r = .25 to .44, p = .01 to .04), and exposure to postpartum maternal distress predicted increased waist-to-hip ratio in girls at age 9–11 years (r = .29, p = .0016; Kozyrskyj et al., 2011; G. T. Liu, Dancause, Elgbeili, Laplante, & King, 2016). In other studies, increased waist-to-height ratio has been associated with peer problems in 5- to 10-year-old girls and low parent education levels in adolescents (Costa de Oliveira Forkert et al., 2016; Vanaelst et al., 2014). Measures of central adiposity in children have also been associated with hair cortisol levels in caregivers of 11- to 20-year-olds with disabilities, morning salivary cortisol levels in children aged 6–12 years, and blood pressure reactivity in adolescents aged 14–16 years (X. Chen et al., 2015; Goldbacher, Matthews, & Salomon, 2005; Hill, Eisenmann, Gentile, Holmes, & Walsh, 2011). In other studies, however, researchers have reported no significant associations between measures of central adiposity and childcare attendance in 4-year-olds, serum cortisol levels in 8- to 13-year-olds, or exposure to racism in 16- to 20-year-olds (Priest, Paradies, Gunthorpe, Cairney, & Sayers, 2011; Weigensberg, Toledo-Corral, & Goran, 2008; Zahir, Heyman, & Wojcicki, 2013).
Growth and stature
Glucocorticoids inhibit the production and activity of osteoblasts, increase osteoblast apoptosis, and increase osteoclast catabolic activity, leading to a decrease in bone formation and loss of bone mineral density (Olney, 2009). Chronically elevated glucocorticoid levels also suppress growth hormone secretion and contribute to linear growth failure through effects on growth-plate function (Giustina & Wehrenberg, 1992; Olney, 2009). The relationship between chronic stress and stunted growth in children is most profoundly demonstrated in studies of children exposed to extremely stressful environments such as institutionalization, foster care, or homelessness (Dobrova-Krol, van IJzendoorn, Bakermans-Kranenburg, Cyr, & Juffer, 2008; Fierman et al., 1991; Gunnar, Frenn, Wewerka, & Van Ryzin, 2009; A. E. Johnson, Bruce, Tarullo, & Gunnar, 2011). However, delayed growth has also been detected in infants and toddlers with exposure to maternal depression and early placement in childcare, suggesting growth measures may represent a sensitive indicator of exposure to chronic stress (Patel, DeSouza, & Rodrigues, 2003; Surkan et al., 2008; Zmiri, Rubin, Akons, Zion, & Shaoul, 2011).
Discussion
A wide range of biomarkers is available to help pediatric researchers explore the pathways linking exposure to chronic stress with lifelong disease. However, the field is still very much in its infancy, with most studies having been published only in the last decade. Thoughtful and theoretically based approaches to the selection and measurement of chronic stress biomarkers will be crucial for the quality of future biobehavioral research studies and ultimately lead to a better understanding of the effects of chronic stress in childhood and adolescence.
Selecting Appropriate Biomarkers
The physiologic response to chronic stress is complex and nonlinear and involves multiple physiological systems. Thus, selection of measures for a research study must include a theoretical basis such as the allostatic load model described in Figure 1. One robust approach might include a combination of both primary mediators and secondary outcomes of the stress response that reflects both HPA-axis and SNS activity, as these two systems are highly coordinated and interconnected (Rotenberg & McGrath, 2016). Evaluating and comparing responses to stress among multiple physiological systems may also provide important insight into the overall efficiency and functioning of the stress response system in the presence of chronic stress (Gordis, Granger, Susman, & Trickett, 2006; Koss et al., 2014).
An alternative approach to studying biomarkers across multiple physiological systems would be to focus on specific pathways of the stress response that lead to tertiary end points of interest. This approach might include examination of cortisol, cytokines, and sIGA to explore immune pathways; cortisol, insulin, and BMI to explore metabolic pathways; or catecholamines, blood pressure, and HRV to explore cardiac pathways. With this approach, chronic stress pathways can be examined with respect to interfering risk and protective factors or variations by child sex or age. It might also be useful for identifying sensitive periods where children are most vulnerable to the effects of chronic stress or windows of development where there are opportunities for repair.
Although incorporating biomarkers into pediatric research represents an innovative approach to understanding the effects of chronic stress, investigators must use caution when adding biomarkers to a research study, avoiding the temptation to select biomarkers based on convenience or novelty. Given the complexity of the physiologic response to chronic stress, interpretation of results presents a particular challenge. Failure to consider compensatory mechanisms or negative feedback loops could result in misleading findings, and blunted or suppressed responses to chronic stress could be misinterpreted or overlooked (Juster et al., 2010). Thus, researchers must select biomarkers a priori and on the basis of a strong theoretical rationale in order to produce the most meaningful results and make valuable contributions to the literature.
Measurement Considerations
Collection methods
Methods for measuring biomarkers include collection of serum, saliva, urine, or hair as well as electrocardiography and neuroimaging studies. In order to select the best method to both obtain accurate results and reduce participant burden, researchers must consider the invasiveness of the collection method, the setting for sample collection, and sample storage requirements. As diurnal rhythms are established in early infancy, researchers should also consider whether analysis requires collection of multiple samples across one or more days to capture diurnal rhythms, such as in collection of salivary cortisol, sAA, sIGA, or inflammatory cytokines (Adam & Kumari, 2009; Granger, Kivlighan, Fortunato, et al., 2007; Riis et al., 2015; Rivkees, 2003; Watamura et al., 2010). Table 4 contains a summary of the advantages and disadvantages of each biomarker collection method.
Table 4.
Summary of Biomarker Collection Methods.
| Method | Advantages | Disadvantages | Other Considerations |
|---|---|---|---|
| Serum | Reliable and valid, direct assessment of biomarker circulation in bloodstream | Invasive, may induce acute stress response | Some biomarkers can be analyzed in dried blood spots, offering a less-invasive alternative to whole blood samples |
| Saliva | Noninvasive, simple to collect | May require multiple samples to capture diurnal rhythms; validity for inflammatory markers unclear | Recent tooth brushing, food ingestion, dental issues, or illnesses may impact results |
| Urine | Noninvasive, reflects levels over 12- or 24-hr period | Burden related to 24-hr urine collection in outpatient setting | Must also correct for child’s body surface area and ensure adequacy of volume collected |
| Hair | Noninvasive, reflects chronic stress exposure over time | Reference ranges and confounders require further investigation | Potential variation related to sampling site, hair color, cosmetic treatments, and personal hygiene habits |
| Neuroimaging | Direct assessment of brain-matter volume and function | Participant burden, expensive | May use MRI to assess gray or white matter volume or fMRI to assess brain activity/function |
| Electrocardiography | Noninvasive, sensitive measure of cardiac electrical activity | Requires child cooperation, may be time consuming | Also consider ambulatory Holter monitoring |
| Anthropometric measures | Noninvasive, simple to collect | May not accurately reflect adiposity; measurement of waist circumference particularly prone to error | Also consider DEXA scan for more accurate measure of total adiposity |
Note. DEXA = dual-energy X-ray absorptiometry; fMRI = functional magnetic resonance imaging; MRI = magnetic resonance imaging.
Feasibility
When working with pediatric participants, researchers should carefully consider the feasibility of biomarker collection. For biomarkers that require collection of multiple samples, child nap times, school attendance, or parent work schedules may interfere with feasibility and accurate timing of sample collection (Condon, 2016). Additionally, although many available biomarkers are noninvasive, young children may still find it difficult to cooperate or follow directions for various collection methods. For example, certain salivary biomarkers require collection of passive drool, which may prove challenging in research with young children for whom collection with a cotton swab may be more appropriate. Urine collection might also be compromised with children who are not toilet trained, and hair collection might be unfeasible in children with absent or very short hair on the scalp. See Rodriguez et al. (2016) for a more detailed review of the developmental and physiologic considerations required when collecting biomarkers for research with children and adolescents.
Laboratory analysis
For many of the biomarkers reviewed, selecting a laboratory, method, or assay for sample analysis may present a significant challenge. While commonly used biomarkers like salivary cortisol are well validated, many others lack methodological gold standards or established reference ranges for use in pediatric research (Juster et al., 2010). For example, hair cortisol can be analyzed using a number of methods including enzyme-linked immunosorbent assays (ELISA), radioimmunoassays, and high-performance liquid chromatography–mass spectrometry (Gow, Thomson, Rieder, Van Uum, & Koren, 2010; Russell et al., 2012). While the majority of published studies have used the ELISA method, there is as yet no consensus on the most precise and accurate method for analysis (Gow et al., 2010). Similarly, a variety of immunoassays are available for analysis of cytokines either in isolation or as a panel, but the optimal method for cytokine analysis in children and adolescents is currently unknown (Dossus, Becker, Achaintre, Kaaks, & Rinaldi, 2009; Leng et al., 2008). Until further research is completed, investigators must exercise due diligence in selecting a method that is most likely to be reliable and reproducible. This selection may be based on expert opinion, laboratory consultation, or current recommendations in the literature. Choosing a laboratory also requires careful consideration, and researchers should determine whether quality control processes and safeguards are in place to ensure safety of samples as well as reliability and validity of results (Marton & Weiner, 2013).
Ethical considerations
When incorporating biomarkers into a study with children or adolescents, researchers must also carefully consider the ethical implications of biomarker collection. This consideration is particularly important for research with families who identify as members of an ethnic minority and who may mistrust medical research or attribute specific cultural meanings to collection of certain biomarkers in children (Corbie-Smith, Thomas, & George, 2002; Ford, Boch, & McCarthy, 2016). For example, in American Indian and Alaska native communities, hair cutting is often reserved for specific occasions such as periods of mourning (Ford et al., 2016). In research with young children, collection of a hair sample may be the child’s first haircut and thus may be an emotionally laden experience for the parent or caregiver. Participants may have apprehensions or concerns about invasive or radiological testing, and parents may experience uncertainty or misconceptions about the intended use of collected biomarker samples (Condon, 2016). As with all pediatric research, researchers must carefully weigh potential risks and benefits to participants when including biomarkers in a study (Rodriguez et al., 2016). Open communication and adequate time for questions are essential to ensure that families fully understand the type and extent of testing to be performed and can provide truly informed consent. Investigating the perceptions and preferences of families in future qualitative or community-based participatory research studies will also provide greater insight into the ethical implications of pediatric biobehavioral research.
