Abstract
Background: Despite the proven efficacy and advantages of absorbable implants, their use for metacarpal shaft fixation has been limited. This is likely due to the high reported complication rates in early studies with polyglycolic acid (PGA) implants, notably high rates of noninfectious inflammatory reaction (5%-25%), occurring up to 30 weeks after fixation. The objective of this study was to assess the clinical outcomes of newer absorbable plates and screws in the treatment of metacarpal shaft fractures. Methods: The authors performed a systematic search of the PubMed, Ovid MEDLINE, and EMBASE databases dating from 1946 to 2017. Primary outcome measures were the development of noninfectious inflammatory reaction and implant failure. Results: A total of 42 metacarpal shaft fractures in 35 patients were included. The average follow-up time was 20.4 months (n = 24; range: 3.6-61 months). Only 1 case (2.4%) of noninfectious inflammatory reaction was reported with polylactic acid (PLA) plates and PLA/PGA compounds. Noninfectious inflammatory reaction was observed in 4 out of the 9 patients (44.4%) with a trimethylene carbonate/PLA compound. Symptoms appeared after an average time of 15.8 months (range: 12-19 months) post-fixation. Painless prolonged inflammation that resolved spontaneously within 6 months was reported in 7.1% of cases (n = 3). Implant failure with loss of fracture reduction was reported in 9.5% of cases (n = 4). Conclusions: Newer absorbable materials appear to have significantly lower rates of noninfectious inflammatory reaction than previously reported. When compared with metallic fixation of the metacarpal shaft, absorbable fixation appears to have comparable complication rates and biomechanical properties.
Keywords: metacarpal shaft, hand, absorbable, plates, fracture, fixation, treatment
Introduction
Metacarpal (MC) fractures are common entities that account for 10% of all fractures and up to 50% of all hand fractures.1,2 Simple, nondisplaced transverse fractures of the shaft heal well with closed reduction and plaster immobilization or percutaneous pinning.3 Plate and screw stabilization is occasionally the optimal treatment of choice in complex MC fractures. Indications include multiple fractures with soft tissue injury or bone loss, markedly displaced shaft fractures (particularly border MCs), and reconstruction for nonunion or malunion.4 Rigid and stable fixation with plate and screws is often achieved with titanium or stainless steel implants. Despite the proven efficacy and advantages of absorbable implants, their use for MC shaft fixation has been limited.5 This is likely due to the high reported complication rates in early studies, notably high rates of noninfectious inflammatory reaction (5%-25%), occurring up to 30 weeks after fixation.6 This propensity to cause a delayed noninfectious inflammatory reaction in response to particles released during the implant degradation and absorption process has been documented with older first-generation polyglycolic acid (PGA) implants.6 To date, few studies have explored the clinical outcomes of modern polylactide-based implants in the treatment of MC shaft fractures. Herein, the authors conducted a systematic review of the literature regarding the use of newer absorbable implants for the internal fixation of MC shaft fractures.
Methods
The authors of this article conducted a search of the PubMed, OVID MEDLINE, and EMBASE databases to identify clinical studies involving the use of absorbable implants in the fixation of MC shaft fractures. An example of the complete search strategy is provided in Figure 1. Different spellings and versions of the following key words were searched: [(“biodegradable” or “resorbable” or “absorbable” or “bioabsorbable”) and (“implant” or “plate” or “fixation”) and “metacarpal”]. The search was limited to studies involving human subjects, published in peer-reviewed journals and written in the English language. Two independent reviewers assessed the eligibility of the studies using the same strict inclusion and exclusion criteria. Studies were selected based on the relevance of the title and/or abstract of retrieved records (Figure 2). The initial screen excluded studies with evidently irrelevant titles or abstracts. If content was unclear in the initial screen based on abstract review, a formal article review was undertaken. Studies of MC shaft fractures fixated with absorbable plates were included. A strict exclusion criterion was the use of pure PGA plates. Inclusion was further based on the evaluation of implant-related outcomes, with documentation of one or more of the following primary endpoints: noninfectious inflammatory response and implant failure. Secondary endpoints included range of motion; local soft tissue swelling; grip strength; Disabilities of the Arm, Shoulder and Hand (DASH) score; pain scores; and radiologic outcomes. The systematic review followed the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.7
Figure 1.

