Abstract
Objective:
To perform a comparative analysis of infiltrating immune cells in a newly developed C57BL/6 background syngeneic transplantable mouse oral cancer (MOC) model.
Study Design/Setting:
Scientific study in an academic medical center.
Methods:
Use of carcinogen-induced tumorigenesis, tissue culture, cell line transplantation, and flow cytometric analysis techniques.
Results:
Previously, we established a series of cell line models that displayed dichotomous growth phenotypes when transplanted into immunocompetent mice. We now show that the indolent growth pattern of the MOC1 generated tumors is associated with increased baseline and inducible MHC class I expression and increased CD8+ T-cell infiltration into the tumor microenvironment. Conversely, the aggressive and metastatic pattern of MOC2 generated tumors has decreased basal and inducible class I expression and is associated with FOXP3+CD4+ regulatory T-cell infiltration. Delayed primary tumor growth after targeted monoclonal antibody therapy of these FOXP3+ regulatory cells further suggests that these immune cells contribute to the aggressive phenotype of MOC2.
Conclusions:
These data validate that key infiltrating immune cells identified here parallel findings in human head and neck cancer, making this newly developed syngeneic model a critical platform for the continued dissection of tumor-host interactions in head and neck cancer.
Keywords: syngeneic model, oral cancer, tumor infiltrating lymphocytes
Introduction
Despite characterization of intracellular pro-growth and pro-survival signaling pathways that drive head and neck squamous cell carcinoma (HNSCC) oncogenesis, meaningful change in patient outcomes utilizing this knowledge has not occurred1. Patients with carcinogen-associated HNSCC demonstrate high loco-regional failure and distant metastasis rates and poor disease-specific survival despite aggressive treatments2. Conversely, patients with HPV-associated oropharyngeal cancer have high rates of cure. A natural host anti-tumor response to strong viral antigens may contribute to these improved outcomes3. Based on this concept and other lines of evidence, there is growing interest in enhancing natural anti-tumor responses to augment current approaches in the treatment of carcinogen-associated HNSCC.
Early evidence implicating the presence of a productive host anti-tumor immune response was derived from studies in mice4. Evidence of such responses in humans comes in part from elevated rates of malignancy in immunocompromised patients5. In HNSCC patients, tumor infiltration of cells involved in and a gene expression profile indicative of an adaptive immune response predicted improved outcomes6–10. In contrast, recent evidence has supported a pro-tumorigenic role for the immune system involving cells that suppress cellular immunity both locally and systemically in patients with HNSCC10.
One major void in the pre-clinical study of immune responses to HNSCC is the lack of syngeneic transplantable models in mouse strains9. The majority of transplantable HNSCC models to date are xenograft models, which recapitulate local growth and metastasis, but require immunodeficient hosts that lack components of adaptive immunity11,12. To address this deficiency, we generated carcinogen-induced oral cancer cell lines that are transplantable into widely used immunocompetent C57BL/6 mice13,14. With this syngeneic oral cancer model, we are able to study components of the host immune response to transplanted cancer cells.
Previously, we established C57BL/6 Mouse Oral Cancer (MOC) lines that demonstrate either indolent or aggressive in-vivo growth phenotypes. We hypothesized that differences in these growth phenotypes are influenced by immune cell infiltration. To address this hypothesis we compared MOC line tumor growth in immunocompent (wild-type, WT) and immunodeficient (RAG2−/−) mice. We compared the immunogenicity of the aggressive and indolent MOC lines by analyzing levels of cell surface MHC class I expression. We also characterized immune cells infiltrating into the tumor microenvironment, regional lymph nodes, and spleens of mice transplanted with both indolent and aggressive MOC cells. Further, we characterized the functionality of a subset of tumor-infiltrating immune cells via antibody-mediated knockdown and subsequent tumor growth analysis.
Materials and Methods:
Animals:
C57BL/6 and RAG2−/− mice were obtained from Taconic (Hudson, NY). Animal Studies and Research Ethics Committees of Washington University in St. Louis approved all animal studies and experimental protocols.
Antibodies:
Anti-CD45, -CD45.2, -CD4, -CD-8, -GR1, -CD11b, -F4/80, -Rat IgG2B, -Class I-Kb, -Class I-Kd and -Class I-Db were from Biolegend (San Diego, CA). Intracellular APC-FoxP3 (eBioscience, San Diego, CA) was used according to manufacturer’s recommendations. Treg cell depletion was performed as previously described15.
