Abstract
Matrix metalloproteinase-1 (MMP-1) and neutrophil elastase are proteolytic enzymes involved in tissue remodeling, but a role for them as uterotonic agents has not been considered. However, both these proteases activate protease-activated receptor 1 (PAR-1) that mediates thrombin-induced contractions. Matrix metalloproteinase-1 and elastase are products of neutrophils that infiltrate intrauterine tissues at the time of labor, so we tested the hypothesis that these proteases might be novel uterotonic agents acting via PAR-1. Decidual tissue was collected from fetal membranes of term not-in-labor (TNL), term labor (TL), and preterm labor (PTL) women and analyzed for gene and protein expression of MMP-1 and neutrophil elastase. Contractile effects of MMP-1 and elastase were tested with uterine strips of day 19 and 20 gestation rats. Expression of MMP-1 and neutrophil elastase was increased in TL and PTL as compared to TNL. Expression of both the pro- and active enzymes of MMP-1 increased progressively from TNL to TL to PTL. Tumor necrosis factor-α, a neutrophil product, increased MMP-1 in decidual and myometrial cells. Both MMP-1 and elastase stimulated strong contractions of myometrial strips, which were prevented by inhibition of PAR-1 and inhibition of inositol trisphosphate receptor or calcium channel blockade. Indomethacin did not prevent protease-induced contractions. These data suggest that MMP-1 and neutrophil elastase may be important but heretofore unrecognized players in stimulating uterine contractions at the time of labor, and they may explain why indomethacin delays, but does not prevent, PTL because indomethacin inhibits the prostaglandin component but not the protease component of labor.
Keywords: matrix metalloproteinase-1, elastase, protease-activated receptor 1, neutrophils, labor
Introduction
Matrix metalloproteinase-1 (MMP-1), also known as interstitial collagenase, is a proteolytic enzyme recognized as an important contributor to labor because of its ability to degrade extracellular matrix and, as such, has been implicated in cervical ripening and preterm premature rupture of the membranes.1–4 A role of MMP-1 as a uterotonic agent has not been considered. Labor is associated with extensive infiltration of neutrophils into decidua and myometrium.4–6 Neutrophils produce MMP-1 but also release pro-inflammatory agents, such as tumor necrosis factor-α (TNF-α), which could increase MMP-1 production in decidua and myometrium. Neutrophils also release another proteolytic enzyme, neutrophil elastase.
Recently, it has been shown that MMP-1 activates protease-activated receptor 1 (PAR-1),7,8 also known as thrombin receptor. Thrombin is a known activator of uterine contractility and implicated in preterm labor (PTL) associated with hemorrhage.9–12 Neutrophil elastase is a serine protease, like thrombin, and so actives PAR-1.13 Activation of PAR-1 results in downstream signaling mechanisms that include activation of RhoA kinase and activation of inositol trisphosphate (IP3)–induced Ca++ release from smooth endoplasmic reticulum.14–16 Inositol trisphosphate calcium release is a recognized mechanism for myometrial contraction during labor.17
We tested the hypothesis that MMP-1 and neutrophil elastase are novel uterotonic agents acting via PAR-1. If so, they may be important players in the onset of labor acting by a mechanism that is independent of prostaglandins.
Materials and Methods
Study Patients
Patient groups were term not-in-labor (TNL, n = 12), term labor (TL, n = 13), and PTL (n = 15). Preterm labor placentas were collected from pregnant women who gave birth prior to 37 weeks of gestation and had no clinical signs of infection. Infection was defined as fever ≥38°C with 1 or more of the following: uterine tenderness, malodorous vaginal discharge, and maternal or fetal tachycardia. Term labor placentas were collected from women with normal pregnancies who gave birth between 37 and 40 weeks of gestation. Pitocin was used in 1 TL patient and misoprostol in 1. Cesarean delivery in TL patients was due to the failure of trial of labor after a previous cesarean delivery. Term not-in-labor placentas were collected from women who underwent non-emergency cesarean deliveries between 38 and 39 weeks of gestation for a previous cesarean delivery or other clinical reason. Demographic patient data are given in Table 1. The only statistically significant differences were for gestational age at delivery and birth weight of the baby, which were less for PTL patients as compared to TNL or TL patients. Exclusion criteria were smokers, drug/alcohol users, pregnancies with stillborn babies, multiple fetuses, lupus, congenital abnormalities, hemorrhage, prior diagnosis of incompetent cervix, diabetes, HIV, or other active infection. Consent was obtained prior to delivery, and the study was approved by the Virginia Commonwealth University Office of Research Subjects Protection.
Table 1.
Clinical Characteristics of Patient Groups.a
| Variable | TNL, n = 12 | TL, n = 13 | PTL, n = 15 |
|---|---|---|---|
| Maternal age, years | 28.0 (5.7) | 23.8 (5.8) | 25.5 (4.1) |
| Prepregnancy BMI, kg/m2 | 28.2 (6.0) | 23.8 (5.8) | 21.5 (3.3) |
| BMI at sample collection, kg/m2 | 33.7 (6.3) | 28.4 (5.4) | 25.5 (4.1) |
| Race | |||
| White | 1 | 3 | 5 |
| Black | 10 | 8 | 10 |
| Asian | 1 | ||
| Hispanic | 1 | ||
| Other | 1 | ||
| Primiparous | 2 | 6 | 3 |
| Multiparous | 10 | 7 | 12 |
| Gestational age, weeks | 38.9 (0.3) | 39.5 (0.8) | 32.5 (2.3)b |
| Infant birth weight, g | 3215 (323) | 3308 (280) | 2081 (553)b |
| Delivery method | |||
| Vaginal | 0 | 11 | 11 |
| Cesarean delivery | 12 | 2 | 4 |
Abbreviations: BMI, body mass index; PTL, preterm labor; SD, standard deviation; TL, term labor; TNL, term not-in-labor.
aValues are mean (SD).
bP < .0001 compared to TNL or TL.