Potential Moderators
Researchers should also consider potential moderators when collecting and analyzing biomarker data in children, as the strength and direction of relationships may be altered by a number of individual characteristics. Important moderators include, but are not limited to, child sex, age, and pubertal status.
Child sex
Sex differences have been noted in studies of both primary mediators and secondary outcomes of chronic stress in children. For example, researchers have consistently reported differences in serum and salivary cortisol levels between boys and girls and have also noted sex differences in studies of catecholamines and inflammatory cytokines (Doom, Cicchetti, Rogosch, & Dackis, 2013; Hatzinger et al., 2013; Östberg et al., 2015; Riis et al., 2015; Trainor, 2011). Differences in these primary mediators may also impact secondary outcomes, as sex differences in amygdala volume, CRP, blood pressure, and waist-to-hip ratio have been noted in studies of children experiencing chronic stress (Buss et al., 2012; Kozyrskyj et al., 2011; Martinovic et al., 2014; Pirkola et al., 2010). However, determining whether these differences are strictly biological or reflect differential experiences of stress and coping requires further exploration (Panagiotakopoulos & Neigh, 2014).
Child age
Susceptibility to chronic stress varies by age. For example, the brain develops in multiple stages throughout childhood, and its vulnerability to the effects of chronic stress varies over the course of development (Andersen, 2003). Thus, researchers must consider and further explore potentially sensitive periods in development in studies of chronic stress in children. In addition, expected biomarker values and appropriate reference ranges may differ by child age or developmental status such as in the cases of serum cortisol, sAA, and CRP (Barra, Silva, Rodrigues, Santos, & Colosimo, 2015; Strahler, Mueller, Rosenloecher, Kirschbaum, & Rohleder, 2010; Wener, Daum, & McQuillan, 2000). Researchers must also be careful to consider appropriate sources of stress for the selected age-group, as the type of stressors experienced by children often vary according to chronological or developmental age. For example, while younger children may be more vulnerable to maternal stress or parenting behaviors, older children may be more affected by school- or peer-related stressors.
Pubertal status
As children reach adolescence, the type and frequency of stressors they encounter may change dramatically as they spend more time with peers and strive toward independence (Klein & Romeo, 2013). Physiological responses to stress also change over the course of development, with children demonstrating increases in HPA-axis reactivity in response to stress following puberty (Blumenthal, Leen-Feldner, Badour, Trainor, & Babson, 2014; Klein & Romeo, 2013; Romeo, 2013). While the mechanisms that mediate puberty-related changes in HPA-axis activity remain unclear, evidence suggests that changes in secretion of sex hormones (testosterone, estradiol, and progesterone) altered sensitivity to adrenocorticotropic hormone and CRH, and differential levels of neural activation may each play a role (Klein & Romeo, 2013). As such, in studies of chronic stress in children, researchers have noted the moderating effect of pubertal status on cortisol and sAA levels (Barra et al., 2015; Granger, Kivlighan, Fortunato, et al., 2007; Negriff, Saxbe, & Trickett, 2015; Zhang et al., 2016). Normal developmental changes that occur during adolescence also require consideration, such as increased amygdala activity in response to emotional stimuli, increased dopamine system activity, and altered gray and white matter volume (Blakemore & Choudhury, 2006; Romeo, 2013; Wahlstrom, White, & Luciana, 2010). Exposure to chronic stress may also accelerate maturation, and thus investigators must precisely assess pubertal maturation when conducting research with older children and adolescents (Negriff et al., 2015).
Future Directions
While some biomarkers, such as salivary cortisol, have been widely measured in pediatric research studies on chronic stress, others, such as leptin and fibrinogen, have been examined in only a handful of studies. Given this gap in the literature, there is enormous opportunity for pediatric researchers to further uncover the complex physiological pathways through which chronic stress “gets under the skin.” Measurement of biomarkers across multiple age groups, as well as during the prenatal period, will provide valuable insight into periods of vulnerability or resilience across the course of development. Further research is also required to understand the impacts of a broader range of stressors that occur in childhood and adolescence. While the impacts of severe stressors such as violence, abuse, and neglect have been widely studied (Hillis, Mercy, & Saul, 2017; McCrory, De Brito, & Viding, 2011), the physiological impacts of prevalent stressors such as racism and bullying require further investigation. Further research in these areas will not only improve understanding of the physiological mechanisms underlying the chronic stress response, it will also inform future policies and targeted interventions that seek to improve health outcomes for children across all stages of development.
In studies of adults, standardized indexes of allostatic load have been used to evaluate the multisystemic effects of chronic stress and compare results across populations (Juster et al., 2010). While researchers have developed composite indexes of allostatic load for pediatric research studies, these indexes have often been limited by the use of only neuroendocrine and cardiovascular measures (Evans et al., 2007). As research on pediatric biomarker measurement continues to expand and validity and reference ranges of biomarkers are established, the next step will be to determine common data elements and develop comprehensive, standardized indexes of chronic stress for use in pediatric research studies. This task will best be accomplished by collaboration among researchers and laboratories as well as the publishing of negative findings to further advance the science. Ultimately, these efforts will create an opportunity for big data analysis, so that the causes, mechanisms, and consequences associated with chronic stress in childhood and adolescence can be fully explored.
Limitations
This review was limited by the lack of pediatric research conducted with many of the biomarkers outlined, particularly the immune and metabolic secondary outcomes associated with a chronic stress response. While the biomarkers I have discussed in this review offer promise for expanding our understanding of chronic stress and health, further research is required to identify the specific biomarkers that best reflect a chronic stress response in children and adolescents. Additionally, it is important to note that due to the complex nature of chronic stress in childhood, the amount of variance explained by biomarker data is often very small. Thus, although chronic stress biomarkers may be significantly associated with variables of interest in a given study, this association may not represent clinical significance for pediatric samples. However, such foundational physiological research is likely to provide important insight into the mechanisms linking stress and disease going forward.
Conclusion
The biomarkers outlined in this review represent important physiological measures with the potential to significantly contribute to research on chronic stress and lifelong health. Although the field remains in its infancy and further research related to measurement is required, thoughtfully incorporating these biomarkers into pediatric research studies will help to elucidate the pathways through which the physical and social environments “get under the skin” in childhood and adolescence. With a more comprehensive and nuanced understanding of these complex physiological pathways, future researchers can develop protective interventions and policies to promote health and reduce inequities among children exposed to chronic stress (Fisher et al., 2016; Gunnar et al., 2006; Slopen, McLaughlin, & Shonkoff, 2014).
Acknowledgments
The author would like to thank Lois Sadler, Linda Mayes, Arietta Slade, Margaret Holland, and Nancy Redeker for their thoughtful comments and suggestions during the development of this article.
Footnotes
Author Contribution: Condon E. contributed to conception and design; contributed to conception, design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Nursing Research of the National Institutes of Health (F31NR016385 and T32NR008346), the NAPNAP Foundation, the Connecticut Nurses Foundation, the Jonas Nurse Leaders Scholars Program, and the Alpha Nu chapter of Sigma Theta Tau International.
