Abstract
Prenatal alcohol exposure often results in an array of fetal developmental abnormalities termed fetal alcohol spectrum disorders (FASDs). Despite the high prevalence of FASDs, the pathophysiology of fetal damage by alcohol remains poorly understood. One of the major obstacles in studying fetal development in response to alcohol exposure is the inability to standardize the amount, pattern of alcohol consumption, and peak blood alcohol levels in pregnant mothers. In the present study, we used Doppler ultrasonography to assess fetal growth and cardiovascular parameters in response to alcohol exposure in pregnant baboons. Baboons were subjected to gastric alcohol infusion 3 times during the second trimester equivalent to human pregnancy, with maternal blood alcohol levels reaching 80 mg/dL within 30 to 60 minutes following alcohol infusion. The control group received a drink that was isocaloric to the alcohol-containing one. Doppler ultrasonography was used for longitudinal assessment of fetal biometric parameters and fetal cardiovascular indices. Fetal abdominal and head circumferences, but not femur length, were significantly decreased in alcohol-exposed fetuses near term. Peak systolic velocity of anterior and middle cerebral arteries decreased during episodes of alcohol intoxication, but there was no difference in Doppler indices between groups near term. Acute alcohol intoxication affected fetal cerebral blood flow independent of changes in the fetal cardiac output. Unlike fetal growth parameters, changes in vascular indices did not persist over gestation. In summary, alcohol effects on fetal growth and on fetal vascular function have different time courses.
Keywords: maternal drinking, fetal cerebral artery, fetal growth restriction, Doppler ultrasound, fetal alcohol spectrum disorders
Introduction
Alcohol consumption during pregnancy is a serious public health problem and a leading preventable cause of birth defects and developmental disabilities (www.cdc.gov). Alcohol’s adverse effects on the developing fetus result in a spectrum of structural abnormalities, behavioral defects, and neurocognitive disabilities termed fetal alcohol spectrum disorders (FASDs). The most severe cases are diagnosed as fetal alcohol syndrome (FAS). In the United States and Western European countries, 2% to 5% of children have FASD (www.cdc.gov).1,2 In some regions of the world, however, up to 29% of live births are with FAS/FASD.3 It is widely documented that prenatal alcohol (ethanol) exposure causes morphological and developmental delays that are accompanied by abnormalities in several key organs of the body, such as brain, heart, kidney, liver, gastrointestinal tract, and the endocrine and immune systems.4–6 Thus, studies on the phenomenology and mechanisms of fetal damage by prenatal alcohol exposure represent a rapidly expanding field of research.
About one-third of women who consume alcohol during pregnancy engage in binge-drinking that results in a rapid rise of blood alcohol concentration (BAC) reaching 80 mg/dL within 2 hours (http://www.cdc.gov/media/releases/2015/p0924-pregnant-alcohol.html). As documented by numerous studies, high but intermittent BAC produces the much more negative effects on a developing fetus when compared to those evoked by low and steady BAC.2,7,8 Thus, it comes as not a surprise that the births with FASD are most frequent in geographic areas with a high prevalence of alcohol binge-drinking.1–2,9 One of the major obstacles in studying fetal development in response to alcohol exposure is the inability to standardize the amount, pattern of alcohol consumption, and peak blood alcohol levels in pregnant mothers.
In the present study, we utilized ultrasound and Doppler sonography to study the consequences of prenatal alcohol exposure on fetal growth and the vascular system in a baboon model of pregnancy. The benefit of using an animal model is the ability to obtain results that are standardized for the timing, amount, and pattern of alcohol exposure. The baboon model of pregnancy has been proven useful in studies of fetal vascular function with maternal BAC that qualifies as a binge alcohol level.10 In addition, baboons are nonhuman primates evolutionarily close to humans and share the critical stages of human fetal development.11–13 Considering that the total length of pregnancy in baboons is 180 days (ie, about two-thirds of the length of pregnancy in humans), the selected timing of fetal alcohol exposure in our work corresponds to the second trimester of pregnancy in humans.14 The second trimester is a critical time for major vascular and neurogenic changes in fetal development as well as fetal growth.15 During this time of gestation, the fetal brain undergoes massive neurogenesis. Rapid increase in the number of neurons is accompanied by invasion of the brain parenchyma by blood vessels and development of the microvessel network required to support the growing demand for oxygen and nutrients.16,17 Thus, we targeted fetuses at the second trimester equivalent of pregnancy with intermittent alcohol exposure to model a high-risk scenario for triggering alcohol-driven developmental abnormalities.