Isolation and Culture of Decidual and Myometrial Cells
Gene and protein expression
Decidual tissue was collected by gentle scraping of the decidual cells from the chorion after delivery into a conical 50 mL tube filled with ice-cold phosphate-buffered saline (PBS). After collection, cells were centrifuged at 4°C for 10 minutes at 300g, PBS was aspirated, and the tissue was washed with 25 mL ice-cold PBS, briefly vortexed, and respun for another 10 minutes. The pellet contained both decidual tissue and residual erythrocytes. Contaminating red blood cells were lysed with 3 mL of ice-cold double-distilled H2O for 30 seconds followed by an addition of 1 mL of 0.6 M solution of KCl to restore tonicity. The tissue was centrifuged again, the supernatant removed, and decidual tissue either frozen at −80°C for Western blot or extracted for total RNA for gene expression.
Primary decidual cell culture
Decidual cells for primary culture were isolated with the method of Lockwood et al.18 After decidual tissue was gently scraped from the chorion, it was digested with collagenase, treated with DNase 3 times, and the remaining cell clusters were dissociated by passing the mixture through a 25-gauge needle. The entire procedure was repeated twice more. The resulting cells were centrifuged in 2 different Percoll gradients and quantified by Trypan blue exclusion assay to assess decidual cell number and viability (>96%). Cells were grown to confluence on culture dishes, harvested, and evaluated for leukocyte contamination by flow cytometry with anti-CD45 and anti-CD14 antibodies. Cells were passaged 3 more times, at which time they were all vimentin positive and both cytokeratin and CD45 negative by flow cytometry, which assured that cells in culture were decidual cells. Treatments were done on cells passaged a maximum of 6 times.
Myometrial cell culture
A human telomerase reverse transcriptase (hTERT) immortalized human myometrial cell line19 was provided by Ruth Ann Word (UT Southwestern Medical Center, Dallas, Texas). Cell culture was maintained in DMEM/F12 medium containing 10% fetal bovine serum, 1% antibiotics and antimycotics (100 U/mL penicillin, 100 μg/mL streptomycin, 25 μg/mL amphotericin B), and incubated at 37°C in 95% air and 5% CO2. Medium was replaced every 48 hours.
For culture, isolated decidual cells or hTERT myometrial cells were suspended in 5 mL DMEM/F12 medium with 1% antibiotic–antimycotic and 10% FBS and seeded at 250 000 cells into T-25 tissue culture flasks. After 24 hours of incubation at 37°C in 5% CO2, attached decidual cells were washed 3× with PBS and fresh media was added. Once cells reached 70% confluency, cells were treated with TNF-α (1 ng/mL) in DMEM/F12 medium for 24 hours. After treatment, the medium was aspirated, cells were washed 2 times with 5 mL PBS and scraped, and total RNA was extracted for gene expression.
Quantitative Reverse Transcription Polymerase Chain Reaction
RNA extraction from decidual tissue was performed using QuickGene RNA tissue kit with QuickGene Mini-80 system (AutoGen, Holliston, Massachusetts). DNase treatment was performed using Turbo DNAse kit (Ambion, Austin, Texas). RNA (1 μg) was reverse transcribed to complementary DNA (cDNA) using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, California). Quantitative RT-PCR reactions were performed with RT2 SYBR Green qPCR Mastermix (SABiosciences, Frederick, Maryland) on an Eppendorf Realplex Thermal Cycler. For each reaction, 8 ng of cDNA was used. Primers for MMP-1 were purchased commercially (SABiosciences, Valencia, California), and primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), used as a housekeeping gene, were purchased from Integrated DNA Technologies, Coralville, Iowa (GAPDH F: 5’-GAT TCC ACC CAT GGC AAA TT-3’, R: 5’-AGA TGG TGA TGG GAT TTC CAT T-3’). Data were normalized to GAPDH by the ▵▵Ct method. Melting curve analysis confirmed specificity of the primers.
