Abstract
The oviduct/fallopian tube is a crucial organ in the mammalian reproductive tract; it plays a critical role in gamete transportation and early embryo development. In women, torsion of the fallopian tubes can cause ischemia and reperfusion (IR) injury. In this study, we tested the effect of this injury on recruitment of bone marrow–derived cells (BMDCs) to the oviducts of reproductive age female mice. Bone marrow–derived cells were collected from ubiquitin–green fluorescent protein–positive male mice and transplanted into wild-type female mice. Ischemia and reperfusion injury was performed in half of the mice, while controls received equivalent surgery without oviduct injury. Two weeks following injury, recruitment of BMDCs to the oviducts was analyzed in both groups. Ischemia and reperfusion injury caused a greater than 2-fold increase in BMDC recruitment to the injured oviducts compared to those without injury. Specifically, the recruitment of BMDCs was localized to the stroma of the oviduct. We demonstrate that IR injury to oviduct recruits BMDCs to this tissue and suggest that BMDCs have function in the healing process.
Keywords: bone marrow derived cells (BMDCs), ischemia and reperfusion injury (IR injury), oviduct, fallopian tube
Introduction
The oviducts or fallopian tubes (human) are the long, slender, narrow ducts lined with ciliated epithelia, connecting the ovaries to the uterus via the uterotubal junction in the female reproductive system in mammals. They have a crucial role in reproductive function in transporting sperm, oocytes, and embryos. Oocytes fertilize in this organ and the early embryo is carried to the uterus by peristaltic movement of the fallopian tube while undergoing the first stages of development. The oviduct is not simply a transport organ, it also provides the appropriate environment and nutrition for the embryo.
Injuries to the fallopian tubes in humans are common and result in tubal dysfunction and infertility. Pelvic infections, endometriosis, and ectopic pregnancies are the most common causes of tubal damage and infertility.1 Adnexal torsion is less common, however may also cause tubal dysfunction and reduced fertility. In order to repair damaged fallopian tubes, several regenerating processes occur to sustain the tubal function and tissue integrity. Resident stem cells found in the fallopian tube are thought to have a role in the healing and regeneration process. The oviduct/fallopian tube contains a population of mesenchymal stem cells (MSCs),2,3 and these MSCs have been successfully used in the treatment of several pathologies other than tubal injury because of their tissue regenerative capacities.4,5
Bone marrow derived cells (BMDCs), including MSCs, have been detected in several organs in the pathological conditions. They mobilize from bone marrow to support resident reparative cells where they contribute to tissue repair and regeneration in these organs.6,7 These cells are found in the endometrium in both human and animal studies.8,9 Uterine injury induces recruitment of these cells in the process of tissue regeneration.10 Therefore, we hypothesized that tissue damage to the oviducts would also mobilize the BMDCs to aid in repair of tissue damage and play a critical role in the regeneration of this tissue.
Materials and Methods
Animals
Six- to 8-week-old C57BL/6J wild-type female mice and transgenic ubiquitin–green fluorescent protein (GFP) male mice were purchased from the Charles River Laboratories (Wilmington, Massachusetts) and Jackson Laboratory (Bar Harbor, Maine), respectively. All mice were housed with free access to food and water and were maintained in a room (21°C ± 1°C) with a regular 12-hour light/dark cycle (7:00 am to 7:00 pm) in the Yale Animal Resources Center at Yale University School of Medicine. All animals were treated under an approved protocol by Institutional Animal Care and Used Committee. Mice were acclimated at least 1 week before starting study.
Submyeloablation
Submyeloablation was performed in all animals (n = 12) to determine BMDC trafficking according to the method described by Tal et al.11 5-Fluorouracil (5-FU) was purchased from Sigma-Aldrich (St Louis, Missouri). Briefly, 2 doses of 5-FU (125 mg/kg) dissolved in phosphate-buffered saline (PBS; Gibco, Grand Island, New York) were injected by intraperitoneal route. The second dose was injected 5 days after the first dose. Additionally, stem cell factor (SCF; R&D Systems, Minneapolis, MN) was used for improving bone marrow engraftment (150 mg/kg body weight in 2 divided doses). These SCF doses were administered at 21 and 9 hours before the second dose of 5-FU. The day after the second dose, BMDCs obtained from GFP male mice were transplanted into each 5-FU-treated mouse (25 × 106 cells/mice) by retro-orbital injection. Due to the toxicity of 5-FU, lethality was assessed by inspecting cages daily; however, all animals survived the submyeloablation process.