References
- Aardal E., Holm A. (1995). Cortisol in saliva-reference ranges and relation to cortisol in serum. Clinical Chemistry and Laboratory Medicine, 33, 927–932. [DOI] [PubMed] [Google Scholar]
- Adam E. K., Kumari M. (2009). Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology, 34, 1423–1436. [DOI] [PubMed] [Google Scholar]
- Addison S., Stas S., Hayden M. R., Sowers J. R. (2008). Insulin resistance and blood pressure. Current Hypertension Reports, 10, 319–325. [DOI] [PubMed] [Google Scholar]
- Alkon A., Boyce W. T., Tran L., Harley K. G., Neuhaus J., Eskenazi B. (2014). Prenatal adversities and Latino children’s autonomic nervous system reactivity trajectories from 6 months to 5 years of age. PLoS One, 9, e86283 doi:10.1371/journal.pone.0086283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S. L. (2003). Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience & Biobehavioral Reviews, 27, 3–18. [DOI] [PubMed] [Google Scholar]
- Apter-Levi Y., Pratt M., Vakart A., Feldman M., Zagoory-Sharon O., Feldman R. (2016). Maternal depression across the first years of life compromises child psychosocial adjustment; relations to child HPA-axis functioning. Psychoneuroendocrinology, 64, 47–56. doi:10.1016/j.psyneuen.2015.11.006 [DOI] [PubMed] [Google Scholar]
- Barra C. B., Silva I. N., Rodrigues T. M. B., Santos J. L. S., Colosimo E. A. (2015). Morning serum basal cortisol levels are affected by age and pubertal maturation in school-aged children and adolescents. Hormone Research in Paediatrics, 83, 55–61. doi:10.1159/000369801 [DOI] [PubMed] [Google Scholar]
- Belujon P., Grace A. A. (2015). Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proceedings of the Royal Society B: Biological Sciences, 282 doi:10.1098/rspb.2014.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S., Choudhury S. (2006). Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry, 47, 296–312. [DOI] [PubMed] [Google Scholar]
- Blumenthal H., Leen-Feldner E. W., Badour C. L., Trainor C. D., Babson K. A. (2014). Pubertal maturation and cortisol level in response to a novel social environment among female adolescents. Journal of Adolescence, 37, 893–900. doi:10.1016/j.adolescence.2014.06.005 [DOI] [PubMed] [Google Scholar]
- Bothe D. A., Grignon J. B., Olness K. N. (2014). The effects of a stress management intervention in elementary school children. Journal of Developmental and Behavioral Pediatrics, 35, 62–67. doi:10.1097/DBP.0000000000000016 [DOI] [PubMed] [Google Scholar]
- Brady S. S., Matthews K. A. (2006). Chronic stress influences ambulatory blood pressure in adolescents. Annals of Behavioral Medicine, 31, 80–88. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. (2009). Mucosal immunity: Induction, dissemination, and effector functions. Scandinavian Journal of Immunology, 70, 505–515. [DOI] [PubMed] [Google Scholar]
- Brody G. H., Yu T., Chen E., Miller G. E. (2014). Prevention moderates associations between family risks and youth catecholamine levels. Health Psychology, 33, 1435–1439. doi:10.1037/hea0000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. T., Staiano A. E., Drazba K. T., Gupta A. K., Sothern M., Katzmarzyk P. T. (2012). Elevated C-reactive protein in children from risky neighborhoods: Evidence for a stress pathway linking neighborhoods and inflammation in children. PLoS One, 7, e45419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücker J., Fries G. R., Kapczinski F., Post R. M., Yatham L. N., Vianna P.…Kauer-Sant’Anna M. (2015). Brain-derived neurotrophic factor and inflammatory markers in school-aged children with early trauma. Acta Psychiatrica Scandinavica, 131, 360–368. doi:10.1111/acps.12358 [DOI] [PubMed] [Google Scholar]
- Buss C., Davis E. P., Shahbaba B., Pruessner J. C., Head K., Sandman C. A. (2012). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America, 109, E1312–E1319. doi:10.1073/pnas.1201295109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagni E., Elenkov I. (2006). Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Annals of the New York Academy of Sciences, 1069, 62–76. [DOI] [PubMed] [Google Scholar]
- Calkins S. D., Graziano P. A., Berdan L. E., Keane S. P., Degnan K. A. (2008). Predicting cardiac vagal regulation in early childhood from maternal–child relationship quality during toddlerhood. Developmental Psychobiology, 50, 751–766. doi:10.1002/dev.20344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson E., Frostell A., Ludvigsson J., Faresjo M. (2014). Psychological stress in children may alter the immune response. Journal of Immunology, 192, 2071–2081. doi:10.4049/jimmunol.1301713 [DOI] [PubMed] [Google Scholar]
- Carrión V. G., Haas B. W., Garrett A., Song S., Reiss A. L. (2010). Reduced hippocampal activity in youth with posttraumatic stress symptoms: An fMRI study. Journal of Pediatric Psychology, 35, 559–569. doi:10.1093/jpepsy/jsp112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll-Scott A., Gilstad-Hayden K., Rosenthal L., Peters S. M., McCaslin C., Joyce R., Ickovics J. R. (2013). Disentangling neighborhood contextual associations with child body mass index, diet, and physical activity: The role of built, socioeconomic, and social environments. Social Science and Medicine, 95, 106–114. doi:10.1016/j.socscimed.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. S., Dellucci T., Turek C., Mir S. (2015). Predicting depressive symptoms and weight from adolescence to adulthood: Stressors and the role of protective factors. Journal of Youth and Adolescence, 44, 2122–2140. doi:10.1007/s10964-015-0301-5 [DOI] [PubMed] [Google Scholar]
- Caserta M. T., Wyman P. A., Wang H., Moynihan J., O’Connor T. G. (2011). Associations among depression, perceived self-efficacy, and immune function and health in preadolescent children. Development and Psychopathology, 23, 1139–1147. doi:10.1017/S0954579411000526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandola T., Brunner E., Marmot M. (2006). Chronic stress at work and the metabolic syndrome: Prospective study. BMJ (Clinical Research Ed.), 332, 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E., Tsigos C., Chrousos G. (2005). Endocrinology of the stress response. Annual Review of Physiology, 67, 259–284. doi:10.1146/annurev.physiol.67.040403.120816 [DOI] [PubMed] [Google Scholar]
- Chen E., Fisher E. B., Bacharier L. B., Strunk R. C. (2003). Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosomatic Medicine, 65, 984–992. doi:10.1097/01.PSY.0000097340.54195.3C [DOI] [PubMed] [Google Scholar]
- Chen E., Lee W. K., Cavey L., Ho A. (2013). Role models and the psychological characteristics that buffer low-socioeconomic-status youth from cardiovascular risk. Child Development, 84, 1241–1252. doi:10.1111/cdev.12037 [DOI] [PubMed] [Google Scholar]
- Chen E., Strunk R. C., Trethewey A., Schreier H. M. C., Maharaj N., Miller G. E. (2011). Resilience in low-socioeconomic-status children with asthma: Adaptations to stress. Journal of Allergy and Clinical Immunology, 128, 970–976. doi:10.1016/j.jaci.2011.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. R., Raine A., Soyfer L., Granger D. A. (2015). Interaction of adrenocortical activity and autonomic arousal on children’s externalizing and internalizing behavior problems. Journal of Abnormal Child Psychology, 43, 189–202. doi:10.1007/s10802-014-9900-y [DOI] [PubMed] [Google Scholar]
- Chen X., Gelaye B., Velez J. C., Barbosa C., Pepper M., Andrade A.…Williams M. A. (2015). Caregivers’ hair cortisol: A possible biomarker of chronic stress is associated with obesity measures among children with disabilities. BMC Pediatrics, 15, 9 doi:10.1186/s12887-015-0322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Armstead C. (2000). Family conflict predicts blood pressure changes in African-American adolescents: A preliminary examination. Journal of Adolescence, 23, 355–358. doi:10.1006/jado.2000.0320 [DOI] [PubMed] [Google Scholar]
- Coelho R., Viola T. W., Walss-Bass C., Brietzke E., Grassi-Oliveira R. (2014). Childhood maltreatment and inflammatory markers: A systematic review. Acta Psychiatrica Scandinavica, 129, 180–192. doi:10.1111/acps.12217 [DOI] [PubMed] [Google Scholar]
- Colburn W., DeGruttola V., DeMets D., Downing G., Hoth D., Oates J.…Woodcock J. (2001). Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology & Therapeutics, 69, 89–95. [DOI] [PubMed] [Google Scholar]
- Condon E. M. (2016). Psychosocial influences on acceptability and feasibility of salivary cortisol collection from community samples of children. Research in Nursing & Health, 39, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbie-Smith G., Thomas S. B., George D. M. M. S. (2002). Distrust, race, and research. Archives of Internal Medicine, 162, 2458–2463. [DOI] [PubMed] [Google Scholar]
- Costa de Oliveira Forkert E., de Moraes A. C. F., Carvalho H. B., Kafatos A., Manios Y., Sjöström M.…Moreno L. A. (2016). Abdominal obesity and its association with socioeconomic factors among adolescents from different living environments. Pediatric Obesity, 12, 110–119. doi:10.1111/ijpo.12116 [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Asevedo E., Mansur R. B., Zugman A., Pan P. M., Gadelha A.…Brietzke E. (2016). Inflammation, neurotrophism and oxidative stress and childhood psychopathology in a large community sample. Acta Psychiatrica Scandinavica, 133, 122–132. doi:10.1111/acps.12453 [DOI] [PubMed] [Google Scholar]
- Curcio R., Stettler H., Suter P. M., Aksözen J. B., Saleh L., Spanaus K.…von Eckardstein A. (2016). Reference intervals for 24 laboratory parameters determined in 24-hour urine collections. Clinical Chemistry and Laboratory Medicine (CCLM), 54, 105–116. [DOI] [PubMed] [Google Scholar]
- Danese A., Dove R., Belsky D. W., Henchy J., Williams B., Ambler A., Arseneault L. (2014). Leptin deficiency in maltreated children. Translational Psychiatry, 4, e446 doi:10.1038/tp.2014.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J., Wheeler J. G., Hirschfield G. M., Eda S., Eiriksdottir G., Rumley A.…Gudnason V. (2004). C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. New England Journal of Medicine, 350, 1387–1397. [DOI] [PubMed] [Google Scholar]
- Davis H., Treiber F. A. (2007). Perceived stress, heart rate, and blood pressure among adolescents with family members deployed in operation Iraqi freedom. Military Medicine, 172, 40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M. D., Baum A. S., Birmaher B., Ryan N. D. (1997). Urinary catecholamine excretion in childhood overanxious and posttraumatic stress disorders. Annals of the New York Academy of Sciences, 821, 451–455. doi:10.1111/j.1749-6632.1997.tb48303.x [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Lefter L., Trickett P. K., Putnam F. W. (1994). Urinary catecholamine excretion in sexually abused girls. Journal of the American Academy of Child & Adolescent Psychiatry, 33, 320–327. [DOI] [PubMed] [Google Scholar]