Although many organs are affected by prenatal alcohol exposure, the brain is the most sensitive; alcohol exposure results in a wide range of structural and functional abnormalities, including fetal cerebral artery function.10,18 Whether deleterious effect of alcohol on the overall fetal growth has different time frame when compared to the effect of alcohol on cerebrovascular and cardiac parameters remains unknown. Our aim is to describe the short- and long-term effects of second-trimester alcohol exposure using a fetal baboon model on fetal cerebrovascular, cardiac, and umbilical Doppler indexes. We also sought to determine the longitudinal changes in fetal growth parameters and vascular indexes associated with this exposure.
Materials and Methods
Study Approval
The care of animals and experimental protocols were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, which is an Association for Assessment and Accreditation of Laboratory Animal Care international–accredited institution.
Fetal Alcohol Exposure Procedure
The study was performed on adult pregnant female baboons (Papio) ranging from 7 to 20 years of age. Baboons were alcohol naive yet had been used in other research studies of different nature; the diversity of baboon medical history and environmental exposures are likely representative of diversity in human populations.
A total of 18 pregnant dams were enrolled into the study; all pregnancies were singleton. Animals were randomly divided into 2 groups that were receiving either control (9 dams) or alcohol-containing (9 dams) infusion (see below). The infusion procedures took place at 90, 100, and 110 days of gestation as confirmed by an ultrasound examination. In humans these time points correspond to the second trimester of pregnancy. A 10 day-long interval between repeated alcohol exposures was chosen to allow full recovery from the previous alcohol exposure episode. Prior to the infusion procedure, the animals were fasted for 12 hours. On the day of the experiment, the animals were sedated with ketamine hydrochloride (Ketaset, Zoetis Inc, Kalamazoo, MI, 10 mg/kg of body weight) and transported to a procedure room. Throughout the procedure, anesthesia was maintained with isoflurane (1.5%-2.0%). A rectal suppository of indomethacin (25 mg) was used to prevent labor during the alcohol/control infusion. An IV catheter was placed into the saphenous vein for blood collection and another catheter was placed into the cephalic vein for IV sodium chloride infusion. Animal monitoring of vital signs and depth of anesthesia consisted of electrocardiography, pulse oximetry, capnography, noninvasive blood pressure, and temperature measurements. A gastric catheter was introduced into the stomach and the drink mixture was administered over 10 minutes. For the experimental group, the drink contained 1.8 g/kg ethanol diluted using reverse osmosis purified drinking water. The control group of animals received an isocaloric solution containing orange-flavored Tang powder (Kraft Foods, Northfield, IL). In both cases, the total volume of infused liquid was equal to 200 mL. Experimental blood collection at 10 time points over 180 minutes confirmed that blood alcohol levels reached on average 80 mg/dL within the first 30 to 60 minutes and remained steady up to 180 minutes following alcohol infusion. Blood alcohol levels in baboons that were receiving infusion of control liquid were undetectable. Prior to anesthetic recovery, but after the last blood collection, both groups of animals received a single intramuscular injection of carprofen (Rimadyl, Zoetis Inc., Kalamazoo, MI) at a dose of 4.4 mg/kg of body weight, which was aimed to alleviate symptoms of hangover following alcohol drinking.
Cesarean deliveries were performed using the standard methodology at 165 days of gestation, corresponding to the equivalent of the end of third trimester of human pregnancy. Delivered fetuses were euthanized by exsanguination while still under the influence of maternal anesthesia.