Extraction of BMDCs and Transplantation
Bone marrow–derived cells were obtained from 3-month-old male GFP mice as described previously.10 Briefly, bones (femur, tibia, and humerus) were flushed by using cold PBS. All bones were flushed until color of the bone turn to white and collected cells were counted by using light microscope (BX41; Olympus, Tokyo, Japan). The 25 × 106 unfractionated GFP + BMDCs within 100 µL PBS were injected to 6- to 8-week-old 5-FU-treated C57BL/6J wild-type recipient female mice by retro-orbital injection.
Blood Collection and Flow Cytometry Analysis
Peripheral blood was collected by retro-orbital venipuncture from the mice 2 weeks after transplantation of BMDCs to determine the percentage of peripheral blood cells expressing GFP and confirm the success of BMDC transplantation and engraftment in recipient mice. Blood was collected into EDTA-coated tubes on ice and diluted with PBS followed by centrifugation at 1500 rpm for 5 minutes. The cell pellet was resuspended and incubated for 10 minutes in red blood cell lysis buffer as per the manufacturer’s protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). The samples were diluted by PBS to stop the reaction and centrifuged at 1500 rpm for 5 minutes. The pellets were resuspended in PBS and filtered through 70-µm mesh into tubes specific for flow cytometry analysis. Flow cytometry was performed on a fluorescence-activated cell sorting Beckman Coulter MoFlo machine (Beckman Coulter, Brea, CA) using the corresponding excitation wavelength for GFP. Gates were applied to forward/side scatter dot plots to exclude non-viable cells and cell debris. Data were analyzed using the software FlowJo V10 (FlowJo, Ashland, OR).
Ischemia and Reperfusion Injury Model in the Fallopian Tube
Ischemia and reperfusion (IR) injury was performed 3 weeks following BMDC transplantation in the study group (n = 7). A midline abdominal incision was made under sterile conditions, then both sides of the oviduct and mesosalpinx were clamped by using a traumatic vascular clips for 60 minutes (Figure 1). The oviduct was then reperfused by release of the clips. Both oviducts were injured in each mouse in the study group. Control animals received an identical abdominal excision and the oviducts were identified but not clamped.
Figure 1.
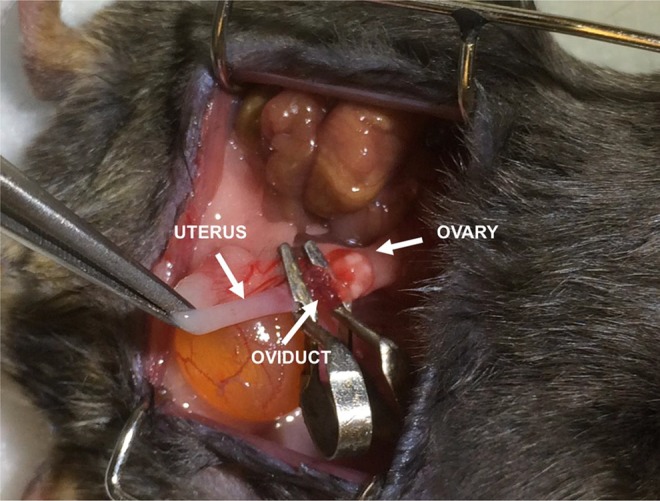
Experimentally induced ischemia of the oviduct. A blue-black appearance was seen after 60 minutes of ischemia.