Western Blotting
Decidual tissues (50 mg each) were homogenized and lysed in radioimmunoprecipitation assay buffer (RIPA) buffer containing 150 mM sodium chloride, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 50 mM Tris (pH 8.0), and 1× Halt protease inhibitor (Thermo Scientific, Pittsburgh, Pennsylvania) using a tip sonicator (Active Motif, Carlsbad, California). Protein concentrations were measured using BCA assay (Thermo Fisher Scientific, Rockford, Illinois). Denatured protein lysates (100 μg) were resolved by electrophoresis on 10% agarose gel and electrotransferred to polyvinylidene fluoride membrane (Immobilon-FL; EMD Millipore, Billerica, Massachusetts). Primary antibodies used were rabbit anti-human MMP-1 (0.2 μg/mL; R&D Systems, Minneapolis, Minnesota), rabbit anti-human neutrophil elastase (1:500; Abcam, Cambridge, Massachusetts), mouse anti-human β-actin (1:10 000; Sigma-Aldrich, St Louis, Missouri), and rabbit anti-human cyclophilin A (1:1000; EMD Millipore). Cyclophilin A was used as the housekeeping gene for MMP-1 because of the similarity in molecular weights between MMP-1 and β-actin. Secondary antibodies used included Alexa Fluor 680 donkey anti-rabbit (1:10 000, Invitrogen, Carlsbad, California) for the detection of primary antibodies raised in rabbit and IRDye800 goat anti-mouse (1:20 000, Rockland Immunochemicals, Gilbertsville, Pennsylvania) for the detection of primary antibody raised in mouse. LI-COR Odyssey Infrared Imaging System (Thermo Scientific) was used to detect the immunoreactive proteins. Image J software (imagej.nih.gov/ij) was used to determine the density of immunoblots for statistical analysis.
Uterine Contractility Experiments
Sprague Dawley timed pregnant rats (200 grams), days 19 to 20 of gestation, were used for experiments to assess effects of exogenous MMP-1 and neutrophil elastase on uterine contraction. A total of 21 animals were used. The study was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and in compliance with the National Institutes of Health guidelines for the humane treatment of laboratory animals. Animals were housed with temperature and humidity control under a 12/12-hour day/night cycle, and their health and well-being was monitored daily. Animals were afforded at least a 72-hour recovery period from shipping before use in experimentation and were fed ad libitum. On the day of experimentation, animals were euthanized and the uterus quickly harvested. Longitudinal myometrial strips (2 mm × 2 cm) were mounted in muscle strip chambers on force transducers with 0.5 g tension in Krebs Ringer bicarbonate buffer equilibrated at 37°C, bubbled with 95% O2 and 5% CO2 as we previously described.20 Four strips were mounted for each animal. Strips fastened to the force transducers were connected to a computerized data acquisition system to measure contractions (Lab Chart Pro; ADInstuments, Colorado Springs, Colorado). Contractile response was quantitated by the area under the tension trace in grams per minute utilizing the Lab Chart Pro software. The average measurement duration was 1067 ± 113 seconds. Matrix metalloproteinase-1 catalytic domain peptide (0.1-1 μg/mL) or elastase (0.003-0.3 U/mL) were added to the bath in 10-fold increments to establish dose-response curves. Once optimal doses were established, mechanism of action was studied by using specific pathway inhibitors: PAR-1 inhibitor (10 and 50 μM, SCH-79797; Tocris, Ellisville, Missouri), IP3 receptor (IP3R) blocker (50 μM, 2-APB; Tocris), nifedipine (20 and 100 μM; Tocris), indomethacin (100 μM; Sigma-Aldrich), and RhoA kinase inhibitor (3 μM Y-27632 dihydrochloride; Tocris). Protease stimulation was tested in quiescent myometrial strips, so each strip provided its own baseline for control. Some strips were also run as untreated controls. Inhibitors were sometimes given before and sometimes after protease stimulation. KCl (40 mM) was added at the end of each experiment to verify tissue viability.
Statistical Analysis
Mann-Whitney U test was used to make comparisons of parameters between 2 groups, and 1-way analysis of variance (ANOVA) with Bonferroni multiple comparisons test was used to make comparisons of parameters between more than 2 groups. For ANOVA, if variances were not equal, data were first transformed using Y2 = Log (y1). Demographic data are presented as mean (standard deviation). Quantitative data are presented as mean ± standard error. A probability level of P < .05 was considered significant. A statistical software application was used (Prism 6.0 for Macintosh; GraphPad Software, Inc, San Diego, California).
Results
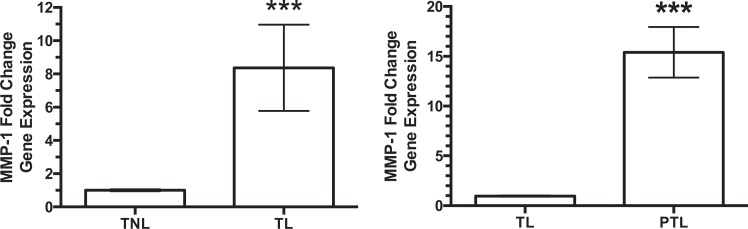
Gene expression of MMP-1 was 8-fold higher in decidual tissue of TL women as compared to TNL women (Figure 1), but expression was increased even more in women with PTL. When compared to the already increased levels in women with TL, women with PTL had a further 15-fold increase in MMP-1.
Figure 1.
Gene expression of matrix metalloproteinase-1 (MMP-1) in decidual tissue. There was an 8-fold increase in the gene expression of MMP-1 in decidual tissue of women with term labor (TL, n = 7) as compared to women at term not-in-labor (TNL, n = 7). However, when MMP-1 gene expression of women with preterm labor (PTL, n = 6) was compared with the already increased expression of women with TL, there was a further increase in expression of 15-fold. ***P < .001, TL versus TNL and PTL versus TL.