- Dixon D., Meng H., Goldberg R., Schneiderman N., Delamater A. (2009). Stress and body mass index each contributes independently to tumor necrosis factor-α production in prepubescent Latino children. Journal of Pediatric Nursing, 24, 378–388. doi:10.1016/j.pedn.2008.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan S. N., Dich N., Evans G. W. (2014). Childhood cumulative risk and later allostatic load: Mediating role of substance use. Health Psychology, 33, 1402–1409. [DOI] [PubMed] [Google Scholar]
- Doan S. N., Evans G. W. (2011). Maternal responsiveness moderates the relationship between allostatic load and working memory. Development and Psychopathology, 23, 873–880. [DOI] [PubMed] [Google Scholar]
- Dobrova-Krol N. A., van IJzendoorn M. H., Bakermans-Kranenburg M. J., Cyr C., Juffer F. (2008). Physical growth delays and stress dysregulation in stunted and non-stunted Ukrainian institution-reared children. Infant Behavior and Development, 31, 539–553. doi:10.1016/j.infbeh.2008.04.001 [DOI] [PubMed] [Google Scholar]
- Doom J. R., Cicchetti D., Rogosch F. A., Dackis M. N. (2013). Child maltreatment and gender interactions as predictors of differential neuroendocrine profiles. Psychoneuroendocrinology, 38, 1442–1454. doi:10.1016/j.psyneuen.2012.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossus L., Becker S., Achaintre D., Kaaks R., Rinaldi S. (2009). Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: Comparison with ELISA. Journal of Immunological Methods, 350, 125–132. [DOI] [PubMed] [Google Scholar]
- Drummond P. D., Hewson-Bower B. (1997). Increased psychosocial stress and decreased mucosal immunity in children with recurrent upper respiratory tract infections. Journal of Psychosomatic Research, 43, 271–278. doi:10.1016/S0022-3999(97)00002-0 [DOI] [PubMed] [Google Scholar]
- Du Clos T. W., Mold C. (2004). C-reactive protein. Immunologic Research, 30, 261–277. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M., Buckhalt J. A., Granger D. A., Erath S. A., Acebo C. (2007). The association between children’s sleep disruption and salivary interleukin-6. Journal of Sleep Research, 16, 188–197. doi:10.1111/j.1365-2869.2007.00593.x [DOI] [PubMed] [Google Scholar]
- El-Sheikh M., Hinnant J. B. (2011). Marital conflict, respiratory sinus arrhythmia, and allostatic load: Interrelations and associations with the development of children’s externalizing behavior. Development and Psychopathology, 23, 815–829. doi:10.1017/S0954579411000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland C. G., Hugo F. N., Hilgert J. B., Nascimento G. G., Junges R., Lim H.-J.…Bosch J. A. (2016). Psychological distress and salivary secretory immunity. Brain, Behavior, and Immunity, 52, 11–17. doi:10.1016/j.bbi.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex M. J., Klein M. H., Cho E., Kalin N. H. (2002). Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry, 52, 776–784. doi:10.1016/S0006-3223(02)01553-6 [DOI] [PubMed] [Google Scholar]
- Evans B. E., Greaves-Lord K., Euser A. S., Tulen J. H. M., Franken I. H. A., Huizink A. C. (2013). Determinants of physiological and perceived physiological stress reactivity in children and adolescents. PLoS One, 8 doi:10.1371/journal.pone.0061724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. W. (2003). A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology, 39, 924–933. [DOI] [PubMed] [Google Scholar]
- Evans G. W. (2013, November). Residential noise exposure and chronic stress in children. Paper presented at the annual conference of the Australian acoustical society 2013: Science, Technology and Amenity, Victor Harbor, Australia Retrieved from https://www.acoustics.asn.au/conference_proceedings/AAS2013/papers/p85.pdf [Google Scholar]
- Evans G. W., Exner-Cortens D., Kim P., Bartholomew D. (2013). Childhood poverty and blood pressure reactivity to and recovery from an acute stressor in late adolescence: The mediating role of family conflict. Psychosomatic Medicine, 75, 691–700. doi:10.1097/PSY.0b013e31829f9823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. W., Fuller-Rowell T. E. (2013). Childhood poverty, chronic stress, and young adult working memory: The protective role of self-regulatory capacity. Developmental Science, 16, 688–696. [DOI] [PubMed] [Google Scholar]
- Evans G. W., Fuller-Rowell T. E., Doan S. N. (2012). Childhood cumulative risk and obesity: The mediating role of self-regulatory ability. Pediatrics, 129, e68–e73. doi:10.1542/peds.2010-3647 [DOI] [PubMed] [Google Scholar]
- Evans G. W., Kim P. (2012). Childhood poverty and young adults’ allostatic load: The mediating role of childhood cumulative risk exposure. Psychological Science, 23, 979–983. doi:10.1177/0956797612441218 [DOI] [PubMed] [Google Scholar]
- Evans G. W., Kim P., Ting A. H., Tesher H. B., Shannis D. (2007). Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology, 43, 341–351. doi:10.1037/0012-1649.43.2.341 [DOI] [PubMed] [Google Scholar]
- Farr O. M., Ko B.-J., Joung K. E., Zaichenko L., Usher N., Tsoukas M.…Mantzoros C. S. (2015). Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutrition, Metabolism and Cardiovascular Diseases, 25, 479–488. doi:10.1016/j.numecd.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierman A. H., Dreyer B. P., Quinn L., Shulman S., Courtlandt C. D., Guzzo R. (1991). Growth delay in homeless children. Pediatrics, 88, 918–925. [PubMed] [Google Scholar]
- Fisher P. A., Beauchamp K. G., Roos L. E., Noll L. K., Flannery J., Delker B. C. (2016). The neurobiology of intervention and prevention in early adversity. Annual Review of Clinical Psychology, 12, 331–357. doi:10.1146/annurev-clinpsy-032814-112855 [DOI] [PubMed] [Google Scholar]
- Ford J. L., Boch S. J., McCarthy D. O. (2016). Feasibility of hair collection for cortisol measurement in population research on adolescent health. Nursing Research, 65, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S. E., Levitt P., Nelson C. A., III (2010). How the timing and quality of early experiences influence the development of brain architecture. Child Development, 81, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis L. A., Granger D. A., Susman E. J. (2013). Adrenocortical regulation, eating in the absence of hunger and BMI in young children. Appetite, 64, 32–38. doi:10.1016/j.appet.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D. S., Sherry B. (2009). The validity of BMI as an indicator of body fatness and risk among children. Pediatrics, 124, S23–S34. doi:10.1542/peds.2008-3586E [DOI] [PubMed] [Google Scholar]
- Friedman E. M., Karlamangla A. S., Gruenewald T. L., Koretz B., Seeman T. E. (2015). Early life adversity and adult biological risk profiles. Psychosomatic Medicine, 77(2), 176–185. doi:10.1097/PSY.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries A. B. W., Shirtcliff E. A., Pollak S. D. (2008). Neuroendocrine dysregulation following early social deprivation in children. Developmental Psychobiology, 50, 588–599. doi:10.1002/dev.20319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni A. J., Telzer E. H., Bower J., Cole S. W., Kiang L., Irwin M. R. (2009). A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosomatic Medicine, 71, 329–333. doi:10.1097/PSY.0b013e3181921b1f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariup M., Gonzalez A., Lázaro L., Torres F., Serra-Pagès C., Morer A. (2015). IL-8 and the innate immunity as biomarkers in acute child and adolescent psychopathology. Psychoneuroendocrinology, 62, 233–242. doi:10.1016/j.psyneuen.2015.08.017 [DOI] [PubMed] [Google Scholar]
- Garner A. S., Shonkoff J. P., Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, & Section on Developmental and Behavioral Pediatrics. (2012). Early childhood adversity, toxic stress, and the role of the pediatrician: Translating developmental science into lifelong health. Pediatrics, 129, e224–e2231. doi:10.1542/peds.2011-2662 [DOI] [PubMed] [Google Scholar]
- Giustina A., Wehrenberg W. B. (1992). The role of glucocorticoids in the regulation of growth hormone secretion mechanisms and clinical significance. Trends in Endocrinology & Metabolism, 3, 306–311. [DOI] [PubMed] [Google Scholar]
- Goldbacher E. M., Matthews K. A., Salomon K. (2005). Central adiposity is associated with cardiovascular reactivity to stress in adolescents. Health Psychology, 24, 375–384. doi:10.1037/0278-6133.24.4.375 [DOI] [PubMed] [Google Scholar]
- Goldman-Mellor S., Brydon L., Steptoe A. (2010). Psychological distress and circulating inflammatory markers in healthy young adults. Psychological Medicine, 40, 2079–2087. doi:10.1017/S0033291710000267 [DOI] [PubMed] [Google Scholar]
- Gomez M. T., Malozowski S., Winterer J., Vamvakopoulos N. C., Chrousos G. P. (1991). Urinary free cortisol values in normal children and adolescents. Journal of Pediatrics, 118, 256–258. [DOI] [PubMed] [Google Scholar]
- Goodman E., Daniels S. R., Dolan L. M. (2007). Socioeconomic disparities in insulin resistance: Results from the Princeton school district study. Psychosomatic Medicine, 69, 61–67. doi:10.1097/01.psy.0000249732.96753.8f [DOI] [PubMed] [Google Scholar]
- Goodman E., Must A., Daniels S. R., Dolan L. M. (2010). Hostility and adiposity mediate disparities in insulin resistance among adolescents and young adults. Journal of Pediatrics, 157, 572–577.e1 doi:10.1016/j.jpeds.2010.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis E. B., Granger D. A., Susman E. J., Trickett P. K. (2006). Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology, 31, 976–987. [DOI] [PubMed] [Google Scholar]
- Gow R., Thomson S., Rieder M., Van Uum S., Koren G. (2010). An assessment of cortisol analysis in hair and its clinical applications. Forensic Science International, 196, 32–37. doi:10.1016/j.forsciint.2009.12.040 [DOI] [PubMed] [Google Scholar]
- Granger D. A., Johnson S. B., Szanton S. L., Out D., Schumann L. L. (2012). Incorporating salivary biomarkers into nursing research: An overview and review of best practices. Biological Research for Nursing, 14, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. A., Kivlighan K. T., El-Sheikh M., Gordis E. B., Stroud L. R. (2007). Salivary a-amylase in biobehavioral research: Recent developments and applications. Annals of the New York Academy of Sciences, 1098, 122–144. doi:10.1196/annals.1384.008 [DOI] [PubMed] [Google Scholar]
- Granger D. A., Kivlighan K. T., Fortunato C., Harmon A. G., Hibel L. C., Schwartz E. B., Whembolua G. (2007). Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology & Behavior, 92, 583–590. [DOI] [PubMed] [Google Scholar]