Immunohistochemistry and Immunofluorescence Studies
In 2 mice, tissues were harvested 24 hours following IR injury in order to determine the acute effect of injury. The remaining mice in both groups (n = 5, study group; n = 5 control group) were killed at 2 weeks from injury to evaluate the recruitment of transplanted cells to the oviduct. The oviducts were rapidly fixed in 4% paraformaldehyde overnight and then embedded into paraffin. Five-micrometer tissue sections were mounted on slides. Sections were stained with hematoxylin and eosin for general morphologic analysis. Immunohistochemistry (IHC) was performed to detect GFP and caspase-3 expression. Sections for IHC were deparaffinized in xylene and rehydrated through a series of ethanol washes. Slides were boiled in pH 6 sodium citrate to antigen retrieval and incubated with 5% rabbit serum for GFP and 5% goat serum for caspase-3 antibody to block nonspecific antigen expression for 30 minutes. Then, slides were incubated at 4°C overnight with 1:2000 dilution of goat anti-GFP primary antibody (ab5450; Abcam, Cambridge, Massachusetts) to identify GFP-positive BMDCs and 1:300 dilution of rabbit anti-caspase-3 primary antibody (ab13847; Abcam) to exhibit oxidative damage. The day following, slides were incubated with biotinylated rabbit anti-goat IgG and goat anti-rabbit (1:200 dilution; Vector Laboratories, Burlingame, California) secondary antibodies for 60 minutes at room temperature, respectively. ABC Vectastain Elite reagents with DAB (3, 3'-diaminobenzidine) plus hydrogen peroxide (H2O2; Vector Laboratories) was used for detection of antibody binding. Tissue sections were counterstained with hematoxylin (Sigma-Aldrich). Stained sections were captured using Nikon (Shinagawa, Japan) Eclipse 80i microscope. Colocalization of GFP and CD45 antibodies in BMDCs recruited to the fallopian tube was detected by immunofluorescence studies. Tissue sections mounted on glass slides were incubated with goat anti-GFP (1:1000 dilution, ab5450; Abcam) and rat anti-CD45 (1:200 dilution; ab25386; Abcam) as primary antibodies for overnight at 4°C. Alexa Fluor 568-conjugated donkey anti-goat IgG (1:200 dilution, A11057; Life Technologies, Carlsbad, California) and Alexa Fluor 488-conjugated donkey anti-rat IgG (1:200 dilution, A21208; Life Technologies) were used as secondary antibodies. Sections were covered with coverslips using Vectashield fluorescent mounting media with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Oviduct from GFP transgenic mice was used as a positive control for the GFP antibody and wild-type spleen for CD45 antibody. Fluorescent images were captured with a laser scanning confocal microscope (Leica TCS SP5) and analyzed with Leica LAS-X software program (Buffalo Grove, IL).
Tissue Analysis and Cell Quantification
Bone marrow–derived GFP-positive cells from the donors localized in both epithelial and stromal regions of recipient’s oviducts were counted. Multiple high-power confocal microscopy fields were assessed in each oviduct section, and 3 sections were evaluated separately from each animal. For evaluation, the total number of DAPI-positive cells with nuclei was counted in each section. At least 500 to 1500 cells were counted. Both GFP-positive and CD45-negative cells were also counted in the same sections to calculate the proportion of these cells in each animal. Two independent observes blinded to the treatment group assessed slides from each animal.
Statistical Analysis
Statistical analysis was performed via GraphPad Prism 6 software (GraphPad (La Jolla, CA)). The Shapiro-Wilk test was used to determine normal distribution. The nonparametric Mann-Whitney U test was used to compare the percentage of GFP-positive cells in recipient mice bloodstream as well as the percentage of GFP-positive/CD45-negative cells in the oviduct. P values <.05 were considered statistically significant.