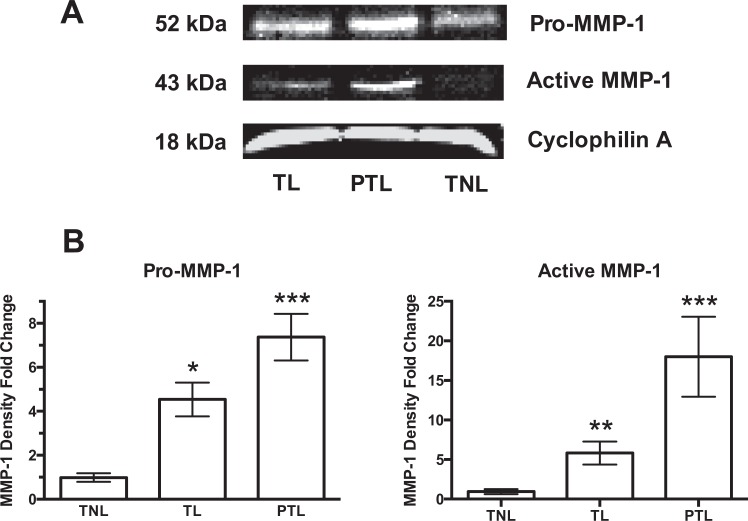
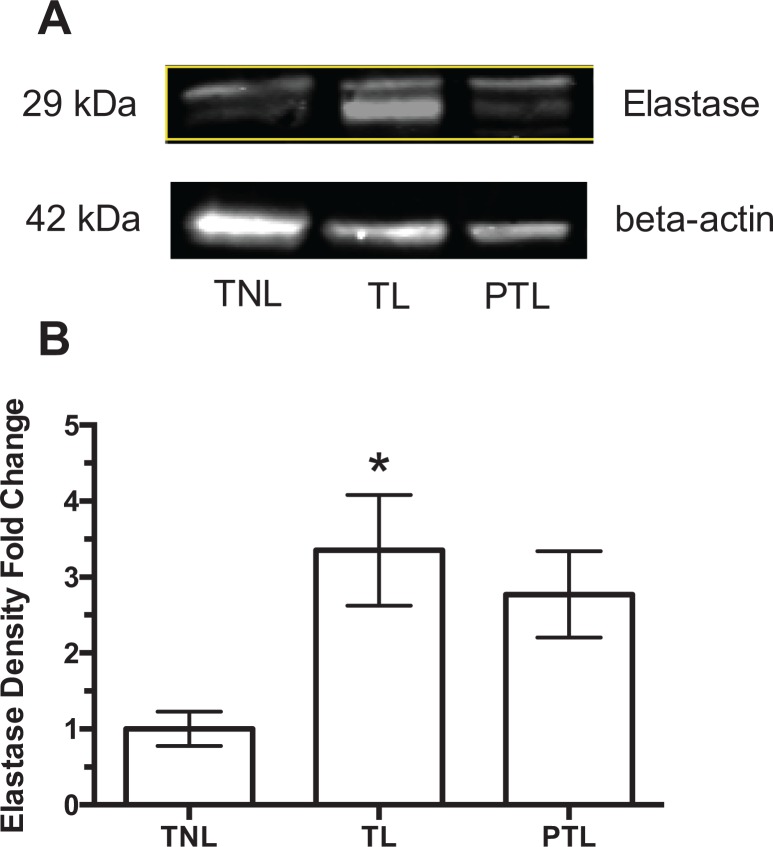
Protein expression of MMP-1 in decidual tissue paralleled the changes in gene expression (Figure 2). A representative Western blot for pro- and active MMP-1 is shown in Figure 2A. Figure 2B shows the fold-change in Western blot density normalized to TNL. Both pro-MMP-1 and active MMP-1 increased significantly in women with TL as compared to women at TNL, and both were increased further in women with PTL. Protein expression of neutrophil elastase also increased with labor. Neutrophil elastase was significantly increased in women with TL; however unlike MMP-1, expression was not further increased in PTL (Figure 3).
Figure 2.
Protein expression of pro-matrix metalloproteinase-1 (MMP-1) and active MMP-1 in decidual tissue of term not-in-labor (TNL), term labor (TL), and preterm labor (PTL) women. A, Representative Western blots. B, Fold-change in Western blot density normalized to TNL. Protein expression of pro-MMP-1 (n = 6) and active MMP-1 (n = 4) were significantly increased in TL as compared to TNL and further increased in PTL. *P < .05, **P < .01, ***P < .001 as compared to TNL.
Figure 3.
Protein expression of neutrophil elastase in decidual tissue in term not-in-labor (TNL), term labor (TL), and preterm labor (PTL) women. A, Representative Western blots. B, Fold-change in Western blot density normalized to TNL. Protein expression of neutrophil elastase was significantly increased in TL (n = 6) as compared to TNL (n = 6) but was not further increased in PTL (n = 6). Two bands were present for neutrophil elastase, most likely due to posttranslational glycosylation. *P < .05 as compared to TNL.
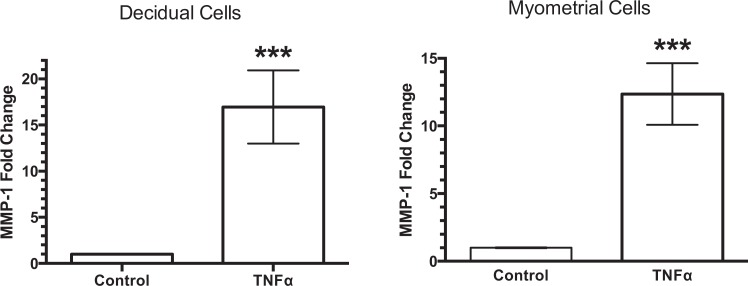
To determine whether neutrophil products could stimulate decidual and myometrial MMP-1, decidual and myometrial cell cultures were treated with TNF-α (1 ng/mL). Tumor necrosis factor-α stimulated significant increases in MMP-1 in both decidual cells (17-fold increase) and myometrial cells (12-fold increase) (Figure 4).