Cell lines:
Using repeated carcinogen exposure to induce transformation, we generated syngeneic C57BL/6 mouse oral cancer (MOC) cell lines as described previously13. Cells were cultured in IMDM/F12 at a 2:1 mixture with 5% FCS (Fisher Scientific, Houston, TX), 1% penicillin/streptomycin, 1% amphotericin, 5 ng/mL EGF (Millipore, Billerica, MA), 400 ng/mL hydrocortisone and 5 mg/mL insulin (Sigma Chemical, St. Louis, MO).
Flow Cytometry:
Transplanted flank tumors were harvested and digested to single cell suspension with Collagenase Type IA (1 mg/ml, Sigma Chemical, St. Louis, MO). Draining lymph nodes and spleen were harvested into single cell suspensions by crushing between frosted glass slides. Splenocytes were treated with Red Blood Cell Lysis Buffer (Sigma Chemical, St. Louis, MO). Cells were washed twice and blocked with rat anti-mouse CD16/CD32 (BD Biosciences, San Jose, CA) for 15 minutes. Staining was done with appropriate antibodies at 4°C for 30 minutes. Cells were further washed and flow cytometry was performed using a FACSCalibur (BD Biosciences, San Jose, CA). Data was analyzed using FloJo software (Tree Star, Ashland, OR).
MHC Class I Expression:
Cultured MOC1 and MOC2 cells were treated with vehicle control or interferon-γ (1000 units) for 72 hours. Cells were harvested with 0.25% Trypsin (Hyclone, Logan, UT), washed twice, stained and analyzed as above.
Tumor transplantation:
MOC cells were harvested, washed twice in D-PBS (Fisher, Houston, TX), resuspended at appropriate concentration and injected into the right subcutaneous flanks of WT C57BL/6 and RAG2−/− mice. Mice were monitored for tumor growth biweekly and tumor size was recorded as the average of the two largest diameters. Tumors, draining lymph nodes, and spleens were harvested for FACS evaluation and H&E staining.
Statistics:
Tumor growth was analyzed by single day comparison analysis using Mann-Whitney U-test (nonparametric equivalent of independent samples t-test). All other analyses used the independent samples t-test.
Results:
MOC cell lines have different growth phenotypes in vivo
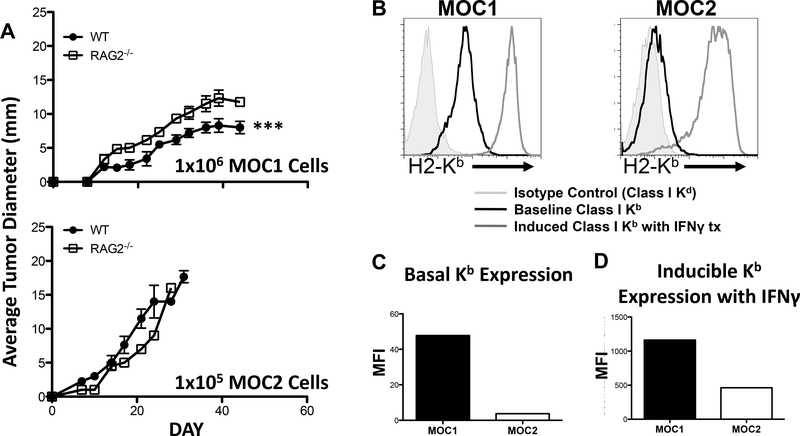
As representative models for the two growth phenotypes, we compared MOC1, which is less aggressive, and MOC2, which is a highly invasive cell line that spontaneously metastasizes to draining lymph nodes following flank transplantation. Previous work demonstrated that both MOC1 and MOC2 formed tumors when 1×106 cells were transplanted into the flanks of WT mice, and that similar growth phenotypes were observed between flank and orthotopically transplanted tumors13. We found that the kinetics of tumor growth was more rapid at a 10-fold decreased inoculum of 1×105 MOC2 cells/mouse when compared to MOC1 at 1×106 cells/mouse (Figure 1A), demonstrating the more aggressive phenotype of MOC2 cells. When parallel transplantation of these same cell lines into flanks of immunodeficient RAG2−/− mice was performed, MOC2 produced tumors that also grew faster than MOC1 (Figure 1A). Interestingly, whereas there was no difference in MOC2 tumor growth between RAG2−/− and WT mice, tumors formed after MOC1 cell transplantation grew slower in WT compared to RAG2−/− mice (Figure 1A).
Figure 1: Growth rates and class I expression of MOC lines.