Results
Engraftment of GFP-Positive Cells in the Oviduct
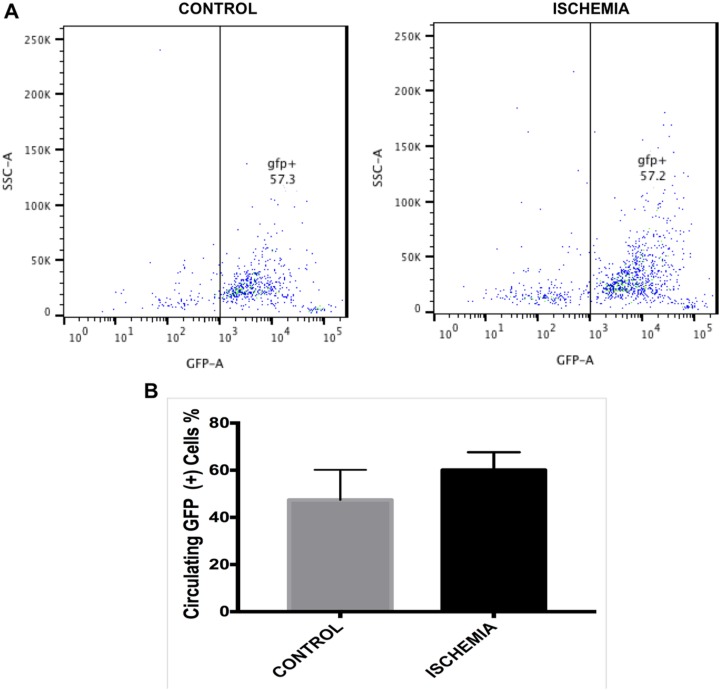
The percentage of GFP-positive cells in the circulation of recipient mice was determined by flow cytometry analysis 2 weeks after transplantation of BMDCs from male GFP mice. Flow cytometry analysis evaluated the engraftment of donor cells in recipient mice and assured adequate and equivalent engraftment after bone marrow transplantation. Two mice were excluded from the study because of transplantation failure (<20% GFP-positive cells) after flow cytometry analysis. After confirmation of successful GFP-positive BMDC transplantation, mice were randomly divided into 2 groups of equal number (n = 5 per group). As shown in Figure 2, the number of GFP-positive cells in the circulation of each group was compared and there were no significant differences.
Figure 2.
Engraftment of green fluorescent protein (GFP)-positive cells in bone marrow (BM) as detected in the circulation after bone marrow transplant. Flow cytometry analysis of recipient circulating blood cells at 14 days after bone marrow–derived cells transplantation (A). Histogram showing the number of GFP-positive cells in circulation of recipient’s blood. B, Percentage of GFP-positive cells recruited in mice in each group (n = 5). Data presented as mean ± standard error of the mean (SEM). No significance difference was noted between the 2 groups.
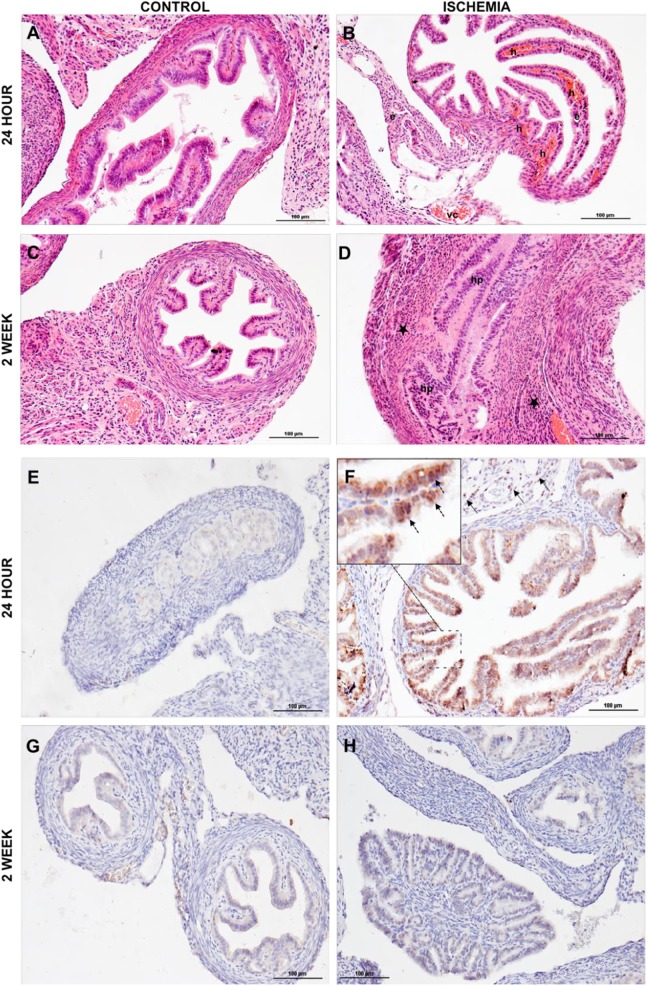
Tissues were harvested 2 weeks after IR surgery and sections were subjected to hematoxylin and eosin staining for general morphological changes in both the groups. Additionally, acute effect of IR injury was evaluated after 24 hours. Increased vascular congestion, parenchymal hemorrhages, and edema were detected in acute tissue sections, but these findings were diminished by 2 weeks after IR injury. Increased inflammatory cell infiltration to parenchyma was observed in epithelium after 2 weeks of IR injury, as shown in Figure 3A to D. This injury also induced apoptosis. Expression of caspase-3, a protease that mediates apoptosis, was increased at 24 hours after the ischemic insult and was again decreased to normal levels comparable to the control group by 2 weeks postinjury, as summarized in Figure 3E to H.