Figure 4.
Stimulation of matrix metalloproteinase-1 (MMP-1) gene expression by tumor necrosis factor-α. Tumor necrosis factor-α (TNF-α) significantly stimulated gene expression of MMP-1 in primary cultures of decidual cells (n = 12) and in an human telomerase reverse transcriptase (hTERT) myometrial cell line (n = 7), demonstrating the ability of a neutrophil product to increase MMP-1 in intrauterine tissues. ***P < .001 as compared to control.
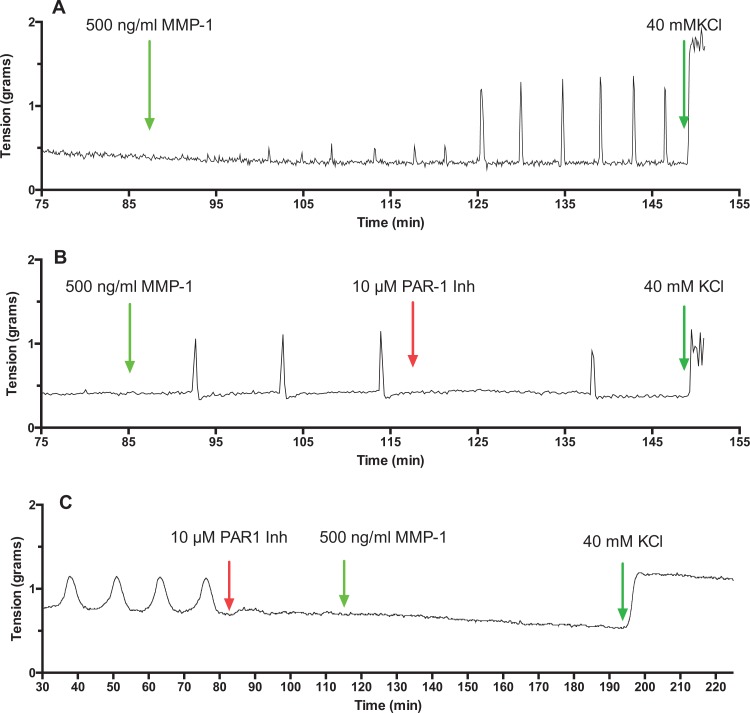
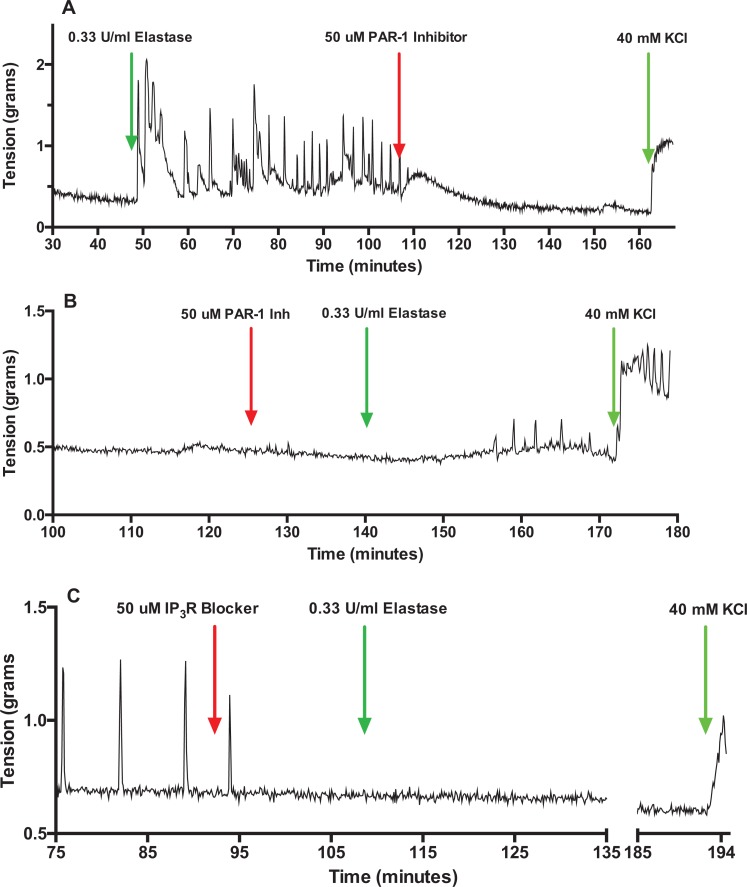
Figure 5 shows representative tracings of rat myometrium contractions induced by MMP-1. Matrix metalloproteinase-1 produced powerful myometrial contractions (Figure 5A) that were inhibited by PAR-1 inhibitor (Figure 5B and C). Figure 6 shows elastase also caused strong contractions of rat myometrium (Figure 6A), which similar to MMP-1 were inhibited by PAR-1 inhibitor (Figure 6A and B). Figure 6C shows that blockade of the IP3R prevents elastase from inducing uterine contraction. Nifedipine also inhibited elastase-induced contraction (data not shown).