Doppler Ultrasonography of Fetal Growth and Cardiovascular Parameters
The drink mixture was infused at 90, 100, and 110 days of gestation and ultrasounds were performed immediately before and at 120 minutes postinfusion at each gestational age. Ultrasounds were again performed at 135, 155, and 165 days of gestation. The latter corresponds to near term in humans. The ultrasounds were performed using the SonoAce R3 (Samsung, Medison Co, Gumi-si, Republic of Korea). Middle cerebral artery (MCA) was interrogated using pulse wave Doppler just distal to the circle of Willis, and the angle of insonation was maintained near 0 degrees. Peak systolic velocity (PSV) and pulsatility index (PI) were calculated. The anterior cerebral artery (ACA) was probed just distal to the circle of Willis, and attempts were made at maintaining the angle of insonation near 0 degrees. If the angle exceeded 15 degrees, the Doppler waves were not included in the analysis. A free loop of umbilical artery was tested with pulse wave Doppler and the PIs were calculated. Each measurement was repeated 3 times, and the mean value was utilized in the analysis as a single datapoint.
Cardiac Tei index was measured by obtaining the apical 5-chamber cardiac view and placing the Doppler gate over the mitral and aortic valves. The Tei index was calculated by the following formula: isovolumetric relaxation time + isovolumetric contraction time/ejection time of aortic outflow. Each measurement was obtained 3 times, and the average was utilized for the statistical analysis. Fetal biometric parameters were obtained once during each ultrasound session, including the abdominal circumference, head circumference, and femur length (in millimeters).
Chemicals
Ultra pure, 200 proof ethanol was purchased from American Bioanalytical (Natick, MA). Unless stated otherwise, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Data Analysis
Final plotting, fitting, and statistical analysis of the data were conducted using Origin 8.5.1 (OriginLab), InStat 3.0 (GraphPad), and SAS/STATv14.1 (SAS Institute Inc). Data are expressed as mean ± standard error of the mean (SEM), and n stands for the number of observations. Unless stated otherwise, the number of observations corresponds to the number of examined fetuses. This number is usually smaller than the number of animals enrolled in each experimental group due to the inability to obtain high-quality Doppler records in some cases and also due to the fact that not all pregnancies lasted throughout the duration of the study. Comparison of means was tested using either analysis of variance (ANOVA) or student t test, depending on the number of independent variable levels. Following ANOVA, multiple comparison testing with Tukey adjustments for P values was performed. A simple linear regression model accounting for clustering (repeated measures) of experimental units was performed, in order to test the outcome differences between the experimental groups at 100 to 110 versus 155 to 165 dGa. Due to multiple testing, we conservatively consider the results statistically significant at the α level of .01.
Results
Effect of Fetal Alcohol Exposure on Overall Fetal Growth
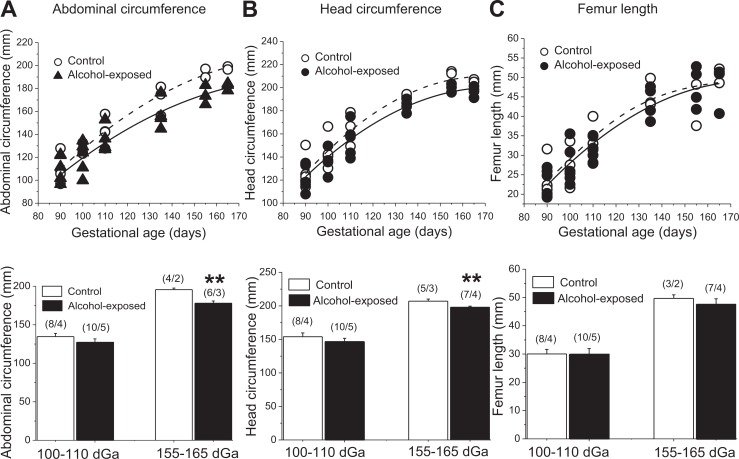
To perform detailed fetal morphometry throughout gestation, fetal biometric parameters were obtained via ultrasound at different time points during pregnancy. Although no difference in fetal abdominal circumference was detected between groups at the time of alcohol exposure episodes (100-110 days), abdominal circumference measurements were significantly reduced in alcohol-exposed fetuses closer to the end of gestation (155-165 days) as compared to the control group at the same time point (P = .0033; Figure 1A). Similarly, the head circumference was significantly smaller in the alcohol-exposed group at 155 to 165 (P = .0005) but not at 100 to 110 days of gestation (Figure 1B). In contrast, fetal femur length was statistically indistinguishable between groups at any time point during gestation (Figure 1C).