Figure 3.
Histological and immunohistochemical analysis. Histological studies showed that (A) no pathologic changes were detected in control animals at 24 hours postischemia. B, Vascular congestion, edema, and severe intraparenchymal hemorrhage were observed in ischemia and reperfusion (IR) group at 24 hours postischemia. C, Normal histology was observed in the control group at 2 weeks postischemia. D, Increased inflammatory cell infiltration (the majority are leucocytes and macrophages) and epithelial cell proliferation determined at 2 weeks postischemia. Vascular congestion (vc), hemorrhage (h), edema (e), hyperplasia (hp), and inflammatory cells infiltration (*). Immunohistochemical studies showed (E) caspase 3 expression in the control group at 24 hours after ischemia. F, Increased caspase-3 expression was observed in epithelial (dashed arrow in magnified view) and stromal (arrow) cells of IR group at 24 hours after ischemia. G, Caspase-3 expression in the control group 2 weeks after ischemia. H, Caspase-3 expression gradually decreased and comparable levels with the control group were seen at 2 weeks after ischemia.
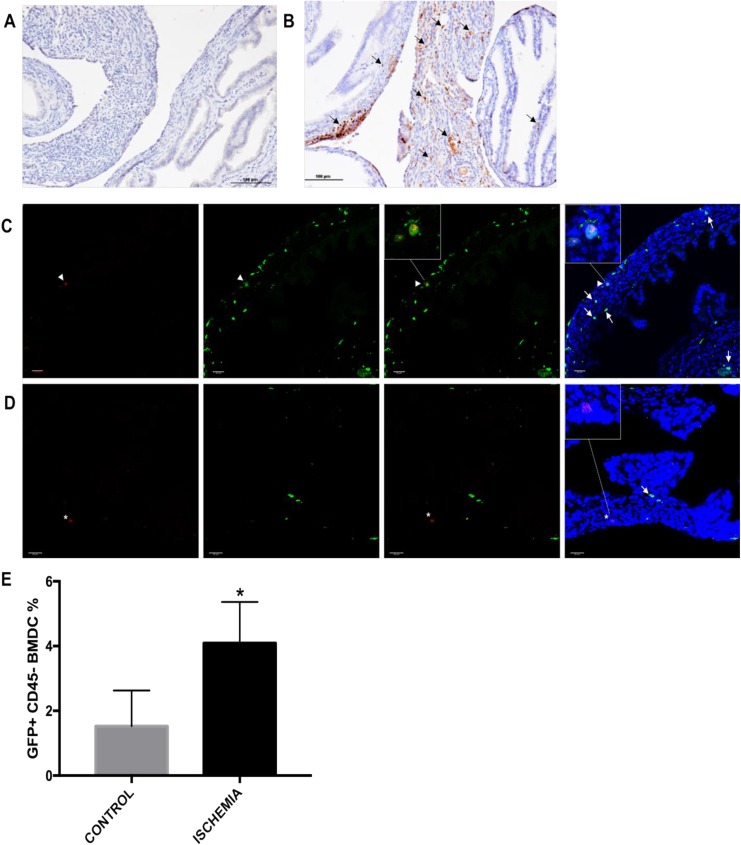
Recruitment of donor BMDCs to the oviduct was evaluated by IHC. Both control and experimental groups were compared to quantify recruitment of GFP-positive cells. We observed an increase in GFP-positive cells recruited to oviduct in the IR injury group compared to the control group. Further, the GFP-positive cells were recruited specifically to the stromal region of the oviduct, as shown in Figure 4A and B. Bone marrow–derived cells contain both immune cells, expected to infiltrate any tissue after injury, and MSCs. To identify the nonimmune MSCs, we carried out immunostaining for the pan-leukocyte marker CD45 on these tissue sections. CD45 colocalization studies identified GFP-positive cells that were CD45 negative. Both injury and noninjury groups were compared for the number of GFP-positive/CD45-negative cells recruited to the oviduct. Greater than 2-fold more GFP-positive/CD45-negative cells were recruited to and engrafted the injured oviduct compared to the noninjured controls (P = .02), as shown in Figure 4C and E.
