Abstract
We report biomolecular evidence of dolphin morbillivirus in 4 wild Eurasian otters (Lutra lutra) from southern Italy; 2 animals showed simultaneous immunohistochemical reactivity against morbilliviral antigen. These cases add further concern and support to the progressively expanding host range of dolphin morbillivirus in the western Mediterranean Sea.
Keywords: dolphin morbillivirus, Morbillivirus, Eurasian otter, Lutra lutra, infection, host range, Mediterranean, Italy, viruses
The genus Morbillivirus comprises several lympho-epithelio-neurotropic, highly pathogenic RNA viruses of domestic and wild vertebrates, including aquatic mammals. Among them, cetacean morbillivirus (CeMV) has been responsible since the 1980s for dramatic epidemics in free-ranging cetaceans worldwide (1). Specifically, the CeMV strain termed dolphin morbillivirus (DMV) has caused at least 4 unusual mortality events (UMEs) among striped dolphins (Stenella coeruleoalba) in the western Mediterranean Sea and, to a lesser extent, long-finned pilot whales (Globicephala melas) and other wild cetaceans from the same region (1–3).
We report evidence of DMV infection in 4 wild Eurasian otters (Lutra lutra) from southern Italy (Apulia and Basilicata regions). The animals, all adult females, belonged to a group of 7 individuals found dead at Parco Nazionale del Cilento, a large national park that extends to the coastline of southwestern Italy. The animals underwent necropsy at Istituto Zooprofilattico Sperimentale della Puglia e della Basilicata (Foggia, Italy) during 2016 and 2017, according to an official agreement between the institute and the park aimed at assessing the health and conservation status of the otter population.
Within a multidisciplinary approach framework, we conducted in-depth histopathologic, microbiologic, parasitologic, and ecotoxicologic analyses on the 7 otters, along with biomolecular (reverse transcription PCR [RT-PCR]) and immunohistochemical (IHC) investigations for Morbillivirus spp. After using a technique amplifying a highly conserved fragment of the Morbillivirus nucleoprotein (NP) gene (4), we applied 2 additional methods aimed at detecting DMV-specific hemagglutinin (HA) (5) and NP gene sequences (6) for more detailed analysis. To increase the biomolecular results’ reliability, we performed all the extraction, amplification, and sequencing steps in 3 different laboratories. We also conducted the histopathological and IHC analyses in 3 different laboratories. For IHC analysis, we used a commercial monoclonal antibody (VMRD, https://www.vmrd.com) against the NP antigen of canine distemper virus (CDV), including adequate morbillivirus-positive and -negative control tissues in each run.
At necropsy, we found multiple traumatic injuries, probably caused by motor vehicles and deemed the putative cause of death, in all 7 otters. Microscopically, we observed a bilateral, subacute-to-chronic, broncho- or bronchiolo-interstitial pneumonia, showing endobronchial, endobronchiolar, or endoalveolar macrophage infiltration, thickening of alveolar septa, and lympho-histiocytic septal infiltration, in 2 otters; 1 also showed a multifocal, portal and lobular, nonsuppurative hepatitis. Lungs and kidneys from these 2 animals were molecularly positive for DMV (Figure, panel A). Their pulmonary tissue showed positive morbilliviral antigen immunostaining in vascular walls and endothelial cells, as well as in alveolar epithelial cells (consistent, or not, with hyperplastic type II pneumocytes); we also found positive immunostaining in thickened alveolar septa and endoalveolar macrophages (Figure, panel B). Although the advanced postmortem autolysis did not enable us to obtain reliable histopathological information from the 5 remaining otters, biomolecular analyses yielded positive results from several tissues (submandibular lymph node, parotidal gland, lung, brain, heart, kidney, urinary bladder, and liver) in 2 of them (Figure, panel A).
Figure.
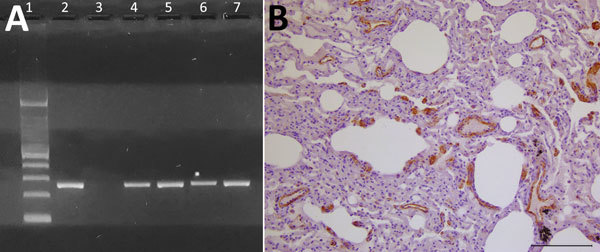
Evidence of dolphin morbillivirus infection in Eurasian otters (Lutra lutra), southwestern Italy. A) Comparison of nucleoprotein gene amplification products from infected otters, obtained by reverse transcription PCR. A specific band at the expected molecular weight of 287 bp is shown. Lane 1, molecular weight marker (Tracklt 100bp DNA Ladder; Invitrogen, http://www.thermofisher.com); lane 2, positive control (lung tissue from an infected striped dolphin, Stenella coeruleoalba); lane 3, negative control (distilled water); lanes 4–7, samples from morbillivirus-positive Eurasian otters: LL-290, lung (lane 4); LL-291, kidney (lane 5); LL-3380, lung (lane 6); LL-7318, lung (lane 7). B) Mayer’s hematoxylin counterstain of lung tissue shows marked and widespread immunohistochemical labeling for morbillivirus antigen (dark areas), particularly evident at the level of vascular walls and endothelial cells and, to a lesser extent, of alveolar epithelial cells (morphologically consistent, or not, with hyperplastic type II pneumocytes) as well as of thickened alveolar septa. Scale bar indicates 100 μm.