Figure 1.
Effect of fetal alcohol exposure on the overall fetal growth. A, Changes in abdominal circumference throughout gestation of control (hollow labels) versus alcohol-exposed (black labels) fetuses. Bottom bar graph is showing average of 2 repeated measures at 100 and 110 versus 155 and 165 dGa. **Statistically significant from control fetuses at 155 to 165 dGa; P < .01. B, Changes in head circumference throughout the gestation of control (hollow labels) versus alcohol-exposed (black labels) fetuses. Bottom bar graph is showing average of 2 repeated measures at 100 and 110 versus 155 and 165 dGa. **Statistically significant from control fetuses at 155 to 165 dGa; P < .01. C, Changes in femur length throughout the gestation of control (hollow labels) versus alcohol-exposed (black labels) fetuses. Bottom graph is showing average of 2 repeated measures at 100 and 110 versus 155 and 165 dGa.
Effect of Alcohol Exposure on Fetal Cerebral Artery Flow Velocity Waveforms
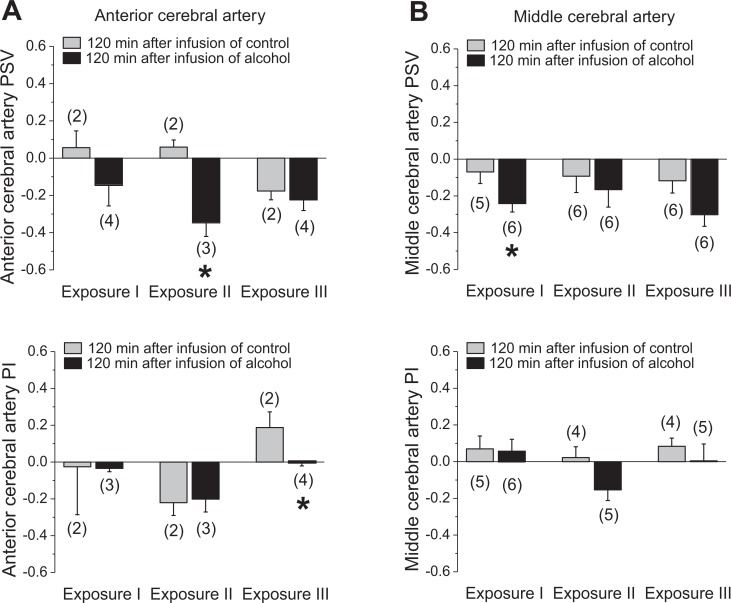
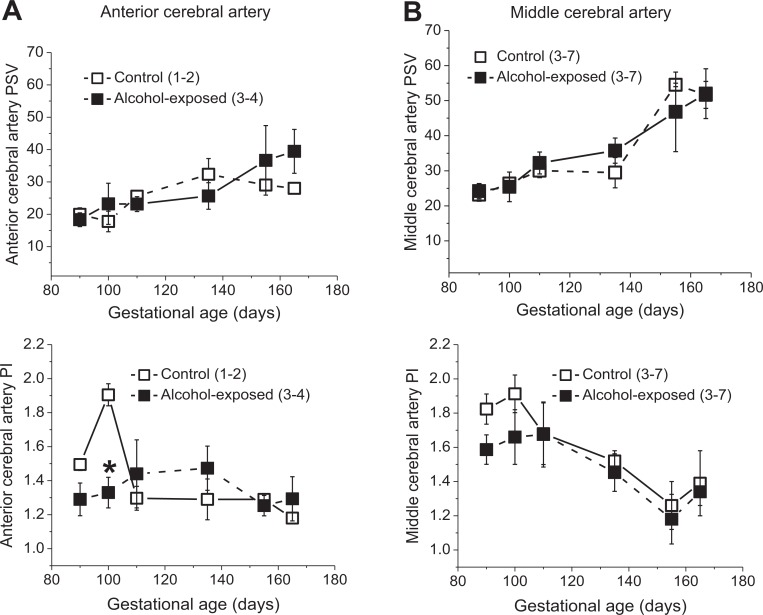
Considering that fetal cerebrovascular effects of alcohol may be localized to a specific brain region,19 we assessed flow velocity waveforms in anterior versus middle cerebral arteries (ACAs and MCAs). There was an overall trend for reduced PSV during the time of alcohol intoxication for both cerebral vessels. However, a decrease in PSV reached statistical significance for ACAs during the second exposure and for MCAs during the first exposure (Figure 2). Although both arteries exhibited a trend toward decreased PI during the third alcohol exposure, statistical significance was reached only for ACA (Figure 2). Longitudinal evaluation of ACAs and MCAs throughout the second (measurements obtained immediately before the infusion of control or alcohol drink) and third trimesters revealed a progressive increase in PSV accompanied by a decrease in PIs. Despite the fact that alcohol intoxication was accompanied by changes in the fetal cerebral arteries (Figure 2), we did not detect significant differences between groups throughout the gestation (Figure 3). The only exception was a statistically significant drop in the ACA PI around 100 days of gestation, yet this change was not present at later stages of gestation (Figure 3A).