Figure 4.
Detection of green fluorescent protein (GFP)-positive cells in tissue from the fallopian tube by immunohistochemistry. A, The GFP-positive cells staining in oviducts of the control group. B, Increased GFP-positive cell recruitment (arrow) was observed localized to stroma of ischemia and reperfusion (IR) injured oviducts. C, Immune cells originated from donor bone marrow were excluded by colocalization of CD45 staining along with GFP staining. CD45 and GFP-positive cells (white arrowhead) were excluded from GFP-positive and CD45-negative cells (white arrow) in IR injured oviducts. D, Fewer GFP-positive and CD45-negative cells (white arrow) were recruited in the control group. Additionally, CD45-positive but GFP-negative immune cells (asterisk) originated from host bone marrow were observed in tissue sections. E, Bar graph showing the percentage of GFP-positive and CD45 negative cells recruited to the oviducts of the IR injured and control groups. Original magnification: ×20, scale bar: 100 μm. Green represents GFP, red represents CD45, blue represents nuclei 4′,6-diamidino-2-phenylindole (DAPI). Original magnification ×40, scale bar 20 μm. Data presented as mean ± standard error of the mean (SEM). *P = .02.
Discussion
We describe a role of BMDCs in oviduct tissue repair, perhaps contributing to healing after IR injury. These BMDCs likely support tissue healing process. Bone marrow–derived cells comprise 2 stem cell populations: hematopoietic stem cells, which can differentiate into all mature blood cell lineages, and MSCs, which have a high level of plasticity. Bone marrow–derived mesenchymal stem cells (BMDSCs) can generate mesodermal, ectodermal, and endodermal cells, including skeletal, cardiac muscle, lung, liver, and neuronal cells.12 These cells circulate in the bloodstream and can be recruited to multiple organs. They play a role in the normal cell turnover of many organs and are recruited in higher numbers in response to disease. Evidence to support this comes from both animal models and humans transplanted with allogeneic bone marrow stem cells. Donor cells have been identified in several organs after male to female or HLA (human leukocyte antigen) mismatch bone marrow stem cell transplantation.13–15 Our group was the first to identify a the role of BMDSCs in uterine repair.8 However, BMDSCs likely have a role in the repair of multiple organs. Amelioration of several diseases has been reported after bone marrow transplantation.16 This therapeutic effect of BMDCs arises from not only plasticity of these cells but also stimulating the activity of endogenous progenitor cells by secreting growth factors, immunomodulatory cytokines, and exosomes.17,18 The healing process typically is immediately triggered after tissue injury. Injury and blood clot formation initially triggers platelet activation and the release of a variety of cytokines and growth factors that in turn trigger the migration and infiltration of immune cells to the lesions.19 This inflammatory process and released factors direct circulating BMDCs to the injured tissues and increase mobilizing of BMDCs from bone marrow.20 Tissue expression levels of stromal-derived factor 1 (also termed as CXCL12) and C-X-C chemokine receptor type 4, in injured tissue and in bone marrow, respectively, are critical factors in the mobilization and chemotaxis of BMDCs.21 Regulation of these factors by injury, inflammation, chemotherapy, or clinical treatment modalities such as G-CSF (granulocyte-colony stimulating factor) results in BMDC mobilization and homing.22,23
Studies investigating BMDC recruitment into the uterus revealed that circulating BMDCs primarily engraft the stroma. These cells are often initially located in close proximity to blood vessels, but not in the vascular wall,24,25 in a manner similar to what was observed in our study. Donor BMDCs were initially localized in oviductal stromal area, close to vascular structures. Samples obtained at a longer time after tissue injury also contained BMDCs localized in the epithelium, similar to the long-term results of uterine studies.8,10 Bone marrow–derived cells reaching the uterus or oviduct through the bloodstream after injury increases these cells’ viability.26 A decrease in cell engraftment and cell viability is seen after local injections in injured tissues.25 This route likely is needed to reach the primary stem cell niche in these tissues, followed by differentiation into mature or progenitor cells that survive outside of that niche.