- Grassi G., Ram V. S. (2016). Evidence for a critical role of the sympathetic nervous system in hypertension. Journal of the American Society of Hypertension, 10, 457–466. doi:10.1016/j.jash.2016.02.015 [DOI] [PubMed] [Google Scholar]
- Groeneveld M. G., Vermeer H. J., Linting M., Noppe G., van Rossum E. F., van IJzendoorn M. H. (2013). Children’s hair cortisol as a biomarker of stress at school entry. Stress, 16, 711–715. [DOI] [PubMed] [Google Scholar]
- Grossman P., Taylor E. W. (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74, 263–285. [DOI] [PubMed] [Google Scholar]
- Gunnar M. R., Fisher P. A., Dozier M., Fox N., Levine S., Neal C.…Vazquez D. (2006). Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology, 18, 651–677. doi:10.1017/S0954579406060330 [PubMed] [Google Scholar]
- Gunnar M. R., Frenn K., Wewerka S. S., Van Ryzin M. J. (2009). Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology, 34, 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M. R., Morison S. J., Chisholm K., Schuder M. (2001). Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology, 13, 611–628. doi:10.1017/S095457940100311X [DOI] [PubMed] [Google Scholar]
- Hair N. L., Hanson J. L., Wolfe B. L., Pollak S. D. (2015). Association of child poverty, brain development, and academic achievement. JAMA Pediatrics, 169, 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan K., McCarthy A. M., Kleiber C., Lutgendorf S., Tsalikian E. (2006). Strategies for salivary cortisol collection and analysis in research with children. Applied Nursing Research, 19, 95–101. [DOI] [PubMed] [Google Scholar]
- Hanson J. L., Chandra A., Wolfe B. L., Pollak S. D. (2011). Association between income and the hippocampus. PLoS One, 6, e18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. L., Nacewicz B. M., Sutterer M. J., Cayo A. A., Schaefer S. M., Rudolph K. D.…Davidson R. J. (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77, 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzinger M., Brand S., Perren S., Von Wyl A., Stadelmann S., von Klitzing K., Holsboer-Trachsler E. (2013). In pre-school children, cortisol secretion remains stable over 12 months and is related to psychological functioning and gender. Journal of Psychiatric Research, 47, 1409–1416. doi:10.1016/j.jpsychires.2013.05.030 [DOI] [PubMed] [Google Scholar]
- Hertzman C., Boyce T. (2010). How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health, 31, 329–347. [DOI] [PubMed] [Google Scholar]
- Hill E. E., Eisenmann J. C., Gentile D., Holmes M. E., Walsh D. (2011). The association between morning cortisol and adiposity in children varies by weight status. Journal of Pediatric Endocrinology and Metabolism, 24, 709–713. doi:10.1515/JPEM.2011.267 [DOI] [PubMed] [Google Scholar]
- Hillis S. D., Mercy J. A., Saul J. R. (2017). The enduring impact of violence against children. Psychology, Health & Medicine, 22, 393–405. [DOI] [PubMed] [Google Scholar]
- Hinnant J. B., El-Sheikh M., Keiley M., Buckhalt J. A. (2013). Marital conflict, allostatic load, and the development of children’s fluid cognitive performance. Child Development, 84, 2003–2014. doi:10.1111/cdev.12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnant J. B., Erath S. A., El-Sheikh M. (2015). Harsh parenting, parasympathetic activity, and development of delinquency and substance use. Journal of Abnormal Psychology, 124, 137–151. doi:10.1037/abn0000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel A. S., Hunt R. H., Cowell R. A., Van Den Heuvel S. E., Gunnar M. R., Thomas K. M. (2015). Duration of early adversity and structural brain development in post-institutionalized adolescents. NeuroImage, 105, 112–119. doi:10.1016/j.neuroimage.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollar D., Lombardo M., Lopez-Mitnik G., Hollar T. L., Almon M., Agatston A. S., Messiah S. E. (2010). Effective multi-level, multi-sector, school-based obesity prevention programming improves weight, blood pressure, and academic performance, especially among low-income, minority children. Journal of Health Care for the Poor and Underserved, 21, 93–108. [DOI] [PubMed] [Google Scholar]
- Holman E. A. (2015). Psychological distress and susceptibility to cardiovascular disease across the lifespan: Implications for future research and clinical practice. Journal of the American College of Cardiology, 66, 1587–1589. doi:10.1016/j.jacc.2015.08.026 [DOI] [PubMed] [Google Scholar]
- Hostinar C. E., Johnson A. E., Gunnar M. R. (2015). Early social deprivation and the social buffering of cortisol stress responses in late childhood: An experimental study. Developmental Psychology, 51, 1597–1608. doi:10.1037/dev0000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S., Shargill N. S., Spiegelman B. M. (1993). Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science, 259, 87–91. [DOI] [PubMed] [Google Scholar]
- Houseknecht K. L., Baile C. A., Matteri R. L., Spurlock M. E. (1998). The biology of leptin: A review. Journal of Animal Science, 76, 1405–1420. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2010). Evaluation of biomarkers and surrogate endpoints in chronic disease. Washington, DC: National Academies Press; doi:10.17226/12869 [PubMed] [Google Scholar]
- Jednoróg K., Altarelli I., Monzalvo K., Fluss J., Dubois J., Billard C.…Ramus F. (2012). The influence of socioeconomic status on children’s brain structure. PLoS One, 7, e42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop D. S., Turner-Cobb J. M. (2008). Measurement and meaning of salivary cortisol: A focus on health and disease in children: Review. Stress, 11, 1–14. [DOI] [PubMed] [Google Scholar]
- Johansson S., Norman M., Legnevall L., Dalmaz Y., Lagercrantz H., Vanpée M. (2007). Increased catecholamines and heart rate in children with low birth weight: Perinatal contributions to sympathoadrenal overactivity. Journal of Internal Medicine, 261, 480–487. doi:10.1111/j.1365-2796.2007.01776.x [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Bruce J., Tarullo A. R., Gunnar M. R. (2011). Growth delay as an index of allostatic load in young children: Predictions to disinhibited social approach and diurnal cortisol activity. Development and Psychopathology, 23, 859–871. doi:10.1017/S0954579411000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. B., Riis J. L., Noble K. G. (2016). State of the art review: Poverty and the developing brain. Pediatrics, 137, e20153075–e20153075. doi:10.1542/peds.2015-3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. B., Riley A. W., Granger D. A., Riis J. (2013). The science of early life toxic stress for pediatric practice and advocacy. Pediatrics, 131, 319–327. doi:10.1542/peds.2012-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung K. E., Park K.-H., Zaichenko L., Sahin-Efe A., Thakkar B., Brinkoetter M.…Mantzoros C. S. (2014). Early life adversity is associated with elevated levels of circulating leptin, irisin, and decreased levels of adiponectin in midlife adults. Journal of Clinical Endocrinology and Metabolism, 99, E1055–E1060. doi:10.1210/jc.2013-3669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R., McEwen B. S., Lupien S. J. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35, 2–16. [DOI] [PubMed] [Google Scholar]
- Karlen J., Frostell A., Theodorsson E., Faresjo T., Ludvigsson J. (2013). Maternal influence on child HPA axis: A prospective study of cortisol levels in hair. Pediatrics, 132, e1333–e1340. doi:10.1542/peds.2013-1178 [DOI] [PubMed] [Google Scholar]
- Karlen J., Ludvigsson J., Hedmark M., Faresjo A., Theodorsson E., Faresjo T. (2015). Early psychosocial exposures, hair cortisol levels, and disease risk. Pediatrics, 135, e1450–e1457. doi:10.1542/peds.2014-2561 [DOI] [PubMed] [Google Scholar]
- Keeshin B. R., Strawn J. R., Out D., Granger D. A., Putnam F. W. (2015). Elevated salivary alpha amylase in adolescent sexual abuse survivors with posttraumatic stress disorder symptoms. Journal of Child and Adolescent Psychopharmacology, 25, 344–350. doi:10.1089/cap.2014.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil M. F. (2012). Salivary cortisol: A tool for biobehavioral research in children. Journal of Pediatric Nursing, 27, 287–289. doi:10.1016/j.pedn.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P. S., El-Sheikh M., Vaughn B., Granger D. A. (2010). Relations between mucosal immunity and children’s mental health: The role of child sex. Physiology and Behavior, 101, 705–712. doi:10.1016/j.physbeh.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny M. E., Schedlowski M. (2007). Understanding the interaction between psychosocial stress and immune-related diseases: A stepwise progression. Brain, Behavior, and Immunity, 21, 1009–1018. [DOI] [PubMed] [Google Scholar]
- Kepper M., Sothern M., Zabaleta J., Ravussin E., Velasco-Gonzalez C., Leonardi C.…Scribner R. (2016). Prepubertal children exposed to concentrated disadvantage: An exploratory analysis of inflammation and metabolic dysfunction. Obesity, 24, 1148–1153. doi:10.1002/oby.21462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Yoon K. S. (1998). Stress: Metaplastic effects in the hippocampus. Trends in Neurosciences, 21, 505–509. [DOI] [PubMed] [Google Scholar]
- Klein Z. A., Romeo R. D. (2013). Changes in hypothalamic-pituitary-adrenal stress responsiveness before and after puberty in rats. Hormones and Behavior, 64, 357–363. doi:10.1016/j.yhbeh.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Kohlboeck G., Romanos M., Tiesler C., Koletzko S., Kratzsch J., Thiery J.…Heinrich J. (2014). Peer problems are associated with elevated serum leptin levels in children. Psychological Medicine, 44, 255–265. doi:10.1017/S003329171300069X [DOI] [PubMed] [Google Scholar]
- Koss K. J., George M. R., Cummings E. M., Davies P. T., El-Sheikh M., Cicchetti D. (2014). Asymmetry in children’s salivary cortisol and alpha-amylase in the context of marital conflict: Links to children’s emotional security and adjustment. Developmental Psychobiology, 56, 836–849. doi:10.1002/dev.21156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozyrskyj A. L., Zeng Y., Colman I., Hayglass K. T., Sellers E. A. C., Becker A. B., MacNeil B. J. (2011). Maternal distress in early life predicts the waist-to-hip ratio in schoolchildren. Journal of Developmental Origins of Health and Disease, 2, 72–80. doi:10.1017/S2040174410000723 [DOI] [PubMed] [Google Scholar]
- Krause-Parello C. A., Friedmann E. (2014). The effects of an animal-assisted intervention on salivary alpha-amylase, salivary immunoglobulin a, and heart rate during forensic interviews in child sexual abuse cases. Anthrozoos, 27, 581–590. doi:10.2752/089279314X14072268688005 [Google Scholar]