Figure 2.
Effect of fetal alcohol exposure on fetal cerebral artery Doppler indices during alcohol exposure. A, Average changes in anterior cerebral artery (ACA) peak systolic velocity (PSV) and pulsatility index (PI) by repeated alcohol exposure. Data are normalized to the values that were obtained immediately before the infusion of control or alcohol-containing drink. Zero level represents no change in the measured parameter as observed at 120 minutes following the infusion of the assigned drink as compared to the preinfusion level in each group. Changes in the positive direction reflect increases in the measured parameter upon drink infusion, whereas changes in the negative direction are equivalent to decreases in the measured parameter upon drink infusion. Here and in B, *statistically significant difference in changes in fetal artery function parameters in response to maternal gastric infusion of control versus alcohol-containing solution, P < .05. B, Middle cerebral artery data; annotation is similar to A.
Figure 3.
Effect of fetal alcohol exposure on fetal cerebral artery Doppler indices throughout gestation. Peak systolic velocity (PSV) and pulsatility index (PI) values throughout gestation in control versus alcohol-exposed group of anterior (A) and middle (B) cerebral arteries. *Statistically significant difference when compared to control at the same time point of gestation.
Effect of Fetal Alcohol Exposure on Umbilical Artery and Fetal Heart Function
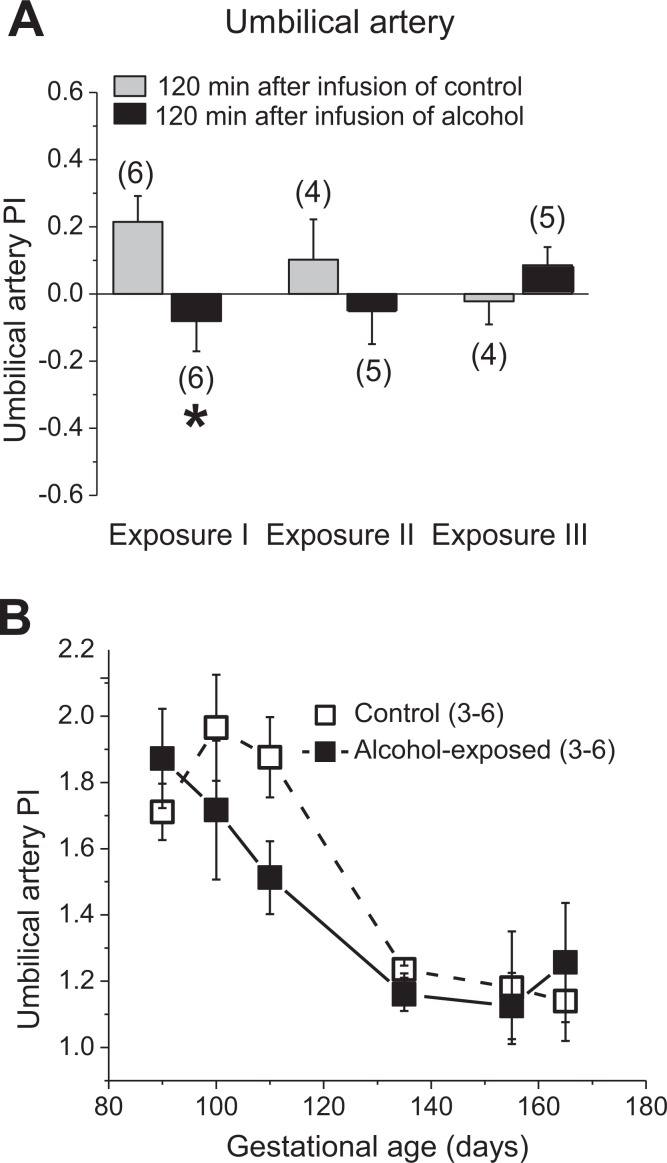
In alcohol-exposed fetuses, a decrease in the umbilical artery PI was observed during the first alcohol exposure (Figure 4A). Longitudinal evaluation of the umbilical artery revealed a progressive decline in PI throughout gestation in both groups. During the period of alcohol exposure in the treatment group, the umbilical artery PIs were decreased by a greater magnitude; this difference was not as pronounced in the third trimester equivalent that was during the time when fetuses were not repeatedly exposed to alcohol (P = .0962; Figure 4B). Although alcohol’s effect on the umbilical artery was apparent, there was no significant difference in fetal heart Tei index between the control and alcohol-exposed groups (data not shown).