A. Growth curves of transplanted MOC lines. B. Class I Kb expression at baseline and after IFN-γ stimulation. C. Baseline expression of Kb on MOC lines. D. Induced expression of Kb on MOC lines.
Higher MHC Class I expression in MOC1 compared to MOC2
Having observed growth rate differences between MOC1 and MOC2 tumors in WT mice and a decreased growth rate of MOC1 in RAG2−/− mice compared to MOC2 cells, we next evaluated the cell-surface MHC Class I expression of the two cell lines as a possible explanation. Comparison of constitutive H2-Kb showed that MOC1 had a twelve-fold increased expression relative to MOC2 (Figure 1B). In addition, MOC1 also had slightly elevated levels of constitutive H2-Db expression (data not shown). To assess induction of Class I components, cells were treated with IFN-γ (1000 units) for 72 hours. FACS analysis demonstrated that MOC1 cells show a 2.5-fold increase in inducible H2-Kb (Figure 1B, C and D) and a two-fold increase in inducible H2-Db expression (data not shown) compared to MOC2 cells. Thus, differences in constitutive and inducible MHC Class I component expression between MOC1 and MOC2 cells correlated with growth phenotypes.
Tumor infiltrating CD11b+/Gr1+ cells
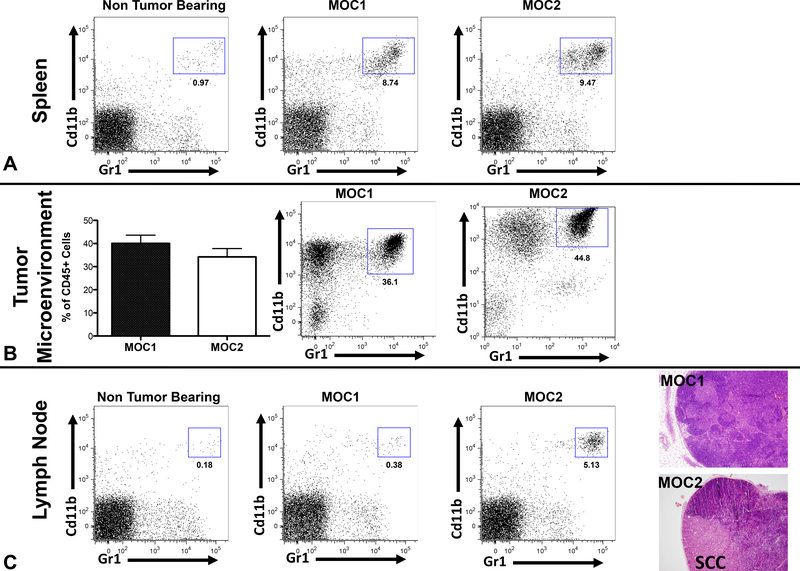
We next analyzed tumor infiltrating immune cells. When MOC1 and MOC2 generated tumors reached a diameter of 8mm, tumors, draining lymph nodes, and spleens were harvested and infiltrating immune cells were evaluated via flow cytometry. Non-tumor-bearing spleens and lymph nodes served as controls. There was an 8–9-fold increase in CD11b+/Gr1+ cells present in the spleen of tumor-bearing mice compared to non-tumor-bearing controls (Figure 2A). However, there were no significant differences in CD11b+/Gr1+ cells between the spleens of MOC1 and MOC2 transplanted mice (statistical data not shown). Significant levels of CD11b+/Gr1+ cells were present in the tumor microenvironment, and no significant differences in infiltrating CD11b+/Gr1+ cells between tumors generated from MOC1 and MOC2 transplantation were observed (Figure 2B).
Figure 2: Expanded CD11b+/Gr1+ cells in MOC lines.
A. Representative FACS data from mouse spleens. B. Representative data from MOC generated tumors. C. FACS data from regional lymph nodes. H&E stained sections of tumor-draining lymph nodes.
Increased CD11b+/Gr1+ cell infiltration in metastatic lymph nodes
As previously described, MOC2 generated tumors spontaneously metastasize to draining lymph nodes, whereas MOC1 generated tumors do not demonstrate a metastatic phenotype13. There was minimal CD11b+/Gr1+ cell infiltration in draining lymph nodes of non-tumor-bearing mice and MOC1-tumor-bearing mice. However, increased infiltration or expansion of CD11b+/Gr1+ cells was observed in lymph nodes containing metastatic disease from MOC2 generated flank tumors (Figure 2B). This CD11b+/Gr1+ cell infiltrate was not correlated with primary tumor growth.