OVID EMBASE search history.
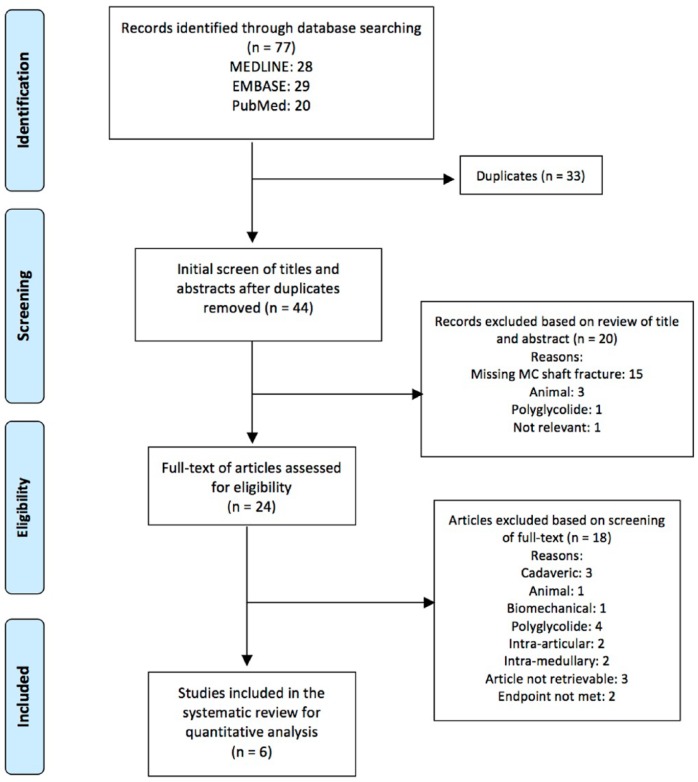
Figure 2.
Preferred reporting items for systematic reviews and meta-analyses flow diagram representing results from PubMed, EMBASE, and OVID databases.
Note. MC = metacarpal.
Results
The MEDLINE, EMBASE, and PubMed preliminary search yielded 28, 29, and 20 articles, respectively. After duplicates were removed, a total of 44 articles published between the years 1989 and present remained. A total of 6 articles were ultimately acceptable for inclusion into the current systematic review according to the eligibility criteria outlined above.2,8-12 Due to the paucity in available literature, 5 of these articles were therapeutic studies of level IV evidence in the form of case reports (n = 3) and case series (n = 2) that did not include a control group. One article was a prospective comparative study of level II evidence (Table 1). A flow diagram outlining the systematic search can be found in Figure 2.
Table 1.
Summary of Selected Studies.