Figure 4.
Effect of fetal alcohol exposure on umbilical artery Doppler indices. A, Average changes in pulsatility index (PI) by repeated alcohol exposure. Data are normalized to the values that were obtained immediately before the infusion of control or alcohol-containing drink. Zero level represents no change in measured parameter as observed at 120 minutes following the infusion of the assigned drink when compared to preinfusion levels in each group. Changes in the positive direction reflect the average increase in the measured parameter upon drink infusion, whereas changes in the negative direction are equivalent to the average decrease in the measured parameter upon drink infusion. *Statistically significant difference in changes in fetal artery Doppler indices in response to maternal gastric infusion of control versus alcohol-containing solution, P < .05. B, The mean umbilical artery PI throughout the gestation in control versus alcohol-exposed groups.
Discussion
In the present study, we described for the first time the consequences of alcohol exposure during the second trimester equivalent of human pregnancy on fetal vascular function in pregnant baboons, and compared the timing of their appearance with the timing of fetal growth delay. After just 3 infusion episodes, alcohol exposure resulted in reduction in morphometric indexes (abdominal and head circumference measurements) in the fetuses (Figure 1). These changes were not demonstrated immediately after alcohol exposure (100-110 days of gestation) but became statistically significant at later gestational ages (155-165 dGa; Figure 1).
This period is equivalent to the third trimester of pregnancy in humans. Remarkably, our finding is consistent with previous reports pointing to an association between prenatal alcohol exposure and third-trimester growth restriction.8,20 Thus, our fetal morphometric data validate the suitability of the baboon model to study the consequences of prenatal alcohol exposure on fetal development.