- Krenichyn K., Saegert S., Evans G. W. (2001). Parents as moderators of psychological and physiological correlates of inner-city children’s exposure to violence. Journal of Applied Developmental Psychology, 22, 581–602. doi:10.1016/S0193-3973(01)00095-8 [Google Scholar]
- Kushner M. R., Barrios C., Smith V. C., Dougherty L. R. (2015). Physiological and behavioral vulnerability markers increase risk to early life stress in preschool-aged children. Journal of Abnormal Child Psychology, 44, 859–870. doi:10.1007/s10802-015-0087-7 [DOI] [PubMed] [Google Scholar]
- Kwok M. K., Subramanian S. V., Leung G. M., Schooling C. M. (2015). Household income and adolescent blood pressure in a Chinese birth cohort: “Children of 1997.” Social Science and Medicine, 144, 88–95. doi:10.1016/j.socscimed.2015.09.012 [DOI] [PubMed] [Google Scholar]
- Landstra A. M., Postma D. S., Boezen H. M., Van Aalderen W. M. (2012). Role of serum cortisol levels in children with asthma. American Journal of Respiratory and Critical Care Medicine, 165, 708–712. doi:10.1164/ajrccm.165.5.2102115 [DOI] [PubMed] [Google Scholar]
- Laudat M., Cerdas S., Fournier C., Guiban D., Guilhaume B., Luton J. (1988). Salivary cortisol measurement: A practical approach to assess pituitary-adrenal function. Journal of Clinical Endocrinology & Metabolism, 66, 343–348. [DOI] [PubMed] [Google Scholar]
- Leng S. X., McElhaney J. E., Walston J. D., Xie D., Fedarko N. S., Kuchel G. A. (2008). ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 63, 879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. H., Snidman N., Leonard A., Meyer J., Tronick E. (2016). Intra-individual stability and developmental change in hair cortisol among postpartum mothers and infants: Implications for understanding chronic stress. Developmental Psychobiology, 58, 509–518. doi:10.1002/dev.21394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. T., Dancause K. N., Elgbeili G., Laplante D. P., King S. (2016). Disaster-related prenatal maternal stress explains increasing amounts of variance in body composition through childhood and adolescence: Project Ice Storm. Environmental Research, 150, 1–7. doi:10.1016/j.envres.2016.04.039 [DOI] [PubMed] [Google Scholar]
- Lopez-Castejon G., Brough D. (2011). Understanding the mechanism of IL-1ß secretion. Cytokine and Growth Factor Reviews, 22, 189–195. doi:10.1016/j.cytogfr.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. A., Matthews K. A., Hall M. (2013). Elevated C-reactive protein in adolescents: Roles of stress and coping. Psychosomatic Medicine, 75, 449–452. doi:10.1097/PSY.0b013e31828d3f1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J., Belden A., Botteron K., Marrus N., Harms M. P., Babb C.…Barch D. (2013). The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatrics, 167, 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J. L., Barch D. M., Belden A., Gaffrey M. S., Tillman R., Babb C.…Botteron K. N. (2012). Maternal support in early childhood predicts larger hippocampal volumes at school age. Proceedings of the National Academy of Sciences of the United States of America, 109, 2854–2859. doi:10.1073/pnas.1118003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S. J., King S., Meaney M. J., McEwen B. S. (2000). Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry, 48, 976–980. doi:10.1016/S0006-3223(00)00965-3 [DOI] [PubMed] [Google Scholar]
- Lupien S. J., Parent S., Evans A. C., Tremblay R. E., Zelazo P. D., Corbo V.…Seguin J. R. (2011). Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America, 108, 14324–1432. doi:10.1073/pnas.1105371108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakasioti G., Alexopoulos E. I., Varlami V., Chaidas K., Liakos N., Gourgoulianis K., Kaditis A. G. (2013). Low morning serum cortisol levels in children with tonsillar hypertrophy and moderate-to-severe OSA. Sleep, 36, 1349–1354. doi:10.5665/sleep.2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis N. J., Rol N., Corthésy B. (2011). Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunology, 4, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros C. S., Magkos F., Brinkoetter M., Sienkiewicz E., Dardeno T. A., Kim S. Y.…Koniaris A. (2011). Leptin in human physiology and pathophysiology. American Journal of Physiology, Endocrinology and Metabolism, 301, E567–E584. doi:10.1152/ajpendo.00315.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinovic M., Belojevic G., Evans G. W., Asanin B., Lausevic D., Kovacevic N. D.…Pantovic S. (2014). Blood pressure among rural Montenegrin children in relation to poverty and gender. European Journal of Public Health, 24, 385–389. doi:10.1093/eurpub/ckt181 [DOI] [PubMed] [Google Scholar]
- Marton M. J., Weiner R. (2013). Practical guidance for implementing predictive biomarkers into early phase clinical studies. BioMed Research International, 2013, 9 doi:10.1155/2013/891391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E., De Brito S. A., Viding E. (2011). The impact of childhood maltreatment: A review of neurobiological and genetic factors. Frontiers in Psychiatry, 2, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. (2003). Interacting mediators of allostasis and allostatic load: Towards an understanding of resilience in aging. Metabolism, 52, 10–16. [DOI] [PubMed] [Google Scholar]
- McEwen B. S. (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology, 583, 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. (2012). Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America, 109, 17180–17185. doi:10.1073/pnas.1121254109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S., Wingfield J. C. (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior, 43, 2–15. [DOI] [PubMed] [Google Scholar]
- McGrath J. J., Matthews K. A., Brady S. S. (2006). Individual versus neighborhood socioeconomic status and race as predictors of adolescent ambulatory blood pressure and heart rate. Social Science and Medicine, 63, 1442–1453. doi:10.1016/j.socscimed.2006.03.019 [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Novak M. A. (2012). Minireview: Hair cortisol: A novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology, 153, 4120–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels N., Sioen I., Clays E., De Buyzere M., Ahrens W., Huybrechts I.…De Henauw S. (2013). Children’s heart rate variability as stress indicator: Association with reported stress and cortisol. Biological Psychology, 94, 433–440. doi:10.1016/j.biopsycho.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Miller A. L., Clifford C., Sturza J., Rosenblum K., Vazquez D. M., Kaciroti N., Lumeng J. C. (2013). Blunted cortisol response to stress is associated with higher body mass index in low-income preschool-aged children. Psychoneuroendocrinology, 38, 2611–2617. doi:10.1016/j.psyneuen.2013.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. L., Sturza J., Rosenblum K., Vazquez D. M., Kaciroti N., Lumeng J. C. (2015). Salivary alpha amylase diurnal pattern and stress response are associated with body mass index in low-income preschool-aged children. Psychoneuroendocrinology, 53, 40–48. doi:10.1016/j.psyneuen.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E., Chen E., Zhou E. S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. doi:10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Mills N. T., Scott J. G., Wray N. R., Cohen-Woods S., Baune B. T. (2013). Research review: The role of cytokines in depression in adolescents: A systematic review. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54, 816–835. doi:10.1111/jcpp.12080 [DOI] [PubMed] [Google Scholar]
- Moore G. A. (2010). Parent conflict predicts infants’ vagal regulation in social interaction. Development and Psychopathology, 22, 23–33. doi:10.1017/S095457940999023X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey J. N., Boggero I. A., Scott A. B., Segerstrom S. C. (2015). Current directions in stress and human immune function. Current Opinion in Psychology, 5, 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Errington S., Szabo C. P., Pitts N., Jacklin L. (2014). Cortisol and IL-6 responses to stress in female children presenting at a sexual abuse clinic. Journal of Child and Adolescent Trauma, 7, 185–191. doi:10.1007/s40653-014-0019-7 [Google Scholar]
- Murali R., Chen E. (2005). Exposure to violence and cardiovascular and neuroendocrine measures in adolescents. Annals of Behavioral Medicine, 30, 155–163. doi:10.1207/s15324796abm3002_8 [DOI] [PubMed] [Google Scholar]
- Mushtaq M. U., Gull S., Abdullah H. M., Shahid U., Shad M. A., Akram J. (2011). Waist circumference, waist-hip ratio and waist-height ratio percentiles and central obesity among Pakistani children aged five to twelve years. BMC Pediatrics, 11, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakavachara P., Viprakasit V. (2013). Adrenal insufficiency is prevalent in HbE/ß-thalassaemia paediatric patients irrespective of their clinical severity and transfusion requirement. Clinical Endocrinology, 79, 776–783. doi:10.1111/cen.12235 [DOI] [PubMed] [Google Scholar]
- Nater U. M., Rohleder N. (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology, 34, 486–496. doi:10.1016/j.psyneuen.2009.01.014 [DOI] [PubMed] [Google Scholar]
- Negriff S., Saxbe D. E., Trickett P. K. (2015). Childhood maltreatment, pubertal development, HPA axis functioning, and psychosocial outcomes: An integrative biopsychosocial model. Developmental Psychobiology, 57, 984–993. doi:10.1002/dev.21340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neovius M., Linne Y., Rossner S. (2005). BMI, waist-circumference and waist-hip-ratio as diagnostic tests for fatness in adolescents. International Journal of Obesity, 29, 163–169. [DOI] [PubMed] [Google Scholar]
- Noble K. G., Houston S. M., Brito N. H., Bartsch H., Kan E., Kuperman J. M.…Libiger O. (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K. G., Houston S. M., Kan E., Sowell E. R. (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15, 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney R. C. (2009). Mechanisms of impaired growth: Effect of steroids on bone and cartilage. Hormone Research, 72, 30–35. doi:10.1159/000229761 [DOI] [PubMed] [Google Scholar]
- Olstad D. L., Ball K., Wright C., Abbott G., Brown E., Turner A. I. (2016). Hair cortisol levels, perceived stress and body mass index in women and children living in socioeconomically disadvantaged neighborhoods: The READI study. Stress, 19, 158–167. doi:10.3109/10253890.2016.1160282 [DOI] [PubMed] [Google Scholar]