| Article | Age/sex | MC No. (hand) | Fracture description | Diagnosed on | Type of absorbable implant | Follow-up | Primary endpoints | Secondary endpoints | Prognosis | Study description | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noninfectious inflammatory response | ROM | Other | Grip strength | Radiologic measures | Other | |||||||||
| Choi et al10 2015 | 30/M | 5th (L) | Comminuted | CT and XR | PLAa | 14 | Yes (12 months post-op) | Implant failureb | Refracture | Repeated trauma and refracture of 5th MC, ORIF using metallic plates 16 months post-op | Case report, therapy IV | |||
| Dumont et al11 2007 | 37 | 2nd | Short oblique | XR | PLA-PGA | 3.67 | No | +10°/80° | 117.86% | |||||
| 41 | 4th | Wedge | XR | PLA-PGA | 3.67 | No | +10°/90° | 94.83% | ||||||
| 21 | 4th | Spiral | XR | PLA-PGA | 3.67 | No | 0°/90° | 84.62% | ||||||
| 18 | 4th | Long oblique | XR | PLA-PGA | 3.67 | No | +10°/80° | 100.00% | ||||||
| 29 | 3rd | Long oblique | XR | PLA-PGA | 3.67 | No | +10°/90° | 81.25% | ||||||
| 33 | 5th | Transverse | XR | PLA-PGA | 3.67 | No | 0°/80° | 68.18% | ||||||
| 26 | 3rd/4th | Short/long oblique | XR | MC3: PLA-PGA; MC4: PLA-PGA | 3.67 | No | 0°/80° | 85.71% | ||||||
| 22 | 5th | Comminuted | XR | PLA-PGA | 3.67 | No | +10°/90° | Implant failure | 87.50% | ORIF with metallic plate | ||||
| 21 | 5th | Transverse | XR | PLA-PGA | 3.67 | No | +10°/90° | 95.65% | ||||||
| 18 | 4th | Short oblique | XR | PLA-PGA | 3.67 | No | +10°/80° | 95.24% | ||||||
| 17 | 2nd | Transverse | XR | PLA-PGA | 3.67 | No | 0°/80° | 95.45% | ||||||
| 43 | 4th | Long oblique | XR | PLA-PGA | 3.67 | No | 0°/80° | 105.26% | ||||||
| Averaged data | 27.2 (range: 18-43) | Displaced, unstable | All XR | PLA-PGA | 3.67 | None | +5.8°/84.2° | Implant failurec (n = 2) Prolonged local swelling (n = 3) over 6 weeks but resolved after 6 months |
Only 1 patient had a persistent loss of >20% after 6 months | DASH (n = 14) 1.5 months: 30 3 months: 13 6.5 months: 3 VAS pain scale (n = 14) 1.5 months: 18 3 months: 2 6.5 months: 0.2 |
Case series, therapy IV | |||
| Givissis et al2 2010 | 32/M | 3rd (L) | NA | Trimethylene carbonate and PLA | 61 | Yes (15 months post-op) | Healed fracture within 6 weeks | Surgical debridement to remove implant 18 months post-op | ||||||
| 18/M | 5th (R) | NA | Trimethylene carbonate and PLA | 55 | Transient local swelling (at 22 months post-op), disappeared after 2 weeks | |||||||||
| 27/M | 5th (L) | NA | Trimethylene carbonate and PLA | 50 | Yes (19 months post-op) | Surgical debridement to remove implant 22 months post-op | ||||||||
| 56/F | 4th (L) | NA | Trimethylene carbonate and PLA | 49 | ||||||||||
| 29/M | 5th (R) | NA | Trimethylene carbonate and PLA | 47 | ||||||||||
| 75/F | 2nd (L) | NA | Trimethylene carbonate and PLA | 45 | Yes (14 months post-op) | Surgical debridement to remove implant 17 months post-op | ||||||||
| 57/M | 4th (L) | NA | Trimethylene carbonate and PLA | 35 | Transient local swelling (at 13 months post-op), disappeared after 2 weeks | |||||||||
| 20/F | 4th (L) | NA | Trimethylene carbonate and PLA | 35 | ||||||||||
| 32/M | 4th/5th (R) | NA | Trimethylene carbonate and PLA | 34 | Yes (15 months post-op) | Surgical debridement to remove implant 18 months post-op | ||||||||
| Averaged data | 36.5 (range: 18-75) | Displaced, unstable | NA | Trimethylene carbonate and PLA | 45.7 (range: 34-61) |
N = 4 | Transient local swelling under 2 weeks (n = 2) | VAS score 2.25 (range: 2-3) | Case series, therapy IV | |||||
| Lionelli et al12 2001 | 19/M | 4th (R) | Displaced | XR | PLA-PGA | No | Implant failure | Subjective pain | Implant removal and ORIF with metallic plate | Case report, therapy IV | ||||
| Sakai et al9 2012 | 32/M | 3rd/4th (R) | XR and CT | u-HA/PLA | 6 | No | +10°/74° | 80.20% | DASH: 0 | |||||
| Averaged data | 35.1 (range: 20-78), 10 M, 1 F | N = 16 | None severely comminuted | u-HA/PLA | 7.5 | None | 92.7% ±19.7% |
Prospective comparative study, therapy II | ||||||
| Waris et al13 2004 | 22 | 3rd (R) | Displaced, comminuted | XR | PLA | 15 | No | +20°/60° | Scar contracture on thumb | Case report, therapy IV | ||||
Note. M = males; F = females; L = left; R = right; MC = metacarpal fracture; ROM = range of motion; CT = computed tomography; XR = x-ray; PLA = polylactic acid; ORIF = open reduction and internal fixation; PGA = polyglycolic acid; DASH = Disabilities of the Arm, Shoulder and Hand score; VAS = visual analogue scale; u-HA = hydroxyapatite.