Fallopian tube torsion is a clinical condition that results in ischemia and is often due to concomitant ovarian torsion and ovarian pathology. Isolated fallopian tube torsion has been reported.26 The blood supply to the fallopian tubes ceases after torsion, resulting in ischemia as modeled here. Tubal detorsion restores blood supply causing reperfusion damage. Reactive oxygen species such as superoxide anion (O2 −), H2O2, hypochlorous acid, nitric oxide–derived peroxynitrite, and hydroxyl radical (OH) damage cell membrane proteins and phospholipids. The damaged cells induce the activation of chemotaxis and endothelial adhesion of leucocytes27 and BMDCs.28 Cell death is inevitable after IR injury. This occurs through both cell necrosis and apoptosis. As necrosis in response to IR injury and the role of stem cells have been previously characterized, here we chose to evaluate the role of apoptosis and determined that IR injury affects apoptosis in the oviduct. To exhibit cell damage of IR injury, we used apoptotic pathway protease caspase-3. Apoptosis is induced after reperfusion damage,29 as modeled in our study. We found that increased apoptosis and high levels of caspase-3 expression after 24-hour ischemia; however, caspase expression decreased within 2 weeks after ischemia. An inflammatory response was also clearly observed at 2 weeks after ischemia in the parenchymal area of oviducts. This inflammatory response is a major trigger for recruitment of BMDC.28
In cardiac IR studies, reduced ischemic area and improved cardiac function were observed after BMDC transplantation.30 Similarly, the effect of the bone marrow–derived stem cells on tubal healing was investigated by Almasry et al; they showed improved healing in chemically injured tubal mucosa after local injection of BMDSCs.31 This healing process was attributed to the role of BMDSCs in stimulation of resident stem cells, increasing tissue vascular endothelial growth factor and proliferating cell nuclear antigen levels and reducing apoptotic activity. In addition, they demonstrated to reduce caspase-3 expression levels after BMDSC injection. Similar to our results, these findings support the antiapoptotic activity of BMDSCs. Bone marrow–derived cells recruitment to damaged tubal tissue is part of the normal response to injury and likely facilitates the healing process.
Conclusion
Ischemia and reperfusion injury increases the recruitment of BMDCs in oviduct. These cells support tissue healing processes and are associated with reduced apoptotic activity. Stem cell treatment after ischemic injury may be useful in restoring tubal function in women as well.
Acknowledgments
The authors thank Shafiq Shaikh and Aya Tal for their technical support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH U54 HD052668 and R01 HD076422 and The Scientific and Technological Research Council of Turkey (TUBITAK) postdoc research program (1059B191500864).