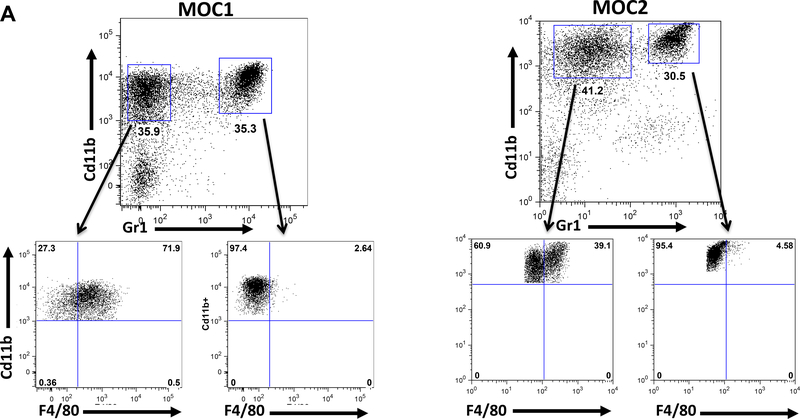
Infiltrating CD11b+/Gr1- cells are F4/80+
In the microenvironment of both MOC1 and MOC2 tumors, there was a large infiltration of CD11b+/Gr1- cells in addition to CD11b+/Gr1+ cells (Figure 3). Flow cytometric analysis of this population showed that a significant portion of these cells were F4/80+ (Figure 3). There was no significant difference in the tumor infiltration of CD11b+/Gr1-/F4/80+ myeloid cells between MOC1 and MOC2 generated tumors (data not shown). Of note, the CD11b+/Gr1+ infiltrating cells were F4/80-, establishing a population of cells used as a negative control. Interestingly, there were very few CD11b+/Gr1- cells present in either tumor-bearing or non-tumor-bearing draining lymph nodes in MOC1 or MOC2 transplanted mice (Figure 2C).
Figure 3: CD11b+/Gr1- cells are F4/80+.
A. Representative FACS plots of MOC1 or MOC2 generated tumor microenvironments. CD11b+/Gr1+ cells represent an F4/80 negative control.
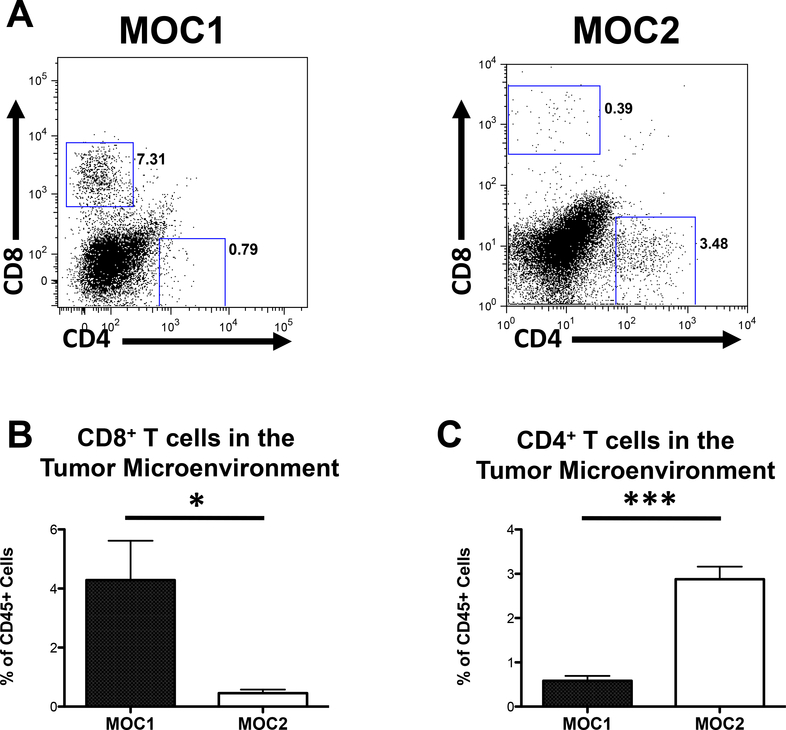
T-cell infiltration corresponds to tumor growth phenotype
While no apparent correlation between myeloid cell infiltration and growth phenotype was seen, there were significant differences in CD4+ and CD8+ T-cell infiltration in the tumor microenvironment. MOC1 generated tumors demonstrated elevated CD8+ T-cell infiltration (Figures 4A, B) and decreased CD4+ T-cell infiltration into the tumor microenvironment (Figures 4A, C) compared to MOC2 generated tumors. In contrast, MOC2 generated tumors demonstrated elevated CD4+ T-cell infiltration and decreased CD8+ T-cell infiltration relative to MOC1 generated tumors. These data suggest that the less aggressive growth pattern of MOC1 generated tumors may be associated with an increased presence of CD8+ T-cells, or conversely, that elevated CD4+ T-cell populations may contribute to the more aggressive growth pattern of MOC2 generated tumors.