Polymer is not specified in the article; however, the commercial company (Linvatec biomaterials Ltd, Tampere, Finland) uses polylactic acid implants.
Implant failure secondary to refracture at the same site caused by trauma.
One patient required secondary operation for ORIF with a titanium plate. The other patient had a secondary loss of reduction, but did not need reoperation.
Clinical Presentation
A total of 35 patients presented with 42 cases of MC shaft fractures. One patient died in a car accident shortly after surgery and was excluded from this study. The mean age of patients was 31 (n = 25, range: 17-78 years). Males comprised 83% (n = 19) of patients, while females comprised of only 17% (n = 4) of patients. Sex was not indicated in 52% (n = 12) of patients. When documented, the right hand was injured in 46% (n = 6) of patients, compared with 54% (n = 7) for the left hand. The fourth and fifth MC bones had the highest fracture rates, accounting for 41.4% (n = 12) and 27.6% (n = 8) of all cases, respectively. The third MC was involved in 17.2% (n = 5) and the second in 13.8% (n = 4) of all cases. Though infrequently documented, the mechanisms of injury were described as falls, crush injuries, and motor vehicle accidents.
Investigations
Metacarpal fractures were diagnosed on plain radiographs alone in 68.5% of patients (n = 24). Computed tomography scan in addition to plain films was utilized in 5.7% of patients (n = 2). The imaging modality utilized for initial fracture diagnosis was omitted in 54.3% of all patients (n = 9).
Implant Material
The type of implant used varied across studies. Copolymers of polylactic acid (PLA) and PGA were used as the fixation device in 33.3% of MC shaft fractures (n = 14). Mixed isomers of PLA-based implants were used in 28.5% (n = 12) of fractures, among which 10 also contained trimethylene carbonate. Novel bioabsorbable implants made of hydroxyapatite/PLA (u-HA/PLA) were used in 11 patients for internal fixation of 16 MC shaft fractures.9 First-generation implants consisting of purely PGA were not used in any of the studies included in the current review.