In contrast to fetal morphometric parameters, vascular indices dropped in the presence of alcohol, yet this change did not persist throughout gestation. Therefore, alcohol effects on fetal growth and on fetal vascular function have different time courses. The fact that the vascular effect of alcohol precedes fetal growth restriction suggests that the developmental delay (including neurological abnormalities) observed in FAS/FASD may be triggered by alcohol-induced alterations in vascular function. The mechanisms that link alcohol-induced dilation of cerebral artery with the delayed growth remain to be uncovered. They may not be linked specifically to alcohol but rather represent a nonspecific response of growth to the vascular insufficiency. An intriguing example is the case of spontaneous intracranial hypotension. During this condition, alcohol-related component is absent, yet noticeable morphological abnormalities of the skull have been reported and attributed to an abnormal drop in the cerebral artery tone.21,22 The idea that alcohol-driven alterations in cerebral blood flow, yet not the presence of alcohol per se, may underlie neuronal damage is also in unison with the studies on alcohol effect using the ovine model of pregnancy during third trimester equivalent of human pregnancy. In this model, fetal alcohol intoxication increased blood flow (consistent with cerebral artery dilation), this effect being selective to cerebellum.19 As a result, a decrease in the Purkinje cell number was observed in cerebellum, but there was no loss in neurons in the hippocampus or the olfactory bulb; these regions did not experience alterations in cerebral blood flow.19
Our findings on decreased fetal cerebral artery Doppler indices during alcohol intoxication are consistent with other reports describing vascular consequences of low to moderate alcohol levels during second trimester equivalent of human pregnancy. Indeed, a recent study utilizing a mouse model of pregnancy demonstrated that single as well as repeated alcohol exposure episodes during mid-pregnancy resulted in a significant decrease in blood flow velocity (consistent with the artery dilation) in MCAs and posterior cerebral artery areas for at least 24 hours.23 In a baboon model of pregnancy, it was suggested that the effect of maternal drinking on the fetal cerebral artery stemmed from the alcohol-induced dilation of fetal cerebral arteries.24 This notion is supported by a recent study from our group that documents decrease in baboon fetal MCA Doppler indices under the presence of alcohol during second trimester equivalent of human pregnancy.10 This decrease is consistent with alcohol-induced dilation of the fetal cerebral artery.10 Our present work expands previous findings by showing the vulnerability of ACAs to the presence of alcohol (Figure 2). The fact that alcohol exposure in utero results in cerebral artery dilation in both murine and nonhuman primate models of pregnancy underscores the consistency of alcohol-induced dilation of fetal cerebral arteries across species and thus, increases confidence regarding the applicability of laboratory findings to human disease.
In a sheep model, however, blood alcohol levels of 85 mg/dL, which are comparable to BACs reached in our experimental model, did not evoke significant changes in fetal cerebral blood flow during third trimester equivalent of human pregnancy.19 The discrepancy with our data may be explained by the different timing of fetal alcohol exposure (third trimester equivalent in ovine model versus second trimester equivalent in our experiments). It is tempting to speculate that second trimester may represent a window of vulnerability for alcohol-induced effect on the cerebrovascular system. It is noteworthy that the drop in fetal cerebral artery Doppler indices observed in our experiments in the presence of 80 mg/dL alcohol in maternal bloodstream is consistent with the cerebral vasodilation described in adult humans in the presence of similar (90 mg/dL) blood alcohol level.25 Thus, disregarding the age (prenatal period or adulthood), 80 to 90 mg/dL blood alcohol levels in primates trigger cerebral vasodilation.
In summary, our work demonstrates that fetal alcohol exposure in the second trimester equivalent of human pregnancy results in vasodilation of fetal cerebral arteries. However, this effect is not sustained throughout the entirety of gestation. In contrast, the consequences of alcohol exposure on morphometric parameters are not immediate but delayed. Further work is needed to determine whether different amounts and durations of fetal alcohol exposure have similar effects on the time course of alcohol-induced changes in fetal artery Doppler indices when compared to the ethanol effects on growth restriction.
Acknowledgments
The authors thank the Laboratory Animal Care Unit personnel at UT HSC for their assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH R21 AA022433 (ANB) and in part by the Office of the Director, National Institutes of Health under Award Number P40OD010988.