Figure 4: T-cell infiltration in the tumor microenvironment.
A. CD4+ and CD8+ cells in MOC generated tumors. B. % live CD45+, CD8+ cells in the tumor microenvironment (*=p<0.05). C. % live CD45+ CD4+ in the tumor microenvironment (***=p<0.001).
Tumor infiltrating CD4+ T-cells include Tregs that contribute to the aggressive growth phenotype of MOC2 generated tumors
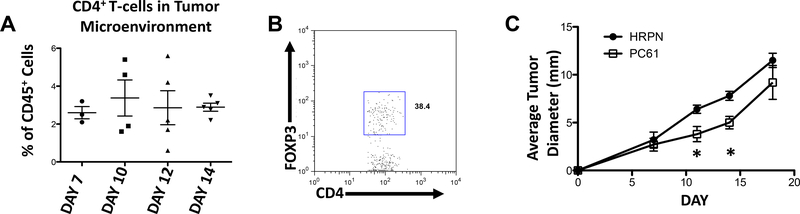
CD4+ T cells infiltrated the microenvironment of MOC2 generated tumors early and did not significantly fluctuate throughout growth (Figure 5A). Intracellular flow cytometric analysis for FoxP3, the critical transcription factor driving Treg development, was used to further characterize these CD4+ T cells. A significant percentage of these cells were found to be FoxP3+, suggesting their functional role as Tregs (Figure 5B and data not shown). To assess the functional contribution of these cells, we used an established Treg depleting regimen utilizing an anti-CD25 (PC61) monoclonal antibody (mAb). Treatment with PC61 showed a modest but significant decrease in growth rate of MOC2 generated tumors compared to control antibody at days 11 and 14 after transplantation (Figure 5C). These data suggest that in our syngeneic model, Treg infiltration of the tumor microenvironment occurs early and at least in part contributes to the aggressive growth phenotype observed with MOC2 generated tumors.
Figure 5: Depletion with anti-CD25 (PC61) attenuates MOC2 primary tumor growth.
A. CD4+ T-cells in the tumor microenvironment. B. FoxP3 in the tumor microenvironment. C. MOC2 tumors treated with HRPN or PC61. (*=p<0.05)
Discussion:
Here, we characterize infiltration of immune cells into the tumor microenvironment using a syngeneic, transplantable model of oral squamous cell carcinoma developed on a C57BL/6 genetic background13. Our approach was to address the fidelity of this system in relation to human HNSCC and to identify key immune components that could serve as a basis for further investigation to delineate therapeutic targets. Similar to human HNSCC, we demonstrated that baseline immunogenicity and variable immune cell infiltration correlated with tumor aggressiveness.
The interaction between tumor cells and infiltrating immune and inflammatory cells is complex. Key mediators of this interaction have been correlated to clinical outcomes in HNSCC9. Downregulation of cell surface MHC class I proteins, which are necessary for CD8+ T-cell detection of tumor-associated antigenic material, is associated with tumor progression and poor survival in patients with HNSCC10,16–18. Here, we demonstrated significantly elevated basal and inducible expression of MHC class I molecules on cells from the more indolent MOC1 derived tumors. MOC1 cells with higher MHC class I expression demonstrated slower growth in immunocompetent WT mice compared to immunodeficient RAG2−/− mice, suggesting that one or more components of adaptive immunity that are lacking in RAG2−/− mice act to suppress tumor growth. Given our current understanding of MHC class I restriction of tumor antigen specific CD8+ cytotoxic T-cells, we evaluated for and confirmed significantly elevated levels of CD8+ T-cells in MOC1 derived tumors compared to MOC2 derived tumors in WT mice.
This association between increased CD8+ T-cells and a more indolent growth pattern with MOC1 generated tumors parallels the improved outcomes associated with increased tumor infiltrating CD8+ T-cells in patients with HNSCC. Patients whose primary and metastatic tumor deposits have higher levels of CD8+ T-cells demonstrated more favorable outcomes6,7. Yet, while CD8+ T-cells are crucial for anti-tumor immune responses, CD8+ T-cell functional inhibition in the tumor microenvironment is the rule rather than the exception in patients with HNSCC10. These data have prompted investigation into the role of CD4+ T-cell subsets in the HNSCC microenvironment.