Sequencing of all the 200-bp NP gene viral amplicons obtained (GenBank accession nos. MG836265–8) showed remarkable differences from the available CDV sequences but high homology (99%) with 2 DMV sequences (GenBank accession nos. EF469546.1, KU720625.1). We compared an additional 456-bp HA gene fragment (GenBank provisional accession no. MG905831), obtained from the kidney of 1 of the otters we tested, with 2 DMV isolates recovered from Mediterranean striped dolphins in 2007; this fragment displayed 100% homology with 1 DMV isolate (GenBank accession no. HQ829973) and 99.56% homology with the other (accession no. AJ608288).
Susceptibility to morbilliviruses is not new to L. lutra otters; CDV infection has been documented, with subsequent risk for viral spillover to coastal pinnipeds (7). In this respect, the endemic behavior and the progressively expanding host range of DMV in the western Mediterranean Sea are a matter of concern (2,3,8), as exemplified by a peculiar case of infection in a captive harbor seal (Phoca vitulina), a species with a mixed marine–terrestrial ecology (9).
How the otters in this study may have acquired DMV infection is unknown. Of note, 8 cases of DMV infection were detected during 2016 and 2017 in dolphins stranded along the Ionian Sea coast, in an area geographically consistent with that in which the 4 DMV-infected otters were found. It is possible that >1 dolphins with DMV-associated brain lesions could have entered the rivers or lagoons inhabited by the otters and transmitted the virus to them. Alternatively, the otters’ movement toward and placement close to the sea, clearly documented for 1 of them (A. Petrella, unpub. data), could underlie a subsequent encounter with DMV-infected dolphins. As an additional or complementary option, susceptible invertebrate hosts might have acted as DMV reservoirs, similarly to what has been reported for Baicalia carinata and Limnaea auricularia mollusks, which probably were a source of infection during the CDV epidemic among Baikal seals (Pusa sibirica) in 1987 (10).
In conclusion, we identified DMV infection among Eurasian otters in southwestern Italy, along the coast of the western Mediterranean Sea. The effect of DMV infection on the health and conservation of the threatened Eurasian otter populations warrants further investigation.
Acknowledgments
We thank Antonio Lo Polito, Maria Luigia Prencipe, and Fabio Gisoldo for their valuable technical support. We also thank Gabriella Di Francesco, Alessio Lorusso, Daniela Malatesta, and Guendalina Zaccaria for their valuable support in performing histopathological, immunohistochemical, and biomolecular analyses as well as for their greatly appreciated contribution to fruitful and insightful discussions on the data reported in the manuscript.
Biography
Dr. Padalino works at the Virology Department of Istituto Zooprofilattico Sperimentale della Puglia e della Basilicata, Foggia, Italy, where she is in charge of the Molecular Biology and Cell Culture Laboratory. Her research is mainly focused on the molecular diagnosis and epidemiologic surveillance of viral infectious diseases in domestic and wild animals.
Footnotes
Suggested citation for this article: Padalino I, Di Guardo G, Carbone A, Troiano P, Parisi A, Galante D, et al. Dolphin morbillivirus in Eurasian otters, Italy. Emerg Infect Dis. 2019 Feb [date cited]. https://doi.org/10.3201/eid2502.180256
References
- 1.Van Bressem MF, Duignan PJ, Banyard A, Barbieri M, Colegrove KM, De Guise S, et al. Cetacean morbillivirus: current knowledge and future directions. Viruses. 2014;6:5145–81. 10.3390/v6125145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzariol S, Centelleghe C, Beffagna G, Povinelli M, Terracciano G, Cocumelli C, et al. Mediterranean fin whales (Balaenoptera physalus) threatened by dolphin morbillivirus. Emerg Infect Dis. 2016;22:302–5. 10.3201/eid2202.150882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzariol S, Centelleghe C, Di Provvido A, Di Renzo L, Cardeti G, Cersini A, et al. Dolphin morbillivirus associated with a mass stranding of sperm whales, Italy. Emerg Infect Dis. 2017;23:144–6. 10.3201/eid2301.160239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frisk AL, König M, Moritz A, Baumgärtner W. Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. J Clin Microbiol. 1999;37:3634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centelleghe C, Beffagna G, Zanetti R, Zappulli V, Di Guardo G, Mazzariol S. Molecular analysis of dolphin morbillivirus: A new sensitive detection method based on nested RT-PCR. J Virol Methods. 2016;235:85–91. 10.1016/j.jviromet.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 6.Verna F, Giorda F, Miceli I, Rizzo G, Pautasso A, Romano A, et al. Detection of morbillivirus infection by RT-PCR RFLP analysis in cetaceans and carnivores. J Virol Methods. 2017;247:22–7. 10.1016/j.jviromet.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 7.Mos L, Ross PS, McIntosh D, Raverty S. Canine distemper virus in river otters in British Columbia as an emergent risk for coastal pinnipeds. Vet Rec. 2003;152:237–9. 10.1136/vr.152.8.237 [DOI] [PubMed] [Google Scholar]
- 8.Jo WK, Osterhaus AD, Ludlow M. Transmission of morbilliviruses within and among marine mammal species. Curr Opin Virol. 2018;28:133–41. 10.1016/j.coviro.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 9.Mazzariol S, Peletto S, Mondin A, Centelleghe C, Di Guardo G, Di Francesco CE, et al. Dolphin morbillivirus infection in a captive harbor seal (Phoca vitulina). J Clin Microbiol. 2013;51:708–11. 10.1128/JCM.02710-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondratov IG, Denikina NN, Belikov SI, Durymanova AA, Ustinova EN, Shestopalov AM. Mollusks as a natural reservoir of morbilliviruses. Dokl Biol Sci. 2003;389:154–6. 10.1023/A:1023435312334 [DOI] [PubMed] [Google Scholar]