References
- 1. May PA, Gossage JP, Brooke LE, et al. Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. Am J Public Health. 2005;95(7):1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. May PA, Gossage JP. Maternal risk factors for fetal alcohol spectrum disorders: not as simple as it might seem. Alcohol Res Health. 2011;34(1):15–26. [PMC free article] [PubMed] [Google Scholar]
- 3. Olivier L, Curfs LM, Viljoen DL. Fetal alcohol spectrum disorders: prevalence rates in South Africa. S Afr Med J. 2016;106(6 suppl 1):S103–S106. [DOI] [PubMed] [Google Scholar]
- 4. Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25(3):185–191. [PMC free article] [PubMed] [Google Scholar]
- 5. Gauthier TW. Prenatal alcohol exposure and the developing immune system. Alcohol Res. 2015;37(2):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caputo C, Wood E, Jabbour L. Impact of fetal alcohol exposure on body systems: a systematic review. Birth Defects Res C Embryo Today. 2016;108(2):174–180. [DOI] [PubMed] [Google Scholar]
- 7. Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25(3):168–174. [PMC free article] [PubMed] [Google Scholar]
- 8. West JR, Kelly SJ, Pierce DR. Severity of alcohol-induced deficits in rats during the third trimester equivalent is determined by the pattern of exposure. Alcohol Alcohol Suppl. 1987;1:461–465. [PubMed] [Google Scholar]
- 9. Viljoen DL, Gossage JP, Brooke L, et al. Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. J Stud Alcohol. 2005;66(5):593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seleverstov O, Tobiasz A, Jackson JS, et al. Maternal alcohol exposure during mid-pregnancy dilates fetal cerebral arteries via endocannabinoid receptors. Alcohol. 2017;61:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart CB, Disotell TR. Primate evolution—in and out of Africa. Curr Biol. 1998;8(16):R582–R588. [DOI] [PubMed] [Google Scholar]
- 12. Pillay P, Manger PR. Order-specific quantitative patterns of cortical gyrification. Eur J Neurosci. 2007;25(9):2705–2712. [DOI] [PubMed] [Google Scholar]
- 13. Bauer C. The baboon (Papio sp.) as a model for female reproduction studies. Contraception. 2015;92(2):120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stevens VC. Some reproductive studies in the baboon. Hum Reprod Update. 1997;3(6):533–540. [DOI] [PubMed] [Google Scholar]
- 15. Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci. 2010;33:379–408. [DOI] [PubMed] [Google Scholar]
- 16. Norman MG, O’Kusky JR. The growth and development of microvasculature in human cerebral cortex. J Neuropathol Exp Neurol. 1986;45(3):222–232. [PubMed] [Google Scholar]
- 17. Fowden AL, Forhead AJ. Endocrine regulation of feto-placental growth. Horm Res. 2009;72(5):257–265. [DOI] [PubMed] [Google Scholar]
- 18. Nunez CC, Roussotte F, Sowell ER. Focus on: structural and functional brain abnormalities in fetal alcohol spectrum disorders. Alcohol Res Health. 2011;34(1):121–131. [PMC free article] [PubMed] [Google Scholar]
- 19. Parnell SE, Ramadoss J, Delp MD, et al. Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions: ovine model. Exp Physiol. 2007;92(5):933–943. [DOI] [PubMed] [Google Scholar]
- 20. Sawant OB, Wu G, Washburn SE. Maternal L-glutamine supplementation prevents prenatal alcohol exposure-induced fetal growth restriction in an ovine model. Amino Acids. 2015;47(6):1183–1192. [DOI] [PubMed] [Google Scholar]
- 21. Gordon N. Spontaneous intracranial hypotension. Dev Med Child Neurol. 2009;51(12):932–935. [DOI] [PubMed] [Google Scholar]
- 22. Yoon MK, Parsa AT, Horton JC. Skull thickening, paranasal sinus expansion, and sella turcica shrinkage from chronic intracranial hypotension. J Neurosurg Pediatr. 2013;11(6):667–672. [DOI] [PubMed] [Google Scholar]
- 23. Bake S, Tingling JD, Miranda RC. Ethanol exposure during pregnancy persistently attenuates cranially directed blood flow in the developing fetus: evidence from ultrasound imaging in a murine second trimester equivalent model. Alcohol Clin Exp Res. 2012;36(5):748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kochunov P, Castro C, Davis DM, et al. Fetal brain during a binge drinking episode: a dynamic susceptibility contrast MRI fetal brain perfusion study. Neuroreport. 2010;21(10):716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blaha M, Aaslid R, Douville CM, Correra R, Newell DW. Cerebral blood flow and dynamic cerebral autoregulation during ethanol intoxication and hypercapnia. J Clin Neurosci. 2003;10(2):195–198. [DOI] [PubMed] [Google Scholar]