Figure 5.
Matrix metalloproteinase-1 (MMP-1)-induced contractions of pregnant rat myometrial strips. Active MMP-1-induced powerful contractions of rat myometrium (A), which were prevented by inhibition of protease-activated receptor 1 (PAR-1; B and C), demonstrating MMP-1 stimulates uterine contractility by activating PAR-1.
Figure 6.
Elastase-induced contractions of pregnant rat myometrial strips. Similar to matrix metalloproteinase-1 (MMP-1), elastase stimulated powerful contractions of the rat myometrium (A). B, Elastase-induced contractions were prevented by inhibition of protease-activated receptor 1 (PAR-1). Elastase-induced contractions were abolished by pretreatment with the inositol trisphosphate receptor (IP3R) blocker (C), as well as calcium channel blockade (data not shown), demonstrating that the downstream signaling mechanism for PAR-1-mediated contraction was IP3-induced release of calcium.
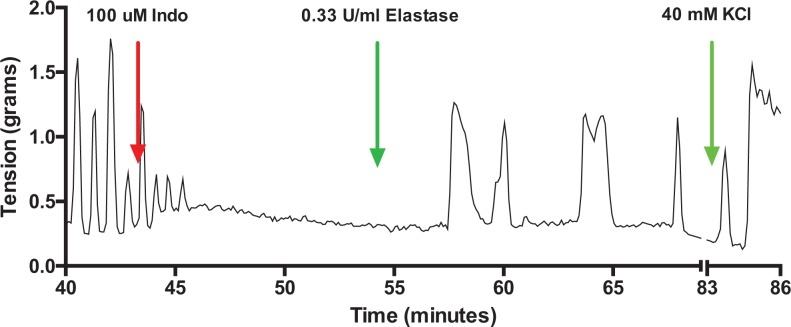
Figure 7 shows a representative tracing of a uterine strip treated with indomethacin. Indomethacin inhibited spontaneous uterine activity but did not inhibit the ability of elastase to cause uterine contraction, indicating that protease-induced contraction is not mediated by prostaglandins.
Figure 7.
Lack of effect of indomethacin on elastase-induced contractions. Treatment of a spontaneously contracting uterine strip with indomethacin completely abolished spontaneous contractions, but it did not prevent elastase from causing uterine contractions, demonstrating that protease-induced contractions are not mediated by prostaglandins.
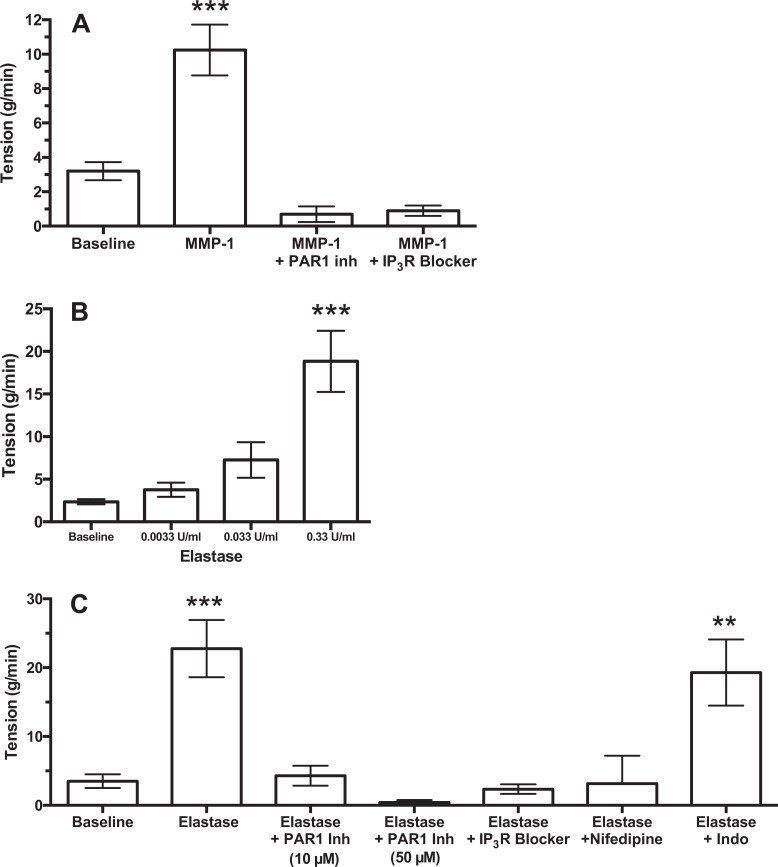
Figure 8 shows summarized results for MMP-1 and elastase. Both MMP-1 and elastase stimulated uterine contractility and a dose-response was demonstrated for elastase. Inhibition of PAR-1, as well as blockade of the IP3R, inhibited the ability of both MMP-1 and elastase to induce uterine contractions. Blockade of Ca++ channels prevented elastase from inducing contraction, but inhibition of RhoA kinase did not (data not shown). These data demonstrate that proteases stimulate uterine contractility by activating PAR-1, and the downstream signaling pathway utilized is IP3 release of calcium. Treatment with indomethacin did not prevent elastase from contracting the myometrium, demonstrating that prostaglandins did not mediate the response.
Figure 8.