Prognosis After Treatment
The average follow-up time for patients was 20.4 months (n = 25, range: 3.6-61 months). Treatment of noninfectious inflammatory reaction required surgical debridement of the absorbable implant in all cases. Subsequent internal fixation with metallic implants depended on the integrity of the involved bone after debridement. Noninfectious inflammatory reaction was observed in 4 out of the 9 patients (44.4%) with a trimethylene carbonate/PLA compound. These patients sought medical attention due to local swelling and tenderness persisting for at least 1 month. They were also found to have a positive ballottement sign on exam, indicative of contained fluid accumulation.2 An initial trial of oral analgesics (nonsteroidal anti-inflammatory drugs) and activity limitation failed to show improvement after 2 months. Ultimately, all four of these patients underwent surgical debridement and removal of free remnants of the implanted material. Intraoperatively, implant remnants were enveloped in reactive tissue and the presence of aseptic pus or serum was noted. Extensor tendons showed no signs of inflammation or irritation. Although screw parts retained inside the bone were left in place, none of the patients experienced a recurrence of any type of soft tissue reaction at the final follow-up. No additional fixation methods were needed.1 In the remaining studies, only 1 case (2.4%) of noninfectious inflammatory reaction was reported using a PLA implant.10 Unfortunately, this patient refused further investigation despite experiencing swelling around the operative site, and later returned with refracture of the same MC bone over a year after the first operation. A 2 × 1 cm well-demarcated mass in the subperiosteal layer located just above the original screw holes was excised prior to open reduction and internal fixation (ORIF) with metallic plates.10 During surgical debridement, there was no mention of residual absorbable implant material. However, given that the repeat surgery occurred over a year after insertion of the absorbable implants, it is likely that they had resorbed by this time. Overall, noninfectious inflammatory reactions occurred after an average delay of 15 months (n = 5, range: 12-19 months) from the initial operation.2,10 The initial presentation was typically described as painful erythematous fluctuating papules emerging over the implant site.2 Histopathology of foreign body granulomas was described as predominately histiocytes and multinucleated giant cells surrounding particles derived from implant degradation.2,10
All complications and reported study endpoints are detailed in Table 1. Implant failure with loss of fracture reduction was reported in 9.5% of all cases (n = 4). Among them, 75% (n = 3) required implant removal and repeat ORIF with metallic plates, while 25% (n = 1) did not require reoperation.10-12 None of the studies mention whether the use of absorbable implants impacted the integrity of the fixation construct when revision with metallic implants was required. Although Lionelli et al mention that the absorbable screws were well anchored into the bone at the time of repeat ORIF, they do not state whether these screws were left in place or removed.12 Nevertheless, no further complications were noted, and union was achieved with metallic implants. Similarly, Dumont et al do not clearly state whether the absorbable implant remnants or screws were removed before secondary fixation with metallic implants.11 After experiencing implant failure in 2 patients in their study, Dumont et al changed their postoperative regimen to include a 3-week orthosis composed of semirigid/hardcast rigid casting tape to support the MCs, while allowing free range of movement to the wrist and fingers.11 Since implementing this orthosis, they have not experienced implant failure.
Painless transient local swelling, which resolved spontaneously after 2 weeks, was reported in 5.7% of all patients (n = 2).2 In these two patients, the swelling appeared at roughly 13 and 22 months post-operatively, respectively, and neither had to seek medical attention. Prolonged soft tissue swelling lasting more than 6 weeks but less than 6 months was reported in 8.6% of all patients (n = 3). Overall, the average grip strength compared with the contralateral hand was 91.7% (n = 13, range: 68%-117%), with only one patient having a persistent loss of >30% after 6 months.9,11 With regard to mobility, there was no significant difference in postoperative stiffness between metallic and absorbable implants.9,10 The total active range of motion at the metacarpophalangeal joint of the involved digit was +7°/82° (range: +20°/60° to 0°/90°; n = 14).8-10 Only two studies reported on the DASH score. The first study comprised of 14 patients who noted a DASH score of 30 after 1.5 months, 13 after 3 months, and 3 after 6.5 months.11 The second study reported on 1 patient who had a DASH score of 0 after 6 months post-operation.9