Functionally unique from their CTL-inducing Th1 and humoral immunity-inducing Th2 counterparts, CD4+FOXP3+ Tregs secrete immunosuppressive cytokines and inhibit anti-tumor CD8+ T-cell responses10,19,20. Tregs play both a physiologic role in limiting autoimmunity and a pathologic role in many cancer types including HNSCC21. While the majority of studies have suggested that increased levels of tumor-associated or circulating Tregs are associated with poor survival22–24, others indicate improved prognosis in patients with elevated Treg levels25. Here, we demonstrated elevated levels of CD4+ T-cells present in tumors generated from the more aggressive MOC2 cells and characterized a subset of these to be FOXP3+, suggesting their functional role as Tregs. Demonstrating the tumor-promoting role of Tregs in our model, we recorded growth inhibition of MOC2 generated tumors following antibody-mediated Treg depletion.
Interestingly, subpopulations of CD44+ HNSCC tumor cells, shown to possess cancer stem cell properties and resistance to standard anti-cancer therapies26,27, more effectively induce immunosuppressive Treg responses in vitro27. Our lab has previously reported that MOC2 cells, which generate tumors with elevated levels of infiltrating Tregs, also express high levels of CD44 compared to MOC1 cells13, further demonstrating biologic differences between our variably aggressive cell lines and immunologic similarities between our syngeneic mouse model and human HNSCC.
In addition to lymphoid immune cells, cells of myeloid origin also contribute to immunomodulation in HNSCC patients28. Tumor cells as well as infiltrating immune cells create a cytokine milieu that can influence immature myeloid cells to display either pro-tumor or anti-tumor phenotypes28–30. A heterogenous but closely related group of immature myeloid cells in which terminal differentiation is inhibited by tumor and stromal factors are called myeloid derived suppressor cells (MDCSs) and contribute to immune suppression in several tumor systems10,31. In patients with HNSCC, a subset of this immature myeloid population, identified by the marker CD34+, secrete immunosuppressive cytokines, inhibit T-lymphocyte function32,33 and are associated with tumor progression34.
In mice, MDSCs are identified by cell surface antigens Gr1+ and CD11b+31. Here, we demonstrated robust recruitment of CD11b+/Gr1+ cells into the spleen and tumor microenvironment of both MOC1 and MOC2 generated tumors. Interestingly, we also demonstrated significantly elevated recruitment of CD11b+/Gr1+ cells into the microenvironment of regional metastatic disease arising from MOC2 generated tumors. To our knowledge, this is the first report of recruitment of immature myeloid cells into the tumor draining lymph node in a model of spontaneous metastasis from an epithelial tumor. Watanabe et al. documented recruitment of CD11b+/Gr1+ MDSCs into regional metastatic disease in a sarcoma model, and showed that this MDSC population inhibited T-lymphocyte activation independent of Tregs35. Studies evaluating the presence of immature myeloid suppressor cells in deposits of regional metastasis with patients with HNSCC are lacking. Future research is needed to determine whether these immature myeloid cells are recruited into the lymph node by metastatic deposits of tumor or whether primary tumors promote a niche favorable for metastasis via cytokine-driven recruitment of immature myeloid cells into regional lymph nodes prior to regional metastasis.
More mature myeloid cells, such as macrophages, are present in the tumor microenvironment of patients with HNSCC as well36,37. Macrophage accumulation in HNSCC specimens is associated with advanced tumor stage at diagnosis and tumor progression36,37. Tumor infiltrating macrophages (TAMs) can be functionally classified as anti-tumor (M1) and pro-tumor (M2) variants and have been extensively studied in many cancer types38. Differentiating M1 or M2 phenotypes based upon surface marker expression profiles was not performed here. We identified that CD11b+/Gr1-/F4/80+ cells, consistent with TAMs, are present in both MOC1 and MOC2 generated tumors and represent a majority of the CD11b+/Gr1- cell population. Of note, CD11b+/Gr1-/F4/80+ TAMs were not present in metastatic tumor deposits arising from MOC2 generated tumors, suggesting these cells play a biologic role in primary but not metastatic disease in our model.