- Oosterman M., De Schipper J. C., Fisher P., Dozier M., Schuengel C. (2010). Autonomic reactivity in relation to attachment and early adversity among foster children. Development and Psychopathology, 22, 109–118. doi:10.1017/S0954579409990290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östberg V., Almquist Y. B., Folkesson L., Låftman S. B., Modin B., Lindfors P. (2015). The complexity of stress in mid-adolescent girls and boys: Findings from the multiple methods school stress and support study. Child Indicators Research, 8, 403–423.doi:10.1007/s12187-014-9245-7 [Google Scholar]
- Pabst O. (2012). New concepts in the generation and functions of IgA. Nature Reviews Immunology, 12, 821–832. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J. L., Bogdan R., Agrawal A., Gaffrey M. S., Belden A. C.…Barch D. M. (2014). Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology, 39, 1245–1253. doi:10.1038/npp.2013.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer F. B., Anand K. J., Graff J. C., Murphy L. E., Qu Y., Völgyi E.…Tylavsky F. A. (2013). Early adversity, socioemotional development, and stress in urban 1-year-old children. Journal of Pediatrics, 163, 1733–1739.e1. [DOI] [PubMed] [Google Scholar]
- Panagiotakopoulos L., Neigh G. N. (2014). Development of the HPA axis: Where and when do sex differences manifest? Frontiers in Neuroendocrinology, 35, 285–302. doi:10.1016/j.yfrne.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Patel V., DeSouza N., Rodrigues M. (2003). Postnatal depression and infant growth and development in low income countries: A cohort study from Goa, India. Archives of Disease in Childhood, 88, 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaston R. T., Weinkove C. (2004). Measurement of catecholamines and their metabolites. Annals of Clinical Biochemistry, 41, 17–38. doi:10.1258/000456304322664663 [DOI] [PubMed] [Google Scholar]
- Pedersen J. M., Lund R., Andersen I., Clark A. J., Prescott E., Rod N. H. (2016). Psychosocial risk factors for the metabolic syndrome: A prospective cohort study. International Journal of Cardiology, 215, 41–46. doi:10.1016/j.ijcard.2016.04.076 [DOI] [PubMed] [Google Scholar]
- Pervanidou P., Chrousos G. P. (2012). Metabolic consequences of stress during childhood and adolescence. Metabolism, 61, 611–619. [DOI] [PubMed] [Google Scholar]
- Pervanidou P., Margeli A., Lazaropoulou C., Papassotiriou I., Chrousos G. P. (2008). The immediate and long-term impact of physical and/or emotional stress from motor vehicle accidents on circulating stress hormones and adipo-cytokines in children and adolescents. Stress, 11, 438–447. doi:10.1080/10253890801890622 [DOI] [PubMed] [Google Scholar]
- Phelps E. A., LeDoux J. E. (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48, 175–187. doi:10.1016/j.neuron.2005.09.025 [DOI] [PubMed] [Google Scholar]
- Pirkola J., Vääräsmäki M., Ala-Korpela M., Bloigu A., Canoy D., Hartikainen A.-L.…Pouta A. (2010). Low-grade, systemic inflammation in adolescents: Association with early-life factors, gender, and lifestyle. American Journal of Epidemiology, 171, 72–82. doi:10.1093/aje/kwp320 [DOI] [PubMed] [Google Scholar]
- Potasz C., Varela M. J. V. D., Carvalho L. C. D., Prado L. F. D., Prado G. F. D. (2013). Effect of play activities on hospitalized children’s stress: A randomized clinical trial. Scandinavian Journal of Occupational Therapy, 20, 71–79. doi:10.3109/11038128.2012.729087 [DOI] [PubMed] [Google Scholar]
- Powell L. M., Wada R., Krauss R. C., Wang Y. (2012). Ethnic disparities in adolescent body mass index in the United States: The role of parental socioeconomic status and economic contextual factors. Social Science and Medicine, 75, 469–476. doi:10.1016/j.socscimed.2012.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretty C., O’Leary D. D., Cairney J., Wade T. J. (2013). Adverse childhood experiences and the cardiovascular health of children: A cross-sectional study. BMC Pediatrics, 13, 208 doi:10.1186/1471-2431-13-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest N. C., Paradies Y. C., Gunthorpe W., Cairney S. J., Sayers S. M. (2011). Racism as a determinant of social and emotional wellbeing for aboriginal Australian youth. Medical Journal of Australia, 194, 546–550. [DOI] [PubMed] [Google Scholar]
- Pumprla J., Howorka K., Groves D., Chester M., Nolan J. (2002). Functional assessment of heart rate variability: Physiological basis and practical applications. International Journal of Cardiology, 84, 1–14. [DOI] [PubMed] [Google Scholar]
- Rao U., Chen L.-A., Bidesi A. S., Shad M. U., Thomas M. A., Hammen C. L. (2010). Hippocampal changes associated with early-life adversity and vulnerability to depression. Biological Psychiatry, 67, 357–364. doi:10.1016/j.biopsych.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U., Hammen C. L., Poland R. E. (2010). Longitudinal course of adolescent depression: Neuroendocrine and psychosocial predictors. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 141–151. doi:10.1097/00004583-201002000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash J. A., Campbell T. S., Letourneau N., Giesbrecht G. F. (2015). Maternal cortisol during pregnancy is related to infant cardiac vagal control. Psychoneuroendocrinology, 54, 78–89. doi:10.1016/j.psyneuen.2015.01.024 [DOI] [PubMed] [Google Scholar]
- Reinhart W. H. (2003). Fibrinogen—marker or mediator of vascular disease? Vascular Medicine, 8, 211–216. doi:10.1191/1358863x03vm494ra [DOI] [PubMed] [Google Scholar]
- Riis J. L., Granger D. A., DiPietro J. A., Bandeen-Roche K., Johnson S. B. (2015). Salivary cytokines as a minimally-invasive measure of immune functioning in young children: Correlates of individual differences and sensitivity to laboratory stress. Developmental Psychobiology, 57, 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis J. L., Granger D. A., Minkovitz C. S., Bandeen-Roche K., DiPietro J. A., Johnson S. B. (2016). Maternal distress and child neuroendocrine and immune regulation. Social Science and Medicine, 151, 206–214. doi:10.1016/j.socscimed.2015.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe R. C. A., Noppe G., Windhorst D. A., Tiemeier H., van Rossum E. F. C., Jaddoe V. W. V.…van den Akker E. L. T. (2016). Splitting hair for cortisol? Associations of socio-economic status, ethnicity, hair color, gender and other child characteristics with hair cortisol and cortisone. Psychoneuroendocrinology, 66, 56–64. doi:10.1016/j.psyneuen.2015.12.016 [DOI] [PubMed] [Google Scholar]
- Rivkees S. A. (2003). Developing circadian rhythmicity in infants. Pediatrics, 112, 373–381. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. (1982). Cortisol-induced insulin resistance in man: Impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action. Journal of Clinical Endocrinology and Metabolism, 54, 131–138. [DOI] [PubMed] [Google Scholar]
- Rodriguez J., Huntington-Moskos L., Johnson A., Williams S., Gulledge E., Feeley C., Rice M. (2016). Collecting biological measures for research with children and adolescents. Journal of Pediatric Health Care, 30, 279–283. [DOI] [PubMed] [Google Scholar]
- Rogosch F. A., Dackis M. N., Cicchetti D. (2011). Child maltreatment and allostatic load: Consequences for physical and mental health in children from low-income families. Development and Psychopathology, 23, 1107–1124. doi:10.1017/S0954579411000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo R. D. (2013). The teenage brain: The stress response and the adolescent brain. Current Directions in Psychological Science, 22, 140–145. doi:10.1177/0963721413475445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg S., McGrath J. J. (2016). Inter-relation between autonomic and HPA axis activity in children and adolescents. Biological Psychology, 117, 16–25. doi:10.1016/j.biopsycho.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski A., Blumenthal J. A., Kaplan J. (1999). Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation, 99, 2192–2217. [DOI] [PubMed] [Google Scholar]
- Russell E., Koren G., Rieder M., Van Uum S. (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37, 589–601. [DOI] [PubMed] [Google Scholar]
- Ruttle P. L., Klein M. H., Slattery M. J., Kalin N. H., Armstrong J. M., Essex M. J. (2014). Adolescent adrenocortical activity and adiposity: Differences by sex and exposure to early maternal depression. Psychoneuroendocrinology, 47, 68–77. doi:10.1016/j.psyneuen.2014.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman K. M., Holden G. W., Holahan C. J. (2005). The psychobiology of children exposed to marital violence. Journal of Clinical Child and Adolescent Psychology, 34, 129–139. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M. (1996). Stress, glucocorticoids, and damage to the nervous system: The current state of confusion. Stress, 1, 1–19. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M. (2002). Endocrinology of the stress-response In Becker J. B., Breedlove S. M., Crews D., McCarthy M. M. (Eds.), Behavioral endocrinology (pp. 409–450). Cambridge, MA: MIT Press. [Google Scholar]
- Sapolsky R. M., Romero L. M., Munck A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21, 55–89. doi:10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Sauvé B., Koren G., Walsh G., Tokmakejian S., Van Uum S. H. (2007). Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical & Investigative Medicine, 30, 183–191. [DOI] [PubMed] [Google Scholar]
- Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. (2011). The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta—Molecular Cell Research, 1813, 878–888. doi:10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- Sesso M. L. T., Borges M. C. L., Ferriani V. P. L., Geraldo-Martins V. R., Rodrigues D. B. R., Nogueira R. D. (2014). Prospective evaluation of cytokine in saliva of preterm and fullterm neonates. Immunobiology, 219, 830–835. doi:10.1016/j.imbio.2014.07.015 [DOI] [PubMed] [Google Scholar]
- Shonkoff J. P., Garner A. S., Siegel B. S., Dobbins M. I., Earls M. F., McGuinn L.,…Committee on Early Childhood, Adoption, and Dependent Care. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129, e232–e246. [DOI] [PubMed] [Google Scholar]
- Simmons J. G., Badcock P. B., Whittle S. L., Byrne M. L., Mundy L., Patton G. C.…Allen N. B. (2016). The lifetime experience of traumatic events is associated with hair cortisol concentrations in community-based children. Psychoneuroendocrinology, 63, 276–281. doi:10.1016/j.psyneuen.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Slopen N., Kubzansky L. D., Koenen K. C. (2013). Internalizing and externalizing behaviors predict elevated inflammatory markers in childhood. Psychoneuroendocrinology, 38, 2854–2862. doi:10.1016/j.psyneuen.2013.07.012 [DOI] [PubMed] [Google Scholar]