Discussion
Metallic plates allow rigid and stable fixation of MC shaft fractures and adequately restore length. Disadvantages include extensive periosteal dissection, albeit less significant with new generation locking plates, and the possible necessity for future plate removal, reported in ~15% of MC fractures in more recent studies.14-16 The most important complications to consider when dealing with metallic implants are plate prominence, stiffness, tendon irritation and rupture, infection, bone atrophy, and osteoporosis due to stress shielding.2,12,17 On the contrary, absorbable implants incrementally transfer load to a healing fracture thereby limiting stress shielding, while promoting bone union.2,9,10,12,17 Their physical properties make them magnetic resonance imaging-compatible and radiolucent, facilitating postoperative radiological evaluation.2,10 In addition, they offer the theoretical advantage of circumventing the need for a second operation for hardware extraction.2
The biomechanical properties of absorbable implants are comparable with their metallic counterparts, allowing them to achieve adequate fracture stabilization.1 Comparable torsional rigidity and failure torque has been reported between bioabsorbable miniplating and metallic fixation for MC fractures.16 Sakai et al compared the mechanical properties of novel bioabsorbable plates with those of titanium plates for MC fractures. The average grip strength measured as a percentage of the contralateral hand was 92.7% (range: 56%-120%) for bioabsorbable plates and 86.4% (range: 57-119) for titanium plates. The total active range of active motion of fractured fingers was 267° (range: 254°-270°) for bioabsorbable plates and 250° (range: 200°-270°) for titanium plates. They concluded that there were no significant differences between the 2 groups in terms of total finger motion or grip strength. Moreover, all fractures in their cohort united without any postoperative complications following treatment with either bioabsorbable or titanium plates.9
Despite the aforementioned advantages, absorbable implants have yet to replace metallic devices as common practice for osteosynthesis. From a practical point of view, absorbable implants are less favorable due to their significantly higher costs compared with their metallic counterparts. An e-mail correspondence with a manufacturer company in 2015 showed the following price difference: titanium plate 1.5 mm €68, titanium plate 2.0 mm €34.50, and screw €9. Absorbable plates and screws were associated with the following costs: plate 2.0 mm €80, plate 2.5 mm €88, and screw €24.18 A cost-minimizing analysis by Böstman showed that absorbable implants were financially comparable with metallic implants for treatment of MC fractures.19 In their study, the total cost for fixation of MC fractures using absorbable implants was $3537. This was not significantly higher than the combined cost of metallic implant fixation and removal, with a break-even point at a hardware removal rate of 19%. More recent studies are needed to compare the financial impact of metallic with newer absorbable implants.
A feared limitation associated with absorbable implants is the propensity for a noninfectious inflammatory reaction to ensue.6 Such foreign body reactions (FBRs) are an inherent biological tissue response provoked by implant degradation and absorption. The tissue’s response to foreign material ranges from clinically insignificant mild reactions to persistent swelling that may eventually progress to a painful inflammatory response requiring surgical debridement of the foreign particles.6 The immediate postoperative period is usually uneventful. Foreign body reactions tend to occur after a delay, once the implant decompensation phase commences.2 In the current review, the average time from initial operation to development of a noninfectious inflammatory reaction occurred after 15 months (n = 5, range: 12-19 months).2,10 Other limitations noted in the literature include implant