Summary of matrix metalloproteinase-1 (MMP-1) and elastase results. Myometrial uterine contractility induced by MMP-1 (n = 13) was prevented by protease-activated receptor 1 (PAR-1) inhibition (n = 4) or inositol trisphosphate receptor (IP3R) blockade (n = 5; A). Elastase produced a dose-dependent increase in uterine contractility (B, n = 4). Elastase-induced increases in uterine contractility (n = 15) were prevented by inhibition of PAR-1 (n = 10 low dose, n = 3 high dose), IP3R blockade (n = 7) and nifedipine (n = 6) but not by indomethacin (n = 7; C). ***P < .001 and **P < .01 compared to baseline.
Discussion
More than a decade ago, thrombin was shown to be a potent activator of uterine contractility and implicated in PTL associated with hemorrhage.9–12 In this study, we show that 2 other proteases, MMP-1 and neutrophil elastase, are also potent activators of uterine contractility. Matrix metalloproteinase-1 and neutrophil elastase may play a role in labor not associated with hemorrhage because of the extensive infiltration of neutrophils into intrauterine tissues at the time of labor.4–6 Neutrophils not only produce elastase and MMP-121 but also secrete inflammatory cytokines, such as TNF-α, that could stimulate additional MMP-1 from decidua and myometrium. Consistent with this, we show TNF-α stimulated MMP-1 production in cultured decidual and myometrial cells, and in vivo expression of MMP-1 and neutrophil elastase was increased in TL as compared to TNL with MMP-1 even further increased in PTL possibly due to additional MMP-1 from intrauterine tissues. Expression of both the pro- and active enzymes of MMP-1 increased progressively from TNL to TL to PTL.
The mechanism whereby MMP-1 and elastase increased uterine contractility was via activation of PAR-1 because treatment with a PAR-1 inhibitor prevented their ability to cause contraction. This is the same as thrombin, which also acts via PAR-1.12 Two of the downstream intracellular signaling pathways of PAR-1 are RhoA kinase and activation of IP3-induced release of Ca++ from smooth endoplasmic reticulum.14–16 Inhibition of RhoA kinase did not prevent protease contractility, but inhibition of the IP3R and Ca++ channel blockade did, so the downstream signaling pathway utilized by protease activation of PAR-1 was IP3 release of Ca++ to cause uterine smooth muscle contraction.
We also tested indomethacin as an inhibitor to evaluate whether prostaglandins mediated the contractile response. Indomethacin did not inhibit protease-induced uterine contraction, demonstrating that uterine contractions induced by MMP-1 and elastase are not mediated by prostaglandins. This is the same as the lack of effect of indomethacin on thrombin-induced contraction.11 The fact that indomethacin does not inhibit protease-induced contraction has clinical implications. This may explain why clinical treatment with indomethacin delays, but does not prevent, PTL. Indomethacin inhibits the prostaglandin component, but not the protease component. With regard to the latter, the Food and Drug Administration recently approved a PAR-1 inhibitor.22 Based on the findings of this study, PAR-1 inhibition may be beneficial for the treatment of PTL.
A weakness in our approach is that we used rat myometrium, which may respond differently than human myometrium to MMP-1 and elastase. The amino acid sequence of the N-terminal exodomain of PAR-1 is different between the rat and human (ncbi.nlm.nih.gov). The exodomain contains a tethered ligand, which is inactive when the N-terminal domain is intact. Activation occurs when the N-terminal tail is cleaved, which allows the tethered ligand to bind to an activation site on the membrane portion of the receptor.14–16 In the human, MMP-1 cleaves the N-terminal tail just 2 amino acids distal to the thrombin cleavage site,7,8 which is adjacent to the tethered ligand, resulting in full activation of the receptor. In the rat, the MMP-1 cleavage site is 20 amino acids distal to the thrombin cleavage site, so the N-terminal tail is longer after MMP-1 cleavage in the rat than in the human. The longer N-terminal tail may have decreased the potency of MMP-1 in our study. However, other investigators have shown that the human myometrium contracts to thrombin and PAR-1-activating peptide similar to the rat,23 so protease-induced contractility of the myometrium is most likely similar between the rat and the human and the rat can be used as a model to study mechanisms.
The data presented in this study implicate protease activation of PAR-1 as a critical node in myometrial contraction (Figure 9). In the case of placental abruption and decidual hemorrhage, thrombin is generated as blood clots, resulting in PAR-1 activation. The findings of the present study extend PAR-1-mediated myometrial contraction to increases in MMP-1 and elastase as neutrophils infiltrate intrauterine tissues at the time of labor. Matrix metalloproteinase-1 and elastase were elevated in both TL and PTL, so these proteases may be important but heretofore unrecognized players in stimulating uterine contractions at term, as well as preterm.
Figure 9.