ORCID iD: Ramanaiah Mamillapalli, PhD  http://orcid.org/0000-0002-4022-8910
http://orcid.org/0000-0002-4022-8910
References
- 1. Patil M. Assessing tubal damage. J Hum Reprod Sci. 2009;2(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang J, Zhao Y, Wu X, Yin S, Chuai Y, Wang A. The utility of human fallopian tube mucosa as a novel source of multipotent stem cells for the treatment of autologous reproductive tract injury. Stem Cell Res Ther. 2015;6(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Snegovskikh V, Mutlu L, Massasa E, Taylor HS. Identification of putative fallopian tube stem cells. Reprod Sci. 2014;21(12):1460–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jazedje T, Bueno DF, Almada BV, et al. Human fallopian tube mesenchymal stromal cells enhance bone regeneration in a xenotransplanted model. Stem Cell Rev. 2012;8(2):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kadam S, Patki S, Bhonde R. Human fallopian tube as a novel source of multipotent stem cells with potential for islet neogenesis. J Stem Cells Regen Med. 2009;5(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poulsom R, Alison MR, Cook T, et al. Bone marrow stem cells contribute to healing of the kidney. J Am Soc Nephrol. 2003;14(suppl 1):S48–S54. [DOI] [PubMed] [Google Scholar]
- 7. Grompe M. The role of bone marrow stem cells in liver regeneration. Semin Liver Dis. 2003;23(4):363–372. [DOI] [PubMed] [Google Scholar]
- 8. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–2086. [DOI] [PubMed] [Google Scholar]
- 9. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85. [DOI] [PubMed] [Google Scholar]
- 10. Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 2012;21(18):3324–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tal R, Liu Y, Pluchino N, Shaikh S, Mamillapalli R, Taylor HS. A murine 5-fluorouracil-based submyeloablation model for the study of bone marrow-derived cell trafficking in reproduction. Endocrinology. 2016;157(10):3749–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22(4):487–500. [DOI] [PubMed] [Google Scholar]
- 13. Theise ND, Nimmakayalu M, Gardner R, et al. Liver from bone marrow in humans. Hepatology. 2000;32(1):11–16. [DOI] [PubMed] [Google Scholar]
- 14. Körbling M, Katz RL, Khanna A, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346(10):738–746. [DOI] [PubMed] [Google Scholar]
- 15. Sahin Ersoy G, Zolbin MM, Cosar E, Moridi I, Mamillapalli R, Taylor HS. CXCL12 promotes stem cell recruitment and uterine repair after injury in Asherman’s syndrome. Mol Ther Methods Clin Dev. 2017;4:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gussoni E, Bennett RR, Muskiewicz KR, et al. Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J Clin Invest. 2002;110(6):807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel SA, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz). 2008;56(1):1–8. [DOI] [PubMed] [Google Scholar]
- 18. Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–88. [DOI] [PubMed] [Google Scholar]
- 19. Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(suppl 1):S13–S33. [DOI] [PubMed] [Google Scholar]
- 20. Stagg J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007;69(1):1–9. [DOI] [PubMed] [Google Scholar]
- 21. Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34(8):967–975. [DOI] [PubMed] [Google Scholar]
- 22. Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30(9):973–981. [DOI] [PubMed] [Google Scholar]
- 23. Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLoS One. 2014;9(5):e96662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cervelló I, Gil-Sanchis C, Santamaría X, et al. Human CD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil Steril. 2015;104(6):1552–1560. [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Tal R, Pluchino N, Mamillapalli R, Taylor HS. Systemic administration of bone marrow-derived cells leads to better uterine engraftment than use of uterine-derived cells or local injection. J Cell Mol Med. 2018;22(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bertozzi M, Magrini E, Riccioni S, Giovenali P, Appignani A. Isolated fallopian tube torsion with hydrosalpinx: review of a debated management in a pediatric population. J Pediatr Surg. 2017;52(10):1553–1560. [DOI] [PubMed] [Google Scholar]
- 27. Toyokuni S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int. 1999;49(2):91–102. [DOI] [PubMed] [Google Scholar]
- 28. Khatun M, Sorjamaa A, Kangasniemi M, et al. Niche matters: the comparison between bone marrow stem cells and endometrial stem cells and stromal fibroblasts reveal distinct migration and cytokine profiles in response to inflammatory stimulus. Camussi G, ed. PLoS One. 2017;12(4):e0175986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Umansky SR, Cuenco GM, Khutzian SS, Barr PJ, Tomei LD. Post-ischemic apoptotic death of rat neonatal cardiomyocytes. Cell Death Differ. 1995;2(4):235–241. [PubMed] [Google Scholar]
- 30. DeSantiago J, Bare DJ, Banach K. Ischemia/reperfusion injury protection by mesenchymal stem cell derived antioxidant capacity. Stem Cells Dev. 2013;22(18):2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Almasry SM, Elfayomy AK, El-Sherbiny MH. Regeneration of the fallopian tube mucosa using bone marrow mesenchymal stem cell transplantation after induced chemical injury in a rat model. Reprod Sci. 2017. January 1;1933719117725824. [DOI] [PubMed] [Google Scholar]