failure, seen in 9.5% of all cases.5,17 These findings are comparable with the average metallic implant failure rate of 8%.20 However, given the paucity of literature on implant failure rates, it is difficult to make a thorough comparison. Future studies are required to further evaluate failure rate as new generation implants (eg, locking plates) are becoming increasingly used. Biomechanical studies have demonstrated that implant failure is less concerning with the advent of newer generation absorbable implants that have a greater crystalline component. A biomechanical study by Sakai et al concluded that the strength of u-HA/PLA plates is sufficient for maintaining anatomical reduction of displaced MC fractures.9 Similarly, Waris et al showed that PLA miniplates have mechanical fixation properties comparable with that of titanium plates and Kirschner wires.16
First-generation implants created for biomedical applications in the 1960s consisted mostly of PGA homopolymers. Its mechanical strength is usually lost within 6 weeks, and it is completely resorbed by the body within a few months.21 Due to their rapid degradation rate and loss of strength in vivo, PGA implants had higher rates of refracture and FBRs as compared with metallic devices.10 In a review by Böstman et al in 1991, PGA implants resulted in noninfectious inflammatory response in up to 25% of patients.6,12 The highest incidence was observed in fractures of the distal radius and scaphoid. This led to the development of second-generation implants with greater biostability. Second-generation implants are made of PLA and its isoforms, poly-L-lactide (PLLA), and poly-D-lactide. The nomenclature of PLA is misleading in that it is not a polyacid, but rather a polyester. PLLA can remain detectible in tissues from 18 months to 5 years post-implantation.9 Racemic mixtures of both the D- and L-isomers to form poly-DL-lactide widen the polymer chains, resulting in a less crystalline material that is more rapidly degraded.21 The mechanical strength of poly-DL-lactide is lost in approximately 4 months, and it is completely resorbed within 2 to 3 years.5 PLA can also be combined with PGA to form varying ratios of PLA/PGA copolymers. The mechanical strength of PLA/PGA in a 4:1 ratio is around 70% to 80% within 6 weeks, and complete resorption usually occurs within 1 year.12 Generally, the degradation rate correlates with the content of PGA, whereby the faster resorption occurs with greater PGA. The exception is a 50:50 ratio of PGA:PLA, which actually demonstrates the fastest degradation.21
The challenge with in vivo degradation remains in generating an absorbable implant that degrades to allow tissue regeneration and healing while avoiding the activation of immune responses caused by the release of degraded implant particles. Newer generation of absorbable implants are not as well described in the literature and there exists a greater degree of heterogeneity in their chemical composition. Givissis et al utilized a more modern type of implant consisting of trimethylene carbonate, L-lactide, and D,L-lactide.2 Unfortunately, they observed delayed FBRs requiring surgical debridement in 44.4% (n = 4) of their patients.2 They argue that modern absorbable implants with slower degradation rates simply postpone the occurrence of FBR to a later time, rather than eliminate the propensity of this complication. In our opinion, trimethylene carbonate/PLA compounds are to be avoided given the high rates of noninfectious inflammatory reaction. When excluding the aforementioned material, only 1 case (2.4%) of noninfectious inflammatory reaction was reported with newer absorbable material. Although noninfectious inflammatory reaction was previously a significant concern, newer absorbable material, particularly pure PLA or PLA/PGA compounds, appears to have significantly lower rates than previous reported.
Study limitations include the heterogeneity in absorbable implant material, indications for surgery, surgical technique, surgeon experience, and patient population. Performing a meta-analysis was challenging due to heterogeneity and paucity of data. Future advancements in the field must include comparative prospective studies to compare newer absorbable implants when compared with metallic fixation, particularly in the pediatric population.
Conclusion