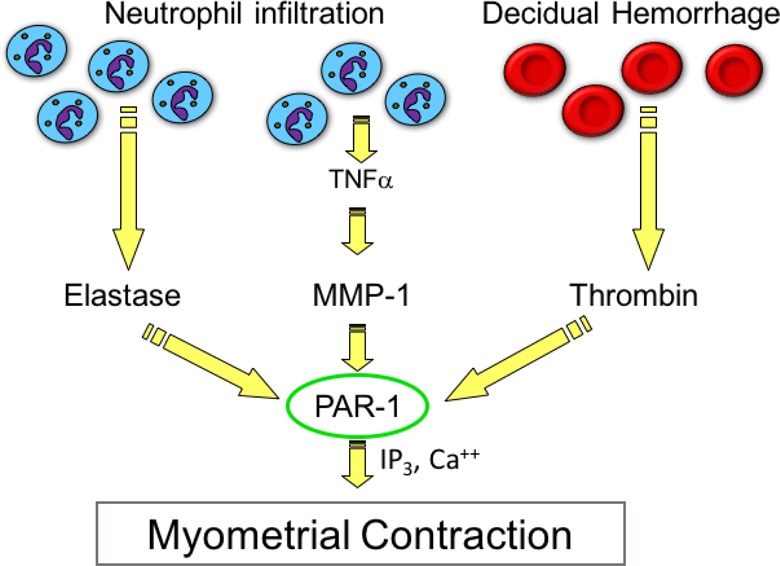
Protease-activated receptor 1 (PAR-1) as a mediator of uterine contraction. The data presented in this study implicate PAR-1 as a critical node in protease-induced myometrial contraction not only by hemorrhage-induced elevations in thrombin but also by increases in matrix metalloproteinase-1 (MMP-1) and elastase as neutrophils infiltrate intrauterine tissues in term and preterm labor. Protease activation of PAR-1 may explain why indomethacin is not able to prevent preterm labor.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by grants from NHLBI, R01 HL069851 (SWW), and NCMHD P60 MD002256 (JFS, SWW).
References
- 1. Maymon E, Romero R, Pacora P, et al. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183(4):914–920. [DOI] [PubMed] [Google Scholar]
- 2. Strauss JF., III Extracellular matrix dynamics and fetal membrane rupture. Reprod Sci. 2013;20(2):140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang H, Ogawa M, Wood JR, et al. Genetic and epigenetic mechanisms combine to control MMP1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet. 2008;17(8):1087–1096. [DOI] [PubMed] [Google Scholar]
- 4. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, III, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16(2):206–215. [DOI] [PubMed] [Google Scholar]
- 5. Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20(2):154–167. [DOI] [PubMed] [Google Scholar]
- 6. Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–236. [PubMed] [Google Scholar]
- 7. Ahn HS, Chackalamannil S, Boykow G, Graziano MP, Foster C. Development of proteinase-activated receptor 1 antagonists as therapeutic agents for thrombosis, restenosis and inflammatory diseases. Curr Pharm Des. 2003;9(28):2349–2365. [DOI] [PubMed] [Google Scholar]
- 8. Trivedi V, Boire A, Tchernychev B, et al. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137(2):332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elovitz MA, Ascher-Landsberg J, Saunders T, Phillippe M. The mechanisms underlying the stimulatory effects of thrombin on myometrial smooth muscle. Am J Obstet Gynecol. 2000;183(3):674–681. [DOI] [PubMed] [Google Scholar]
- 10. Elovitz MA, Saunders T, Ascher-Landsberg J, Phillippe M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am J Obstet Gynecol. 2000;183(4):799–804. [DOI] [PubMed] [Google Scholar]
- 11. Phillippe M, Elovitz M, Saunders T. Thrombin-stimulated uterine contractions in the pregnant and nonpregnant rat. J Soc Gynecol Investig. 2001;8(5):260–265. [DOI] [PubMed] [Google Scholar]
- 12. Shintani Y, Hirano K, Nishimura J, Nakano H, Kanaide H. Enhanced contractile response to thrombin in the pregnant rat myometrium. Br J Pharmacol. 2000;131(8):1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki T, Moraes TJ, Vachon E, et al. Proteinase-activated receptor-1 mediates elastase-induced apoptosis of human lung epithelial cells. Am J Respir Cell Mol Biol. 2005;33(3):231–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–264. [DOI] [PubMed] [Google Scholar]
- 15. Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost. 2001;86(1):298–307. [PubMed] [Google Scholar]
- 16. Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3(8):1800–1814. [DOI] [PubMed] [Google Scholar]
- 17. Smith R. Parturition. N Engl J Med. 2007;356(3):271–283. [DOI] [PubMed] [Google Scholar]
- 18. Lockwood CJ, Arcuri F, Toti P, et al. Tumor necrosis factor-alpha and interleukin-1beta regulate interleukin-8 expression in third trimester decidual cells: implications for the genesis of chorioamnionitis. Am J Pathol. 2006;169(4):1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW. Telomerase immortalization of human myometrial cells. Biol Reprod. 2002;67(2):506–514. [DOI] [PubMed] [Google Scholar]
- 20. Anderson CD, Jr, Kendig DM, Al-Qudah M, Mahavadi S, Murthy KS, Grider JR. Role of various kinases in muscarinic M3 receptor-mediated contraction of longitudinal muscle of rat colon. J Smooth Muscle Res. 2014;50:103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mousa AA, Cappello RE, Estrada-Gutierrez G, et al. Preeclampsia is associated with alterations in DNA methylation of genes involved in collagen metabolism. Am J Pathol. 2012;181(4):1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poole RM, Elkinson S. Vorapaxar: first global approval. Drugs. 2014;74(10):1153–1163. [DOI] [PubMed] [Google Scholar]
- 23. O’Sullivan CJ, Allen NM, O’Loughlin AJ, Friel AM, Morrison JJ. Thrombin and PAR1-activating peptide: effects on human uterine contractility in vitro. Am J Obstet Gynecol. 2004;190(4):1098–1105. [DOI] [PubMed] [Google Scholar]