- Slopen N., Kubzansky L. D., McLaughlin K. A., Koenen K. C. (2013). Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology, 38, 188–200. doi:10.1016/j.psyneuen.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N., McLaughlin K. A., Shonkoff J. P. (2014). Interventions to improve cortisol regulation in children: A systematic review. Pediatrics, 133, 312–326. doi:10.1542/peds.2013-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smy L., Shaw K., Smith A., Russell E., Van Uum S., Rieder M.…Koren G. (2015). Hair cortisol as a novel biomarker of HPA suppression by inhaled corticosteroids in children. Pediatric Research, 78, 44–47. [DOI] [PubMed] [Google Scholar]
- Snider S. R., Kuchel O. (1983). Dopamine: An important neurohormone of the sympathoadrenal system. Significance of increased peripheral dopamine release for the human stress response and hypertension. Endocrine Reviews, 4, 291–309. [DOI] [PubMed] [Google Scholar]
- Sorota S. (2014). The sympathetic nervous system as a target for the treatment of hypertension and cardiometabolic diseases. Journal of Cardiovascular Pharmacology, 63, 466–476. doi:10.1097/FJC.0000000000000064 [DOI] [PubMed] [Google Scholar]
- Spruill T. M. (2010). Chronic psychosocial stress and hypertension. Current Hypertension Reports, 12, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A., Kivimäki M. (2013). Stress and cardiovascular disease: An update on current knowledge. Annual Review of Public Health, 34, 337–354. [DOI] [PubMed] [Google Scholar]
- Strahler J., Mueller A., Rosenloecher F., Kirschbaum C., Rohleder N. (2010). Salivary α-amylase stress reactivity across different age groups. Psychophysiology, 47, 587–595. [DOI] [PubMed] [Google Scholar]
- Stumvoll M., Tataranni P. A., Stefan N., Vozarova B., Bogardus C. (2003). Glucose allostasis. Diabetes, 52, 903–909. [DOI] [PubMed] [Google Scholar]
- Surkan P. J., Kawachi I., Ryan L. M., Berkman L. F., Carvalho Vieira L. M., Peterson K. E. (2008). Maternal depressive symptoms, parenting self-efficacy, and child growth. American Journal of Public Health, 98, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Luby J. L., Botteron K. N., Dietrich R., McAvoy M. P., Barch D. M. (2014). Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. Journal of the American Academy of Child & Adolescent Psychiatry, 53, 800–813.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate E. B., Wood W., Liao Y., Dunton G. F. (2015). Do stressed mothers have heavier children? A meta-analysis on the relationship between maternal stress and child body mass index. Obesity Reviews, 16, 351–361. doi:10.1111/obr.12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. L., Machacek D., Singh R. J. (2002). Validation of a high-throughput liquid chromatography-tandem mass spectrometry method for urinary cortisol and cortisone. Clinical Chemistry, 48, 1511–1519. [PubMed] [Google Scholar]
- Thomas C., Nightingale C. M., Donin A. S., Rudnicka A. R., Owen C. G., Sattar N.…Whincup P. H. (2012). Socio-economic position and type 2 diabetes risk factors: Patterns in UK children of south Asian, Black African-Caribbean and White European origin. PLoS One, 7 doi:10.1371/journal.pone.0032619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M. A. (2009). A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience, 3, 68 doi:10.3389/neuro.09.068.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor B. C. (2011). Stress responses and the mesolimbic dopamine system: Social contexts and sex differences. Hormones and Behavior, 60, 457–469. doi:10.1016/j.yhbeh.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka A. R., Parade S. H., Valentine T. R., Eslinger N. M., Seifer R. (2015). Adversity in preschool-aged children: Effects on salivary interleukin-1ß. Development and Psychopathology, 27, 567–576. doi:10.1017/S0954579415000164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A., Blair C. (2015). Children’s cortisol and salivary alpha-amylase interact to predict attention bias to threatening stimuli. Physiology and Behavior, 138, 266–272. doi:10.1016/j.physbeh.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaghri Z., Guhn M., Weinberg J., Grunau R. E., Yu W., Hertzman C. (2013). Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology, 38, 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den Berg G., Van Eijsden M., Vrijkotte T. G. M., Gemke R. J. B. J. (2012). Socioeconomic inequalities in lipid and glucose metabolism in early childhood in a population-based cohort: The ABCD-study. BMC Public Health, 12, 591 doi:10.1186/1471-2458-12-591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk A. E., van Eijsden M., Stronks K., Gemke R. J. B. J., Vrijkotte T. G. M. (2012). Prenatal stress and balance of the child’s cardiac autonomic nervous system at age 5-6 years. PLoS One, 7, e30413 doi:10.1371/journal.pone.0030413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk A. E., van Eijsden M., Stronks K., Gemke R. J. B. J., Vrijkotte T. G. M. (2015). No associations of prenatal maternal psychosocial stress with fasting glucose metabolism in offspring at 5-6 years of age. Journal of Developmental Origins of Health and Disease, 5, 361–369. doi:10.1017/S2040174414000300 [DOI] [PubMed] [Google Scholar]
- Vanaelst B., Huybrechts I., Bammann K., Michels N., Vriendt T., Vyncke K.…Molnar D. (2012). Intercorrelations between serum, salivary, and hair cortisol and child—reported estimates of stress in elementary school girls. Psychophysiology, 49, 1072–1081. [DOI] [PubMed] [Google Scholar]
- Vanaelst B., Michels N., Clays E., Herrmann D., Huybrechts I., Sioen I.…De Henauw S. (2014). The association between childhood stress and body composition, and the role of stress-related lifestyle factors—cross-sectional findings from the baseline ChiBS survey. International Journal of Behavioral Medicine, 21, 292–301. doi:10.1007/s12529-013-9294-1 [DOI] [PubMed] [Google Scholar]
- Vanaelst B., Michels N., De Vriendt T., Huybrechts I., Vyncke K., Sioen I.…Molnar D. (2013). Cortisone in hair of elementary school girls and its relationship with childhood stress. European Journal of Pediatrics, 172, 843–846. [DOI] [PubMed] [Google Scholar]
- Vanderas A. P., Menenakou M., Kouimtzis T., Papagiannoulis L. (1999). Urinary catecholamine levels and bruxism in children. Journal of Oral Rehabilitation, 26, 103–110. [DOI] [PubMed] [Google Scholar]
- Veldhorst M. A., Noppe G., Jongejan M. H., Kok C. B., Mekic S., Koper J. W.…van den Akker E. L. (2013). Increased scalp hair cortisol concentrations in obese children. Journal of Clinical Endocrinology & Metabolism, 99, 285–290. [DOI] [PubMed] [Google Scholar]
- Vermeer H. J., van IJzendoorn M. H., Groeneveld M. G., Granger D. A. (2012). Downregulation of the immune system in low-quality child care: The case of Secretory Immunoglobulin A (SIgA) in toddlers. Physiology and Behavior, 105, 161–167. doi:10.1016/j.physbeh.2011.08.017 [DOI] [PubMed] [Google Scholar]
- Vignali D. A. A. (2000). Multiplexed particle-based flow cytometric assays. Journal of Immunological Methods, 243, 243–255. doi:10.1016/S0022-1759(00)00238-6 [DOI] [PubMed] [Google Scholar]
- Vining R. F., McGinley R. A., Maksvytis J. J., Ho K. Y. (1983). Salivary cortisol: A better measure of adrenal cortical function than serum cortisol. Annals of Clinical Biochemistry, 20, 329–335. [DOI] [PubMed] [Google Scholar]
- Vitaliano P. P., Scanlan J. M., Zhang J., Savage M. V., Hirsch I. B., Siegler I. C. (2002). A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosomatic Medicine, 64, 418–435. [DOI] [PubMed] [Google Scholar]
- Vliegenthart J., Noppe G., van Rossum E. F. C., Koper J. W., Raat H., van den Akker E. L. T. (2016). Socioeconomic status in children is associated with hair cortisol levels as a biological measure of chronic stress. Psychoneuroendocrinology, 65, 9–14. doi:10.1016/j.psyneuen.2015.11.022 [DOI] [PubMed] [Google Scholar]
- Wahlstrom D., White T., Luciana M. (2010). Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience & Biobehavioral Reviews, 34, 631–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watamura S. E., Coe C. L., Laudenslager M. L., Robertson S. S. (2010). Child care setting affects salivary cortisol and antibody secretion in young children. Psychoneuroendocrinology, 35, 1156–1166. doi:10.1016/j.psyneuen.2010.02.001 [DOI] [PubMed] [Google Scholar]
- Waynforth D. (2007). The influence of parent-infant cosleeping, nursing, and childcare on cortisol and SIgA immunity in a sample of British children. Developmental Psychobiology, 49, 640–648. doi:10.1002/dev.20248 [DOI] [PubMed] [Google Scholar]
- Weigensberg M. J., Toledo-Corral C. M., Goran M. I. (2008). Association between the metabolic syndrome and serum cortisol in overweight Latino youth. Journal of Clinical Endocrinology and Metabolism, 93, 1372–1378. doi:10.1210/jc.2007-2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wener M. H., Daum P. R., McQuillan G. M. (2000). The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. Journal of Rheumatology, 27, 2351–2359. [PubMed] [Google Scholar]
- Willemen A. M., Schuengel C., Koot H. M. (2009). Physiological regulation of stress in referred adolescents: The role of the parent-adolescent relationship. Journal of Child Psychology and Psychiatry and Allied Disciplines, 50, 482–490. doi:10.1111/j.1469-7610.2008.01982.x [DOI] [PubMed] [Google Scholar]
- Wilson D. K., Kliewer W., Plybon L., Sica D. A. (2000). Socioeconomic status and blood pressure reactivity in healthy Black adolescents. Hypertension, 35, 496–500. [DOI] [PubMed] [Google Scholar]
- Winning A., Glymour M. M., McCormick M. C., Gilsanz P., Kubzansky L. D. (2015. Psychological distress across the life course and cardiometabolic risk findings from the 1958 British birth cohort study. Journal of the American College of Cardiology, 66, 1577–1586. doi:10.1016/j.jacc.2015.08.021 [DOI] [PubMed] [Google Scholar]
- Yasuma F., Hayano J. (2004). Respiratory sinus arrhythmia: Why does the heartbeat synchronize with respiratory rhythm? Chest Journal, 125, 683–690. [DOI] [PubMed] [Google Scholar]
- Zahir N., Heyman M. B., Wojcicki J. M. (2013). No association between childcare and obesity at age 4 in low-income Latino children. Pediatric Obesity, 8, E24–E28. doi:10.1111/j.20I47-6T310.2012.Y00125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Lam S.-P., Kong A. P. S., Ma R. C. W., Li S. X., Chan J. W. Y.…Wing Y.-K. (2016). Family conflict and lower morning cortisol in adolescents and adults: Modulation of puberty. Scientific Reports, 6, 22531 doi:10.1038/srep22531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmiri P., Rubin L., Akons H., Zion N., Shaoul R. (2011). The effect of day care attendance on infant and toddler’s growth. Acta Paediatrica, International Journal of Paediatrics, 100, 266–270. doi:10.1111/j.1651-2227.2010.01999.x [DOI] [PubMed] [Google Scholar]