Although noninfectious inflammatory reactions were previously a significant concern, newer absorbable materials (PLA or PLA/PGA) appear to have significantly lower rates than previously reported. When compared with metallic fixation of the MC shaft, absorbable plates and screws appear to have comparable complication rates and biomechanical properties. Absorbable implants are promising and with the advent of newer, more sustainable polymers, they may soon be an attractive alternative to metallic implants, though additional prospective controlled studies are required.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: Informed consent was not required as there were no human subjects in this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jessica Hazan  http://orcid.org/0000-0003-3832-6818
http://orcid.org/0000-0003-3832-6818
References
- 1. Bernstein M, Chung K. Hand fractures and their management: an international view. Injury. 2006;37(11):1043-1048. [DOI] [PubMed] [Google Scholar]
- 2. Givissis PK, Stavridis SI, Papagelopoulos PJ, et al. Delayed foreign-body reaction to absorbable implants in metacarpal fracture treatment. Clin Orthop Relat Res. 2010;468(12):3377-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinstein L, Hanel D. Metacarpal fractures. J Am Soc Surg Hand. 2002;2(4):168-180. [Google Scholar]
- 4. Wolfe SW, Pederson WC, Hotchkiss RN, et al. Green’s Operative Hand Surgery. Philadelphia, PA: Elsevier Health Sciences; 2010. [Google Scholar]
- 5. Waris E, Ashammakhi N, Kaarela O, et al. Use of bioabsorbable osteofixation devices in the hand. J Hand Surg Am. 2004;29(6):590-598. [DOI] [PubMed] [Google Scholar]
- 6. Böstman OM. Absorbable implants for the fixation of fractures. J Bone Joint Surg Am. 1991;73(1):148-53. [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2010;8(5):336-341. [DOI] [PubMed] [Google Scholar]
- 8. Waris E, Ashammakhi N, Raatikainen T, et al. Self-reinforced bioabsorbable versus metallic fixation systems for metacarpal and phalangeal fractures: a biomechanical study. J Hand Surg Am. 2002;27(5):902-909. [DOI] [PubMed] [Google Scholar]
- 9. Sakai A, Oshige T, Zenke Y, et al. Mechanical comparison of novel bioabsorbable plates with titanium plates and small-series clinical comparisons for metacarpal fractures. J Bone Joint Surg Am. 2012;94(17):1597-1604. [DOI] [PubMed] [Google Scholar]
- 10. Choi JS, Lee JH, Kim SM, et al. Foreign-body granuloma after metacarpal fracture treatment with absorbable implants. Arch Plast surg. 2015;42(4):505-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dumont C, Fuchs M, Burchhardt H, et al. Clinical results of absorbable plates for displaced metacarpal fractures. J Hand Surg Am. 2007;32(4):491-496. [DOI] [PubMed] [Google Scholar]
- 12. Lionelli GT, Korentager RA. Biomechanical failure of metacarpal fracture resorbable plate fixation. Ann Plast Surgery. 2002;49(2):202-206. [DOI] [PubMed] [Google Scholar]
- 13. Waris E, Ninkovic M, Harpf C, et al. Self-reinforced bioabsorbable miniplates for skeletal fixation in complex hand injury: three case reports. J Hand Surg Am. 2004;29(3):452-457. [DOI] [PubMed] [Google Scholar]
- 14. Ozer K, Gillani S, Williams A, et al. Comparison of intramedullary nailing versus plate-screw fixation of extra-articular metacarpal fractures. J Hand Surg Am. 2008;33(10):1724-1731. [DOI] [PubMed] [Google Scholar]
- 15. Stern P, Wieser M, Reilly D. Complications of plate fixation in the hand skeleton. Clinorthop Relat Res. 1987;(214):59-65. [PubMed] [Google Scholar]
- 16. Waris E, Ashammakhi N, Happonen H, et al. Bioabsorbable miniplating versus metallic fixation for metacarpal fractures. Clin Orthop Relat Res. 2003;410:310-319. [DOI] [PubMed] [Google Scholar]
- 17. Bozic KJ, Perez LE, Wilson DR, et al. Mechanical testing of bioresorbable implants for use in metacarpal fracture fixation. J Hand Surg Am. 2001;26(4):755-761. [DOI] [PubMed] [Google Scholar]
- 18. van Bakelen NB, Vermeulen KM, Buijs GJ, et al. Cost-effectiveness of a biodegradable compared to a titanium fixation system in maxillofacial surgery: a multicenter randomized controlled trial. PLoS One. 2015;10(7):e0130330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Böstman OM. Metallic or absorbable fracture fixation devices: a cost minimization analysis. Clin Orthop Relat Res. 1996;329(1):233-239. [PubMed] [Google Scholar]
- 20. Fusetti C, Meyer H, Borisch N, et al. Complications of plate fixation in metacarpal fractures. J Trauma. 2002;52(3):535-539. [DOI] [PubMed] [Google Scholar]
- 21. Pina S, Ferreira JM. Bioresorbable plates and screws for clinical applications: a review. J Healthc Eng. 2012;3(2):243-260. [Google Scholar]