A limitation of this study, excluding the Treg depletion studies, is the lack of functional analysis reported for the immune cell types identified. While cell surface markers suggest functional roles for each cell type identified10, including those for Tregs and MDSCs, verification of the roles of each cell type identified with functional analysis is necessary and is a current focus of our laboratory. In addition, our analysis excluded several immune cell types of potential importance including gamma-delta T-lymphocytes, natural killer cells, natural killer T-cells or CD4+ Th17 cells, all shown to play variable roles in human HNSCC biology10.
The few syngeneic HNSCC models currently available have been developed artificially in vitro39,40, lack locally aggressive or metastatic potential39,40 or are of questionable cellular origin11,41. This newly established mouse model parallels carcinogen-associated human HNSCC in mechanism of cellular transformation, intracellular signaling aberrations, aggressive growth and metastatic potential13. Furthermore, the malleability of the C57BL/6 mouse genetic background allows for dissection of other facets of tumor-host interactions. With the use of this syngeneic model, correlations between dysregulated intracellular signaling pathways and abrogated immune signaling and dissection of tumor-immune cell interactions within the tumor microenvironment may allow for the development of therapeutics specifically aimed and modulating both processes.
In summary, we have described the first comparative infiltrating immune cell analysis of two cell lines that display either aggressive or indolent growth phenotypes. We identified increased major histocompatibility complex (MHC) class I expression and CD8+ T-lymphocyte infiltration in tumors generated from the less aggressive cell line. Additionally, we found that a significant portion of CD4+ T-cells infiltrating tumors generated from the more aggressive cell line were immunosuppressive FOXP3+ T-regulatory cells (Tregs), and that antibody-mediated inhibition of these Tregs lead to attenuated growth. Primary tumors generated from both cell lines, as well as regional lymph nodes containing metastatic disease from the more aggressive cell line, demonstrate robust infiltration of Cd11b+Gr1+ cells. These data recapitulate the infiltrating lymphocyte profile observed in human HNSCCs and validate the utility of using this syngeneic oral cancer model to study tumor-host interactions within the tumor and metastatic microenvironment.
Supplementary Material
Comparison of MFI of expression of cell surface class I Db after treatment with IFN-γ for 72 hours on indolent MOC1 (black bar) and aggressive MOC2 (white bar).
Acknowledgments
Grant support: RU was supported by grants from the NCI (K08CA090403), the American Academy of Otolaryngology/Head and Neck Surgery (AAO/HNS) and the Veteran’s Affairs Research Service. NPJ was supported by NIH-T32DC00022 and the AAO/HNS.
References
- 1.Schmitz S, Machiels JP. Molecular biology of squamous cell carcinoma of the head and neck: relevance and therapeutic implications. Expert Rev Anticancer Ther 2010;10:1471–84. [DOI] [PubMed] [Google Scholar]
- 2.Hauswald H, Simon C, Hecht S, et al. Long-term outcome and patterns of failure in patients with advanced head and neck cancer. Radiat Oncol 2011;6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen CT, Lewis JS Jr., El-Mofty SK, et al. Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope 2010;120:1756–72. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001;410:1107–11. [DOI] [PubMed] [Google Scholar]
- 5.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59–67. [DOI] [PubMed] [Google Scholar]
- 6.Snyderman CH, Heo DS, Chen K, et al. T-cell markers in tumor-infiltrating lymphocytes of head and neck cancer. Head Neck 1989;11:331–6. [DOI] [PubMed] [Google Scholar]
- 7.Pretscher D, Distel LV, Grabenbauer GG, et al. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer 2009;9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurlow JK, Pena Murillo CL, Hunter KD, et al. Spectral clustering of microarray data elucidates the roles of microenvironment remodeling and immune responses in survival of head and neck squamous cell carcinoma. J Clin Oncol 2010;28:2881–8. [DOI] [PubMed] [Google Scholar]
- 9.Uppaluri R, Dunn GP, Lewis JS, Jr. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in head and neck cancers. Cancer Immun 2008;8:16. [PMC free article] [PubMed] [Google Scholar]
- 10.Allen C, Judd N, Bui J, et al. The Clinical Implications of Anti-Tumor Immunity in Head and Neck Cancer. Laryngoscope 2011;In Press. [DOI] [PubMed] [Google Scholar]
- 11.Kim S Animal models of cancer in the head and neck region. Clin Exp Otorhinolaryngol 2009;2:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sano D, Myers JN. Xenograft models of head and neck cancers. Head Neck Oncol 2009;1:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Judd NP, Winkler AE, Murillo-Sauca OJ, et al. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer Res 2011;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skarnes WC, Rosen B, West AP, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 2011;474:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bui JD, Uppaluri R, Hsieh CS, et al. Comparative analysis of regulatory and effector T cells in progressively growing versus rejecting tumors of similar origins. Cancer Res 2006;66:7301–9. [DOI] [PubMed] [Google Scholar]
- 16.Bandoh N, Ogino T, Katayama A, et al. HLA class I antigen and transporter associated with antigen processing downregulation in metastatic lesions of head and neck squamous cell carcinoma as a marker of poor prognosis. Oncol Rep 2010;23:933–9. [DOI] [PubMed] [Google Scholar]
- 17.Ogino T, Shigyo H, Ishii H, et al. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res 2006;66:9281–9. [DOI] [PubMed] [Google Scholar]
- 18.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res 2006;12:3890–5. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann C, Strauss L, Wang Y, et al. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clin Cancer Res 2008;14:3706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chikamatsu K, Sakakura K, Whiteside TL, et al. Relationships between regulatory T cells and CD8+ effector populations in patients with squamous cell carcinoma of the head and neck. Head Neck 2007;29:120–7. [DOI] [PubMed] [Google Scholar]
- 21.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol 2011;11:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strauss L, Bergmann C, Gooding W, et al. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 2007;13:6301–11. [DOI] [PubMed] [Google Scholar]
- 23.Strauss L, Bergmann C, Szczepanski M, et al. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res 2007;13:4345–54. [DOI] [PubMed] [Google Scholar]
- 24.Schott AK, Pries R, Wollenberg B. Permanent up-regulation of regulatory T-lymphocytes in patients with head and neck cancer. Int J Mol Med 2010;26:67–75. [DOI] [PubMed] [Google Scholar]
- 25.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res 2006;12:465–72. [DOI] [PubMed] [Google Scholar]
- 26.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A 2007;104:973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chikamatsu K, Takahashi G, Sakakura K, et al. Immunoregulatory properties of CD44+ cancer stem-like cells in squamous cell carcinoma of the head and neck. Head Neck 2011;33:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008;27:5904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pries R, Wollenberg B. Cytokines in head and neck cancer. Cytokine Growth Factor Rev 2006;17:141–6. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka K, Jinhua P, Omura K, et al. Multipotency of CD11bhighGr-1+ immature myeloid cells accumulating in oral squamous cell carcinoma-bearing mice. Oral Oncol 2007;43:586–92. [DOI] [PubMed] [Google Scholar]
- 31.Nagaraj S, Collazo M, Corzo CA, et al. Regulatory myeloid suppressor cells in health and disease. Cancer Res 2009;69:7503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young MR. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head Neck 2006;28:462–70. [DOI] [PubMed] [Google Scholar]
- 33.Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol 1999;21:241–52. [DOI] [PubMed] [Google Scholar]
- 34.Young MR, Wright MA, Lozano Y, et al. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer 1997;74:69–74. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe S, Deguchi K, Zheng R, et al. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol 2008;181:3291–300. [DOI] [PubMed] [Google Scholar]
- 36.Marcus B, Arenberg D, Lee J, et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma. Cancer 2004;101:2779–87. [DOI] [PubMed] [Google Scholar]
- 37.Ritta M, De Andrea M, Mondini M, et al. Cell cycle and viral and immunologic profiles of head and neck squamous cell carcinoma as predictable variables of tumor progression. Head Neck 2009;31:318–27. [DOI] [PubMed] [Google Scholar]
- 38.Sica A, Larghi P, Mancino A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol 2008;18:349–55. [DOI] [PubMed] [Google Scholar]
- 39.Thomas GR, Chen Z, Oechsli MN, et al. Decreased expression of CD80 is a marker for increased tumorigenicity in a new murine model of oral squamous-cell carcinoma. Int J Cancer 1999;82:377–84. [DOI] [PubMed] [Google Scholar]
- 40.Hoover AC, Spanos WC, Harris GF, et al. The role of human papillomavirus 16 E6 in anchorage-independent and invasive growth of mouse tonsil epithelium. Arch Otolaryngol Head Neck Surg 2007;133:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Malley BW Jr., Cope KA, Johnson CS, et al. A new immunocompetent murine model for oral cancer. Arch Otolaryngol Head Neck Surg 1997;123:20–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of MFI of expression of cell surface class I Db after treatment with IFN-γ for 72 hours on indolent MOC1 (black bar) and aggressive MOC2 (white bar).